Abstract
Salmonella is a major cause of food-borne disease in many countries. Serotype determination of Salmonella is important for disease assessment, infection control, and epidemiological surveillance. In this study, a microarray system that targets the O antigen-specific genes was developed for simultaneously detecting and identifying all 46 Salmonella O serogroups. Of these, 40 serogroups can be confidently identified, and the remaining 6, in three pairs (serogroups O67 and B, E1 and E4, and A and D1), need to be further distinguished from each other using PCR methods or conventional serotyping methods. The microarray was shown to be highly specific when evaluated against 293 Salmonella strains, 186 Shigella strains, representative Escherichia coli strains, and 10 strains of other bacterial species. The assay correctly identified 288 (98%) of the Salmonella strains. The detection sensitivity was determined to be 50 ng genomic DNA per sample. By testing simulated samples in a tomato background, 2 to 8 CFU per gram inoculated could be detected after enrichment. This newly developed microarray assay is the first molecular protocol that can be used for the comprehensive detection and identification of all 46 Salmonella O serogroups. Compared to the traditional serogrouping method, the microarray provides a reliable, high-throughput, and sensitive approach that can be used for rapid identification of multiple Salmonella O serogroups simultaneously.
INTRODUCTION
Salmonella is a facultative anaerobic Gram-negative bacterium belonging to the family Enterobacteriaceae (14). The genus Salmonella is composed of two species, Salmonella enterica and Salmonella bongori, seven subgroups, and more than 2,600 defined serotypes (29). Salmonella is widely distributed in nature, and it is transmitted via contaminated food, including meat, poultry, eggs, dairy products, and fresh produce, such as tomato and lettuce, thereby gaining entry into almost every aspect of the human food chain (2, 15, 24). It is recognized as the leading cause of food-borne outbreaks and infections in many countries, causing human illnesses such as typhoid fever, paratyphoid fever, and other salmonelloses (10, 25, 35). From May to November 2010, a multistate outbreak of human infection across the United States was attributed to the consumption of eggs contaminated with Salmonella enterica serovar Enteritidis, which resulted in 1,939 reported illnesses (30). It is estimated that there are 1.3 million cases of salmonellosis, 15,000 hospitalizations, and 400 deaths annually in the United States (9).
Salmonella isolates are serotyped according to the White-Kauffmann-Le Minor scheme on the basis of surface antigen identification of O (somatic) and H (flagellar) antigenic epitopes, permitting the characterization of 46 O serogroups and 119 H antigens and, thereby, more than 2,500 different serotypes. The White-Kauffmann-Le Minor scheme is utilized by public health organizations worldwide and is considered the gold standard for the determination of Salmonella serotypes (29, 32). The O antigen is the outermost component of the lipopolysaccharide (LPS), and it is extremely polymorphic due to variations in the types of sugars present, arrangements, and linkages, and it contributes major antigenic diversity to the cell surface. The genes required for O antigen biosynthesis are organized mainly in a large regulon on the chromosome, which is located between the galF and gnd genes (39). The O antigen variation among the 46 Salmonella O serogroups is mainly due to the extensive genetic diversity present within their respective O antigen gene clusters. Typically, three different types of genes are present within O antigen gene clusters: (i) nucleotide sugar synthesis genes that encode enzymes involved in the synthesis of the sugars that form the O subunit; (ii) sugar transferase genes that assemble the sugar substituents into the O subunit; and (iii) O unit-processing genes that encode proteins involved in the processing and assembly steps to build the O antigen from the O subunit, such as the O antigen flippase (wzx) and O antigen polymerase (wzy) (7). While the sequences of sugar synthesis genes within the O antigen gene cluster are relatively conserved, the processing genes wzx and wzy are highly variable and are considered to be specific to each O serogroup (18).
Both the virulence and host range of Salmonella isolates are serotype specific (5). Therefore, the rapid and accurate determination of Salmonella serotype is essential for human disease surveillance and outbreak control of this important food-borne pathogen (38). The highly variable nature of the O antigen provides the basis for serotyping. Despite its widespread utility, the traditional serotyping method has deficiencies. The method is carried out by assessing agglutination reactions using antisera raised against the O-standard reference strains. It requires well-trained technicians and high-quality antisera, both of which may be difficult to obtain consistently and are very costly in resource-limited settings (19). Furthermore, it is time-consuming and tedious, as well as subjective in interpretation due to cross-reactivity. In recent years, molecular typing methods that target the O antigen-specific genes have been developed, and these have become an attractive alternative to traditional approaches for O serogroup determination (3, 7, 22, 37). Fitzgerald et al. developed an O group-specific Bio-Plex array to detect six serogroups of Salmonella in the United States (6). However, these methods only target a very small subset of the Salmonella serogroups, which are inadequate for comprehensive and reliable detection of Salmonella. DNA microarrays allow a large number of specific DNA sequences to be detected simultaneously, and it has been shown to be useful as a rapid and sensitive pathogen detection strategy that overcomes the disadvantages of conventional PCR and multiplex PCR methods.
In this study, a DNA microarray assay was developed to identify and detect all 46 Salmonella O serogroups. PCR primers and probes were designed based on the specific DNA markers of the O antigen gene clusters, which were retrieved either from the GenBank database or from our in-house databases. The specificity and sensitivity assessments using pure cultures and simulated food samples demonstrated that this new DNA microarray assay is specific and reliable, and it could serve as an effective alternative to traditional serotyping approaches.
MATERIALS AND METHODS
Bacterial strains examined and genomic DNA extraction.
The bacterial strains used in this study, including 290 Salmonella strains, 186 representative strains of all Shigella and Escherichia coli serotypes, and 10 strains from other bacterial species, are listed in Table 1. All strains were inoculated into Luria-Bertani (LB) medium and incubated overnight at 37°C. Genomic DNA was prepared using a phenol-chloroform method as described previously (1).
Table 1.
Salmonella strains and other bacterial species used in this study
| Genus/species and serogroup | Serotype | No. of strains from each source | Total no. |
|---|---|---|---|
| Salmonella (n = 293) | |||
| A(O2) | Paratyphi A, Nitra, Kiel, Koessen | 3,a 2b | 5 |
| B(O4) | Hessarek, Drogana, Essen, Kingston, II, Derby, Hato, Agona, Chester, Stanley, Saintpaul, Huettwilen, Banana, Coeln, Albert, Ball, Haduna, Clackamas, Brezany, Kiambu, Indiana | 35,a 2b | 37 |
| C1(O6,7) | Choleraesuis, IIIb, Sanjuan, Montevideo, II, Livingstone, Oranienburg, Birkenhead, VI | 12a | 12 |
| C2(O8) | Muenchen, Glostrup, Newport, Tallahassee, Bovismorbificans, Litchfield, Kottbus, Takoradi | 8,a 2b | 10 |
| D1(O9) | Dublin, II | 2,a 2b | 4 |
| D2(O9,46) | Strasbourg, Marylebone, Bergedorf | 5a | 5 |
| D3(O9,46,27) | II | 5a | 5 |
| E1(O3,10) | Anatum, Westhampton, II | 4a | 4 |
| E4(O1,3,19) | Senftenberg | 1a | 1 |
| F(O11) | II, VI, IIIb, IIIa, Marseille, Gallen, Luciana, Aberdeen | 11,a 5b | 16 |
| G(O13) | Durham, Raus, II, IIIa, IIIb, Havana, Poona | 10a | 10 |
| H(O6,14) | Carrau, II, IIIb, Soahanina | 5a | 5 |
| I(O16) | Hannover, Brazil, Hvittingfoss, II, IV | 5a | 5 |
| J(O17) | Jangwani, IIIb, IV, IIIa, Bonames | 8a | 8 |
| K(O18) | Cerro, IIIa, IIIb, II, Blukwa, IV | 5,a 3b | 8 |
| L(O21) | Assen, Ghana, II, IIIa, IIIb | 6a | 6 |
| M(O28) | Dakar, II, IIIb, Brisbane, Solna | 6a | 6 |
| N(O30) | Urbana, Sternschanze, Overvecht, II | 4a | 4 |
| O(O35) | Adelaide, Umhlatazana, Monschaui, II | 6a | 6 |
| P(O38) | Lansing, IV, IIIa, IIIb | 5a | 5 |
| Q(O39) | Champaign, II, Wandsworth | 3a | 3 |
| R(O40) | Karamoja, Shikmonah, II, IIIa, IIIb, IV, V | 8a | 8 |
| S(O41) | Burundi, Waycross, II, IV, VI | 11a | 11 |
| T(O42) | Faji, Ursenbach, II, IIIa | 7a | 7 |
| U(O43) | Berkeley, II, IIIb, IV | 5a | 5 |
| V(O44) | Niakhar, Niarembe, Tiergarten, II, IIIa, V | 8a | 8 |
| W(O45) | Dugbe, Meekatharra, Karachi, IIIa, VI, II | 8a | 8 |
| X(O47) | II, Bootle, Sya | 4a | 4 |
| Y(O48) | II, IIIa, IIIb, IV, V, VI, Buckeye, Dahlem | 13a | 13 |
| Z(O50) | II, IIIa, IIIb, IV, VI | 13a | 13 |
| O51 | Dan, II, IIIa, IIIb, Karaya | 7a | 7 |
| O52 | Utrecht, Ord, II | 4a | 4 |
| O53 | II, IIIa, Leda | 6a | 6 |
| O54 | Uccle | 1a | 1 |
| O55 | II | 2a | 2 |
| O56 | II | 1a | 1 |
| O57 | II, IIIb, Antonio, Maryland | 6a | 6 |
| O58 | II | 1a | 1 |
| O59 | II, IIIa, IIIb | 4a | 4 |
| O60 | II, IIIb | 4a | 4 |
| O61 | II, IIIb | 3a | 3 |
| O62 | IIIa | 2a | 2 |
| O63 | IIIa | 2a | 2 |
| O65 | IIIb | 1a | 1 |
| O66 | V | 3a | 3 |
| O67 | Crossness | 1a | 1 |
| Other (n = 196) | |||
| Shigella and E. coli | 165,a 20,c 1f | 186 | |
| Pseudomonas aeruginosa | 1d | 1 | |
| Staphylococcus aureus | 1e | 1 | |
| Klebsiella pneumoniae | 1e | 1 | |
| Yersinia enterocolitica | 1a | 1 | |
| Enterococcus faecalis | 1d | 1 | |
| Enterococcus faecium | 1f | 1 | |
| Enterococcus cloacae | 1d | 1 | |
| Vibrio parahaemolyticus | 1b | 1 | |
| Vibrio cholerae | 1a | 1 | |
| Citrobacter freundii | 1g | 1 |
Institute of Medical and Veterinary Science, Adelaide, Australia.
Center for Disease Control and Prevention, Shanghai, China.
Federal Institute for Risk Assessment, Berlin, Germany.
Institute of Microbiology, Chinese Academy of Sciences, China.
National Center for Medical Culture Collection, China.
Chinese Center for Disease Control and Prevention.
Tianjin Entry-Exit Inspection and Quarantine Bureau.
Oligonucleotide primers and probes.
The oligonucleotide primers and probes used in this study are listed in Table S1 in the supplemental material, and these were designed based on the GenBank sequences and our in-house database information of O antigen gene clusters for all 46 Salmonella O serogroups (B. Liu, Y. A. Knirel, L. Feng, A. V. Perepelov, S. N. Senchenkova, P. R. Reeves, and L. Wang, unpublished data). Two primers and three to four probes were designed against the targeted genes using Primer Premier 5.0 (Premier Boost International, Palo Alto, CA) and OligoArray 2.0 software, respectively (31). The primer pair used to amplify 16S rRNA genes has been reported previously (17). A probe based on the region of 16S rRNA that is conserved for all bacteria was used as the positive control. To enhance hybridization yield, each probe was 5′-amino modified and contained 15 poly(T) oligonucleotides at the 5′ end. A probe containing 40 poly(T) oligonucleotides was used as the negative control, and a 3′-Cy3-labeled probe was used as the positional reference and printing control.
Multiplex PCR.
Multiplex PCR, used to amplify the target genes, was carried out in five groups (see Table S1 in the supplemental material). Each multiplex PCR was performed in a 30-μl reaction mixture containing 100 ng of DNA; 1× PCR buffer (50 mM KCl, 10 mM Tris-HCl [pH 8.3]); 2.5 mM MgCl2; 0.16 mM (each) dATP, dCTP, dGTP, and dTTP; 0.05 to 0.2 μM respective primers based on each of targeted genes; 0.06 μM (each) two primers based on the 16S rRNA gene; and 2.5 U Taq DNA polymerase (TaKaRa Biotechnology, Dalian, China). The PCR was performed with an initial denaturation step of 95°C for 5 min, followed by 30 cycles at 95°C of 30 s, 55°C for 45 s, and 72°C for 1 min, with a final elongation step of 72°C for a 5-min elongation step. PCR products were purified using the Microcon centrifugal filter devices kit (Millipore Corporation, MA). Two microliters of each PCR product was run on an agarose gel to confirm the presence of appropriate amplicons (Fig. 1).
Fig 1.
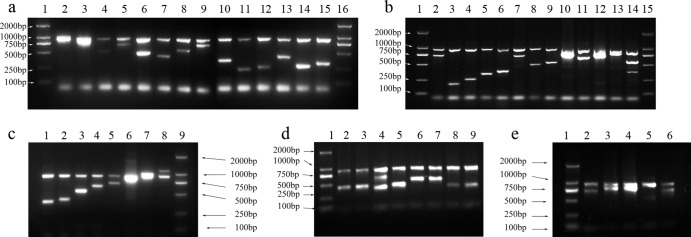
Agarose gel electrophoresis of multiplex PCR products for all 46 Salmonella serogroups. (a) Group A. Lanes 1 and 16, DL2000 DNA markers; lanes 2 to 15, DNA templates from Salmonella serogroups F, G, H, I, J, K, L, M, S, R, Q, P, O, and N, respectively. (b) Group B. Lanes 1 and 15, DL2000 DNA markers; lanes 2 to 14, DNA templates from Salmonella serogroups O57, O55, O54, O53, O52, O51, Z, Y, X, W, V, U, and T, respectively. (c) Group C. Lane 9, DL2000 DNA marker; lanes 1 to 8, DNA templates from Salmonella serogroups O66, O65, O63, O62, O61, O60, O59, and O58, respectively. (d) Group D. Lane 1, DL2000 DNA marker; lanes 2 to 9, DNA templates from Salmonella serogroups A, D1, D3, D2, B, O67, E1, and E4. (e) Group E. Lane 1, DL2000 DNA marker; lanes 2 to 6, the DNA templates from Salmonella serogroups C1, E1, E4, O56, and C2.
Target DNA labeling.
PCR products were labeled using the PCR conditions described above, except that only reverse primers were used, and 0.3 μl of 25 nM Cy3-dUTP (Amersham Biosciences UK Ltd., Little Chalfont, England) was included. Five microliters of purified amplification product generated from the multiplex PCR described above was added as the template. Thermal cycling conditions were the same as those for the multiplex PCR described above. All labeled DNA was purified using the Microcon centrifugal filter device kit and then stored at −20°C in the dark.
Oligonucleotide probe sets and microarray construction.
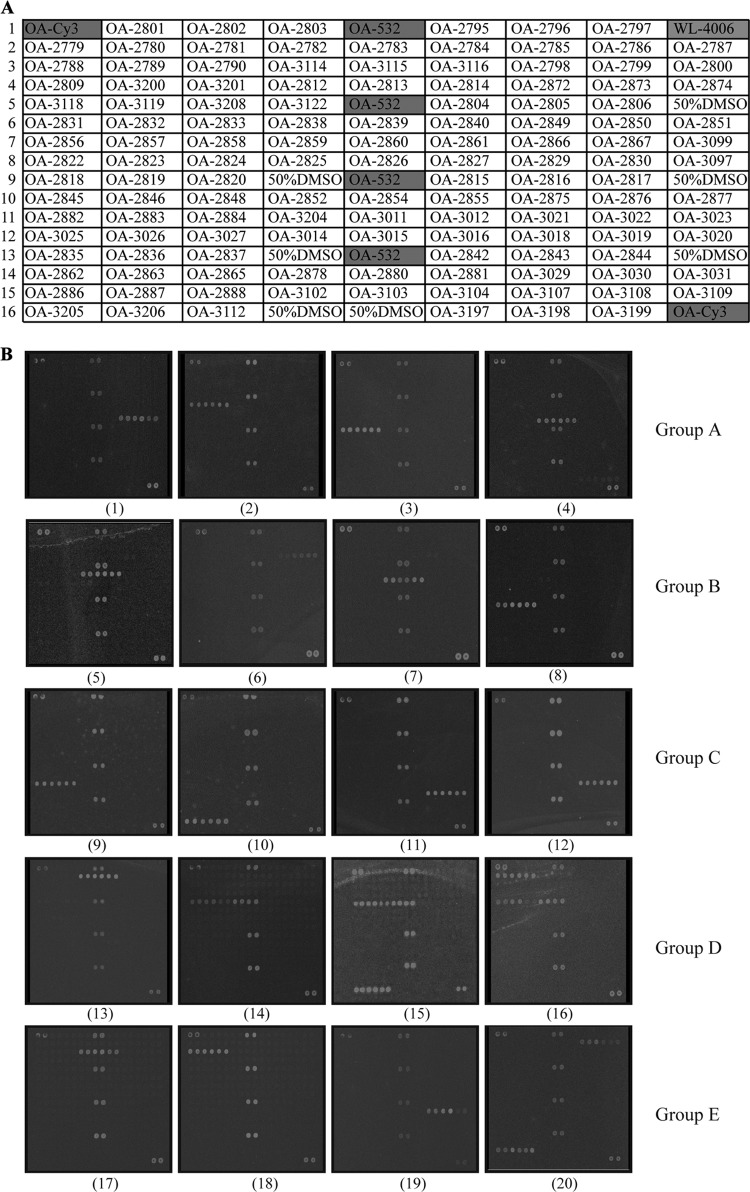
Probes were dissolved in 50% dimethyl sulfoxide (DMSO) to a final concentration of 1 μg/μl and then spotted onto aldehyde group-modified glass slides (CapitalBio Corporation, Beijing, China) using a SpotArray 72 instrument (Perkin-Elmer Corporation, CA). Each probe was spotted in duplicate to eliminate irregular data due to physical defects on the glass slides. Printed slides were dried for 24 h at room temperature in the dark and scanned at 532 nm to assess and confirm spotting quality. A schematic diagram of the respective probe positions is shown in Fig. 2A.
Fig 2.
(A) Probe positions on the microarray slide. OA-532 is the positive-control probe based on the bacterial 16S rRNA gene. WL-4006 is the negative-control probe. OA-Cy3 is the positional reference and printing control probe. The rest of the probes are specific probes for the target strains. (B) Microarray hybridization patterns for representative Salmonella serogroup strains. Pattern 1, Salmonella serogroup P; 2, Salmonella serogroup S; 3, Salmonella serogroup M; 4, Salmonella serogroup O; 5, Salmonella serogroup U; 6, Salmonella serogroup O55; 7, Salmonella serogroup O51; 8, Salmonella serogroup W; 9, Salmonella serogroup O58; 10, Salmonella serogroup O59; 11, Salmonella serogroup 62; 12, Salmonella serogroup O63; 13, Salmonella serogroup B; 14, Salmonella serogroup A; 15, Salmonella serogroup D2; 16, Salmonella serogroup D3; 17, Salmonella serogroup C1; 18, Salmonella serogroup C2; 19, Salmonella serogroup O56; and 20, Salmonella serogroup E1.
DNA microarray hybridization.
Eight microliters of the labeled target DNA was mixed with an equal volume of preheated (50°C) hybridization buffer (25% formamide, 0.1% sodium dodecyl sulfate, 6× SSPE [1× SSPE consists of 0.18 M NaCl, 10 mM NaH2PO4, and 1 mM EDTA, pH 7.7]). The mixture was applied to a hybridization chamber and incubated at 50°C for 1.5 h in a water bath. After hybridization, the slide was washed with solution A (1× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate] and 0.1% SDS) for 3 min, followed by a wash with 0.05× SSC for 3 min and a final wash with 95% ethanol for 1.5 min. The slide was dried under a gentle airstream prior to scanning.
Simulated food samples.
Fresh tomatoes were purchased from a local market, fully cleaned, and homogenized by a sterile blender. Bacterial cultures were serially diluted and quantified by the plate-counting method. Aliquots (100 μl) of each dilution were used to seed 25 g of tomato homogenate in 225 ml buffered peptone water (BPW). A total of 10 Salmonella serogroup strains were selected randomly and mixed with tomato samples, including serogroups E1, C1, B, D3, O61, O65′O52, O53, F, and M. The spiked tomato samples were incubated at 37°C overnight with shaking. After the enrichment, DNA was extracted from each sample (1 ml) and tested on the DNA microarray.
Fluorescence scanning and data analysis.
The hybridized microarray was scanned using a GenePix personal 4100A microarray scanner (Axon Instruments, CA) with a laser beam at 532 nm, the photomultiplier tube (PMT) gain set to 650, and a pixel size of 5 μm. Two files were generated with GenePix Pro 6.0, one with the images saved as a TIF file and the other, for signal intensities, was saved as a GPR file. The signal-to-noise ratio was calculated for each spot using Bactarray Analyzer 1.0, developed in our laboratory, with the signal-to-noise ratio threshold set at 3.0. A serotype was confirmed and reported when the positive-control probes, the negative-control probes, and the printing control probes generated were correct and all of the probes of the given serogroup generated positive signals above the threshold.
RESULTS
Identification of target genes.
The O antigen gene clusters of all 46 Salmonella serogroups were retrieved from GenBank and our in-house databases. Multiple-sequence alignments of O antigen genes were carried out to select specific genes for different O serogroups. The highly divergent O-unit-processing genes wzx and wzy were selected as target genes for all of the Salmonella O serogroups, with the exception of serogroup O54 and serogroups A and D1, for which the glycosyltransferase gene wbbE and the O antigen synthesis gene prt (CDP-paratose synthase) were selected, respectively.
Primer screening.
Specific primers were designed based on the selected target genes for each serogroup. These primers were screened against the representative strains from each of the 46 Salmonella O serogroups with the capacity to generate single PCR products of the anticipated sizes of the corresponding strains, while negative results were generated consistently from the strains belonging to other serogroups or other species. The identifications based on PCR products were validated with all the serogroups except for three pairs of serogroups, A/D1, B/O67, and E1/E4. Each of these three pairs generated the same PCR product from the same primers due to the high levels of similarities between their O antigen gene clusters, and a further serological test was needed for their differentiation. Nonspecific PCR products were detected from the serogroups D2 and D3 with the primer pair of serogroups A/D1 and also from the serogroups E1/E4 with the primer pair of serogroup D2 (Table 2). The serogroup O54 strain used in our study was subtyped as serotype Uccle (O3, 54) and produced positive results with both O54 and E1/E4 primers. Since the serogroups D2, D3, E1/E4, and O54 have their own serogroup-specific probes, these serogroups could still be correctly identified. Therefore, 43 specific primer pairs were selected from the 81 primer pairs initially designed for the multiplex PCR amplification and labeling (see Table S1 in the supplemental material).
Table 2.
Molecular characterization of six closely related serogroups
| Serogroup | Positive probes by microarray |
Serogroup by microarray determination | |
|---|---|---|---|
| Serogroup specific | Nonspecific | ||
| A | OA-3118, OA-3119, OA-3208, OA-3122 | A or D1 | |
| D1 | OA-3118, OA-3119, OA-3208, OA-3122 | A or D1 | |
| D2 | OA-3205, OA-3206, OA-3112 | OA-3118, OA-3119, OA-3208, OA-3122 | D2 |
| D3 | OA-2779, OA-2780, OA-2781 | OA-3118, OA-3119, OA-3208, OA-3122 | D3 |
| E1 | OA-2785, OA-2786, OA-2787 | OA-3205, OA-3206, OA-3112 | E1 or E4 |
| E4 | OA-2785, OA-2786, OA-2787 | OA-3205, OA-3206, OA-3112 | E1 or E4 |
Multiplex PCR to amplify the target genes.
Multiplex PCR was used to amplify and label the target genes in five groups. Group A targeted serogroups F, G, H, I, J, K, L, M, N, O, P, Q, R, and S; group B targeted serogroups T42, U43, V44, W45, X47, Y48, Z50, O51, O52, O53, O54, 055, and O57; group C targeted serogroups O58, O59, O60, O61, O62, O63, O65, and O66; group D targeted serogroups B/O67, A/D1, D2, and D3; and group E targeted serogroups C1, C2, O56, and E1/E4. We initially used the same concentration of 0.2 μM for all primers; however, several serogroups failed to generate expected hybridization signals under these conditions, which was probably due to the competition or interference between different primer pairs in the multiplex PCR. Therefore, the primer concentrations were adjusted in the range of 0.03 to 0.58 μM, and these were evaluated as the optimized amplification conditions (see Table S1 in the supplemental material). For each target strain, at least two bands were generated by the multiplex PCR, one for the 16S rRNA primer pair and the other for the O serogroup-specific primer pair. PCR products ranging in size from 205 to 1,132 bp were amplified (Fig. 1).
Specificity of the probes.
The DNA microarray was tested against 148 Salmonella strains representing all 46 O serogroups, 186 Shigella and E. coli strains, and 10 strains from other bacterial species (Table 1). From the 183 oligonucleotide probes initially designed, 133 probes were selected for use, including 130 specific probes, one positive-control probe, one negative-control probe, and one printing and reference control probe (see Table S1 in the supplemental material). All representative Salmonella strains tested against the probes on the microarray hybridized consistently to their corresponding probes and could be successfully differentiated into the correct serogroups. The isolates belonging to Shigella and E. coli and the other bacterial species reacted only to the 16S rRNA gene probe, and none of them bound to the serogroup-specific probes on the microarray. The microarray hybridization patterns observed for 20 representative Salmonella O serogroups are shown in Fig. 2B.
Sensitivity of detection with DNA.
To determine the sensitivity of the assay, serial dilutions of genomic DNA from strains representing each of the 46 serogroups were prepared in volumes of 1, 10, and 50 to 100 ng and used as the templates for multiplex PCR. The positive signals were obtained with DNA levels of 10 ng or above, while the negative results or very weak fluorescence signals were detected at lower DNA amounts. However, 50 ng DNA provided consistent identification results; thus, this amount was chosen as the base amount for the microarray. At this concentration, all 46 Salmonella serogroups could be detected accurately. Therefore, the sensitivity of the assay was determined as 50 ng DNA.
Blind test.
The specificity and sensitivity of the designed microarray system was further tested using a double-blind approach. A total of 142 strains were randomly selected (Table 1) and used to hybridize to the microarrays without any prior knowledge of their identity, and agglutination tests with specific antisera were performed separately. All of the detection results from the microarrays were consistent with those obtained by the conventional serotyping methods with five exceptions, three for serogroup O28 and two for serogroup O59 (Table 3). To evaluate the simultaneous detection efficacy of multiple strains, mixed cultures containing two Salmonella O serogroups strains (i.e., serogroups G and O50, Q and O57, O61 and O66, D1 and D3, or C1 and C2) were prepared and tested with the microarray. All of the samples gave the correct results. The consistency of the double-blinded test with the conventional serotyping method was 96.48%.
Table 3.
Properties of Salmonella serogroups O28 and O59
| Serogroup | Serotype | Antigenic formula | Microarray determination |
|---|---|---|---|
| O28 | Dakar | 28:a:1,6 | Positive |
| O28 | Dakar | 28:a:1,6 | Positive |
| O28 | IIIb | 28:z10:z | Positive |
| O28 | Brisbane | 28:z;e,n,z15 | Negative |
| O28 | Solna | 28:a:1,5 | Negative |
| O28 | II | 28:a:e,n,x | Negative |
| O59 | II | 59:k:(z) | Positive |
| O59 | II | 59:k:z65 | Positive |
| O59 | IIIa | 59:z4,z23:- | Negative |
| O59 | IIIb | 59:c:e,n,x,z15 | Negative |
Test of the simulated food samples.
Artificially contaminated tomato samples were evaluated using the microarray. A total of 10 Salmonella O serogroup strains, including serogroups E1, C1, B, D3, O61, O65′O52, O53, F, and M, were cultured and mixed with tomato samples. After overnight enrichment, DNA was extracted from the samples and tested using the microarray assay. As few as 2 to 8 CFU (initial inoculum) of Salmonella was detected in 1 g tomato sample, and all serotypes inoculated were successfully identified. The unspiked tomato sample failed to give a positive signal. Representative hybridization patterns using DNA extracted from tomato samples are shown in Fig. 3.
Fig 3.
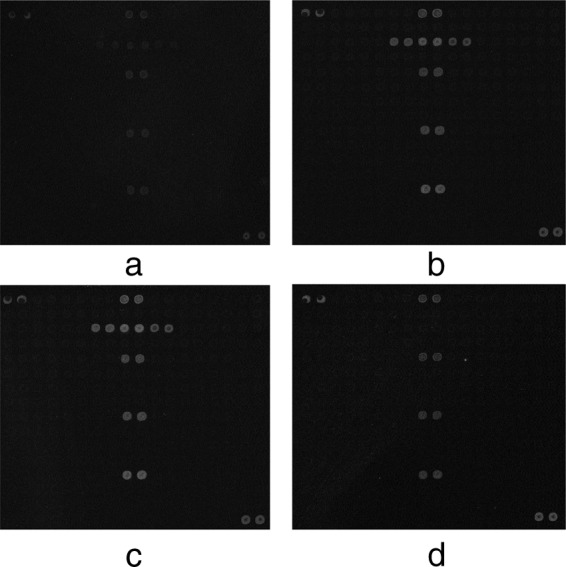
Microarray hybridization patterns representative of a Salmonella serogroup from DNA extracted from spiked tomato samples. (a) Salmonella serogroup B, 2 CFU/g; (b) Salmonella serogroup B, 11 CFU/g; (c) Salmonella serogroup B, 102 CFU/g; (d) Salmonella serogroup B, unspiked tomato sample.
DISCUSSION
In this present study, we describe a microarray method for the determination of Salmonella O serogroups based on the O serogroup-specific genes. This is the first report to describe a molecular method for the comprehensive detection and identification of all 46 Salmonella O serogroups. Compared to other molecular-based detection methods, such as PCR and DNA sequencing, microarrays are more applicable for high throughput, are highly specific in combination with PCR, and are more efficient than DNA sequencing. PCR is rapid and easy to process, but it is hard to differentiate similar amplicon sizes from different serotypes. The DNA sequencing is very accurate; however, it requires more expensive equipment, has higher testing costs, and takes a lot of time and work for library preparation and sequencing (34).
In this study, the O unit-processing genes wzx and wzy were selected to be the target genes for the majority of Salmonella serogroups. In the cases where other genes were selected, Salmonella O54, for which the transferase gene wbbE was selected as a specific gene (13), has its O antigen gene cluster on a small mobilizable plasmid which lacks wzx and wzy genes, and it usually has a chromosomally encoded O antigen which is coexpressed with the O54 factor (12). The O54 strain used in our study also produced positive results with E1/E4 primers, as it is subtyped as serotype Uccle (O3, 54) and expresses additional O3 (E) factor. The O antigen gene clusters of serogroups A and D1 shared high levels of homology to serogroups B, D2, D3, E1, and E4. The wzx genes had 94 to 99% identities among serogroups A, D1, D2, and D3, while the wzy genes of serogroups A, D1, and B are identical (4, 26). Therefore, the O antigen synthesis gene prt was used for molecular differentiation of serogroups A and D1 from other serogroups (22). The serogroups D2 and D3 also produced positive results with the primer pair and probes for serogroup A/D1, as they have identical prt genes (4) and were distinguished by their own serogroup-specific primer pair and probes (Table 2). The wzy genes of serogroups E1/E4 shared 98% identities with serogroup D2; therefore, E1/E4 also produced positive results with the primer pair and probes for D2 (40). The serogroups E1/E4 were distinguished from D2 by their own serogroup-specific primer pair and probes (Table 2).
The microarray assay provided serogroup designations comparable to those of traditional methods in the vast majority of cases. However, this method alone was unable to differentiate three closely related serogroup pairs: serogroups E1 and E4, serogroups A and D1, and serogroups O67 and B. The O antigen gene cluster of serogroup E1 shares great similarity to that of serogroup E4 (39). The O antigen gene cluster of serogroup A is identical to that of serogroup D1 except for a frameshift mutation in the tyv gene (21), and there is not a serogroup-specific probe that can distinguish them (8, 22). Molecular markers for the differentiation of those three pairs are currently unavailable, and further serological tests are needed for the differentiation of these serogroups. Serogroup O67 is very rare and appears to be a variant of serogroup B (6, 18), and the region between galF and gnd in Salmonella serogroup O67 is the same as that in the serogroup B O antigen gene cluster. However, there is no cross-reaction for group B and O67 antisera, and their structural analysis revealed that the O67 gene cluster is not located between galF and gnd. The draft genome of the Salmonella O67 type strain was obtained by our laboratory recently, and a potential O67 O antigen gene cluster was found elsewhere in the genome, which is consistent with its O antigen chemical structure (Liu et al., unpublished). Based on this gene cluster, we designed an additional PCR primer pair targeting the wejV gene (see Table S2 in the supplemental material). PCR assays showed that this primer pair was specific to serogroup O67, as none of the serogroup B strains tested gave positive results. In future work, probes based on the O67-specific gene can be incorporated into the microarrays to distinguish between the serogroups O67 and B.
The unexpected negative results were obtained with 5 of the 290 Salmonella isolates tested, including three serogroup O28 strains (six in total) and two serogroup O59 strains (four in total) (Table 3). These might be due to variations of Salmonella O antigen clusters within a serogroup, as such cases were reported previously (6). The two serogroup O59 strains that failed to be identified by the microarray belong to subspecies IIIa and IIIb serogroup O59. It has been reported that O59 in subspecies IIIb had different O-polysaccharide structures than O59 in subspecies II, although both of their O antigens include a common disaccharide, which could provide a common epitope responsible for their serological relatedness (28). Since O antigen gene cluster sequence information for those two subspecies are not available, the specific primers and probes designed based on the O antigen gene cluster of subspecies II serogroup O59 might not be suitable for subspecies IIIa and IIIb. We are currently in the process of sequencing the O antigen clusters of serogroup O59 subspecies IIIa and IIIb to reveal any sequence variations that can be used for the identification of these two subspecies. Salmonella serogroup O28 was originally divided into three subfactors (O281, O282, and O283) without structural differences being ascribed (16). The wzx and wzy genes from the O antigen gene clusters of serotype Dakar (O281 and O283) and serotype Pomona (O281 and O282) share 51 and 48% identity, respectively (11). The specific primers and probes for O28 were targeted on the wzy gene of S. enterica serotype Dakar in our microarray. An additional PCR primer pair was designed against the wzy gene of S. enterica serotype Pomona (see Table S2 in the supplemental material). The three O28 strains initially giving negative results were positive when tested using this additional primer pair (data not shown). Thus, the extra primer pair could be incorporated into the microarray in further studies to increase the power of the assay.
Salmonella and E. coli are known to be evolutionarily related (27). There are several pairs of Salmonella and E. coli strains that are known to have identical or closely related O antigen organizations, such as serotype O35 and E. coli O111, serotype O50 and E. coli O55, and serotype O30 and E. coli O157 (33, 36). Nonetheless, their gene clusters at the nucleotide level are divergent, and the average identities of wzx and wzy are 70 and 63%, respectively, which is enough difference to discriminate them from each other. Our microarray was tested against the 186 Shigella and E. coli representative strains to validate the specificity of the new assay, and each of these strains produced a negative signal against the Salmonella-specific probes.
In conclusion, this study presents a new multiplex PCR-based microarray assay for the comprehensive detection and identification of all 46 Salmonella O serogroups. This new method provides an accurate and reliable approach for differentiating Salmonella at the serogroup level and contributes significantly to large-scale epidemiology studies, and it could be employed to monitor local, regional, and national trends in human salmonellosis. Although the microarrays developed here allow the analysis of all Salmonella O serogroups simultaneously, serotyping of Salmonella based on flagellar antigens (H antigens) still needs to be done using the conventional method. Currently, the sequences of flagellin alleles representing 67 out of the 114 known Salmonella flagellar antigenic types have been reported (23), with more to come. In future work, it will be worthwhile to incorporate flagellar antigen-specific probes into the microarrays for more comprehensive analysis and practical uses.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Key Program for Infectious Diseases of China (2013ZX10004216-001-001), the National 863 Program of China (2012AA020103 and 2011AA100901-2), the National 973 Program of China (2011CB504900), National Natural Science Foundation of China (NSFC) Program (31170094, 31030002, and 31270003), the Tianjin Research Program of Application Foundation and Advanced Technology (10JCYBJC10000), the Research Fund for the Doctoral Program of Higher Education of China (20090031120023), the Fundamental Research Fund for the Central Universities (65020121 and 65020061), and the Public Health Key Disciplines in Shanghai Health Microbiology (12GWZX0801).
Footnotes
Published ahead of print 22 March 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.00225-13.
REFERENCES
- 1. Bastin DA, Reeves PR. 1995. Sequence and analysis of the O antigen gene (rfb) cluster of Escherichia coli O111. Gene 164: 17–23 [DOI] [PubMed] [Google Scholar]
- 2. Behravesh CB, Blaney D, Medus C, Bidol SA, Phan Q, Soliva S, Daly ER, Smith K, Miller B, Taylor T, Nguyen T, Perry C, Hill TA, Fogg N, Kleiza A, Moorhead D, Al-Khaldi S, Braden C, Lynch MF. 2012. Multistate outbreak of Salmonella serotype Typhimurium infections associated with consumption of restaurant tomatoes, U. S. A., 2006: hypothesis generation through case exposures in multiple restaurant clusters. Epidemiol. Infect. 140: 2053–2061 [DOI] [PubMed] [Google Scholar]
- 3. Brown PK, Romana LK, Reeves PR. 1992. Molecular analysis of the rfb gene cluster of Salmonella serovar Muenchen (strain M67): the genetic basis of the polymorphism between groups C2 and B. Mol. Microbiol. 6: 1385–1394 [DOI] [PubMed] [Google Scholar]
- 4. Curd H, Liu D, Reeves PR. 1998. Relationships among the O-antigen gene clusters of Salmonella enterica groups B, D1, D2, and D3. J. Bacteriol. 180: 1002–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fierer J, Guiney DG. 2001. Diverse virulence traits underlying different clinical outcomes of Salmonella infection. J. Clin. Investig. 107: 775–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fitzgerald C, Collins M, van Duyne S, Mikoleit M, Brown T, Fields P. 2007. Multiplex, bead-based suspension array for molecular determination of common Salmonella serogroups. J. Clin. Microbiol. 45: 3323–3334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fitzgerald C, Sherwood R, Gheesling LL, Brenner FW, Fields PI. 2003. Molecular analysis of the rfb O antigen gene cluster of Salmonella enterica serogroup O:6,14 and development of a serogroup-specific PCR assay. Appl. Environ. Microbiol. 69: 6099–6105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Franklin K, Lingohr EJ, Yoshida C, Anjum M, Bodrossy L, Clark CG, Kropinski AM, Karmali MA. 2011. Rapid genoserotyping tool for classification of Salmonella serovars. J. Clin. Microbiol. 49: 2954–2965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hardnett FP, Hoekstra RM, Kennedy M, Charles L, Angulo FJ. 2004. Epidemiologic issues in study design and data analysis related to FoodNet activities. Clin. Infect. Dis. 38(Suppl. 3): S121–S126 [DOI] [PubMed] [Google Scholar]
- 10. Heissenhuber A, Hautmann W, Arenz S, Kugler R, Kleih W, Ludwig S, Wildner M. 2005. Accumulated occurrence of illnesses with Salmonella Enteritidis in hospitals and nursing homes in the district Oberallgaeu, Bavaria, in July 2004. Gesundheitswesen 67: 845–852 [DOI] [PubMed] [Google Scholar]
- 11. Hu B, Perepelov AV, Liu B, Shevelev SD, Guo D, Senchenkova SN, Shashkov AS, Feng L, Knirel YA, Wang L. 2010. Structural and genetic evidence for the close relationship between Escherichia coli O71 and Salmonella enterica O28 O-antigens. FEMS Immunol. Med. Microbiol. 59: 161–169 [DOI] [PubMed] [Google Scholar]
- 12. Keenleyside WJ, Perry M, Maclean L, Poppe C, Whitfield C. 1994. A plasmid-encoded rfbO:54 gene cluster is required for biosynthesis of the O:54 antigen in Salmonella enterica serovar Borreze. Mol. Microbiol. 11: 437–448 [DOI] [PubMed] [Google Scholar]
- 13. Keenleyside WJ, Whitfield C. 1996. A novel pathway for O-polysaccharide biosynthesis in Salmonella enterica serovar Borreze. J. Biol. Chem. 271: 28581–28592 [DOI] [PubMed] [Google Scholar]
- 14. Kim S, Frye JG, Hu JX, Fedorka-Cray PJ, Gautom R, Boyle DS. 2006. Multiplex PCR-based method for identification of common clinical serotypes of Salmonella enterica subsp. enterica. J. Clin. Microbiol. 44: 3608–3615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kuhn KG, Sorensen G, Torpdahl M, Kjeldsen MK, Jensen T, Gubbels S, Bjerager GO, Wingstrand A, Porsbo LJ, Ethelberg S. 2012. A long-lasting outbreak of Salmonella Typhimurium U323 associated with several pork products, Denmark, 2010. Epidemiol. Infect. 26: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kumirska J, Szafranek J, Czerwicka M, Paszkiewicz M, Dziadziuszko H, Kunikowska D, Stepnowski P. 2007. The structure of the O-polysaccharide isolated from the lipopolysaccharide of Salmonella Dakar (serogroup O:28). Carbohydr. Res. 342: 2138–2143 [DOI] [PubMed] [Google Scholar]
- 17. Lane DJ, Pace B, Olsen GJ, Stahl DA, Sogin ML, Pace NR. 1985. Rapid determination of 16S ribosomal RNA sequences for phylogenetic analyses. Proc. Natl. Acad. Sci. U. S. A. 82: 6955–6959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li Q, Reeves PR. 2000. Genetic variation of dTDP-L-rhamnose pathway genes in Salmonella enterica. Microbiology 146(Pt 9): 2291–2307 [DOI] [PubMed] [Google Scholar]
- 19. Lim BK, Thong KL. 2009. Application of PCR-based serogrouping of selected Salmonella serotypes in Malaysia. J. Infect. Dev. Ctries. 3: 420–428 [DOI] [PubMed] [Google Scholar]
- 20. Liu B, Knirel YA, Feng L, Perepelov AV, Senchenkova SN, Reeves PR, Wang L. 2008. Structural diversity in Salmonella O antigens and its genetic basis. FEMS Microbiol. Rev. 32: 627–653. (Erratum, 34:606, 2010) [DOI] [PubMed] [Google Scholar]
- 21. Liu D, Verma NK, Romana LK, Reeves PR. 1991. Relationships among the rfb regions of Salmonella serovars A, B, and D. J. Bacteriol. 173: 4814–4819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Luk JM, Kongmuang U, Reeves PR, Lindberg AA. 1993. Selective amplification of abequose and paratose synthase genes (rfb) by polymerase chain reaction for identification of Salmonella major serogroups (A, B, C2, and D). J. Clin. Microbiol. 31: 2118–2123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McQuiston JR, Parrenas R, Ortiz-Rivera M, Gheesling L, Brenner F, Fields PI. 2004. Sequencing and comparative analysis of flagellin genes fliC, fljB, and flpA from Salmonella. J. Clin. Microbiol. 42: 1923–1932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mertens E, Kreher H, Rabsch W, Bornhofen B, Alpers K, Burckhardt F. 2012. Severe infections caused by Salmonella Enteritidis PT8/7 linked to a private barbecue. Epidemiol. Infect. 19: 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Muhlenberg W. 1993. Epidemiologic public health conclusions from observations of illnesses caused by Salmonella Enteritidis. Gesundheitswesen 55: 21–27 [PubMed] [Google Scholar]
- 26. Naide Y, Nikaido H, Maekelae PH, Wilkinson RG, Stocker BA. 1965. Semirough strains of Salmonella. Proc. Natl. Acad. Sci. U. S. A. 53: 147–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ochman H, Wilson AC. 1987. Evolution in bacteria: evidence for a universal substitution rate in cellular genomes. J. Mol. Evol. 26: 74–86 [DOI] [PubMed] [Google Scholar]
- 28. Perepelov AV, Liu B, Senchenkova SN, Shashkov AS, Guo D, Feng L, Knirel YA, Wang L. 2011. Structures of the O-polysaccharides of Salmonella enterica O59 and Escherichia coli O15. Carbohydr. Res. 346: 381–383 [DOI] [PubMed] [Google Scholar]
- 29. Popoff MY, Bockemuhl J, Gheesling LL. 2003. Supplement 2001 (no. 45) to the Kauffmann-White scheme. Res. Microbiol. 154: 173–174 [DOI] [PubMed] [Google Scholar]
- 30. Centers for Disease Control and Prevention 2010. Investigation update: multistate outbreak of human Salmonella Enteritidis infections associated with shell eggs. CDC, Atlanta, GA [Google Scholar]
- 31. Rouillard JM, Zuker M, Gulari E. 2003. OligoArray 2.0: design of oligonucleotide probes for DNA microarrays using a thermodynamic approach. Nucleic Acids Res. 31: 3057–3062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. WHO Collaborating Center for Reference and Research on Salmonella 2007. Antigenic formulae of the Salmonella serovars, 9th ed. Institut Pasteur, Paris, France [Google Scholar]
- 33. Samuel G, Hogbin JP, Wang L, Reeves PR. 2004. Relationships of the Escherichia coli O157, O111, and O55 O-antigen gene clusters with those of Salmonella enterica and Citrobacter freundii, which express identical O antigens. J. Bacteriol. 186: 6536–6543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shallom SJ, Tae H, Sarmento L, Preston D, McIver L, Franck C, Dickerman A, Adams LG, Garner HR. 2012. Comparison of genome diversity of Brucella spp. field isolates using Universal Bio-signature detection array and whole genome sequencing reveals limitations of current diagnostic methods. Gene 509: 142–148 [DOI] [PubMed] [Google Scholar]
- 35. Somily AM, Adam MH, Gad El Rab MO, Morshed MG, Shakoor Z. 2011. Detection of Salmonella typhi agglutinins in sera of patients with other febrile illnesses and healthy individuals. Ann. Afr. Med. 10: 41–44 [DOI] [PubMed] [Google Scholar]
- 36. Wang L, Reeves PR. 2000. The Escherichia coli O111 and Salmonella enterica O35 gene clusters: gene clusters encoding the same colitose-containing O antigen are highly conserved. J. Bacteriol. 182: 5256–5261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang L, Romana LK, Reeves PR. 1992. Molecular analysis of a Salmonella enterica group E1 rfb gene cluster: O antigen and the genetic basis of the major polymorphism. Genetics 130: 429–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wollin R. 2007. A study of invasiveness of different Salmonella serovars based on analysis of the Enter-net database. Euro Surveill. 12: E070927.3. [DOI] [PubMed] [Google Scholar]
- 39. Xiang SH, Haase AM, Reeves PR. 1993. Variation of the rfb gene clusters in Salmonella enterica. J. Bacteriol. 175: 4877–4884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xiang SH, Hobbs M, Reeves PR. 1994. Molecular analysis of the rfb gene cluster of a group D2 Salmonella enterica strain: evidence for its origin from an insertion sequence-mediated recombination event between group E and D1 strains. J. Bacteriol. 176: 4357–4365 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.