Abstract
Herpes simplex virus 1 (HSV-1) and HSV-2 establish latency in different neuronal subtypes (A5+ and KH10+) in murine trigeminal ganglia, results which correlate with restricted productive infection in these neurons in vitro. HSV-2 latency-associated transcript (LAT) contains a cis-acting regulatory element near the transcription start site that promotes productive infection in A5+ neurons and a second element in exon 1 that inhibits productive infection in KH10+ neurons. HSV-1 contains no such regulatory sequences, demonstrating different mechanisms for regulating productive HSV infection in neurons.
TEXT
Herpes simplex virus 1 (HSV-1) and HSV-2 express productive cycle genes in some neurons and establish latent infection in others. We previously demonstrated that HSV-1 and HSV-2 preferentially establish latency in different neuronal subtypes in murine sensory ganglia: HSV-1 in A5+ neurons and HSV-2 in KH10+ neurons (1, 2). In dissociated adult murine trigeminal cultures (AMTC), we previously found that HSV-1 productively infected KH10+ but not A5+ neurons while HSV-2 productively infected A5+ but not KH10+ neurons (Fig. 1A) (3). Thus, the neuronal subtypes that restrict productive HSV infection in vitro are the same neurons that harbor latent infection in vivo. Both host and viral factors may play roles in regulating neuronal specificity (1, 2, 4–7). To determine if the latency-associated transcript (LAT) region regulates productive infection in A5+ and KH10+ neurons, we assayed HSV infection of AMTC using a series of LAT deletion and chimeric viruses (5).
Fig 1.
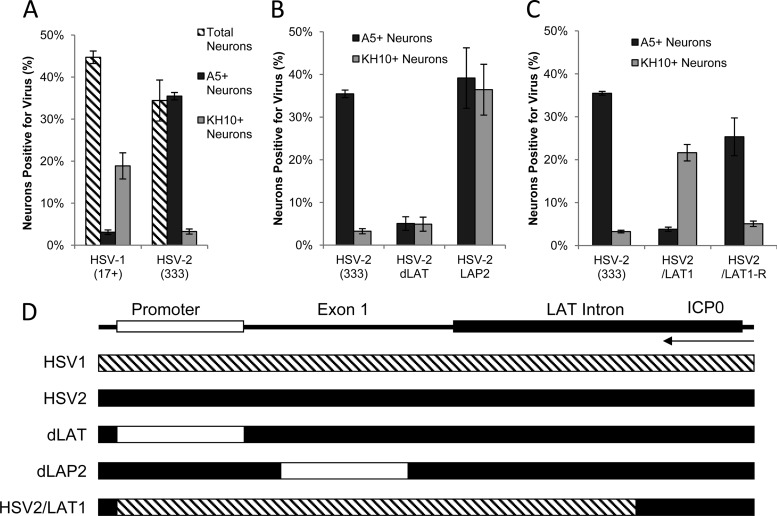
Productive infection of cultured primary adult murine TG neurons with HSV-2 LAT region deletion and chimeric viruses (multiplicity of infection [MOI], 30; 10 h postinoculation). (A) Percentages of total (hatched bars), A5+ (black bars), and KH10+ (gray bars) neurons productively infected with HSV-1 and HSV-2, as identified by MAbs A5 and KH10, and polyclonal antisera against HSV-1 or HSV-2. (These data were published previously [3] and are shown here for reference). (B) Percentages of A5+ and KH10+ neurons productively infected with HSV-2 wild type (333) and HSV-2 LAT region deletion viruses (dLAT and LAP2). (C) Percentages of A5+ and KH10+ neurons productively infected with HSV-2 wild type (333), chimeric virus HSV2/LAT1, and its rescuant, HSV2/LAT1-R. (D) Maps illustrating HSV-2 LAT region deletion and chimeric sequence swap. Deletions are indicated by white boxes on the HSV-2 LAT region (black), and the HSV-1 sequence placed into HSV-2 is indicated by a hatched box on the HSV-2 background (black). ICP0 transcript is shown for reference.
The HSV-2 LAT region contains two regulatory sequences for productive infection of neurons.
HSV-2 (333) productively infects 36% of A5+ neurons in AMTC but only 3% of KH10+ neurons (Fig. 1B). In contrast, dLAT (NotI-NotI deletion of the HSV-2 LAT promoter) (Fig. 1D) (8) productively infects only 5% of A5+ neurons (Fig. 1B). The LAP2 virus (LAT promoter 2 deletion in HSV-2 exon 1) (Fig. 1D) (9) productively infects A5+ and KH10+ neurons equivalently (39% and 36%, respectively) (Fig. 1B). Thus, two distinct functional elements within HSV-2 LAT regulate productive infection: the NotI-NotI region promotes productive infection in A5+ neurons, and the LAP2 region inhibits productive infection in KH10+ neurons.
HSV-2/LAT1 is a chimera in which 2.8 kb of the HSV-2 LAT region (promoter, exon 1, and ∼1.5 kb of the intron, excluding ICP0 coding sequences) was replaced by the same region from HSV-1 (10). HSV-2/LAT1 preferentially establishes latency in A5+ neurons in vivo, suggesting that HSV-1 LAT in the context of HSV-2 changes the viral phenotype to that of HSV-1 (1). In AMTC, HSV-2/LAT1 productively infects 22% of KH10+ neurons but only 4% of A5+ neurons, in contrast to HSV-2 or the rescuant, HSV-2/LAT1-R (Fig. 1C). Thus, the pattern of productive infection with HSV-2/LAT1 resembles that of HSV-1, with productive infection in KH10+ neurons but restricted infection in A5+ neurons. Since the HSV-1 LAT region in HSV-2/LAT1 replaces sequences that were deleted in dLAT and LAP2, the observed phenotype of HSV-2/LAT1 might be due to either deletions of HSV-2 LAT or inserted HSV-1 LAT-associated functions. To distinguish between these possibilities, we studied the effect of HSV-1 LAT deletions on the pattern of productive infection in AMTC. Unlike HSV-2, HSV-1 LAT mutations involving the promoter, exon 1, and the intron had no effect on the neuronal pattern of infection in AMTC compared to wild-type HSV-1 (Fig. 2A).
Fig 2.
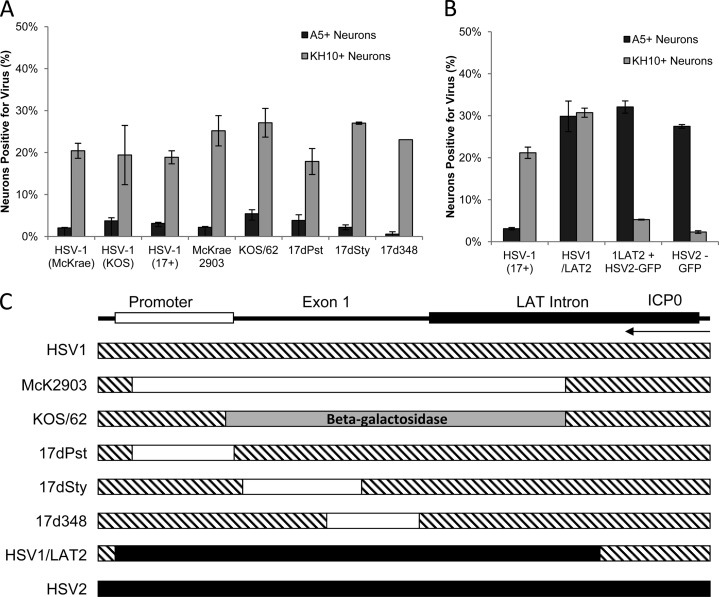
Productive infection of cultured primary adult murine TG neurons with HSV-1 LAT region deletion and chimeric viruses (MOI, 30; 10 h postinoculation). (A) Percentages of A5+ and KH10+ neurons productively infected with HSV-1 wild-type (McKrae, KOS, 17+) and HSV-1 LAT region deletion (McKrae 2903, KOS/62, 17dPst, 17dSty, 17d348) viruses. (B) Percentages of A5+ and KH10+ neurons productively infected with HSV-1, chimeric virus HSV1/LAT2, or HSV2-GFP or coinfected with both HSV1/LAT2 and HSV2-GFP, evaluated for productive infection using polyclonal antisera to detect HSV-1 and HSV1/LAT2 and GFP expression to detect HSV2-GFP. (C) Maps illustrating HSV-1 LAT region deletions and chimeric sequence swap. Deletions are indicated by white boxes, and beta-galactosidase is indicated by a gray box on the HSV-1 LAT region (hatched); HSV-2 sequence placed into HSV-1 is indicated by a black box on the HSV-1 background (hatched). ICP0 transcript is shown for reference.
HSV-1/LAT2, the reciprocal of HSV-2/LAT1 (Fig. 2C) (11), has a latent phenotype similar to that of wild-type (WT) HSV-2 in vivo (6). In AMTC, HSV-1/LAT2 productively infects A5+ and KH10+ neurons equivalently (30% and 31%, respectively), in contrast to HSV-1 and the rescuant, which infect 3% of A5+ neurons and 22% of KH10+ neurons (Fig. 2B). Thus, only the function that promotes productive infection in A5+ neurons was transferred from HSV-2 to HSV-1. This suggests that the HSV-2 LAT NotI-NotI region, which promotes productive infection in A5+ neurons, contains a transferrable cis-acting element, while the HSV-2 LAP2 region, which contains a function that restricts productive infection in KH10+ neurons, is not transferrable to HSV-1 as an independent functional element.
We hypothesized that the HSV-2 LAP2 sequence requires an additional factor to maintain functionality in the context of HSV-1. Therefore, we coinfected AMTC with HSV-1/LAT2 and HSV-2-green fluorescent protein (GFP) (12) and assayed the pattern of productive infection of each virus. Only 5% of KH10+ neurons were productively infected with HSV-1/LAT2, similar to percentages with either HSV-2 or HSV-2-GFP alone (Fig. 1 and 2B), in contrast to HSV-1 or HSV-1/LAT2 alone (Fig. 2B). Thus, either a viral factor from outside the HSV-2 LAT region or a cellular factor produced during HSV-2 infection is required for the HSV-2 LAP2 region to restrict productive infection in KH10+ neurons.
Mapping regulatory domains in LAT regions.
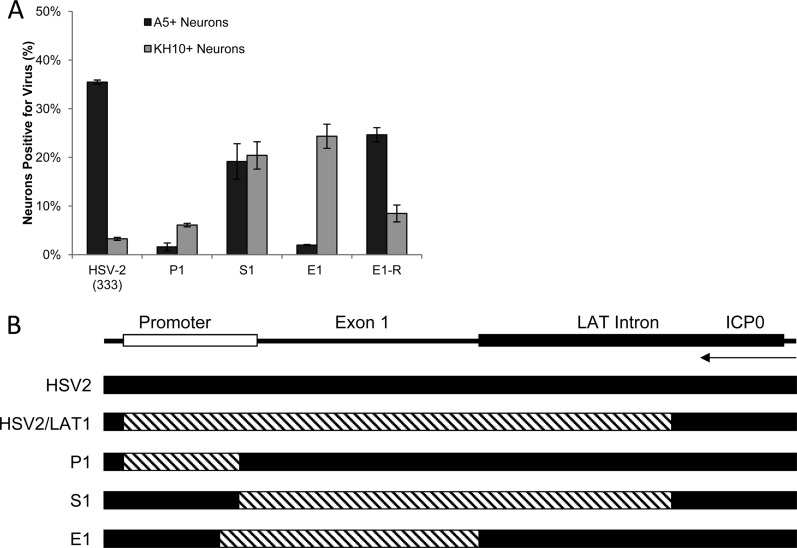
We next used previously characterized HSV-2/HSV-1 chimeras (P1, S1, and E1) containing HSV-1 (17+) LAT regions in an HSV-2 (333) background (Fig. 3B) (4, 13) to map functional domains of the LAT region that regulate productive infection in A5+ and KH10+ neurons. In P1, the HSV-1 LAT promoter (NotI-PvuI) drives expression of HSV-2 LAT (13). In S1, the HSV-2 LAT promoter drives expression of HSV-1 LAT (PvuI-XhoI) (13). The PvuI site is just downstream of the HSV LAT TATA box (Fig. 4). P1 productively infected only 1.6% of A5+ neurons (Fig. 3A), similar to dLAT (Fig. 1B), while S1 productively infected A5+ and KH10+ neurons equivalently (19% and 22%, respectively), similar to LAP2 (Fig. 1B). These results suggest that transfer of HSV-1 LAT sequences into HSV-2 does not transfer a function from HSV-1 to HSV-2 but simply deletes the functional regions from HSV-2 that stimulate productive infection in A5+ neurons (NotI-PvuI) or inhibit productive infection in KH10+ neurons (LAP2). Thus, studies with chimeric viruses must be carefully interpreted since functional elements can be both added and deleted during viral construction.
Fig 3.
Productive infection of cultured primary adult murine TG neurons with HSV-2, the chimeric viruses P1, S1, and E1, and the E1 rescuant (MOI, 30; 10 h postinoculation). (A) Percentages of A5+ and KH10+ neurons productively infected with HSV-2 (333), the chimeric viruses P1, S1, and E1, and the E1 rescuant E1-R. (B) Maps illustrating LAT region chimeric sequence swaps. HSV-1 LAT sequences (hatched) replaced HSV-2 LAT sequences (black). P1 contains the HSV-1 LAT promoter from NotI to the PvuI site just downstream of the TATA box (the 5′ end of the region replaced in HSV2/LAT1); S1 contains the HSV-1 LAT sequence from the PvuI site through ∼1.5 kb downstream of the 5′ splice site of the intron (the 3′ end of the region replaced in HSV2/LAT1) but excludes ICP0 coding sequences; E1 contains HSV-1 LAT exon 1 from the TATA box to the 5′ splice site of the intron. ICP0 transcript is shown for reference.
Fig 4.
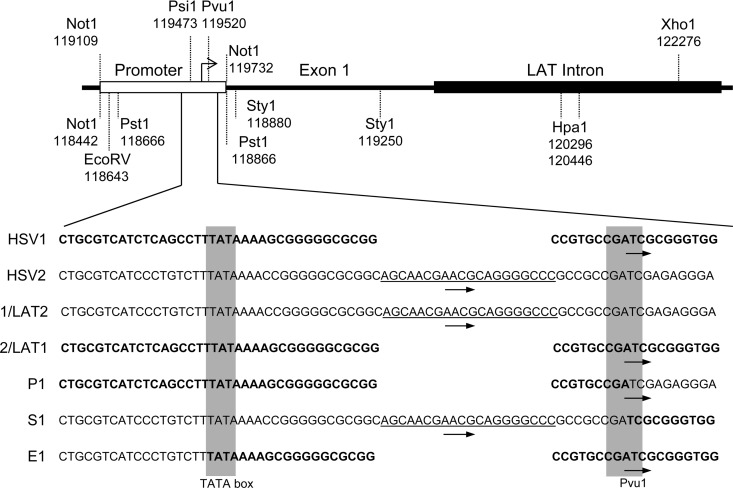
Alignment of HSV-1, HSV-2, and chimeric viruses. Sequences of HSV-1, HSV-2, HSV1/LAT2, HSV2/LAT1, P1, S1, and E1 are aligned at the TATA box and the PvuI restriction enzyme site just downstream (gray boxes). HSV-1 sequences are bold, and HSV-2 sequences are normal font. Arrows indicate LAT transcription start sites. Underlined sequences are HSV-2 sequences present in wild-type HSV-2, HSV1/LAT2, and S1; this sequence is necessary for productive infection in A5+ neurons.
E1, in which the HSV-2 LAT region from TATA to the intron 5′ splice site was replaced by the same region from HSV-1 (exon 1) (4), produced a similar pattern of infection in AMTC as HSV-1, with productive infection of KH10+ neurons (22%) but minimal productive infection in A5+ neurons (2%). Thus, transfer of HSV-1 LAT exon 1 into HSV-2 effectively deletes the regions responsible for both productive infection of A5+ neurons and restricted infection of KH10+ neurons. Sequence analysis suggests that the HSV-2 function that promotes productive infection in A5+ neurons maps between the LAT TATA box and the PvuI site 44 bp downstream. This region contains multiple putative regulatory sequences and is nearly identical in HSV-1 and HSV-2 except for an apparent 20-bp deletion in HSV-1 (Fig. 4). We conclude that these 20 bp are required for productive HSV infection of A5+ neurons since viruses containing them productively infect A5+ neurons (HSV-2, HSV-1/LAT2, and S1), and those lacking them (HSV1, HSV-2/LAT1, P1, and E1) are restricted for productive infection in A5+ neurons.
HSV-1 and HSV-2 productive infections are regulated by different mechanisms in neurons.
Monoclonal antibodies (MAbs) A5+ and KH10+ recognize two distinct populations of murine nociceptive neurons. Nerve growth factor (NGF)-responsive A5+ neurons are largely calcitonin gene-related peptide (CGRP) positive with Aδ fibers, while glial cell line-derived neurotrophic factor (GDNF)-responsive KH10+ C-fiber neurons colabel with isolectin-B4 (IB4) (2, 14, 15). Similar, but not identical, Aδ and C-fiber neuronal populations are found in human sensory ganglia and innervating human skin (16, 17). A5+ neurons restrict productive HSV-1 infection and are the major site of HSV-1 latency. KH10+ neurons restrict productive HSV-2 infection and are the major site of HSV-2 latency. Our studies demonstrate that the mechanisms that regulate these phenotypes are mediated in part by two regulatory elements in the HSV-2 LAT region: a 20-bp sequence near the TATA box contains a cis-acting element that promotes productive infection in A5+ neurons, while LAP2 inhibits productive infection in KH10+ neurons but requires an additional factor. HSV-1 LAT contains no such regulatory elements, demonstrating that HSV-1 and HSV-2 neuron specificity is regulated by different mechanisms.
ACKNOWLEDGMENTS
We thank the following people for their kind gift of viruses for these studies: Philip Krause, David Bloom, and Steve Wechsler. The monoclonal antibodies FE-A5, developed by B. Fenderson, and KH10, developed by T. M. Jessell and J. Dodd, were obtained from the Developmental Studies Hybridoma Bank, developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biological Sciences, IA. We also thank Kathleen Apakupakul for editing.
This study was supported in part by NIH grant PN2-EY018241 and the Littlefield Foundation and Trust.
We have no competing financial interest in the research presented.
Footnotes
Published ahead of print 20 March 2013
REFERENCES
- 1. Margolis TP, Imai Y, Yang L, Vallas V, Krause PR. 2007. Herpes simplex virus type 2 (HSV-2) establishes latent infection in a different population of ganglionic neurons than HSV-1: role of latency-associated transcripts. J. Virol. 81:1872–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yang L, Voytek CC, Margolis TP. 2000. Immunohistochemical analysis of primary sensory neurons latently infected with herpes simplex virus type 1. J. Virol. 74:209–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bertke AS, Swanson SM, Chen J, Imai Y, Kinchington PR, Margolis TP. 2011. A5-positive primary sensory neurons are nonpermissive for productive infection with herpes simplex virus 1 in vitro. J. Virol. 85:6669–6677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bertke AS, Patel A, Imai Y, Apakupakul K, Margolis TP, Krause PR. 2009. Latency-associated transcript (LAT) exon 1 controls herpes simplex virus species-specific phenotypes: reactivation in the guinea pig genital model and neuron subtype-specific latent expression of LAT. J. Virol. 83:10007–10015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bloom DC. 2004. HSV LAT and neuronal survival. Int. Rev. Immunol. 23:187–198 [DOI] [PubMed] [Google Scholar]
- 6. Imai Y, Apakupakul K, Krause PR, Halford WP, Margolis TP. 2009. Investigation of the mechanism by which herpes simplex virus type 1 LAT sequences modulate preferential establishment of latent infection in mouse trigeminal ganglia. J. Virol. 83:7873–7882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Margolis TP, Dawson CR, LaVail JH. 1992. Herpes simplex viral infection of the mouse trigeminal ganglion. Immunohistochemical analysis of cell populations. Invest. Ophthalmol. Vis. Sci. 33:259–267 [PubMed] [Google Scholar]
- 8. Krause PR, Stanberry LR, Bourne N, Connelly B, Kurawadwala JF, Patel A, Straus SE. 1995. Expression of the herpes simplex virus type 2 latency-associated transcript enhances spontaneous reactivation of genital herpes in latently infected guinea pigs. J. Exp. Med. 181:297–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yoshikawa T, Stanberry LR, Bourne N, Krause PR. 1996. Downstream regulatory elements increase acute and latent herpes simplex virus type 2 latency-associated transcript expression but do not influence recurrence phenotype or establishment of latency. J. Virol. 70:1535–1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yoshikawa T, Hill JM, Stanberry LR, Bourne N, Kurawadwala JF, Krause PR. 1996. The characteristic site-specific reactivation phenotypes of HSV-1 and HSV-2 depend upon the latency-associated transcript region. J. Exp. Med. 184:659–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hill J, Patel A, Bhattacharjee P, Krause P. 2003. An HSV-1 chimeric containing HSV-2 latency associated transcript (LAT) sequences has significantly reduced adrenergic reactivation in the rabbit eye model. Curr. Eye Res. 26:219–224 [DOI] [PubMed] [Google Scholar]
- 12. Bertke AS, Apakupakul K, Ma A, Imai Y, Gussow AM, Wang K, Cohen JI, Bloom DC, Margolis TP. 2012. LAT region factors mediating differential neuronal tropism of HSV-1 and HSV-2 do not act in trans. PLoS One 7:e53281 doi:10.1371/journal.pone.0053281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bertke AS, Patel A, Krause PR. 2007. Herpes simplex virus latency-associated transcript sequence downstream of the promoter influences type-specific reactivation and viral neurotropism. J. Virol. 81:6605–6613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dodd J, Jessell TM. 1986. Cell surface glycoconjugates and carbohydrate-binding proteins: possible recognition signals in sensory neurone development. J. Exp. Biol. 124:225–238 [DOI] [PubMed] [Google Scholar]
- 15. Dodd J, Jessell TM. 1985. Lactoseries carbohydrates specify subsets of dorsal root ganglion neurons projecting to the superficial dorsal horn of rat spinal cord. J. Neurosci. 5:3278–3294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nolano M, Provitera V, Caporaso G, Stancanelli A, Leandri M, Biasiotta A, Cruccu G, Santoro L, Truini A. 2013. Cutaneous innervation of the human face as assessed by skin biopsy. J. Anat. 222:161–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pan A, Wu H, Li M, Lu D, He X, Yi X, Yan XX, Li Z. 2012. Prenatal expression of purinergic receptor P2X3 in human dorsal root ganglion. Purinergic Signal. 8:245–254 [DOI] [PMC free article] [PubMed] [Google Scholar]