Abstract
Contradictory studies report either pro- or anti-inflammatory endothelial cell (EC) responses to human cytomegalovirus (hCMV) infection, hindering the validation of a potential link between this virus and associated inflammatory pathologies. Clarifying this issue, we report that hCMV induces a biphasic response. Early after inoculation, hCMV promoted lymphocyte and, to a lesser extent, neutrophil capture under in vivo relevant shear stresses. In contrast, later stages of infection rendered EC refractory to basal, or cytokine-induced, leukocyte recruitment.
TEXT
Endothelial cells (EC), the master regulators of leukocyte extravasation, are critical sites of human cytomegalovirus (hCMV) infection in vivo (for a review, see reference 1). hCMV antigens are found in EC in inflammatory pathologies, such as atherosclerosis (2) and inflammatory bowel disease (3), but the role of the virus in their development remains highly controversial. Studies report that hCMV induces production of the EC proinflammatory mediators interleukin 8 (IL-8) (4, 5) and IL-6 (6) and expression of the leukocyte adhesion receptor ICAM-1 (7, 8). In addition, reports have shown that hCMV-infected EC (hCMV-EC) supported enhanced leukocyte recruitment (5, 9–11). These heavily cited studies, particularly those demonstrating a role for hCMV-induced IL-8 in neutrophil transmigration (4, 5), support the idea that hCMV contributes to the development or exacerbation of inflammatory pathologies. However, these same studies used static adhesion assays involving prolonged coculture of leukocytes with hCMV-EC or hCMV-EC supernatants before quantification of recruitment. Thus, these systems circumvent components of the leukocyte recruitment paradigm that are critical in vivo, i.e., initial capture from flowing blood and rolling on the EC surface (for a review, see reference 12). Therefore, these results do not determine if hCMV-EC can induce leukocyte recruitment under vascular shear forces. Nevertheless, investigators have shown that hCMV can induce EC VCAM-1 and E-selectin expression, important capture receptors involved in leukocyte tethering from blood flow (11), implying that hCMV-mediated recruitment could occur under shear forces. These results, however, contradict an earlier study that claimed hCMV induces no such increase (8). Furthermore, other data indicate that hCMV renders EC refractory to cytokine-mediated adhesion molecule induction (8) and IL-8 secretion (13), suggesting that hCMV can induce an anti-inflammatory phenotype, although there are no reports of the subsequent effects on leukocyte recruitment. Here, we addressed these contradictions in the literature by examining the ability of hCMV to induce a pro- or anti-inflammatory EC phenotype. Importantly, we examined the functional relevance of infection by studying leukocyte recruitment under shear stress in an in vivo relevant flow-based recruitment assay. We found that hCMV induced a biphasic inflammation response, which may explain many of the inconsistencies in the current literature and also has important implications regarding the biological and clinical relevance of hCMV in inflammatory pathologies.
We isolated EC from umbilical veins (14) and seeded them into channel slides (μ-Slide VI; Ibidi) (15) or multiwell plates (16). EC were untreated or inoculated with a clinical strain of hCMV (VR1814) at a multiplicity of infection (MOI) of 5 (hCMV-EC), before centrifugation at 450 × g for 30 min. EC were incubated for 90 min at 37°C and 5% CO2, medium was replaced, and cells were cultured for 1 or 7 days postinoculation (dpi). VR1814 contains the gH/gL/pUL (128, 130, 131A) complex and thus is both endothelial and fibroblast tropic. In some experiments, we treated EC with 100 U/ml tumor necrosis factor alpha (TNF-α) (4 h). The percentages of hCMV-positive EC were similar at both time points, although late antigen (LA) was only evident at 7 dpi (at 1 dpi, hCMV immediate early protein [IE] was detected in 72.1 ± 12.8% [mean ± standard deviation] EC, while LA was undetectable [n = 3 experiments]; at 7 dpi, IE was detected in 56.2% ± 23.7% and LA in 63.2% ± 10.5% EC [n = 6 experiments]). hCMV-EC were not cytolytic at 1 or 7 dpi, and trypan blue exclusion confirmed equivalent viabilities of EC and hCMV-EC at both time points.
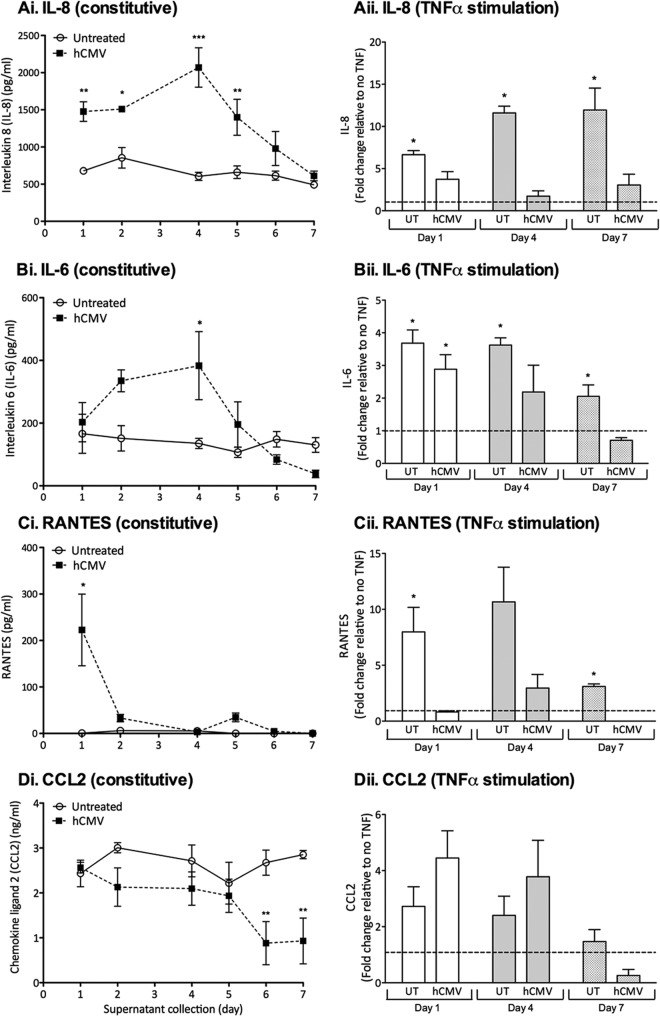
First, we determined if hCMV modulated EC chemokine expression. We collected cell culture supernatants every 24 h and measured human IL-8, IL-6, RANTES, or CCL2 by enzyme-linked immunosorbent assay (ELISA; Duoset; R&D Systems). hCMV significantly enhanced the expression of the neutrophil chemoattractant IL-8 (17) up to 4 dpi (Fig. 1Ai) and enhanced IL-6 secretion with similar kinetics (Fig. 1Bi), observations consistent with previous studies demonstrating enhanced IL-6 (6, 18, 19) and IL-8 (4, 5, 18) secretion up to 5 dpi. However, by 7 dpi, we found no difference between hCMV-EC and EC (Fig. 1Ai and Bi). At 1 dpi, hCMV induced the expression of the lymphocyte chemoattractant RANTES (Fig. 1Ci), although this increase was not observed subsequently. In contrast to the induction of IL-8, IL-6, and RANTES, hCMV did not modulate the expression of the lymphocyte chemoattractant CCL2 (20) until 6 dpi, when it was actually markedly depressed (Fig. 1Di). Previous studies showed that hCMV similarly inhibited fibroblast CCL2 expression (21). These data reveal that hCMV modulated constitutive EC chemokine secretion, in a time- and chemokine-specific manner. Importantly, induction of IL-6, IL-8, and RANTES was transient, a dynamic that should be considered when evaluating the possible role of hCMV in chronic inflammatory pathologies. Furthermore, there was no evidence that the virus induced a sustained EC proinflammatory chemokine secretory profile. To determine if hCMV could exacerbate external inflammatory cues, we measured IL-8, IL-6, RANTES, and CCL2 secretion in response to 4 h of TNF-α treatment. As expected, TNF-α treatment of uninfected EC at 1, 4, and 7 days induced secretion of IL-8 (Fig. 1Aii), IL-6 (Fig. 1Bii), and RANTES (Fig. 1Cii) but not CCL2 (Fig. 1Dii). Interestingly, hCMV-EC were largely refractory to TNF-α treatment, even at early time points, with no significant induction of IL-8 (Fig. 1Aii) or RANTES (Fig. 1Cii) at 1, 4, or 7 dpi. As for uninfected EC, TNF-α treatment of hCMV-EC did not significantly modulate CCL2 secretion, compared to non-cytokine-stimulated hCMV-EC (Fig. 1Dii). Previous studies demonstrated that signaling through the IL-8 receptor, CXCR2, was essential for neutrophil transmigration through TNF-stimulated EC (22), therefore suggesting that hCMV could inhibit neutrophil transmigration, as TNF-α failed to induce IL-8, and constitutive levels were no longer elevated. TNF-α treatment induced IL-6 secretion from hCMV-EC at 1 dpi (Fig. 1Bii) but not beyond that seen for the uninfected control and not at later time points. Thus, we found no evidence to suggest hCMV could exacerbate cytokine-induced inflammatory chemokine secretion. As modulation in EC inflammatory chemokine expression alone cannot induce leukocyte recruitment under in vivo/flow conditions (23) (as expression of EC adhesion receptors are necessary for capture [24, 25]), we examined the effect of hCMV on leukocyte adhesion receptor expression. We stained EC and hCMV-EC with ICAM-1 (fluorescein isothiocyanate [FITC]; Dako), E-selectin (allophycocyanin [APC]; Biolegend), or VCAM-1 (FITC; Bioscience) antibodies, with or without prior stimulation with the inflammatory cytokine TNF-α. TNF-α treatment of uninfected EC induced E-selectin, VCAM-1, and ICAM-1, at both 1 and 7 dpi (Fig. 2A, B, and C), as expected. hCMV inoculation alone did not significantly induce E-selectin (Fig. 2A) or VCAM-1 (Fig. 2B) at either time point, unlike some previous reports that showed hCMV induced an increase in these adhesion receptors up to 4 dpi (11, 26) but in agreement with others that claimed infection did not modulate their expression (27). In the study by Bentz et al. (11), microvascular EC were used, rather than HUVEC, which could introduce a source of variability and provide an explanation for some of the different observations between studies. Indeed, it is likely that the effect of hCMV on adhesion molecule expression could vary between macrovascular and microvascular EC from different sites. We found that hCMV significantly inhibited TNF-α-induced expression of E-selectin and VCAM-1 at 7 dpi (Fig. 2A and B), with a downward trend also evident at 1 dpi. A previous study reported a similar observation at early time points postinoculation (8). hCMV inoculation alone significantly enhanced ICAM-1 expression at 7 dpi (Fig. 2C), with an upward trend also evident at 1 dpi, similar to that previously reported in microvascular EC (11), although this was not statistically significant. However, as for E-selectin and VCAM-1, hCMV almost completely inhibited subsequent TNF-α-induced ICAM-1 expression at 7 dpi (Fig. 2C). Previous studies have shown that TNF-α-induced ICAM-1/VCAM-1 expression is necessary for the induction of neutrophil/peripheral blood lymphocyte [PBL] recruitment from flow in this system (24, 25). Thus, our observations suggest that TNF-α-induced leukocyte recruitment would be inhibited by hCMV at 7 dpi.
Fig 1.
Effect of hCMV inoculation on EC IL-6, IL-8, and CCL2 secretion. Supernatants from uninfected EC or hCMV-EC were analyzed for IL-8 (A), IL-6 (B), CCL2 (C), or RANTES (D) after collection, over 24-h periods between 0 and 7 dpi (i) or from a 4-h incubation period with or without TNF-α at 1, 4, or 7 dpi (ii). Data are means ± standard errors of the means (SEM) from 3 independent experiments; each individual experiment was performed using EC from a different donor. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Fig 2.
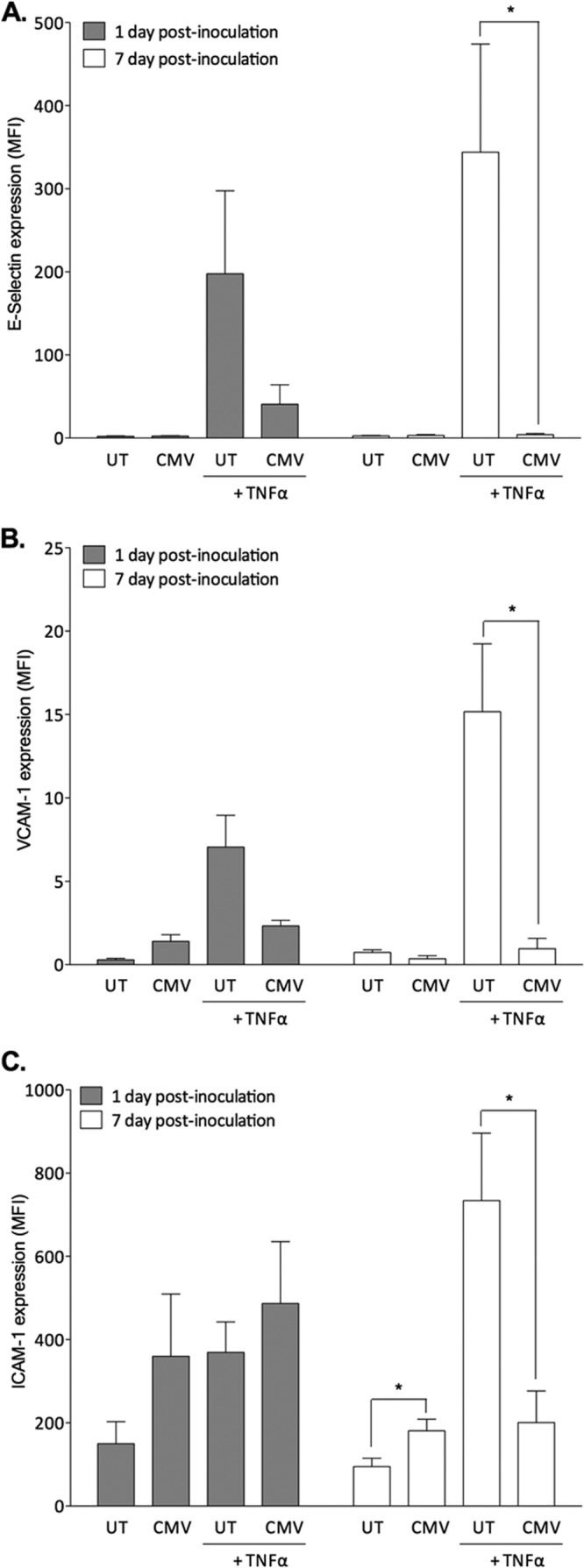
Effect of hCMV on constitutive and TNF-α-induced EC adhesion molecule expression. EC were treated with or without hCMV, cultured for 1 or 7 days prior to treatment with or without TNF-α for 4 h, and then stained for E-selectin (A), VCAM-1 (B), or ICAM-1 (C). Data are means ± SEM from 3 independent experiments; each individual experiment was performed using EC from a different donor. *, P < 0.05.
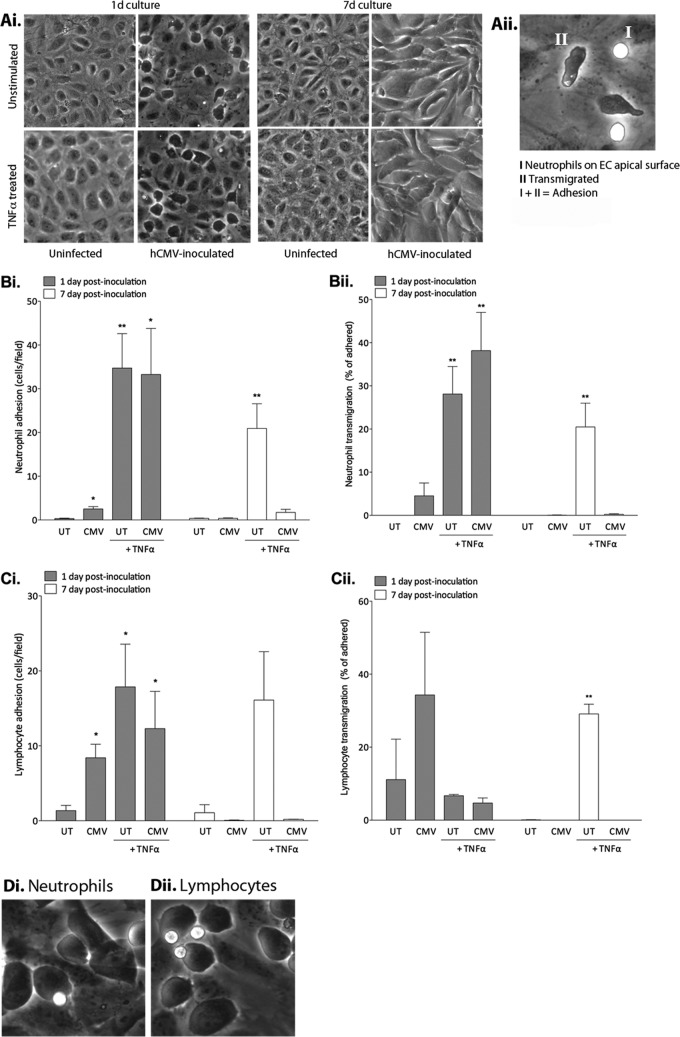
To determine if our observations corresponded to the anticipated functional consequences, we measured leukocyte recruitment under shear stress. We isolated neutrophils or PBL from whole blood (28) and adjusted the cells to 1 × 106/ml in PBS-albumin (0.15% bovine serum). We perfused leukocytes through the channel slides at a wall shear stress of 0.05 Pa and observed the cells by phase-contrast video microscopy (15). hCMV-EC had modified morphology, but monolayers remained intact and were not disrupted by TNF-α treatment (Fig. 3Ai). Leukocytes were classified as adherent if they were on the apical surface (phase bright) or transmigrated underneath the EC (phase dark) (Fig. 3Aii). As we anticipated, TNF-α induced neutrophil (Fig. 3Bi) and PBL (Fig. 3Ci) adhesion on uninfected EC at both time points. At 1 dpi, hCMV inoculation alone significantly enhanced neutrophil (Fig. 3Bi) and PBL (Fig. 3Ci) adhesion compared to uninfected EC. However, the hCMV-induced neutrophil adhesion was minor in comparison to that induced by TNF-α stimulation of uninfected EC (Fig. 3Bi). hCMV inoculation, in the absence of TNF treatment, induced the capture of almost 3 times more PBL than neutrophils (Fig. 3Ci) (2.5 ± 0.6 compared to 8.4 ± 2.1 cells/field, neutrophils compared to PBL, respectively; P = 0.02). This lymphocyte bias could have implications when considering a possible role for hCMV in the development of inflammatory pathologies. Lymphocytes have a key role in the development of chronic inflammatory disorders previously linked to the presence of hCMV, such as atherosclerosis (29), whereas neutrophils are less frequently observed (29). A subset of hCMV-infected EC were enlarged and protruding from the monolayer at 1 dpi, but this phenotype was not observed at 7 dpi, when infected cells had a more flattened uniform appearance (Fig. 3Ai). The modest neutrophil and PBL capture seen on hCMV-EC at 1 dpi tended to be onto the protruding surface of these enlarged cells (illustrated in Fig. 3Di and ii), and thus it is possible that rheological factors could have contributed to increased capture. hCMV did not enhance neutrophil transmigration through the EC monolayer at this time point, in conflict with highly cited previous reports (4, 5). Critically, in these two studies, the investigators exposed neutrophils to supernatants containing IL-8 for a prolonged period before measuring transmigration, which could lead to neutrophil activation and adhesion receptor-independent migration. Indeed, the authors showed that hCMV-EC supernatant-induced neutrophil transmigration was independent of EC adhesion molecule expression, an unlikely scenario in vivo. The flow conditions used in our recruitment assay did not allow prolonged neutrophil exposure to secreted IL-8, and thus the hCMV-induced IL-8 secretion at 1 dpi (Fig. 1A) was not sufficient to induce transmigration in the absence of other recruitment cues. hCMV did not enhance PBL transmigration though the EC monolayer at this time point either, revealing that there was no functional consequence of the increased RANTES secretion observed (Fig. 1Di), in the absence of essential adhesion receptor expression (Fig. 2). At 1 dpi, hCMV did not modulate TNF-α-induced neutrophil (Fig. 3Bi) or PBL (Fig. 3Ci) adhesion, suggesting that the trend of inhibition in TNF-α-induced expression of E-selectin and VCAM-1 seen at 1 dpi (Fig. 2A and B) was not sufficient to disrupt adhesion. There was no enhancement of neutrophil (Fig. 3Bi and ii) or PBL (Fig. 3Ci and 1Cii) recruitment on hCMV-EC compared to that of the uninfected control at 7 dpi. Importantly, there was a significant inhibition of neutrophil (Fig. 3Bi and ii) and PBL (Fig. 3Ci and 1Cii) adhesion and transmigration in response to TNF-α, consistent with our observations that hCMV-EC were refractory to TNF-α stimulation, as measured by chemokine (Fig. 1) and adhesion molecule (Fig. 2) expression.
Fig 3.
Effect of hCMV on EC leukocyte recruitment in a flow-based assay. (Ai) Phase-contrast images of uninfected EC and hCMV-EC at 1 and 7 dpi, before and after TNF-α treatment, prior to the addition of leukocytes. (Aii) Image of uninfected EC treated with TNF-α for 4 h to show phase bright surface-adherent neutrophils captured from flow (I) and phase dark neutrophils transmigrated below the EC layer (II). Uninfected or hCMV-EC were cultured for 1 or 7 dpi, prior to treatment with or without TNF-α, before analysis of neutrophil (B) or PBL (C) adhesion (i) or transmigration (ii). Data are means ± SEM from 3 independent experiments; each individual experiment was performed using EC from a different donor. *, P < 0.05; **, P < 0.01. (D) Neutrophils (i) and lymphocytes (ii) tended to be recruited to enlarged protruding hCMV-infected EC at 1 dpi.
In conclusion, we show that hCMV-induced modulation in EC inflammatory status can be biphasic, and, overall, our data argue that hCMV may in fact be less proinflammatory than several earlier highly cited studies proposed. In addition, we show that hCMV-infected EC preferentially capture lymphocytes rather than neutrophils. We suggest that the role of hCMV in inflammatory pathologies is likely to be context dependent, with factors such as time postinfection likely influencing the functional outcomes.
ACKNOWLEDGMENTS
This work was supported by the Swedish Heart and Lung Foundation (grant no. 20100614, 20100259), Swedish Medical Research Foundation (K2010-56X-12615-13-3, K2012-64X-10857-19-5), Swedish Cancer Foundation (110480), CERIC, and the Cardiovascular Program and Stockholm County Council.
Footnotes
Published ahead of print 27 March 2013
REFERENCES
- 1. Jarvis MA, Nelson JA. 2007. Human cytomegalovirus tropism for endothelial cells: not all endothelial cells are created equal. J. Virol. 81:2095–2101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nerheim PL, Meier JL, Vasef MA, Li WG, Hu L, Rice JB, Gavrila D, Richenbacher WE, Weintraub NL. 2004. Enhanced cytomegalovirus infection in atherosclerotic human blood vessels. Am. J. Pathol. 164:589–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lawlor G, Moss AC. 2010. Cytomegalovirus in inflammatory bowel disease: pathogen or innocent bystander? Inflamm. Bowel Dis. 16:1620–1627 [DOI] [PubMed] [Google Scholar]
- 4. Grundy JE, Lawson KM, MacCormac LP, Fletcher JM, Yong KL. 1998. Cytomegalovirus-infected endothelial cells recruit neutrophils by the secretion of C-X-C chemokines and transmit virus by direct neutrophil-endothelial cell contact and during neutrophil transendothelial migration. J. Infect. Dis. 177:1465–1474 [DOI] [PubMed] [Google Scholar]
- 5. Craigen JL, Yong KL, Jordan NJ, MacCormac LP, Westwick J, Akbar AN, Grundy JE. 1997. Human cytomegalovirus infection up-regulates interleukin-8 gene expression and stimulates neutrophil transendothelial migration. Immunology 92:138–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Visseren FL, Verkerk MS, Bouter KP, Diepersloot RJ, Erkelens DW. 1999. Interleukin-6 production by endothelial cells after infection with influenza virus and cytomegalovirus. J. Lab. Clin. Med. 134:623–630 [DOI] [PubMed] [Google Scholar]
- 7. Burns LJ, Pooley JC, Walsh DJ, Vercellotti GM, Weber ML, Kovacs A. 1999. Intercellular adhesion molecule-1 expression in endothelial cells is activated by cytomegalovirus immediate early proteins. Transplantation 67:137–144 [DOI] [PubMed] [Google Scholar]
- 8. Knight DA, Waldman WJ, Sedmak DD. 1999. Cytomegalovirus-mediated modulation of adhesion molecule expression by human arterial and microvascular endothelial cells. Transplantation 68:1814–1818 [DOI] [PubMed] [Google Scholar]
- 9. Shahgasempour S, Woodroffe SB, Garnett HM. 1998. Modulation of HLA class I antigen and ICAM-2 on endothelial cells after in vitro infection with human cytomegalovirus. Immunol. Cell Biol. 76:217–221 [DOI] [PubMed] [Google Scholar]
- 10. Span AH, Van Boven CP, Bruggeman CA. 1989. The effect of cytomegalovirus infection on the adherence of polymorphonuclear leucocytes to endothelial cells. Eur. J. Clin. Invest. 19:542–548 [DOI] [PubMed] [Google Scholar]
- 11. Bentz GL, Jarquin-Pardo M, Chan G, Smith MS, Sinzger C, Yurochko AD. 2006. Human cytomegalovirus (HCMV) infection of endothelial cells promotes naive monocyte extravasation and transfer of productive virus to enhance hematogenous dissemination of HCMV. J. Virol. 80:11539–11555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ley K. 1996. Molecular mechanisms of leukocyte recruitment in the inflammatory process. Cardiovasc. Res. 32:733–742 [PubMed] [Google Scholar]
- 13. Jarvis MA, Borton JA, Keech AM, Wong J, Britt WJ, Magun BE, Nelson JA. 2006. Human cytomegalovirus attenuates interleukin-1beta and tumor necrosis factor alpha proinflammatory signaling by inhibition of NF-kappaB activation. J. Virol. 80:5588–5598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cooke BM, Usami S, Perry I, Nash GB. 1993. A simplified method for culture of endothelial cells and analysis of adhesion of blood cells under conditions of flow. Microvasc. Res. 45:33–45 [DOI] [PubMed] [Google Scholar]
- 15. Butler LM, Jeffery HC, Wheat RL, Rae PC, Townsend K, Alkharsah KR, Schulz TF, Nash GB, Blackbourn DJ. 2011. Kaposi's sarcoma-associated herpesvirus infection of endothelial cells inhibits neutrophil recruitment through an interleukin-6-dependent mechanism: a new paradigm for viral immune evasion. J. Virol. 85:7321–7332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Butler LM, Rainger GE, Rahman M, Nash GB. 2005. Prolonged culture of endothelial cells and deposition of basement membrane modify the recruitment of neutrophils. Exp. Cell Res. 310:22–32 [DOI] [PubMed] [Google Scholar]
- 17. Bickel M. 1993. The role of interleukin-8 in inflammation and mechanisms of regulation. J. Periodontol. 64:456–460 [PubMed] [Google Scholar]
- 18. Botto S, Streblow DN, DeFilippis V, White L, Kreklywich CN, Smith PP, Caposio P. 2011. IL-6 in human cytomegalovirus secretome promotes angiogenesis and survival of endothelial cells through the stimulation of survivin. Blood 117:352–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Almeida GD, Porada CD, St Jeor S, Ascensao JL. 1994. Human cytomegalovirus alters interleukin-6 production by endothelial cells. Blood 83:370–376 [PubMed] [Google Scholar]
- 20. Carr MW, Roth SJ, Luther E, Rose SS, Springer TA. 1994. Monocyte chemoattractant protein-1 acts as a T-lymphocyte chemoattractant. Proc. Natl. Acad. Sci. U. S. A. 91:3652–3656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hirsch AJ, Shenk T. 1999. Human cytomegalovirus inhibits transcription of the CC chemokine MCP-1 gene. J. Virol. 73:404–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tull SP, Yates CM, Maskrey BH, O'Donnell VB, Madden J, Grimble RF, Calder PC, Nash GB, Rainger GE. 2009. Omega-3 fatty acids and inflammation: novel interactions reveal a new step in neutrophil recruitment. PLoS Biol. 7:e1000177 doi:10.1371/journal.pbio.1000177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ley K, Laudanna C, Cybulsky MI, Nourshargh S. 2007. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 7:678–689 [DOI] [PubMed] [Google Scholar]
- 24. Stone PC, Lally F, Rahman M, Smith E, Buckley CD, Nash GB, Rainger GE. 2005. Transmigrated neutrophils down-regulate the expression of VCAM-1 on endothelial cells and inhibit the adhesion of flowing lymphocytes. J. Leukoc. Biol. 77:44–51 [DOI] [PubMed] [Google Scholar]
- 25. Yang L, Froio RM, Sciuto TE, Dvorak AM, Alon R, Luscinskas FW. 2005. ICAM-1 regulates neutrophil adhesion and transcellular migration of TNF-alpha-activated vascular endothelium under flow. Blood 106:584–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dengler TJ, Raftery MJ, Werle M, Zimmermann R, Schonrich G. 2000. Cytomegalovirus infection of vascular cells induces expression of pro-inflammatory adhesion molecules by paracrine action of secreted interleukin-1beta. Transplantation 69:1160–1168 [DOI] [PubMed] [Google Scholar]
- 27. Sedmak DD, Knight DA, Vook NC, Waldman JW. 1994. Divergent patterns of ELAM-1, ICAM-1, and VCAM-1 expression on cytomegalovirus-infected endothelial cells. Transplantation 58:1379–1385 [PubMed] [Google Scholar]
- 28. Buttrum SM, Hatton R, Nash GB. 1993. Selectin-mediated rolling of neutrophils on immobilized platelets. Blood 82:1165–1174 [PubMed] [Google Scholar]
- 29. Libby P. 2012. Inflammation in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 32:2045–2051 [DOI] [PMC free article] [PubMed] [Google Scholar]