Abstract
Griffithsin, which binds N-linked glycans on gp120 to prevent HIV entry, has the most potent HIV-1 inhibitory activity described for any antiviral lectin and is being developed for topical preexposure prophylaxis. The current studies were designed to further assess its potential by exploring its activity against herpes simplex virus 2 (HSV-2), a cofactor for HIV acquisition, in vitro and in a murine model. Safety was evaluated by examining its impact on epithelial barrier integrity in polarized cultures and testing whether repeated intravaginal dosing potentiates the susceptibility of mice to genital herpes. Griffithsin displayed modest inhibitory activity against HSV-2 if present during viral entry but completely blocked plaque formation if present postentry, reduced plaque size, and prevented cell-to-cell spread. These in vitro findings translated to significant protection against genital herpes in mice treated with 0.1% griffithsin gel. Griffithsin, but not placebo gel, prevented viral spread (visualized with a luciferase-expressing virus), significantly reduced disease scores, and resulted in greater survival (P < 0.05, log rank test). Protection persisted when HSV-2 was introduced in seminal plasma. Although griffithsin triggered a small decline in transepithelial electrical resistance in polarized cultures, this did not translate to any significant increase in the ability of HIV to migrate from the apical to the basolateral chamber nor to an increase in susceptibility to HSV-2 in mice treated with griffithsin gel for 7 days. These findings demonstrate that griffithsin inhibits HSV-2 by a unique mechanism of blocking cell-to-cell spread and support its further development for HIV and HSV-2 prevention.
INTRODUCTION
There has been exciting progress in the development of oral and topical products for the prevention of HIV and herpes simplex virus 2 (HSV-2). The recent CAPRISA 004 trial, in which a 39% (95% confidence interval [CI] of 6 to 61%) reduction in HIV and a 54% reduction in HSV-2 acquisition were observed in women who applied 1% tenofovir gel before and after sex, illustrated the potential for delivering safe and effective vaginal prevention products (1). However, the finding that tenofovir vaginal gel applied daily provided no protection against HIV acquisition in the recently discontinued arm of the Microbicide Trials Network 003 (VOICE) trial highlights the complexities of delivering preexposure prophylactic (PrEP) strategies (2). Similarly, there have been conflicting results with oral PrEP clinical trials. For example, efficacies of 66% and 73% were observed among women taking daily oral tenofovir disoproxil fumarate (TDF) (Viread) or the combination of TDF and emtricitabine (FTC) (Truvada), respectively, in the Partners in Prevention trial, whereas the VOICE oral TDF arm and the FEMPReP Truvada study were discontinued early for futility (3–6). The differences in outcomes likely reflect a combination of behavioral and biomedical factors, including adherence to product, differences in the populations studied (e.g., discordant couples in Partners), and mucosal inflammation. Inflammation, which may be triggered in response to hormonal contraception, other sexually transmitted infections, and changes in vaginal microbiota, may promote HIV acquisition and modulate drug efficacy and pharmacokinetics (7).
The discrepant clinical trial outcomes highlight the need to develop additional products that could be used either alone or in combination with tenofovir to improve the efficacy of prevention strategies. One such candidate is griffithsin (GRFT). GRFT is a small homodimeric lectin that was isolated from a red alga (Griffithsia sp.) and binds to clusters of oligomannose N-linked glycans on the HIV-1 envelope glycoprotein gp120 to prevent viral entry. GRFT has the most potent and broad-spectrum HIV-1 inhibitory activity yet described for any antiviral lectin and showed synergistic activity with tenofovir in vitro (8–11). Importantly, it is unlikely that GRFT will be developed as part of any antiretroviral treatment regimen because of its poor oral bioavailability, rendering it ideal for topical prophylaxis without promoting drug resistance and compromising population-level antiretroviral efficacy. This differentiates GRFT from other protein- and peptide-based candidate PrEP drugs (12). An efficient, scalable manufacturing system for bulk production of the GRFT active pharmaceutical ingredient was developed using Nicotiana plants (11). In contrast to cyanovirin-N (CV-N), which was the first lectin formulated as a candidate vaginal prevention product, plant-produced GRFT causes no mitogenic stimulation of peripheral blood mononuclear cells (PBMC) (11), induces minimal secretion of inflammatory cytokines and chemokines by epithelial cells or human PBMC, and has no measureable effect on cell viability or gene expression in human ectocervical cells (13). GRFT was also safe in the rabbit vaginal irritation model (11).
In addition to these measures of safety, topically applied products may inadvertently increase HIV acquisition by disrupting the epithelium, which serves as a barrier to HIV. We previously published results with two synergistic preclinical safety models: a dual-chamber culture model, which examines the impact drugs have on epithelial cell integrity and on the ability of HIV to traverse a multilayered epithelial barrier (14), and a murine model that evaluates whether formulated gels potentiate the susceptibility of mice to genital HSV-2 infection (15–17). We hypothesize that increased susceptibility to genital herpes in the mouse reflects changes in mucosal integrity and immune environment and serves as a surrogate of increased HIV risk. We have used these models to test several products in clinical development, and the results obtained have predicted, for example, that cellulose sulfate (which was ineffective in clinical trials [18]) may disrupt the epithelial barrier to facilitate HIV and HSV infection (14, 16, 17). Building from the experience with these models, the current studies were designed to further evaluate the safety of GRFT. As a prelude to testing GRFT in the murine HSV susceptibility model, we first evaluated its antiviral activity against HSV-2. Previous studies with CV-N have yielded conflicting results with respect to its anti-HSV-1 activity (19, 20), and there are no published studies examining the activity of GRFT in models of HSV-2 infection. Oligomannose glycans appear to be more prevalent on viral envelope glycoproteins than in the host cellular glycome, playing a vital role in virus attachment, entry, and immune evasion and providing an attractive target for broad-spectrum antiviral prophylaxis and therapy (21). The HIV envelope glycoproteins are very heavily glycosylated, with almost all N-linked glycosylation sites occupied by oligomannose structures (22, 23). The structure of the N-linked glycans on HSV glycoproteins has not been investigated in detail in recent years, but a 1982 study by Wenske et al. reported that, based on sensitivity to endoglycosidase EndoH, the N-linked glycans on the HSV-2 envelope glycoproteins were predominantly of the complex form (24). Thus, we hypothesized that GRFT would have only modest activity against HSV-2, reflecting the presence of fewer oligomannose N-linked glycans on its envelope glycoproteins.
MATERIALS AND METHODS
Cells and viral strains.
CaSki (human cervical epithelial), HEC-1-A (human endometrial), VK2/E6E7 (human papillomavirus [HPV] E6/E7 immortalized vaginal epithelial), Vero (monkey kidney), and 3T3 (murine fibroblast) cells were obtained from the American Tissue Culture Collection. TZM-bl cells (an engineered HeLa cell clone that expresses human CD4, CCR5, and CXCR4 and contains HIV-1 Tat-regulated reporter genes for firefly luciferase and β-galactosidase) were obtained from the AIDS Research and Reference Reagent Program. Jurkat-Tat-CCR5 (JT-CCR5) cells were provided by Quentin Sattentau (Sir William Dunn School of Pathology, University of Oxford, Oxford, United Kingdom). Cells were cultured as previously described (25–27). Peripheral blood mononuclear cells (PBMC) were isolated from human leukopacks (New York Blood Center) by density gradient centrifugation using Ficoll-Histopaque (Sigma-Aldrich, St. Louis, MO). CD14+ monocytes were isolated using CD14 magnetic cell separation (Easysep; StemCell Technologies, Vancouver, BC, Canada), and immature monocyte-derived dendritic cells (moDC) were generated by culturing the cells for 7 to 8 days in RPMI 1640, 10% fetal bovine serum (FBS), 2 mM glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin supplemented with 800 U/ml recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF), and 800 U/ml recombinant human interleukin 4 (IL-4) (R&D Systems Inc., Minneapolis, MN) (28). HIV-1BaL (R5-utilizing strain) was grown as previously described (26). HSV-2(G) (29) was grown on Vero cells. HSV-2(4674), a clinical isolate obtained from the clinical virology laboratory at Montefiore Hospital, Bronx, NY, was propagated on human keratinocytes (HaCaT) (provided by David Johnson, Oregon Health & Sciences University, Portland, OR). HSV-2-MS-luciferase, expressing firefly luciferase under the control of a cytomegalovirus (CMV) promoter with a nonessential latency-associated transcript (LAT) gene as the locus of insertion, was propagated on Vero cells (30). HSV-2(333)ZAG, a recombinant virus expressing green fluorescent protein (GFP) under the control of a CMV promoter (the construct was inserted in an intergenic region between UL3 and UL4), was propagated on Vero cells (gift from P. Spear, Northwestern University). All viral stocks were stored at −80°C.
Drugs.
Recombinant GRFT was produced in Nicotiana benthamiana plants as described previously (11) and formulated as 0.1% gel using Carbopol 974P, glycerin, EDTA, methylparaben, and propylparaben (Magee Women's Research Institute, Pittsburgh PA). A matched Carbopol placebo gel containing all excipients except GRFT was used as a negative control. Nonoxynol-9 (N-9) was included as a positive toxicity control; 4% N-9 gel was obtained from Encare, manufactured by Blairex Laboratories, Inc., Columbus, IN. PRO 2000 was provided by Indevus Pharmaceuticals, and acyclovir (ACV) was purchased from Bedford Laboratories (Bedford, OH).
Antiviral activity in vitro.
CaSki, 3T3, and VK2/E6E7 cells were grown in 24-well plates to near confluence and then exposed to GRFT (10, 100, or 500 μg/ml, equivalent to 0.392, 3.92, and 19.58 nmol, respectively, in phosphate-buffered saline [PBS]) or 200 μg/ml PRO 2000 for 15 min prior to exposure to HSV-2(4674) at an inoculum designed to yield 75 to 100 PFU in control wells. Cells were incubated for 1 h at 37°C and then washed with PBS and overlaid with medium containing methyl cellulose. Alternatively, the cells were first infected with virus, and then GRFT was added to the overlay medium after 1 h of incubation. Plaques were counted after 48 h of incubation, and the percentages of plaques relative to plaques formed on control wells (no drug) were calculated. Immature moDCs (1 × 106 cells) were pretreated with GRFT (10, 100, or 500 μg/ml) overnight at 37°C and were then challenged with HSV-2(333)ZAG (multiplicity of infection [MOI] of 1 PFU/cell) or mock infected by being exposed to RPMI with 10% FBS. After incubation for 1 h at 37°C, the cells were washed three times with PBS and were then cultured in fresh medium for 4 h. GFP expression, as a marker of viral infection, was quantified by flow cytometry after gating on the live populations using a fixable violet Live/Dead marker (Invitrogen, Carlsbad, CA). Ten thousand live events were acquired per sample. To determine if GRFT blocked release of infectious progeny from DCs, the DCs were infected with HSV-2 for 1 h in the absence or presence of GRFT, and then after being washed as described above, the cells were cultured for 24 h in medium containing GRFT or medium alone, and viral release was quantified by determining the titer of the culture supernatants on Vero cells.
Single-step and multistep growth of virus.
To measure single-step growth of virus, CaSki cells were infected with HSV-2(4674) (MOI of 5 PFU/cell) for 1 h at 37°C. Cells were washed for 2 min with a low-pH citrate buffer (40 mM citric acid, 10 mM KCl, 135 mM NaCl, pH 3.0) for 2 min to inactivate extracellular virus and then were washed three times with media prior to being overlaid with 199V alone or supplemented with GRFT (100 μg/ml) or ACV (100 μg/ml). Supernatants and cells were harvested separately 4, 8, and 16 h postinfection (hpi) and stored at −80°C until plating on Vero cells to measure infectious virus. Cells were frozen and thawed three times to cause lysis prior to infecting Vero cells. For multistep growth assays, the CaSki cells were infected with HSV-2(4674) (MOI of 0.01 PFU/cell) for 1 h at 37°C as described above; supernatants and cells were harvested at 12, 24, 36, 48, and 72 hpi; and the titer was determined on Vero cells as described for single-step growth experiments.
Measurement of viral DNA levels using RT-qPCR.
CaSki cells were infected with virus at an MOI of 1 for 1 h at 37°C. Cells were washed with citrate buffer once and with medium three times and then cultured in 199V alone or supplemented with 100 μg/ml GRFT or ACV. At 8 and 12 hpi, cells were harvested and DNA was extracted using a DNeasy blood and tissue kit (Qiagen, Valencia, CA). Viral replication was assessed by real-time quantitative PCR (RT-qPCR) using primers for ICP4 and RPLPO from Applied Biosystems (Carlsbad, CA).
Infectious center assay.
CaSki cells (donor cells) were exposed to virus at 37°C to allow entry (MOI of 5 PFU/cell). Four hours after infection, the infected cells were detached with trypsin-EDTA and counted, and ∼500 infected cells were plated onto duplicate monolayers of uninfected cells in medium containing pooled human immunoglobulin G (IgG; Sigma) in the presence of GRFT or medium alone. The pooled human immunoglobulin neutralizes infection by virus released into the medium. Plaques were quantified by Giemsa staining after 48 h of incubation (31).
Dual-chamber safety assay.
HEC-1-A cells were grown in Transwell inserts and allowed to polarize to mimic the genital tract epithelial barrier, which is relatively impervious to HIV (14). Cells were apically exposed to GRFT (100 and 1,000 μg/ml) or 0.1% N-9 for 16 h. Drug was removed by washing thrice with serum-free medium before the addition of HIV-1BaL (40 ng of p24 antigen) in medium and JT-CCR5 cells to the apical (AP) and basolateral (BL) chambers, respectively. Cultures were maintained for an additional 5 days, and BL supernatants were collected at different times and tested for p24 content as previously described (14).
Drug toxicity and cell apoptosis analysis.
Cells (CaSki, VK2/E6E7, 3T3, and immature moDCs) were exposed to 10, 100, or 500 μg/ml of GRFT or medium as a negative-control overnight (moDCs) or for 48 h (CaSki, VK2/E6E7, and 3T3) at 37°C, and cell viability was assessed using an MTS assay (Promega, Madison, WI). In addition, the DCs were harvested and analyzed for early and late signs of apoptosis by using an annexin V and 7AAD staining protocol (BD Pharmingen, San Diego, CA). Cells were analyzed by flow cytometry, acquiring 10,000 events per sample (28). To assess whether GRFT had an impact on DC maturation or function, DCs were treated with GRFT (10, 100, or 500 μg/ml), lipopolysaccharide (LPS) (10 μg/ml) (positive control), or medium (negative control) for 24 h at 37°C. Cells were stained with anti-CD83-allophycocyanin (APC) (BD Pharmingen) for 30 min, washed twice in PBS, and fixed in 4% paraformaldehyde (BD Biosciences, Franklin Lakes, NJ). Flow cytometry was performed on a Becton, Dickinson LSR II analyzer, and analysis was carried out using FlowJo version 9.3.1 software (Tree Star, Inc., Ashland, OR). Ten thousand live events were acquired per sample.
Murine models.
Studies were conducted with approval from the Albert Einstein College of Medicine Institutional Animal Care and Use Committee. Female BALB/c mice (6 to 8 weeks), purchased from Jackson Laboratory (Bar Harbor, ME), were pretreated subcutaneously with 2.5 mg of medroxyprogesterone acetate (MPA; Sicor Pharmaceuticals, Irvine, CA) 5 days prior to intravaginal application of 20 μl of 0.1% GRFT, Carbopol gel, or no gel. Fifteen minutes later, mice were challenged with 30 μl of virus diluted either in PBS or in pooled human seminal plasma (SP) (Lee BioSolutions, St. Louis, MO) at a dose that typically causes disease in 50% and 90% (LD50 and LD90) of mice treated with placebo gel. Mice were monitored for 14 days and scored (scale of 0 to 5) for evidence of erythema, edema, or genital ulcers (genital disease score) or for evidence of urinary or fecal retention and hind-limb paralysis (neurological score). Mice were euthanized for genital tract or neurologic disease scores of 4 or more (32). Vaginal washes were collected on days 0, 1, and 2 postinfection for measurement of cytokines and chemokines. In parallel experiments, mice were sacrificed at days 1, 3, and 7 postinfection, and genital tract tissue was excised to assess for infectious virus by performing plaque assay on tissue homogenates and for expression of cytokines and chemokines.
To visualize the spread of viral infection, MPA-treated mice received 20 μl of gel intravaginally 15 min prior to challenge with 30 μl HSV-2-MS-luciferase diluted in PBS (equivalent to ∼106 PFU). Mice were imaged on days 1 and 4 postinfection. Mice were anesthetized with isoflurane and then treated with an intraperitoneal injection of 1.6 mg d-luciferin potassium salt (Gold Biotechnology, St. Louis, MO) in 100 μl PBS. Images were captured using an IVIS Lumina II bioluminescent imager (Caliper Instruments, Hopkinton, MA) up to 20 min after substrate injection. Images were analyzed using Living Image version 4.2 software (Caliper Instruments). Identical regions of interests (ROIs) were placed on each infected mouse to determine total flux.
To evaluate whether exposure to GRFT gel increased the susceptibility to infection, 40 μl of GRFT or Carbopol gel was delivered intravaginally daily for 7 days to MPA-treated mice. Approximately 12 h after the seventh application, a vaginal wash was performed with 150 μl of saline to remove any drug remaining in the vaginal vault, and the mice were then intravaginally challenged with HSV-2(4674) (equivalent to approximately an LD10, LD50, and LD90) and monitored daily for 14 days as described above. The vaginal washes were pooled (2 mice per pool) and evaluated for anti-HIV activity in a TZM-bl assay (33).
Detection of cytokines and chemokines.
Protease inhibitors (Roche Applied Science, Indianapolis, IN) were added to each vaginal wash sample before centrifugation at 210 g for 10 min at 4°C, to remove mucous and cellular debris. The supernatants were stored at −80°C, pooled in groups varying from 2 to 5 mice, and assayed for IL-6, gamma interferon (IFN-γ), IL-1β, monocyte chemoattractant protein 1 (MCP-1), macrophage inflammatory protein 1 beta (MIP-1β), MIP-2, RANTES, and tumor necrosis factor alpha (TNF-α) release using the Fluorokine MultiAnalyte profiling system (R&D Systems, Minneapolis, MN), measured with a Luminex 100 system, and analyzed with StarStation version 2.3 (Applied Cytometry Systems, Dinnington, South Yorkshire, United Kingdom).
Gene expression by RT-qPCR.
Total RNA was extracted from the genital tract using an Absolutely RNA reverse transcriptase PCR (RT-PCR) prep minikit (Stratagene, La Jolla, CA). Reverse transcription and RT-PCR were performed as described previously (17). Relative gene expression levels of cellular genes, normalized to β-actin RNA levels as an endogenous housekeeping gene, were compared to those of untreated mice. Primers for β-actin, IFN-γ, IRF-7, Mx1, and Oas2 were obtained from Applied Biosystems (Carlsbad, CA).
HSV-2 titer in mouse tissue.
Genital tract tissue was weighed, homogenized in serum-free Dulbecco's modified Eagle's medium (DMEM) using RNase-free pestles, sonicated for 30 s, and centrifuged at 10,000 × g for 5 min. Supernatants were assayed for viral titer on Vero cells (2 × 105 cells/well of a 48-well plate), and plaques were quantified using Giemsa stain in standard plaque assay.
Statistical analyses.
Responses were compared by analysis of variance (ANOVA) or unpaired t tests, using GraphPad Prism version 5 (San Diego, CA). Kaplan-Meier survival curves were compared by log rank tests. A P value of <0.05 was considered significant.
RESULTS
GRFT has modest effects on HSV entry but inhibits cell-to-cell spread.
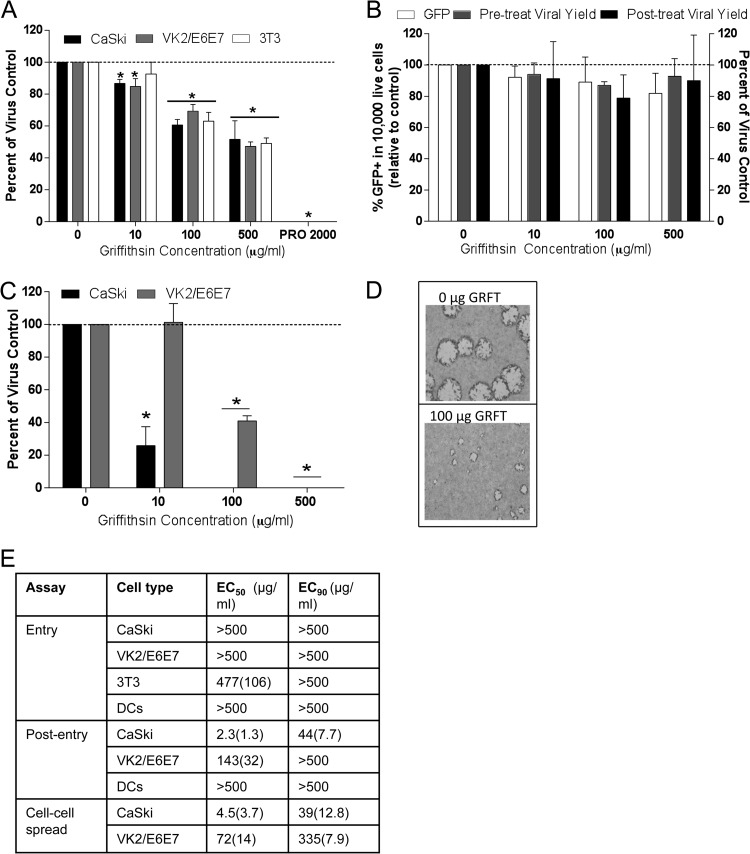
Human cervical (CaSki) and vaginal (VK2/E6E7) cells or murine fibroblasts (3T3) were infected with HSV-2(4674) in the presence of increasing concentrations of GRFT (0, 10, 100, or 500 μg/ml) or PRO 2000 (200 μg/ml), a known inhibitor of HSV binding and entry (31) for 1 h, and then the inoculum and drug were removed by washing and the cells were overlaid with fresh medium. Plaques were counted after 24 to 48 h. GRFT had only modest effects on HSV plaque formation and reduced the number of plaques by less than 50% even at the highest concentration (500 μg/ml), when drug was present during viral entry, while PRO 2000 completely blocked plaque formation (Fig. 1A). Similarly, GRFT failed to block infection of immature moDCs when cells were pretreated with the drug; infection was monitored by quantifying GFP expression 4 h postexposure to HSV-2(333) (GFP) (Fig. 1B). However, when GRFT was added postentry to CaSki or VK2/E6E7 cells, there was a significant reduction in the number and size of viral plaques formed, resulting in mean concentrations that inhibit 50% of viral plaques (EC50) of 2.3 (standard error of the mean [SEM], 1.3) μg/ml and 143 (SEM, 32) μg/ml in CaSki and VK2/E6E7 cells, respectively (Fig. 1C to E). These findings suggest that GRFT may prevent the first round of viral replication, HSV release, and/or cell-to-cell spread.
Fig 1.
Griffithsin inhibits HSV-2 infection post entry. (A) CaSki, VK2/E6E7, and 3T3 cells were exposed to GRFT or PRO 2000 for 15 min, followed by challenge with HSV-2(4674) for 1 h. The cells were washed and then overlaid with medium, and plaques were counted after 24 to 48 h; results are presented as PFU/well as percentages of control (no drug) and are means + SEM from 2 to 4 experiments conducted in duplicate. (B) Immature moDCs were exposed to GRFT overnight and then challenged with HSV-2(333)ZAG (MOI of 1 PFU/cell) for 1 h at 37°C. Infection was assessed by quantifying GFP expression (10,000 live event acquisition) by flow cytometry 4 hpi. Results are presented as percentage of GFP+ cells and are means + SEM from six (pretreat) independent experiments. To monitor effects of GRFT on the release of viral progeny, the DCs were exposed to GRFT either before or after initial entry, and supernatants were harvested 24 hpi and overlaid onto Vero cells; viral plaques were counted after Giemsa staining and are presented as percentages relative to DCs infected in the absence of GRFT from 2 independent experiments, each conducted in duplicate. (C) CaSki or VK2/E6E7 cells were first exposed to HSV-2 for 1 h, washed with PBS, and then cultured in medium supplemented with the indicated concentration of GRFT; results are presented as percentages of the control and are the means + SEM from 3 experiments performed in duplicate. (D) Representative photograph of viral plaques in VK2/E6E7 cells when GRFT was added postentry. (E) EC50 and EC90 (μg/ml) were calculated for GRFT using data from panels A to C and Fig. 2A. Asterisks indicate P values of <0.05 compared to no drug (t test).
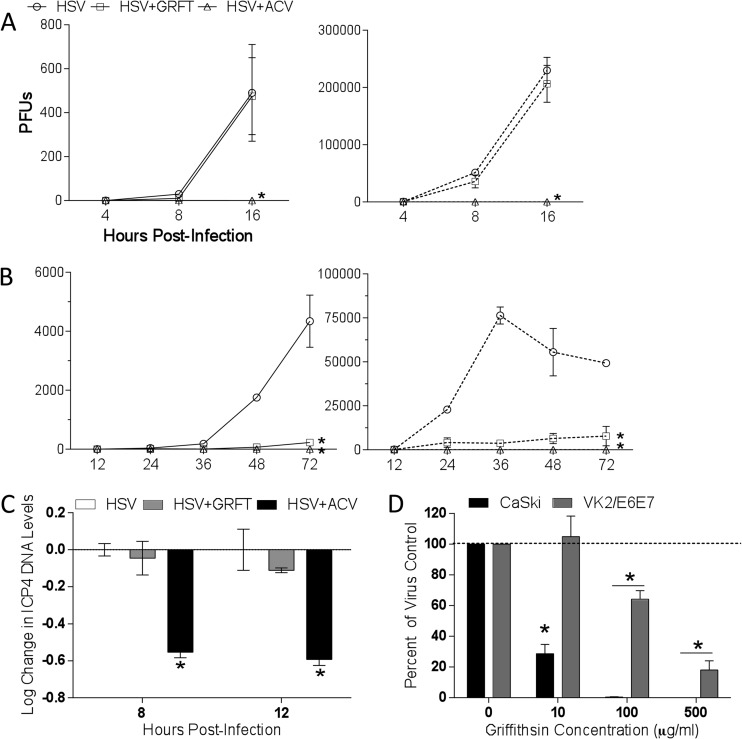
To address these possibilities, single-step and multistep viral growth were assessed by adding GRFT or acyclovir (ACV), an inhibitor of HSV DNA replication, to the cultures postentry. The titers of supernatants and cells were determined separately to exclude any effect of residual extracellular GRFT in supernatants or of intracellular ACV in the harvested cells that might impact the subsequent titer determination of viral progeny. There was no difference in single-step growth curve when cells were treated with GRFT (Fig. 2A), but GRFT almost completely blocked multistep growth of virus (Fig. 2B). As expected, ACV blocked both single-step and multistep HSV growth. Consistent with the absence of any effects on the single-step growth curve, GRFT had little or no effect on HSV DNA levels measured by RT-qPCR of ICP4 DNA 8 or 12 h postinfection, whereas ACV significantly reduced viral DNA levels (Fig. 2C). Moreover, GRFT had no impact on the release of infectious progeny from moDCs when drug was added pre- or postentry, as monitored by titer determination of the DC culture supernatants on Vero cells 24 hpi (Fig. 1B). Taken together, these findings indicate that GRFT likely interferes with cell-to-cell spread of HSV-2.
Fig 2.
Griffithsin inhibits cell-to-cell spread of HSV-2. (A) One-step growth of HSV was quantified by infecting CaSki cells with an MOI of 5 and then adding GRFT or ACV to the overlay media. At 4, 8, and 16 hpi, supernatants and cells were harvested and, after cells were freeze-thawed three times, viral growth was quantified by determining the titers of the supernatants (solid lines) or cells (dotted lines) on Vero cells. (B) To assess multistep growth, CaSki cells were infected with an MOI of 0.01, and drug was added to overlay media as described for panel A, and then at 12, 24, 36, 48, and 72 hpi, supernatants and cells were again harvested and titers were determined on Vero cells. Data shown are from two experiments conducted in duplicate. Asterisks indicate significance compared to no drug using two-way ANOVA. (C) CaSki cells were infected with HSV-2 at an MOI of 1 with GRFT, ACV, or no drug (control) added to the overlay media. Cells were harvested 8 or 12 hpi, and DNA was extracted and run in real-time PCR. Data are presented as means ± SEM of log change in ICP4 DNA levels relative to infected cells without drug, controlling for RPLPO as the housekeeping gene. Asterisk represents significance compared to HSV without drug using unpaired t test, P < 0.05. (D) Cells were infected with HSV-2 for 4 to 5 h, after which time they were trypsinized and cocultured with uninfected cells in medium supplemented with pooled human IgG and GRFT for 48 h to allow plaques to form. Data are shown as means + SEM of percentage of virus control from three independent experiments. Asterisks indicates significance from virus control (no GRFT) using unpaired t test, P < 0.05.
To further address this, infectious center assays were conducted (31). Uninfected cells were cocultured with ∼500 infected cells in the presence of pooled human immunoglobulin. The pooled immunoglobulin neutralizes any released virus, restricting viral spread to local fusion events between the plasma membranes of infected and uninfected cells. The addition of GRFT to the cocultures completely blocked cell-to-cell spread in CaSki and VK2/E6E7 cells at 100 and 500 μg/ml, respectively (Fig. 2D).
GRFT inhibits viral infection in the murine model.
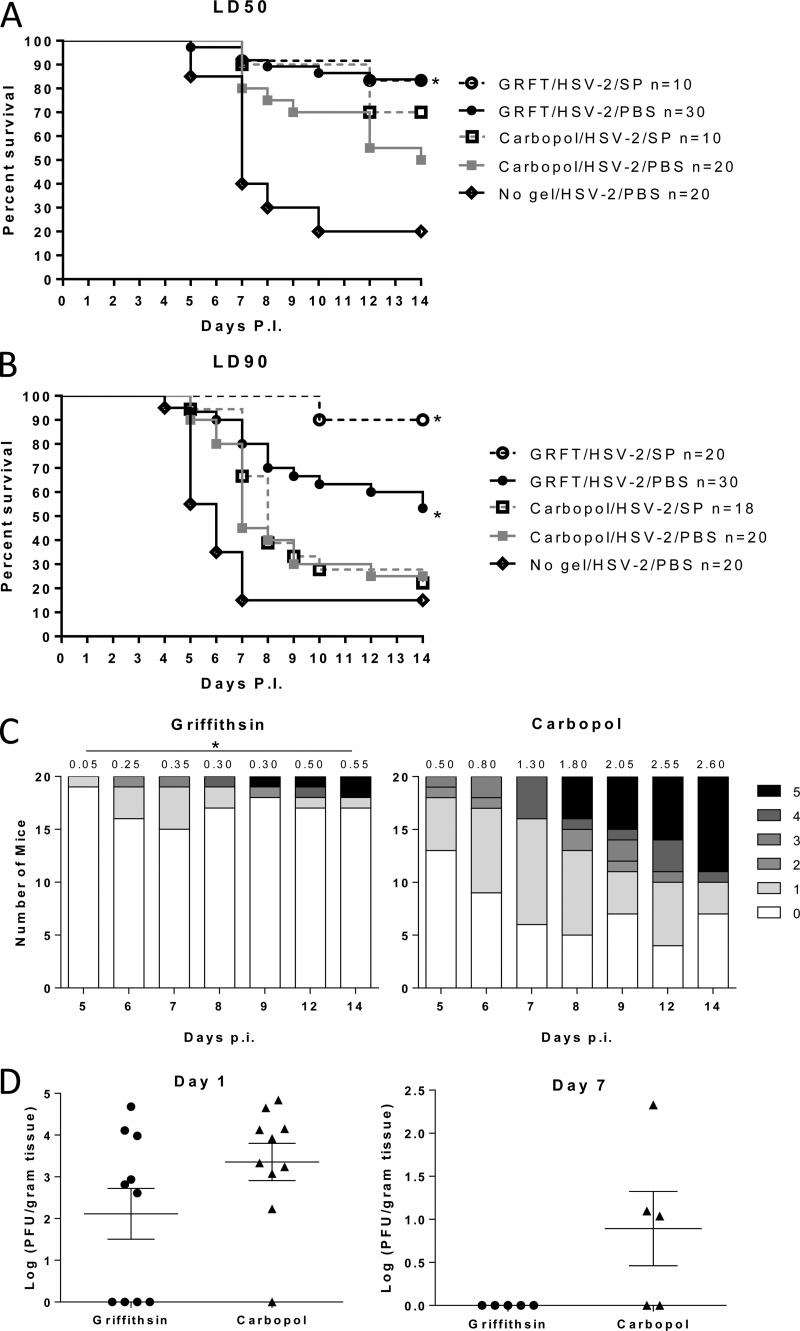
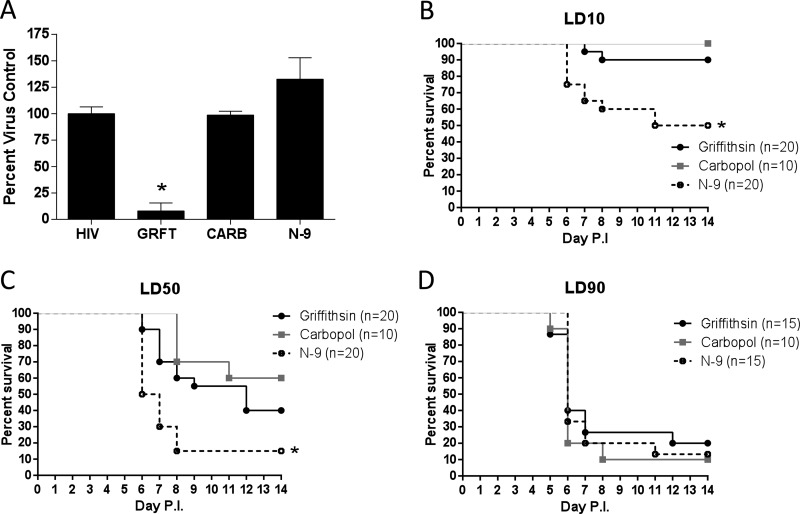
The observation that GRFT prevents cell-to-cell spread suggests that it could be an effective agent against viral dissemination and mitigate genital herpes disease. To evaluate this, medroxyprogesterone-treated mice received a single application of GRFT (0.1% gel), placebo Carbopol gel, or no gel 15 min prior to challenge with an LD50 or LD90 of HSV-2 (based on prior studies with placebo gels). Virus was delivered in PBS or pooled human seminal plasma to simulate what may happen clinically. Mice were monitored and scored for 14 days for signs of disease. GRFT protected the mice from HSV-2 disease compared to Carbopol-treated and untreated (no gel) animals, regardless of whether virus was introduced in PBS or seminal plasma (Fig. 3A to C; P < 0.05). Notably, at the higher inoculum (LD90), greater protection was observed when mice were challenged with HSV-2 in seminal plasma than when virus was introduced in PBS (Fig. 3B).
Fig 3.
Griffithsin protects mice from genital herpes infection. Medroxyprogesterone acetate-treated mice received a single dose of 0.1% GRFT or Carbopol gel intravaginally (20 μl). Fifteen minutes later, the mice were intravaginally inoculated with an LD50 (A) or LD90 (B) of HSV-2(4674) diluted in either PBS or pooled human seminal plasma (SP). Mice were scored for disease daily and were sacrificed if the score reached 4. Additional controls included mice that received no gel prior to challenge with virus in PBS. The number of mice in each group is indicated in the figure legend of the Kaplan-Meier survival curves; asterisks indicate P values of <0.05 (log rank test) (A and B). Disease scores for the 20 mice treated with Carbopol or GRFT gel and challenged with an LD50 of HSV-2 in PBS are shown in panel C, with average daily disease scores indicated above each bar. The asterisks denote that the difference in disease scores was significantly lower for GRFT- than for Carbopol-treated mice at each time point (P < 0.05; t test). Genital tract tissue was harvested 1 and 7 days postinfection, weighed, and homogenized, and supernatants were cultured on Vero cells to determine the amount of infectious HSV-2 present. Plaques were counted after 48 h; each dot represents an individual animal, and the line indicates mean ± SEM; day 1, P = 0.12; day 7, P = 0.07, unpaired t test.
The in vivo protective effects of GRFT were further evaluated by harvesting genital tract tissue and assaying for infectious virus. HSV-2 was recovered from excised homogenized genital tract tissue in 9/10 mice that received Carbopol (median log titer of 3.92 PFU/g tissue) and 6/10 mice who received GRFT (median log titer of 3.46 PFU/g tissue) 1 day postinfection (P = 0.11). Virus was recovered from 3/5 mice that received Carbopol gel (median log titer of 1.10 PFU/g tissue) compared to 0/5 mice that received GRFT 7 days postinfection (P = 0.07, unpaired t test) (Fig. 3D).
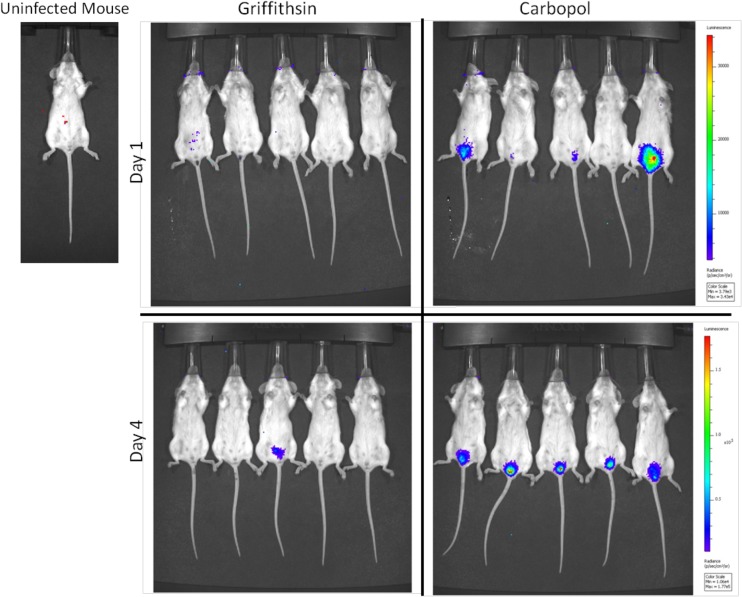
To further examine the impact of GRFT on genital herpes, we took advantage of an HSV-2 variant, MS-luciferase, in which a luciferase expression cassette was inserted into the LAT locus (30), and monitored viral spread using bioluminescent imaging. Mice were pretreated with Carbopol or GRFT gel and then challenged with 106 PFU of MS-luciferase. In the Carbopol-treated mice, luciferase activity increased by an average of 2.7-fold and 4.7-fold compared to the background (uninfected mouse treated with d-luciferin) on days 1 and 4 postviral challenge, respectively (Fig. 4). GRFT-treated mice showed no increase in luciferase activity compared to an uninfected mouse. Luciferase was detected in one GRFT-treated mouse on day 4, but none of the GRFT-treated mice developed significant clinical disease over a 10-day observation period.
Fig 4.
GRFT treatment prevents viral dissemination in mice. Medroxyprogesterone-treated mice received a single dose of GRFT or Carbopol gel and 15 min later were intravaginally infected with HSV-2-MS-luciferase (106 PFU in 30 μl). Mice were imaged on days 1 and 4 postinfection, 15 min after receiving an intraperitoneal dose of d-luciferin. Total flux from identical ROIs were compared to total flux from ROI on the control mouse pretreated with medroxyprogesterone, not infected with HSV-2, 15 min after intraperitoneal injection of d-luciferin.
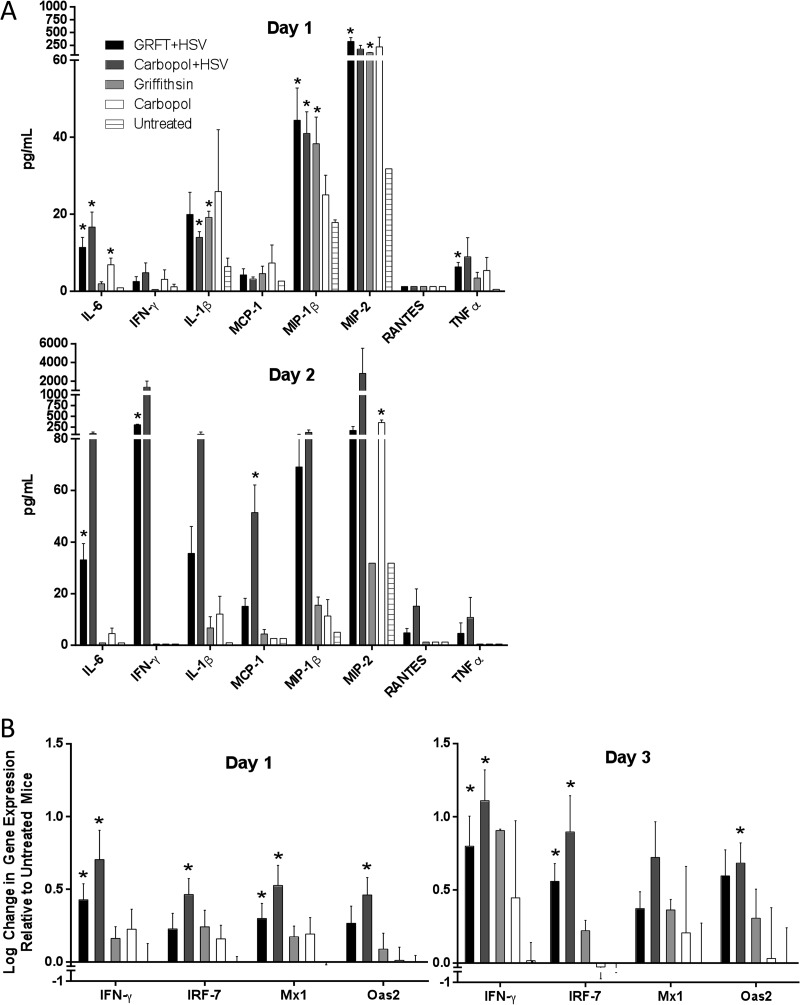
IFN responses to HSV-2 are preserved in GRFT-pretreated mice.
Genital herpes triggers mucosal inflammation and an IFN-γ response (34, 35). Strong IFN-γ responses are associated with viral clearance, and individuals with recurrent disease have impaired virus-specific IFN-γ responses compared with those of asymptomatic HSV-2 carriers (36). To explore the impact of GRFT on the mucosal response to virus, vaginal washes were collected 1 or 2 days after vaginal challenge from groups of mice pretreated with GRFT or Carbopol gel. Controls included mice treated with GRFT or Carbopol gel that were not infected and untreated, uninfected mice. Consistent with prior studies (15, 17), there was an increase in vaginal wash levels of IFN-γ and several proinflammatory mediators, including IL-6, IL-1β, MIP-1β, and MIP-2, in HSV-2-infected compared to uninfected mice, independent of gel exposure 1 and/or 2 days postinfection (Fig. 5A). In the absence of virus, GRFT was associated with an increase in IL-1β, MIP-1β, and MIP-2 on day 1 compared to levels in untreated mice, and Carbopol was associated with higher levels of IL-6 on day 1 and MIP-1 on day 2 than GRFT-treated mice (P < 0.05). The biological significance of these modest changes requires further study.
Fig 5.
HSV induces proinflammatory and interferon responses in the genital tract independent of griffithsin treatment. (A) Concentrations of cytokines and chemokines were measured in vaginal washes on days 1 and 2 postinfection. Results are presented as pg/ml in vaginal washes and are means + SEM from 2 to 4 pools of washes from 2 to 5 mice per pool. Asterisks represent significance compared to untreated, uninfected mice using unpaired t test. (B) Gene expression was measured by qRT-PCR on genital tract tissue harvested from 2 to 10 mice on days 1 and 3 postinfection; results are normalized to β-actin and expressed as means + SEM log change relative to control untreated, uninfected mice (*, P < 0.05).
To further explore the impact of GRFT on the IFN response to virus, RNA was isolated from genital tract tissue and analyzed by RT-qPCR on days 1 and 3 postinfection. HSV-2 induced upregulation of several IFN genes, including IFN-γ, Oas2, IRF-7, and Mx1 genes, with a small reduction in these responses in mice pretreated with GRFT (Fig. 5B). GRFT itself had no impact on IFN responses compared to untreated mice. These findings are consistent with the in vitro observations that GRFT has only modest effects on HSV entry (Fig. 1) and that viral entry may be sufficient to induce an initial IFN response (37, 38).
Evaluation of the safety of 0.1% GRFT gel.
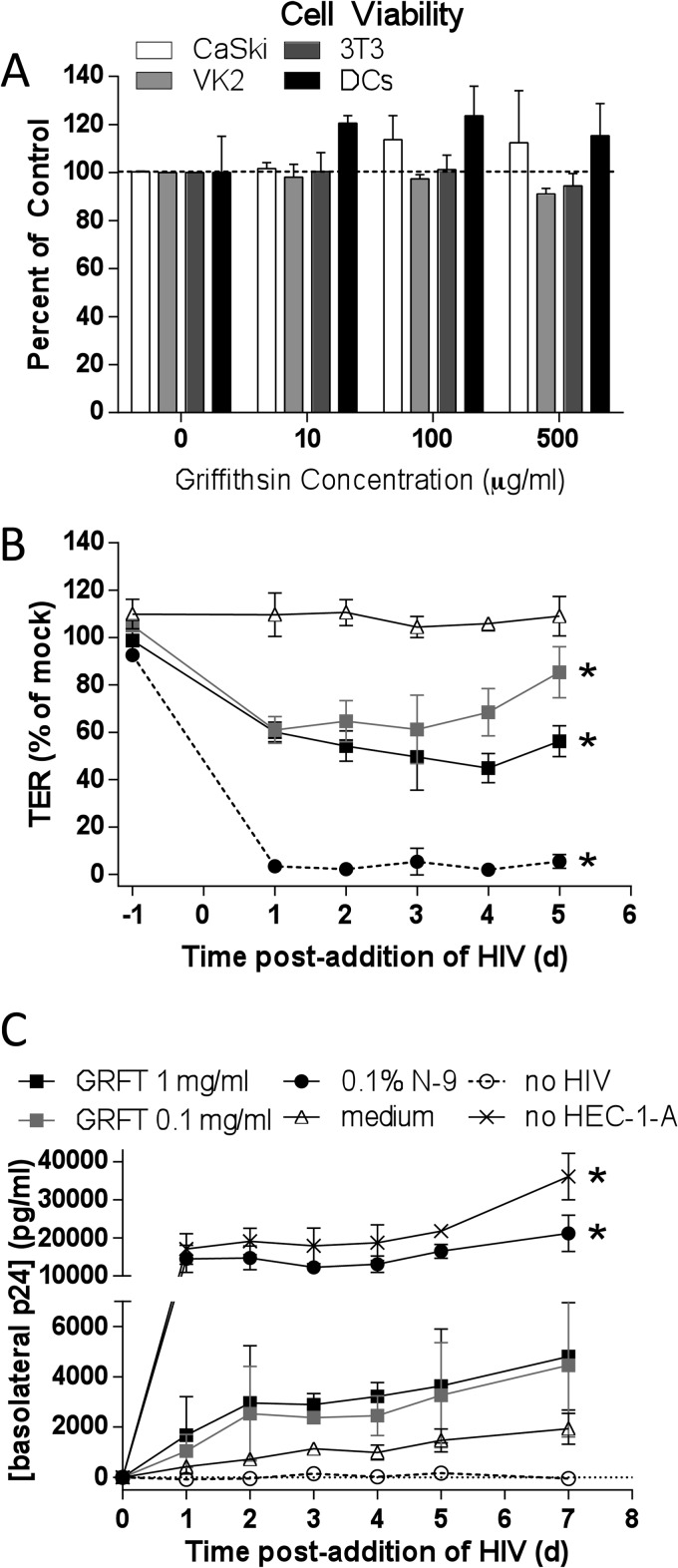
There was no reduction in CaSki, VK2/E6E7, 3T3, or moDC viability in response to 48 h of exposure to GRFT in vitro (Fig. 6A). To further explore safety, we took advantage of two additional preclinical safety models that focus on the impact of drugs on mucosal integrity. HEC-1-A cells were cultured on Transwell inserts until the transepithelial electrical resistance (TER) reached a steady state of ∼300 Ω · cm2 (typically day 4 or 5 after plating cells). The polarized cells were exposed apically to a single dose of GRFT (0.1 and 1 mg/ml), N-9 (0.1% [vol/vol]), or culture medium (negative control) for 18 h and then washed three times. HIV-1BaL was added to the AP chamber and JT-CCR5 cells to the BL chamber. The TER and p24 contents in BL culture supernatants were measured daily (Fig. 6B and C). The former provides a measure of barrier integrity and the latter of HIV transmigration across the epithelium. Additional controls included inserts in which no cells were seeded, a measure of the maximal migration of HIV across the insert pores (positive control), and wells in which no HIV was added (p24 background). After application of N-9, the TER fell rapidly and HIV freely traversed the disrupted epithelium, resulting in p24 levels comparable to those observed in the absence of any cells (positive control). Exposure to both concentrations of GRFT resulted in a modest and less sustained decline in TER, which differed significantly from medium alone. This was associated with a modest increase in HIV p24 levels in the BL compartment that did not reach statistical significance compared to medium alone; only a small amount of HIV traverses the barrier when cells are cultured in medium (Fig. 6B).
Fig 6.
Griffithsin is not cytotoxic but reduces transepithelial electrical resistance in a dual-chamber model. (A) Cells were exposed to GRFT for 48 h (CaSki, VK2/E6E7, 3T3) or overnight (moDCs), and cell viability was assessed by MTS; results are means + SEM from at least 2 independent experiments. (B) HEC-1-A cells were cultured in Transwells (0.5 × 105 to 1 × 105 cells/insert), and TER was monitored daily. After the TER reached a plateau (4 or 5 days), cells were exposed to 0.1 or 1 mg/ml GRFT, 0.1% (vol/vol) N-9, or medium alone for 18 h. After removal of drug by washing, HIV-1BaL (40 ng p24) and JT-CCR5 cells (105 cells/well) were added to the upper and lower chambers, respectively. The TER was monitored prior to drug addition (t = −1 day) and then daily after addition of HIV. (C) Supernatants were collected from the basolateral chambers at the indicated times and tested for p24 content by enzyme-linked immunosorbent assay (ELISA). Results are means ± SD from at least two independent experiments, where each condition was tested in duplicate. Controls included wells in which no drug was added (medium), no cells were added, and no HIV was added. Asterisks indicate significance compared to medium (P < 0.05) using two-way ANOVA with Tukey's multiple comparisons test.
GRFT does not increase susceptibility to HSV-2 in a mouse safety model.
To determine if the modest decline in TER and concomitant increase in HIV p24 levels in the dual-chamber model translated to increased susceptibility to HSV-2 in mice, mice were treated once daily for 7 consecutive days with 0.1% GRFT gel, Carbopol gel, or 4% N-9 gel, and then 12 to 24 h after the last dose, vaginal washes were collected to remove any residual luminal drug and subsequently assayed for anti-HIV activity. There was sufficient GRFT within the vaginal washes to inhibit HIV-1 infection in a TZM-bl assay; N-9 washes had no antiviral activity (Fig. 7A). The mice were then challenged with an LD10, LD50, and LD90 of virus. Consistent with prior studies, there was an increase in mortality in N-9-treated compared to Carbopol-treated mice following challenge with the LD10 or LD50 dose of virus (P < 0.05) (Fig. 7B and C). In contrast, survival rates between GRFT- and Carbopol-treated mice did not differ significantly at any of the challenge doses. All of the mice succumbed to infection following exposure to an LD90 (Fig. 7D), indicating that when GRFT drug is removed, protection against HSV-2 is lost.
Fig 7.
Repeat exposure to griffithsin does not increase susceptibility to HSV-2 in a murine model. Mice were pretreated with medroxyprogesterone acetate; 5 days later, mice received one 20-μl dose of 0.1% GRFT, Carbopol, or 4% N-9 gel intravaginally daily for 7 days. On the eighth day, a vaginal wash was taken to remove residual drug and to test for residual anti-HIV activity in a TZM-bl assay; results are means (SEM) from 3 pools of vaginal washes, each pool containing washes from 2 mice, conducted in triplicate. (A) Data are plotted as percentages of virus control. Asterisk indicates P values of <0.05 compared to washes from Carbopol-treated mice (unpaired t test). Mice were then challenged with HSV-2(4674) diluted in 30 μl PBS at LD10 (B), LD50 (C), or LD90 (D), with 10 to 20 mice per group. Mice were scored for disease daily and were sacrificed if indicated by disease scores. Kaplan-Meier survival curves are shown; asterisks indicate P values of <0.05 (log rank test), compared to Carbopol treatment.
Exposure to GRFT has no deleterious impact on human DCs.
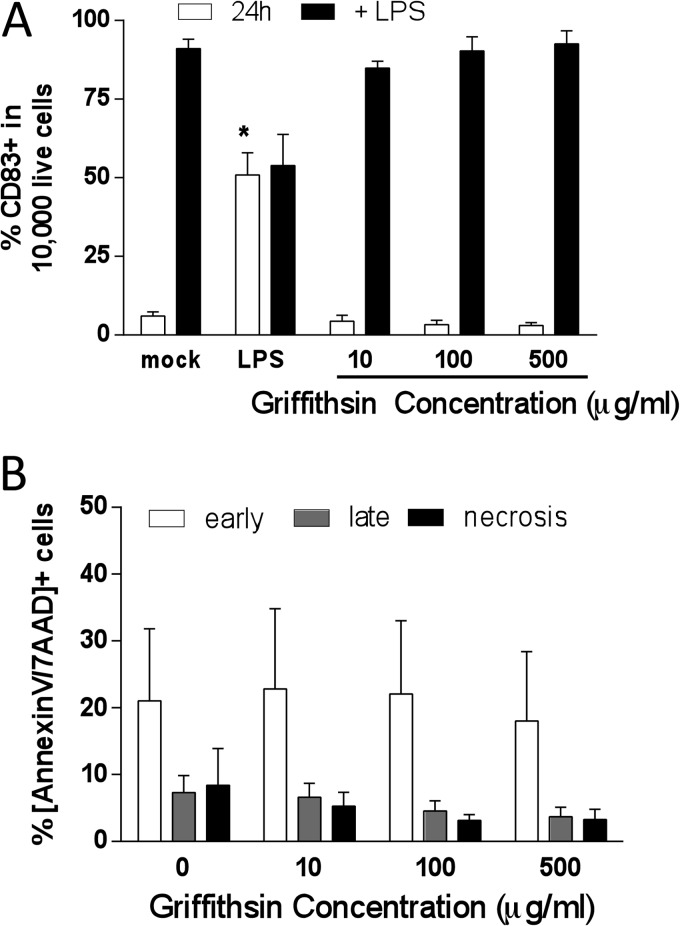
Lectins such as GRFT may modulate DC function by interacting with carbohydrates on the DC surface. To assess whether GRFT induced DC maturation or interfered with the maturation response to LPS, cells were exposed to medium or GRFT drug for 24 h and were then stained and analyzed for the expression of CD83, a cell surface marker of DC maturation. There was no increase in CD83 expression in response to GRFT compared to mock (medium)-treated cells. However, LPS induced significant upregulation of CD83 expression in mock- and GRFT-treated cells, indicating that GRFT has no deleterious impact on the ability of DCs to respond to maturation signals (LPS) (Fig. 8A). In addition, GRFT did not induce apoptosis in DCs compared to the mock-exposed cells (Fig. 8B). Together these findings indicate that GRFT does not adversely impact DC function.
Fig 8.
Griffithsin does not induce DC maturation or induce apoptosis. (A) Immature moDCs were exposed to medium (mock) or LPS (10 μg/ml) for 24 h at 37°C, and then one set each was stimulated with LPS (10 μg/ml) for 1 h. The cells were then analyzed by flow cytometry for surface marker CD83 expression. Data are mean percentages of CD83+ + SEM from six (24 h) and two (+LPS) independent experiments. (B) Immature moDCs were exposed to the indicated concentrations of GRFT overnight and analyzed by flow cytometry for percentage of cells that are annexin V+/7AAD− (early apoptosis) and annexin V+/7AAD+ (late apoptosis). Data are means + SEM from two independent experiments. Asterisks indicate significance compared to mock-treated cells under each condition (P < 0.05, ANOVA).
DISCUSSION
The current studies were initially designed to further evaluate the safety of GRFT, which is being advanced as a candidate vaginal product to prevent HIV infection. An unexpected finding was that GRFT inhibited HSV-2 infection postentry by blocking cell-to-cell spread (Fig. 1 and 2) and protected mice from genital herpes disease (Fig. 3). There was little dissemination of virus in mice treated with a single dose of gel prior to vaginal inoculation, as evidenced by the imaging studies with a luciferase-reporter virus (Fig. 4). Importantly, GRFT retained its protective activity in the mice when virus was introduced in human seminal plasma. These results contrast with studies of PRO 2000 and cellulose sulfate, polyanionic vaginal products that failed to prevent HIV or HSV-2 in clinical trials (18, 39). Seminal proteins interfered with the antiviral activity of these entry inhibitors by competing with their ability to bind to the viral envelope (16, 40). Thus, GRFT appears to inhibit HSV-2 by a unique postentry mechanism distinct from other candidate topical PrEP agents.
HSV disseminates primarily by direct cell-to-cell spread, a process that enables virus to escape neutralizing antibody. The more potent in vitro activity of GRFT against cell-to-cell spread compared to entry is somewhat surprising, as both fusion events require cellular receptors/coreceptors and glycoproteins B (gB), gD, and hetero-oligomers of gH-gL. However, several lines of evidence suggest that entry and cell-to-cell spread are not identical processes. First, mutants selected for growth on U(S)11cl19.3 cells, a cell line resistant to entry and cell-to-cell spread because of the absence of a gD receptor, exhibited a marked increase in cell-to-cell spread without a concomitant increase in efficiency of entry of free virus, indicating that these processes are distinct (41). Second, gE contributes to cell-to-cell spread in epithelial and neuronal cell models but is not required for viral entry (42, 43). Third, in studies of HSV-1 infection in Ric21 cells, a BHK cell line defective in the enzymes of the Golgi system which add terminal sugars to N-linked glycans, there was a reduction in plaque size and the number of viral progeny released into the culture supernatant, but the infectivity of released virions from both cell types was similar (44). These findings are consistent with the observation here that GRFT, which targets N-linked glycans, has only modest effects on entry but prevents cell-to-cell spread. The more potent activity against cell-to-cell spread is also reminiscent of the finding that GRFT is the most potent antiretroviral of any class for preventing transmission of cell-associated HIV-1 (45).
GRFT may target glycans on the viral envelope glycoproteins and/or cellular receptors/coreceptors involved in cell-to-cell spread. The most extensively characterized HSV glycoprotein, with respect to its N- and O-linked glycosylations, is HSV-1 gC (gC-1), which contains nine sites for N-linked glycosylation and numerous clusters of O-linked glycans (46). However, while gC-1 mediates binding of HSV-1 to heparan sulfate and is involved in immune evasion through its interactions with complement components, it is not required for cell-to-cell spread (47). Moreover, for HSV-2, gB-2, not gC-2, plays the major role in promoting viral binding, and gC-2 is dispensable for viral infection in cell culture (48). Thus, it seems unlikely that GRFT targets gC. It is also possible that GRFT targets carbohydrates on heparan sulfate proteoglycans or other cell-surface components that participate in cell-to-cell spread (31). The specific structural features of heparan sulfate required for cell-to-cell spread may differ from those required for binding and have not yet been elucidated (49). Future studies with deletion viruses, recombinant glycoproteins (presuming that they are properly glycosylated), and cell surface molecules are needed to identify precisely what viral and/or cellular components and glycans GRFT targets to prevent cell-to-cell spread.
The other key findings from this study add to the growing safety profile of GRFT. First, mice responded to HSV-2 exposure with a potent IFN response that was similar in both the Carbopol- and GRFT-treated mice (Fig. 5). Thus, GRFT does not appear to prevent protective mucosal responses. Second, although GRFT induced a modest decrease in the TER and a concomitant statistically not significant increase in HIV migration across the epithelial barrier to infect T cells cultured in the lower chamber (Fig. 6), this did not translate to increased susceptibility to HSV-2 when mice were treated for 7 days with GRFT and then challenged with sublethal doses of HSV-2. This contrasts with results obtained here and in previous studies with N-9, which triggered a rapid and profound drop in TER and increased the susceptibility of the mice to HSV-2 following intravaginal challenge with either an LD10 or LD50 (Fig. 7) (15, 17). The results also differ from our studies with cellulose sulfate, which triggered a more sustained decrease in TER and promoted HSV-2 infection in the murine model (14, 16). Third, GRFT was not cytotoxic and had no deleterious effects on DCs. The drug did not induce DC apoptosis or maturation nor did it prevent DCs from responding appropriately to a maturation signal (LPS). Maintenance of DC function is critical, as these cells play a sentinel role in mucosal defense and link innate and adaptive immune responses.
The protective effects of GRFT against HSV disease, a major cofactor in the HIV epidemic, suggest that GRFT may contribute both directly and indirectly to HIV protection. Despite efforts to limit the spread of HSV, the worldwide prevalence and public health impact of genital herpes continues to increase; HSV-2 seroprevalence rates approach 90 to 95% among HIV-infected individuals and female sex workers in developing countries, where HSV-2 remains the dominant cause of genital ulcerative disease (50). Two meta-analyses support the conclusion that prevalent HSV-2 infection increases the risk of HIV acquisition 2- to 4-fold, and incident HSV-2 further increases the risk and contributes more to the spread of HIV-1 than number of sex partners or other sexually transmitted infections (STI) (51, 52). Thus, the protective effects of GRFT against HSV-2 should further augment its potential as a topical PrEP product.
While the concentration of GRFT needed to inhibit HSV in vitro (high nanomolar to low micromolar) is greater than that observed for other viruses where GRFT is effective in the low nanomolar to mid-picomolar range (e.g., HIV, severe acute respiratory syndrome coronavirus [SARS-CoV]) (53), the concentrations delivered in topical gels clearly exceed the concentration needed to protect against HSV and were effective in the murine model even with only a single dose and in the presence of human seminal plasma. The higher concentrations may reflect differences in carbohydrates expressed by HSV envelope glycoproteins. GRFT binds to select monosaccharides (mannose, glucose, and N-acetylglucosamine) in a multivalent manner via its three independent carbohydrate-binding domains and is thus ideally designed to engage multiple triantennary arms of specific high-mannose oligosaccharides, including those expressed by HIV gp120 (54, 56). Repeated gel applications or alternative sustained release formulations of the drug could provide even greater protection against both HSV and HIV and indicate that GRFT is a strong candidate for further advancement, either alone or in combination with other antiretroviral drugs, as topical PrEP.
ACKNOWLEDGMENTS
This work was supported by grants from the National Institutes of Health, R01AI076169, U19AI076980, and R21AI81072 (W.H.), and the Center for AIDS Research at the Albert Einstein College of Medicine and Montefiore Medical Center (NIH AI-051519).
Footnotes
Published ahead of print 27 March 2013
REFERENCES
- 1. Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, Kharsany AB, Sibeko S, Mlisana KP, Omar Z, Gengiah TN, Maarschalk S, Arulappan N, Mlotshwa M, Morris L, Taylor D. 2010. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 329:1168–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. MTN 25 November 2011, posting date MTN statement on decision to discontinue tenofovir gel in VOICE, a major HIV prevention study in women. http://www.mtnstopshiv.org/node/3909 MTN, Pittsburgh, PA [Google Scholar]
- 3. MTN 28 September 2011, posting date MTN statement on decision to discontinue use of oral tenofovir tablets in VOICE, a major HIV prevention study in women. http://www.mtnstopshiv.org/node/3619 MTN, Pittsburgh, PA [Google Scholar]
- 4. Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, Tappero JW, Bukusi EA, Cohen CR, Katabira E, Ronald A, Tumwesigye E, Were E, Fife KH, Kiarie J, Farquhar C, John-Stewart G, Kakia A, Odoyo J, Mucunguzi A, Nakku-Joloba E, Twesigye R, Ngure K, Apaka C, Tamooh H, Gabona F, Mujugira A, Panteleeff D, Thomas KK, Kidoguchi L, Krows M, Revall J, Morrison S, Haugen H, Emmanuel-Ogier M, Ondrejcek L, Coombs RW, Frenkel L, Hendrix C, Bumpus NN, Bangsberg D, Haberer JE, Stevens WS, Lingappa JR, Celum C. 2012. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N. Engl. J. Med. 367:399–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Celum C, Baeten JM. 2012. Tenofovir-based pre-exposure prophylaxis for HIV prevention: evolving evidence. Curr. Opin. Infect. Dis. 25:51–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, Malahleha M, Owino F, Manongi R, Onyango J, Temu L, Monedi MC, Mak'Oketch P, Makanda M, Reblin I, Makatu SE, Saylor L, Kiernan H, Kirkendale S, Wong C, Grant R, Kashuba A, Nanda K, Mandala J, Fransen K, Deese J, Crucitti T, Mastro TD, Taylor D. 2012. Preexposure prophylaxis for HIV infection among African women. N. Engl. J. Med. 367:411–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kiser PF, Mesquita PM, Herold BC. 2012. A perspective on progress and gaps in HIV prevention science. AIDS Res. Hum. Retroviruses 28:1373–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alexandre KB, Gray ES, Lambson BE, Moore PL, Choge IA, Mlisana K, Karim SS, McMahon J, O'Keefe B, Chikwamba R, Morris L. 2010. Mannose-rich glycosylation patterns on HIV-1 subtype C gp120 and sensitivity to the lectins, griffithsin, cyanovirin-N and scytovirin. Virology 402:187–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Emau P, Tian B, O'Keefe RB, Mori T, McMahon JB, Palmer KE, Jiang Y, Bekele G, Tsai CC. 2007. Griffithsin, a potent HIV entry inhibitor, is an excellent candidate for anti-HIV microbicide. J. Med. Primatol. 36:244–253 [DOI] [PubMed] [Google Scholar]
- 10. Ferir G, Palmer KE, Schols D. 2011. Synergistic activity profile of griffithsin in combination with tenofovir, maraviroc and enfuvirtide against HIV-1 clade C. Virology 417:253–258 [DOI] [PubMed] [Google Scholar]
- 11. O'Keefe BR, Vojdani F, Buffa V, Shattock RJ, Montefiori DC, Bakke J, Mirsalis J, d'Andrea AL, Hume SD, Bratcher B, Saucedo CJ, McMahon JB, Pogue GP, Palmer KE. 2009. Scaleable manufacture of HIV-1 entry inhibitor griffithsin and validation of its safety and efficacy as a topical microbicide component. Proc. Natl. Acad. Sci. U. S. A. 106:6099–6104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hurt CB, Eron JJ, Jr, Cohen MS. 2011. Pre-exposure prophylaxis and antiretroviral resistance: HIV prevention at a cost? Clin. Infect. Dis. 53:1265–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kouokam JC, Huskens D, Schols D, Johannemann A, Riedell SK, Walter W, Walker JM, Matoba N, O'Keefe BR, Palmer KE. 2011. Investigation of griffithsin's interactions with human cells confirms its outstanding safety and efficacy profile as a microbicide candidate. PLoS One 6:e22635 doi:10.1371/journal.pone.0022635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mesquita PM, Cheshenko N, Wilson SS, Mhatre M, Guzman E, Fakioglu E, Keller MJ, Herold BC. 2009. Disruption of tight junctions by cellulose sulfate facilitates HIV infection: model of microbicide safety. J. Infect. Dis. 200:599–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Galen BT, Martin AP, Hazrati E, Garin A, Guzman E, Wilson SS, Porter DD, Lira SA, Keller MJ, Herold BC. 2007. A comprehensive murine model to evaluate topical vaginal microbicides: mucosal inflammation and susceptibility to genital herpes as surrogate markers of safety. J. Infect. Dis. 195:1332–1339 [DOI] [PubMed] [Google Scholar]
- 16. Segarra TJ, Fakioglu E, Cheshenko N, Wilson SS, Mesquita PM, Doncel GF, Herold BC. 2011. Bridging the gap between preclinical and clinical microbicide trials: blind evaluation of candidate gels in murine models of efficacy and safety. PLoS One 6:e27675 doi:10.1371/journal.pone.0027675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wilson SS, Cheshenko N, Fakioglu E, Mesquita PM, Keller MJ, Herold BC. 2009. Susceptibility to genital herpes as a biomarker predictive of increased HIV risk: expansion of a murine model of microbicide safety. Antivir. Ther. 14:1113–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Van Damme L, Govinden R, Mirembe FM, Guedou F, Solomon S, Becker ML, Pradeep BS, Krishnan AK, Alary M, Pande B, Ramjee G, Deese J, Crucitti T, Taylor D. 2008. Lack of effectiveness of cellulose sulfate gel for the prevention of vaginal HIV transmission. N. Engl. J. Med. 359:463–472 [DOI] [PubMed] [Google Scholar]
- 19. O'Keefe BR, Smee DF, Turpin JA, Saucedo CJ, Gustafson KR, Mori T, Blakeslee D, Buckheit R, Boyd MR. 2003. Potent anti-influenza activity of cyanovirin-N and interactions with viral hemagglutinin. Antimicrob. Agents Chemother. 47:2518–2525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tiwari V, Shukla SY, Shukla D. 2009. A sugar binding protein cyanovirin-N blocks herpes simplex virus type-1 entry and cell fusion. Antiviral Res. 84:67–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Balzarini J. 2007. Targeting the glycans of glycoproteins: a novel paradigm for antiviral therapy. Nat. Rev. Microbiol. 5:583–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bonomelli C, Doores KJ, Dunlop DC, Thaney V, Dwek RA, Burton DR, Crispin M, Scanlan CN. 2011. The glycan shield of HIV is predominantly oligomannose independently of production system or viral clade. PLoS One 6:e23521 doi:10.1371/journal.pone.0023521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Doores KJ, Bonomelli C, Harvey DJ, Vasiljevic S, Dwek RA, Burton DR, Crispin M, Scanlan CN. 2010. Envelope glycans of immunodeficiency virions are almost entirely oligomannose antigens. Proc. Natl. Acad. Sci. U. S. A. 107:13800–13805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wenske EA, Bratton MW, Courtney RJ. 1982. Endo-beta-N-acetylglucosaminidase H sensitivity of precursors to herpes simplex virus type 1 glycoproteins gB and gC. J. Virol. 44:241–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fichorova RN, Rheinwald JG, Anderson DJ. 1997. Generation of papillomavirus-immortalized cell lines from normal human ectocervical, endocervical, and vaginal epithelium that maintain expression of tissue-specific differentiation proteins. Biol. Reprod. 57:847–855 [DOI] [PubMed] [Google Scholar]
- 26. Madan RP, Mesquita PM, Cheshenko N, Jing B, Shende V, Guzman E, Heald T, Keller MJ, Regen SL, Shattock RJ, Herold BC. 2007. Molecular umbrellas: a novel class of candidate topical microbicides to prevent human immunodeficiency virus and herpes simplex virus infections. J. Virol. 81:7636–7646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mesquita PM, Wilson SS, Manlow P, Fischetti L, Keller MJ, Herold BC, Shattock RJ. 2008. Candidate microbicide PPCM blocks human immunodeficiency virus type 1 infection in cell and tissue cultures and prevents genital herpes in a murine model. J. Virol. 82:6576–6584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stefanidou M, Ramos I, Mas Casullo V, Trepanier JB, Rosenbaum S, Fernandez-Sesma A, Herold BC. 2013. HSV-2 prevents dendritic cell maturation, induces apoptosis and triggers release of pro-inflammatory cytokines: potential links to HSV-HIV synergy. J. Virol. 87:1443–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ejercito PM, Kieff ED, Roizman B. 1968. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J. Gen. Virol. 2:357–364 [DOI] [PubMed] [Google Scholar]
- 30. Halford WP, Puschel R, Gershburg E, Wilber A, Gershburg S, Rakowski B. 2011. A live-attenuated HSV-2 ICP0 virus elicits 10 to 100 times greater protection against genital herpes than a glycoprotein D subunit vaccine. PLoS One 6:e17748 doi:10.1371/journal.pone.0017748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cheshenko N, Keller MJ, MasCasullo V, Jarvis GA, Cheng H, John M, Li JH, Hogarty K, Anderson RA, Waller DP, Zaneveld LJ, Profy AT, Klotman ME, Herold BC. 2004. Candidate topical microbicides bind herpes simplex virus glycoprotein B and prevent viral entry and cell-to-cell spread. Antimicrob. Agents Chemother. 48:2025–2036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hendrickson BA, Guo J, Brown I, Dennis K, Marcellino D, Hetzel J, Herold BC. 2000. Decreased vaginal disease in J-chain-deficient mice following herpes simplex type 2 genital infection. Virology 271:155–162 [DOI] [PubMed] [Google Scholar]
- 33. Keller MJ, Mesquita PM, Torres NM, Cho S, Shust G, Madan RP, Cohen HW, Petrie J, Ford T, Soto-Torres L, Profy AT, Herold BC. 2010. Postcoital bioavailability and antiviral activity of 0.5% PRO 2000 gel: implications for future microbicide clinical trials. PLoS One 5:e8781 doi:10.1371/journal.pone.0008781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Keller MJ, Madan RP, Shust G, Carpenter CA, Torres NM, Cho S, Khine H, Huang ML, Corey L, Kim M, Herold BC. 2012. Changes in the soluble mucosal immune environment during genital herpes outbreaks. J. Acquir. Immune Defic. Syndr. 61:194–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Peng T, Zhu J, Klock A, Phasouk K, Huang ML, Koelle DM, Wald A, Corey L. 2009. Evasion of the mucosal innate immune system by herpes simplex virus type 2. J. Virol. 83:12559–12568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Singh R, Kumar A, Creery WD, Ruben M, Giulivi A, Diaz-Mitoma F. 2003. Dysregulated expression of IFN-gamma and IL-10 and impaired IFN-gamma-mediated responses at different disease stages in patients with genital herpes simplex virus-2 infection. Clin. Exp. Immunol. 133:97–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Collins SE, Noyce RS, Mossman KL. 2004. Innate cellular response to virus particle entry requires IRF3 but not virus replication. J. Virol. 78:1706–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rasmussen SB, Sorensen LN, Malmgaard L, Ank N, Baines JD, Chen ZJ, Paludan SR. 2007. Type I interferon production during herpes simplex virus infection is controlled by cell-type-specific viral recognition through Toll-like receptor 9, the mitochondrial antiviral signaling protein pathway, and novel recognition systems. J. Virol. 81:13315–13324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. McCormack S, Ramjee G, Kamali A, Rees H, Crook AM, Gafos M, Jentsch U, Pool R, Chisembele M, Kapiga S, Mutemwa R, Vallely A, Palanee T, Sookrajh Y, Lacey CJ, Darbyshire J, Grosskurth H, Profy A, Nunn A, Hayes R, Weber J. 2010. PRO2000 vaginal gel for prevention of HIV-1 infection (Microbicides Development Programme 301): a phase 3, randomised, double-blind, parallel-group trial. Lancet 376:1329–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Patel S, Hazrati E, Cheshenko N, Galen B, Yang H, Guzman E, Wang R, Herold BC, Keller MJ. 2007. Seminal plasma reduces the effectiveness of topical polyanionic microbicides. J. Infect. Dis. 196:1394–1402 [DOI] [PubMed] [Google Scholar]
- 41. Rauch DA, Rodriguez N, Roller RJ. 2000. Mutations in herpes simplex virus glycoprotein D distinguish entry of free virus from cell-cell spread. J. Virol. 74:11437–11446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Polcicova K, Goldsmith K, Rainish BL, Wisner TW, Johnson DC. 2005. The extracellular domain of herpes simplex virus gE is indispensable for efficient cell-to-cell spread: evidence for gE/gI receptors. J. Virol. 79:11990–12001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Saldanha CE, Lubinski J, Martin C, Nagashunmugam T, Wang L, van Der Keyl H, Tal-Singer R, Friedman HM. 2000. Herpes simplex virus type 1 glycoprotein E domains involved in virus spread and disease. J. Virol. 74:6712–6719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Serafini-Cessi F, Dall'Olio F, Scannavini M, Campadelli-Fiume G. 1983. Processing of herpes simplex virus-1 glycans in cells defective in glycosyl transferases of the Golgi system: relationship to cell fusion and virion egress. Virology 131:59–70 [DOI] [PubMed] [Google Scholar]
- 45. Selhorst P, Grupping K, Bourlet T, Delezay O, Arien KK, Vanham G. 2012. In vitro activities of candidate microbicides against cell-associated HIV. Antimicrob. Agents Chemother. 56:805–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Biller M, Mardberg K, Hassan H, Clausen H, Bolmstedt A, Bergstrom T, Olofsson S. 2000. Early steps in O-linked glycosylation and clustered O-linked glycans of herpes simplex virus type 1 glycoprotein C: effects on glycoprotein properties. Glycobiology 10:1259–1269 [DOI] [PubMed] [Google Scholar]
- 47. Herold BC, WuDunn D, Soltys N, Spear PG. 1991. Glycoprotein C of herpes simplex virus type 1 plays a principal role in the adsorption of virus to cells and in infectivity. J. Virol. 65:1090–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gerber SI, Belval BJ, Herold BC. 1995. Differences in the role of glycoprotein C of HSV-1 and HSV-2 in viral binding may contribute to serotype differences in cell tropism. Virology 214:29–39 [DOI] [PubMed] [Google Scholar]
- 49. Herold BC, Gerber SI, Polonsky T, Belval BJ, Shaklee PN, Holme K. 1995. Identification of structural features of heparin required for inhibition of herpes simplex virus type 1 binding. Virology 206:1108–1116 [DOI] [PubMed] [Google Scholar]
- 50. Nagot N, Ouedraogo A, Foulongne V, Konate I, Weiss HA, Vergne L, Defer MC, Djagbare D, Sanon A, Andonaba JB, Becquart P, Segondy M, Vallo R, Sawadogo A, Van de Perre P, Mayaud P. 2007. Reduction of HIV-1 RNA levels with therapy to suppress herpes simplex virus. N. Engl. J. Med. 356:790–799 [DOI] [PubMed] [Google Scholar]
- 51. Chen L, Jha P, Stirling B, Sgaier SK, Daid T, Kaul R, Nagelkerke N. 2007. Sexual risk factors for HIV infection in early and advanced HIV epidemics in sub-Saharan Africa: systematic overview of 68 epidemiological studies. PLoS One 2:e1001 doi:10.1371/journal.pone.0001001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. 2006. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS 20:73–83 [DOI] [PubMed] [Google Scholar]
- 53. O'Keefe BR, Giomarelli B, Barnard DL, Shenoy SR, Chan PK, McMahon JB, Palmer KE, Barnett BW, Meyerholz DK, Wohlford-Lenane CL, McCray PB., Jr 2010. Broad-spectrum in vitro activity and in vivo efficacy of the antiviral protein griffithsin against emerging viruses of the family Coronaviridae. J. Virol. 84:2511–2521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Moulaei T, Shenoy SR, Giomarelli B, Thomas C, McMahon JB, Dauter Z, O'Keefe BR, Wlodawer A. 2010. Monomerization of viral entry inhibitor griffithsin elucidates the relationship between multivalent binding to carbohydrates and anti-HIV activity. Structure 18:1104–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ziolkowska NE, Shenoy SR, O'Keefe BR, McMahon JB, Palmer KE, Dwek RA, Wormald MR, Wlodawer A. 2007. Crystallographic, thermodynamic, and molecular modeling studies of the mode of binding of oligosaccharides to the potent antiviral protein griffithsin. Proteins 67:661–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hansen JE, Nielsen C, Vestergaard BF. 1991. Inhibition of human immunodeficiency virus 1 (HIV-1) and herpes simplex virus 1 (HSV-1) infectivity with a broad range of lectins. Scand. J. Infect. Dis. 23:425–430 [DOI] [PubMed] [Google Scholar]