Abstract
Plant viruses overcome the barrier of the plant cell wall by encoding cell-to-cell movement proteins (MPs), which direct newly replicated viral genomes to, and across, the wall. The paradigm for how a single MP regulates and coordinates these activities is the Tobacco mosaic virus (TMV) 30-kDa protein (MPTMV). Detailed studies demonstrate that TMV multiplies exclusively in the cytoplasm and have documented associations of MPTMV with endoplasmic reticulum (ER) membrane, microtubules, and plasmodesmata throughout the course of infection. As TMV poorly infects Arabidopsis thaliana, Turnip vein clearing virus (TVCV) is the tobamovirus of choice for studies in this model plant. A key problem, which has contributed to confusion in the field, is the unproven assumption that the TVCV and TMV life cycles are identical. We engineered an infectious TVCV replicon that expressed a functional fluorescence-tagged MPTVCV and report here the unexpected discovery that MPTVCV, beyond localizing to ER membrane and plasmodesmata, targeted to the nucleus in a nuclear localization signal (NLS)-dependent manner, where it localized to novel F-actin-containing filaments that associated with chromatin. The MPTVCV NLS appeared to be conserved in the subgroup 3 tobamoviruses, and our mutational analyses showed that nuclear localization of MPTVCV was necessary for efficient TVCV cell-to-cell movement and systemic infection in Nicotiana benthamiana and Arabidopsis thaliana. Our studies identify a novel nuclear stage in TVCV infection and suggest that nuclear MP encoded by TVCV and other subgroup 3 tobamoviruses interacts with F-actin and chromatin to modulate host defenses or cellular physiology to favor virus movement and infection.
INTRODUCTION
The plant cell wall is a barrier to virus exit from and entry into cells. Thus, successful infection requires that plant viruses encode movement proteins to transport the viral genome locally cell to cell within a leaf and on into the vascular system, through which the virus will systemically invade the plant. Cell-to-cell movement occurs via a common pathway: plant viruses exploit plasmodesmata (PD), membranous transwall channels that connect adjacent plant cells. Cell-to-cell movement proteins (hereafter designated MP, with the specific virus indicated) alter PD permeability and thereby enable transport of the viral genome into adjacent cells (1). Beyond this essential role in intercellular trafficking, movement proteins act to coordinate replication of the viral genome with its directed transport to PD. Depending on their replication strategy, plant viruses may encode additional movement proteins (hereafter referred to by their virus-specific names) to bind the viral genome and coordinate intracellular trafficking events with the cell-to-cell MP. However, many plant viruses utilize a single MP to coordinate intracellular and intercellular trafficking events, as typified by the 30-kDa MPTMV encoded by the tobamovirus Tobacco mosaic virus (TMV) (2, 3). In general, virion assembly is not essential for cell-to-cell movement. Indeed, for TMV and most other plant viruses, viral coat protein (CP) is not required for intercellular trafficking, although it is essential for systemic spread (2).
Much of our understanding of movement protein function is based on pioneering studies of TMV, a positive-sense RNA (+RNA) virus that, like most RNA viruses, multiplies entirely within the cytoplasm of the infected cell. Upon virion uncoating, the TMV +RNA genome is first translated into the viral replicase, which initiates an early phase of viral RNA replication at sites associated with endoplasmic reticulum (ER)-derived membranes. This includes synthesis of two distinct subgenomic RNAs that encode MPTMV and CP (4). As the viral life cycle progresses, TMV genomes associate with additional ER sites to amplify replication and eventually traffic to and through PD. MPTMV shares two hallmark features with other +RNA virus so-called “30K-like” MPs, namely, the ability to bind nucleic acids and to alter the gating properties of PD (5), which suggests a potential role in coordinating these intracellular trafficking events with cell-to-cell movement. Such a key role for MPTMV in distributing viral RNA within infected cells is indicated by studies employing an infectious clone of TMV that encodes a functional fusion of MPTMV to the green fluorescent protein (GFP). This TMV replicon has been used in confocal microscopy studies to examine the associations of MPTMV with cellular components during the time course of virus infection in individual expanding infection sites, visualized as fluorescent foci on inoculated leaves of Nicotiana benthamiana, which is a permissive host (6). Early (leading edge of foci) and throughout the infection site, MPTMV-GFP localizes to discrete punctae at the cell periphery, which appear to mark the location of PD (6). Early in infection, MPTMV-GFP is also on motile cytosolic vesicles and accumulates at sites derived from ER membranes. The latter appear to be sites of viral replication and protein synthesis as they contain replicase, viral RNA, and CP (6–8). These replication sites enlarge as infection progresses, during which the reticulate ER network collapses. At late times during infection (center of infection sites), MPTMV-GFP appears to redistribute from replication sites to microtubules and is then degraded, at which point MPTMV-GFP is seen only in small punctae at the periphery (PD) (6). This last finding and other studies (9–12) have led to the suggestion that microtubules direct MPTMV to be degraded via proteasomes. However, the role of microtubules in TMV infection remains controversial (1, 12).
Turnip vein clearing virus (TVCV) is in Tobamovirus subgroup 3, which also includes Ribgrass mosaic virus (RMV) and Youcai mosaic virus (YoMV, reported to be the same as Oilseed rape mosaic virus [ORMV]) (13). In contrast to subgroup 1, which includes TMV and other tobamoviruses isolated from solanaceous plants, and also the orchid-infecting Odontoglossum ringspot virus (ORV) (14), subgroup 3 viruses were isolated from the Brassicaceae family (formerly called crucifers) and can all infect the model plant Arabidopsis thaliana. In contrast to TMV, TVCV efficiently infects the Arabidopsis Col-0 ecotype and has become the model tobamovirus for virus-host interaction studies that take advantage of the genetic, genomic, and molecular resources afforded by this model plant. It has generally been assumed that the TVCV life cycle, including MPTVCV distribution during infection, follows the same stages as that of TMV, but this has not been proven. Indeed, host factors identified in Arabidopsis using TVCV are often studied in the context of TMV infection and interactions in N. benthamiana, in which transient assays and cellular studies can be easier to do.
In order to realize the full potential of TVCV for investigating virus-host interactions and cell-to-cell transport in Arabidopsis, we engineered a TVCV replicon that encodes a functional form of MPTVCV fused to GFP or red fluorescent protein (RFP) and used these infectious clones to investigate the subcellular localization of MPTVCV during the time course of TVCV infection in Arabidopsis Col-0 and N. benthamiana, both permissive hosts. As reported here, we unexpectedly discovered a nuclear stage in TVCV infection. Similarly to TMV, we found that MPTVCV localized to PD and what appear to be ER-derived replication sites during the time course of virus infection in expanding infection sites. However, in striking contrast to TMV, we observed that MPTVCV did not associate with microtubules but did accumulate in what appeared to be novel F-actin-containing nuclear filaments. We further identified a nuclear localization signal (NLS) in MPTVCV that is conserved in the subgroup 3 tobamoviruses. We showed, through mutational analyses, that this NLS both was required for the nuclear localization of MPTVCV and was necessary for efficient TVCV cell-to-cell spread and systemic infection in both N. benthamiana and Arabidopsis. Our studies identify a novel nuclear stage in TVCV infection and suggest that nuclear targeting of MP encoded by TVCV and potentially other subgroup 3 tobamoviruses may play a role in modulating host defense or cellular physiology to favor virus movement and infection.
MATERIALS AND METHODS
Generation of fusion proteins and TVCV replicons.
We used Gateway technology to construct MPTVCV C-terminal fusions to GFP (MPTVCV-GFP) or monomeric RFP (MPTVCV-RFP). We amplified the MPTVCV coding sequence located between HindIII and NsiI sites in pBluescript KS(+)-MPTVCV using PCR and cloned it into the donor vector pDONR207 and then into the pSITE 2NB or pSITE 4NB destination vector (15) to generate pSITE2NB::MPTVCV-GFP and pSITE4NB::MPTVCV-RFP, respectively. To construct the viral replicon clones pTVCV::MP-GFP and pTVCV::MP-RFP, the C-terminal GFP/RFP-fused coding sequence of MPTVCV starting at nucleotide (nt) 580 (an internal EcoRI site) was PCR amplified from each of these vectors to add a 3′-terminal PpuMI site following the GFP or RFP stop codon, and each fragment was then cloned between the EcoRI and PpuMI sites of pTVCV50, which contains the infectious TVCV full-length genomic cDNA clone under the control of the SP6 promoter (16). The resulting TVCV replicons TVCV::MP-GFP and TVCV::MP-RFP each expressed MPTVCV (the GFP or RFP fusion) from the endogenous MP promoter but were deleted for the CP gene, which overlaps the 3′ end of the MP coding region (14).
To generate the NLS virus [TVCVMP(K210A/K212A)] and replicon (TVCV::MPK210A/K212A-GFP) mutants, we used PCR mutagenesis (17) to change nt 628 to 630 from AAG to GCT and nt 634 to 635 from AA to GC in the MPTVCV coding sequence in pTVCV50 and pTVCV::MP-GFP, respectively, thereby substituting Ala residues for Lys at positions 210 and 212 in MPTVCV. In brief, each plasmid was amplified with Pfu ultra-high-fidelity DNA polymerase (Agilent, CA) using the complementary primers 5′-GCGGTTGACAATTTCAGGGCTAGGGCAAAGAAGGTTGAAGAAAGGGATG-3′ and 5′-CATCCCTTTCTTCAACCTTCTTTGCCCTAGCCCTGAAATTGTCAACCGC-3′, followed by template digestion with DpnI. To construct the N-terminal glutathione S-transferase (GST) fusions GST-MPTVCV and GST-MPTVCV(K210A/K212A), the coding sequences for each were amplified from pTVCV50 and pTVCVMP(K210A/K212A), respectively, using primers that contain 5′ BamHI and 3′ XhoI restriction sites, and each was then cloned between the BamHI and XhoI sites in pGEX-5X-1 (GE Healthcare Biosciences, Piscataway, NJ). To generate the TagRFP-UtrCH fusion, we PCR amplified the calponin homology domain of utrophin (18) from pCS2(+)-GFP-UtrCH (Addgene, Cambridge, MA). This was cloned into pDONR207 and then into the pSITEII 6C1 destination vector (19) to generate pSITEII6C1::TagRFP-UtrCH.
All constructs were confirmed by DNA sequence analysis performed on an Applied Biosystems automated 3730 DNA analyzer at the Cornell University Life Sciences Core DNA Sequencing Lab.
Plant infectivity and transient expression assays.
For transient expression studies using agroinfiltration, N. benthamiana plants were grown and maintained under greenhouse conditions of 22°C with 16-h supplemental lighting. For local infection site and infectivity assays, N. benthamiana plants were grown and maintained in growth chambers at 25°C under long-day (16-h-light/8-h-dark) conditions. Arabidopsis ecotype Col-0 plants were grown in growth rooms or chambers at 22°C. These plants were initially grown under short-day (8-h-light/16-h-dark) conditions for local infection site studies or under long-day (16-h-light/8-h-dark) cycle for infectivity studies. Following inoculations, Col-0 plants were maintained under long-day conditions.
For nuclear localization studies of MPTVCV, pSITE2NB::MPTVCV-GFP or pSITE2NB::MPTVCV(K210A/K212A)-GFP was agroinfiltrated into 4- to 5-week transgenic N. benthamiana lines that expressed either histone 2B-RFP to label chromatin (19) or fibrillarin-RFP to label nucleoli (19), using cultures of Agrobacterium tumefaciens GV2260 at an optical density at 600 nm (OD600) of 0.05 as previously described (20), and plants were imaged at 40 h postinfiltration (p.i.). To coexpress MPTVCV-GFP and the actin probe TagRFP-UtrCH, GV2260 cultures at an OD600 of 0.01 for pSITE2NB::MPTVCV-GFP and pSITEII6C1::TagRFP-UtrCH were mixed in equal volumes and coinfiltrated into wild-type (wt) N. benthamiana. Cell-to-cell movement of MPTVCV-GFP and MPTVCV (K210A/K212A)-GFP was assayed by transient expression in N. benthamiana leaf epidermal cells, as previously described (21). In brief, pSITE2NB::MPTVCV-GFP or pSITE2NB::MPTVCV(K210A/K212A)-GFP was agroinfiltrated into N. benthamiana leaves at an OD600 of 0.0005. To control for leaf variability, plasmid pairs were agroinfiltrated into different sectors on the same leaf for direct comparisons. We used confocal microscopy to count cells in individual fluorescent foci at 40 to 44 h p.i. and analyzed the results by t test.
For local infection site studies, plants were inoculated with in vitro-synthesized infectious genomic +RNA transcripts. Plasmids pTVCV::MPTVCV-GFP and pTVCV::MPTVCV-RFP carrying the viral replicons TVCV::MP-GFP and TVCV::MP-RFP, respectively, were linearized with KpnI, and RNA was transcribed using the SP6 mMessage Machine kit (Life Technologies, Grand Island, NY). Leaves from 4- to 5-week-old wild-type (wt) N. benthamiana plants or transgenic lines that expressed compartment-specific markers (ER, GFP-HDEL [22]; actin cytoskeleton, talin-GFP [19]; chromatin, histone 2B-RFP [19]; microtubules, TUA-GFP [23]) were rub inoculated with the infectious +RNA transcripts using Celite (Sigma, St. Louis, MO) (16), and expanding fluorescent foci were imaged at 4 days p.i. by confocal microscopy. To directly compare the relative cell-to-cell spread of TVCV::MP-GFP and that of TVCV::MPK210A/K212A-GFP, half of each leaf was inoculated with a standardized amount of +RNA for each replicon (20% of the +RNA produced by in vitro transcription of 1 μg DNA template) and the sizes of infection sites were scored at 5 days p.i. using a stereomicroscope and ImageJ software (http://rsbweb.nih.gov/ij/). Arabidopsis Col-0 plants were inoculated at the 12- to 14-leaf stage as described for N. benthamiana and imaged by confocal microscopy at 7 days p.i.
For infectivity assays, plants were inoculated with leaf extracts from N. benthamiana plants infected with wt p50TVCV or pTVCVMP(K210A/K212A). Systemically infected leaves were frozen and ground in 10 ml phosphate-buffered saline (PBS) and 0.1 g Celite per gram of leaf tissue, and extracts were clarified by centrifugation at 5,000 × g for 5 min prior to use. To quantify the relative amounts of virus in these extracts, CP intensities in 2, 3, 4, and 5 μl were determined by SDS-PAGE and, based on this, plants were rub inoculated with equivalent amounts of virus. To determine infectivity in N. benthamiana, 4- to 5-week-old plants were inoculated with virus-containing extract or mock inoculated with PBS, and systemic symptoms were scored daily after infection. To detect CP in systemically infected leaves, extracts from a leaf disc (1-cm diameter) punched from the third leaf from the top of infected plants were prepared and CP was detected by SDS-PAGE and Coomassie blue staining, as previously described (24). For infectivity assays in Arabidopsis, Col-0 plants at the 8-leaf stage were inoculated with appropriate amounts of virus-containing extracts or with PBS. Infectivity was determined based on the levels of viral CP in extracts of systemic leaves (0.5 g tissue/ml buffer), as determined by SDS-PAGE and Coomassie blue staining. Statistical analyses were done using JMPPro 0.0.2 software (SAS, Cary, NC). Local cell-to-cell spread was analyzed using a t test, and the Wilcoxon test was applied to infectivity assays.
Replication assays.
Leaf mesophyll protoplasts were isolated from leaves of 4- to 5-week-old N. benthamiana plants and transfected with 2 μg of TVCV or TVCV::MP(K210A/K212A) +RNA transcripts, as previously described (21). Total RNA was isolated at 0 h, 4 h, and 20 h posttransfection using TRIzol (Life Technologies, Grand Island, NY), and following incubation with DNase I (Life Technologies, Grand Island, NY), reverse transcription was performed using Omniscript reverse transcriptase (Qiagen, Hilden, Germany) and the following primers: TVCV minus strand (−RNA), 5′-ATGAGGCCGTTGCCGAG-3′; 18S rRNA internal control, 5′-AGTCTGTCAATCCTTACTAT-3′. Semiquantitative reverse transcription-PCR (sqRT-PCR) analyses were performed as described previously (21), using primers 5′-CTGATGAATTCGTCGATTCGGTTGCAGCA-3′ and 5′-TATCAAGGGACCCTGTATCTGGGAA-3′ to amplify the reverse-transcribed TVCV RNA (350 bp) and primers 5′-CTGGCGACGCATCATTC-3′ and 5′-GAATTACCGCGGCTGCT-3′ to amplify 18S rRNA (301 bp).
RNA binding assays.
The MPTVCV and MPK210A/K212A coding sequences were each cloned as translational fusions to the glutathione S-transferase (GST) coding sequence in pGEX-5X-1 (GE, Piscataway, NJ). GST-MPTVCV, GST-MPTVCV(K210A/K212A), or GST was expressed in BL21 Rosetta cells by induction in 0.4 mM isopropyl-β-thiogalactopyranoside (IPTG) at 28°C, and cell lysates were prepared in lysis buffer (50 mM Tris-HCl, pH 8, 200 mM NaCl, 1 mM dithiothreitol [DTT], 1 mM phenylmethylsulfonyl fluoride [PMSF], 1 mg/ml lysozyme) as previously described (21). Insoluble GST-MPTVCV and GST-MPTVCV(K210A/K212A) were obtained by centrifugation at 10,000 × g at 4°C for 10 min, following which pellets were washed twice by resuspension in 400 μl of wash buffer (50 mM Tris-HCl, pH 8, 200 mM NaCl, 10 mM EDTA, 0.5% Triton X-100, 1 mM DTT, 1 mM PMSF) and centrifugation at 10,000 × g at 4°C. Clarified lysates containing GST were used directly without purification on glutathione-Sepharose. To test for RNA binding, 1 μg of GST-MPTVCV or GST-MPTVCV(K210A/K212A) pellets, or GST lysate, was resolved on 13.5% SDS-PAGE gels, and proteins were blotted to nitrocellulose and incubated with RNA as previously described (25). Fluorescein-labeled RNA was transcribed in vitro from the 1.85-kb Xenopus laevis EF1α gene, expressed from the SP6 promoter in pTRIPLEscript using the SP6 mMessage Machine kit (Life Technologies, Grand Island NY) and fluorescein RNA labeling mix (Roche, Mannheim, Germany) per the manufacturers' protocols. Following incubation of renatured protein blots with fluorescein-labeled RNA, RNA binding was visualized using the Typhoon system (GE, Piscataway, NJ) with 532-nm excitation and 526-nm short-pass emission filters.
Microscopy.
Confocal laser scanning microscopy (CLSM) was performed using a Leica TCS SP5 spectral imaging system (Leica Microsystems, Wetzlar, Germany). GFP fluorescence was excited with a 488-nm argon laser, and emission was detected at 500 to 530 nm. RFP fluorescence was excited with a 561-nm diode-pumped solid-state (DPSS) laser, and emission was detected at 590 to 630 nm. Z series were collected at ∼1-μm intervals and imaged using Leica software (Leica Microsystems). Three-dimensional (3D) imaging was done using Velocity software (Improvision, Coventry, United Kingdom). Virus infection sites on inoculated leaves were identified using an Olympus SZX-12 stereomicroscope (Olympus Corporation, Center Valley, PA). Green fluorescence was excited with a 480-/30-nm filter, and an emission 535-/40-nm filter was used for detection.
RESULTS
MPTVCV localizes to PD and ER replication sites but not to microtubules.
As a tool to investigate MP trafficking in Arabidopsis during virus infection, we constructed an infectious TVCV replicon, modeled after the approach used with TMV (6). To do this, we created a translational fusion of the MPTVCV and GFP (or RFP) coding regions to express a C-terminal MPTVCV-GFP fusion from the viral MP promoter. As in TMV, this fusion deleted the CP gene (Fig. 1A, TVCV::MP-GFP). Based on studies of the analogous TMV::MP-GFP (6), we expected TVCV::MP-GFP to be infectious and move cell to cell in inoculated leaves, thereby allowing us to examine the subcellular localization of MPTVCV-GFP during the time course of virus infection in infection sites. However, without CP, TVCV::MP-GFP, like TMV::MP-GFP, would not systemically infect plants. To demonstrate the infectious nature of our TVCV::MP-GFP replicon clone and the function of the encoded MPTVCV-GFP, we inoculated N. benthamiana leaves with TVCV::MP-GFP and used confocal microscopy to compare the resulting expanding fluorescent foci to those produced by the well-characterized TMV::MP-GFP (Fig. 1).
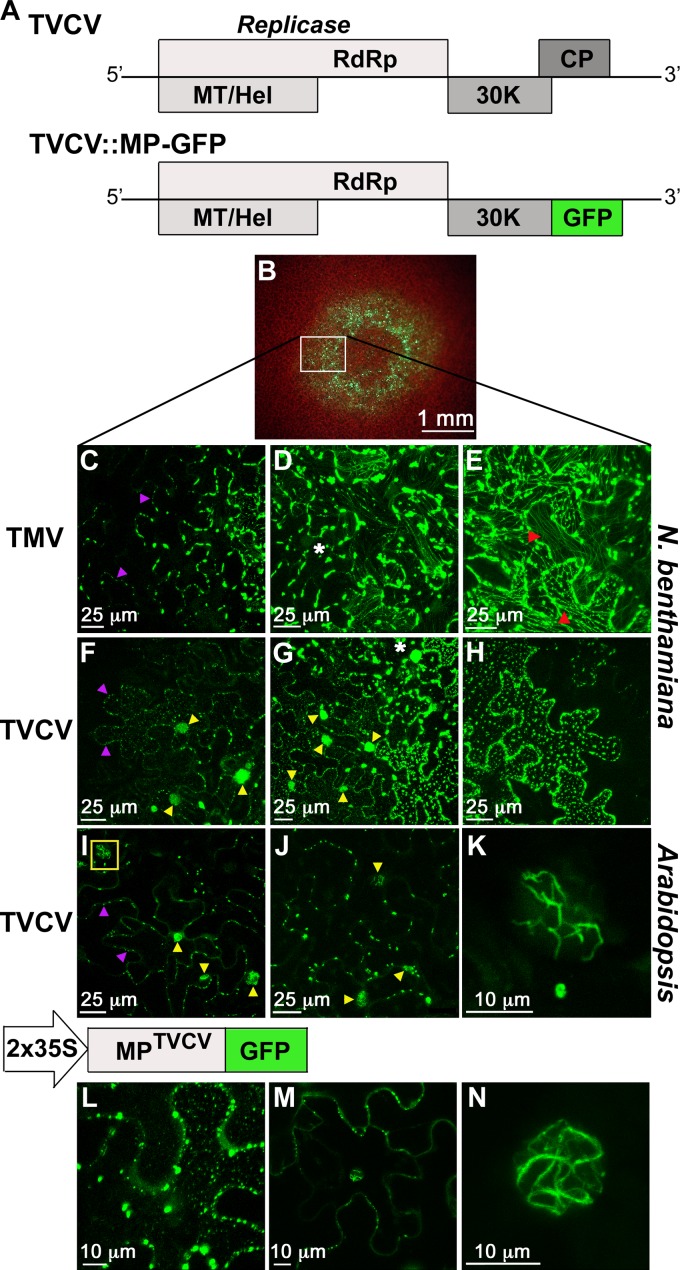
Fig 1.
MPTVCV localizes to the nucleus during virus infection. (A) Schematic representation of TVCV +RNA genome and the replicon TVCV::MP-GFP. (B) TVCV::MP-GFP expanding fluorescent foci on inoculated N. benthamiana leaf at 4 days p.i. are evident as a fluorescent ring and similar to infection sites produced by TMV::MP-GFP (6). The boxed area is the region magnified (left to right) in panels F to H. Equivalent regions of TMV::MP-GFP infection sites in N. benthamiana are shown in panels C to E. (C to J) CLSM-projected Z series of cells in fluorescent foci at 4 days p.i. on N. benthamiana leaves (C to H) or 7 days p.i. on Arabidopsis Col-0 leaves (I to K) inoculated with TMV::MP-GFP (C to E) or TVCV::MP-GFP (F to K). Cells at early (leading edge) (C, F, and I), middle (D, G, and J), and late (E and H) stages of infection are shown. (H) Black areas at right and bottom (E and H) are cells in which MPTVCV-GFP was degraded and remained only at PD. Examples of MPTVCV-GFP-containing nuclei (yellow arrowheads) and PD (purple arrowheads), MPTMV-GFP colocalizing with microtubules (red arrowheads), and intracellular peripheral bodies (white asterisks) are marked. See the text for details. (K) Higher magnification of nucleus boxed in panel I showing MPTVCV-GFP localizing to nuclear filaments. (L to N) Transient expression of MPTVCV-GFP in agroinfiltrated N. benthamiana leaf cells at 40 h. CLSM-projected Z series show MPTVCV-GFP localizing to PD and intracellular bodies at the cell periphery (L), PD and the nucleus at a midplane section (M), and a filamentous structure inside the nucleus (N).
Similarly to TMV::MP-GFP (6, 26), TVCV::MP-GFP produced infection sites that appeared as fluorescent rings on inoculated leaves, in which GFP fluorescence was absent from the centers (late times in infection) (Fig. 1B). This suggested that MPTVCV, like MPTMV, accumulated during the course of infection but was degraded at late times in the TVCV life cycle. When we compared TVCV and TMV directly in N. benthamiana, confocal examination of MPTVCV-GFP subcellular localization across these infection sites showed that MPTVCV-GFP recapitulated a number of the features of MPTMV-GFP (Fig. 1C to H). Early in infection (first cells at the leading edge), MPTVCV-GFP, like MPTMV-GFP, localized to discrete punctae at the cell wall, which appear to be PD (27), and to motile intracellular vesicles, with the latter being more numerous in TVCV-infected cells (Fig. 1C and F). At this early time, both MPTVCV-GFP and MPTMV-GFP also localized to ER membrane-derived replication sites, which appeared to enlarge and accumulate MP at later stages of infection, based on their levels of fluorescence (Fig. 1C, D, F, and G). To show this, we inoculated a TVCV::MP-RFP replicon onto an N. benthamiana transgenic line that expressed the ER marker GFP-HDEL (22). As reported for MPTMV (28), MPTVCV-RFP colocalized with ER in small intracellular and larger peripheral bodies, which were formed at junctions of cortical ER tubules (Fig. 1D and G and 2A). As infection progressed, the ER network collapsed and these replication sites enlarged (Fig. 2B, D, G, H, and I). Consistent with these being replication sites, the peripheral bodies consisted of MPTVCV-RFP engulfed by ER membrane (Fig. 2C). Upon complete collapse of the ER, these MPTVCV-RFP replication sites remained associated with the fragmented ER (Fig. 2G, H, and I). Also like MPTMV, MPTVCV appeared to be degraded at the latest times in infection (focus centers) (Fig. 1H), at which point the ER network re-formed and MPTVCV was found only at PD (punctae at the cell wall) (Fig. 1H and 2E and data not shown).
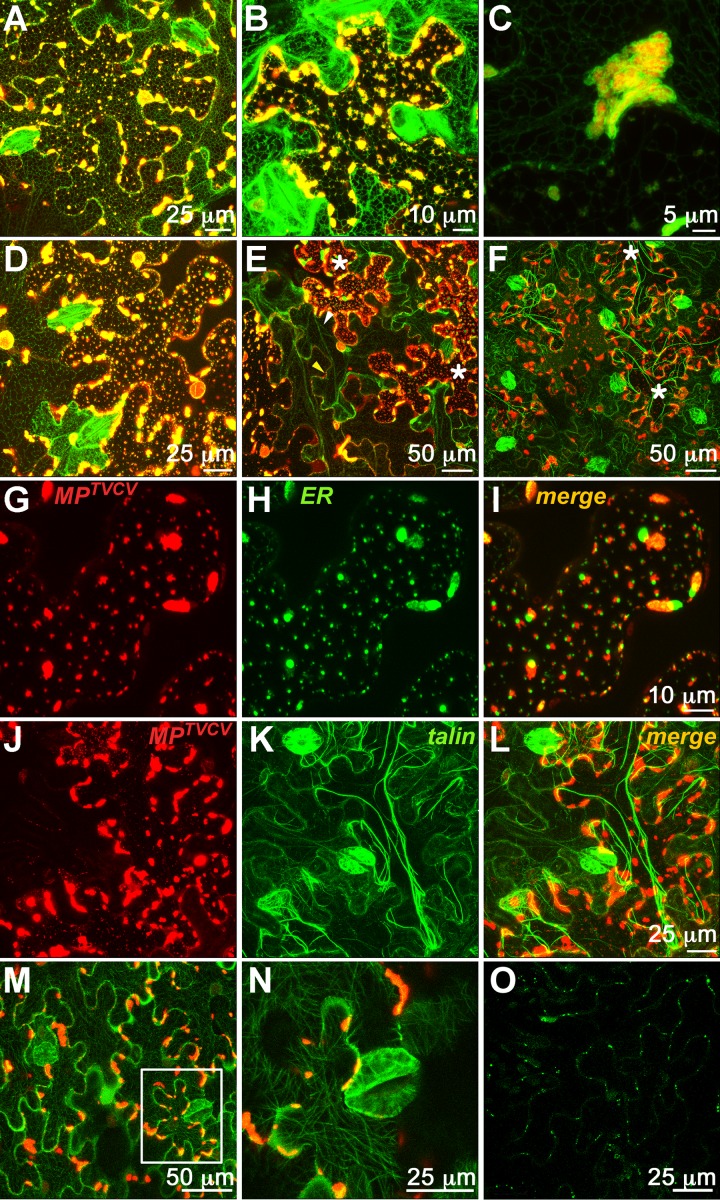
Fig 2.
TVCV infection leads to collapse of the ER network and disruption of the actin cytoskeleton. (A to N) CLSM-projected Z series of cells from TVCV::MP-RFP-infected expanding fluorescent foci at 4 days p.i. on transgenic N. benthamiana lines expressing GFP-HDEL to label ER (A to E and G to I), talin-GFP to label the actin cytoskeleton (F, J, K, and L), or TUA-GFP to label microtubules (M and N). Cells at middle (A to C) or late (D to N) stages of infection are shown. Football-shaped ∼25- by 30-μm areas densely labeled with GFP-HDEL (A and D), talin-GFP (F, K, and L), or TUA-GFP (M and N) are stomatal guard cells. (A) ER sites containing MPTVCV-RFP at nodes in the reticular ER and at the cell periphery. (B and D) Progressive collapse of the reticulate ER and the subsequent enlargement of MPTVCV-RFP ER sites in the cortical regions of the cell. (C) High-magnification image of MPTVCV-RFP-containing ER site. (E) ER network re-forms (white arrowhead) at latest stages of infection when MPTVCV is degraded and remains only at PD (yellow arrowhead). (F) Disruption of the actin cytoskeleton late in infection. Asterisks in panels E and F mark examples of cells that contain enlarged peripheral MPTVCV-ER sites at late-stage infection before ER re-forms. (G to I) MPTVCV-RFP and ER (GFP-HDEL) sites partition into visibly distinct compartments but remain associated with each other late in infection. (J to L) The actin cytoskeleton is disrupted late in TVCV infection, with actin filaments (talin-GFP) appearing to be distorted near, and bundle around, MPTVCV-RFP-containing ER sites, but MPTVCV-RFP does not distribute to microtubules, and the microtubule network is not perturbed (M and N). (N) Enlargement of boxed area in panel M. (O) CLSM-projected Z series of cells at center of TVCV::MP-GFP infection site on inoculated Col-0 leaves at 7 days p.i. As in TMV infection, MPTVCV-GFP is downregulated at the center of infection sites and remains only at what appear to be PD (punctate spots along the cell periphery).
Despite these similarities to MPTMV, we found that MPTVCV differed from MPTMV in three important respects. First, in contrast to the well-documented association of MPTMV with microtubules (12, 29) (Fig. 1E), we found no association of MPTVCV with microtubules when we inoculated TVCV::MP-RFP onto N. benthamiana transgenic plants that expressed a GFP fusion of Arabidopsis α-tubulin (TUA-GFP) (23), nor did we observe any rearrangement of microtubules (Fig. 1F to H and 2M and N). Second, when we inoculated TVCV::MP-RFP onto an N. benthamiana transgenic line expressing a talin-GFP fusion (19), we observed, at late stages of infection, distortion of the actin cytoskeleton and bundling of filamentous actin (F-actin) near and around MPTVCV-marked replication sites that appeared to parallel changes in the ER membrane (Fig. 2F, J, K, and L). Third, in striking contrast to MPTMV, MPTVCV-GFP localized to nuclei in infected cells at the earliest stages and throughout infection up to the point of MPTVCV degradation at the center of infection sites (Fig. 1F to H). To show that this unexpected nuclear accumulation of MPTVCV-GFP was due to nuclear import of MPTVCV and not diffusion of free GFP produced by truncation or protein degradation, we analyzed protein extracts from N. benthamiana leaves infected with our TVCV::MP-GFP and TMV::MP-GFP replicons on immunoblots using anti-GFP antibodies and did not detect any free GFP (data not shown). In addition, we found that MPTVCV targets to nuclei in an NLS-dependent manner (see below).
The localization pattern of MPTVCV-GFP in TVCV infection sites on Arabidopsis was the same as that on N. benthamiana, differing only in the temporal aspects of MPTVCV-GFP distribution. In Col-0, MPTVCV-GFP localized to intracellular and peripheral ER replication sites, in addition to PD and nuclei, at all stages in infection, from the earliest stages in cells at the infection front through the late stages up to the point of MPTVCV-GFP degradation at the infection site center (Fig. 1I to K). As in N. benthamiana, MPTVCV-GFP-associated peripheral ER sites expanded as infection progressed (Fig. 1I and J), MPTVCV-GFP was found only at PD at the latest stage in infection (centers of foci) when MPTVCV-GFP was degraded (Fig. 2O), and MPTVCV-GFP did not distribute to microtubules (Fig. 1I and J).
Nuclear MPTVCV localizes to novel F-actin-containing filaments that associate with chromatin.
We observed that MPTVCV-GFP localized to a novel filamentous nuclear compartment in TVCV-infected cells in both Arabidopsis and N. benthamiana (Fig. 1F, G, I, and K and data not shown), in which, based on fluorescence intensity, it continued to accumulate as infection progressed, up to the latest stages in infection just prior to its being degraded (Fig. 1F to H). To show that this novel nuclear localization, as well as other differences from MPTMV (Fig. 1F to H compared to C to E), was an intrinsic property of MPTVCV per se, we transiently expressed MPTVCV-GFP or MPTMV-GFP, each driven by the 35S promoter, in N. benthamiana leaf cells using agroinfiltration (30). As we found for the early stages in TVCV infection, MPTVCV-GFP, when expressed alone, localized to PD, numerous motile vesicles, intracellular and peripheral bodies, and the same nuclear filaments as those seen in TVCV-infected cells (Fig. 1L to N; see also Movie S1 in the supplemental material) and did not localize to microtubules. In contrast, MPTMV-GFP, when expressed alone, localized to PD, intracellular bodies, and microtubules but did not localize to nuclei (data not shown), as previously reported (31). Importantly, we observed the same MPTVCV-GFP-containing nuclear filaments in both Col-0 and N. benthamiana plants that were infected with our TVCV replicon or transiently expressed MPTVCV-GFP (Fig. 1K and N). Thus, the localization of MPTVCV to these novel nuclear filaments, as well as to PD, motile vesicles, and ER sites, was an intrinsic characteristic of this tobamovirus movement protein that did not require viral RNA or other viral components.
To more precisely localize these MPTVCV-GFP-containing filaments within the nucleus, we used agroinfiltration to transiently express MPTVCV-GFP in transgenic N. benthamiana lines that expressed RFP fusions of either histone 2B (H2B-RFP) to label chromatin or fibrillarin (FBL-RFP) to label nucleoli and Cajal bodies (19). MPTVCV-GFP did not localize to nucleoli or to discrete FBL-RFP-labeled speckles (Cajal bodies) (Fig. 3A to C and data not shown). Rather, we found that MPTVCV-GFP-containing filaments were associated with H2B-RFP-labeled chromatin, where MPTVCV-GFP appeared to be in a filamentous network that contacted discrete regions of chromatin (Fig. 3D to L). These filaments bore a striking resemblance to polymerized nuclear actin that, in a recent study from the Gurdon lab, was shown to be required for transcriptional reactivation of the pluripotency gene Oct4 when mouse cell nuclei are transplanted into the Xenopus laevis oocyte giant nucleus (germinal vesicle [GV]) (32). Thus, to determine whether MPTVCV-GFP colocalized with F-actin in these nuclear filaments, we used agroinfiltration to transiently coexpress MPTVCV-GFP and the calponin binding domain of utrophin (UtrCH) fused to TagRFP (TagRFP-UtrCH), a probe reported to faithfully mark F-actin without altering the balance of actin assembly/disassembly (18). TagRFP-UtrCH, when expressed alone, labeled the actin cytoskeleton and, when found in the nucleus, was diffuse throughout the nucleoplasm (Fig. 3Q). When it was coexpressed with MPTVCV-GFP, TagRFP-UtrCH still labeled the actin cytoskeleton, but the nuclear TagRFP-UtrCH now colocalized with MPTVCV-GFP in filaments (Fig. 3M to O). Thus, these chromatin-associated nuclear filaments contain both MPTVCV and F-actin.
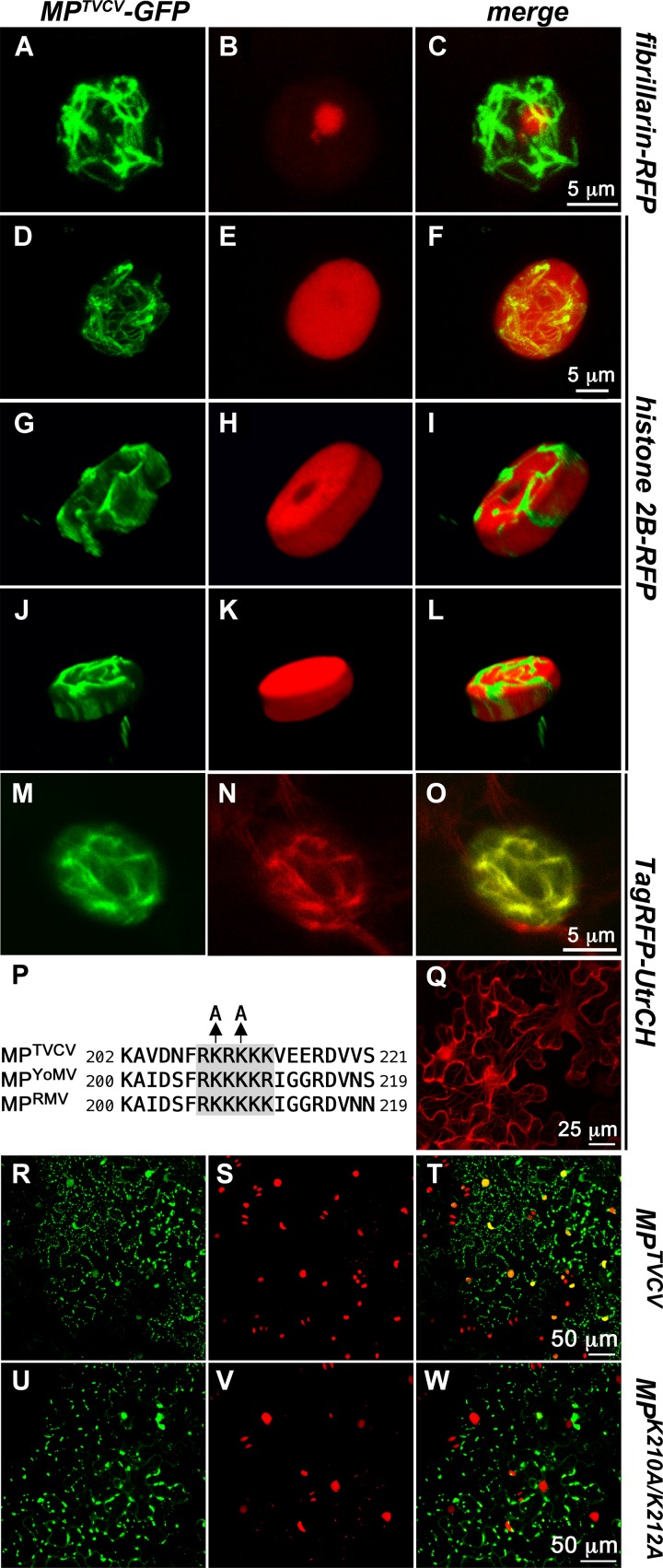
Fig 3.
MPTVCV-GFP localizes in an NLS-dependent manner to actin-containing nuclear filaments that appear to contact discrete regions of chromatin. (A to O) CLSM-projected Z series of nuclei from agroinfiltrated N. benthamiana leaf cells transiently expressing MPTVCV-GFP. Shown are transgenic N. benthamiana plants expressing fibrillarin-RFP (nucleolus and Cajal bodies) (A to C) or histone 2B (H2B)-RFP (chromatin) (D to L) or wt plants agroinfiltrated to coexpress TagRFP-UtrCH (M to O). MPTVCV-GFP (green), fibrillarin-RFP, H2B-RFP, TagRFP-UtrCH (red), and the corresponding superimposed images are shown. (G to L) Projected Z-plane 5-μm sections from the middle (G to I) and bottom (J to L) of the nucleus shown in panels D to F. (M to O) MPTVCV-GFP and TagRFP-UTR colocalize to the nuclear filaments in MPTVCV-expressing cells. (Q) TagRFP-UtrCH transiently expressed alone in N. benthamiana leaf cells labels the actin cytoskeleton and, when found in nuclei, is diffuse in the nucleoplasm. (P) Alignment of MPTVCV, MPYoMV, and MPRMV protein sequences showing conservation of basic residues (gray shading) of the monopartite NLS at the position corresponding to residues 209 to 214 of MPTVCV. Locations of the two Lys-to-Ala mutations in MPTVCV(K210A/K212A) are shown. (R to W) CLSM-projected Z series of leaves from transgenic N. benthamiana plants expressing histone 2B-RFP and inoculated with the replicon TVCV::MP-GFP (R to T) or TVCV::MPK210A/K212A-GFP (U to W). Shown are MPTVCV-GFP (green) (R), MPTVCV(210A/212A)-GFP (green) (U), and histone 2B-RFP (red) (S and V) and the corresponding superimposed images (T and W). MPTVCV-GFP localizes to nuclei (yellow-orange in panel T), but MPTVCV(210A/212A)-GFP does not (nuclei are red only in panel W).
MPTVCV contains an NLS that is required for import into the nucleus.
Our inspection of the MPTVCV sequence identified a potential classic basic monopartite nuclear localization sequence at amino acid (aa) residues 209 to 214 (RKRKKK) (Fig. 3P and 4). Aligning the MP sequences from different tobamoviruses, we found that this putative NLS appeared to be conserved in all subgroup 3 tobamoviruses but was not present in the viruses in subgroup 1 or 2, including TMV, save for Tobacco mild green mosaic virus (TMGMV) and Sunn-hemp mosaic virus (SHMV), both in subgroup 1 (Fig. 4; see also Fig. S1 in the supplemental material; other data not shown).
Fig 4.
The MPTVCV NLS appears to be conserved among subgroup 3 tobamoviruses. Alignment of MP protein sequences from the subgroup 3 tobamoviruses (TVCV, YoMV, and RMV) and representative subgroup 1 tobamoviruses, including TMV (shown at bottom). The basic residues in the MPTVCV monopartite NLS are shaded in gray, with the two missense mutations in MPTVCV(K210A/K212A) indicated. The corresponding predicted monopartite NLSs in YoMV and RMV are also shown with basic residues shaded in gray, as is a predicted monopartite NLS in TMGMV. Broken lines indicate additional residues predicted to be part of the NLS interaction domain with importin-α (cNLS Mapper, http://nls-mapper.iab.keio.ac.jp/cgi-bin/NLS_Mapper_form.cgi//). Boxed in SHMV are residues predicted to comprise a potential extended bipartite NLS. Single and double lines beneath the MPTMV sequence mark the two extended RNA binding regions identified in TMV (33). Sequence similarity is indicated below the aligned sequences. TVCV, Turnip vein clearing virus (accession no. Q88921); YoMV, Youcai mosaic virus (same as ORMV, Oilseed rape mosaic virus; accession no. Q66221); RMV, Holmes ribgrass mosaic virus (accession no. Q9QDI8); SHMV, Sunn-hemp mosaic virus (accession no. P03585); ORSV, Odontoglossum ringspot virus, strain Japan (accession no. P22590); TMGMV, Tobacco mild green mosaic virus (TMV U2 strain; accession no. P18338); PMMVJ, Pepper mild mottle virus, strain Japan (accession no. P89658); TMOB, Tobamovirus Ob (also known as Obuda pepper virus; accession no. Q83485); ToML, Tomato mosaic virus, strain L (also known as TMV strain tomato; accession no. P69513); TMV, Tobacco mosaic virus, strain U1 (accession no. P03583). Numbers to the right of each sequence indicate residue position within the protein.
To determine if MPTVCV amino acid residues 209 to 214 were, in fact, an NLS, we mutated the Lys residues at positions 210 and 212 to Ala and introduced this mutation into our infectious replicon to create TVCV::MPK210A/K212A-GFP (Fig. 3P). We then inoculated the transgenic N. benthamiana line that expressed H2B-RFP with this or with our wt TVCV::MP-GFP replicon. We found that TVCV::MPK210A/K212A-GFP was still infectious, producing expanding fluorescent foci on inoculated leaves (Fig. 5C). In wt TVCV::MP-GFP-infected sites, MPTVCV-GFP clearly localized with H2B-RFP in nuclei throughout the infection sites, in addition to its cytoplasmic distribution among PD, motile vesicles, and ER replication sites (Fig. 3R to T). In contrast, MPTVCV(K210A/K212A)-GFP did not enter the nucleus, as evident from our finding that it did not localize to H2B-RFP-marked nuclei (Fig. 3U to W). Thus, MPTVCV residues 209 to 214 act as a functional NLS that is essential for MPTVCV import into the nucleus. Our MPTVCV NLS mutant still localized to PD, vesicles, and ER replication sites in TVCV::MPK210A/K212A-GFP-infected sites. The only difference that we observed from wt MPTVCV-GFP was that the number of intracellular bodies and ER sites, and the accumulation of MPTVCV(K210A/K212A)-GFP at these sites, as assessed by fluorescence intensities, appeared to be increased (Fig. 3R and U).
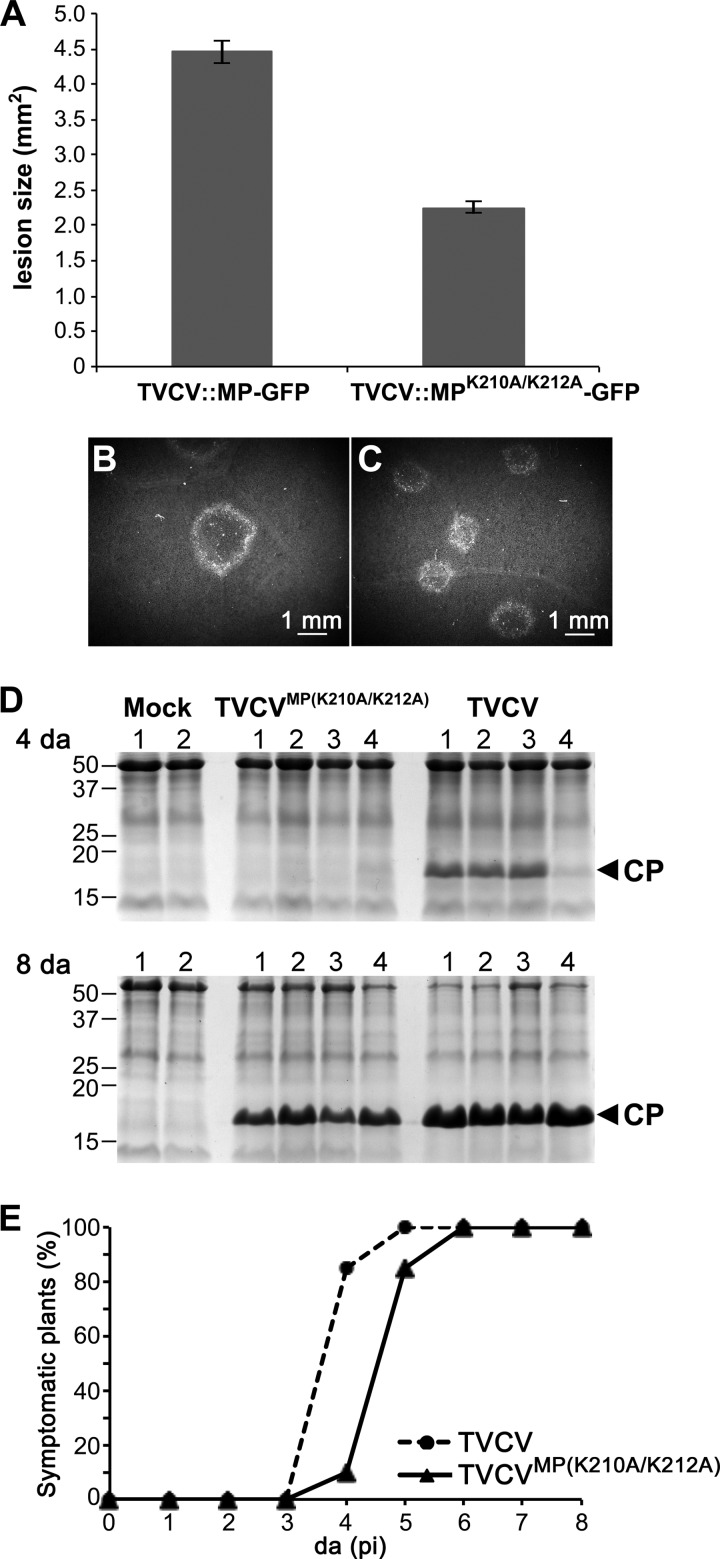
Fig 5.
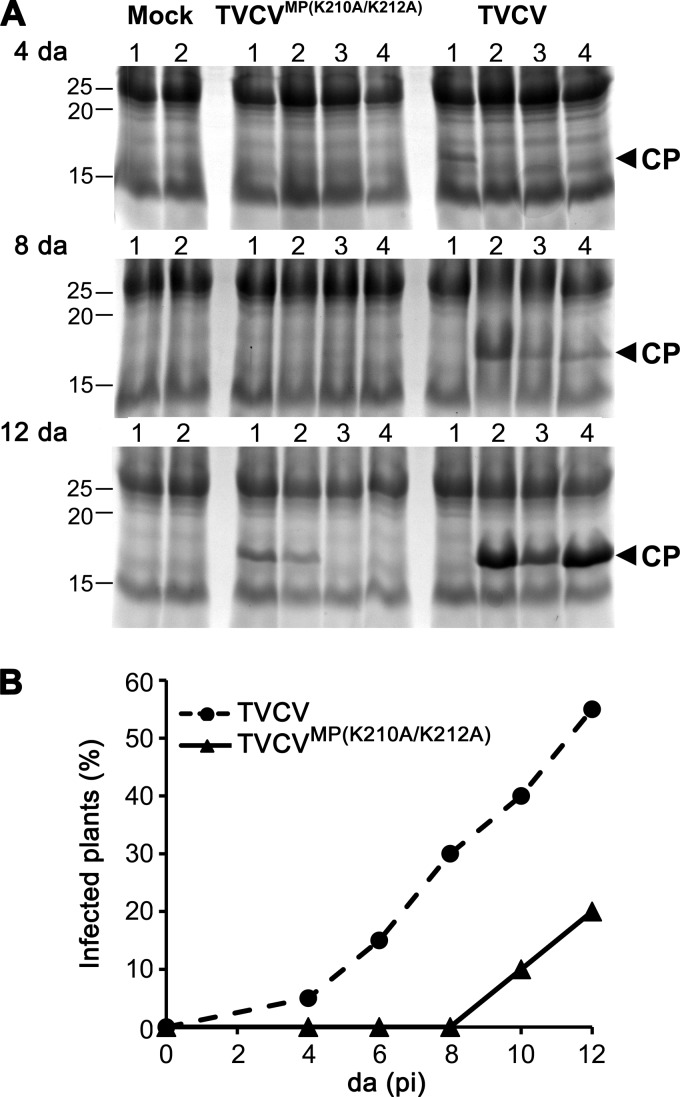
The NLS mutant TVCVMP(K210A/K212A) is delayed in local spread and systemic infection in N. benthamiana. (A) Areas of infection sites at 5 days p.i. on N. benthamiana leaves inoculated with replicon TVCV::MP-GFP or TVCV::MPK210A/K212A-GFP. The areas of 102 TVCV::MP-GFP and 131 TVCV::MPK210A/K212A-GFP infection sites on leaves inoculated with a standardized amount of RNA (see Materials and Methods) were measured. (B and C) Representative infection sites produced by TVCV::MP-GFP (B) or TVCV::MPK210A/K212A-GFP (C) on N. benthamiana leaves at 5 days p.i. (D) Coomassie blue-stained SDS-PAGE gels of systemic leaf extracts from N. benthamiana plants inoculated with full-length infectious TVCVMP(K210A/K212A) (NLS mutant) or with wt TVCV or mock inoculated at 4 days and 8 days p.i. Equal amounts of extract from 1-cm punches of systemic leaves were analyzed in each gel (see Materials and Methods). Positions of CP (17 kDa) and of protein markers (kDa) are marked. (E) Time course of appearance of systemic disease symptoms on N. benthamiana plants inoculated with equal amounts of wt TVCV or the NLS mutant TVCVMP(K210A/K212A) (Table 1, trial 1).
Nuclear MPTVCV is necessary for efficient TVCV infection.
TVCV::MPK210A/K212A-GFP infection sites displayed the same progression of infection as we observed in wt TVCV sites, with MPTVCV(K210A/K212A)-GFP being degraded at the latest stage of infection. However, when we inoculated N. benthamiana plants with our MPTVCV NLS mutant TVCV::MPK210A/K212A-GFP, it was evident that the expanding foci were smaller than those produced by wt TVCV::MP-GFP (Fig. 5B and C). To quantify this, we measured the sizes of expanding TVCV::MP-GFP and TVCV::MPK210A/K212A-GFP foci on inoculated leaves at 5 days p.i. Wild-type TVCV::MP-GFP infection sites were about twice the area of TVCV::MPK210A/K212A-GFP infection sites (4.5 ± 0.2 mm2 versus 2.2 ± 0.1 mm2, P < 0.0001) (Fig. 5A). This suggested that a functional MPTVCV NLS was necessary for efficient TVCV cell-to-cell spread.
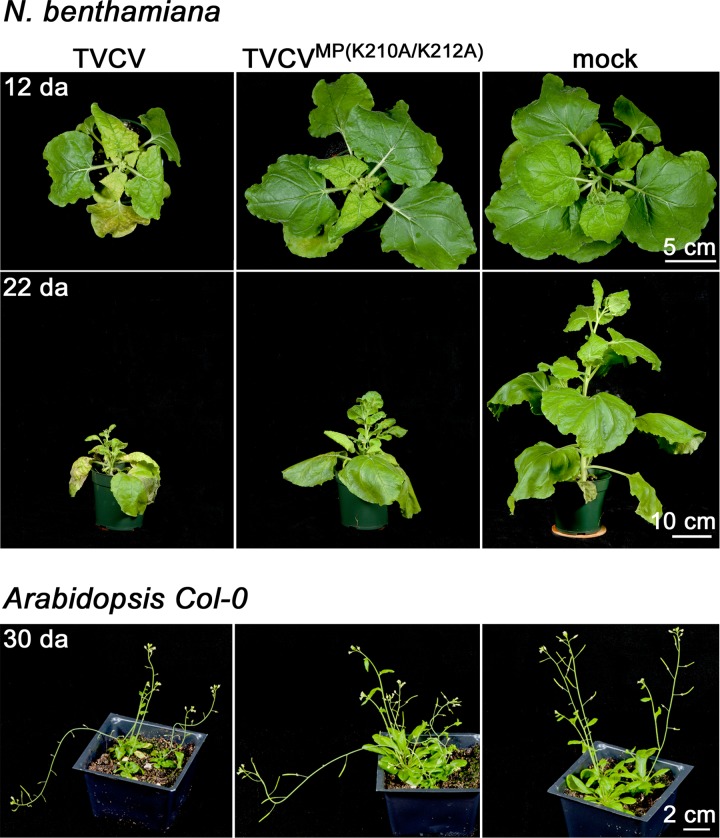
To examine this further, we analyzed the consequences of mutating the MPTVCV NLS for TVCV systemic infection in N. benthamiana and Arabidopsis. To do this, we introduced the NLS mutation into the fully infectious wt TVCV genome that expressed untagged MP to produce TVCVMP(K210A/K212A). As shown in Fig. 5, TVCVMP(K210A/K212A) systemic spread was inhibited in N. benthamiana, based on both the percentage of infected plants and the systemic accumulation of TVCV CP at different days p.i. TVCVMP(K210A/K212A)-infected plants exhibited a significant delay (P < 0.0001) in the onset of systemic disease symptoms, compared to plants infected with wt TVCV (Fig. 5E; Table 1), and displayed attenuated disease symptoms (Fig. 6). Fitting with this, the systemic accumulation of CP was also delayed. Viral CP was clearly detected at 4 days and 8 days p.i. in systemic leaf extracts from ∼75% and 100%, respectively, of wt TVCV-infected N. benthamiana plants. In contrast, CP was rarely detected, and then at only very low levels, in TVCVMP(K210A/K212A)-infected N. benthamiana systemic leaves at 4 days p.i., and by 8 days p.i., although present in all plants tested, CP had accumulated to lower levels than those in wt TVCV-infected plants (Fig. 5D). We obtained the same results in Arabidopsis. As TVCV produces mild disease symptoms in Col-0 plants, we assessed systemic infection in this host by assaying the accumulation of TVCV CP in extracts of systemic leaves. TVCVMP(K210A/K212A) systemic spread, compared to that of wt TVCV, was significantly delayed in Col-0 plants, based on the systemic accumulation of TVCV CP at different days p.i. (Fig. 7; Table 2; P < 0.001), and this NLS mutant also produced attenuated disease symptoms, compared to wt TVCV, in Col-0 plants (Fig. 6).
Table 1.
TVCV infectivity on N. benthamianaa
| Trial | Inoculum | % of total inoculated plants at day postinoculation: |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||
| 1 | wt | 0 | 0 | 0 | 85 | 100 | 100 | 100 | 100 |
| TVCVMP(K210/K212A) | 0 | 0 | 0 | 10 | 85 | 100 | 100 | 100 | |
| Mock | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 2 | wt | 0 | 0 | 10 | 85 | 100 | 100 | 100 | 100 |
| TVCVMP(K210/K212A) | 0 | 0 | 0 | 10 | 80 | 100 | 100 | 100 | |
| Mock | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 3 | wt | 0 | 0 | 0 | 90 | 100 | 100 | 100 | 100 |
| TVCVMP(K210/K212A) | 0 | 0 | 0 | 15 | 90 | 100 | 100 | 100 | |
| Mock | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
Symptomatic plants as percentage of total inoculated plants (20 per assay). Nonparametric survival analysis with right censoring (Wilcoxon test statistic), P < 0.0001 for 3 trials shown.
Fig 6.
The NLS mutant TVCVMP(K210A/K212A) produces attenuated disease symptoms in N. benthamiana and Arabidopsis Col-0. N. benthamiana (top) or Col-0 (bottom) plants at 12 days or 22 days (N. benthamiana) or 30 days (Col-0) postinoculation with wt TVCV or TVCVMP(K210A/K212A), or mock inoculated, as indicated.
Fig 7.
The NLS mutant TVCVMP(K210A/K212A) is delayed in systemic infection in Arabidopsis Col-0. (A) Coomassie blue-stained SDS-PAGE gels of systemic leaf extracts from Col-0 plants inoculated with full-length infectious TVCVMP(K210A/K212A) (NLS mutant) or with wt TVCV or mock inoculated at 4 days, 8 days, and 12 days p.i. Equal amounts of systemic leaf tissue extracts (0.5 g/ml) were analyzed in each gel (see Materials and Methods). Positions of CP (17 kDa) and of protein markers (kDa) are marked. (B) Development of systemic disease as assessed by CP accumulation in Col-0 plants inoculated with equal amounts of wt TVCV or the NLS mutant TVCVMP(K210A/K212A) (Table 2, trial 1).
Table 2.
TVCV infectivity on Arabidopsis Col-0a
| Trial | Inoculum | % total inoculated plants at day postinoculation: |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 6 | 8 | 10 | 12 | ||
| 1 | wt | 0 | 0 | 0 | 5 | 15 | 30 | 40 | 55 |
| TVCVMP(K210/K212A) | 0 | 0 | 0 | 0 | 0 | 0 | 10 | 20 | |
| Mock | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 2 | wt | 0 | 0 | 0 | 6.25 | 25 | 50 | 75 | NDb |
| TVCVMP(K210/K212A) | 0 | 0 | 0 | 0 | 0 | 12.5 | 25 | ND | |
| Mock | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ND | |
| 3 | wt | 0 | 0 | 0 | 0 | 15 | 35 | 55 | 75 |
| TVCVMP(K210/K212A) | 0 | 0 | 0 | 0 | 0 | 5 | 25 | 35 | |
| Mock | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
Infected plants as percentage of total inoculated plants (20 per assay), based on presence of TVCV CP in extracts of systemic leaves (see Materials and Methods). Nonparametric survival analysis with right censoring (Wilcoxon test statistic), P < 0.001 for 3 trials shown.
ND, not determined.
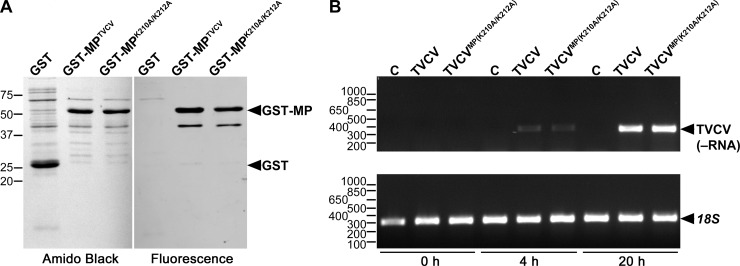
To show that this delay in local and systemic virus spread was specifically due to the absence of a nuclear function of MPTVCV, and not the result of an effect of the NLS mutation in MPTVCV(K210A/K212A) on virus replication or cell-to-cell movement per se, we examined the ability of MPTVCV(K210A/K212A) to bind RNA and traffic cell to cell, the latter as a means to assess its effects on PD gating, and assayed the replication of TVCVMP(K210A/K212A) in N. benthamiana protoplasts. The C-terminal half of MPTMV contains two extended RNA binding domains (33). Based on our sequence alignments, the MPTVCV NLS (residues 209 to 214) is within the second of these domains (Fig. 4). To exclude the possibility that our NLS mutant MPTVCV(K210A/K212A) was defective in RNA binding, we used a membrane binding assay to test GST fusions of both MPTVCV(K210A/K212A) and wt MPTVCV for their ability to bind single-stranded RNA (ssRNA) in vitro. As shown in Fig. 8, MPTVCV(K210A/K212A) bound ssRNA in vitro to the same extent as did wt MPTVCV. This binding was specific for MPTVCV, as free GST did not bind ssRNA when tested in this same assay (Fig. 8A).
Fig 8.
The NLS mutant MPTVCV(K210A/K212A) is not defective in ssRNA binding in vitro or in virus replication. (A) SDS-PAGE renatured gel blots of E. coli-expressed GST-MPTVCV, GST-MPTVCV(K210A/K212A) or GST, as labeled, incubated with fluorescein-labeled EF1α RNA in vitro and then stained with amido black to detect proteins. Positions of GST-MP (56 kDa) and GST (26 kDa) and of protein markers (kDa) are marked. (B) Replication of wt TVCV and TVCVMP(K210A/K212A) (NLS mutant) in N. benthamiana protoplasts. Virus replication at 0, 4, and 20 h posttransfection, as indicated, was detected by RT-PCR using primers specific for the TVCV −RNA strand (see Materials and Methods). Positions of amplified TVCV −RNA fragment and 18S RNA loading control, and of DNA markers (nt), are marked. C, mock-transfected control.
To directly examine the ability of the NLS mutant to traffic between cells, we used agroinfiltration to transiently express MPTVCV(K210A/K212A)-GFP and wt MPTVCV-GFP in N. benthamiana leaf epidermal cells, using an established assay (21). We infiltrated a low concentration of each A. tumefaciens culture so that each movement protein would be expressed in individual isolated cells (∼1 in 10) by ∼24 h p.i. (21), and then we assessed MP cell-to-cell trafficking over time by using CLSM to count the numbers of cells in individual foci at 44 to 46 h p.i. As shown in Table 3, the NLS mutant MPTVCV(K210A/K212A) -GFP trafficked cell to cell at least as well as did wt MPTVCV-GFP, if not slightly more efficiently (Table 3). Thus, this NLS mutation did not impair the two key activities of MPTVCV(K210A/K212A) that are essential for its function in virus movement, namely, its ability to bind ssRNA (Fig. 8A) and to traffic cell to cell (Table 3).
Table 3.
Cell-to-cell trafficking of MPTVCV and MPTVCV(K210A/K212A)
| Trial | Proteina | Total focib (total no. of fluorescent cells) | No. of focic (% total foci) |
Pd | |||
|---|---|---|---|---|---|---|---|
| 1 cell | 2–7 cells | 8–13 cells | ≥14 cells | ||||
| 1 | MPTVCV | 30 (249) | 1 (3.3) | 12 (40.0) | 15 (50.0) | 2 (6.7) | |
| MPTVCV(K210A/K212A) | 29 (329) | 1 (3.4) | 4 (13.8) | 17 (58.6) | 7 (24.1) | <0.05 | |
| 2 | MPTVCV | 31 (252) | 1 (3.2) | 14 (45.2) | 12 (38.7) | 4 (12.9) | |
| MPTVCV(K210A/K212A) | 32 (340) | 0 (0.0) | 8 (25.0) | 18 (56.3) | 6 (18.8) | <0.05 | |
Vectors encoding GFP fusions of MPTVCV or MPTVCV(K210A/K212A) were agroinfiltrated into N. benthamiana leaves. Shown are 2 separate trials.
Total number of fluorescent foci counted.
The number of foci in which each movement protein was in a single cell (1 cell) or had moved to 2 to 7, 8 to 13, or ≥14 cells.
P, t test statistic.
To exclude the possibility that the NLS mutation in MPTVCV(K210A/K212A) affected virus replication, we transfected N. benthamiana protoplasts with full-length genomic (+RNA) transcripts of wt TVCV or the NLS mutant TVCVMP(K210A/K212A) and assayed virus replication by sqRT-PCR, using reverse transcription primers specific for the TVCV −RNA strand to ensure that we quantified virus replication and not the subsequent transcription of viral mRNA. We detected no viral RNA by sqRT-PCR immediately following transfection, which established both the baseline for our assay and the specificity of our reverse transcription primer for TVCV −RNA (Fig. 8B, 0 h). At 4 h and 20 h posttransfection, we detected increasing amounts of viral RNA, with the NLS mutant TVCVMP(K210A/K212A) and wt TVCV replicating to the same levels (Fig. 8B). Thus, the NLS mutant MPTVCV(K210A/K212A) does not have a direct effect on TVCV replication, and MPTVCV(K210A/K212A) properly localizes to ER sites and PD, traffics cell to cell, and binds RNA (Fig. 3U to W and 8; Table 3). Furthermore, the phenotype of MPTVCV(K210A/K212A) in delaying TVCV spread is independent of virus encapsidation since this delay is exhibited by both the replicon TVCV::MPK210A/K212A, which does not encode CP, and by the mutated full-length virus TVCVMP(K210A/K212A) (Fig. 5 to 7). Based on our results, we conclude that MPTVCV targets to the nucleus, where it localizes to F-actin-containing filaments that associate with chromatin to provide a function that is necessary for efficient TVCV spread and systemic infection.
DISCUSSION
MPTMV is one of the most extensively characterized plant virus movement proteins and has been the model for understanding how a single movement protein can execute multiple functions to regulate virus genome transport within and between cells (7, 34). However, Arabidopsis thaliana is, in general, a poor host for TMV. In contrast, TVCV efficiently infects Arabidopsis, including the Col-0 ecotype, and hence is the tobamovirus of choice for studies in this model plant to take advantage of its genetic, genomic, and molecular and cell biological resources. A key problem, which contributes to current confusion in the field, is the unproven assumption that the TVCV and TMV life cycles, including the localization of MPTVCV and MPTMV to virus replication sites and subcellular compartments, are the same. We have used an infectious clone of TVCV that encodes a functional MPTVCV-GFP fusion to directly compare the distributions of MPTVCV and MPTMV during virus infection. Our results, reported here, showed that MP association with PD and with ER sites and its degradation at late stages were common features of infection by both TVCV and TMV, thereby underscoring the importance of these associations, and the regulation of MP levels, for tobamovirus replication and intercellular trafficking (Fig. 1 and 2). Importantly, we identified three striking features that distinguished TVCV infection from that of TMV.
Unlike MPTMV, which redistributes from viral ER-associated replication sites to microtubules at late stages in infection, MPTVCV accumulated in an unknown compartment that remained adjacent to these ER sites, and it did not redistribute to microtubules (Fig. 1 and 2E, G, H, and I). A second difference from TMV was that these late changes in ER were accompanied by a marked rearrangement and bundling of the actin cytoskeleton near and around these ER/MPTVCV sites (Fig. 2F, J, K, and L). Thus, while the role of microtubules in promoting TMV cell-to-cell movement, or targeting MPTMV for degradation, late in infection is still debated (29, 35), our studies did not identify a role for MPTVCV-microtubule associations in TVCV infection, at least in terms of a redistribution of MPTVCV to microtubules or obvious tracking of MPTVCV along microtubules. In contrast, our findings did demonstrate distortion of the actin cytoskeleton late in infection at times when apparent TVCV replication sites enlarged to form compartments that remained adjacent to sites of collapsed ER membrane (Fig. 1 and 2). This, again, is distinct from TMV, where disruption of the microfilament network has not been observed (29). These differences between TVCV and TMV are consistent with other studies that indicate differing requirements for microtubules and microfilaments in infection by these two tobamoviruses. MPTMV has been reported to bind to F-actin and to tubulin in vitro (36). Although the actin cytoskeleton is not obviously rearranged or morphologically altered during TMV infection, and the significance of MPTMV interactions with microfilaments remains uncharacterized, imaging studies, combined with gene silencing and the use of inhibitory drugs such as latrunculin B, do point to a role for the actin cytoskeleton and myosin motors in promoting TMV intercellular movement, in terms of both the TMV genome complexed with the viral 126-kDa replicase and host factors trafficking along microfilaments, and MPTMV reaching PD (12, 37–39). In particular, TMV cell-to-cell spread, as assessed by the sizes of infection sites produced on N. benthamiana leaves, is inhibited by latrunculin B, which inhibits actin polymerization, or by silencing host actin or myosin XI-2 (37–39). In this same assay, latrunculin B also inhibits the intercellular spread of the potexvirus Potato virus X and the tombusvirus Tomato bushy stunt virus. However, neither latrunculin B nor silencing of myosin XI-2 or three other myosin isoforms was found to inhibit the cell-to-cell spread of TVCV. Similarly, the microtubule inhibitor oryzalin does not inhibit TVCV cell-to-cell spread (37). The cytoskeletal rearrangements that we observed during TVCV infection are intriguing in light of our finding that MPTVCV localizes to nuclear F-actin filaments (Fig. 3). They may be related to MPTVCV inducing or stabilizing nuclear F-actin filaments and consequently perturbing the balance of cytoplasmic G- and F-actin and would be consistent with the lack of an effect of latrunculin B on TVCV cell-to-cell spread (37). Fitting with this suggestion, we found that expressing very high levels of the TagRFP-UtrCH probe, which strongly binds to actin, interfered with the formation of nuclear MPTVCV-containing F-actin filaments (data not shown). Whatever the explanation, our findings further underscore important differences in the involvement of microtubules and microfilaments in TMV and TVCV infection.
The most conspicuous and surprising difference from MPTMV was that MPTVCV targeted, in an NLS-dependent manner, to the nuclei of infected cells at all stages of infection, where it accumulated in novel F-actin-containing filaments that were associated with chromatin (Fig. 1 and 3). Our mutational analyses identified a classic basic monopartite NLS in MPTVCV at amino acid residues 209 to 214, which was necessary and sufficient for targeting MPTVCV to nuclei (Fig. 3 and 4), and also necessary for efficient TVCV local spread and systemic infection in both Arabidopsis Col-0 and N. benthamiana plants (Fig. 5 to 7). This NLS appears to be conserved among the subgroup 3 tobamoviruses comprising TVCV, YoMV/ORMV, and RMV, all of which infect plants in the Brassicaceae family, such as Arabidopsis and turnips. In addition, our comparison of the MP sequences for all reported subgroup 3 virus isolates showed that any amino acid substitutions within this sequence are Arg/Lys and Lys/Arg swaps that would preserve its character as a basic monopartite NLS (Fig. 4; see also Fig. S1 in the supplemental material).
Tobamoviruses appear to be ancient virus lineages. Based on phylogenetic analyses, it has been suggested that subgroup 1 and 2 tobamoviruses may have codiverged with their hosts (solanaceous plants for subgroup 1 and cucurbits and legumes for subgroup 2), but the subgroup 3 viruses likely arose from a subgroup 1 virus jumping into Brassica species (14). In contrast to the apparently conserved NLS, which we identified among the subgroup 3 tobamoviruses, our in silico analyses and use of three different algorithms (cNLS Mapper, WoLF PSort, and NucPred) did not predict any NLSs in the MPs encoded by the subgroup 1 or 2 viruses, including the well-characterized U1 strain of TMV (Fig. 4 and data not shown), save for SHMV (amino acid residues 211 to 214, 230 to 240, and 242 to 248) and one isolate of TMGMV (also known as TMV U2 strain, P18338, amino acid residues 214/215 to 224) (40, 41) (Fig. 4; see also Fig. S2 in the supplemental material). Intriguingly, the SHMV basic residues at positions 211 to 214, which may be part of a putative bipartite NLS, and the predicted TMGMV NLS are also conserved in terms of their position within the viral MP (Fig. 4). SHMV appears to be a recombinant between subgroup 1 and 3 viruses (14), which could explain the presence of the predicted NLS. The situation is more complex for TMGMV in that the MP encoded by three other isolates, referred to as strains U2 (EMBL/GenBank/DDBJ accession no. A4LAL8), Japanese (42), and HP (accession no. A3QVG8), is nearly identical in sequence to that reported by Nejidat et al. (98.8% similar and 96.1% identical) (40), except for what appears to be a deletion of the predicted NLS (see Fig. S2). Thus, although this sequence does not appear to be essential for TMGMV infection and viability, it does raise the question of whether a virus similar to the TMGMV strain that encodes the MP with a putative NLS (40, 41) was the progenitor of the subgroup 3 tobamoviruses.
Our extensive characterization of the NLS mutant MPTVCV(K210A/K212A) identified a single clear defect in this mutated MP: MPTVCV(K210A/K212A) was defective in nuclear targeting (Fig. 3). Compared to wild-type MPTVCV, MPTVCV(K210A/K212A) was not altered in its localization to PD, motile vesicles, and ER replication sites (Fig. 3). MPTVCV(K210A/K212A) also was not defective in its two essential functions for virus movement, namely, binding ssRNA and its ability to alter PD, as assessed by its trafficking between cells (Fig. 8A; Table 3), nor did this NLS mutation directly affect virus replication (Fig. 8B). In addition, the effect of MPTVCV(K210A/K212A) on virus infection was independent of encapsidation since both the mutant replicon TVCV::MPK210A/K212A-GFP, which does not encode CP, and the full-length mutant virus TVCVMP(K210A/K212A) encoding CP exhibited a significant delay in virus spread (Fig. 5 to 7; Tables 1 and 2). Thus, having eliminated direct effects on ER and PD targeting, (viral) RNA binding, cell-to-cell transport, replication, and encapsidation, our results strongly argue that MPTVCV provides an important function in the nucleus, one which is necessary to promote virus spread and systemic infection, and appears to have evolved in the subgroup 3 tobamoviruses. Furthermore, this nuclear function of MPTVCV does not require viral RNA or other TVCV components since MPTVCV-GFP targeted to the same novel nuclear filaments in TVCV-infected Arabidopsis Col-0 and N. benthamiana leaf cells or when it was transiently expressed alone (Fig. 1 and 3).
What could be this nuclear function of MPTVCV? Independent of their essential roles in genome trafficking, movement proteins can also redirect cellular activities to favor virus multiplication, including acting as virulence factors to block host defenses and thereby determining pathogenicity on different hosts (43, 44). One movement protein (TGB1) of Potato virus X acts to suppress gene silencing to block the host small interfering RNA (siRNA) defense (3, 45). The tobamovirus Tomato mosaic virus (ToMV) MPToMV can act as an effector to block pathogen-associated molecular pattern (PAMP)-triggered immunity or to induce effector-triggered immunity in plants with the Tm-2 resistance gene (46). The latter may involve MPToMV interacting with the transcription factors KELP and MBF1 (47, 48). The nuclear shuttle movement protein NSP encoded by the geminivirus Cabbage leaf curl virus, which transports viral genomes between the nucleus and cytoplasm, can interact with plasma membrane receptor-like kinases to suppress host defense (49, 50). It also redirects a host nuclear acetyltransferase to regulate genome encapsidation versus nuclear export, which may reprogram cells to support virus replication as well (51, 52). Our results suggest that nuclear MPTVCV behaves like a virulence factor. It was necessary, but not sufficient, for virus infection: TVCV that expressed MPTVCV(K212A/K212A) was infectious. Yet, without directly affecting ER and PD localization, RNA binding, cell-to-cell trafficking, replication, or encapsidation, this NLS mutation reduced TVCV cell-to-cell spread, delayed TVCV systemic spread, and attenuated disease symptoms (Fig. 5 to 8; Tables 1 and 2). Such features typify mutations in viral, bacterial, and fungal proteins that suppress host defenses (53–55).
Pertinent to how nuclear MPTVCV might act as a virulence factor is our demonstration that nuclear MPTVCV localizes to F-actin filaments that appear to associate with discrete regions of chromatin (Fig. 3). Recent studies in yeast, animal cells, and plants point to roles for nuclear actin and actin-related proteins (ARPs) in many aspects of genetic and epigenetic regulation, including transcription, mRNA processing, gene movement, and chromatin remodeling (56, 57). Most of these functions relate to actin-ARP dimers binding to the ATPase subunit of SWI/SNF- and INO80-family chromatin remodeling complexes, where they positively regulate ATPase activity and are essential for remodeler complex assembly to reposition, reconfigure, or eject nucleosomes. As such, they coordinate with histone modifiers (e.g., histone acetyltransferases [HATs]) to recruit DNA-binding proteins to activate or repress transcription or remodel chromosome regions (58–60). The forms of nuclear actin remain enigmatic. In the current view, nuclear actin exists as a dynamic pool of monomeric (G), oligomeric, and polymeric forms. Polymerized nuclear actin has been implicated in selective gene activation during cellular differentiation, the inflammatory response, and retinoic acid-induced transcription (57, 61). Indeed, recent studies using synthetic inducible gene arrays, or of estrogen-induced interactions among genes on different chromosomes, indicate a role for polymerized actin in gene positioning and large-scale chromatin organization (62–64). But, the recent study of Oct4 reactivation in mouse cell nuclei transplanted into GVs provides the first clear visualization of nuclear F-actin during transcriptional activation and evidence for its role in binding BAF remodeling complexes to enhance transcription (32). We propose that the nuclear function of MPTVCV is to induce or stabilize F-actin filaments to spatially reorganize chromatin and thereby affect the expression of antiviral defense-related host genes.
Our results establish a nuclear function for MPTVCV that is necessary for efficient TVCV cell-to-cell movement and systemic infection and which involves MPTVCV localizing to F-actin filaments that associate with chromatin. Several observations suggest that nuclear localization does not simply serve to downregulate MPTVCV cytoplasmic levels late in infection, either by diverting MPTVCV to the nucleus or by targeting it for degradation: nuclear MPTVCV was present at the earliest stages of and throughout infection, infection with the NLS mutant TVCV::MPK210A/K212A did not affect the distribution and subcellular associations of cytoplasmic MPTVCV (Fig. 3), and MPTVCV was degraded in the absence of nuclear targeting (Fig. 5; the relative sizes of the dark centers in TVCV::MPK210A/K212A versus wt TVCV infection rings were unchanged). Based on the roles of nuclear actin and ARPs in structurally organizing chromatin and regulating gene transcription (62–64), we propose that MPTVCV-containing F-actin nuclear filaments associate with actin and/or ARPs in chromatin remodeling complexes to spatially organize chromatin and thereby coordinately reprogram the transcription of host genes to favor virus infection. Whether the transcription of host defense-related genes, as we propose, or that of host genes that generally affect cellular metabolism and function is altered is now an important question to be answered.
Supplementary Material
ACKNOWLEDGMENTS
We thank Asako Uchiyama, Greg Ray, and Parth Jarvia in our lab for rousing discussions and helpful suggestions. We also thank Asako Uchiyama for providing pSITE2NB::MPTVCV-GFP and pSITE4NB::MPTVCV-RFP; Greg Ray for help with protoplast isolation; Michael Goodin (University of Kentucky, KY, USA) for pSITE vectors and for the histone 2B-RFP-, fibrillarin-RFP-, and talin-GFP-expressing N. benthamiana lines; Karl Oparka (University of Edinburgh, Edinburgh, United Kingdom) for the TMV::MP-GFP infectious clone and N. benthamiana plants expressing TUA-GFP; Shoko Ueki and Vitaly Citovsky (SUNY Stony Brook) for pBluescript KS(+)-TVCV-MP and pTVCV50; and Jeffrey Roberts (Cornell University) for use of his Typhoon system.
This research was supported by Public Health Service grant AI-066054 from the National Institute of Allergy and Infectious Diseases to S.G.L.
Footnotes
Published ahead of print 27 March 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.03390-12.
REFERENCES
- 1. Schoelz JE, Harries PA, Nelson RS. 2011. Intracellular transport of plant viruses: finding the door out of the cell. Mol. Plant 4:813–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lazarowitz SG, Beachy RN. 1999. Viral movement proteins as probes for intracellular and intercellular trafficking in plants. Plant Cell 11:535–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Verchot-Lubicz J, Torrance L, Solovyev AG, Morozov SY, Jackson AO, Gilmer D. 2010. Varied movement strategies employed by triple gene block-encoding viruses. Mol. Plant Microbe Interact. 23:1231–1247 [DOI] [PubMed] [Google Scholar]
- 4. Lazarowitz SG. 2001. The plant viruses, p 533–598 In Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE. (ed), Fields virology, 4th ed Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 5. Waigmann E, Lucas WJ, Citovsky V, Zambryski P. 1994. Direct functional assay for tobacco mosaic virus cell-to-cell movement protein and identification of a domain involved in increasing plasmodesmal permeability. Proc. Natl. Acad. Sci. U. S. A. 91:1433–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Heinlein M, Padgett HS, Gens JS, Pickard BG, Casper SJ, Epel BL, Beachy RN. 1998. Changing patterns of localization of the tobacco mosaic virus movement protein and replicase to the endoplasmic reticulum and microtubules during infection. Plant Cell 10:1107–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mas P, Beachy RN. 1999. Replication of tobacco mosaic virus on endoplasmic reticulum and role of the cytoskeleton and virus movement protein in intracellular distribution of viral RNA. J. Cell Biol. 147:945–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Asurmendi S, Berg RH, Koo JC, Beachy RN. 2004. Coat protein regulates formation of replication complexes during tobacco mosaic virus infection. Proc. Natl. Acad. Sci. U. S. A. 101:1415–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Curin M, Ojangu EL, Trutnyeva K, Ilau B, Truve E, Waigmann E. 2007. MPB2C, a microtubule-associated plant factor, is required for microtubular accumulation of tobacco mosaic virus movement protein in plants. Plant Physiol. 143:801–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kragler F, Curin M, Trutnyeva K, Gansch A, Waigmann E. 2003. MPB2C, a microtubule-associated plant protein binds to and interferes with cell-to-cell transport of tobacco mosaic virus movement protein. Plant Physiol. 132:1870–1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Reichel C, Beachy RN. 2000. Degradation of tobacco mosaic virus movement protein by the 26S proteasome. J. Virol. 74:3330–3337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wright KM, Wood NT, Roberts AG, Chapman S, Boevink P, Mackenzie KM, Oparka KJ. 2007. Targeting of TMV movement protein to plasmodesmata requires the actin/ER network: evidence from FRAP. Traffic 8:21–31 [DOI] [PubMed] [Google Scholar]
- 13. Aguilar I, Sanchez F, Martin AM, Martinez-Herrera D, Ponz F. 1996. Nucleotide sequence of Chinese rape mosaic virus (oilseed rape mosaic virus), a crucifer tobamovirus infectious on Arabidopsis thaliana. Plant Mol. Biol. 30:191–197 [DOI] [PubMed] [Google Scholar]
- 14. Lartey RT, Voss TC, Melcher U. 1996. Tobamovirus evolution: gene overlaps, recombination, and taxonomic implications. Mol. Biol. Evol. 13:1327–1338 [DOI] [PubMed] [Google Scholar]
- 15. Chakrabarty R, Banerjee R, Chung SM, Farman M, Citovsky V, Hogenhout SA, Tzfira T, Goodin M. 2007. pSITE vectors for stable integration or transient expression of autofluorescent protein fusions in plants: probing Nicotiana benthamiana-virus interactions. Mol. Plant Microbe Interact. 20:740–750 [DOI] [PubMed] [Google Scholar]
- 16. Zhang Y, Lartey RT, Hartson SD, Voss TC, Melcher U. 1999. Limitations to tobacco mosaic virus infection of turnip. Arch. Virol. 144:957–971 [DOI] [PubMed] [Google Scholar]
- 17. Weiner MP, Costa GL, Schoettlin W, Cline J, Mathur E, Bauer JC. 1994. Site-directed mutagenesis of double-stranded DNA by the polymerase chain reaction. Gene 151:119–123 [DOI] [PubMed] [Google Scholar]
- 18. Burkel BM, von Dassow G, Bement WM. 2007. Versatile fluorescent probes for actin filaments based on the actin-binding domain of utrophin. Cell Motil. Cytoskeleton 64:822–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Martin K, Kopperud K, Chakrabarty R, Banerjee R, Brooks R, Goodin MM. 2009. Transient expression in Nicotiana benthamiana fluorescent marker lines provides enhanced definition of protein localization, movement and interactions in planta. Plant J. 59:150–162 [DOI] [PubMed] [Google Scholar]
- 20. Levy A, Erlanger M, Rosenthal M, Epel BL. 2007. A plasmodesmata-associated beta-1,3-glucanase in Arabidopsis. Plant J. 49:669–682 [DOI] [PubMed] [Google Scholar]
- 21. Lewis JD, Lazarowitz SG. 2010. Arabidopsis synaptotagmin SYTA regulates endocytosis and virus movement protein cell-to-cell transport. Proc. Natl. Acad. Sci. U. S. A. 107:2491–2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Haseloff J, Siemering KR, Prasher DC, Hodge S. 1997. Removal of a cryptic intron and subcellular localization of green fluorescent protein are required to mark transgenic Arabidopsis plants brightly. Proc. Natl. Acad. Sci. U. S. A. 94:2122–2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gillespie T, Boevink P, Haupt S, Roberts AG, Toth R, Valentine T, Chapman S, Oparka KJ. 2002. Functional analysis of a DNA-shuffled movement protein reveals that microtubules are dispensable for the cell-to-cell movement of tobacco mosaic virus. Plant Cell 14:1207–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lartey R, Ghoshroy S, Ho J, Citovsky V. 1997. Movement and subcellular localization of a tobamovirus in Arabidopsis. Plant J. 12:537–545 [DOI] [PubMed] [Google Scholar]
- 25. Pascal E, Sanderfoot AA, Ward BM, Medville R, Turgeon R, Lazarowitz SG. 1994. The geminivirus BR1 movement protein binds single-stranded DNA and localizes to the cell nucleus. Plant Cell 6:995–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Szecsi J, Ding XS, Lim CO, Bendahmane M, Cho MJ, Nelson RS, Beachy RN. 1999. Development of tobacco mosaic virus infection sites in Nicotiana benthamiana. Mol. Plant Microbe Interact. 12:143–152 [Google Scholar]
- 27. Oparka KJ, Prior DA, Santa Cruz S, Padgett HS, Beachy RN. 1997. Gating of epidermal plasmodesmata is restricted to the leading edge of expanding infection sites of tobacco mosaic virus (TMV). Plant J. 12:781–789 [DOI] [PubMed] [Google Scholar]
- 28. Beachy RN, Heinlein M. 2000. Role of P30 in replication and spread of TMV. Traffic 1:540–544 [DOI] [PubMed] [Google Scholar]
- 29. Harries PA, Schoelz JE, Nelson RS. 2010. Intracellular transport of viruses and their components: utilizing the cytoskeleton and membrane highways. Mol. Plant Microbe Interact. 23:1381–1393 [DOI] [PubMed] [Google Scholar]
- 30. Frederick RD, Thilmony RL, Sessa G, Martin GB. 1998. Recognition specificity for the bacterial avirulence protein AvrPto is determined by Thr-204 in the activation loop of the tomato Pto kinase. Mol. Cell 2:241–245 [DOI] [PubMed] [Google Scholar]
- 31. Kotlizky G, Katz A, vander Laak J, Boyko V, Lapidot M, Beachy RN, Heinlein M, Epel BL. 2001. A dysfunctional movement protein of tobacco mosaic virus interferes with targeting of wild-type movement protein to microtubules. Mol. Plant Microbe Interact. 14:895–904 [DOI] [PubMed] [Google Scholar]
- 32. Miyamoto K, Pasque V, Jullien J, Gurdon JB. 2011. Nuclear actin polymerization is required for transcriptional reprogramming of Oct4 by oocytes. Genes Dev. 25:946–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Citovsky V, Wong ML, Shaw AL, Prasad BV, Zambryski P. 1992. Visualization and characterization of tobacco mosaic virus movement protein binding to single-stranded nucleic acids. Plant Cell 4:397–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Deom CM, Oliver MJ, Beachy RN. 1987. The 30-kilodalton gene product of tobacco mosaic virus potentiates virus movement. Science 237:389–394 [DOI] [PubMed] [Google Scholar]
- 35. Harries P, Ding BA. 2011. Cellular factors in plant virus movement: at the leading edge of macromolecular trafficking in plants. Virology 411:237–243 [DOI] [PubMed] [Google Scholar]
- 36. McLean BG, Zupan J, Zambryski PC. 1995. Tobacco mosaic virus movement protein associates with the cytoskeleton in tobacco cells. Plant Cell 7:2101–2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Harries PA, Park JW, Sasaki N, Ballard KD, Maule AJ, Nelson RS. 2009. Differing requirements for actin and myosin by plant viruses for sustained intercellular movement. Proc. Natl. Acad. Sci. U. S. A. 106:17594–17599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kawakami S, Watanabe Y, Beachy RN. 2004. Tobacco mosaic virus infection spreads cell to cell as intact replication complexes. Proc. Natl. Acad. Sci. U. S. A. 101:6291–6296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu JZ, Blancaflor EB, Nelson RS. 2005. The tobacco mosaic virus 126-kilodalton protein, a constituent of the virus replication complex, alone or within the complex aligns with and traffics along microfilaments. Plant Physiol. 138:1853–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nejidat A, Cellier F, Holt CA, Gafny R, Eggenberger AL, Beachy RN. 1991. Transfer of the movement protein gene between two tobamoviruses: influence on local lesion development. Virology 180:318–326 [DOI] [PubMed] [Google Scholar]
- 41. Solis I, Garcia-Arenal F. 1990. The complete nucleotide sequence of the genomic RNA of the tobamovirus tobacco mild green mosaic virus. Virology 177:553–558 [DOI] [PubMed] [Google Scholar]
- 42. Morishima N, Ido T, Hamada H, Yoshimoto E, Mizumoto H, Takeuchi S, Kiba A, Hikichi Y, Okuno T. 2003. Infectious in vitro transcripts from a cDNA clone of Tobacco mild green mosaic tobamovirus and its biological activity in host and nonhost plants and in their protoplasts. J. Gen. Plant Pathol. 69:335–338 [Google Scholar]
- 43. Fenczik CA, Padgett HS, Holt CA, Casper SJ, Beachy RN. 1995. Mutational analysis of the movement protein of odontoglossum ringspot virus to identify a host-range determinant. Mol. Plant Microbe Interact. 8:666–673 [DOI] [PubMed] [Google Scholar]
- 44. Ingham DJ, Pascal E, Lazarowitz SG. 1995. Both bipartite geminivirus movement proteins define viral host range, but only BL1 determines viral pathogenicity. Virology 207:191–204 [DOI] [PubMed] [Google Scholar]
- 45. Renovell A, Carmen Vives M, Ruiz-Ruiz S, Navarro L, Moreno P, Guerri J. 2012. The citrus leaf blotch virus movement protein acts as silencing suppressor. Virus Genes 44:131–140 [DOI] [PubMed] [Google Scholar]
- 46. Weber H, Ohnesorge S, Silber MV, Pfitzner AJ. 2004. The tomato mosaic virus 30 kDa movement protein interacts differentially with the resistance genes Tm-2 and Tm-2(2). Arch. Virol. 149:1499–1514 [DOI] [PubMed] [Google Scholar]
- 47. Matsushita Y, Deguchi M, Youda M, Nishiguchi M, Nyunoya H. 2001. The tomato mosaic tobamovirus movement protein interacts with a putative transcriptional coactivator KELP. Mol. Cells 12:57–66 [PubMed] [Google Scholar]
- 48. Matsushita Y, Miyakawa O, Deguchi M, Nishiguchi M, Nyunoya H. 2002. Cloning of a tobacco cDNA coding for a putative transcriptional coactivator MBF1 that interacts with the tomato mosaic virus movement protein. J. Exp. Bot. 53:1531–1532 [PubMed] [Google Scholar]
- 49. Fontes EP, Santos AA, Luz DF, Waclawovsky AJ, Chory J. 2004. The geminivirus nuclear shuttle protein is a virulence factor that suppresses transmembrane receptor kinase activity. Genes Dev. 18:2545–2556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Santos AA, Lopes KV, Apfata JA, Fontes EP. 2010. NSP-interacting kinase, NIK: a transducer of plant defence signalling. J. Exp. Bot. 61:3839–3845 [DOI] [PubMed] [Google Scholar]
- 51. Carvalho MF, Lazarowitz SG. 2004. Interaction of the movement protein NSP and the Arabidopsis acetyltransferase AtNSI is necessary for cabbage leaf curl geminivirus infection and pathogenicity. J. Virol. 78:11161–11171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Carvalho MF, Turgeon R, Lazarowitz SG. 2006. The geminivirus nuclear shuttle protein NSP inhibits the activity of AtNSI, a vascular-expressed Arabidopsis acetyltransferase regulated with the sink-to-source transition. Plant Physiol. 140:1317–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Aliyari R, Ding SW. 2009. RNA-based viral immunity initiated by the Dicer family of host immune receptors. Immunol. Rev. 227:176–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bierne H, Cossart P. 2012. When bacteria target the nucleus: the emerging family of nucleomodulins. Cell. Microbiol. 14:622–633 [DOI] [PubMed] [Google Scholar]
- 55. Cui J, Shao F. 2011. Biochemistry and cell signaling taught by bacterial effectors. Trends Biochem. Sci. 36:532–540 [DOI] [PubMed] [Google Scholar]
- 56. Meagher RB, Kandasamy MK, Smith AP, McKinney EC. 2010. Nuclear actin-related proteins at the core of epigenetic control. Plant Signal. Behav. 5:518–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Visa N, Percipalle P. 2010. Nuclear functions of actin. Cold Spring Harbor Perspect. Biol. 2:a000620 doi:10.1101/cshperspect.a000620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bao Y, Shen X. 2011. SnapShot: chromatin remodeling: INO80 and SWR1. Cell 144:158–158.e2 doi:10.1016/j.cell.2010.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cairns BR. 2009. The logic of chromatin architecture and remodelling at promoters. Nature 461:193–198 [DOI] [PubMed] [Google Scholar]
- 60. Kasten MM, Clapier CR, Cairns BR. 2011. SnapShot: chromatin remodeling: SWI/SNF. Cell 144:310.e311 doi:10.1016/j.cell.2011.01.007 [DOI] [PubMed] [Google Scholar]
- 61. Miyamoto K, Gurdon JB. 2011. Nuclear actin and transcriptional activation. Commun. Integr. Biol. 4:582–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chuang CH, Carpenter AE, Fuchsova B, Johnson T, de Lanerolle P, Belmont AS. 2006. Long-range directional movement of an interphase chromosome site. Curr. Biol. 16:825–831 [DOI] [PubMed] [Google Scholar]
- 63. Dundr M, Ospina JK, Sung MH, John S, Upender M, Ried T, Hager GL, Matera AG. 2007. Actin-dependent intranuclear repositioning of an active gene locus in vivo. J. Cell Biol. 179:1095–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hu Q, Kwon YS, Nunez E, Cardamone MD, Hutt KR, Ohgi KA, Garcia-Bassets I, Rose DW, Glass CK, Rosenfeld MG, Fu XD. 2008. Enhancing nuclear receptor-induced transcription requires nuclear motor and LSD1-dependent gene networking in interchromatin granules. Proc. Natl. Acad. Sci. U. S. A. 105:19199–19204 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.