Abstract
Highly pathogenic avian influenza viruses (HPAIV) of subtypes H5 and H7 have caused numerous outbreaks in diverse poultry species and rising numbers of human infections. Both HPAIV subtypes support a growing concern of a pandemic outbreak, specifically via the avian-human link. Natural reassortment of both HPAIV subtypes is a possible event with unpredictable outcome for virulence and host specificity of the progeny virus for avian and mammalian species. NS reassortment of H5N1 HPAIV viruses in the background of A/FPV/Rostock/1934 (H7N1) HPAIV has been shown to change virus replication kinetics and host cell responses in mammalian cells. However, not much is known about the virus-host interaction of such viruses in avian species. In the present study, we show that the NS segment of A/Vietnam/1203/2004 (FPV NS VN, H5N1) HPAIV significantly altered the characteristics of the H7 prototype HPAIV in tracheal organ cultures (TOC) of chicken and turkey in vitro, with decreased replication efficiency accompanied by increased induction of type I interferon (IFN) and apoptosis. Furthermore, species-specific differences between chicken and turkey were demonstrated. Interestingly, NS-reassortant FPV NS VN showed an overall highly pathogenic phenotype, with increased virulence and replication potential compared to the wild-type virus after systemic infection of chicken and turkey embryos. Our data demonstrate that single reassortment of an H5-type NS into an H7-type HPAIV significantly changed virus replication abilities and influenced the avian host cell response without prior adaptation.
INTRODUCTION
Severe outbreaks of highly pathogenic avian influenza viruses (HPAIV) in poultry have been frequently reported (1). Since the emergence of zoonotic H5N1 HPAIV in Asia in 1997, more than 400 million birds have died or were culled not only in Asia but also in Europe and Africa (2, 3). H5N1 viruses have caused high mortality among various bird species, but H7 subtype HPAIV also are known to cause large numbers of fatalities in poultry populations (4–6). Infection of poultry species with H7 and H5 subtype HPAIV usually causes severe generalized systemic disease, with mortality rates up to 100% (7). Until today, H7 subtype HPAIV have, with one exception, mainly caused mild infections in humans (8–10). H5N1 viruses caused 610 officially confirmed human infections with 59% lethality (by December 2012) (11, 12). A recent meta-analysis of H5N1 seroprevalence studies suggests a much higher number of human infections worldwide (13). Since both H7 and H5 subtype HPAIV are cocirculating among wild birds and poultry, reassortment between the two subtypes is a possible threat (14). Intersubtype reassortment of influenza A viruses (IV) has been frequently observed and was responsible for the evolution of new H5N1 HPAIV genotypes with the potential to infect humans (15, 16).
Previous studies showed that the nonstructural protein 1 (NS1), encoded by the influenza virus NS segment, can change the host response and virulence of the virus in the case of reassortment without prior adaptation (17, 18). As such, it was shown that NS-reassortant viruses in the genetic background of A/FPV/Rostock/1934 (designated FPV wild type [wt]; H7N1) HPAIV carrying NS segments of A/goose/Guangdong/1/1996 (FPV NS GD; H5N1) HPAIV, A/mallard/NL/12/2000 (FPV NS Ma; H7N3) low-pathogenicity avian influenza virus (LPAIV), and of a more recent human isolate, A/Vietnam/1203/2004 (FPV NS VN, H5N1) HPAIV, have different growth kinetics from FPV wt in mammalian cells (19). In particular, FPV NS GD showed higher replication rates than FPV wt, while replication of FPV NS VN and FPV NS Ma was decreased. In contrast, in permanent avian LMH and QT6 cell lines, FPV NS GD and FPV NS Ma showed the same replication kinetics as FPV wt, and growth of only FPV NS VN was decreased (19). Also in an in vivo study in mice, the same reassortant FPV NS GD caused more efficient replication and virulence than the wild-type virus (20).
The major function of the NS1 protein is the inhibition of type I interferon (IFN) production and of antiviral effects of IFN-induced proteins (for a review, see reference 21). The IFN response is a strong antiviral mechanism limiting viral replication. Furthermore, the NS1 protein can increase virus replication by activating the cellular phosphatidylinositol 3-kinase (PI3K) and by downregulation of apoptosis (22, 23). The nuclear export protein (NS2/NEP), which is translated from spliced NS segment mRNA, mediates viral ribonucleoprotein (RNP) export from the nucleus of IV-infected cells via binding to the viral M1 protein (24). Furthermore, the NS2/NEP protein has the ability to modify virus RNA levels by regulation of IV transcription and replication (25).
The role of type I IFN in IV infection of avian species is not clear. A previous study showed that IV-infected primary chicken embryo cells produced a mixture of type I IFNs of usually more than 80% IFN-α and up to 20% IFN-β (26). Recent studies have indicated that in the chicken embryo we already find significant expression of type I IFN in the spleen starting at embryonation day 14 (27).
IV growth may be strongly inhibited in IFN-α-pretreated chicken cell culture, but a recent study revealed that IFN-α pretreatment did not protect chickens in vivo against wild-type (A/Cygnus cygnus/Germany/R65/2006 H5N1 [R65]) or mutant (R65 with a deletion of the NS1 open reading frame [R65-delNS1]) HPAIV infection (28).
Extensive studies in mice showed that the Mx protein plays a key role in the IFN-induced antiviral state in mammalian species (29). Mx function against IV infection in chickens has been controversially discussed (30). However, a recent study demonstrated that the chicken Mx protein lacks GTPase activity and seems to be uninvolved in the antiviral effect of type I IFN in chickens (31).
NS1 proteins with an internal deletion or a truncation of the C-terminal end have been shown to lose the ability to suppress the host's IFN response, which can result in reduced virus replication (32, 33). The NS1 of FPV NS VN HPAIV, carrying a C-terminal 10-amino-acid (aa) truncation, has been shown to induce a high IFN-β response in A549 permanent human alveolar epithelial cells compared to the wild-type FPV HPAIV (19). Furthermore, FPV NS VN showed increased apoptosis in MDCK (Madin-Darby canine kidney) cells (19).
The goal of this study was to compare the growth characteristics of different NS-reassortant HPAIV with NS segments of different subtypes and allele groups in avian hosts for their potential to replicate in tracheal organ cultures (TOC) of chicken (TOC-Ch) and turkey (TOC-Tu) as the primary epithelial target cells and for their ability to suppress the host's innate immune response. Furthermore, we also used an embryo model of chicken and turkey to analyze virus growth kinetics under in vivo conditions. The FPV NS reassortants carrying NS segments of H5N1 HPAIV A/Vietnam/1203/2004 and A/goose/Guangdong/1/1996 as well as H7N3 LPAIV A/mallard/NL/12/2000 were compared with the wild-type A/FPV/Rostock/1934 H7N1 HPAIV.
Our study shows that the NS segment of A/Vietnam/1203/2004 (H5N1) in the background of wild-type A/FPV/Rostock/1934 (H7N1) did not sufficiently suppress the local innate immune response in TOC of chicken and turkey, resulting in increased release of type I IFN and apoptosis rates coinciding with lower virus titers. Interestingly, in vivo infection of chicken and turkey embryos demonstrated, on the other hand, an increased virulence for FPV NS VN compared to the wild-type virus, as demonstrated by faster replication and more severe lesion development. We may speculate that under in vivo conditions in avian species, the HPAIV replication rate is possibly less dependent on the NS1-controlled innate immune response than in mammalian hosts.
MATERIALS AND METHODS
Viruses and titration.
The following IV were used: wild-type A/FPV/Rostock/1934 (FPV wt; H7N1) HPAIV, NS-reassortant viruses with the genetic background of FPV wt and NS segments from A/Vietnam/1203/2004 (FPV NS VN; H5N1) HPAIV, A/goose/Guangdong/1/1996 (FPV NS GD; H5N1) HPAIV, and A/mallard/NL/12/2000 (FPV NS Ma; H7N3) LPAIV. Reassorted viruses had been generated previously using reverse genetics (19). MDCK (Madin-Darby canine kidney) cells were used to amplify the progeny viruses. Rescued viruses had been plaque purified three times, propagated, and titrated on MDCK cells (19). Virus stocks were stored at −70°C, and virus titration was done using a focus-forming assay (reported as focus-forming units [FFU]/ml) (20). The generation of recombinant HPAIV and all experiments were carried out under biosafety level 3 conditions.
Based on phylogenetic analysis, NS1 proteins of A/FPV/Rostock/1934 and A/Vietnam/1203/2004 belong to allele A and those of A/goose/Guangdong/1/1996 and A/mallard/NL/12/2000 belong to allele B (19). The NS1 protein of A/Vietnam/1203/2004 contains a 5-amino-acid (aa) internal deletion as well as a 10-aa C-terminal truncation.
Vesicular stomatitis virus (VSV; kindly provided by Gert Zimmer, Institute for Virology, University of Veterinary Medicine Hannover, Germany) was propagated in chicken embryo fibroblasts (CEF), stored at −70°C, and titrated on CEF to determine the 50% tissue culture infectious dose (TCID50) by the method of Reed and Muench (34).
Embryonated eggs and tissue and cell culture.
Embryonated eggs from chicken (VALO-SPF; Lohmann Tierzucht, Cuxhaven, Germany) and turkey (Moorgut Kartzfehn, Boesel, Germany) were used for organ and cell culture preparation as well as embryo infection studies. They were incubated at 37.8°C and 55% humidity. All parental flocks tested negative for avian IV (AIV) antibodies of subtypes H5, H7, H6, and H9 in a standard hemagglutination inhibition (HAI) test (35). All experiments were performed in accordance with German animal welfare regulations.
Primary embryo fibroblast cultures from chicken (CEF) were prepared from embryonated eggs as previously described (36). CEF were maintained in 1:1 McCoy's 5A modified medium plus L-15 Leibovitz medium (Biochrom, Berlin, Germany) with 10% fetal bovine serum (FBS; Biochrom), 1% l-glutamine (200 mM; Biochrom), and 1% penicillin-streptomycin ([P-S] 10,000 U/ml, 10,000 μg/ml; Biochrom) at 37.5°C and 5% CO2.
TOC from chicken (TOC-Ch) and turkey (TOC-Tu) were prepared from embryonated eggs at incubation days 20 and 26, respectively (37). Briefly, embryos were sacrificed, and the tracheae were removed under sterile conditions. Each trachea was cut manually into approximately 0.8-mm-thick rings using a microtome blade. Individual rings were transferred to 5-ml tubes (Sarstedt, Nuembrecht, Germany) with 0.8 ml of prewarmed Medium 199 with Hanks' salts (Biochrom) including 1% P-S. TOC were cultured at 37.5°C in a rotating shaker (Reax 2; Heidolph, Schwabach, Germany) at lowest rotation speed.
After 24 h, the ciliary activity of the respiratory epithelium of each TOC was assessed using an inverted microscope. Only rings with 100% ciliary activity were used for the experiments.
MDCK cells were maintained in Dulbecco's modified Eagle's medium (DMEM; Biochrom) supplemented with 10% FBS and 1% P-S at 37°C and 5% CO2.
qRT-PCR.
Quantification of the IV matrix (M) gene (38) as well as alpha interferon (IFN-α) mRNA levels was done by quantitative real-time RT-PCR (qRT-PCR) as previously described. Briefly, embryonic tissue samples were homogenized in peqGOLD TriFast (Peqlab, Erlangen, Germany) reagent with ceramic beads (CK14; Peqlab) in a tissue homogenizer (Precellys 24; Peqlab). Total cellular RNA extraction was done according to the manufacturer's instructions. RNA was eluted in 30 μl of RNase-free water and stored at −70°C. RNA purity and concentration were determined (NanoDrop ND-1000; Peqlab), and samples were subsequently diluted 10−3 in RNase-free water for optimal RNA concentration.
IFN-α-specific primers and probes were designed based on partial genomic DNA sequences of chicken and turkey (39, 40) and are described in . M gene- and 28S rRNA gene-specific primers and probes have been described previously (38, 41). Reverse transcription-PCR (RT-PCR) was performed using an Ambion AgPath-ID One-Step RT-PCR kit (Life Technologies, Carlsbad, CA) according to the manufacturer's instructions as follows: the final concentrations of primers were 200 nM for the chicken and turkey IFN-α (Ch/TkIFN-α) and M genes and 400 nM for the 28S gene; the final concentrations of probes were 50 nM for the Ch/TkIFN-α genes, 100 nM for the M gene, and 400 nM for the 28S gene. Five microliters of diluted total RNA was used per 25-μl reaction mixture, and RT-PCR was performed using a Stratagene MX 3005P qRT-PCR detection system (Stratagene, La Jolla, CA) with the following cycle profile: 1 cycle at 45°C for 10 min and 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 57°C for 45 s. Each sample was tested in duplicates. For quantification, cycle threshold (CT) values of the Ch/TkIFN-α and M genes were normalized against the CT values of the constantly expressed 28S rRNA housekeeping gene of the same sample (ΔCT) as described by Powell et al. (42). The data are presented as mean ΔCT values subtracted from 35 or as mRNA fold change in relation to ΔCT values from noninfected groups.
Table 1.
Quantitative real-time RT-PCR primers and probes
| RNA target | Primer or probea | Sequenceb | Accession no.c |
|---|---|---|---|
| Chicken IFN-α | ChIFNα F | 5′-GAC AGC CAA CGC CAA AGC-3′ | U07868 |
| ChIFNα R | 5′-GTC GCT GCT GTC CAA GCA TT-3′ | ||
| ChIFNα P | 5′-(FAM)-CTC AAC CGG ATC CAC CGC TAC ACC-(TAMRA)-3′ | ||
| Turkey IFN-α | TkIFNα F | 5′-GAC AGC CAA CGC CAA AGC-3′ | U28140 |
| TkIFNα R | 5′-GTG GCT GTC CGC CAA GCA TT-3′ | ||
| TkIFNα P | 5′-(FAM)-CTC AAC CAG ATC CAG CGG TAC GCC-(TAMRA)-3′ |
F, forward primer; R, reverse primer; P, probe.
FAM, 6-carboxyfluorescein; TAMRA, carboxytetramethylrhodamine.
Refers to genomic DNA sequence.
IFN bioassay.
Bioactive total interferon (type I and type II) was detected in TOC supernatants and turkey embryo serum using a cytopathic effect (CPE) inhibition assay as previously described (43). To inactivate viruses, supernatants or sera were dialyzed in glycine buffer (100 mM, pH 2.0) by using dialysis tubes (Visking, size 2 with a cutoff of 12 to 14 kDa [Medicell, London, United Kingdom]) for 5 h and, after the buffer was changed, for an additional 16 h at 4°C. Subsequently, virus-inactivated samples were dialyzed in phosphate-buffered saline (PBS; pH 7.4) for 5 h and, after the buffer was changed, dialyzed overnight at 4°C. For IFN bioassays, CEF were seeded overnight on 96-well plates. CEF were incubated with serially diluted samples for 24 h at 37.5°C and 5% CO2. Recombinant chicken IFN-α (kindly provided by Bernd Kaspers, LMU Munich, Germany) was used as a positive control. The test culture fluids were replaced with medium containing VSV (4 × 104 TCID50/well) and incubated for 48 h. Virus-induced CPE was determined microscopically. The IFN activity of the test samples is expressed in units/ml (U/ml). One unit is defined as the highest dilution of the sample that caused 100% protection against VSV-induced CPE.
Apoptosis.
Detection of apoptotic cells in paraffin sections of TOC was performed with a terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assay using an in situ cell death detection kit (with peroxidase [POD]; Roche Applied Sciences, Mannheim, Germany) according to the manufacturer's instructions. Reactions were developed using a DAB (diaminobenzidine) Peroxidase Substrate Kit (Vector Laboratories, Burlingame, CA) according to the manufacturer's instructions. TOC sections were counterstained with Hematoxylin QS (Vector Laboratories) for 5 min and mounted with Aquatex (Merck, Darmstadt, Germany). The number of apoptotic cells was counted microscopically in the respiratory epithelium of each individual TOC and is presented as the mean of total apoptotic cells per complete TOC ring (n = 5 rings per time point and experiment).
Histopathology and immunohistochemistry for viral antigen detection.
Chicken and turkey embryonic tissues were processed following standard procedures, and 2-μm paraffin sections were made from each sample in duplicates. For histopathology, tissue sections were stained with hematoxylin and eosin (HE). The severity of histopathologic lesions of each organ was evaluated as previously described (44) using a modified lesion score of 0 to 3: 0, no lesions; 1, mild lesions with focal inflammation (edema and bleeding); 2, moderate lesions with focal to multifocal inflammation and scarce lymphocytic infiltration; 3, severe lesions with disseminated inflammation, tissue degeneration, and massive lymphocytic infiltration.
Immunohistochemistry was performed to detect IV nucleoprotein (NP) in tissues. Tissue sections were blocked against endogenous peroxidase with 3% hydrogen peroxide in methanol, and antigen retrieval was achieved by microwave treatment in citrate buffer (10 mM, pH 6.0). Normal horse serum and an Avidin/Biotin Blocking Kit (Vector Laboratories) were used to block nonspecific staining. Sections were incubated with anti-NP antibody (mouse anti-influenza A virus nucleoprotein; Southern Biotech, Birmingham, AL) diluted 1:250 in PBS. After a washing step in PBS, sections were processed using a Vectastain Elite ABC kit (mouse IgG; Vector Laboratories). Tissue sections were subsequently treated with reagents of the DAB Peroxidase Substrate kit following the manufacturer's instructions, counterstained with Hematoxylin QS, and mounted with Aquatex.
TOC infection study.
TOC of chicken and turkey were infected 5 days after preparation to avoid negative effects due to early inflammatory responses of the tissue (45).
For infection, TOC were washed with phosphate-buffered saline (PBS) and incubated with 103 FFU of HPAIV FPV wt or reassortant FPV NS VN, FPV NS GD, and FPV NS Ma per individual culture (n = 5 per time point and virus) in PBS plus 0.2% bovine serum albumin (PBS-BSA) (PAA Laboratories, Pasching, Austria). Virus-negative controls were incubated with PBS-BSA. After incubation for 1 h at 37.5°C, the supernatant was removed and TOC were washed with PBS and subsequently cultured in 1 ml of Medium 199 with Hanks' salts supplemented with 1% P-S and 0.2% BSA. For quantification of ciliostasis, TOC were analyzed under an inverted microscope to determine the percentage of remaining ciliary activity, as described previously (46).
Infectious virus titers of TOC supernatants were determined using a focus-forming assay (focus-forming units [FFU]/ml) as previously described (20). Furthermore, TOC supernatants were titrated for bioactive IFN using a VSV CPE inhibition assay. TOC were fixed in 4% paraformaldehyde (PFA), further processed for the preparation of paraffin sections, and analyzed for histopathological lesions as well as for apoptotic cells using an in situ TUNEL assay.
Embryo infection study.
Eggs from chicken and turkey were inoculated with FPV wt or FPV NS VN HPAIV at embryonation days 14 and 18, respectively. FPV NS VN was selected for the embryo infection study since this NS reassortant showed the most significant differences in growth characteristics in TOC compared to the FPV wt in either avian species. Embryonated eggs were prepared for intravenous (i.v.) infection as previously described (47), with modifications. Briefly, eggs were candled to mark the area around the largest vein embedded in the chorioallantoic membrane (preceding the vena umbilicalis) near the air cell. A triangle of eggshell was excised using a diamond-coated cutting wheel mounted on a rotary tool (diameter, 22.2 mm; Dremel Europe, Breda, Netherlands). The generated eggshell windows were sealed with adhesive tape until infection.
Embryos were i.v. inoculated with 103 FFU of FPV wt or FPV NS VN HPAIV in 100 μl of PBS-BSA using a sterile hypodermic needle (27-gauge by 3/4 in.) (Braun, Melsungen, Germany). Eggs were sealed with adhesive tape and incubated at 37.8°C and 90% humidity in an upright position. Analysis of embryo mortality and sampling was done at 8, 16, and 24 h postinfection (hpi) for chicken embryos and at 8, 12, and 24 hpi for turkey embryos. Blood samples from the chorioallantoic vein were collected from turkey embryos at 8 and 12 hpi for detection of circulating IV with the focus-forming assay as well as for detection of bioactive IFN in the VSV CPE inhibition assay. Afterwards, embryos were immediately sacrificed by decapitation, and amnio-allantoic fluids (AAF) were collected and stored at −70°C. Heart, liver, intestine, pectoral muscle, and brains were collected individually with sterile forceps and scissors and stored in peqGOLD TriFast reagent at −20°C for quantification of virus load using qRT-PCR. Based on the different degrees of lesion development, quantification of IFN-α mRNA expression levels by qRT-PCR was done in the most and least affected organs (liver and intestine, respectively). Furthermore, embryonic tissues from chicken and turkey were stored in 4% PFA at 4°C and processed for histopathologic evaluation for turkey and detection of influenza A virus nucleoprotein (NP) antigen for chicken and turkey by immunohistochemical staining.
Statistical analysis.
Statistically significant differences between virus titers were evaluated with a randomized complete block analysis of variance (ANOVA) following a Tukey honestly significant differences (HSD) test for multiple group comparisons using Statistix, version 9.0 (Analytical Software, Tallahassee, FL). Other data were analyzed with a Kruskal-Wallis one-way ANOVA with all-pairwise comparisons or with a Wilcoxon rank sum test (Statistix, version 9.0). Differences were considered significant at a P value of <0.05.
RESULTS
Growth characteristics of HPAIV FPV wt and its NS reassortants in TOC of chicken and turkey.
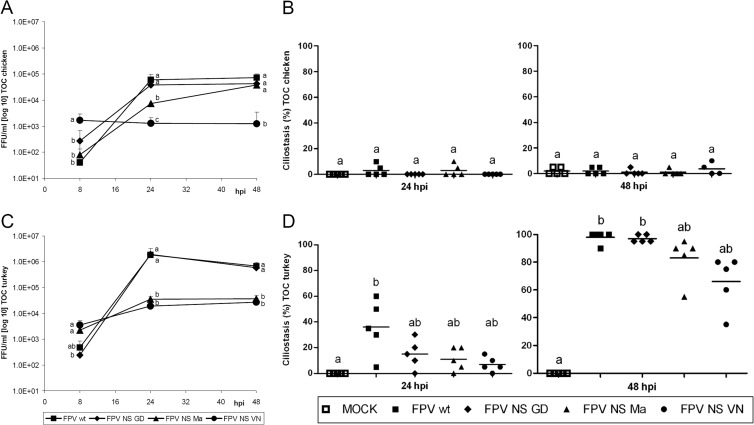
Growth characteristics of FPV wild-type (wt) and NS-reassortant viruses FPV NS GD, FPV NS Ma, and FPV NS VN were compared between tracheal organ cultures (TOC) of chicken (TOC-Ch) and turkey (TOC-Tu). FPV NS VN showed significantly higher virus titers after a single cycle of replication at 8 h postinfection (hpi) than FPV wt and other reassortant viruses in TOC of both avian species (P < 0.05) (Fig. 1A and C). Thereafter, FPV NS VN titers increased only slightly in TOC-Tu and were overall significantly lower than those of FPV wt and FPV NS GD in both species at 24 and 48 hpi (P < 0.05). FPV NS Ma and FPV NS VN replicated to comparable titers in TOC-Tu, whereas in TOC-Ch, FPV NS Ma showed increased replication to significantly higher titers than FPV NS VN at 24 and 48 hpi (P < 0.05). FPV wt and FPV NS GD showed comparable growth kinetics in TOC of both species. Overall, all viruses replicated faster and to significantly higher titers in TOC-Tu than in TOC-Ch (P < 0.05).
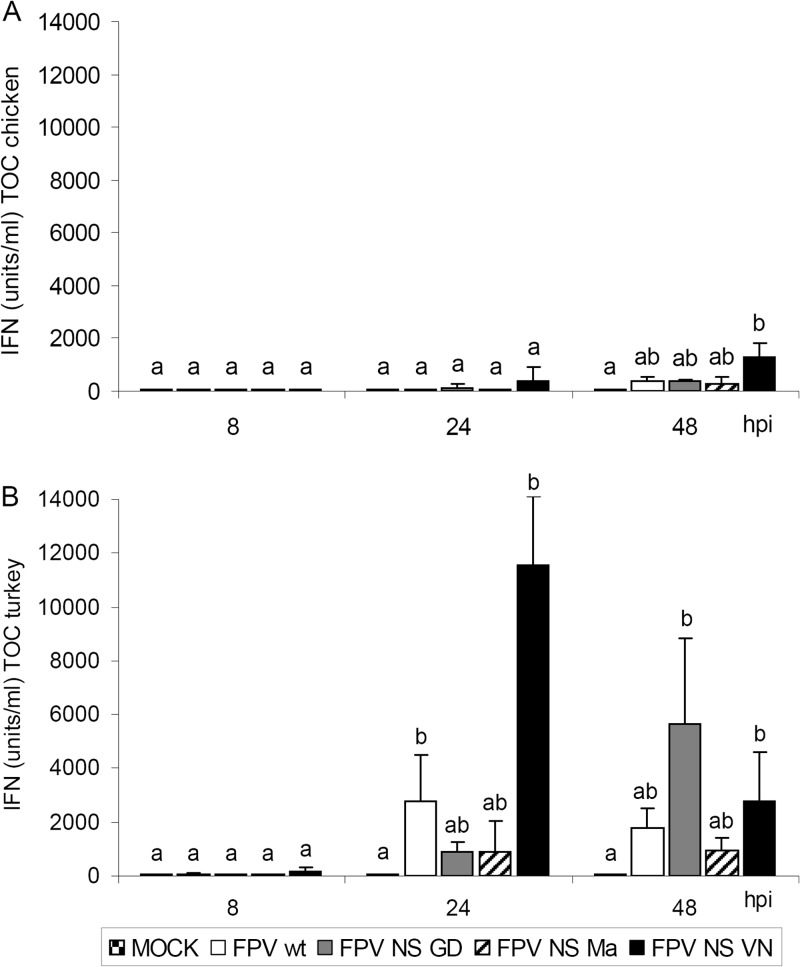
Fig 1.
Replication (A and C) and induction of ciliostasis (B and D) by wild-type and reassortant FPV viruses in TOC of chicken and turkey. Individual TOC (n = 5/virus/time point) were inoculated with 103 FFU of virus. Infectious titers were determined by titration of TOC supernatants on MDCK cells using focus-forming assays (FFU/ml). Ciliostasis was assessed in percent loss of ciliary activity for each TOC by using an inverted microscope (n = 5). Error bars indicate the standard deviations. At 8 h p.i., no ciliostasis was detected in TOC of either avian species. Different letters indicate statistically significant differences between groups at 8, 24, or 48 hpi (P < 0.05; by ANOVA with Tukey HSD [A and C] or Kruskal-Wallis one-way ANOVA [B and D]).
Ciliostasis of the respiratory epithelium was assessed microscopically (Fig. 1B and D). Significant induction of ciliostasis (P < 0.05) was detected only in virus-infected TOC-Tu, with minor differences between FPV wt and NS-reassortant strains (Fig. 1B and D). Although infectious virus titers of FPV NS VN and FPV NS Ma were more than 1 log lower at 24 and 48 hpi than those of other viruses, induction of ciliostasis was only slightly delayed compared that by FPV wt and FPV NS GD. The induction of ciliostasis coincided with the development of histological lesions. Infected TOC-Tu showed more severe cell degeneration and loss of respiratory epithelium than TOC-Ch (data not shown). FPV wt-infected TOC-Tu showed nearly complete loss of the respiratory epithelium at 24 hpi. Other viruses induced comparable lesions at 48 hpi (data not shown).
NS reassortment affected IFN induction and apoptosis of HPAIV FPV in TOC.
FPV NS VN induced overall higher bioactive IFN titers in TOC of either avian species than FPV wt and other NS-reassortant viruses (Fig. 2). IFN was induced earlier and to significantly higher titers in TOC-Tu than TOC-Ch, independent of the infecting viruses (P < 0.05). In TOC-Tu, FPV NS GD showed highest IFN titers at 48 hpi, whereas FPV NS VN showed maximum induction of IFN already at 24 hpi (Fig. 2B).
Fig 2.
IFN induction after infection of TOC from chicken (A) or turkey (B) with wild-type and NS-reassortant FPV HPAIV. Individual TOC were inoculated with 103 FFU of virus (n = 5/virus/time point). TOC supernatants were harvested at different time points, and total bioactive IFN was determined in a VSV CPE inhibition assay (IFN units/ml). Error bars indicate the standard deviations. Different letters indicate statistically significant differences between groups at each time point (P < 0.05; Kruskal-Wallis one-way ANOVA).
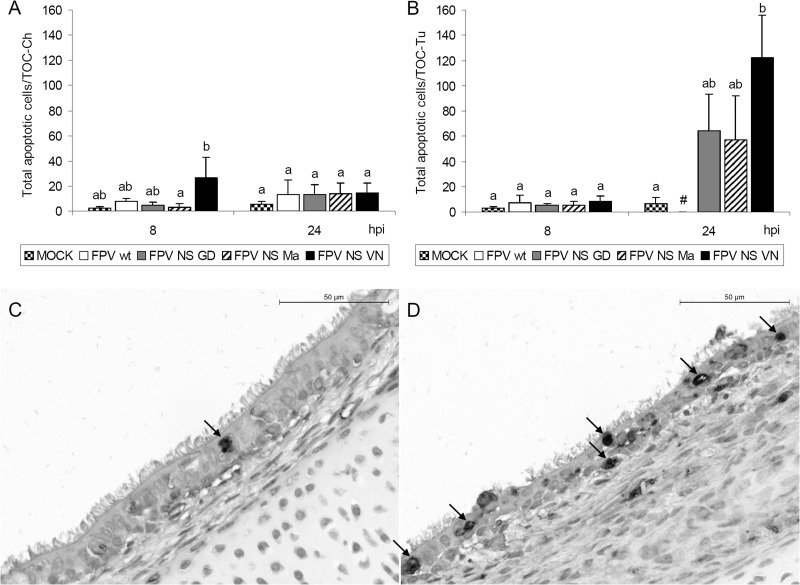
Induction of apoptosis in TOC differed between the tested viruses (Fig. 3). Infection with FPV NS VN led to significantly higher numbers of apoptotic cells in TOC-Tu at 24 hpi than in noninfected controls (P < 0.05) (Fig. 3B). At 48 hpi, apoptotic cell counts of virus-infected TOC-Ch groups were higher than in virus-free controls but did not differ significantly, whereas the number of apoptotic cells in TOC-Tu could not be determined due to consistent loss of respiratory epithelium in virus-infected groups at that time point (data not shown).
Fig 3.
Induction of apoptosis in TOC of chicken (A) and turkey (B to D) after infection with wild-type and NS-reassortant FPV HPAIV. Individual TOC were inoculated with 103 FFU of virus (n = 5/virus/time point). Paraffin sections of TOC were rehydrated and stained for apoptotic cells using an in situ TUNEL method. Values are the total number of apoptotic cells/TOC. Error bars indicate the standard deviations. #, major epithelial cell destruction; no apoptosis evaluation possible. Different letters indicate statistically significant differences between groups at each time point (P < 0.05; Kruskal-Wallis one-way ANOVA). (C) In situ apoptosis TUNEL staining of noninfected TOC of turkey with a random single apoptotic cell at 48 hpi. (D) FPV NS VN-infected TOC of turkey showing multiple apoptotic cells, degeneration, and loss of cilia. Arrows indicate apoptotic cells.
Increased virulence of HPAIV NS-reassortant FPV NS VN in embryos of chicken and turkey.
FPV NS VN was selected for the in vivo infection studies in embryonated eggs of chicken and turkey because it showed the most significant differences in virus replication and failed to suppress the IFN response in TOC compared to FPV wt.
All chicken and turkey embryos infected with either FPV wt or FPV NS VN died within the first 24 hpi. Embryo mortality did not differ between viruses at the time points indicated in the text (n = 5/time point/virus). Pathological examination of chicken and turkey embryos at 8 and 12 to 16 hpi revealed distinct differences in pathogenesis between FPV wt- and FPV NS VN-infected groups. Due to the fast induction of lesions in TOC-Tu, an earlier time point for necropsy was selected for turkey embryos, which were already investigated at 12 hpi instead of the 16 hpi for chicken embryos. Gross lesions were dominated by hemorrhages distributed in various embryonic organs and skin of virus-infected groups. Macroscopically, changes were most prominent in the liver, with more severe lesions in the FPV NS VN-infected groups than in the FPV wt-infected group in either species. FPV NS VN-infected chicken and turkey embryos showed severe swelling of the liver with moderate multifocal petechial bleeding at 16 and 12 hpi, respectively, whereas embryonic livers of FPV wt-infected groups showed only focal petechial bleedings at these time points, and virus-negative embryos were free of any macroscopic lesions (data not shown).
Histopathological lesions of embryonic tissues confirmed the more severe lesion development after infection with FPV NS VN than with FPV wt. Histopathologic lesion scoring of turkey embryos demonstrated that FPV NS VN induced more severe lesions in embryonic liver and heart (Fig. 4). Lesions were restricted to the vascular system, with hemorrhages, edema, and perivascular lymphocytic infiltration (Fig. 4C to E). Furthermore, perivascular cell degeneration of cardiomyocytes and hepatocytes was observed in virus-infected groups.
Fig 4.
Histopathologic examination of intravenously infected turkey embryos. Embryos were inoculated with 103 FFU of FPV wt or recombinant FPV NS VN HPAIV (n = 5/virus/time point). (A and B) Histopathologic lesions of liver and heart were evaluated using following lesion scores (not done for chicken embryos): 0, no lesions; 1, mild lesions with focal inflammation (edema and bleeding); 2, moderate lesions with focal to multifocal inflammation and scarce lymphocytic infiltration; 3, severe lesions with disseminated inflammation, tissue degeneration, and massive lymphocytic infiltration. Error bars indicate the standard deviations. Asterisks indicate statistically significant differences between FPV wt and FPV NS VN groups (P < 0.05; Wilcoxon rank sum test). No lesions were observed in virus-free embryos. (C to E) Histology of turkey embryonic liver at 12 hpi showing representative pictures of noninfected (C), FPV-wt infected (D), and FPV NS VN-infected (E) groups. The arrow indicates lymphocyte aggregation.
Histopathologic examination of the pectoral muscle showed slightly increased petechial bleeding, with a lesion score of 1.3 ± 0.5 (mean ± standard deviation [SD]) at 12 hpi in the FPV NS VN-infected group compared to a lesion score of 0.4 ± 0.6 (n = 5) with FPV wt infection. No differences between virus-infected groups were observed in the brain and intestine of turkey embryos, with only random focal signs of inflammation.
At 24 hpi, infected embryos of chicken and turkey showed macroscopically comparable disseminated hemorrhages in all tissues independent of the virus. Histopathology revealed severe tissue destruction, lymphocytic infiltrations, and disseminated hemorrhages in liver, heart, pectoral muscle, and brain as well as in the intestine's lamina propria, with lesion scores of 2 to 3 in both virus-infected groups. No macroscopic or histopathologic lesions were observed in virus-free control embryos at any time point.
FPV NS VN showed a significantly higher viral load by qRT-PCR than FPV wt in all investigated tissues in turkey embryos at 8 and 12 hpi (P < 0.05) (Fig. 5B). FPV NS VN viral loads in chicken embryos were only significantly higher than those of FPV wt at 24 hpi in three of four investigated tissues (P < 0.05) (Fig. 5A). Highest virus loads were detected in livers of virus-infected turkey embryos, independent of the infecting virus. Virus-infected chicken embryos showed the largest amount of detectable IV mRNA in the heart.
Fig 5.
Virus quantification in intravenously infected embryos of chicken (A and C) and turkey (B and D). Embryos were inoculated with 103 FFU of FPV wt or recombinant VN HPAIV (n = 5/time point). (A and B) Virus quantification in embryonic organs was done with an influenza virus M gene qRT-PCR normalized to 28S rRNA gene expression; CT values were subtracted from 35. (C and D) Amnio-allantoic fluids (AAF) were titrated by a focus-forming assay. Error bars indicate the standard deviations. Asterisks indicate statistically significant differences between FPV wt and FPV NS VN ΔCT values in each organ at the same time point postinfection (P < 0.05; Wilcoxon rank sum test). Different letters indicate statistically significant differences between virus titers (P < 0.05; ANOVA with Tukey HSD).
Immunohistochemical detection of IV antigen-positive cells confirmed qRT-PCR results and demonstrated high viral loads in the heart and liver of both avian species (data not shown). The FPV NS VN-infected group showed a higher rate of positive hepatocytes and cardiomyocytes than the FPV wt-infected group at 16 and 12 hpi in chicken and turkey embryos, respectively. At these early time points, strongest antigen staining was observed in heart and liver tissues adjacent to blood vessels as well as in vascular endothelial cells (data not shown). Furthermore, erythrocytes and endothelial cells as well as occasionally inflammatory mononuclear cells showed strong positive viral antigen staining in all investigated organs. Other cells in the intestine, skeletal muscle, and brain were not positive for viral antigen staining. Overall, the location and amount of influenza virus NP-positive cells in the different organs correlated with histopathologic results.
No infectious virus was detected in the amnio-allantoic fluids (AAF) of embryos at 8 hpi (Fig. 5C and D). FPV NS VN replicated faster and showed higher titers in AAF of chicken and turkey embryos than FPV wt at the other investigated time points. FPV NS VN was also detected in blood samples of infected turkey embryos (not done in chicken), with mean virus titers of 3.6 × 103 FFU/ml (n = 5) as early as 12 hpi, whereas no infectious virus was detected in blood of the FPV wt-infected group at that time point. At 24 hpi, collection of blood was impossible due to the death of all infected embryos.
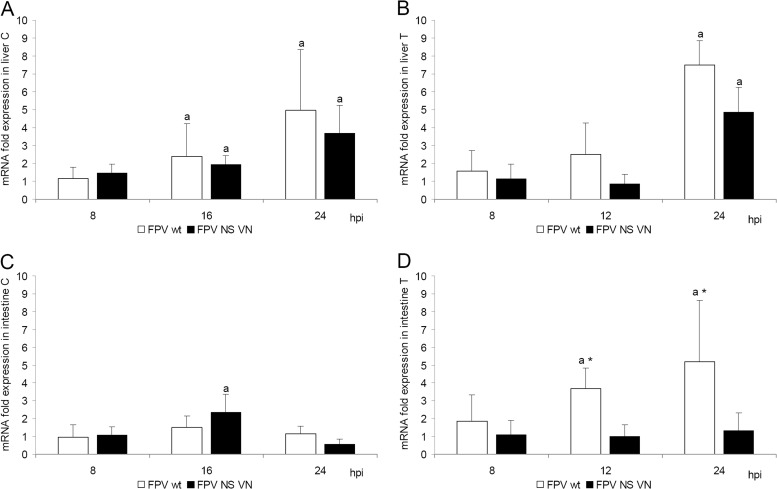
No circulating IFN was detected in the serum samples of turkey embryos at 8 and 12 hpi in the VSV CPE inhibition assay. On the other hand, IFN-α mRNA levels were upregulated in embryonic livers but without differences between the wild-type and NS-reassortant HPAIV after infection of either species (Fig. 6A and B). FPV wt but not FPV NS VN induced upregulation of IFN-α in the intestine of turkey embryos but not in infected chicken embryos (Fig. 6C and D).
Fig 6.
IFN-α mRNA expression levels after intravenous infection of chicken (A and C) and turkey (B and D) embryos with FPV wt and FPV NS VN HPAIV. Embryos were inoculated i.v. with 103 FFU of virus (n = 5/virus/time point). Relative IFN-α quantification was done by qRT-PCR, and values were normalized to 28S rRNA expression. Presented is the fold change in IFN-α mRNA expression compared to that of virus-free controls in embryonic liver (A and B) and intestine (C and D). Error bars indicate the standard deviation. Asterisks indicate statistical significant differences between CT values of FPV wt and FPV NS VN groups at each time point; lowercase letters indicate statistically significant differences with respect to values of the noninfected control groups (P < 0.05; Wilcoxon rank sum test).
DISCUSSION
Single NS reassortment of A/FPV/Rostock/1934 (H7N1) HPAIV with NS segments of two different H5-type HPAIV and one H7-type LPAIV changed the growth kinetics and host cell responses of the reassorted viruses in TOC as well as in embryos of chicken and turkey without the need of previous adaptation.
All examined NS-reassortant viruses showed different growth kinetics in TOC, which varied to some extent between chicken and turkey. Increasing virus titers seemed to correlate positively with induction of ciliostasis in TOC-Tu. Nevertheless, since there was no ciliostasis observed in virus-infected TOC-Ch, a general correlation between successful virus replication and ciliostasis, representing virus-induced death of the ciliated epithelium, cannot be confirmed. Infection of TOC of either species with FPV NS VN resulted in the lowest virus replication accompanied by the highest induction of type I IFN and apoptosis, as previously shown in mammalian cell lines (19). IFN triggers the antiviral response and is suggested to contribute to the control of IV infection in vitro (21). Sequence comparison of the different NS segments indicated that the NS1 protein of A/Vietnam/1203/2004 contains a 5-amino-acid (aa) internal deletion as well as a 10-aa C-terminal truncation (19). Due to the truncation, it lacks a poly(A)-binding protein II (PABII) binding region and the PDZ-binding motif (PBM). By targeting PABII and CPSF30, NS1 protein can inhibit 3′-end processing of cellular pre-mRNAs (48). Since this also includes IFN pre-mRNA, the loss of PABII binding may have contributed to the failure of IFN suppression by FPV NS VN, resulting in decreased virus titers.
The class I PBM of avian IV (ESEV) has been shown to reduce apoptosis during infection by directly binding to the proapoptotic PDZ protein Scribble (49). Therefore, FPV NS VN may be a potent inducer of apoptosis due to the truncation of the PBM at C terminus of NS1.
FPV NS Ma showed the same growth characteristics as FPV NS VN in TOC of turkey but did not induce high titers of IFN or apoptosis rates. Other mechanisms may account for the relatively poor growth of FPV NS Ma in TOC-Tu. Unlike growth in TOC-Tu, FPV NS Ma replicated more effectively than FPV NS VN in TOC-Ch. Previous results in permanent LMH chicken cells support this observation, where FPV NS Ma replicated as effectively as FPV wt and FPV NS GD (19). Interestingly, reassortment of FPV wt with the NS segment of A/goose/Guangdong/1/1996 (H5N1) did not change virus replication rates in TOC of either species although there was a slight delay in lesion development in comparison to the FPV wt. These results in primary avian TOC differ from previous results in permanent human cell lines and primary mouse epithelia cells, where FPV NS GD showed enhanced growth compared to FPV wt, leading to the speculation that genetic reassortment of AIV may have different consequences for different host species (19, 20).
Reassorted allele B NS of H7N1 GD virus and allele A NS of FPV wt revealed comparable viral replication rates, whereas allele B NS of H7N3 Ma as well as allele A NS of H5N1 VN led to reduced replication. It may be speculated that the interaction between NS1 and other viral as well as host proteins may influence the outcome of HPAIV infection, which seems to be unrelated to the subtype or allelic type.
Although TOC are a good model to analyze local HPAIV infection of the upper respiratory tract, they still cannot replace in vivo studies (50). Investigations of the activity and influence on infiltrating innate immune cells but also cells of the specific immune system need an in vivo model.
Interestingly, in vivo infection of chicken and turkey embryos with wild-type FPV and reassortant FPV NS VN HPAIV showed controversial results with respect to our in vitro TOC infection data. FPV NS VN infection of embryos led to more effective virus replication than wild-type FPV infection, in combination with severe induction of lesions in both chicken and turkey, which was not expected based on the in vitro observations in avian TOC. Although FPV NS VN virus growth was reduced in vitro, it replicated more effectively than the wild-type FPV in systemically infected embryos.
An infection study in 15-day-old chickens confirmed the increased virulence of FPV NS VN compared to that of the wild-type FPV in vivo, which was evident by earlier and increased mortality and lesion development in combination with increased expression of the proinflammatory cytokine interleukin-1β (IL-1β) (51).
Furthermore, in contrast to the in vitro observations, expression of IFN-α mRNA upon in vivo infection of chicken and turkey embryos with FPV wt or FPV NS VN did not correlate with virus quantities. In contrast to the results in TOC, the IFN response of HPAIV-infected embryos had no significant effect on virus replication. Although the immune system of a turkey or chicken embryo is expected not to be fully mature at the time of AIV infection, it is known from studies in chicken embryos that the IFN type I response is already functional at embryonation day 14 and develops over time (27, 52). The more mature the innate and also acquired immune responses are, the stronger is the antiviral response expected. Nevertheless, our observations in chicken and turkey embryos coincide with a recent in vivo study (28). Pretreatment of 5-week-old chickens with recombinant chicken IFN-α did not protect against challenge with A/Cygnus cygnus/Germany/R65/2006 H5N1 HPAIV (R65) (28). It was further demonstrated that an R65 mutant with a deletion of NS1 and an NS-reassortant R65 with a C-terminal truncated NS1 of SC35 (H7N7) HPAIV did not induce high IFN levels in chickens, as would have been predicted based on the detection of high IFN levels after in vitro infection studies or observations made in mice.
Our data support the previous hypothesis that the different results between HPAIV infections in vivo and in vitro in the avian system may be due to a potential override of the innate immune response by rapid and efficient virus replication in vivo, possibly due to differences in cell tropism (28).
During viremia, influenza viruses attach to red blood cells and spread to various organs (53). Virus antigen detected in the vessels of all investigated organs support this observation. Large amounts of viral antigen in infected embryo tissues were also associated with colocalized lesions, such as cell degeneration, hemorrhages, and edema, but also mononuclear cells, possibly macrophages, showed strong antigen staining in various tissues. The disseminated hemorrhages in various embryonic organs after infection with FPV wt and FPV NS VN indicate virus tropism for vascular endothelial cells. This strong tropism for the vascular endothelium has been demonstrated with A/FPV/Rostock/1934 and several other H5 and H7 HPAIV viruses before (7, 54, 55).
In summary, single NS reassortment of an H7-type HPAIV with NS segments of different subtypes changed virus replication characteristics and the host cell response in the avian system without prior adaptation. Depending on the NS segment, the NS-reassortant HPAIV varied in their growth characteristics and abilities to counteract the host's innate immune response in the in vitro TOC system. Infection studies in turkey and chicken embryos demonstrated that, despite the inability of the NS-reassortant FPV NS VN HPAIV to suppress the host's antiviral IFN response in vitro, it replicated more efficiently than the wild-type FPV HPAIV under in vivo conditions. Our data seem to correlate with other HPAIV in vivo studies in birds, leading to speculation that the induction of high IFN levels may not allow a prediction about reduced HPAIV virulence in birds, which is possibly different for mammalian species.
ACKNOWLEDGMENTS
We thank M. Stein, M. Riedl, M. Thielking, C. Haase, and C. Winter for their excellent technical assistance and support. In addition, we thank J. Vergara-Alert, A. Darji, and G. Herrler for helpful discussions.
This work was supported by the FluResearchNet, Molecular Signatures Determining Pathogenicity and Species Transmission of Influenza A Viruses, funded by the Federal Ministry of Education and Research (01KI07136 to S.P. and 01KI07133 to S.R.).
Footnotes
Published ahead of print 6 March 2013
REFERENCES
- 1. Swayne DE, Halvorson DA. 2008. Influenza, p 153–184 In Saif YM, Fadly AM, Glisson JR, McDougald LR, Nolan LK, Swane DE. (ed), Diseases of poultry, 12th ed Wiley-Blackwell, Hoboken, NJ [Google Scholar]
- 2. Alexander DJ. 2007. An overview of the epidemiology of avian influenza. Vaccine 25:5637–5644 [DOI] [PubMed] [Google Scholar]
- 3. Food and Agriculture Organization of the United Nations 2012. H5N1 HPAI global overview: January-March 2012. Food and Agriculture Organization of the United Nations, Rome Italy: http://www.fao.org/docrep/015/an388e/an388e.pdf [Google Scholar]
- 4. Iglesias I, Martinez M, Munoz MJ, de la Torre A, Sanchez-Vizcaino JM. 2010. First case of highly pathogenic avian influenza in poultry in Spain. Transbound. Emerg. Dis. 57:282–285 [DOI] [PubMed] [Google Scholar]
- 5. Capua I, Marangon S, dalla Pozza M, Terregino C, Cattoli G. 2003. Avian influenza in Italy 1997–2001. Avian Dis. 47:839–843 [DOI] [PubMed] [Google Scholar]
- 6. Alexander DJ, Brown IH. 2009. History of highly pathogenic avian influenza. Rev. Sci. Tech. 28:19–38 [DOI] [PubMed] [Google Scholar]
- 7. Swayne DE. 2007. Understanding the complex pathobiology of high pathogenicity avian influenza viruses in birds. Avian Dis. 51:242–249 [DOI] [PubMed] [Google Scholar]
- 8. Koopmans M, Wilbrink B, Conyn M, Natrop G, van der Nat H, Vennema H, Meijer A, van Steenbergen J, Fouchier R, Osterhaus A, Bosman A. 2004. Transmission of H7N7 avian influenza A virus to human beings during a large outbreak in commercial poultry farms in the Netherlands. Lancet 363:587–593 [DOI] [PubMed] [Google Scholar]
- 9. Fouchier RA, Schneeberger PM, Rozendaal FW, Broekman JM, Kemink SA, Munster V, Kuiken T, Rimmelzwaan GF, Schutten M, Van Doornum GJ, Koch G, Bosman A, Koopmans M, Osterhaus AD. 2004. Avian influenza A virus (H7N7) associated with human conjunctivitis and a fatal case of acute respiratory distress syndrome. Proc. Natl. Acad. Sci. U. S. A. 101:1356–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alexander DJ. 2006. Avian influenza viruses and human health. Dev. Biol. (Basel) 124:77–84 [PubMed] [Google Scholar]
- 11. Gambotto A, Barratt-Boyes SM, de Jong MD, Neumann G, Kawaoka Y. 2008. Human infection with highly pathogenic H5N1 influenza virus. Lancet 371:1464–1475 [DOI] [PubMed] [Google Scholar]
- 12. WHO 2012. Cumulative number of confirmed human cases for avian influenza A(H5N1) reported to WHO, 2003–2012. World Health Organization, Geneva, Switzerland: www.who.int/influenza/human_animal_interface/EN_GIP_20120116CumulativeNumberH5N1cases.pdf [Google Scholar]
- 13. Wang TT, Parides MK, Palese P. 2012. Seroevidence for H5N1 influenza infections in humans: meta-analysis. Science 335:1463 doi:10.1126/science.1218888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Perez DR, Webby RJ, Hoffmann E, Webster RG. 2003. Land-based birds as potential disseminators of avian mammalian reassortant influenza A viruses. Avian Dis. 47:1114–1117 [DOI] [PubMed] [Google Scholar]
- 15. Li KS, Guan Y, Wang J, Smith GJ, Xu KM, Duan L, Rahardjo AP, Puthavathana P, Buranathai C, Nguyen TD, Estoepangestie AT, Chaisingh A, Auewarakul P, Long HT, Hanh NT, Webby RJ, Poon LL, Chen H, Shortridge KF, Yuen KY, Webster RG, Peiris JS. 2004. Genesis of a highly pathogenic and potentially pandemic H5N1 influenza virus in eastern Asia. Nature 430:209–213 [DOI] [PubMed] [Google Scholar]
- 16. Lei F, Shi W. 2011. Prospective of genomics in revealing transmission, reassortment and evolution of wildlife-borne avian influenza A (H5N1) viruses. Curr. Genomics 12:466–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li Z, Jiang Y, Jiao P, Wang A, Zhao F, Tian G, Wang X, Yu K, Bu Z, Chen H. 2006. The NS1 gene contributes to the virulence of H5N1 avian influenza viruses. J. Virol. 80:11115–11123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sarmento L, Wasilenko J, Pantin-Jackwood M. 2010. The effects of NS gene exchange on the pathogenicity of H5N1 HPAI viruses in ducks. Avian Dis. 54:532–537 [DOI] [PubMed] [Google Scholar]
- 19. Wang Z, Robb NC, Lenz E, Wolff T, Fodor E, Pleschka S. 2010. NS reassortment of an H7-type highly pathogenic avian influenza virus affects its propagation by altering the regulation of viral RNA production and antiviral host response. J. Virol. 84:11323–11335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ma W, Brenner D, Wang Z, Dauber B, Ehrhardt C, Hogner K, Herold S, Ludwig S, Wolff T, Yu K, Richt JA, Planz O, Pleschka S. 2010. The NS segment of an H5N1 highly pathogenic avian influenza virus (HPAIV) is sufficient to alter replication efficiency, cell tropism, and host range of an H7N1 HPAIV. J. Virol. 84:2122–2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hale BG, Randall RE, Ortin J, Jackson D. 2008. The multifunctional NS1 protein of influenza A viruses. J. Gen. Virol. 89:2359–2376 [DOI] [PubMed] [Google Scholar]
- 22. Ehrhardt C, Wolff T, Pleschka S, Planz O, Beermann W, Bode JG, Schmolke M, Ludwig S. 2007. Influenza A virus NS1 protein activates the PI3K/Akt pathway to mediate antiapoptotic signaling responses. J. Virol. 81:3058–3067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ludwig S, Pleschka S, Planz O, Wolff T. 2006. Ringing the alarm bells: signalling and apoptosis in influenza virus infected cells. Cell Microbiol. 8:375–386 [DOI] [PubMed] [Google Scholar]
- 24. Punyadarsaniya D, Liang CH, Winter C, Petersen H, Rautenschlein S, Hennig-Pauka I, Schwegmann-Wessels C, Wu CY, Wong CH, Herrler G. 2011. Infection of differentiated porcine airway epithelial cells by influenza virus: differential susceptibility to infection by porcine and avian viruses. PLoS One 6:e28429 doi:10.1371/journal.pone.0028429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Robb NC, Smith M, Vreede FT, Fodor E. 2009. NS2/NEP protein regulates transcription and replication of the influenza virus RNA genome. J. Gen. Virol. 90:1398–1407 [DOI] [PubMed] [Google Scholar]
- 26. Schwarz H, Harlin O, Ohnemus A, Kaspers B, Staeheli P. 2004. Synthesis of IFN-beta by virus-infected chicken embryo cells demonstrated with specific antisera and a new bioassay. J. Interferon Cytokine Res. 24:179–184 [DOI] [PubMed] [Google Scholar]
- 27. Karpala AJ, Bagnaud-Baule A, Goossens KE, Lowenthal JW, Bean AG. 2012. Ontogeny of the interferon system in chickens. J. Reprod. Immunol. 94:169–174 [DOI] [PubMed] [Google Scholar]
- 28. Penski N, Hartle S, Rubbenstroth D, Krohmann C, Ruggli N, Schusser B, Pfann M, Reuter A, Gohrbandt S, Hundt J, Veits J, Breithaupt A, Kochs G, Stech J, Summerfield A, Vahlenkamp T, Kaspers B, Staeheli P. 2011. Highly pathogenic avian influenza viruses do not inhibit interferon synthesis in infected chickens but can override the interferon-induced antiviral state. J. Virol. 85:7730–7741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Haller O, Staeheli P, Kochs G. 2007. Interferon-induced Mx proteins in antiviral host defense. Biochimie 89:812–818 [DOI] [PubMed] [Google Scholar]
- 30. Wang Y, Brahmakshatriya V, Lupiani B, Reddy S, Okimoto R, Li X, Chiang H, Zhou H. 2012. Associations of chicken Mx1 polymorphism with antiviral responses in avian influenza virus infected embryos and broilers. Poult. Sci. 91:3019–3024 [DOI] [PubMed] [Google Scholar]
- 31. Schusser B, Reuter A, von der Malsburg A, Penski N, Weigend S, Kaspers B, Staeheli P, Hartle S. 2011. Mx is dispensable for interferon-mediated resistance of chicken cells against influenza A virus. J. Virol. 85:8307–8315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kochs G, Koerner I, Thiel L, Kothlow S, Kaspers B, Ruggli N, Summerfield A, Pavlovic J, Stech J, Staeheli P. 2007. Properties of H7N7 influenza A virus strain SC35M lacking interferon antagonist NS1 in mice and chickens. J. Gen. Virol. 88:1403–1409 [DOI] [PubMed] [Google Scholar]
- 33. Soubies SM, Volmer C, Guerin JL, Volmer R. 2010. Truncation of the NS1 protein converts a low pathogenic avian influenza virus into a strong interferon inducer in duck cells. Avian Dis. 54:527–531 [DOI] [PubMed] [Google Scholar]
- 34. Reed LJ, Muench HJ. 1938. A simple method for estimating fifty percent endpoints. Am. J. Hyg. 27:493–497 [Google Scholar]
- 35. World Organisation for Animal Health (OIE) 2009. Avian Manual of diagnostic tests and vaccines for terrestrial animals. World Organisation for Animal Health, Paris, France: http://web.oie.int/fr/normes/mmanual/2008/pdf/2.03.04_AI.pdf [Google Scholar]
- 36. Sekellick MJ, Marcus PI. 1986. Induction of high titer chicken interferon. Methods Enzymol. 119:115–125 [DOI] [PubMed] [Google Scholar]
- 37. Petersen H, Matrosovich M, Pleschka S, Rautenschlein S. 2012. Replication and adaptive mutations of low pathogenic avian influenza viruses in tracheal organ cultures of different avian species. PLoS One 7:e42260 doi:10.1371/journal.pone.0042260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Spackman E, Senne DA, Myers TJ, Bulaga LL, Garber LP, Perdue ML, Lohman K, Daum LT, Suarez DL. 2002. Development of a real-time reverse transcriptase PCR assay for type A influenza virus and the avian H5 and H7 hemagglutinin subtypes. J. Clin. Microbiol. 40:3256–3260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sekellick MJ, Ferrandino AF, Hopkins DA, Marcus PI. 1994. Chicken interferon gene: cloning, expression, and analysis. J. Interferon Res. 14:71–79 [DOI] [PubMed] [Google Scholar]
- 40. Suresh M, Karaca K, Foster D, Sharma JM. 1995. Molecular and functional characterization of turkey interferon. J. Virol. 69:8159–8163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kaiser P, Rothwell L, Galyov EE, Barrow PA, Burnside J, Wigley P. 2000. Differential cytokine expression in avian cells in response to invasion by Salmonella typhimurium, Salmonella enteritidis and Salmonella gallinarum. Microbiology 146:3217–3226 [DOI] [PubMed] [Google Scholar]
- 42. Powell FL, Rothwell L, Clarkson MJ, Kaiser P. 2009. The turkey, compared to the chicken, fails to mount an effective early immune response to Histomonas meleagridis in the gut. Parasite Immunol. 31:312–327 [DOI] [PubMed] [Google Scholar]
- 43. Karaca K, Sharma JM, Tomai MA, Miller RL. 1996. In vivo and in vitro interferon induction in chickens by S −28828, an imidazoquinolinamine immunoenhancer. J. Interferon Cytokine Res. 16:327–332 [DOI] [PubMed] [Google Scholar]
- 44. Laudert E, Hlvorson D, Sivanandan V, Shaw D. 1993. Comparative evaluation of tissue trophism characteristics in turkeys and mallard ducks after intravenous inoculation of type A influenza viruses. Avian Dis. 37:773–780 [PubMed] [Google Scholar]
- 45. Reemers SS, Groot Koerkamp MJ, Holstege FC, van Eden W, Vervelde L. 2009. Cellular host transcriptional responses to influenza A virus in chicken tracheal organ cultures differ from responses in in vivo infected trachea. Vet. Immunol. Immunopathol. 132:91–100 [DOI] [PubMed] [Google Scholar]
- 46. Blaskovic P, Rhodes AJ, Labzoffsky NA. 1972. Infection of chick embryo tracheal organ cultures with influenza A2 (Hong Kong) virus. I. Cytopathology, histopathology, immunofluorescence, hemadsorption, and titration of the released infectious progeny virus. Arch. Gesamte Virusforsch. 37:104–113 [DOI] [PubMed] [Google Scholar]
- 47. Goldsmit L, Barzilai E. 1968. An improved method for the isolation and identification of bluetongue virus by intravenous inoculation of embryonating chicken eggs. J. Comp. Pathol. 78:477–487 [DOI] [PubMed] [Google Scholar]
- 48. Chen Z, Li Y, Krug RM. 1999. Influenza A virus NS1 protein targets poly(A)-binding protein II of the cellular 3′-end processing machinery. EMBO J. 18:2273–2283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liu H, Golebiewski L, Dow EC, Krug RM, Javier RT, Rice AP. 2010. The ESEV PDZ-binding motif of the avian influenza A virus NS1 protein protects infected cells from apoptosis by directly targeting Scribble. J. Virol. 84:11164–11174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. McGee ZA, Woods ML. 1987. Use of organ-cultures in microbiological research. Annu. Rev. Microbiol. 41:291–300 [DOI] [PubMed] [Google Scholar]
- 51. Vergara-Alert J, Wang Z, Busquets N, Petersen H, Rivas R, Chaves A, Valle R, Dolz R, Majo N, Rautenschlein S, Rodriguez F, Pleschka S, Darji A. 2010. NS1 protein from H5N1 avian influenza viruses increase the virulence of H7N1 A/FPV/Rostock/34 in chickens, abstr 170, p 229 In Proceedings of the 4th European Congress for Virology, Cernobbio, Italy [Google Scholar]
- 52. Sekellick MJ, Biggers WJ, Marcus PI. 1990. Development of the interferon system. I. In chicken cells development in ovo continues on time in vitro. In Vitro Cell Dev. Biol. 26:997–1003 [DOI] [PubMed] [Google Scholar]
- 53. Mori I, Komatsu T, Takeuchi K, Nakakuki K, Sudo M, Kimura Y. 1995. Viremia induced by influenza virus. Microb. Pathog. 19:237–244 [DOI] [PubMed] [Google Scholar]
- 54. Jones YL, Swayne DE. 2004. Comparative pathobiology of low and high pathogenicity H7N3 Chilean avian influenza viruses in chickens. Avian Dis. 48:119–128 [DOI] [PubMed] [Google Scholar]
- 55. Feldmann A, Schafer MK, Garten W, Klenk HD. 2000. Targeted infection of endothelial cells by avian influenza virus A/FPV/Rostock/34 (H7N1) in chicken embryos. J. Virol. 74:8018–8027 [DOI] [PMC free article] [PubMed] [Google Scholar]