Abstract
We report the identification of a functional nuclear localization signal (NLS) in the human cytomegalovirus (HCMV) large tegument protein pUL48 that is required for nuclear localization in transfected cells and is essential for viral growth. The NLS was mapped to pUL48 amino acid residues 284 to 302. This sequence contains a bipartite NLS comprising two clusters of basic residues (bC1 and bC2) separated by 9 amino acids. Deletion or mutation of bC1 or mutation of bC2 abrogated the nuclear localization of full-length pUL48 in transiently expressing cells, thus strongly implying a bipartite character of the NLS. Nuclear localization could be restored by fusion of a functional NLS together with enhanced green fluorescent protein (EGFP) to the N terminus of these mutants. In HCMV-infected cells, pUL48 was found in both nuclear and cytoplasmic fractions, supporting a function of the NLS during virus infection. NLS mutant viruses, generated by markerless bacterial artificial chromosome mutagenesis, were not viable in cell culture, whereas coexpression of pUL48 complemented growth of these mutants. The fusion of a functional NLS to the N terminus of pUL48 in a nonviable NLS mutant virus partially rescued the growth defect. Furthermore, the replacement of the bipartite pUL48 NLS by the monopartite pUL36 NLS of herpes simplex virus 1 supported viral growth to some extent but still revealed a severe defect in focus formation and release of infectious virus particles. Together, these results show that nuclear targeting of pUL48 is mediated by a bipartite NLS whose function is essential for HCMV growth.
INTRODUCTION
The human cytomegalovirus (HCMV) belongs to the betaherpesvirus subfamily and is characterized by a very slow replication cycle. Generation and release of infectious herpesvirus particles from infected cells is a complex, multistep process accomplished by many individual viral and cellular proteins that participate in an intricate network of protein-protein interactions. How this process is orchestrated during HCMV infection is poorly understood, and the precise details need to be elucidated. Viral tegument proteins are structural components of the virion connecting the nucleocapsid with the viral envelope. In the case of HCMV, more than 38 viral tegument proteins are detectable in virus particles (1, 2). Aside from their structural functions, tegument proteins fulfill crucial roles during almost all steps of the herpesviral life cycle (summarized in references 3, 4, and 5). Although the tegument layer was initially thought to be mostly unstructured, it can be divided into an inner and an outer tegument depending on the position of the proteins within the virus particle. The inner tegument layer is comprised of those tegument proteins that most closely associate with the capsid. These proteins are thus thought to be important for the stability of the capsid (6, 7) and for its proper trafficking within the cell (8, 9).
One of these inner tegument proteins of HCMV is the large tegument protein pUL48 (also referred to as high-molecular-weight protein [HMWP]), which is highly conserved among herpesviruses (8, 9). It is the largest tegument protein of HCMV, with a size of 2,241 amino acids and a molecular mass of about 253 kDa (2, 8, 10). The exact function of pUL48 during HCMV replication is still unclear. Deletion of the UL48 gene abrogates viral growth, which argues for an essential role of pUL48 during HCMV replication (11). However, two other mutants generated by random transposon mutagenesis were replication competent but impaired in viral growth (12). Identification of N-terminal ubiquitin-specific protease activity of pUL48, which cleaves both Lys48- and Lys63-linked ubiquitin monomers and dimers, suggests an enzymatic role of the large tegument protein (10, 13). This role could be deubiquitination of viral or cellular proteins marked for degradation or, alternatively, interference with cellular signaling pathways. The importance of this activity for virus replication was demonstrated by a 10-fold reduction in the production of new viral progeny of an active-site mutant virus (13). Notably, the deubiquitinating activity appears to be conserved among the large tegument proteins of herpesviruses (14–16). The close association of the large tegument proteins with the capsid has been studied in detail (9, 17–21). It appears to be of particular importance during virus entry into the host cell, as the pUL48 counterparts pUL36 (VP1-2) of herpes simplex virus 1 (HSV-1) and that of pseudorabies virus (PrV) were shown to interact with the microtubule network to facilitate transport of capsids to the nucleus and capsid targeting to the nuclear pore complex and to be involved in releasing the viral genome into the nucleus (21–29). A role of the large tegument protein during viral entry is further supported by a temperature-sensitive HSV-1 pUL36 mutant, which shows a block at the very early stages of infection when incubated at a nonpermissive temperature (30–32). Furthermore, proteolytic cleavage of pUL36 is reportedly required to trigger the release of viral DNA from the capsid into the nucleus (33). Apart from their function during virus entry, the large tegument proteins have a second function during late stages of infection, e.g., during nuclear and cytoplasmic egress of virus particles. Several studies have made use of UL36 deletion mutant viruses to demonstrate an essential function of pUL36 for the generation of infectious particles in both HSV-1 and PrV. In the absence of pUL36, viral nucleocapsids fail to undergo secondary envelopment, and thus, they accumulate in the cytoplasm (34, 35). This defect appears to involve impaired trafficking of progeny nucleocapsids to the sites of secondary envelopment (20). The notion that pUL36 might also function during nuclear egress was at first not well substantiated, since nucleocapsids efficiently accumulated in the cytoplasm of cells infected with UL36 deletion mutants. However, the observed association of HSV-1 pUL36 with intranuclear capsids (17) and the identification of a C-terminal subspecies of PrV pUL36 that selectively enhances nuclear egress of C capsids (36) support a role of the large tegument proteins during nuclear egress.
In order to fulfill these various functions at different steps of the viral replication cycle, the large tegument proteins are assumed to require the ability to traffic efficiently between the nucleus and the cytoplasm. Nuclear and cytoplasmic localization was shown for PrV pUL36 in transfected cells, but in infected cells, pUL36 was exclusively localized to the cytoplasm (37). However, in the case of HSV-1 pUL36, both nuclear and cytoplasmic localizations in HSV-1-infected cells have been reported (38). The latter observation was confirmed with the identification of a functional nuclear localization signal (NLS) in the N terminus of HSV-1 pUL36. The NLS of HSV-1 pUL36 is a classical monopartite basic amino acid-rich motif, which is highly conserved among herpesvirus large tegument proteins (39). Deletion of the 7-amino-acid core NLS of HSV-1 pUL36 revealed a crucial role during virus entry. Viral growth of the HSV-1 pUL36 NLS mutant was abolished due to a block in the routing of capsids to the nuclear pore, thereby preventing infection (22).
Taking into account that many of the identified functions of the large tegument proteins appear to be conserved among herpesviruses, we set out to clarify (i) the intracellular distribution of pUL48, (ii) the presence of nuclear localization signals, and (iii) their possible function(s) during HCMV infection. By applying combined mutational approaches in transfected cells as well as in the context of viral infection, we could demonstrate the presence of a functional bipartite NLS in the large tegument protein of HCMV which is required for viral growth.
MATERIALS AND METHODS
Cells and viruses.
Human foreskin fibroblasts (HFFs) were maintained in minimal essential medium (MEM; Gibco-BRL) supplemented with 10% fetal calf serum (FCS; Gibco-BRL) and 1% nonessential amino acids (Biochrom AG). HFFs were used until passage 23 for infection studies. Human embryonic lung fibroblasts (MRC-5; European Collection of Cell Cultures) and human epithelial carcinoma cells (HeLa) were maintained in Dulbecco's modified Eagle's medium (DMEM; Gibco-BRL) supplemented with 10% FCS and 1% nonessential amino acids. MRC-5 cells were used between passages 24 and 28 for the reconstitution of virus mutants. HeLa cells were used for transfection of expression plasmids and subsequent Western blot analysis. Permanent African green monkey kidney cells (Cos7) were maintained in MEM supplemented with 5% FCS and used in localization experiments. All media were additionally supplemented with 1% penicillin-streptomycin (100-fold; PAA Laboratories) and 1% l-glutamine (200 mM; PAA Laboratories).
Generation of a polyclonal antibody directed against pUL48.
An amino-terminal region comprising amino acid residues 399 to 1150 of pUL48 of HCMV strain TB40-BAC4 (accession no. EF999921 [40]) was fused N terminally to the maltose binding protein (MBP) and C terminally to the His tag and expressed from prokaryotic expression vector pTST101 (kindly provided by Guenther Muth, Tuebingen, Germany) in Escherichia coli strain BL21 (Novagen). Affinity chromatography against MBP was used to purify the MBP-pUL48 fusion protein from crude E. coli lysates. Subsequent enzymatic digestion with factor Xa cleaved the MBP-pUL48 fusion protein into the pUL48 fragment and the MBP fragment. The pUL48 fragment was confirmed in Western blot analysis using an anti-His tag antibody (Penta-His antibody; Qiagen) after purification by SDS-PAGE, Coomassie blue staining, and excision of the band corresponding to pUL48 from the gel. The purified pUL48 fragment was used for the immunization of rabbits by standard protocols. The rabbits received two booster immunizations at intervals of 2 weeks. After a total of 8 weeks, polyclonal sera were collected and used for detection of full-length pUL48.
Cloning of full-length UL48.
The approximately 6.7-kbp UL48 gene was cloned from the viral genome by using a variation of the RED-GAM recombination procedure, also known as gap repair (41), which allows cloning without the risk of introducing errors by PCR amplification. Gap repair was used to generate expression plasmids pEF-UL48 and pEGFP-UL48. Therefore, bacterial plasmid pENTR1A (Invitrogen) was first amplified using primers (see Table S1 in the supplemental material) containing 40-bp homology to the start (forward primer) and the end (reverse primer) of UL48. The resulting linear PCR product was then used as a template for a RED-GAM recombination by electroporating E. coli containing the HCMV bacterial artificial chromosome (BAC) clone TB40-BAC4. Recombination between the homologous sequences of PCR-amplified pENTR1A and the homologous sequences in the UL48 gene of TB40-BAC4 resulted in a pENTR1A-derived plasmid in which the entire UL48 gene was recombined from the BAC DNA. Kanamycin resistance encoded on pENTR1A allowed the selection for the plasmid. Kanamycin-resistant clones were screened for correct recombination and insertion of the UL48 sequence into plasmid pENTR1A. One correct clone was chosen, and the insertion was verified by sequencing the start and end of the insert. The full-length UL48 gene was released using EcoRI and subcloned into the mammalian expression vectors pEF1/Myc-His C (Invitrogen) and pEGFP-C2 (Clontech), resulting in plasmids pEF-UL48 and pEGFP-UL48, respectively.
To generate plasmids expressing NLS mutants of pUL48, a different gap repair plasmid was constructed based on the pEF1/Myc-His C vector into which two fragments of UL48 comprising nucleotides 1 to 329 and 6294 to 6720 were cloned in frame with the myc tag sequence of the vector. This new gap repair plasmid allowed cloning of UL48 sequences directly into an expression vector and detection of expressed proteins using the myc tag. For the gap repair procedure, this plasmid was enzymatically linearized (BamHI/XbaI) and electroporated into E. coli containing TB40-BAC4. Homologous recombination between the UL48 fragments of the gap repair vector and the respective sequences in the TB40-BAC4 DNA resulted in expression plasmid pEF-UL48-myc. For the generation of expression plasmids pEF-UL48del-bC1-myc, pEF-UL48mut-bC1-myc, and pEF-UL48mut-bC2-myc (see Fig. 3A), the gap repair plasmid was electroporated into E. coli containing the respective NLS mutant BACs.
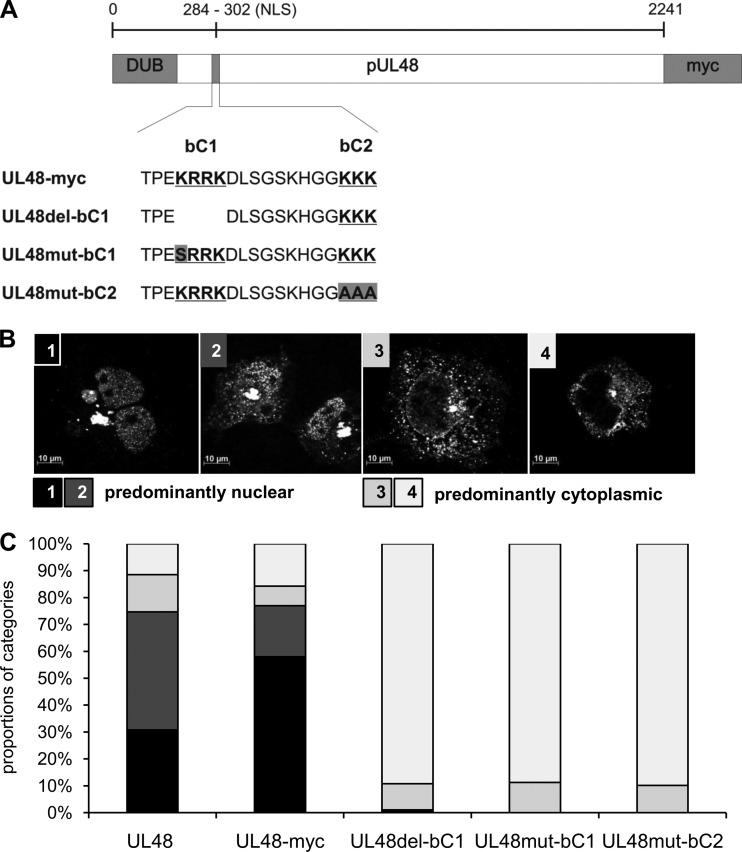
Fig 3.
Functionality of the bipartite NLS in full-length pUL48. (A) Schematic illustration of NLS mutants of full-length pUL48 tagged with a myc epitope tag. The sequence of the bipartite NLS with the two basic clusters (bold and underlined), with the deletion of the first basic cluster (bC1) in UL48del-bC1, and with introduced point mutations in either bC1 or bC2 (gray boxes), is depicted. Plasmids expressing untagged pUL48, pUL48-myc, and the three pUL48 NLS mutants were transfected into HeLa cells. Cells were fixed 16 h posttransfection, and pUL48 was detected with the polyclonal anti-pUL48 serum in indirect immunofluorescence experiments. (B) Intracellular distribution of pUL48 was categorized into four categories: 1, strict nuclear localization; 2, strong nuclear accumulation but also cytoplasmic signals; 3, strong cytoplasmic accumulation and faint signals in the nucleus; 4, strict cytoplasmic localization. Categories 1 and 2, in black and dark gray, respectively, are considered predominantly nuclear distribution, and categories 3 and 4, in light gray and white, respectively, are considered predominantly cytoplasmic localization of pUL48. (C) Summary of the relative proportions of the four categories from four independent experiments. Intracellular distribution of each construct was categorized in each experiment from 100 cells in blinded studies.
To generate NLS rescue plasmids in which an NLS sequence was cloned in front of UL48 separated by an enhanced green fluorescent protein (EGFP) sequence, the EGFP sequence was PCR amplified with forward primers (see Table S1 in the supplemental material) containing the sequence of the pUL48 NLS (corresponding to pUL48 residues 284 to 302), the mutated sequence of UL48NLSmut-bC1, and the sequence of the HSV-1 pUL36 NLS (corresponding to pUL36 residues 426 to 470 [39]), respectively, and was cloned in frame with the start of the UL48 gene into pEF-UL48mut-bC1-myc and pEF-UL48mut-bC2-myc expression vectors, respectively.
To generate the pEF-UL48NLSrepl36 expression plasmid, we exchanged the UL48 NLS sequence (amino acids 284 to 302) with the corresponding UL36 NLS sequence (amino acids 426 to 443 [39]) in the background of the pEF-UL48-myc vector. Therefore, we generated two N-terminal fragments of UL48, aa1-283 with the 3′-fused UL36 NLS sequence and aa303-1218 5′ fused to the UL36 NLS sequence, respectively. In a second PCR, both fragments were fused with the UL36 NLS sequence replacing the UL48 NLS sequence (for all primers, see Table S1 in the supplemental material). By enzymatic digestion with ClaI/BamHI, the N-terminal half of pUL48 in the pEF-UL48-myc vector was replaced with the newly generated one.
Cloning and analysis of the NLS of pUL48.
The sequence of pUL48 was analyzed regarding NLS motifs by using the PSORTII software program (http://psort.hgc.jp/). It predicted two putative monopartite NLS motifs at amino acid positions 1625 and 2124 and one bipartite NLS at position 287. The latter appears to be conserved among herpesviruses, as recently shown in sequence alignments (39). The sequence corresponding to N-terminal residues 1 to 338 of pUL48 was PCR amplified, digested with BamHI and EcoRI, and cloned in frame with the myc tag sequence into pEF1/Myc-His C vector digested with the corresponding enzymes. The resulting plasmid was termed pEF-UL48_1–338. The functionality of the bipartite pUL48 NLS was analyzed by generating a panel of vectors expressing various pUL48-EGFP fusion proteins. The fusion constructs harboring the pUL48 NLS (residues 284 to 302) and those with mutations were generated by PCR amplification of the EGFP sequence using forward primers containing the respective mutated or nonmutated UL48 sequences. The products were digested with BamHI and EcoRI and cloned into pEF1/Myc-His C digested with BamHI and EcoRI. Fusion constructs with larger pUL48 fragments (residues 284 to 328) and the HSV-1 pUL36 NLS (residues 426 to 470) were generated by a combination of PCR amplification and ligation procedure. Briefly, these fragments were first PCR amplified with respective primers, digested with BglII, and ligated to the EGFP sequence that was PCR amplified and digested with BamHI. The resulting fusion constructs were PCR amplified using the forward primers for the fragments and the EGFP reverse primer, digested with BamHI and EcoRI, and cloned into pEF1/Myc-His C digested with BamHI and EcoRI. The resulting plasmids were designated as summarized in Table S1 in the supplemental material.
To analyze the intracellular localization of UL48_1–338 as well as of the generated NLS-EGFP fusion constructs, Cos7 or HeLa cells were seeded on glass coverslips in a 24-well plate (4 × 104 cells per well) and transfected using Lipofectamine LTX (Invitrogen) or Turbofect transfection reagent (Fermentas) according to the manufacturer's protocol. After 16 to 24 h posttransfection, coverslips were washed with phosphate-buffered saline (PBS) and fixed with 4% paraformaldehyde (PFA) for 10 min at 4°C. Cells were permeabilized with 0.1% Triton X-100 for 5 min at room temperature, cell nuclei were stained with DAPI (4′,6′-diamidino-2-phenylindole; Roche), and coverslips were mounted with Ibidi mounting medium (Ibidi, Martinsried, Germany). Representative images were acquired using an inverted fluorescence microscope (Observer.Z1; Zeiss) equipped with the Apotome.
Antibodies.
HCMV-encoded and cellular proteins were detected with monoclonal antibodies (MAbs) in plaque assays, titrations, Western blot analyses, or indirect immunofluorescence studies. The MAbs used in the present study included those directed against pp28 (Santa Cruz Biotech), γ-adaptin (clone 100/3; Sigma-Aldrich), Myc (MAb 9E10), pp150 (UL32; Xp-1) (42), IE1/2 (MAb 63-27), Golgin97 (CDF4; MoBiTec), and LaminB1 (Zymed SF). HCMV tegument protein pUL71 was detected with a previously described polyclonal antibody in Western blot analyses (43). Goat anti-mouse and goat anti-rabbit antibodies conjugated with Alexa Fluor 488 and 555 (Invitrogen) were used for plaque assay and immunofluorescence studies, goat anti-mouse or goat anti-rabbit antibodies conjugated with horseradish peroxidase (HRP; Pierce) were used for Western blot analyses, and an HRP-conjugated rabbit anti-mouse antibody (Dako) was used for titration.
Indirect immunofluorescence.
For indirect immunofluorescence studies of HCMV-infected cells, HFFs were seeded in μ-Slide eight-well plates (Ibidi GmbH, Martinsried, Germany) and synchronized for 48 h by serum starvation. The cells were then infected with wild-type virus at a multiplicity of infection (MOI) between 0.5 and 1 PFU. At 2, 3, 4, and 5 days postinfection (dpi), cells were washed twice with PBS and fixed with 4% PFA in PBS for 10 min at 4°C. For the indirect immunofluorescence of transfected cells, Cos7 cells were grown on glass coverslips in 24-well plates, transfected using Lipofectamine LTX, and fixed with 4% PFA after 16 to 24 h as described above. For the staining procedure, HCMV-infected as well as transfected cells were permeabilized with 0.1% Triton X-100 in PBS for 5 min followed by incubation with blocking solution for 30 min. The blocking solution for transfected cells was PBS supplemented with 5% FCS. To sufficiently block the HCMV-induced Fc receptors in virus-infected cells (44, 45), we used PBS supplemented with 1% bovine serum albumin (BSA) and 10% pooled human serum obtained from HCMV-negative individuals. Human serum has been shown to efficiently block the Fc receptors, which have some affinity for rabbit IgGs (46, 47). Dilutions of primary antibodies in blocking solution were incubated with the cells for at least 45 min. After intensive washing steps (at least three times with PBS supplemented with 1% BSA and 0.1% Triton X-100 for infected cells or with PBS alone for transfected cells) and an incubation with dilutions of secondary antibodies conjugated with either Alexa Fluor 488 or Alexa Fluor 555 (Invitrogen) for another 45 min, cell nuclei were stained with DAPI (4′,6′-diamidino-2-phenylindole; Roche) for 10 min. The coverslips of transfected cells were mounted using Ibidi mounting medium, whereas Ibidi slides allowed direct imaging without prior mounting using the Axio-Observer.Z1 (Zeiss) inverted fluorescence microscope. Confocal images of transfected cells and HCMV-infected cells were acquired by using the 63× objective lens of the microscope with the Apotome and the Axiovision software 4.8 (Zeiss). The images presented in Fig. 4B are nonconfocal images that were acquired without using the Apotome.
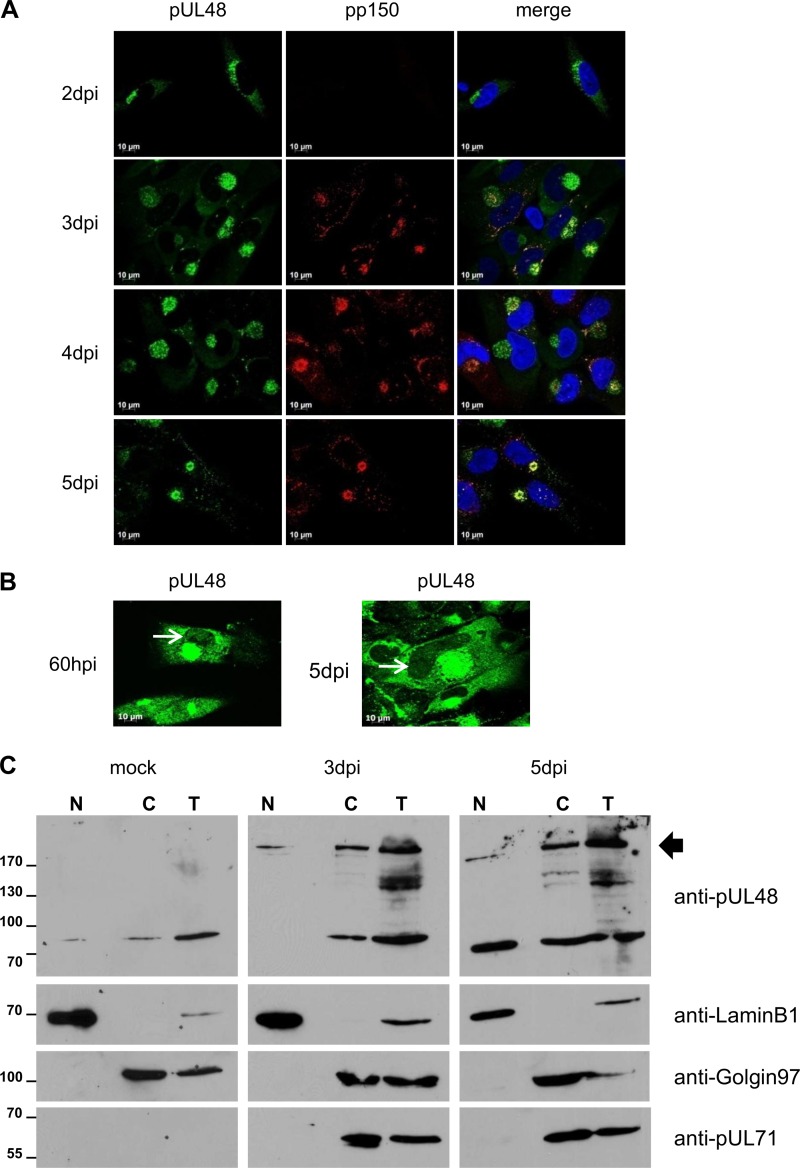
Fig 4.
Intracellular distribution of pUL48 during HCMV infection. (A) Localization of pUL48 in HCMV wild-type virus-infected HFFs was analyzed in indirect immunofluorescence experiments at the indicated day postinfection. pUL48 was detected with the polyclonal anti-pUL48 serum, and HCMV capsid-associated tegument protein pp150 was detected with an anti-pp150 MAb. Nuclei were stained with DAPI. (B) Weak but distinct nuclear signals of pUL48 were detected in some cells after strong enhancement of the pUL48 signals (indicated by the white arrows), representatively shown for HCMV wild-type virus-infected HFFs at 60 h postinfection (hpi) and at 5 dpi. pUL48 was detected with the polyclonal anti-pUL48 serum, and the nuclei were stained with DAPI. (C) Mock-infected cells and HCMV wild-type virus-infected cells at 3 and 5 dpi were fractionated as described in Materials and Methods. Total cell lysates (T), nuclear (N) and cytoplasmic (C) fractions, were separated on a 7.5% SDS-PAGE gel and transferred onto PVDF membranes. The polyclonal anti-pUL48 serum was used for the detection of HCMV pUL48. Cellular fractions were assessed using antibodies recognizing LaminB1 (marker for the nuclear fraction) and Golgin97 (marker for trans-Golgi compartments), respectively. The black arrow indicates bands corresponding to full-length pUL48. Numbers at left are molecular masses in kilodaltons.
Quantification of the intracellular pUL48 distribution.
The intracellular distribution of pUL48, pUL48 NLS mutants, and NLS rescue mutants was analyzed in a semiquantitative manner in Cos7 cells, which were transfected with the respective expression plasmids. The intracellular distribution of pUL48 was analyzed with the Axio-Observer.Z1 fluorescence microscope and the 63× objective lens after fixation and staining for pUL48 using the polyclonal anti-pUL48 antibody. The various distributions of pUL48 were classified into four categories: 1, strict nuclear localization; 2, strong nuclear accumulation but also cytoplasmic signals; 3, strong cytoplasmic accumulation and faint signals in the nucleus; 4, strict cytoplasmic localization (see Fig. 3B). For each experiment and construct, at least 100 randomly selected pUL48-positive cells were categorized in a blindfolded manner. Each experiment was repeated at least three times.
Engineering BAC mutants.
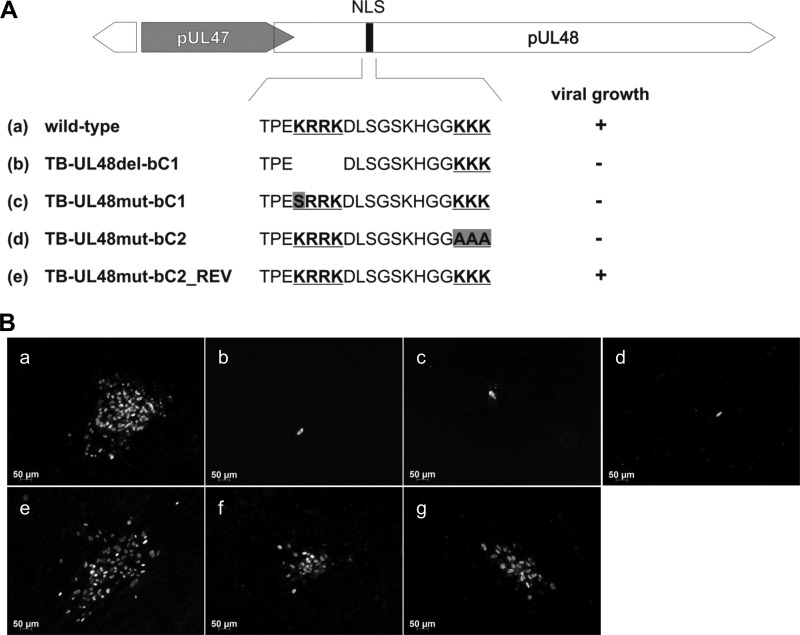
A markerless two-step RED-GAM recombination protocol (48) was used for the generation of all recombinant BAC clones in E. coli as described elsewhere (43). Three mutations of the pUL48 bipartite NLS were introduced into parental BAC TB40-BAC4. The deletion of the first basic cluster (bC1) (pUL48 residues 287 to 290) resulted in mutant BAC TB-UL48del-bC1. Point mutation of the lysine residue 287 of bC1 to serine and point mutations of the three lysine residues 300, 301, and 302 of the second basic cluster (bC2) to alanines resulted in mutant BACs TB-UL48mut-bC1 and TB-UL48mut-bC2, respectively.
In another recombinant BAC mutant, designated TB-EGFP-UL48, the EGFP sequence was inserted in frame with UL48 after nucleotide 9 into TB40-BAC4 using the protocol and template plasmid pEP-EGFP as described in reference 49. The insertion of EGFP after UL48 nucleotide 9 was necessary because the pUL47 coding sequence overlaps the start of pUL48. The same insertion site was used for the generation of NLS rescue mutants. Two identical sets of three mutants were generated in the backbone of recombinant BAC clones TB-UL48mut-bC1 and TB-UL48mut-bC2, respectively. The sequence of the pUL48 NLS (residues 284 to 302) and the mutant sequence UL48mut-bC1, both fused to the start of EGFP, and EGFP alone were inserted by markerless mutagenesis as described for the generation of TB-EGFP-UL48. The templates for the mutagenesis were generated by PCR using specific primers (see Table S2 in the supplemental material) and the template plasmid pEP-EGFP (48). For the UL48NLS-EGFP and the UL48mut-bC1-EGFP insertions, two subsequent amplifications were performed. In the first PCR, the NLS sequence was added to the EGFP sequence, and in the second PCR, the sequences with homology to UL48 defining the site of integration were added. The recombinant clones in the background of TB-UL48mut-bC2 were termed TB-UL48NLS-rsc and TB-UL48NLSmut-bC1-EGFP-UL48mut-bC2 BAC.
To generate recombinant BAC mutant TB-48NLSrepl36, in which the sequence of the UL48 NLS (residues 284 to 302) was exchanged with that of the HSV-1 UL36 NLS (residues 426 to 443), specific primers were used for the markerless RED-GAM mutagenesis of TB40E-BAC4. The resulting recombinant BAC clone was termed TB-48NLSrepl36 BAC.
The correct generation of all recombinant BAC clones was verified by sequencing of the regions that were modified and by restriction fragment length polymorphism.
Virus reconstitution.
HCMV BAC DNA of TB40-BAC4 and BAC DNAs of recombinant BAC clones were isolated from E. coli by using the Nucleobond Xtra midikit (Macherey-Nagel) and used for the electroporation of MRC-5 cells by following previously described protocols (50). In the case of viral growth, MRC-5 cells were used for further virus propagation and generation of virus stocks. Stock virus titers were determined by titration of HFFs as described previously (43). The recombinant virus recovered from TB40-BAC4 DNA was referred to as wild-type virus. The various growth-competent recombinant viruses obtained from their respective BAC DNAs are summarized in Table S2 in the supplemental material.
To compare viral growth rates during reconstitution of the various recombinant viruses and to demonstrate viral growth of growth-deficient mutant viruses under complementing conditions, HCMV-infected cells were detected with an indirect immunofluorescence staining for viral immediate-early antigens 1 and 2 (IE1/2). Briefly, MRC-5 cells (approximately 2 × 106 cells) were electroporated using either 2 μg of BAC DNA together with 1 μg of the pp71 expression plasmid pCMV71 (51) or 2 μg of BAC DNA, 1 μg of pCMV71, and 1 μg of pEF-UL48 in the case of complementation. Cells from two electroporations using the same DNAs were reseeded into one T25 flask. Depending on the cell density the next day, either fresh MRC-5 cells or, in the case of the complementing conditions, MRC-5 cells that were freshly electroporated using 5 μg of pEF-UL48 were added until a confluence of 60 to 80% was reached. After 7 days of culture, cells were fixed with methanol and incubated with an anti-IE1/2 antibody (diluted 1:1 in PBS) for 45 min. The cells were then washed three times with PBS and incubated with the anti-mouse secondary antibody conjugated with Alexa Fluor 555 (Invitrogen). The nuclei were stained with DAPI (Roche). Images were taken by using an Axio-Observer.Z1 fluorescence microscope with a 10× objective lens and the Axiovision software (Zeiss). Reconstitution experiments were repeated at least three times for the growth-deficient mutant viruses using independent BAC DNA preparations and another independent recombinant BAC clone.
Growth analysis.
For the analyses of viral growth, HFFs were infected with the respective viruses at an MOI of 0.01 for 24 h at 37°C. The inocula were removed the next day and replaced with fresh medium after washing with PBS. Equal infection rates were controlled by titration of the inocula, which were termed day 0. Supernatants from virus-infected cells were collected every 3 days until day 18 and stored at −80°C. Virus yields of the supernatants from at least three independent experiments were determined on HFFs by titration as described previously (43).
Focal virus expansion was determined by infecting confluent HFFs with 100 PFU of the respective viruses followed by incubation for 10 days at 37°C under a 0.6 to 0.7% methylcellulose overlay. The overlay was renewed once at 5 dpi. After methanol fixation at 10 dpi, HCMV-infected cells were visualized by indirect immunofluorescence staining for the HCMV IE1/2 antigen. Images of foci of virus-infected cells were acquired by using the Axio-Observer.Z1 confocal microscope with a 10× objective lens and the Axiovision software. For each virus, focus areas of at least 120 foci were determined from three independent experiments by using the software ImageJ (http://rsbweb.nih.gov/ij/index.html). Statistical analysis was performed by applying the Kruskal-Wallis test.
Cell fractionation and Western blotting.
Nuclear and cytoplasmic fractions were isolated from HCMV wild-type virus-infected HFFs using NE-PER nuclear and cytoplasmic extraction reagents (ThermoScientific). Briefly, confluent HFFs were infected with wild-type virus at an MOI of 3 PFU. After 3 and 5 dpi, cells were trypsinized, washed with PBS, and pelleted by centrifugation at 500 × g for 3 min at 4°C. Cytoplasmic fractions were obtained by incubation of the cell pellet with the cytoplasmic extraction buffer according to the manufacturer's protocol, resulting in a final volume of 100 μl for the cytoplasmic fraction. To reduce possible contaminants from the cytoplasm in the nuclear fraction, the cytoplasmic extraction was repeated another time and discarded. Subsequently, nuclear fractions were isolated by incubation with nuclear extraction buffer as recommended in the protocol, resulting in a nuclear fraction of a 50-μl volume. In addition, the cell fractionation was also performed with mock-infected HFFs. Alternatively, cytoplasmic and nuclear fractions of virus-infected cells were isolated with a Dounce homogenizer after swelling in hypotonic buffer followed by an iodixanol gradient purification exactly as previously described (52).
Nuclear, cytoplasmic, and total cell lysates were subjected to SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membranes for Western blot analysis. The nuclear and the cytoplasmic fractions of mock-infected cells and HCMV-infected cells from 3 dpi and 5 dpi were adjusted to the same volume before their subjection to SDS-PAGE. Compartmentalization of proteins into cytoplasmic and nuclear fractions was controlled by Western blotting against fraction-specific cellular proteins. Preparation of total cell lysates of HCMV-infected HFFs and of pEF-UL48-transfected HeLa cells and Western blot analyses were performed as described previously (50).
RESULTS
To evaluate the intracellular localization of HCMV pUL48, a polyclonal antiserum had to be generated by using an amino-terminal fragment comprising pUL48 residues 399 to 1150. The specificity of our antiserum was assessed in Western blot assays and in immunofluorescence experiments. As shown in Fig. 1A, our anti-pUL48 antibody recognized a high-molecular-weight protein with a size of approximately 250 kDa, representing pUL48, in lysates of transfected as well as of HCMV-infected cells. It also recognized some lower-migrating proteins with various intensities in all lysates, including those of mock cells, indicating that these bands are not pUL48 specific (Fig. 1A; see also Fig. 4C). Nevertheless, the reaction of our antiserum was specific, because no signals at the size of pUL48 were observed in lysates of nontransfected 293 cells and noninfected HFFs.
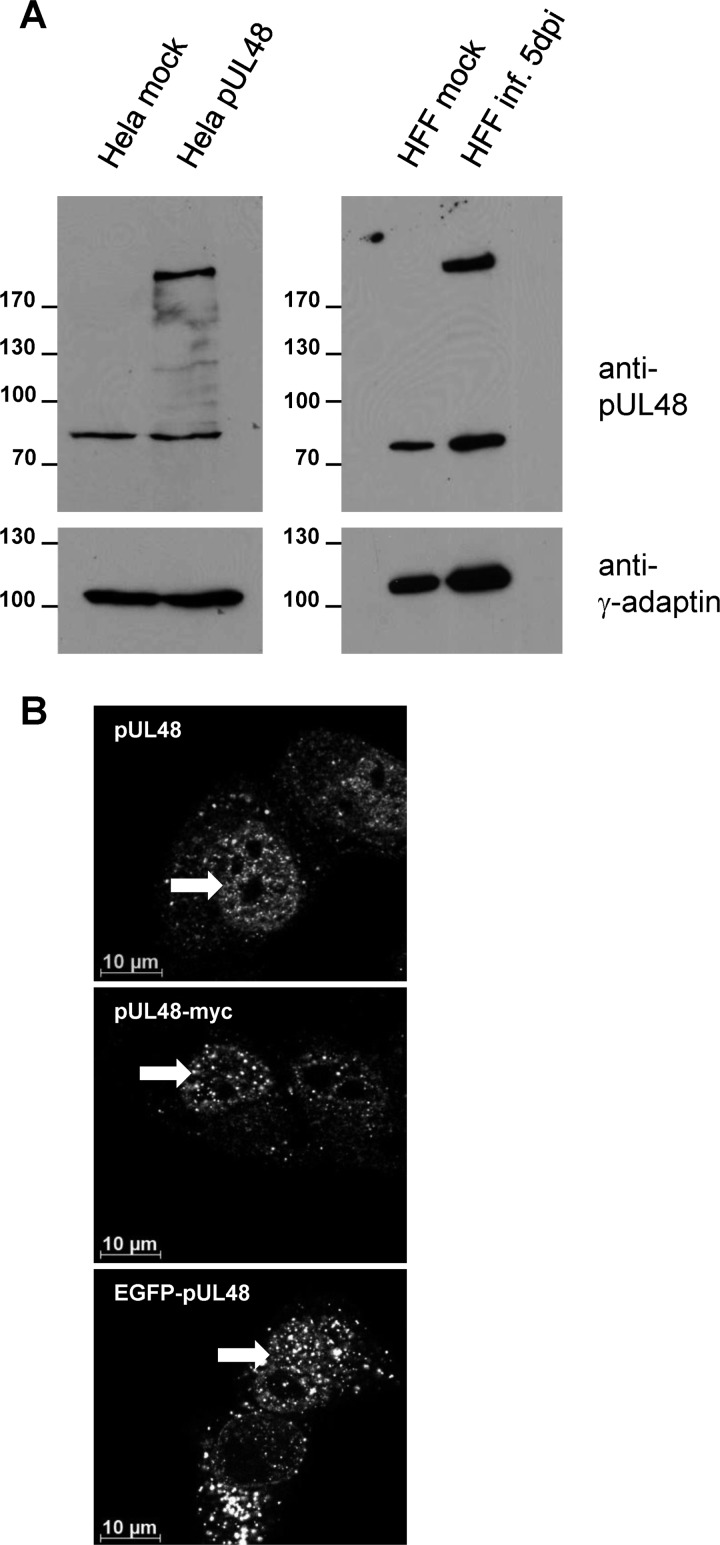
Fig 1.
Validation of a polyclonal anti-pUL48 serum. (A) Cell lysates of nontransfected HeLa cells, HeLa cells transiently expressing pUL48, and mock- and HCMV wild-type virus-infected HFFs 5 days postinfection (dpi) were separated on a 10% polyacrylamide gel. After transfer onto a PVDF membrane, the blot was incubated with a polyclonal anti-pUL48 serum. For a control, the blot was reprobed with an anti-γ-adaptin MAb. (B) Indirect immunofluorescence of HeLa cells transiently expressing the indicated proteins. Intracellular distributions of pUL48, pUL48-myc, and EGFP-pUL48 were compared after staining using the polyclonal anti-pUL48 serum or an anti-myc MAb and by EGFP fluorescence. Images were taken using a 63× objective and the Zeiss Axio-Observer.Z1 fluorescence microscope equipped with the Apotome. White arrows indicate nuclear accumulations of pUL48.
By indirect immunofluorescence, specific signals for pUL48 were detected only in cells expressing full-length pUL48 (Fig. 1B) and not in nontransfected cells (data not shown). These signals were similar to those observed in cells expressing an EGFP-pUL48 fusion protein or a pUL48-myc-tagged protein, indicating that our anti-pUL48 antiserum specifically recognizes the HCMV large tegument protein in indirect immunofluorescence.
pUL48 contains a bipartite NLS.
The detection of HCMV pUL48 in the cytoplasm as well as in the nucleus of transfected cells in indirect immunofluorescence experiments and the large size of approximately 250 kDa suggested active nuclear import of pUL48. Classical nuclear localization signals have been identified in the large tegument counterparts of PrV and HSV-1 (37, 39). Sequence analysis of HCMV pUL48 predicted three putative NLSs. One putative NLS (amino acids 287 to 303) is located adjacent to the pUL48 DUB domain and was of particular interest because it contains a basic amino acid motif (KRRK motif) within its sequence which is conserved in large tegument proteins of herpesviruses (39). For this reason, we decided to focus on this NLS instead of the other two predicted NLSs. In contrast to HSV-1 pUL36, this putative NLS of HCMV pUL48 appears to be a bipartite NLS, characterized by two clusters of basic amino acids separated by a spacer of 9 amino acids (Fig. 2A). To analyze the functionality of this predicted bipartite NLS, fragments of pUL48 containing the putative NLS were fused to the reporter protein EGFP. HeLa cells were transfected, and the localizations of the different EGFP fusion constructs were examined by immunofluorescence (Fig. 2B). Note that EGFP alone and all EGFP fusion constructs have a molecular mass of less than 50 kDa, which allows them to enter the nucleus by diffusion. Taking this into account, we compared the localization of our construct with that of EGFP alone (negative control) and a construct containing the previously identified NLS sequence of HSV-1 pUL36 fused to EGFP. The latter served as a positive control and exhibited a strong nuclear localization as shown in Fig. 2B, panel b. All constructs containing the putative bipartite NLS sequence of pUL48 also exhibited strong nuclear localization (Fig. 2B, panels c, d, and g) comparable to that of the positive control. The specific sequences conferring NLS function were further investigated by introducing different mutations in each of the two basic amino acid clusters (Fig. 2A). We mutated the second arginine residue in the first basic cluster (bC1) to serine and in addition the first lysine residue to alanine in the pUL48 fragment containing amino acids 284 to 328 (Fig. 2A, rows e and f). In the shorter pUL48 fragment, comprising amino acids 284 to 302, we mutated the first lysine residue of bC1 to serine and the three lysine residues of the second basic cluster (bC2) to alanines (Fig. 2A, rows h and i). The EGFP fusion constructs containing the single mutations in bC1 or the mutation of bC2 (Fig. 2B, panels e, f, h, and i) showed an intracellular distribution that was comparable to that of EGFP alone (Fig. 2B, panel a) and clearly different from that of their respective nonmutated fragments. Taken together, the results indicate that pUL48 contains a functional classical bipartite NLS.
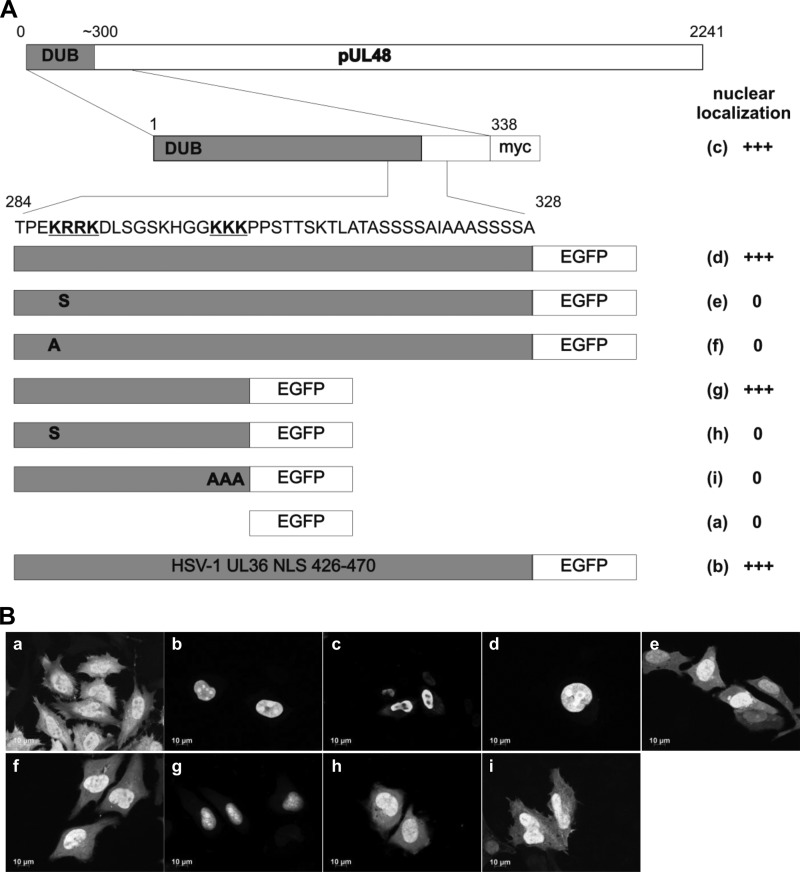
Fig 2.
Identification of a functional bipartite NLS in pUL48 transferable to EGFP. (A) Schematic diagram illustrating the generated pUL48 fragments fused to EGFP (d to i) and an N-terminal fragment containing residues 1 to 338 of pUL48 fused to a myc epitope tag (c). The two basic clusters of the bipartite NLS are highlighted in bold and underlined in the sequence. As a positive control, the previously identified NLS of pUL36 of HSV-1 (residues 426 to 470) was fused to EGFP (b), and EGFP alone (a) served as the negative control. Bold letters in pUL48 fragments indicate point mutations. (B) HeLa cells were transfected with plasmids expressing different pUL48 fragments fused to EGFP, as indicated in panel a. Cells were fixed after 24 h and analyzed for EGFP fluorescence. Only one fragment (c) was detected using the anti-myc MAb. Representative images are shown for each construct. A summary of the nuclear localization efficiency of all constructs is illustrated next to the diagram in panel A. Nuclear localization was evaluated semiquantitatively: +++ indicates a strong nuclear localization similar to that of the positive control (b), and 0 indicates a cellular distribution similar to that of the negative control (a).
Functionality of the bipartite NLS is required for nuclear localization of full-length pUL48.
To address whether the bipartite NLS accounts for the observed nuclear localization of full-length pUL48 in transiently expressing cells, we generated three constructs. In the first construct, the first basic cluster of the bipartite NLS was deleted (UL48del-bC1). In a second construct, we introduced the mutation of the initial lysine residue of the first cluster to serine (UL48mut-bC1). In a third construct, we mutated all lysine residues of the second basic cluster to alanines (UL48mut-bC2) (Fig. 3A). Due to the cloning strategy, all NLS mutants were generated in the backbone of a pUL48-myc construct, which allowed detection of NLS mutants by virtue of the myc epitope tag. The intracellular localization of all three full-length pUL48 NLS mutants was compared to that of the full-length, wild-type pUL48-myc fusion protein and of nontagged full-length pUL48 in indirect immunofluorescence experiments following transient expression. Intriguingly, full-length pUL48 exhibited a somewhat varied localization pattern; certain cells showed weak cytoplasmic and strong nuclear accumulations, and others exhibited pronounced cytoplasmic but only weak nuclear localization. Therefore, we defined 4 distribution categories to facilitate quantification of pUL48 localization (Fig. 3B). The majority of cells expressing wild-type pUL48 exhibited strong nuclear localization with only weak cytoplasmic signals. Deletion or mutation of the NLS had a strong effect on the localization of pUL48, with the majority of cells exhibiting strong cytoplasmic signals and only 10% of cells showing weak nuclear signals (Fig. 3C). These results indicate that the bipartite NLS in the N terminus of HCMV pUL48 is required for nuclear targeting in transiently expressing cells.
pUL48 can be detected in the nuclear fraction of HCMV-infected cells.
To address the intracellular distribution of pUL48 during HCMV infection, we used our pUL48 antibody to stain HCMV-infected cells at different times postinfection. For comparison, we included an antibody recognizing HCMV tegument protein pp150, which is closely associated with cytoplasmic capsids and accumulates at the juxtanuclear assembly complex (AC) (9, 53, 54). Representative images are shown in Fig. 4A. Despite some diffuse cytoplasmic signals, we detected an increased juxtanuclear accumulation of specific pUL48 signals starting at day 2 postinfection. Notably, very weak but distinct nuclear signals in the pUL48 immunofluorescence could be observed already at day 2 postinfection, and they became more apparent at later times of infection (Fig. 4B). However, the majority of pUL48 signals were found in the cytoplasm and not in the nucleus. Signals of pp150 progressively accumulated at the AC starting at 3 dpi and exhibited an increasing colocalization with those of pUL48 at later times. In conclusion, pUL48 is strongly localized to the cytoplasmic AC and was only weakly detectable in the nucleus by indirect immunofluorescence in HCMV-infected cells. The same localization pattern was also observed for an EGFP-pUL48 fusion protein that was expressed in cells infected with mutant virus TB-EGFP-UL48 (data not shown). These results indicated to us that pUL48 shows a different localization pattern during virus infection than that under conditions in which other viral gene products are absent, such as our ectopic expression experiments (Fig. 2 and 3).
To further verify our findings and to clarify whether pUL48 is indeed localized to the nucleus during HCMV infection, we performed cell fractionation experiments. Distribution of pUL48 was examined by Western blotting in cytoplasmic and nuclear fractions of HCMV-infected cells at 3 and 5 dpi. As shown in Fig. 4C, full-length pUL48 was detected in the cytoplasmic fraction but also in the nuclear fraction at both 3 and 5 dpi. The integrity of our fractionated extracts was assessed by probing for the trans-Golgi marker protein Golgin97, to monitor for the presence of proteins expected to be present in the cytosolic extract, and by probing for LaminB1, to monitor for proteins expected to be present in the nuclear fraction. We detected no Golgin97 in the nuclear fraction and no LaminB1 in the cytosolic fraction, indicating that the separation was successful. In addition, we probed for HCMV tegument protein pUL71 which accumulates at the viral AC in infected cells (55; data not shown). Signals for pUL71 were found only in the cytosolic extracts and were absent from nuclear fractions, which for us is further evidence for a successful separation in nuclear and cytosolic fractions. It must be noted that the individual cell fractions are differentially concentrated, such that the nuclear fraction is of higher concentration than the cytoplasmic fraction. Therefore, the signals detected in the Western blot analysis of the individual fractions are not precisely quantitative and, although we adjusted the volumes of both fractions in the Western blot analyses, could not be directly compared with each other for the purpose of making conclusions about relative protein abundance. To increase confidence, fractionation experiments were repeated using a previously described iodixanol gradient purification protocol for separation of the fractions (52). Consistently, pUL48 was also detected in the nuclear fraction in addition to the cytoplasmic fraction (data not shown). From these data, we concluded that the large tegument protein of HCMV is localized to both the cytoplasm and the nucleus during virus infection, which is consistent with our immunofluorescence data.
Functionality of the bipartite NLS is essential for HCMV growth.
Nuclear detection of HCMV pUL48 during virus infection and the presence of a functional NLS prompted us to examine the functional relevance of the pUL48 NLS for HCMV growth. To address this, we generated three mutant viruses by introducing into the backbone of the bacterial artificial chromosome (BAC) TB40-BAC4 (40) each of the following: (i) a deletion of the first basic cluster of the bipartite NLS, resulting in TB-UL48del-bC1; (ii) a point mutation of the initial lysine of the first basic cluster to serine, resulting in TB-UL48mut-bC1; and (iii) mutation of all three lysine residues of the second basic cluster to alanines, resulting in TB-UL48mut-bC2 (Fig. 5A). By repairing the mutations in the BAC TB-UL48mut-bC2, the revertant BAC mutant TB-UL48mut-bC2_REV was generated, which is distinguishable from BAC TB40-BAC4 by silent mutations. Successful generation of the mutant BACs and the revertant BAC was confirmed by sequence analysis of the mutated regions and restriction length polymorphism analysis (data not shown). Next, MRC-5 cells were electroporated with the respective BAC DNAs to reconstitute the mutant and revertant viruses. As summarized in Fig. 5A, only wild-type and revertant viruses were replication competent, whereas all three NLS mutants failed to grow. The reconstitution of the NLS mutants was repeated several times using different conditions (e.g., different DNA preparations or independent BAC clones of these mutants). However, formation of virus plaques was never observed. To exclude the possibility that mutations inadvertently introduced during generation of the NLS mutants account for their replication defect, NLS mutants TB-UL48del-bC1 and TB-UL48mut-bC1 were reconstituted in MRC-5 cells transiently expressing full-length pUL48. The spread of infection was monitored after 7 days of culture by indirect immunofluorescence. Figure 5B shows that parental and revertant viruses formed large foci of immediate-early (IE)-positive cells whereas only single IE-positive cells were observed in the case of the NLS mutants (Fig. 5B). In contrast, formation of several IE-positive foci was observed for TB-UL48del-bC1 and TB-UL48mut-bC1 viruses when complemented with full-length pUL48 (Fig. 5B, panels f and g). The likelihood of a repair of the NLS mutations by a recombination with the wild-type UL48 sequence of the complementing plasmid in this experiment could be excluded because we failed to recover virus from parallel experiments by passaging under noncomplementing conditions after the initial complementation. These results show that the generated NLS mutant viruses are replication competent in the presence of wild-type pUL48 and that no spurious mutations have occurred during mutagenesis.
Fig 5.
Generation and reconstitution of mutant viruses carrying mutations in the pUL48 NLS. (A) Schematic illustration of introduced deletion and mutations of the pUL48 NLS (gray boxes) into TB40-BAC4 (wild type). The wild-type pUL48 NLS is depicted with the two basic clusters of the bipartite NLS in bold and underlined. A revertant virus was generated on the basis of TB-UL48mut-bC2-BAC. Viral growth of each mutant was assessed after electroporation of MRC-5 cells with BAC DNA of the respective mutants. A summary of the viral growth is illustrated next to the schematic illustration as follows: +, plaque formation, and −, no plaques formed within 2 weeks of culture. (B) MRC-5 cells were electroporated with BAC DNAs of wild-type and mutant BACs (a to e) and BAC DNAs of TB-UL48del-bC1 and TB-UL48mut-bC1 together with a plasmid expressing full-length pUL48 (f and g). Cells were fixed 7 days postelectroporation. Virus-infected cells were detected by indirect immunofluorescence against the HCMV immediate-early (IE) antigen.
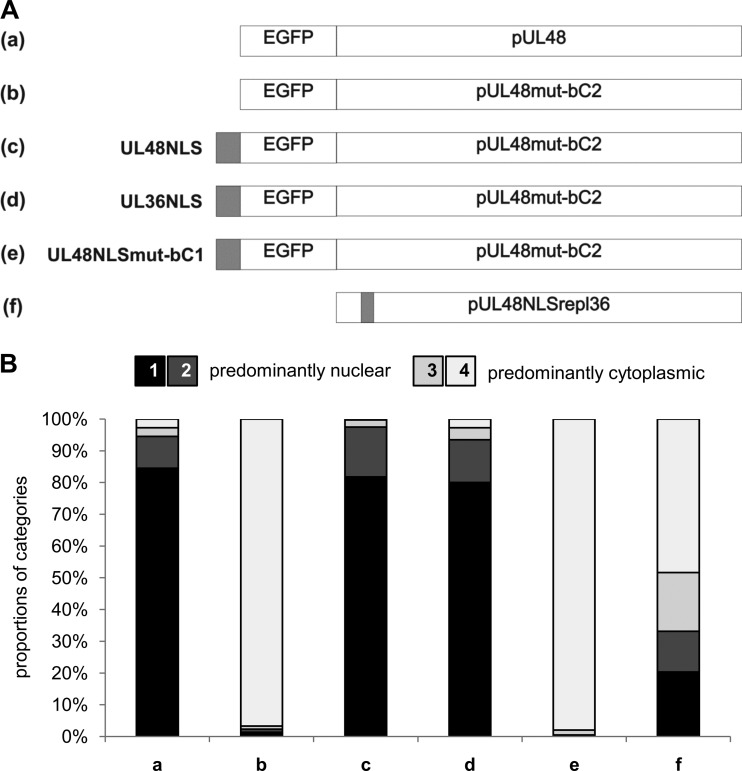
Rescue of nuclear localization of NLS mutants.
Having demonstrated that the NLS in pUL48 is required for nuclear localization in transient expression experiments, we wished to examine whether nuclear localization of a pUL48 NLS mutant could be rescued in NLS transfer experiments. Therefore, we generated a panel of constructs based on the second basic cluster mutant UL48mut-bC2 and fused either the UL48 NLS or the HSV-1 UL36 NLS sequence together with EGFP to the amino terminus (Fig. 6A). Identical constructs were also generated on the basis of the mutant UL48mut-bC1 (data not shown). As controls, constructs with fusion of EGFP alone or together with the mutated UL48NLSmut-bC1 were generated. Intracellular distribution of pUL48 in transiently expressing cells was examined and quantified as described for the results in Fig. 3B. As shown in Fig. 6B, only the fusion of a functional NLS, both the pUL48 NLS and the NLS of pUL36 HSV-1, rescued nuclear localization of the NLS-mutated full-length UL48mut-bC2. Fusion of EGFP alone or fusion of EGFP together with the mutated NLS sequence, UL48NLSmut-bC1, to the amino terminus did not result in significantly altered intracellular distribution of these constructs compared to that of the UL48mut-bC2 mutant. Similar results were also obtained for fusion constructs in the backbone of the point mutant UL48mut-bC1 (data not shown).
Fig 6.
Rescue of nuclear localization of a pUL48 NLS mutant. (A) Schematic illustration of constructs, where the sequences including pUL48 and the pUL48 NLS mutant UL48mut-bC2 were fused with the EGFP sequence (a and b). The UL48 NLS sequence (residues 284 to 302), the HSV-1 UL36 NLS sequence (residues 426 to 470, as positive control), and the UL48NLSmut-bC1 sequence (negative control) were fused together with the EGFP sequence N terminally to the UL48mut-bC2 sequence (c, d, and e). Additionally, the UL48 NLS sequence (residues 284 to 302) was replaced with the UL36 NLS sequence (residues 426 to 443) of HSV-1 (f). (B) The DNAs of plasmids expressing the depicted constructs were transfected into HeLa cells, and their intracellular distribution was evaluated 16 h posttransfection. Categorization of the intracellular distribution was performed as described for Fig. 3 either by EGFP fluorescence or by indirect immunofluorescence using the anti-pUL48 serum in the case of the UL48NLSrepl36 construct. The summary of the relative proportions of the 4 categories from 4 independent experiments for each construct is depicted in the graph. Intracellular distribution of each construct was categorized in each experiment from 100 cells in blinded studies.
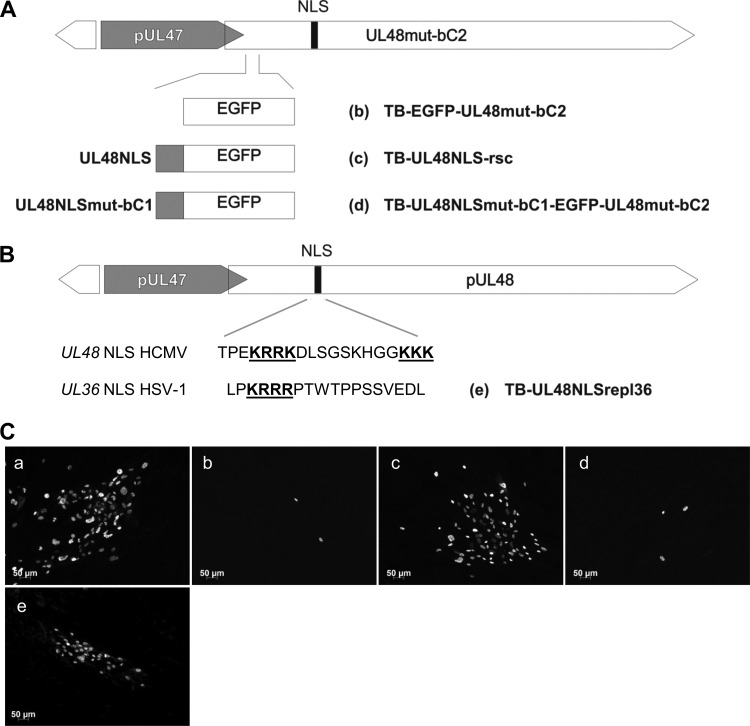
To investigate whether the rescue of nuclear localization by transfer of a functional NLS to the amino terminus of pUL48 is also able to rescue viral growth of NLS mutant viruses, we inserted either a functional NLS or a mutated NLS together with the EGFP sequence or EGFP alone into TB-UL48mut-bC2 by performing markerless BAC mutagenesis (Fig. 7A). The overall integrity of the resulting recombinant BACs and correct incorporation of intended changes were verified by sequencing of the mutated regions and restriction length polymorphism analysis (data not shown). To examine rescue of viral growth, BAC DNA of the generated mutants was electroporated into MRC-5 cells and viral growth was determined by staining of the IE1/2 proteins after 7 days of culture (Fig. 7C). The growth defect of TB-UL48mut-bC2 was rescued by transfer of a functional NLS from wild-type pUL48 to its amino terminus, as shown by the formation of large foci of IE-positive cells (Fig. 7C, panel c) comparable to the results with replication-competent mutant virus TB-EGFP-UL48 (Fig. 7C, panel a). Transfer of EGFP only or together with the mutated pUL48 NLS (UL48NLSmut-bC1) failed to complement the growth defect of TB-UL48mut-bC2, as only single IE-positive cells could be found (Fig. 7C, panels b and d). These observations support our data showing that a functional NLS in pUL48 is required for viral growth and for its efficient nuclear localization in transfected cells.
Fig 7.
Generation and growth of NLS rescue mutant viruses. (A) Illustration of constructed BAC mutants. The EGFP, the UL48NLS-EGFP, and the UL48NLSmut-bC1-EGFP sequences were inserted 9 nucleotides downstream of the start codon of UL48 in TB-UL48mut-bC2-BAC (b, c, and d). (B) In addition, the UL48 NLS sequence (residues 284 to 302) of the HCMV wild-type BAC was replaced with the NLS sequence of HSV-1 UL36 (residues 426 to 443) as illustrated (e). Basic clusters are again depicted in bold and underlined. (C) MRC-5 cells were electroporated with BAC DNAs of TB-EGFP-UL48 (a) and mutants depicted in panels A and B, respectively. Cells were fixed at day 7 postelectroporation. Virus-infected cells were detected by indirect immunofluorescence against the HCMV immediate-early (IE) antigen.
Replacement of the pUL48 NLS with the HSV-1 pUL36 NLS.
The conservation of the NLS in large tegument proteins of herpesviruses raised the question whether the pUL48 NLS can be functionally replaced with the pUL36 NLS of HSV-1. To address this, we first generated a plasmid expressing a full-length pUL48 in which pUL48 residues 284 to 302 were replaced with residues 426 to 443 of HSV-1 pUL36. Intracellular distribution of this construct was analyzed and compared to that of nonmutated full-length pUL48 in transiently expressing cells. The construct with the inserted pUL36 NLS was able to localize to the nucleus, but the number of cells with nuclear localization was clearly reduced compared to that for nonmutated full-length pUL48 (Fig. 6B). This finding showed that the function of the bipartite pUL48 NLS can be only partially replaced by the pUL36 NLS.
To examine whether the HSV-1 pUL36 NLS is also able to replace the function of the pUL48 NLS during virus infection, we generated a mutant virus by performing markerless BAC mutagenesis. As shown in Fig. 7C, panel e, this mutant virus, termed TB-UL48NLSrepl36, was replication competent and formed foci of IE-positive cells.
Growth analysis of the viral NLS rescue mutant and the NLS replacement mutant.
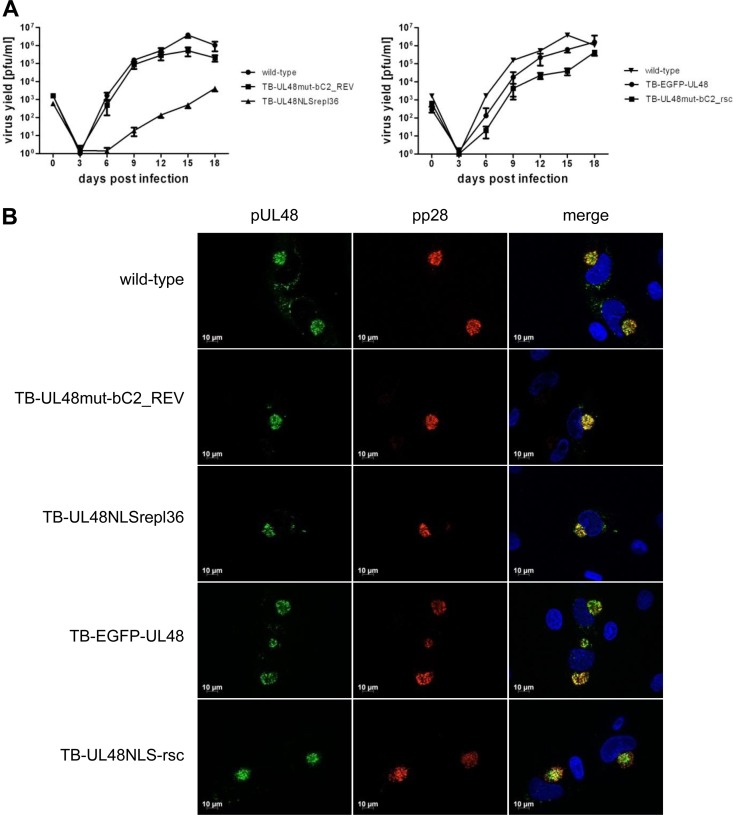
To assess the grade of growth complementation of the NLS rescue mutant virus in comparison to those of the wild-type virus and the TB-EGFP-UL48 virus, we performed multistep replication kinetics in HFFs and determined focal spread efficiency. Notably, insertion of EGFP at the N terminus of pUL48 already resulted in reduced virus yields in the supernatant compared to those for wild-type virus. The NLS rescue virus showed a defect in cell-free virus production of about 100-fold-reduced virus yields in the supernatant at 15 dpi compared to those of wild type or 10-fold-reduced virus yields compared to that of TB-EGFP-UL48 virus (Fig. 8A). Similarly, the TB-UL48NLSrepl36 virus was compared to the wild-type virus and the NLS revertant virus. Replacement of the pUL48 NLS with the pUL36 NLS in TB-UL48NLSrepl36 virus severely affected virus growth, as this mutant exhibited up to 1,000-fold-reduced virus yields in the supernatant compared to the wild type and to the NLS revertant virus at 15 dpi, respectively. We next assessed the focal spread of the NLS rescue mutant virus and the TB-UL48NLSrepl36 virus in HFFs after 10 dpi. Both mutant viruses exhibited a severe defect in focus formation marked by relative plaque areas of approximately 43% for the NLS rescue mutant and approximately 22% for TB-UL48NLSrepl36 virus compared to those of wild-type virus (Table 1). These results showed that both mutant viruses, the NLS rescue mutant and TB-UL48NLSrepl36, were replication competent but exhibited intermediate growth phenotypes.
Fig 8.
Analysis of viral NLS and rescue mutants. (A) Multistep growth kinetics of HCMV wild type, the revertant TB-UL48mut-bC2_REV, and respective virus mutants were performed using HFFs infected at an MOI of 0.01 PFU. Each virus was controlled in triplicate, and each growth kinetic was repeated three times. The virus yield of each supernatant was determined from day 0 every 3 days until day 18 postinfection. Growth curves include the mean of all determined virus yields, including the standard deviation. (B) Intracellular distribution of pUL48 was controlled for the revertant and mutant viruses in comparison to the wild type in indirect immunofluorescence experiments of infected HFFs at 3 dpi. pUL48 was detected by indirect immunofluorescence in wild-type, TB-UL48mut-bC2_REV, and TB-UL48NLSrepl36 virus-infected cells with our polyclonal anti-pUL48 serum and by EGFP fluorescence in cells infected with TB-EGFP-UL48 and TB-UL48NLS-rsc viruses. In addition, the tegument protein pp28 was detected with an anti-pp28 MAb. Nuclei were stained with DAPI.
Table 1.
Focus expansion analyses of pUL48 NLS revertant and mutant viruses compared to HCMV wild type
| Virus | % relative plaque areaa | Pb |
|---|---|---|
| HCMV wild type | 99.99 | |
| TB-UL48mut-bC2_REV | 108.75 | NS |
| TB-UL48NLSrepl36 | 22.21 | *** |
| TB-EGFP-UL48 | 92.55 | NS |
| TB-UL48NLS-rsc | 43.79 | *** |
Relative plaque areas were determined from three independent experiments. All data sets were normalized internally, and the value for wild-type virus was set to 100%.
Significance (P < 0.001) in comparison to the plaque areas of wild-type virus was determined by performing Kruskal-Wallis statistics.
, not significant.
Similar focal spread efficiency and cell-free virus production in multiple replication kinetics of the NLS revertant virus in comparison to wild-type virus indicated that the mutation of the NLS in TB-UL48mut-bC2 had been successfully repaired and that the defect in reconstitution of this mutant was due to the mutation of the NLS and not to some unexpected changes during the generation of this mutant.
Finally, we examined the intracellular localization of pUL48 in cells infected with either the NLS rescue virus or the TB-UL48NLSrepl36 virus at 3 dpi. As shown in Fig. 8B, pUL48 signals were detected at the viral AC in cells infected either with the NLS rescue virus or with the TB-UL48NLSrepl36 virus. The pUL48 localization pattern was similar to what was observed in cells infected with wild-type, revertant, and TB-EGFP-UL48 viruses, respectively, showing that neither the transfer of the NLS to the pUL48 N terminus nor the replacement of the pUL48 NLS with the pUL36 NLS affected distribution of pUL48 at the viral AC.
DISCUSSION
In this study, we have identified a functional NLS in the large tegument protein pUL48 of HCMV and we demonstrated that its function is essential for viral growth. The large tegument protein is highly conserved among herpesviruses, which is highlighted by shared features and functions such as essential roles during the viral replication cycle (8, 20, 30, 31, 34, 35, 38), deubiquitinase activity (10, 13–16), and a strong association with the capsid (9, 17–19, 21). Despite this high conservation, virus-specific functions of the large tegument proteins might also exist, since certain features are not completely clarified for each homolog of the different herpesvirus families. The vast majority of our current knowledge about functional domains, interactions with other viral or cellular proteins, and functions during the various steps of the herpesviral replication cycle comes mainly from investigations of the large tegument protein of alphaherpesviruses, whereas data about functions of the betaherpesvirus homologs are limited. In this study, we report for the first time on the intracellular distribution of HCMV pUL48 during infection and in the absence of other viral proteins. The existence of an active nuclear import of HCMV pUL48 was strongly suggested by our observation of nuclear accumulations in transiently expressing cells. This is consistent with previous reports about the intracellular localization of pUL36 of HSV-1 and PrV in transfected cells (37–39, 56). Interestingly, in HCMV-infected cells pUL48 exhibited an intracellular distribution that is mainly cytoplasmic with only weak nuclear signals in indirect immunofluorescence experiments. This difference in localization in the absence of other viral proteins versus that in virus infection strongly suggests a regulated distribution of pUL48 in virus-infected cells by a direct or indirect effect of other viral proteins. A difference in localization between transfected and infected cells has also been described for pUL36 of PrV (37). In agreement with our data, HSV-1 pUL36 was detected in the nucleus of virus-infected cells in indirect immunofluorescence experiments (38, 39). To gain more confidence about the nuclear presence of pUL48 and to overcome some limitations of single-cell analysis by indirect immunofluorescence, especially in detecting the very weak nuclear signals of pUL48, we performed cell fractionation experiments. With two different fractionation methods, we could clearly detect pUL48 in concentrated nuclear fractions of HCMV-infected cells, thus supporting our immunofluorescence data showing a nuclear localization of pUL48 during HCMV infection. Again, these findings are consistent at least with those for HSV-1 pUL36, which was also found in nuclear fractions of virus-infected cells (39), and thus support the idea that nuclear localization during virus infection is a conserved feature of the large tegument proteins.
Still, the question remains why, in contrast to transfected cells, pUL48 is only weakly detected in virus infection in indirect immunofluorescence experiments with our pUL48 antiserum (Fig. 4B) or with a mutant virus expressing an EGFP-UL48 fusion protein (data not shown). It is possible that nuclear import of pUL48 is less efficient in virus-infected cells or that during infection pUL48 is rapidly exported from the nucleus after import. The high intrinsic nuclear localization activity of pUL48 in transfected cells argues for rapid export during virus infection (Fig. 3). Supportive data for two hypotheses have been reported: nuclear export is facilitated (i) by an interaction with other viral proteins or (ii) together with viral capsids during virus infection. On one hand, there is a strong association of pUL48 with HCMV capsids (8, 9), and on the other hand, a protein complex consisting of pUL48, pUL47, and pUL69 has been reported (57). The latter protein is of special interest as pUL69 has been demonstrated to possess nucleocytoplasmic shuttling activity (58). However, nuclear import of pUL48 is obviously not dependent on the presence of other viral proteins and thus is mediated by an interaction with either cellular proteins or intrinsic NLSs. In our search for NLSs in pUL48, we identified a functional NLS in the N terminus of pUL48. Our discovery of an NLS in the betaherpesvirus large tegument protein is in agreement with previous reports on NLSs in pUL36 of HSV-1 (39) and PrV (37). It is interesting that from experimental data (including ours) as well as from sequence comparisons, this NLS appears to be highly conserved. In contrast to the alphaherpesvirus homologs, the NLS in pUL48 is a classical bipartite NLS with the two clusters of basic residues separated by a spacer of 9 amino acids (59–61). The two basic clusters were shown to be equally important for the functionality of the pUL48 NLS (this study), which is characteristic for this type of NLS and has also been demonstrated for the bipartite NLS of HCMV tegument protein pp65 (62).
Mutation of the NLS in the context of full-length pUL48 led to a virtually complete abrogation of nuclear accumulations of pUL48 in transiently expressing cells, on the one hand demonstrating the functionality of the NLS for nuclear localization and on the other hand suggesting that the two additional NLS sequences, which are predicted in pUL48 by PSORTII computational analysis, are of minor importance. Also, in pUL36 of HSV-1 and PrV three or four NLSs were predicted in total, but only the conserved N-terminal NLS mediated efficiently the nuclear import of pUL36 in transfected cells (37, 63).
The role of the pUL48 NLS in the context of HCMV infection was addressed by the generation and analysis of three viral NLS mutants. All viral NLS mutants were nonviable in cell culture, and their growth could be complemented only by providing pUL48 in trans. A revertant virus generated from the NLS mutant TB-UL48mut-bC2 exhibited growth characteristics that were indistinguishable from those of wild-type virus, demonstrating that the growth deficiency of the NLS mutant was due solely to the mutations in the NLS and not to additional second-site mutations. Taken together, these results clearly show an essential role of the bipartite NLS in pUL48 during HCMV infection, as has been recently reported for the corresponding NLS in pUL36 of HSV-1 (22, 39). While not being a formal proof, the fact that three different mutations in the pUL48 NLS resulted in the same phenotypes strongly suggests that this is caused by a disruption of the NLS function rather than by major conformational changes. This is further supported by our findings that nuclear import of pUL48 NLS mutants in transfected cells could be rescued by translocation of a functional NLS together with EGFP to the N terminus of these mutants. Interestingly, the monopartite pUL36 NLS sequence of HSV-1 (39) also was sufficient to restore nuclear import of the pUL48 NLS mutants in transiently expressing cells, albeit with decreased efficiency. Notably, the pUL36 NLS is very similar to the sequence of the prototypic monopartite NLS of simian virus 40 (SV40) large T antigen (61). Therefore, it is reasonable to assume that also the SV40 NLS would rescue nuclear import of NLS mutated pUL48. The fusion of the pUL48 NLS sequence with EGFP to the N terminus of the viral NLS mutant TB-UL48mut-bC2 partially restored viral growth. Expression and intracellular localization of pUL48 in that mutant virus were undistinguishable from those of HCMV wild-type virus, together suggesting functional rescue. Both the fusion of a functional NLS to the pUL48 N terminus of a NLS mutant and the exchange of the pUL48 NLS with the pUL36 NLS supported viral growth and nuclear localization in transfected cells. Although the exact function of pUL48 during the HCMV replication cycle has not been elucidated yet, the essentiality of the pUL48 NLS is consistent with the observation of a previous genome-wide analysis of HCMV for an essential role of the large tegument protein for the generation of infectious virus progeny (11). Regardless, future studies will be needed to determine the exact function of pUL48 during HCMV replication.
The bipartite pUL48 NLS and the monopartite pUL36 NLS share only the first highly conserved basic cluster. Interestingly, the exchange of the pUL48 NLS with the pUL36 NLS of HSV-1 led to an intermediate growth phenotype, characterized by an impaired viral growth of the respective viral mutant TB-UL48NLSrepl36 in comparison to wild-type virus. This phenotype could well be explained by our observation of reduced nuclear localization of the chimeric construct pUL48NLSrepl36 in transfected cells compared to that of wild-type pUL48. In consequence, it can be argued that efficient nuclear localization of pUL48 seems to directly correlate with viral growth. The growth capacity of the TB-UL48NLSrepl36 virus as such demonstrates the importance of a functional NLS in HCMV pUL48 for virus replication independently of a monopartite or bipartite character of this sequence. In this context, it would also be interesting to address whether the pUL48 NLS is able to replace the function of the pUL36 NLS.
All these results taken together strongly suggest that the nuclear targeting of pUL48 is an essential feature during HCMV morphogenesis. Nevertheless, the question remains what the function of the NLS truly is in the context of the various functions that large tegument proteins possess. Good evidence for an NLS function during virus entry by targeting incoming capsids to the nuclear pores has been recently reported for the pUL36 NLS of HSV-1 (22). However, from our data we cannot discriminate whether the pUL48 NLS has a similar function during HCMV entry or whether pUL48 has a yet-unknown role during nuclear particle assembly or release and thus requires a functional NLS. It is furthermore conceivable that pUL48 has to traffic through the nucleus to either undergo functionally relevant protein modifications or form a protein complex with other viral proteins. Future studies are certainly needed to clarify the specific role of the NLS in HCMV pUL48. Especially, the NLS rescue mutant and the NLS replacement mutant viruses generated in this study, both exhibiting intermediate growth phenotypes, may allow us to further investigate the function of the pUL48 NLS during HCMV entry and egress.
Supplementary Material
ACKNOWLEDGMENTS
The technical assistance of Jutta Hegler, Monika Dürre, and Anke Lüske is gratefully acknowledged. We thank Christian Sinzger (Institute of Virology, Ulm, Germany) and Jeremy Kamil (LSU Health Sciences Center, Shreveport, LA) for their comments and helpful discussions.
This work was supported by the Deutsche Forschungsgesellschaft through SPP1175 (grant ME 1740/2-1). Ivonne Brock was supported by the Graduate School in Molecular Medicine Ulm.
Footnotes
Published ahead of print 20 March 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.03558-12.
REFERENCES
- 1. Baldick CJ, Shenk T. 1996. Proteins associated with purified human cytomegalovirus particles. J. Virol. 70:6097–6105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Varnum SM, Streblow DN, Monroe ME, Smith P, Auberry KJ, Pasa-Tolic L, Wang D, Camp DG, II, Rodland K, Wiley S, Britt W, Shenk T, Smith RD, Nelson JA. 2004. Identification of proteins in human cytomegalovirus (HCMV) particles: the HCMV proteome. J. Virol. 78:10960–10966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guo H, Shen S, Wang L, Deng H. 2010. Role of tegument proteins in herpesvirus assembly and egress. Protein Cell 1:987–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kalejta RF. 2008. Tegument proteins of human cytomegalovirus. Microbiol. Mol. Biol. Rev. 72:249–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mettenleiter TC. 2002. Herpesvirus assembly and egress. J. Virol. 76:1537–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tandon R, Mocarski ES. 2008. Control of cytoplasmic maturation events by cytomegalovirus tegument protein pp150. J. Virol. 82:9433–9444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tandon R, Mocarski ES. 2011. Cytomegalovirus pUL96 is critical for the stability of pp150-associated nucleocapsids. J. Virol. 85:7129–7141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bradshaw PA, Duran-Guarino MR, Perkins S, Rowe JI, Fernandez J, Fry KE, Reyes GR, Young L, Foung SK. 1994. Localization of antigenic sites on human cytomegalovirus virion structural proteins encoded by UL48 and UL56. Virology 205:321–328 [DOI] [PubMed] [Google Scholar]
- 9. Yu X, Shah S, Lee M, Dai W, Lo P, Britt W, Zhu H, Liu F, Zhou ZH. 2011. Biochemical and structural characterization of the capsid-bound tegument proteins of human cytomegalovirus. J. Struct. Biol. 174:451–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang J, Loveland AN, Kattenhorn LM, Ploegh HL, Gibson W. 2006. High-molecular-weight protein (pUL48) of human cytomegalovirus is a competent deubiquitinating protease: mutant viruses altered in its active-site cysteine or histidine are viable. J. Virol. 80:6003–6012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dunn W, Chou C, Li H, Hai R, Patterson D, Stolc V, Zhu H, Liu F. 2003. Functional profiling of a human cytomegalovirus genome. Proc. Natl. Acad. Sci. U. S. A. 100:14223–14231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yu D, Silva MC, Shenk T. 2003. Functional map of human cytomegalovirus AD169 defined by global mutational analysis. Proc. Natl. Acad. Sci. U. S. A. 100:12396–12401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim ET, Oh SE, Lee Y-O, Gibson W, Ahn J-H. 2009. Cleavage specificity of the UL48 deubiquitinating protease activity of human cytomegalovirus and the growth of an active-site mutant virus in cultured cells. J. Virol. 83:12046–12056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Böttcher S, Maresch C, Granzow H, Klupp BG, Teifke JP, Mettenleiter TC. 2008. Mutagenesis of the active-site cysteine in the ubiquitin-specific protease contained in large tegument protein pUL36 of pseudorabies virus impairs viral replication in vitro and neuroinvasion in vivo. J. Virol. 82:6009–6016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kattenhorn LM, Korbel GA, Kessler BM, Spooner E, Ploegh HL. 2005. A deubiquitinating enzyme encoded by HSV-1 belongs to a family of cysteine proteases that is conserved across the family Herpesviridae. Mol. Cell 19:547–557 [DOI] [PubMed] [Google Scholar]
- 16. Schlieker C, Korbel GA, Kattenhorn LM, Ploegh HL. 2005. A deubiquitinating activity is conserved in the large tegument protein of the Herpesviridae. J. Virol. 79:15582–15585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bucks MA, Regan KJO, Murphy MA, Wills JW, Richard J. 2007. Herpes simplex virus type 1 tegument protein Vp1/2 and UL37 are associated with intranuclear capsids. Virology 361:316–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Coller KE, Lee JI, Ueda A, Smith GA. 2007. The capsid and tegument of the alphaherpesviruses are linked by an interaction between the UL25 and VP1/2 proteins. J. Virol. 81:11790–11797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gibson W. 1996. Structure and assembly of the virion. Intervirology 39:389–400 [DOI] [PubMed] [Google Scholar]
- 20. Schipke J, Pohlmann A, Diestel R, Binz A, Rudolph K, Nagel C-H, Bauerfeind R, Sodeik B. 2012. The C terminus of the large tegument protein pUL36 contains multiple capsid binding sites that function differently during assembly and cell entry of herpes simplex virus. J. Virol. 86:3682–3700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhou ZH, Chen DH, Jakana J, Rixon FJ, Chiu W. 1999. Visualization of tegument-capsid interactions and DNA in intact herpes simplex virus type 1 virions. J. Virol. 73:3210–3218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Abaitua F, Hollinshead M, Bolstad M, Crump CM, O'Hare P. 2012. A nuclear localization signal in herpesvirus protein VP1-2 is essential for infection via capsid routing to the nuclear pore. J. Virol. 86:8998–9014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Copeland AM, Newcomb WW, Brown JC. 2009. Herpes simplex virus replication: roles of viral proteins and nucleoporins in capsid-nucleus attachment. J. Virol. 83:1660–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Granzow H, Klupp BG, Thomas C, Mettenleiter TC. 2005. Entry of pseudorabies virus: an immunogold-labeling study. J. Virol. 79:3200–3205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Luxton GWG, Lee JI, Haverlock-Moyns S, Schober JM, Smith GA. 2006. The pseudorabies virus VP1/2 tegument protein is required for intracellular capsid transport. J. Virol. 80:201–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Radtke K, Kieneke D, Wolfstein A, Michael K, Steffen W, Scholz T, Karger A, Sodeik B. 2010. Plus- and minus-end directed microtubule motors bind simultaneously to herpes simplex virus capsids using different inner tegument structures. PLoS Pathog. 6:e1000991 doi:10.1371/journal.ppat.1000991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shanda SK, Wilson DW. 2008. UL36p is required for efficient transport of membrane-associated herpes simplex virus type 1 along microtubules. J. Virol. 82:7388–7394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sodeik B, Ebersold MW, Helenius A. 1997. Microtubule-mediated transport of incoming herpes simplex virus 1 capsids to the nucleus. J. Cell Biol. 136:1007–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wolfstein A, Nagel C-H, Radtke K, Döhner K, Allan VJ, Sodeik B. 2006. The inner tegument promotes herpes simplex virus capsid motility along microtubules in vitro. Traffic 7:227–237 [DOI] [PubMed] [Google Scholar]
- 30. Batterson W, Furlong D, Roizman B. 1983. Molecular genetics of herpes simplex virus. VIII. Further characterization of a temperature-sensitive mutant defective in release of viral DNA and in other stages of the viral reproductive cycle. J. Virol. 45:397–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Batterson W, Roizman B. 1983. Characterization of the herpes simplex virion-associated factor responsible for the induction of alpha genes. J. Virol. 46:371–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Knipe DM, Batterson W, Nosal C, Roizman B, Buchan A. 1981. Molecular genetics of herpes simplex virus. VI. Characterization of a temperature-sensitive mutant defective in the expression of all early viral gene products. J. Virol. 38:539–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jovasevic V, Liang L, Roizman B. 2008. Proteolytic cleavage of VP1-2 is required for release of herpes simplex virus 1 DNA into the nucleus. J. Virol. 82:3311–3319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Desai PJ. 2000. A null mutation in the UL36 gene of herpes simplex virus type 1 results in accumulation of unenveloped DNA-filled capsids in the cytoplasm of infected cells. J. Virol. 74:11608–11618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fuchs W, Klupp BG, Granzow H, Mettenleiter TC. 2004. Essential function of the pseudorabies virus UL36 gene product is independent of its interaction with the UL37 protein. J. Virol. 78:11879–11889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Leelawong M, Lee JI, Smith GA. 2012. Nuclear egress of pseudorabies virus capsids is enhanced by a subspecies of the large tegument protein that is lost upon cytoplasmic maturation. J. Virol. 86:6303–6314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Möhl BS, Böttcher S, Granzow H, Kuhn J, Klupp BG, Mettenleiter TC. 2009. Intracellular localization of the pseudorabies virus large tegument protein pUL36. J. Virol. 83:9641–9651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McNabb DS, Courtney RJ. 1992. Analysis of the UL36 open reading frame encoding the large tegument protein (ICP1/2) of herpes simplex virus type 1. J. Virol. 66:7581–7584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Abaitua F, O'Hare P. 2008. Identification of a highly conserved, functional nuclear localization signal within the N-terminal region of herpes simplex virus type 1 VP1-2 tegument protein. J. Virol. 82:5234–5244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sinzger C, Hahn G, Digel M, Katona R, Sampaio KL, Messerle M, Hengel H, Koszinowski U, Brune W, Adler B. 2008. Cloning and sequencing of a highly productive, endotheliotropic virus strain derived from human cytomegalovirus TB40/E. J. Gen. Virol. 89:359–368 [DOI] [PubMed] [Google Scholar]
- 41. Lee EC, Yu D, Martinez de Velasco J, Tessarollo L, Swing DA, Court DL, Jenkins NA, Copeland NG. 2001. A highly efficient Escherichia coli-based chromosome engineering system adapted for recombinogenic targeting and subcloning of BAC DNA. Genomics 73:56–65 [DOI] [PubMed] [Google Scholar]
- 42. Jahn G, Harthus HP, Broker M, Borisch B, Platzer B, Plachter B. 1990. Generation and application of a monoclonal antibody raised against a recombinant cytomegalovirus-specific polypeptide. Klin. Wochenschr. 68:1003–1007 [DOI] [PubMed] [Google Scholar]
- 43. Schauflinger M, Fischer D, Schreiber A, Chevillotte M, Walther P, Mertens T, von Einem J. 2011. The tegument protein UL71 of human cytomegalovirus is involved in late envelopment and affects multivesicular bodies. J. Virol. 85:3821–3853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lilley BN, Ploegh HL, Tirabassi RS. 2001. Human cytomegalovirus open reading frame TRL11/IRL11 encodes an immunoglobulin G Fc-binding protein. J. Virol. 75:11218–11221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Murayama T, Natsuume-Sakai S, Shimokawa K, Furukawa T. 1986. Fc receptor(s) induced by human cytomegalovirus bind differentially with human immunoglobulin G subclasses. J. Gen. Virol. 67:1475–1478 [DOI] [PubMed] [Google Scholar]
- 46. Antonsson A, Johansson PJ. 2001. Binding of human and animal immunoglobulins to the IgG Fc receptor induced by human cytomegalovirus. J. Gen. Virol. 82:1137–1145 [DOI] [PubMed] [Google Scholar]
- 47. Buchkovich NJ, Maguire TG, Paton AW, Paton JC, Alwine JC. 2009. The endoplasmic reticulum chaperone BiP/GRP78 is important in the structure and function of the human cytomegalovirus assembly compartment. J. Virol. 83:11421–11428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tischer B, von Einem J, Kaufer B, Osterrieder N. 2006. Two-step red-mediated recombination for versatile high-efficiency markerless DNA manipulation in Escherichia coli. Biotechniques 40:191–198 [DOI] [PubMed] [Google Scholar]
- 49. Tischer B, Smith G, Osterrieder N. 2010. En passant mutagenesis: a two step markerless red recombination system. Methods Mol. Biol. 634:421–451 [DOI] [PubMed] [Google Scholar]
- 50. Chevillotte M, Landwehr S, Linta L, Frascaroli G, Lüske A, Buser C, Mertens T, von Einem J. 2009. Major tegument protein pp65 of human cytomegalovirus is required for the incorporation of pUL69 and pUL97 into the virus particle and for viral growth in macrophages. J. Virol. 83:2480–2570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schierling K, Stamminger T, Mertens T, Winkler M. 2004. Human cytomegalovirus proteins ppUL82 (pp71) and ppUL35 interact and cooperatively activate the major immediate-early enhancer. J. Virol. 78:9512–9523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sinzger C, Kahl M, Laib K, Klingel K, Rieger P, Plachter B, Jahn G. 2000. Tropism of human cytomegalovirus for endothelial cells is determined by a post-entry step dependent on efficient translocation to the nucleus. J. Gen. Virol. 81:3021–3035 [DOI] [PubMed] [Google Scholar]
- 53. AuCoin DP, Smith GB, Meiering CD, Mocarski ES. 2006. Betaherpesvirus-conserved cytomegalovirus tegument protein ppUL32 (pp150) controls cytoplasmic events during virion maturation. J. Virol. 80:8199–8210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sampaio KL, Cavignac Y, Sinzger C, Stierhof Y-D. 2005. Human cytomegalovirus labeled with green fluorescent protein for live analysis of intracellular particle movements. J. Virol. 79:2754–2767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Womack A, Shenk T. 2010. Human cytomegalovirus tegument protein pUL71 is required for efficient virion egress. mBio 1(5):e00282–10 doi:10.1128/mBio.00282-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lee JI-H, Luxton GWG, Smith GA. 2006. Identification of an essential domain in the herpesvirus VP1/2 tegument protein: the carboxy terminus directs incorporation into capsid assemblons. J. Virol. 80:12086–12094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bechtel JT, Shenk T. 2002. Human cytomegalovirus UL47 tegument protein functions after entry and before immediate-early gene expression. J. Virol. 76:1043–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lischka P, Rosorius O, Trommer E, Stamminger T. 2001. A novel transferable nuclear export signal mediates CRM1-independent nucleocytoplasmic shuttling of the human cytomegalovirus transactivator protein pUL69. EMBO J. 20:7271–7283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Christophe D, Christophe-Hobertus C, Pichon B. 2000. Nuclear targeting of proteins: how many different signals? Cell. Signal. 12:337–341 [DOI] [PubMed] [Google Scholar]
- 60. Fontes MRM, Teh T, Jans D, Brinkworth RI, Kobe B. 2003. Structural basis for the specificity of bipartite nuclear localization sequence binding by importin-alpha. J. Biol. Chem. 278:27981–27987 [DOI] [PubMed] [Google Scholar]
- 61. Hicks GR, Raikhel NV. 1995. Protein import into the nucleus: an integrated view. Annu. Rev. Cell Dev. Biol. 11:155–188 [DOI] [PubMed] [Google Scholar]
- 62. Schmolke S, Drescher P, Jahn G, Plachter B. 1995. Nuclear targeting of the tegument protein pp65 (UL83) of human cytomegalovirus: an unusual bipartite nuclear localization signal functions with other portions of the protein to mediate its efficient nuclear transport. J. Virol. 69:1071–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ojala PM, Sodeik B, Ebersold MW, Kutay U, Helenius A. 2000. Herpes simplex virus type 1 entry into host cells: reconstitution of capsid binding and uncoating at the nuclear pore complex in vitro. Mol. Cell. Biol. 20:4922–4931 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.