Abstract
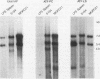
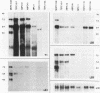
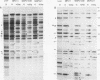
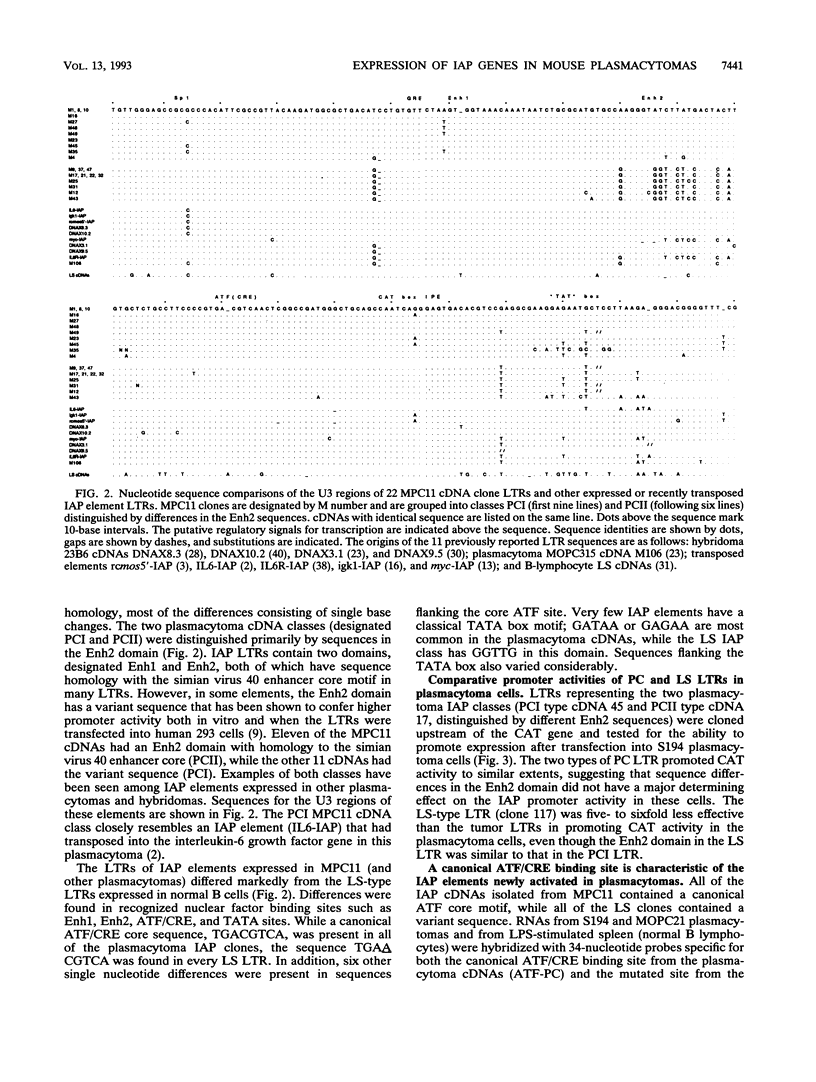
Mouse plasmacytomas generally express higher levels of RNA transcripts from endogenous intracisternal A-particle (IAP) proviral elements than do lipopolysaccharide-stimulated normal lymphocytes. Lymphocytes express a limited and highly characteristic set of IAP elements (lymphocyte-specific [LS] elements). In this study, we examined whether LS elements are expressed at higher levels after transformation of the cells and/or whether new IAP elements are activated. The IAP elements expressed in plasmacytoma MPC11 were characterized by sequence analysis of 22 cDNA clones. The long terminal repeats (LTRs) of the tumor cDNAs proved to be highly related in sequence. None of the clones was of the LS cDNA type. The MPC11 LTRs were five- to sixfold more active than an LS cDNA LTR when tested for promoter activity by transfection into plasmacytoma cells. The LTRs of the tumor-derived cDNAs contained a canonical ATF core sequence (ATF-PC), while the LS cDNAs contained an altered sequence (ATF-LS). An ATF-PC oligonucleotide probe detected multiple IAP transcripts on Northern (RNA) blots of RNA from several plasmacytoma but gave no reaction with RNA from stimulated B lymphocytes. In contrast, an ATF-LS probe detected higher levels of RNA in lymphocyte than in tumor RNAs. Thus, expression of IAP elements in transformed B cells is selective for a different set of regulatory sequence variants than those expressed in normal B cells. Other oligonucleotide probes representing LS- and PC-specific sequence variants detected multiple common hypomethylated IAP proviral loci in three independently derived plasmacytomas. Overall, the results show that established plasmacytomas exhibit a characteristic pattern of IAP proviral hypomethylation and regulatory sequence selection.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergsagel P. L., Victor-Kobrin C., Timblin C. R., Trepel J., Kuehl W. M. A murine cDNA encodes a pan-epithelial glycoprotein that is also expressed on plasma cells. J Immunol. 1992 Jan 15;148(2):590–596. [PubMed] [Google Scholar]
- Blankenstein T., Qin Z. H., Li W. Q., Diamantstein T. DNA rearrangement and constitutive expression of the interleukin 6 gene in a mouse plasmacytoma. J Exp Med. 1990 Mar 1;171(3):965–970. doi: 10.1084/jem.171.3.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canaani E., Dreazen O., Klar A., Rechavi G., Ram D., Cohen J. B., Givol D. Activation of the c-mos oncogene in a mouse plasmacytoma by insertion of an endogenous intracisternal A-particle genome. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7118–7122. doi: 10.1073/pnas.80.23.7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christy R. J., Brown A. R., Gourlie B. B., Huang R. C. Nucleotide sequences of murine intracisternal A-particle gene LTRs have extensive variability within the R region. Nucleic Acids Res. 1985 Jan 11;13(1):289–302. doi: 10.1093/nar/13.1.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christy R. J., Huang R. C. Functional analysis of the long terminal repeats of intracisternal A-particle genes: sequences within the U3 region determine both the efficiency and direction of promoter activity. Mol Cell Biol. 1988 Mar;8(3):1093–1102. doi: 10.1128/mcb.8.3.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clynes R., Wax J., Stanton L. W., Smith-Gill S., Potter M., Marcu K. B. Rapid induction of IgM-secreting murine plasmacytomas by pristane and an immunoglobulin heavy-chain promoter/enhancer-driven c-myc/v-Ha-ras retrovirus. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6067–6071. doi: 10.1073/pnas.85.16.6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falzon M., Kuff E. L. A variant binding sequence for transcription factor EBP-80 confers increased promoter activity on a retroviral long terminal repeat. J Biol Chem. 1990 Aug 5;265(22):13084–13090. [PubMed] [Google Scholar]
- Falzon M., Kuff E. L. Binding of the transcription factor EBP-80 mediates the methylation response of an intracisternal A-particle long terminal repeat promoter. Mol Cell Biol. 1991 Jan;11(1):117–125. doi: 10.1128/mcb.11.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falzon M., Kuff E. L. Isolation and characterization of a protein fraction that binds to enhancer core sequences in intracisternal A-particle long terminal repeats. J Biol Chem. 1989 Dec 25;264(36):21915–21922. [PubMed] [Google Scholar]
- Falzon M., Kuff E. L. Multiple protein-binding sites in an intracisternal A particle long terminal repeat. J Virol. 1988 Nov;62(11):4070–4077. doi: 10.1128/jvi.62.11.4070-4077.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feenstra A., Fewell J., Lueders K., Kuff E. In vitro methylation inhibits the promotor activity of a cloned intracisternal A-particle LTR. Nucleic Acids Res. 1986 May 27;14(10):4343–4352. doi: 10.1093/nar/14.10.4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galien R., Mercier G., Garcette M., Emanoil-Ravier R. ras oncogene activates the intracisternal A particle long terminal repeat promoter through a c-AMP response element. Oncogene. 1991 May;6(5):849–855. [PubMed] [Google Scholar]
- Grossman Z., Mietz J. A., Kuff E. L. Nearly identical members of the heterogeneous IAP gene family are expressed in thymus of different mouse strains. Nucleic Acids Res. 1987 May 11;15(9):3823–3834. doi: 10.1093/nar/15.9.3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley R. G., Shulman M. J., Hozumi N. Transposition of two different intracisternal A particle elements into an immunoglobulin kappa-chain gene. Mol Cell Biol. 1984 Dec;4(12):2565–2572. doi: 10.1128/mcb.4.12.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojman-Montes de Oca F., Lasneret J., Dianoux L., Canivet M., Ravicovitch-Ravier R., Périès J. Regulation of intracisternal A particles in mouse teratocarcinoma cells: involvement of DNA methylation in transcriptional control. Biol Cell. 1984;52(3):199–204. doi: 10.1111/j.1768-322x.1985.tb00337.x. [DOI] [PubMed] [Google Scholar]
- Kuff E. L., Fewell J. W. Intracisternal A-particle gene expression in normal mouse thymus tissue: gene products and strain-related variability. Mol Cell Biol. 1985 Mar;5(3):474–483. doi: 10.1128/mcb.5.3.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuff E. L. Intracisternal A particles in mouse neoplasia. Cancer Cells. 1990 Dec;2(12):398–400. [PubMed] [Google Scholar]
- Kuff E. L., Lueders K. K. The intracisternal A-particle gene family: structure and functional aspects. Adv Cancer Res. 1988;51:183–276. doi: 10.1016/s0065-230x(08)60223-7. [DOI] [PubMed] [Google Scholar]
- Lamb B. T., Satyamoorthy K., Solter D., Basu A., Xu M. Q., Weinmann R., Howe C. C. A DNA element that regulates expression of an endogenous retrovirus during F9 cell differentiation is E1A dependent. Mol Cell Biol. 1992 Nov;12(11):4824–4833. doi: 10.1128/mcb.12.11.4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiter E. H., Kuff E. L. Intracisternal Type A particles in murine pancreatic B cells. Immunocytochemical demonstration of increased antigen (p73) in genetically diabetic mice. Am J Pathol. 1984 Jan;114(1):46–55. [PMC free article] [PubMed] [Google Scholar]
- Lueders K. K., Fewell J. W., Kuff E. L., Koch T. The long terminal repeat of an endogenous intracisternal A-particle gene functions as a promoter when introduced into eucaryotic cells by transfection. Mol Cell Biol. 1984 Oct;4(10):2128–2135. doi: 10.1128/mcb.4.10.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueders K. K., Frankel W. N., Mietz J. A., Kuff E. L. Genomic mapping of intracisternal A-particle proviral elements. Mamm Genome. 1993;4(2):69–77. doi: 10.1007/BF00290429. [DOI] [PubMed] [Google Scholar]
- Lueders K. K., Kuff E. L. Intracisternal A-particle genes: identification in the genome of Mus musculus and comparison of multiple isolates from a mouse gene library. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3571–3575. doi: 10.1073/pnas.77.6.3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens C. L., Huff T. F., Jardieu P., Trounstine M. L., Coffman R. L., Ishizaka K., Moore K. W. cDNA clones encoding IgE-binding factors from a rat-mouse T-cell hybridoma. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2460–2464. doi: 10.1073/pnas.82.8.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M. W. Base composition-independent hybridization in dried agarose gels: screening and recovery for cloning of genomic DNA fragments. Biotechniques. 1988 May;6(5):444–447. [PubMed] [Google Scholar]
- Mietz J. A., Fewell J. W., Kuff E. L. Selective activation of a discrete family of endogenous proviral elements in normal BALB/c lymphocytes. Mol Cell Biol. 1992 Jan;12(1):220–228. doi: 10.1128/mcb.12.1.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mietz J. A., Grossman Z., Lueders K. K., Kuff E. L. Nucleotide sequence of a complete mouse intracisternal A-particle genome: relationship to known aspects of particle assembly and function. J Virol. 1987 Oct;61(10):3020–3029. doi: 10.1128/jvi.61.10.3020-3029.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mietz J. A., Kuff E. L. Intracisternal A-particle-specific oligonucleotides provide multilocus probes for genetic linkage studies in the mouse. Mamm Genome. 1992;3(8):447–451. doi: 10.1007/BF00356154. [DOI] [PubMed] [Google Scholar]
- Mietz J. A., Kuff E. L. Tissue and strain-specific patterns of endogenous proviral hypomethylation analyzed by two-dimensional gel electrophoresis. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2269–2273. doi: 10.1073/pnas.87.6.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan R. A., Christy R. J., Huang R. C. Murine A type retroviruses promote high levels of gene expression in embryonal carcinoma cells. Development. 1988 Jan;102(1):23–30. doi: 10.1242/dev.102.1.23. [DOI] [PubMed] [Google Scholar]
- Sugita T., Totsuka T., Saito M., Yamasaki K., Taga T., Hirano T., Kishimoto T. Functional murine interleukin 6 receptor with the intracisternal A particle gene product at its cytoplasmic domain. Its possible role in plasmacytomagenesis. J Exp Med. 1990 Jun 1;171(6):2001–2009. doi: 10.1084/jem.171.6.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama Y., O'Mara M. A., Spilsbury K., Thwaite R., Rowe P. B., Symonds G. Stage-specific expression of intracisternal A-particle sequences in murine myelomonocytic leukemia cell lines and normal myelomonocytic differentiation. J Virol. 1991 Apr;65(4):2149–2154. doi: 10.1128/jvi.65.4.2149-2154.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ymer S., Tucker W. Q., Campbell H. D., Young I. G. Nucleotide sequence of the intracisternal A-particle genome inserted 5' to the interleukin-3 gene of the leukemia cell line WEHI-3B. Nucleic Acids Res. 1986 Jul 25;14(14):5901–5918. doi: 10.1093/nar/14.14.5901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zierler M., Christy R. J., Huang R. C. Nuclear protein binding to the 5' enhancer region of the intracisternal A particle long terminal repeat. J Biol Chem. 1992 Oct 15;267(29):21200–21206. [PubMed] [Google Scholar]