Abstract
Progressive multifocal leukoencephalopathy (PML) is the main adverse effect of natalizumab. Detectable JC virus-specific effector memory T-cell (TEM) responses may indicate ongoing JCV replication. We detected JCV-specific TEM responses in blood of patients with multiple sclerosis (MS) treated with natalizumab, including 2 patients with PML. The frequency of detection of these responses increased with the time on natalizumab. Thus, a subset of MS patients exhibit immunological hallmarks of JCV replication during prolonged natalizumab therapy.
TEXT
Natalizumab, an antibody used to treat relapsing multiple sclerosis (MS), prevents trafficking of activated lymphocytes, including autoreactive lymphocytes, through the blood-brain barrier (BBB). The main adverse effect of natalizumab is progressive multifocal leukoencephalopathy (PML), a devastating demyelinating disease caused by replication of human JC polyomavirus (JCV) in oligodendrocytes and astrocytes (1, 2). Natalizumab inhibition of effector T-cell trafficking from blood to the central nervous system (CNS) might favor local JCV replication (3–7).
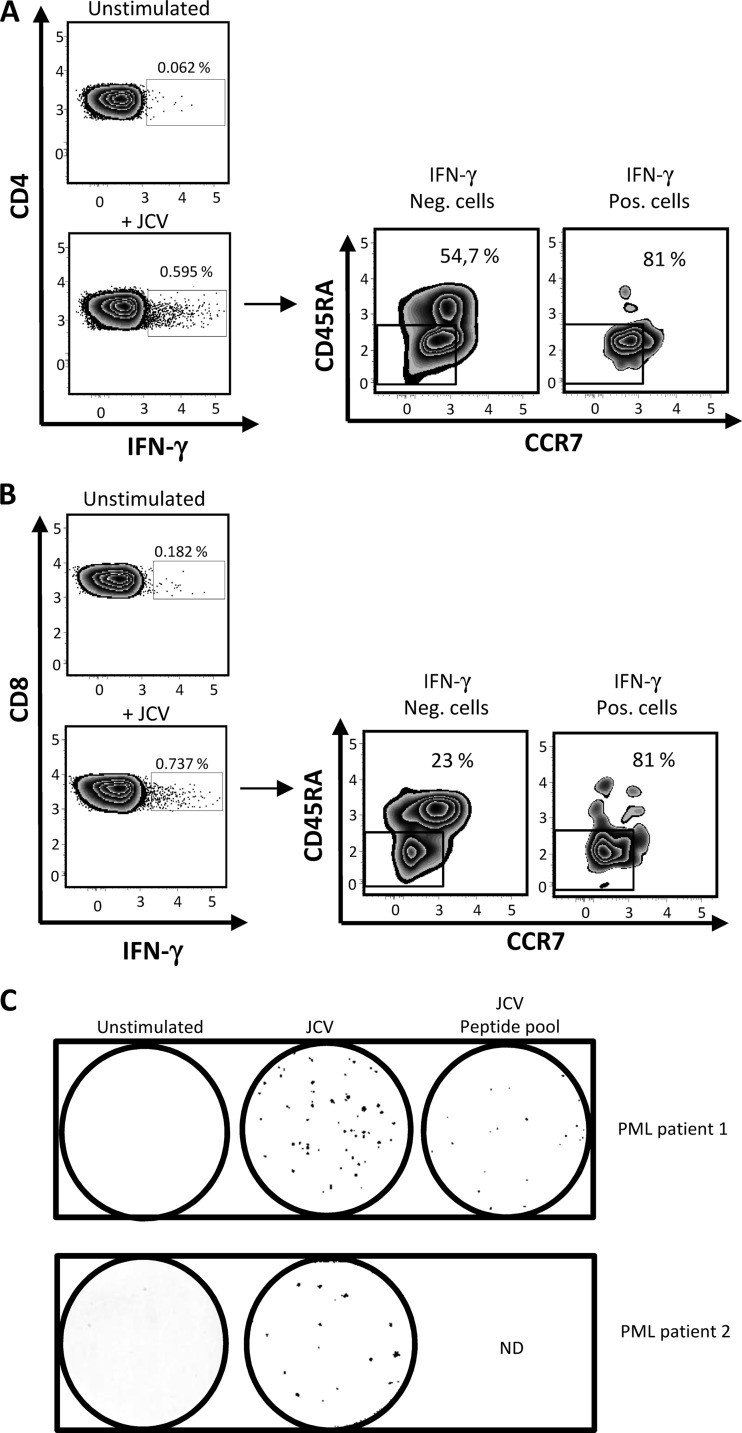
Here, we examined the presence of JCV-specific effector memory T cells (TEM) in blood of MS patients after overnight antigen activation ex vivo. In vivo, specific TEM numbers decline once the cognate antigen is cleared (8–10), and their presence may thus point to ongoing JCV replication. TEM rapidly release cytokines such as gamma interferon (IFN-γ) when reexposed to antigen. A previous enzyme-linked immunosorbent spot assay (ELISPOT) examined IFN-γ expression in T cells following long-term activation with JCV peptides for up to 14 days (11). However, prolonged activation allows long-term quiescent T-cell memory to be reactivated and to expand, meaning that a positive response does not necessarily signify an ongoing immune response. Here, we first examined the presence of JCV-specific TEM in two MS patients who developed PML. The first patient was diagnosed with PML after 39 months on natalizumab (39 infusions) (Department of Neurology, Pitié-Salpétrière Hospital). She was 39 years old and had a 10-year history of relapsing-remitting MS. Prior to natalizumab, she had received cyclophosphamide and mitoxantrone. PML was confirmed by MRI and JCV PCR on cerebrospinal fluid (CSF). When tested for JCV-specific TEM, 8 weeks after PML symptom onset, she had 1,040 JCV genome copies per ml of CSF (JCV Q-PCR Alert kit; Nanogen Advanced Diagnostics). Her peripheral blood mononuclear cells (PBMC) were activated in vitro in AIM-V and Albumax medium (Invitrogen) for 16 h with purified JCV (strain MAD-4; LGC Promochem; 104.5 50% tissue culture infective dose [TCID50]/0.2 ml). No recombinant interleukin 2 (IL-2) was added. An amount of virus corresponding to 5,534 PFU was added to each well. This corresponded to a multiplicity of infection (MOI) of 0.02. Cells were then tested for intracellular IFN-γ by flow cytometry. Cell mortality following overnight activation was less than 5%. As shown in Fig. 1A and B, responding cells were detected in both the CD4 and CD8 T-cell subsets, and most of them had the CCR7− CD45RA− phenotype characteristic of memory effectors (12). Anti-JCV TEM were also detected by IFN-γ ELISPOT (capture and detection antibodies, clones 1-D1 and K7-B6-1, respectively; Mabtech) following 16 h of activation with purified JCV or VP-1 peptides (a mix of 14 pools of overlapping 15-amino-acid peptides covering the entire JCV VP-1 protein [Neosystem]) (Fig. 1C). The second patient was 46 years old and had an 11-year history of relapsing-remitting MS. Prior to natalizumab, she had received beta interferon, glatiramer acetate, azathioprine, and mitoxantrone. She was referred to the Tenon Hospital MS center for reevaluation after 24 natalizumab infusions. A brain MRI performed in May 2012 detected a small, linear, T2-hyperintense suspect lesion in the juxtacortical right frontal region. The first CSF sample was negative for JCV. Repeat MRI after natalizumab withdrawal revealed an increase in lesion size, leading to two further CSF examinations, both of which were negative for JCV. JCV PCR on plasma was performed 2 and 4 months after the onset of PML. Both plasma samples were negative. Brain biopsy performed in September 2012 finally confirmed the diagnosis of PML.
Fig 1.
Detection of JCV-specific effector memory T cells (TEM) in blood of two natalizumab-treated patients with PML. In panels A and B, PBMC from one patient who developed PML following natalizumab treatment (39 infusions) were activated with purified JC virus overnight and then tested for intracellular IFN-γ by flow cytometry in the CD4 and CD8 T-cell subsets (A and B, respectively). CCR7 and CD45RA expression on IFN-γ-positive and -negative cells is also shown for CD4 T cells (A) and CD8 T cells (B). In panel C, PBMC from 2 patients who developed PML on natalizumab (patient 1 and patient 2 received 39 and 24 infusions, respectively) were activated overnight with purified JC virus and/or with a pool of VP1 peptides prior to IFN-γ ELISPOT. The numbers of IFN-γ spots per 0.25 × 106 PBMC after overnight activation with purified JCV and JCV peptide pools were 54 and 21, respectively (untreated well, 0 spots) in patient 1. In patient 2, purified JCV yielded 15 spots (untreated well, 0 spots).
ELISPOT analysis of the IFN-γ response to JCV, performed 2 days after the last natalizumab infusion, showed the presence of JCV-specific TEM in blood (Fig. 1C). Interestingly, circulating JCV-specific TEM numbers were lower than in the first PML patient, who may have had a higher level of JCV replication in the brain, as JCV PCR on CSF was positive in the first patient and negative in the second.
We also examined the presence of JCV-specific TEM by ELISPOT in a series of 62 MS patients with relapsing-remitting MS treated with natalizumab according to the European marketing terms, as well as in 35 MS patients who were not receiving any disease-modifying therapy, and a group of 40 healthy donors matched for age and sex with MS patients. Characteristics of the MS patients are shown in Table 1. The patients and healthy donors gave their written consent to participate in the study, which was approved by the ethics committee of Pitié-Salpêtrière Hospital, Paris. An ELISPOT response was considered positive if the corrected number of spots (number of spots in activated wells minus number of spots in untreated wells) was at least 10, with fewer than 10 spots in untreated wells.
Table 1.
Clinical features of the patients with multiple sclerosis
| Feature | Value |
|
|---|---|---|
| Natalizumab (n = 64 MS patients) | Control (n = 35 MS patients) | |
| Median age | 40 | 44 |
| Sex ratio (female to male) | 1.74 | 1.69 |
| Median Expanded Disability Status Scale score (range) | 3.5 (1–7.5) | 3.5 (1–6.5) |
| Duration of the disease ± SD (yrs) | 11.4 ± 5.2 | 10 ± 4.8 |
| No. (range) of natalizumab infusions | 24 (1–48) | |
| No. of patients sampled twice (interval between 1st and 2nd sample) | 41 (10.6 ± 2.8 mo) | |
| No. of patients sampled 3 times or more (interval between 2nd and 3rd sample) | 6 (10 ± 0.3 mo) | |
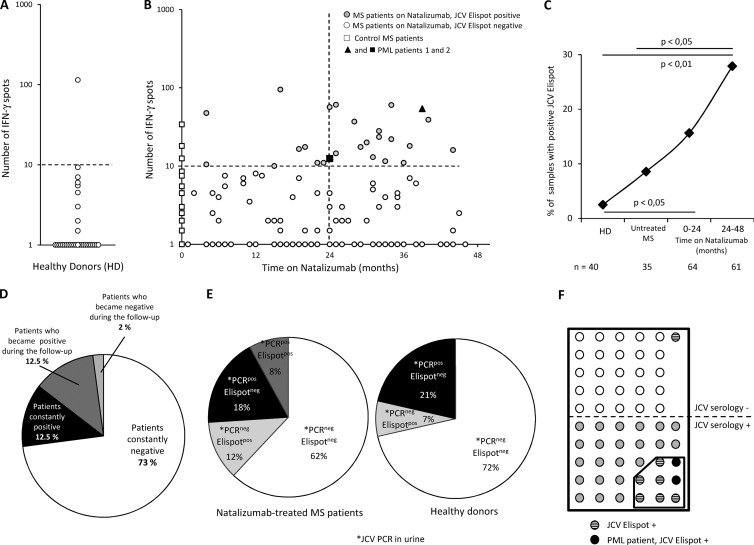
The proportion of positive samples was 2.5% in healthy donors (Fig. 2A). In the MS group, the percentage of positive patients rose with the time on natalizumab (Fig. 2B and C), from 8.8% in untreated MS patients to 15.9% among patients treated with natalizumab for less than 24 months and 27% among patients treated for more than 24 months (Fig. 2C) (significant for patients treated for more than 24 months versus untreated MS [Fisher's exact test, P < 0.05] and versus control subjects [P < 0.01]). This increase in the frequency of detection of JCV TEM responses suggested that prolonged natalizumab treatment favored JCV reactivation.
Fig 2.
Increased detection of JCV-specific TEM in patients on natalizumab. Panels A and B represent the number of IFN-γ spots per 0.25 × 106 PBMC after overnight activation with purified JC virus in 40 healthy donors (A) and in 99 MS patients according to the time on natalizumab (B). In panel B, the 2 patients with PML are indicated by a closed triangle and a closed square. The dashed line represents the positivity cutoff (see the text). Panel C represents the percentage of JCV-ELISPOT-positive samples in healthy donors (HD), control patients (Untreated MS), and patients treated with natalizumab for up to 2 years (0–24 months) or more (24–48 months). Some patients treated with natalizumab were tested at 2 or 3 time points. The number of blood samples analyzed is indicated below. Statistical analysis used Fisher's test. Panel D shows JCV ELISPOT results for patients who were tested at 2 or 3 time points. Panel E shows the results of urinary JCV PCR and JCV ELISPOT in 50 MS patients treated with natalizumab and in 14 healthy donors tested with both assays. Panel F shows results of JCV serology and JCV ELISPOT in 56 MS patients on natalizumab tested with both assays.
A subset of natalizumab-treated patients were sampled at least twice (see Table 1). As shown in Fig. 2D, most of these patients either became or remained positive during the sampling period.
The kidney is a major reservoir for JCV. A significant percentage of the general population excretes the virus in their urine but has no evidence of JCV in their blood (13–15). In addition, the presence of JCV DNA in the brain appears to be independent of its presence in the kidney (16). As shown in Fig. 2E, 60% of MS patients with detectable JCV TEM in blood had no detectable JCV in their urine, and 69% of patients with positive JCV PCR in urine had no detectable JCV TEM. JCV urinary excretion without detectable JCV TEM was also observed in healthy donors (Fig. 2E). This suggests that the presence of JCV replication in kidney does not necessarily lead to detectable JCV TEM in blood. Conversely, this raises the possibility that JCV TEM detection in blood may reflect ongoing extrarenal JCV replication.
In a study using a novel JCV enzyme-linked immunosorbent assay (ELISA) method (17), the estimated seroprevalence of JCV in MS patients treated with natalizumab was 53.6% (17). Seropositivity does not necessarily correlate with the presence of TEM responses, which is related to virus reactivation. Long-lived plasma cells may persist in bone marrow for long time periods, perhaps for decades, maintaining sustained serum antibody titers without active pathogen replication (18, 19). We used the same ELISA method to test 56 of our natalizumab-treated MS patients who had been tested by JCV ELISPOT, including the 2 PML patients. As shown in Fig. 2F, 53.6% of the patients were seropositive and 16.1% were ELISPOT positive. Twenty-seven percent of JCV-seropositive patients had detectable JCV TEM in their blood. Only one patient who was JCV ELISPOT positive was seronegative. According to Gorelik et al., the false-negative rate of the JCV serologic assay is 2.5% (17).
Our results show that the frequency of detection of JCV TEM responses in MS patients increases with the time on natalizumab. A previous study also showed an increase in T cell responses to JCV after 12 months of natalizumab therapy, but this study was limited to 18 months of treatment (20), whereas we found that the strongest increase in effector T cell responses occurred after 24 months.
Our results thus point to JCV reactivation during natalizumab therapy, including in extrarenal sites. JCV has been detected in the brain of HIV-seronegative individuals without PML (21). The diminished CNS immunosurveillance induced by natalizumab might favor local virus reactivation that may start as asymptomatic intermittent virus replication but could evolve toward sustained virus replication and then to PML. Virus reactivation could activate peripheral specific T lymphocytes, via antigen-presenting cells, in locations such as brain-draining cervical lymph nodes (22–24). As natalizumab targets the VLA-4 receptor, these specific cells cannot efficiently cross the blood-brain barrier and might thus accumulate in blood, facilitating their detection. PML is also a complication of late-stage HIV infection. Whether detection of JCV-specific effector CD4 and CD8 T cells may precede AIDS-associated PML is a point of interest that deserves further investigation. However, AIDS-associated PML is clearly different from natalizumab-induced PML. Most HIV-infected patients who develop PML have profound CD4 lymphopenia, which also alters CD8 T cell functionality through the lack of CD4 help (25). Moreover, unlike patients on natalizumab, there is no blockade of T cell trafficking through the blood-brain barrier, and this may prevent JCV-specific TEM accumulation in blood.
Together, our results suggest that functional assays capable of detecting JCV-specific TEM responses might help to identify patients at risk of developing PML during treatment with antibodies that inhibit lymphocyte trafficking through the blood-brain barrier.
Footnotes
Published ahead of print 20 March 2013
REFERENCES
- 1. Brew BJ, Davies NW, Cinque P, Clifford DB, Nath A. 2010. Progressive multifocal leukoencephalopathy and other forms of JC virus disease. Nat. Rev. Neurol. 6:667–679 [DOI] [PubMed] [Google Scholar]
- 2. Gasnault J, Taoufik Y. 2006. New trends in progressive multifocal leukoencephalopathy. Rev. Neurol. (Paris) 162:43–56 [DOI] [PubMed] [Google Scholar]
- 3. Clifford DB, De Luca A, Simpson DM, Arendt G, Giovannoni G, Nath A. 2010. Natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: lessons from 28 cases. Lancet Neurol. 9:438–446 [DOI] [PubMed] [Google Scholar]
- 4. Kleinschmidt-DeMasters BK, Tyler KL. 2005. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis. N. Engl. J. Med. 353:369–374 [DOI] [PubMed] [Google Scholar]
- 5. Langer-Gould A, Atlas SW, Green AJ, Bollen AW, Pelletier D. 2005. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N. Engl. J. Med. 353:375–381 [DOI] [PubMed] [Google Scholar]
- 6. Major EO. 2010. Progressive multifocal leukoencephalopathy in patients on immunomodulatory therapies. Annu. Rev. Med. 61:35–47 [DOI] [PubMed] [Google Scholar]
- 7. Van Assche G, Van Ranst M, Sciot R, Dubois B, Vermeire S, Noman M, Verbeeck J, Geboes K, Robberecht W, Rutgeerts P. 2005. Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn's disease. N. Engl. J. Med. 353:362–368 [DOI] [PubMed] [Google Scholar]
- 8. Keating SM, Bejon P, Berthoud T, Vuola JM, Todryk S, Webster DP, Dunachie SJ, Moorthy VS, McConkey SJ, Gilbert SC, Hill AV. 2005. Durable human memory T cells quantifiable by cultured enzyme-linked immunospot assays are induced by heterologous prime boost immunization and correlate with protection against malaria. J. Immunol. 175:5675–5680 [DOI] [PubMed] [Google Scholar]
- 9. Reece WH, Pinder M, Gothard PK, Milligan P, Bojang K, Doherty T, Plebanski M, Akinwunmi P, Everaere S, Watkins KR, Voss G, Tornieporth N, Alloueche A, Greenwood BM, Kester KE, McAdam KP, Cohen J, Hill AV. 2004. A CD4(+) T-cell immune response to a conserved epitope in the circumsporozoite protein correlates with protection from natural Plasmodium falciparum infection and disease. Nat. Med. 10:406–410 [DOI] [PubMed] [Google Scholar]
- 10. Todryk SM, Pathan AA, Keating S, Porter DW, Berthoud T, Thompson F, Klenerman P, Hill AV. 2009. The relationship between human effector and memory T cells measured by ex vivo and cultured ELISPOT following recent and distal priming. Immunology 128:83–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen Y, Bord E, Tompkins T, Miller J, Tan CS, Kinkel RP, Stein MC, Viscidi RP, Ngo LH, Koralnik IJ. 2009. Asymptomatic reactivation of JC virus in patients treated with natalizumab. N. Engl. J. Med. 361:1067–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401:708–712 [DOI] [PubMed] [Google Scholar]
- 13. Kitamura T, Aso Y, Kuniyoshi N, Hara K, Yogo Y. 1990. High incidence of urinary JC virus excretion in nonimmunosuppressed older patients. J. Infect. Dis. 161:1128–1133 [DOI] [PubMed] [Google Scholar]
- 14. Markowitz RB, Thompson HC, Mueller JF, Cohen JA, Dynan WS. 1993. Incidence of BK virus and JC virus viruria in human immunodeficiency virus-infected and -uninfected subjects. J. Infect. Dis. 167:13–20 [DOI] [PubMed] [Google Scholar]
- 15. Rossi A, Delbue S, Mazziotti R, Valli M, Borghi E, Mancuso R, Calvo MG, Ferrante P. 2007. Presence, quantitation and characterization of JC virus in the urine of Italian immunocompetent subjects. J. Med. Virol. 79:408–412 [DOI] [PubMed] [Google Scholar]
- 16. Bayliss J, Karasoulos T, Bowden S, Glogowski I, McLean CA. 2011. Immunosuppression increases latent infection of brain by JC polyomavirus. Pathology 43:362–367 [DOI] [PubMed] [Google Scholar]
- 17. Gorelik L, Lerner M, Bixler S, Crossman M, Schlain B, Simon K, Pace A, Cheung A, Chen LL, Berman M, Zein F, Wilson E, Yednock T, Sandrock A, Goelz SE, Subramanyam M. 2010. Anti-JC virus antibodies: implications for PML risk stratification. Ann. Neurol. 68:295–303 [DOI] [PubMed] [Google Scholar]
- 18. Amanna IJ, Carlson NE, Slifka MK. 2007. Duration of humoral immunity to common viral and vaccine antigens. N. Engl. J. Med. 357:1903–1915 [DOI] [PubMed] [Google Scholar]
- 19. Manz RA, Hauser AE, Hiepe F, Radbruch A. 2005. Maintenance of serum antibody levels. Annu. Rev. Immunol. 23:367–386 [DOI] [PubMed] [Google Scholar]
- 20. Jilek S, Jaquiery E, Hirsch HH, Lysandropoulos A, Canales M, Guignard L, Schluep M, Pantaleo G, Du Pasquier RA. 2010. Immune responses to JC virus in patients with multiple sclerosis treated with natalizumab: a cross-sectional and longitudinal study. Lancet Neurol. 9:264–272 [DOI] [PubMed] [Google Scholar]
- 21. Tan CS, Ellis LC, Wuthrich C, Ngo L, Broge TA, Jr, Saint-Aubyn J, Miller JS, Koralnik IJ. 2010. JC virus latency in the brain and extraneural organs of patients with and without progressive multifocal leukoencephalopathy. J. Virol. 84:9200–9209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de Vos AF, van Meurs M, Brok HP, Boven LA, Hintzen RQ, van der Valk P, Ravid R, Rensing S, Boon L, BAt Hart Laman JD. 2002. Transfer of central nervous system autoantigens and presentation in secondary lymphoid organs. J. Immunol. 169:5415–5423 [DOI] [PubMed] [Google Scholar]
- 23. Karman J, Ling C, Sandor M, Fabry Z. 2004. Initiation of immune responses in brain is promoted by local dendritic cells. J. Immunol. 173:2353–2361 [DOI] [PubMed] [Google Scholar]
- 24. Walter L, Albert ML. 2007. Cutting edge: cross-presented intracranial antigen primes CD8+ T cells. J. Immunol. 178:6038–6042 [DOI] [PubMed] [Google Scholar]
- 25. Gasnault J, Costagliola D, Hendel-Chavez H, Dulioust A, Pakianather S, Mazet AA, de Goer de Herve MG, Lancar R, Lascaux AS, Porte L, Delfraissy JF, Taoufik Y. 2011. Improved survival of HIV-1-infected patients with progressive multifocal leukoencephalopathy receiving early 5-drug combination antiretroviral therapy. PLoS One 6:e20967 doi:10.1371/journal.pone.0020967 [DOI] [PMC free article] [PubMed] [Google Scholar]