Abstract
Mucosal surfaces are not targeted by most human immunodeficiency virus type 1 (HIV-1) vaccines, despite being major routes for HIV-1 transmission. Here we report a novel vaccination regimen consisting of a mucosal prime with a modified replicating vaccinia virus Tiantan strain (MVTTSIVgpe) and an intramuscular boost with a nonreplicating adenovirus strain (Ad5SIVgpe). This regimen elicited robust cellular immune responses with enhanced magnitudes, sustainability, and polyfunctionality, as well as higher titers of neutralizing antibodies against the simian immunodeficiency virus SIVmac1A11 in rhesus monkeys. The reductions in peak and set-point viral loads were significant in most animals, with one other animal being protected fully from high-dose intrarectal inoculation of SIVmac239. Furthermore, the animals vaccinated with this regimen were healthy, while ∼75% of control animals developed simian AIDS. The protective effects correlated with the vaccine-elicited SIV-specific CD8+ T cell responses against Gag and Pol. Our study provides a novel strategy for developing an HIV-1 vaccine by using the combination of a replicating vector and mucosal priming.
INTRODUCTION
Mucosal tissues are the major entry points and first line of host defense against human immunodeficiency virus/simian immunodeficiency virus (HIV/SIV) transmission (1–3). Entry can occur in only a few hours and leads to a productive infection in activated memory CD4+ CCR5+ T cells, which are abundant in the mucosal lymph tissues (1, 4, 5). Profound CD4+ T cell lymphopenia soon develops, with viral persistence in the gut-associated lymphoid tissues (5–7). Therefore, a successful HIV/SIV vaccine must induce protective immunity to block initial acquisition and subsequent replication (8–10). The most important characteristics of sufficient protective immunity are the sustainable responses of cell-mediated and broadly neutralizing antibodies (bnAbs) (11–30). Several breakthroughs have been achieved based on nonhuman primate models in studies of various immunologic approaches against SIV challenge. For example, studies have shown passively infused bnAbs to be significantly more effective than nonneutralizing antibodies in reducing viral replication and protecting against viral transmission (31–36). However, candidate immunogens that could elicit such bnAbs are still being explored (37–39). Similar animal models have also been used to demonstrate that vector-based AIDS vaccines can induce robust, sustainable T cell and antibody responses, as well as effectively blocking viral acquisition, replication, and disease progression (12–15, 18, 22).
The strategies used in these studies, however, did not include replicating vectors with direct mucosal vaccination. Earlier work by the Robert-Guroff group showed that a mucosal prime with live recombinant adenovirus 5 (Ad5) plus protein or vector boosts can elicit potent T cell immunity, systemic and mucosal antibody-mediated neutralization, and antibody-dependent cellular cytotoxicity and transcytosis inhibition (30, 40–49). Unlike nonreplicating adenovirus vectors, which have a limited anatomic distribution, the replicating Ad5 vector can disseminate across multiple mucosal sites irrespective of delivery route (44). The major identified cell targets of the replicating Ad5 vaccine were tissue macrophages and myeloid dendritic cells (mDCs) (44). These and some of our earlier findings led us to postulate that initial vaccination with a replicating vector that engages the mucosal system in concert with a potent boosting agent may significantly bolster protective immunity against HIV/SIV mucosal transmission. To test this, we generated a modified replicating vaccinia virus Tiantan (MVTT) strain from its parental VTT strain through targeted gene knockout. The parental VTT strain was used from the 1950s to the 1980s to immunize approximately 300 million individuals during the smallpox eradication campaign in China. Compared with its parental VTT strain, MVTT has reduced neurotoxicity and pathogenicity (50–52). Furthermore, single-dose intranasal MVTT vaccination has clearly been shown to be safe and to effectively elicit protective immunity against pathogenic vaccinia virus Western Reverse (WR) challenge (52). Intranasal vaccination with MVTT expressing the severe acute respiratory syndrome coronavirus (SARS-CoV) spike protein (MVTT-S) produced higher titers of neutralizing antibodies than vaccination with the nonreplicating vaccinia virus MVA-S (51, 53). Based on these findings, we developed a recombinant replicating vector, MVTTSIVgpe, which expresses the three SIVmac239 structural proteins, Gag, Pol and Env, and investigated its protective immunogenicity in vivo in combination with an antigen-matched, nonreplicating vector, Ad5SIVgpe.
MATERIALS AND METHODS
Vectors and virus.
A recombinant modified replicating vaccinia virus Tiantan strain (MVTTSIVgpe) and a recombinant, nonreplicating adenovirus type 5 strain (Ad5SIVgpe) expressing the SIVmac239 Gag, Pol, and Env structural proteins were generated using the homologous recombination technology described previously (51, 54, 55). The original SIVmac239 stock was a generous gift of P. A. Marx and was further adapted in Chinese macaques by X. Wu (Chinese Academy of Medical Sciences, Beijing, China) prior to this study. The challenge stock was generated and titrated in Chinese rhesus monkey peripheral blood mononuclear cells (PBMCs).
Animals and vaccination.
A total of 20 Chinese rhesus monkeys that weighed 3 to 5 kg and were 3 to 6 years of age were used in two separate studies: study I and study II (Fig. 1). In study I, 12 macaques were divided into three groups: (i) 4 monkeys received MVTTSIVgpe (109 PFU in 1 ml of phosphate-buffered saline [PBS]) through intraoral (i.o.) (0.5 ml) and intranasal (i.n.) (0.5 ml) routes and Ad5SIVgpe (1011 viral particles [vp] in 1 ml of PBS) through intramuscular (i.m.) injection (MVTTioin+Adim regimen); (ii) 4 monkeys received a homologous prime and boost with the Ad5SIVgpe vaccine (1011 vp in 1 ml of PBS) through intramuscular injection (Adim+Adim regimen); and (iii) 4 monkeys received PBS through intramuscular injection, as a sham control group. Study II was then conducted to support study I outcomes and to rule out vector-mediated, nonspecific protective effects. In the second study, eight monkeys were divided into two groups: (i) four monkeys received the MVTTioin+Adim testing regimen as in study I, and (ii) four monkeys received an empty MVTT control vector (109 PFU) through intraoral (0.5 ml) and intranasal (0.5 ml) routes and an empty Ad5 control vector (1011 vp in 1 ml of PBS) through intramuscular injection (vMVTTioin+vAdim regimen) (Fig. 1). At either week 30 after the initial vaccination or week 24 after the final vaccination, each animal was challenged intrarectally with 5 × 105 50% tissue culture infective doses (TCID50) of Chinese rhesus monkey-adapted and neutralization-resistant SIVmac239. In all cases, the challenge virus stock was administered in 1 ml of PBS. Sequential peripheral blood samples were collected to evaluate the virologic and immunologic responses, following the experimental scheme highlighted in Fig. 1.
Fig 1.
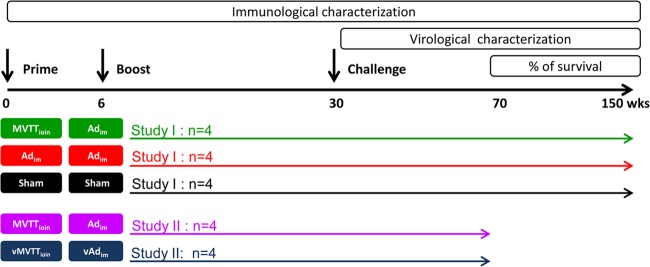
Immunization and challenge schedule for Chinese rhesus monkeys for study I and study II. Three groups (n = 4 per group) were used in study I, while two groups (n = 4 per group) were used in study II. Each group was color coded, and each animal is represented by a unique symbol in the other figures: emerald symbols represent the four animals in the MVTTioin+Adim group, red symbols represent the four animals in the Adim+Adim group, black symbols represent the four animals in the sham control group, purple symbols represent the four animals in the second MVTTioin+Adim group, and blue symbols represent the four animals in the vector control group.
All animals were housed in the Animal Experimental Center of the Guangzhou Institute of Biomedicine and Health, Chinese Academy of Sciences, and were free of infection with simian immunodeficiency virus (SIV), simian T cell lymphotropic virus (STLV-1), and simian retrovirus (SRV) before the initiation of experiments. The experimental protocols were all approved by the Institutional Animal Care and Use Committee (IACUC). The monkeys with simian AIDS were sacrificed humanely by euthanasia in accordance with our IACUC protocols. The symptoms that defined simian AIDS were severe diarrhea, wasting, weight loss, muscular atrophy, severe ulceration, and inverted CD4/CD8 ratios. Additional indicators included huddled posture, immobility, ruffled fur, failure to eat, and hypothermia (colonic temperature of <34°C). The method of euthanasia was intravenous injection of pentobarbital sodium (80 mg/kg of body weight).
SIV peptide pools and cellular immune assays.
Pools of overlapping SIVmac239-specific peptides covering the entire sequences of Gag, Pol, Env, Nef, Vif, Vpx, Vpr, Rev, and Tat were obtained through the AIDS Research and Reference Reagent Program, National Institutes of Health (NIH). These peptide fragments are 15 amino acids in length, with 11 overlapping residues. Peptide pools containing Gag, Pol, and Env peptides were used to evaluate immune responses against vaccine antigens throughout the vaccination and after live SIVmac239 challenge. Peptide pools of Nef, Vif, Vpx, Vpr, Rev, and Tat fragments were used to assess the immune responses against nonvaccine antigens after challenge. Pools were dissolved in dimethyl sulfoxide (DMSO) to a final concentration of 0.4 mg/peptide/ml before use. The cellular immune assays, conducted as previously described, included a gamma interferon enzyme-linked immunosorbent spot (IFN-γ ELISPOT) assay, multicolor intracellular cytokine staining (ICS) for T lymphocyte polyfunctionality, and carboxyfluorescein diacetate succinimidyl ester (CFSE) staining for ex vivo T cell proliferation (56). Multicolor ICS assays and detection of phenotypic markers of T central memory (CD28+ CD95+ cells) and T effector memory (TEM; CD28− CD95+ cells) were performed with the following monoclonal antibodies: anti-CD3–Pacific Blue, anti-CD4–AmCyan, anti-CD8–allophycocyanin (APC)–Cy7, anti-CD28–fluorescein isothiocyanate (FITC), anti-CD95–phycoerythrin (PE)–Cy5, anti-IFN-γ–PE, anti-tumor necrosis factor alpha (anti-TNF-α)–PE–Cy7, and anti-interleukin-2 (anti-IL-2)–APC (BD Pharmingen). Samples were analyzed with a FACSAria flow cytometer (BD Biosciences) and FlowJo software (version 7.6; Tree Star, Inc.). Numbers of circulating CD4+ and CD8+ T lymphocytes were determined using BD TruCount tubes according to the manufacturer's instructions (BD Biosciences).
Humoral immune assays.
The serum-binding antibodies against SIVmac239 were determined by an enzyme-linked immunosorbent assay targeting lysed SIVmac239 particles as described previously (56). nAbs against SIVmac239 and SIVmac1A11 were evaluated as a function of reductions in Tat-regulated luciferase reporter expression after a single round of infection of TZM-bl cells (AIDS Research and Reference Reagent Program, NIH), as described previously (54). Levels of nAbs against the Ad5 and MVTT vectors were measured as a 50% reduction in either reporter-secreted alkaline phosphatase (SEAP) or green fluorescent protein (GFP) activity, as described previously (52, 57).
Viral quantitation assay.
Plasma and PBMCs were collected following standard protocols. Levels of SIV RNA in plasma were quantified by real-time PCR as described previously (56). The assay detection limit was 100 copies per 1 ml plasma.
Data analysis.
Flow cytometry software analysis was performed using FlowJo 7.6 (Tree Star Inc.). Graphical representations were generated with GraphPad Prism 5.01 (GraphPad Software Inc., La Jolla, CA). The survival curves were analyzed by log rank (Mantel-Cox) tests, and immune correlates of protection were determined by Spearman rank correlation tests. Two-tailed P values were calculated for all analyses, and differences were considered statistically significant when P values were <0.05.
RESULTS
MVTTioin+Adim regimen elicits potent immune responses in rhesus monkeys.
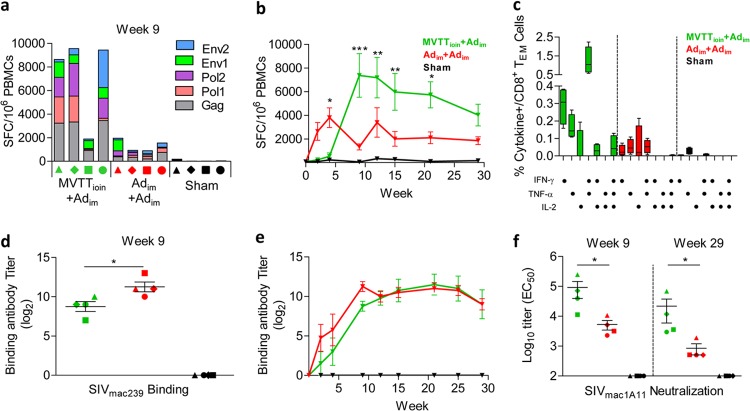
The MVTTioin+Adim regimen contained a combined intraoral (i.o) and intranasal (i.n.) mucosal prime with MVTTSIVgpe and an intramuscular (i.m.) Ad5SIVgpe boost, an approach designed to induce both cellular and humoral immune responses in Chinese rhesus monkeys. In study I, the MVTTioin+Adim regimen was compared against either the homologous Adim+Adim prime-boost with Ad5SIVgpe or the sham control regimen. All immunized macaques showed tolerance of the vaccination, with no signs of clinical illness. To evaluate cellular immune responses, ELISPOT assays for SIV-specific IFN-γ-secreting cells were performed on PBMCs obtained over the course of immunization (Fig. 2a and b). Four weeks after immunization, the initial priming with MVTTioin induced 538 ± 557 IFN-γ-secreting cells per million PBMCs, whereas that with Adim elicited 3,805 ± 1,664 IFN-γ-secreting cells per million PBMCs (Fig. 2b) (P < 0.05). Following the boost with Adim, however, the frequency of positive responses increased and was maintained at significantly higher levels in MVTTioin-primed macaques than in the Adim-primed group over multiple time points (Fig. 2b) (P < 0.05). The frequency of positive responses in the MVTTioin+Adim group peaked 9 weeks after initial immunization, at an average of 7,382 ± 3,685 IFN-γ-secreting cells per million PBMCs (Fig. 2b). Responses were predominately against Gag and Pol peptides, although an anti-Env response was also detected (Fig. 2a). In contrast, positive IFN-γ responses in the Adim+Adim group declined slightly after boosting (Fig. 2b), likely due to the blocking effect by the high levels of vector-specific neutralizing antibodies induced during the priming step (see Fig. S1 in the supplemental material). Between this point and viral challenge, the frequency of positive responses in the MVTTioin+Adim group remained significantly higher than that in the Adim+Adim group, except for the time point of week 29 after initial priming (Fig. 2b) (P < 0.05). Furthermore, compared with the Adim+Adim group, all animals but monkey 3 in the MVTTioin+Adim group maintained significantly higher levels of Gag-specific CD8+ TEM cell polyfunctionality, particularly for IFN-γ and TNF-α, but not IL-2, release (Fig. 2c) (P < 0.05).
Fig 2.
Immune characterization of the three groups in study I before challenge. (a and b) Temporal comparisons of SIV-specific spot-forming cells (SFC) per million PBMCs as measured by IFN-γ ELISPOT assay. (c) Comparison of percentages of CD8+ TEM cells in peripheral blood that secreted IFN-γ, TNF-α, and IL-2 after stimulation with the SIV Gag peptide pool. (d and e) Temporal comparisons of SIV-binding antibody responses (endpoint titers) against lysed SIVmac239 particles. (f) Comparison of nAb titers against SIVmac1A11 at the peak and 1 week before challenge. EC50, 50% effective concentration. Error bars in panels c to f represent standard errors of the means (SEM). *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Additionally, both the MVTTioin+Adim and Adim+Adim regimens elicited equivalent levels of SIVmac239-specific binding antibodies (Fig. 2d and e). Although higher levels of binding antibodies were found in the Adim+Adim group than in the MVTTioin+Adim group during the initial and boosting immunizations, such differences disappeared 12 weeks after the boost (Fig. 2d and e). However, 9 weeks after initial immunization and immediately preceding viral challenge (week 29), the MVTTioin+Adim regimen generated significantly higher levels of nAbs against the tier 1 virus SIVmac1A11 (Fig. 2f) (P < 0.05). No detectable nAbs against neutralization-resistant strain SIVmac239 were observed. Collectively, these results suggested that the MVTTioin+Adim regimen elicited higher levels of nAbs and cellular immune responses with more enhanced magnitudes, sustainability, and polyfunctionality than those with the Adim+Adim regimen in Chinese rhesus monkeys.
MVTTioin+Adim regimen elicits protective immunity against SIV challenge in rhesus monkeys.
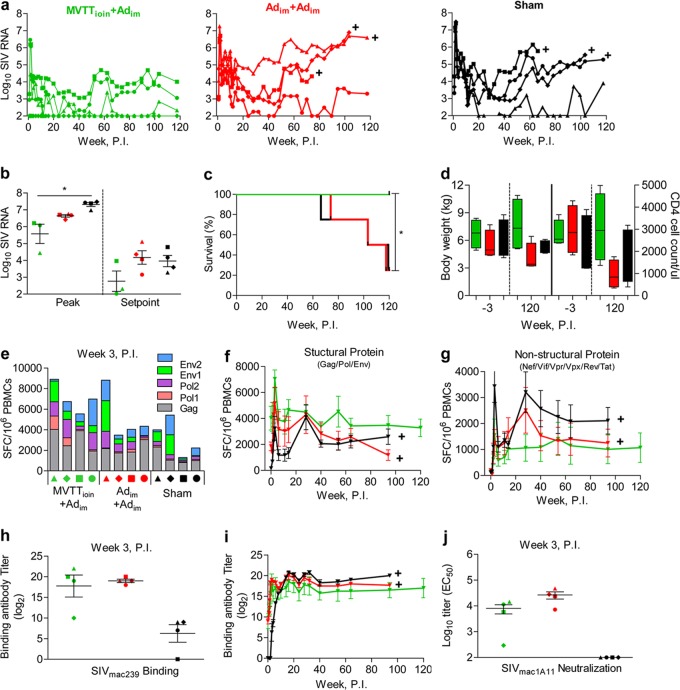
We next sought to determine whether the superior cellular and humoral responses elicited by the MVTTioin+Adim regimen would correlate with better control of pathogenic SIVmac239 infection. Twenty-four weeks after the boost, each monkey was challenged intrarectally with a single high dose of Chinese monkey-adapted pathogenic SIVmac239 (5 × 105 TCID50 per animal) (Fig. 1). All animals became infected, except for monkey 2 in the MVTTioin+Adim group. However, differences in peak and set-point viral loads were observed across all three groups (Fig. 3a). In the MVTTioin+Adim group, the infected monkeys showed average reductions in the peak and set-point viral loads of 1.74 log (5.57 ± 0.98 versus 7.31 ± 0.23 log copies of SIV RNA) and 1.20 log (2.76 ± 1.04 3.96 ± 0.66 log copies of SIV RNA), respectively, compared with sham controls (Fig. 3b) (P < 0.05). No such differences were detected between the Adim+Adim and sham control groups (Fig. 3b) (P > 0.05). This suggested a significant control of replication in the MVTTioin+Adim group during both the acute and chronic phases of infection. More importantly, at 120 weeks postinfection, all four monkeys vaccinated with the MVTTioin+Adim regimen remained clinically healthy, while three-fourths of the animals in the Adim+Adim and sham groups developed simian AIDS (Fig. 3c) (P < 0.05), as defined by symptoms of diarrhea, wasting and body weight loss, and CD4 T cell decline (Fig. 3d).
Fig 3.
Comparison of protective effects among the three groups of animals in study I after challenge. (a and b) Temporal comparisons of plasma SIV RNA copies at peak and set point and over the course of infection, up to week 120 after challenge. (c) Comparison of disease progression and survival up to week 120 after challenge. (d) Changes in body weight and CD4 T cell count 3 weeks before and 120 weeks after challenge. Values for the last time point before sacrifice were used for animals that were euthanized before 120 weeks after challenge. Temporal changes in SIV-specific IFN-γ-secreting cells against the vaccine antigens Gag, Pol, and Env (e and f) or against the nonvaccine antigens Nef, Tat, Rev, Vif, Vpr, and Vpx (g), as measured by ELISPOT assay, are shown. Temporal analyses of SIV-specific binding (h and i) and neutralizing (j) antibody responses after challenge are also shown. Plus symbols represent time points when animals were humanely euthanized after the onset of simian AIDS. The error bars in panels f to j represent SEM. *, P < 0.05. P.I., postinfection.
To further assess immune protection, sequential PBMC and plasma samples were examined for SIV-specific cellular and humoral immune responses after infection. SIVmac239 infection boosted the vaccine-elicited antigen-specific CD8+ T cell ELISPOT responses against the structural proteins Gag, Pol, and Env (Fig. 3e and f). Although the frequencies of positive responses for all three groups peaked at 3 weeks postinfection, response magnitudes were significantly higher in MVTTioin+Adim group animals than in Adim+Adim group animals (Fig. 3e and f). The frequency of positive responses at the peak was, on average, 7,045 ± 1,395 per million PBMCs for the MVTTioin+Adim group, 5,171 ± 2,461 for the Adim+Adim group, and 3,235 ± 1,839 for the sham control group (Fig. 3f) (P < 0.05). In the majority of animals, peak responses were primarily against Gag and Pol peptides, with Env responses detected in several animals (Fig. 3e). Furthermore, despite a decline in antigen-specific CD8+ T cell ELISPOT responses after the peak responses, IFN-γ-secreting cell levels remained relatively stable in the MVTTioin+Adim group, being, on average, above 3,801 ± 1,495 per million PBMCs throughout the subsequent phase of infection (Fig. 3f). It should be noted that there was a transient increase in IFN-γ-secreting cells in both the Adim+Adim and sham groups at 28 weeks postinfection (Fig. 3f). This coincided with a rise in viral plasma loads in both groups of animals (Fig. 3a). Interestingly, such biphasic cellular immune responses were more apparent against the nonvaccine antigens Nef, Vif, Vpr, Vpx, Rev, and Tat (Fig. 3g), reflecting the elevated responses triggered by the rise of viral replication in the Adim+Adim and sham groups. Collectively, these findings suggest that the MVTTioin+Adim and Adim+Adim vaccine regimens generated distinct immune response profiles, which may be a critical factor behind the observed differences in clinical outcomes. Finally, in terms of antibody responses after viral challenge, only minimal differences in anti-SIVmac1A11 binding and neutralizing antibody responses were observed between the two vaccine regimens, except in monkey 2 (Fig. 3h, i, and j). In monkey 2, no viral load (Fig. 3a), anti-SIV ELISPOT responses against nonstructural antigens, or boosted binding and neutralizing antibodies against SIVmac1A11 (Fig. 3h and j) were detectable, indicating a predisposed vaccine-boosted protection from infection.
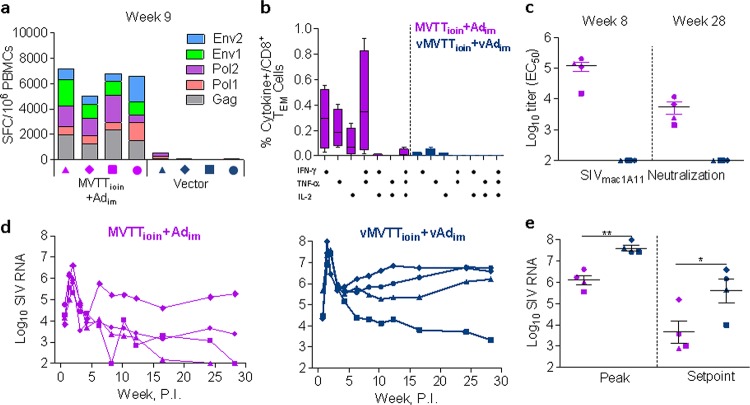
To further assess the enhanced immunogenicity and protective capacity of the MVTTioin+Adim regimen, study II was conducted, with the same vaccination and viral challenge schedule for an additional four macaques (Fig. 1). A second control group of four macaques was inoculated with empty MVTT and Ad5 viral vectors, following the previous routes and dosages (Fig. 1). As in study I, sequential PBMC and plasma samples were collected and used to monitor cellular and humoral immune responses during the trial period. Consistent with our initial results, the MVTTioin+Adim regimen elicited high levels of CD8+ T cell ELISPOT responses against Gag, Pol, and Env (Fig. 4a). The frequency of positive responses in the MVTTioin+Adim group peaked 9 weeks after initial immunization, at an average of 5,336 ± 1,459 IFN-γ-secreting cells per million PBMCs (Fig. 4a). Responses were predominantly targeted to Gag and Pol peptides, with an appreciable portion found against Env (Fig. 4a). Equivalent to the results of study I, high levels of Gag-specific CD8+ TEM cells secreting both IFN-γ and TNF-α were identified (Fig. 4b). In terms of antibody responses, high levels of neutralizing antibody titers against the tier 1 virus SIVmac1A11 were observed again at week 8 after initial immunization and preceding viral challenge (week 28) (Fig. 4c). More importantly, the regimen also reproduced the observed protection against the SIVmac239 intrarectal challenge, as demonstrated by 1.52-log (6.09 ± 0.44 versus 7.61 ± 0.27 log copies of SIV RNA) and 1.9-log (3.65 ± 1.06 versus 5.60 ± 1.14 log copies of SIV RNA) reductions in peak and set-point viral loads, respectively, compared with the vector control group (Fig. 4d and e) (P < 0.05). Together, studies I and II provide strong evidence that the MVTTioin+Adim regimen induces a superior, more potent and antigen-specific immune response against pathogenic SIVmac239 infection in Chinese rhesus monkeys than that induced by the Adim+Adim regimen.
Fig 4.
Immunologic and virologic characterization of two groups before and after challenge in study II. (a) Peak levels of SIV-specific IFN-γ ELISPOT responses to the MVTTioin+Adim or control vector regimen. (b) Comparison of Gag-specific CD8+ TEM polyfunctional responses in the peripheral blood with secretion of IFN-γ, TNF-α, and IL-2 between the two groups before viral challenge. (c) Comparison of neutralizing antibody responses against SIVmac1A11 between the two groups at peak and before viral challenge. (d and e) Temporal comparisons of plasma SIV RNA copies for up to 30 weeks after challenge or at the peak and set point. Error bars in panels b, c, and e represent SEM. *, P < 0.05; **, P < 0.01.
SIV-specific CD8+ T cell-mediated immunity against Gag and Pol is associated with protection.
Finally, we investigated the protective effects of the two regimens against SIVmac239 infection, with eight animals vaccinated with the MVTTioin+Adim regimen and four vaccinated with the Adim+Adim regimen. Regression analysis revealed that prior to viral challenge, the peak SIV-specific CD8+ T cell ELISPOT responses against Gag and Pol, but not Env, were negatively correlated with both the peak viral load (Fig. 5a) (P = 0.0005, P = 0.0074, and P = 0.1667) and the set-point viral load (Fig. 5b) (P = 0.0010, P = 0.0446, and P = 0.1908). The percentage of Gag-specific IFN-γ+ TNF-α+ CD8+ TEM cells before challenge was also associated with virologic control (Fig. 5c and d) (P = 0.0014 and P = 0.031). These findings indicate that SIV-specific CD8+ T cell-mediated immunity against Gag and Pol is associated with the control of SIVmac239 replication and attenuation of disease progression.
Fig 5.
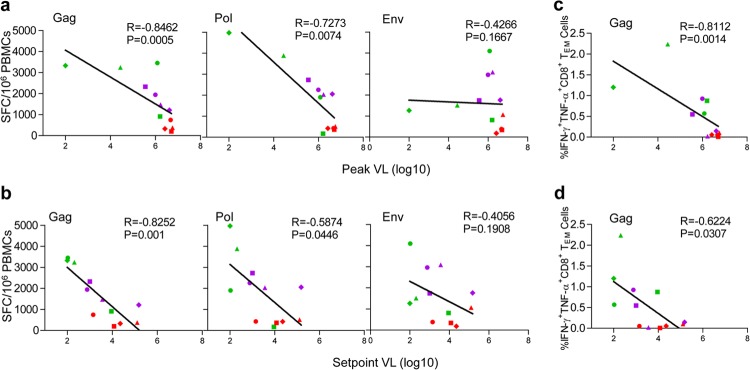
Analysis of immune correlates of protection in MVTTioin+Adim- and Adim+Adim-vaccinated animals. (a and b) Negative correlation between the peak and set-point viral loads and SIV-specific CD8+ T cell ELISPOT responses against Gag and Pol but not Env. (c and d) Negative correlation between the percentages of Gag-specific IFN-γ- and TNF-α-positive CD8+ TEM cells and peak and set-point viral loads.
DISCUSSION
We report here a potent replicating vaccinia virus/adenovirus vector-based vaccine regimen that directly primes the mucosal system for robust and sustainable immunity against neutralization-resistant SIVmac239 challenge in monkeys. The novelty of this regimen lies in the replicating nature of MVTT, an attenuated version of the parental VTT strain that was used for the eradication of smallpox in China. With the proven safety and efficacy of VTT in humans, a replicating vector was hypothesized to improve both the quantity and the quality of vaccine antigens through de novo synthesis and multiple yet limited rounds of replication that highly mimic the early steps in a wild-type infection. This hypothesis is well supported by the results presented in this work and elsewhere for the use of a replicating recombinant Ad5 vector to prime the mucosal system (30, 40–49). Among the eight MVTTioin+Adim-vaccinated monkeys, one was completely protected from infection, while the others displayed significantly lower peak and set-point viral loads than those of the control groups. Although some other vector-based strategies have obtained partial protection against viral acquisition and/or replication (12–15, 25, 58, 59), no previous vaccine candidates have shown efficacy similar to that reported here against a single high-dose mucosal challenge (5 × 105 TCID50 per animal). Indeed, prior studies applied multiple low-dose challenges that were about 1,000- to 10,000-fold lower than that in this report (12, 13, 27–30). The drastic dosage difference between our study and others highlights the exceptionally high levels of protective immunity induced by our vaccine regimen.
We found that SIV-specific CD8+ T cell ELISPOT responses against Gag and Pol, but not Env, and Gag-specific IFN-γ+ TNF-α+ CD8+ TEM cell responses were associated with the virologic control after challenge. These findings are unique compared with other studies, for two reasons. First, the SIV-specific CD8+ T cell ELISPOT responses induced by the MVTTioin+Adim regimen were about 2- to 3-fold higher than those in previous reports (12, 13, 15, 22, 58); second, the MVTTioin+Adim regimen combined key correlates induced by other promising vaccine approaches, such as persistently replicating cytomegalovirus-based vaccines, nonreplicating poxvirus and adenovirus vector-based vaccines (12, 13), and a recombinant yellow fever 17D (rYF17D) prime vaccine boosted with recombinant Ad5 (59). For instance, a SIV vaccine based on persistently replicating cytomegalovirus from rhesus monkeys (RhCMV) was able to induce persistent, high-frequency SIV-specific TEM responses, which is one of the immunologic correlates identified for the robust control of SIVmac239 challenge (13, 14). In particular, the CD8+ TEM cells induced by RhCMV were associated with higher levels of IFN-γ and TNF-α but not IL-2 (13, 14), as in our study. This polarized TEM phenotype may indicate cytotoxic potential upon antigen recognition and stimulation. Furthermore, SIV vaccine regimens based on nonreplicating poxvirus and adenovirus vectors have been used to induce protective immunity in monkeys, with protection against viral acquisition correlated with the Env-specific binding and neutralizing activity, as well as attenuated replication and disease progression with Gag ELISPOT responses (12). These changes are consistent with those induced by our vaccine strategy, although we identified CD8+ T cell ELISPOT responses against Pol as an additional correlate of protection.
Several caveats of our study and related conclusions should be noted. First, the number of animals was relatively small, limiting the statistical power of these findings. Future studies with a reasonably large number of animals are necessary to more concretely characterize the protective immunity associated with the MVTTioin+Adim regimen. Furthermore, it would be ideal to examine the protective potential of the MVTTioin+Adim regimen against virus swarms through repeated low-dose rather than single high-dose challenge, to better mimic viral inoculums during natural HIV/SIV transmission. Second, it is uncertain whether any of the observed SIV-specific immune protection against high-dose viral challenge was due to unique genotypic and/or phenotypic features of Chinese rhesus monkeys. In particular, the relatively low immunogenicity of the Adim+Adim regimen in Chinese monkeys was somewhat surprising. High titers of neutralizing antibody against the Ad5 vector induced in the priming step may have attenuated the boosting effect, resulting in decreased rather than increased SIV-specific immune responses. Why this restriction was biased toward cellular but not humoral responses requires future investigation. Furthermore, clear differences in major histocompatibility complex (MHC) allele compositions (60), as well as rates of disease progression after SIV infection (61), have been reported between Indian and Chinese rhesus monkeys. In general, Indian monkeys have shown progression to AIDS in a relatively short period, whereas Chinese monkeys have demonstrated a more prolonged progression similar to that of HIV-1 infection in humans (61). We should note that none of our animals carried the protective allele, Mamu-A1*01, found in Indian monkeys. Third, it would be ideal to use a replicating recombinant Ad5 intraoral and intranasal prime plus a nonreplicating Ad5 intramuscular boost as an additional control for the MVTTioin+Adim regimen, to more precisely evaluate the attributed immune responses induced by the replicating MVTTSIVgpe strain. Fourth, although intraoral and intranasal immunization was used in this study, we decided not to collect mucosal samples for virologic and immunologic characterization in order to maintain the integrity of mucosal surfaces for subsequent challenge. It is therefore uncertain whether immune responses specific to the mucosal surface correlated with protection and disease attenuation in infected animals. Fifth, although our results clearly show that the MVTTioin+Adim regimen is superior to the Adim+Adim regimen, it is uncertain to what extent this can be attributed to the use of heterologous virus vectors for the prime and boost versus mucosal priming with MVTT per se. Future studies comparing this approach with other routes of initial priming will provide a more definitive answer to this question. Nevertheless, the exceptional protective capability and unique immunologic correlates induced by the MVTTioin+Adim regimen highlight the potential of a combination of mucosal priming with a replicating vaccinia virus and a nonreplicating adenovirus boost. It should serve as an important, novel strategy for the development of an effective vaccine against HIV-1 infection.
Supplementary Material
ACKNOWLEDGMENTS
We thank Yichu Liu, Xuehua Zheng, Maochao Zhang, Pingchao Li, Xin Ma, Li Qin, Haixiang Zhang, Yi Zheng, and the staff at the Animal Center of GIBH for their excellent technical assistance. We also thank Lizhi Qu, Weimin Zhang, Zhi Wang, Xiangjie Feng, and Xiangxing Zhu for their discussions and advice. Many thanks are also given to Sheri A. Dubey and Kara Cox at Merck Co. USA for their technical support with the ELISPOT, ICS, and quantitative adenovirus neutralization assays and to the AIDS Research and Reference Reagent Program of the NIH for providing SIV Gag, Pol, and Env and other peptide pools. We also thank Kelly C. Arledge for her help in editing our manuscript.
This work was supported by the National Science and Technology Major Project on the development of novel mucosal vaccines against HIV-1 infection (grants 2008ZX10001-011 and 2012ZX10001-009), the HKU-UDF and LSK Faculty of Medicine matching fund to the HKU AIDS Institute, Hong Kong RGC (grant HKU 762208 M) and RGC GRF (grant HKU 762811 M), the Knowledge Innovation Program of the Chinese Academy of Sciences (grants KSCX1-YW-10 and KSCX2-EW-J-27), the National Natural Science Foundation of China (grants 81000737 and 30825035), the Tsinghua University Initiative Scientific Research Program and Tsinghua Yue-Yuen Medical Sciences Fund, the Guangdong Natural Science Foundation (grant S2011010004564), and the Bureau of Science and Technology of Guangzhou Municipality S&T Support Program (grant 2010J-E381).
Footnotes
Published ahead of print 13 March 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.03247-12.
REFERENCES
- 1. Haase AT. 2011. Early events in sexual transmission of HIV and SIV and opportunities for interventions. Annu. Rev. Med. 62:127–139 [DOI] [PubMed] [Google Scholar]
- 2. Keele BF, Estes JD. 2011. Barriers to mucosal transmission of immunodeficiency viruses. Blood 118:839–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pope M, Haase AT. 2003. Transmission, acute HIV-1 infection and the quest for strategies to prevent infection. Nat. Med. 9:847–852 [DOI] [PubMed] [Google Scholar]
- 4. Li Q, Skinner PJ, Ha SJ, Duan L, Mattila TL, Hage A, White C, Barber DL, O'Mara L, Southern PJ, Reilly CS, Carlis JV, Miller CJ, Ahmed R, Haase AT. 2009. Visualizing antigen-specific and infected cells in situ predicts outcomes in early viral infection. Science 323:1726–1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Veazey RS, DeMaria M, Chalifoux LV, Shvetz DE, Pauley DR, Knight HL, Rosenzweig M, Johnson RP, Desrosiers RC, Lackner AA. 1998. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280:427–431 [DOI] [PubMed] [Google Scholar]
- 6. Bosinger SE, Sodora DL, Silvestri G. 2011. Generalized immune activation and innate immune responses in simian immunodeficiency virus infection. Curr. Opin. HIV AIDS 6:411–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brenchley JM, Douek DC. 2008. The mucosal barrier and immune activation in HIV pathogenesis. Curr. Opin. HIV AIDS 3:356–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Belyakov IM, Ahlers JD. 2012. Mucosal immunity and HIV-1 infection: applications for mucosal AIDS vaccine development. Curr. Top. Microbiol. Immunol. 354:157–179 [DOI] [PubMed] [Google Scholar]
- 9. Letvin NL. 2006. Progress and obstacles in the development of an AIDS vaccine. Nat. Rev. Immunol. 6:930–939 [DOI] [PubMed] [Google Scholar]
- 10. McMichael AJ, Borrow P, Tomaras GD, Goonetilleke N, Haynes BF. 2010. The immune response during acute HIV-1 infection: clues for vaccine development. Nat. Rev. Immunol. 10:11–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barouch DH. 2008. Challenges in the development of an HIV-1 vaccine. Nature 455:613–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barouch DH, Liu J, Li H, Maxfield LF, Abbink P, Lynch DM, Iampietro MJ, SanMiguel A, Seaman MS, Ferrari G, Forthal DN, Ourmanov I, Hirsch VM, Carville A, Mansfield KG, Stablein D, Pau MG, Schuitemaker H, Sadoff JC, Billings EA, Rao M, Robb ML, Kim JH, Marovich MA, Goudsmit J, Michael NL. 2012. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature 482:89–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, Coyne-Johnson L, Whizin N, Oswald K, Shoemaker R, Swanson T, Legasse AW, Chiuchiolo MJ, Parks CL, Axthelm MK, Nelson JA, Jarvis MA, Piatak M, Jr, Lifson JD, Picker LJ. 2011. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature 473:523–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hansen SG, Vieville C, Whizin N, Coyne-Johnson L, Siess DC, Drummond DD, Legasse AW, Axthelm MK, Oswald K, Trubey CM, Piatak M, Jr, Lifson JD, Nelson JA, Jarvis MA, Picker LJ. 2009. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat. Med. 15:293–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu J, O'Brien KL, Lynch DM, Simmons NL, La Porte A, Riggs AM, Abbink P, Coffey RT, Grandpre LE, Seaman MS, Landucci G, Forthal DN, Montefiori DC, Carville A, Mansfield KG, Havenga MJ, Pau MG, Goudsmit J, Barouch DH. 2009. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature 457:87–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao HX, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH. 2012. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N. Engl. J. Med. 366:1275–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Montefiori DC, Karnasuta C, Huang Y, Ahmed H, Gilbert P, de Souza MS, McLinden R, Tovanabutra S, Laurence-Chenine A, Sanders-Buell E, Moody MA, Bonsignori M, Ochsenbauer C, Kappes J, Tang H, Greene K, Gao H, Labranche CC, Andrews C, Polonis VR, Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Self SG, Berman PW, Francis D, Sinangil F, Lee C, Tartaglia J, Robb ML, Haynes BF, Michael NL, Kim JH. 2012. Magnitude and breadth of the neutralizing antibody response in the RV144 and Vax003 HIV-1 vaccine efficacy trials. J. Infect. Dis. 206:431–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barnett SW, Burke B, Sun Y, Kan E, Legg H, Lian Y, Bost K, Zhou F, Goodsell A, Zur Megede J, Polo J, Donnelly J, Ulmer J, Otten GR, Miller CJ, Vajdy M, Srivastava IK. 2010. Antibody-mediated protection against mucosal simian-human immunodeficiency virus challenge of macaques immunized with alphavirus replicon particles and boosted with trimeric envelope glycoprotein in MF59 adjuvant. J. Virol. 84:5975–5985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Flatz L, Cheng C, Wang L, Foulds K, Ko SY, Kong WP, Roychoudhuri R, Shi W, Bao S, Todd JP, Asmal M, Shen L, Donaldson M, Schmidt SD, Gall J, Pinschewer DD, Letvin NL, Rao S, Mascola JR, Roederer M, Nabel GJ. 2012. Gene-based vaccination with a mismatched envelope protects against simian immunodeficiency virus infection in nonhuman primates. J. Virol. 86:7760–7770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Horton H, Vogel TU, Carter DK, Vielhuber K, Fuller DH, Shipley T, Fuller JT, Kunstman KJ, Sutter G, Montefiori DC, Erfle V, Desrosiers RC, Wilson N, Picker LJ, Wolinsky SM, Wang C, Allison DB, Watkins DI. 2002. Immunization of rhesus macaques with a DNA prime/modified vaccinia virus Ankara boost regimen induces broad simian immunodeficiency virus (SIV)-specific T-cell responses and reduces initial viral replication but does not prevent disease progression following challenge with pathogenic SIVmac239. J. Virol. 76:7187–7202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lakhashe SK, Velu V, Sciaranghella G, Siddappa NB, Dipasquale JM, Hemashettar G, Yoon JK, Rasmussen RA, Yang F, Lee SJ, Montefiori DC, Novembre FJ, Villinger F, Amara RR, Kahn M, Hu SL, Li S, Li Z, Frankel FR, Robert-Guroff M, Johnson WE, Lieberman J, Ruprecht RM. 2011. Prime-boost vaccination with heterologous live vectors encoding SIV gag and multimeric HIV-1 gp160 protein: efficacy against repeated mucosal R5 clade C SHIV challenges. Vaccine 29:5611–5622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Letvin NL, Rao SS, Montefiori DC, Seaman MS, Sun Y, Lim SY, Yeh WW, Asmal M, Gelman RS, Shen L, Whitney JB, Seoighe C, Lacerda M, Keating S, Norris PJ, Hudgens MG, Gilbert PB, Buzby AP, Mach LV, Zhang J, Balachandran H, Shaw GM, Schmidt SD, Todd JP, Dodson A, Mascola JR, Nabel GJ. 2011. Immune and genetic correlates of vaccine protection against mucosal infection by SIV in monkeys. Sci. Transl. Med. 3:81ra36 doi:10.1126/scitranslmed.3002351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yamamoto T, Johnson MJ, Price DA, Wolinsky DI, Almeida JR, Petrovas C, Nason M, Yeh WW, Shen L, Roederer M, Rao SS, McDermott AB, Lefebvre F, Nabel GJ, Haddad EK, Letvin NL, Douek DC, Koup RA. 2012. Virus inhibition activity of effector memory CD8(+) T cells determines simian immunodeficiency virus load in vaccinated monkeys after vaccine breakthrough infection. J. Virol. 86:5877–5884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Genesca M, Miller CJ. 2010. Use of nonhuman primate models to develop mucosal AIDS vaccines. Curr. HIV AIDS Rep. 7:19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Genesca M, Rourke T, Li J, Bost K, Chohan B, McChesney MB, Miller CJ. 2007. Live attenuated lentivirus infection elicits polyfunctional simian immunodeficiency virus Gag-specific CD8+ T cells with reduced apoptotic susceptibility in rhesus macaques that control virus replication after challenge with pathogenic SIVmac239. J. Immunol. 179:4732–4740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stone M, Ma ZM, Genesca M, Fritts L, Blozois S, McChesney MB, Miller CJ. 2009. Limited dissemination of pathogenic SIV after vaginal challenge of rhesus monkeys immunized with a live, attenuated lentivirus. Virology 392:260–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alpert MD, Rahmberg AR, Neidermyer W, Ng SK, Carville A, Camp JV, Wilson RL, Piatak M, Jr, Mansfield KG, Li W, Miller CJ, Lifson JD, Kozlowski PA, Evans DT. 2010. Envelope-modified single-cycle simian immunodeficiency virus selectively enhances antibody responses and partially protects against repeated, low-dose vaginal challenge. J. Virol. 84:10748–10764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Qureshi H, Ma ZM, Huang Y, Hodge G, Thomas MA, DiPasquale J, DeSilva V, Fritts L, Bett AJ, Casimiro DR, Shiver JW, Robert-Guroff M, Robertson MN, McChesney MB, Gilbert PB, Miller CJ. 2012. Low-dose penile SIVmac251 exposure of rhesus macaques infected with adenovirus type 5 (Ad5) and then immunized with a replication-defective Ad5-based SIV gag/pol/nef vaccine recapitulates the results of the phase IIb step trial of a similar HIV-1 vaccine. J. Virol. 86:2239–2250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reynolds MR, Weiler AM, Piaskowski SM, Kolar HL, Hessell AJ, Weiker M, Weisgrau KL, Leon EJ, Rogers WE, Makowsky R, McDermott AB, Boyle R, Wilson NA, Allison DB, Burton DR, Koff WC, Watkins DI. 2010. Macaques vaccinated with simian immunodeficiency virus SIVmac239Delta nef delay acquisition and control replication after repeated low-dose heterologous SIV challenge. J. Virol. 84:9190–9199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xiao P, Patterson LJ, Kuate S, Brocca-Cofano E, Thomas MA, Venzon D, Zhao J, DiPasquale J, Fenizia C, Lee EM, Kalisz I, Kalyanaraman VS, Pal R, Montefiori D, Keele BF, Robert-Guroff M. 2012. Replicating adenovirus-simian immunodeficiency virus (SIV) recombinant priming and envelope protein boosting elicits localized, mucosal IgA immunity in rhesus macaques correlated with delayed acquisition following a repeated low-dose rectal SIVmac251 challenge. J. Virol. 86:4644–4657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Burton DR, Hessell AJ, Keele BF, Klasse PJ, Ketas TA, Moldt B, Dunlop DC, Poignard P, Doyle LA, Cavacini L, Veazey RS, Moore JP. 2011. Limited or no protection by weakly or nonneutralizing antibodies against vaginal SHIV challenge of macaques compared with a strongly neutralizing antibody. Proc. Natl. Acad. Sci. U. S. A. 108:11181–11186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hessell AJ, Rakasz EG, Poignard P, Hangartner L, Landucci G, Forthal DN, Koff WC, Watkins DI, Burton DR. 2009. Broadly neutralizing human anti-HIV antibody 2G12 is effective in protection against mucosal SHIV challenge even at low serum neutralizing titers. PLoS Pathog. 5:e1000433 doi:10.1371/journal.ppat.1000433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mascola JR, Stiegler G, VanCott TC, Katinger H, Carpenter CB, Hanson CE, Beary H, Hayes D, Frankel SS, Birx DL, Lewis MG. 2000. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat. Med. 6:207–210 [DOI] [PubMed] [Google Scholar]
- 34. Parren PW, Marx PA, Hessell AJ, Luckay A, Harouse J, Cheng-Mayer C, Moore JP, Burton DR. 2001. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J. Virol. 75:8340–8347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ruprecht RM, Hofmann-Lehmann R, Smith-Franklin BA, Rasmussen RA, Liska V, Vlasak J, Xu W, Baba TW, Chenine AL, Cavacini LA, Posner MR, Katinger H, Stiegler G, Bernacky BJ, Rizvi TA, Schmidt R, Hill LR, Keeling ME, Montefiori DC, McClure HM. 2001. Protection of neonatal macaques against experimental SHIV infection by human neutralizing monoclonal antibodies. Transfus. Clin. Biol. 8:350–358 [DOI] [PubMed] [Google Scholar]
- 36. Veazey RS, Shattock RJ, Pope M, Kirijan JC, Jones J, Hu Q, Ketas T, Marx PA, Klasse PJ, Burton DR, Moore JP. 2003. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat. Med. 9:343–346 [DOI] [PubMed] [Google Scholar]
- 37. Azoitei ML, Ban YE, Julien JP, Bryson S, Schroeter A, Kalyuzhniy O, Porter JR, Adachi Y, Baker D, Pai EF, Schief WR. 2012. Computational design of high-affinity epitope scaffolds by backbone grafting of a linear epitope. J. Mol. Biol. 415:175–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Azoitei ML, Correia BE, Ban YE, Carrico C, Kalyuzhniy O, Chen L, Schroeter A, Huang PS, McLellan JS, Kwong PD, Baker D, Strong RK, Schief WR. 2011. Computation-guided backbone grafting of a discontinuous motif onto a protein scaffold. Science 334:373–376 [DOI] [PubMed] [Google Scholar]
- 39. Diskin R, Scheid JF, Marcovecchio PM, West AP, Jr, Klein F, Gao H, Gnanapragasam PN, Abadir A, Seaman MS, Nussenzweig MC, Bjorkman PJ. 2011. Increasing the potency and breadth of an HIV antibody by using structure-based rational design. Science 334:1289–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Demberg T, Florese RH, Heath MJ, Larsen K, Kalisz I, Kalyanaraman VS, Lee EM, Pal R, Venzon D, Grant R, Patterson LJ, Korioth-Schmitz B, Buzby A, Dombagoda D, Montefiori DC, Letvin NL, Cafaro A, Ensoli B, Robert-Guroff M. 2007. A replication-competent adenovirus-human immunodeficiency virus (Ad-HIV) tat and Ad-HIV env priming/Tat and envelope protein boosting regimen elicits enhanced protective efficacy against simian/human immunodeficiency virus SHIV89.6P challenge in rhesus macaques. J. Virol. 81:3414–3427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gomez-Roman VR, Grimes GJ, Jr, Potti GK, Peng B, Demberg T, Gravlin L, Treece J, Pal R, Lee EM, Alvord WG, Markham PD, Robert-Guroff M. 2006. Oral delivery of replication-competent adenovirus vectors is well tolerated by SIV- and SHIV-infected rhesus macaques. Vaccine 24:5064–5072 [DOI] [PubMed] [Google Scholar]
- 42. Malkevitch N, Patterson LJ, Aldrich K, Richardson E, Alvord WG, Robert-Guroff M. 2003. A replication competent adenovirus 5 host range mutant-simian immunodeficiency virus (SIV) recombinant priming/subunit protein boosting vaccine regimen induces broad, persistent SIV-specific cellular immunity to dominant and subdominant epitopes in Mamu-A*01 rhesus macaques. J. Immunol. 170:4281–4289 [DOI] [PubMed] [Google Scholar]
- 43. Malkevitch NV, Patterson LJ, Aldrich MK, Wu Y, Venzon D, Florese RH, Kalyanaraman VS, Pal R, Lee EM, Zhao J, Cristillo A, Robert-Guroff M. 2006. Durable protection of rhesus macaques immunized with a replicating adenovirus-SIV multigene prime/protein boost vaccine regimen against a second SIVmac251 rectal challenge: role of SIV-specific CD8+ T cell responses. Virology 353:83–98 [DOI] [PubMed] [Google Scholar]
- 44. Patterson LJ, Kuate S, Daltabuit-Test M, Li Q, Xiao P, McKinnon K, DiPasquale J, Cristillo A, Venzon D, Haase A, Robert-Guroff M. 2012. Replicating adenovirus-simian immunodeficiency virus (SIV) vectors efficiently prime SIV-specific systemic and mucosal immune responses by targeting myeloid dendritic cells and persisting in rectal macrophages, regardless of immunization route. Clin. Vaccine Immunol. 19:629–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Patterson LJ, Malkevitch N, Venzon D, Pinczewski J, Gomez-Roman VR, Wang L, Kalyanaraman VS, Markham PD, Robey FA, Robert-Guroff M. 2004. Protection against mucosal simian immunodeficiency virus SIV(mac251) challenge by using replicating adenovirus-SIV multigene vaccine priming and subunit boosting. J. Virol. 78:2212–2221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhao J, Pinczewski J, Gomez-Roman VR, Venzon D, Kalyanaraman VS, Markham PD, Aldrich K, Moake M, Montefiori DC, Lou Y, Pavlakis GN, Robert-Guroff M. 2003. Improved protection of rhesus macaques against intrarectal simian immunodeficiency virus SIV(mac251) challenge by a replication-competent Ad5hr-SIVenv/rev and Ad5hr-SIVgag recombinant priming/gp120 boosting regimen. J. Virol. 77:8354–8365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhou Q, Hidajat R, Peng B, Venzon D, Aldrich MK, Richardson E, Lee EM, Kalyanaraman VS, Grimes G, Gomez-Roman VR, Summers LE, Malkevich N, Robert-Guroff M. 2007. Comparative evaluation of oral and intranasal priming with replication-competent adenovirus 5 host range mutant (Ad5hr)-simian immunodeficiency virus (SIV) recombinant vaccines on immunogenicity and protective efficacy against SIV(mac251). Vaccine 25:8021–8035 [DOI] [PubMed] [Google Scholar]
- 48. Lubeck MD, Natuk R, Myagkikh M, Kalyan N, Aldrich K, Sinangil F, Alipanah S, Murthy SC, Chanda PK, Nigida SM, Jr, Markham PD, Zolla-Pazner S, Steimer K, Wade M, Reitz MS, Jr, Arthur LO, Mizutani S, Davis A, Hung PP, Gallo RC, Eichberg J, Robert-Guroff M. 1997. Long-term protection of chimpanzees against high-dose HIV-1 challenge induced by immunization. Nat. Med. 3:651–658 [DOI] [PubMed] [Google Scholar]
- 49. Peng B, Wang LR, Gomez-Roman VR, Davis-Warren A, Montefiori DC, Kalyanaraman VS, Venzon D, Zhao J, Kan E, Rowell TJ, Murthy KK, Srivastava I, Barnett SW, Robert-Guroff M. 2005. Replicating rather than nonreplicating adenovirus-human immunodeficiency virus recombinant vaccines are better at eliciting potent cellular immunity and priming high-titer antibodies. J. Virol. 79:10200–10209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fang Q, Yang L, Zhu W, Liu L, Wang H, Yu W, Xiao G, Tien P, Zhang L, Chen Z. 2005. Host range, growth property, and virulence of the smallpox vaccine: vaccinia virus Tian Tan strain. Virology 335:242–251 [DOI] [PubMed] [Google Scholar]
- 51. Chen Z, Zhang L, Qin C, Ba L, Yi CE, Zhang F, Wei Q, He T, Yu W, Yu J, Gao H, Tu X, Gettie A, Farzan M, Yuen KY, Ho DD. 2005. Recombinant modified vaccinia virus Ankara expressing the spike glycoprotein of severe acute respiratory syndrome coronavirus induces protective neutralizing antibodies primarily targeting the receptor binding region. J. Virol. 79:2678–2688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yu W, Fang Q, Zhu W, Wang H, Tien P, Zhang L, Chen Z. 2010. One time intranasal vaccination with a modified vaccinia Tiantan strain MVTT(ZCI) protects animals against pathogenic viral challenge. Vaccine 28:2088–2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Huang X, Lu B, Yu W, Fang Q, Liu L, Zhuang K, Shen T, Wang H, Tian P, Zhang L, Chen Z. 2009. A novel replication-competent vaccinia vector MVTT is superior to MVA for inducing high levels of neutralizing antibody via mucosal vaccination. PLoS One 4:e4180 doi:10.1371/journal.pone.0004180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chen Z, Huang Y, Zhao X, Ba L, Zhang W, Ho DD. 2008. Design, construction, and characterization of a multigenic modified vaccinia Ankara candidate vaccine against human immunodeficiency virus type 1 subtype C/B′. J. Acquir. Immune Defic. Syndr. 47:412–421 [DOI] [PubMed] [Google Scholar]
- 55. Shiver JW, Fu TM, Chen L, Casimiro DR, Davies ME, Evans RK, Zhang ZQ, Simon AJ, Trigona WL, Dubey SA, Huang L, Harris VA, Long RS, Liang X, Handt L, Schleif WA, Zhu L, Freed DC, Persaud NV, Guan L, Punt KS, Tang A, Chen M, Wilson KA, Collins KB, Heidecker GJ, Fernandez VR, Perry HC, Joyce JG, Grimm KM, Cook JC, Keller PM, Kresock DS, Mach H, Troutman RD, Isopi LA, Williams DM, Xu Z, Bohannon KE, Volkin DB, Montefiori DC, Miura A, Krivulka GR, Lifton MA, Kuroda MJ, Schmitz JE, Letvin NL, Caulfield MJ, Bett AJ, Youil R, Kaslow DC, Emini EA. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415:331–335 [DOI] [PubMed] [Google Scholar]
- 56. Sun C, Zhang L, Zhang M, Liu Y, Zhong M, Ma X, Chen L. 2010. Induction of balance and breadth in the immune response is beneficial for the control of SIVmac239 replication in rhesus monkeys. J. Infect. 60:371–381 [DOI] [PubMed] [Google Scholar]
- 57. Sun C, Zhang Y, Feng L, Pan W, Zhang M, Hong Z, Ma X, Chen X, Chen L. 2011. Epidemiology of adenovirus type 5 neutralizing antibodies in healthy people and AIDS patients in Guangzhou, southern China. Vaccine 29:3837–3841 [DOI] [PubMed] [Google Scholar]
- 58. Genesca M, Skinner PJ, Bost KM, Lu D, Wang Y, Rourke TL, Haase AT, McChesney MB, Miller CJ. 2008. Protective attenuated lentivirus immunization induces SIV-specific T cells in the genital tract of rhesus monkeys. Mucosal Immunol. 1:219–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mudd PA, Martins MA, Ericsen AJ, Tully DC, Power KA, Bean AT, Piaskowski SM, Duan L, Seese A, Gladden AD, Weisgrau KL, Furlott JR, Kim YI, Veloso de Santana MG, Rakasz E, Capuano S, 3rd, Wilson NA, Bonaldo MC, Galler R, Allison DB, Piatak M, Jr, Haase AT, Lifson JD, Allen TM, Watkins DI. 2012. Vaccine-induced CD8+ T cells control AIDS virus replication. Nature 491:129–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wambua D, Henderson R, Solomon C, Hunter M, Marx P, Sette A, Mothe BR. 2011. SIV-infected Chinese-origin rhesus macaques express specific MHC class I alleles in either elite controllers or normal progressors. J. Med. Primatol. 40:244–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ling B, Veazey RS, Luckay A, Penedo C, Xu K, Lifson JD, Marx PA. 2002. SIV(mac) pathogenesis in rhesus macaques of Chinese and Indian origin compared with primary HIV infections in humans. AIDS 16:1489–1496 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.