Abstract
Epithelial cells lining mucosal surfaces impose multiple barriers to viral infection. At the ocular surface, the carbohydrate-binding protein galectin-3 maintains barrier function by cross-linking transmembrane mucins on the apical glycocalyx. Despite these defense mechanisms, many viruses have evolved to exploit fundamental cellular processes on host cells. Here, we use affinity assays to show that herpes simplex virus type 1 (HSV-1), but not HSV-2, binds human galectin-3. Knockdown of galectin-3 in human corneal keratinocytes by small interfering RNA significantly impaired HSV-1 infection, but not expression of nectin-1, indicating that galectin-3 is a herpesvirus entry mediator. Interestingly, exposure of epithelial cell cultures to transmembrane mucin isolates decreased viral infectivity. Moreover, HSV-1 failed to elute the biological counterreceptor MUC16 from galectin-3 affinity columns, suggesting that association of transmembrane mucins to galectin-3 provides protection against viral infection. Together, these results indicate that HSV-1 exploits galectin-3 to enhance virus attachment to host cells and support a protective role for transmembrane mucins under physiological conditions by masking viral entry mediators on the epithelial glycocalyx.
INTRODUCTION
Epidemiological data have demonstrated that herpes simplex virus type 1 (HSV-1) is a common human pathogen, with more than 50% of the adult population in the United States testing seropositive (1). Primary infections with HSV-1 are known to cause a wide range of mucosal and cutaneous lesions. In the eye, herpes simplex virus represents the commonest single infectious cause of blindness in the Western world (2). Epithelial keratitis, the most prevalent presentation of ocular infection by herpes simplex virus, appears to be the result of the direct effect of the virus in the superficial epithelial layer of the cornea (3). Herpesvirus infection begins with the interaction of the viral envelope with components of the host cell glycocalyx; thus, elucidating these interactions is critical for understanding disease pathogenesis and ultimately for devising effective treatments.
Epithelia exposed to the external environment are protected from adverse conditions by transmembrane mucins, a group of large and highly O-glycosylated glycoproteins (4). Several transmembrane mucins (MUC1, MUC3A, MUC3B, MUC4, MUC12, MUC13, MUC15, MUC16, MUC17, MUC20, and MUC21) have been identified to date (5). These mucins are major components of individual cell membranes on apical epithelial cells. Their extracellular domain is mainly composed of a variable number of tandem repeats that can extend up to 500 nm above the plasma membrane—far above all other membrane-associated proteins (6). Recent evidence has shown that transmembrane mucins constitute one of the first lines of defense of mucosal surfaces that limit the access of viruses into their hosts (7). In the airway epithelium, MUC1, among other O-glycosylated high-molecular-weight proteins in the apical glycocalyx, is a major contributor to the physical barrier function against virus penetration (8). It has been suggested that transmembrane mucins function as space-filling molecules in the glycocalyx, fully capable of forming dense meshworks that selectively restrict viral infection (9).
Transmembrane mucins can interact with multiple proteins through both the extracellular and intracellular domains (10). Carbohydrate structures present in the highly glycosylated extracellular region make them potential candidates to interact with the galectin family of β-galactoside-specific binding proteins (11). Galectin-3 (Gal-3), the only chimera galectin currently identified, interacts in a carbohydrate-dependent manner with both MUC16 and MUC1 (12, 13). These interactions occur in a multivalent fashion and generate strong molecular lattices on cell surfaces that are resistant to lateral movement (14–16). In corneal epithelium, association of galectin-3 with transmembrane mucins contributes to maintenance of the epithelial barrier (13).
A number of proteins involved in fundamental cellular processes on host cells are exploited by viruses to allow entry and replication and include members of the galectin family (17, 18). To address the possibility that herpesvirus 1 uses galectin-3 to facilitate infection and to determine whether transmembrane mucins, in addition to providing a physical barrier, act as high-affinity cell surface receptors for galectins that block herpesvirus interactions, we performed a series of affinity assays with human galectin-3 in vitro and in a three-dimensional (3D) cell culture system. Our results identify galectin-3 as a herpesvirus 1 entry mediator and indicate that transmembrane mucins in corneal epithelial cells limit herpesvirus 1 recognition of galectin-3.
MATERIALS AND METHODS
Cell culture and viruses.
Telomerase-immortalized human corneal keratinocytes were grown, as previously reported, in a 3D cell culture system with multilayered cells (19). Briefly, cells were grown in keratinocyte serum-free medium (KSFM) (Life Technologies, Carlsbad, CA) to achieve confluence, followed by Dulbecco's modified Eagle's medium (DMEM)/F-12 (Sigma-Aldrich, St. Louis, MO) supplemented with 10% calf serum and 10 ng/ml epidermal growth factor (EGF) for 6 to 7 days to promote stratification and differentiation. Vero cells (CCL-81; ATCC, Manassas, VA) were grown in DMEM supplemented with 10% fetal bovine serum (FBS). The HSV-1 strains used in this study were GHSV-UL46 (VR-1544; ATCC), which contains a VP11/12-green fluorescent protein (GFP) fusion protein (20), and 17 syn+, the syncytial (syn) derivative of HSV-1 strain 17 syn (21). Two herpes simplex virus type 2 (HSV-2) strains, the replication-defective dl5 and dl29 mutants, containing deletions in the viral DNA replication protein UL5 and UL29 genes, respectively, were also used (22). The titers of HSV-1 stocks propagated in Vero cells were determined by plaque assay as previously described (23). All virus stocks were stored at −80°C.
Galectin-3 affinity chromatography.
A pulldown assay for viral particles was conducted in phosphate-buffered saline (PBS), pH 7.5, by incubation of 100 μl recombinant human galectin-3 (rhGal-3)-conjugated agarose beads with 10 μl of GHSV-UL46 (7.6 × 108 PFU/ml), 17 syn+ (5 × 108 PFU/ml), dl5 (7.5 × 107 PFU/ml), or dl29 (1.1 × 108 PFU/ml) for 1 h at 37°C, with gentle mixing every 10 min. The beads were washed with PBS to remove unbound virus and subsequently eluted in sequence with 500 mM sucrose and 500 mM β-lactose. A pulldown assay for protein was conducted by incubation of 50 μl rhGal-3-conjugated agarose beads with 100 μg transmembrane mucin isolates, porcine-stomach-secreted mucin (Sigma-Aldrich), or bovine serum albumin (BSA) (Sigma-Aldrich) for 1 h at 37°C. The beads were pelleted by centrifugation, washed with PBS, and resuspended in SDS-PAGE sample buffer. For the competition pulldown assay, 50 μl rhGal-3-conjugated agarose beads was incubated with 100 μg of transmembrane mucin isolates for 1 h at 37°C. The beads were washed with 100 μl of PBS and sequentially eluted with 500 mM sucrose, followed by 5 μl of GHSV-UL46 (7.6 × 108 PFU/ml) and 500 mM β-lactose. Thirty-microliter aliquots of the wash and eluate fractions were incubated with SDS-PAGE sample buffer before electrophoresis. Binding of GHSV-UL46 to rhGal-3-conjugated agarose beads was imaged by fluorescence microscopy (Nikon Eclipse E-400; Tokyo, Japan) in slides covered with coverslips containing Vectashield mounting medium (Vector Laboratories, Burlingame, CA).
Mucin isolation.
Transmembrane mucin was isolated from multilayered cultures of human corneal keratinocytes as previously described (24, 25). Briefly, protein from cell cultures was extracted using RIPA buffer (150 μM NaCl, 50 μM Tris, pH 8.0, 1% NP 40, 0.5% deoxycholate, 0.1% SDS) supplemented with Complete Protease Inhibitor Cocktail (Roche Biochemical, Indianapolis, IN). After homogenization with a pellet pestle, the protein cell extracts were centrifuged at 12,000 × g for 45 min, and the protein concentration of the supernatant was determined using the Pierce BCA Protein Assay Kit (Thermo Scientific, Rockford, IL). High-molecular-weight mucins were separated by gel chromatography on a Sepharose CL-4B size exclusion column. The void volume containing the mucin fraction was digested with RNase A and DNase I (1 mg nuclease/100 mg protein) for 3 h at room temperature and further purified by isopycnic density gradient centrifugation in cesium chloride.
Electrophoresis and Western/lectin blotting.
Proteins in whole-cell lysates and affinity chromatography fractions were denatured in SDS-PAGE sample buffer, separated in 10% acrylamide gels, and electroblotted onto nitrocellulose membranes (Bio-Rad, Hercules, CA). For analysis of MUC16, proteins were resolved by agarose gel electrophoresis (1% [wt/vol]) and transferred to nitrocellulose membranes by vacuum. Nonspecific binding was blocked overnight at 4°C with 5% nonfat milk in TTBS (0.1% Tween 20 in Tris-buffered saline). The membranes were then incubated with antibodies to galectin-3 (H160; 1:2,000; Santa Cruz Biotechnology, Santa Cruz, CA), MUC16 (M11; 1:3,000; Neomarkers, Fremont, CA), GFP (A11122; 1:1,000; Life Technologies), nectin-1 (H-62; 1:500; Santa Cruz), and herpes simplex virus glycoprotein D (gD) (DL6; 1:2,000; Santa Cruz). Housekeeping protein was detected using antibodies to GAPDH (glyceraldehyde-3-phosphate dehydrogenase) (FL-335; 1:2,000; Santa Cruz). Following incubation with the corresponding peroxidase-conjugated anti-rabbit or anti-mouse IgG (1:2,000; Santa Cruz), positive binding was visualized with chemiluminescence (SuperSignal West Pico substrate; Thermo Scientific). Densitometry was performed using ImageJ software (National Institutes of Health, Bethesda, MD; http://rsb.info.nih.gov/nih-image).
For lectin blotting, the membranes were blocked overnight at 4°C with 1% polyvinylpyrrolidone in TTBS, followed by incubation for 1.5 h at room temperature with biotin-labeled peanut agglutinin (PNA) (1:200; Vector Laboratories, Burlingame, CA) to the mucin-associated T-antigen epitope. The membranes were then developed with an ABC kit (Vectastain ABC Kit; Vector Laboratories), and lectin binding was visualized with chemiluminescence. Albumin blots were stained with Ponceau S.
Transient transfection of siRNAs.
Depletion of galectin-3 in human corneal keratinocytes was achieved using the Silencer Select Predesigned small interfering RNA (siRNA) (s8149; Life Technologies) targeting a sequence of human LGALS3 mRNA (5′-CGGUGAAGCCCAAUGCAAAtt-3′). A nonspecific scrambled siRNA (4390843; Life Technologies) served as the negative control. For knockdown in 3D cell culture systems, keratinocytes were transfected twice—at confluence and 3 days postconfluence—by 6-hour incubation with 500 nM siRNA in Lipofectamine 2000 (Life Technologies; 1 μl/100 mm2) dissolved in Opti-MEM (Life Technologies) reduced-serum medium (GlutaMax; Life Technologies). After the final transfection, the cells were incubated in supplemented DMEM/F-12 for 3 additional days. For knockdown in monolayer cultures, keratinocytes were transfected once, at confluence, followed by incubation in KSFM for 3 days.
Viral infectivity.
Stratified human corneal keratinocytes were incubated for 2 h at 37°C with GHSV-UL46 (7.22 × 103 PFU/ml). For inhibition assays, cells were incubated for 2 h with GHSV-UL46 in the presence of increasing concentrations (0.05 to 0.45 mg/ml) of transmembrane mucin isolates, porcine stomach mucin, or bovine serum albumin. Viral particles bound to the cell surface were inactivated afterwards by incubation with citrate buffer (pH 3.0) for 2 min, followed by washes with PBS (26). The cells were then overlaid with fresh medium, and whole-cell lysates were collected 18 h postinfection for immunoblot analyses.
For plaque assays, monolayer cultures of corneal keratinocytes were incubated for 2 h at 37°C with 10-fold serial dilutions of GHSV-UL46 (7.6 × 108 PFU/ml). The culture medium was then aspirated, and the infected cells were overlaid with 0.5% low-melting-temperature agarose (Sigma-Aldrich) in DMEM supplemented with 2% FBS and 1% penicillin-streptomycin (Life Technologies). Three days postinfection, the agarose was removed, and the cells were fixed with 100% methanol and stained with 0.1% crystal violet in 50% ethanol. Plaques were counted manually using a 10× objective lens, and the plaque area was measured using Spot Advanced imaging software (Spot Imaging Solutions, Sterling Heights, MI).
Statistical analyses.
Data are represented as means and standard deviations (SD). Statistical analyses were performed with Student's t test using InStat3 software (GraphPad Software, La Jolla, CA).
RESULTS
HSV-1 binds to human galectin-3.
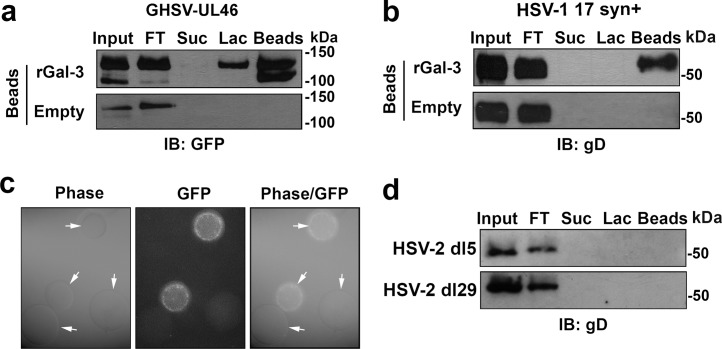
Pathogenic organisms can subvert the recognition of galectins in host cells to ensure successful attachment or invasion (27). An example is the binding of gp120 glycans in human immunodeficiency virus type 1 to galectin-1, which allows rapid association of the virus with susceptible cells, facilitating infection (18, 28). Galectin-3 is a major component of the human cornea, where it localizes to apical membranes of apical epithelial cells both in vivo and in vitro (13). To test whether HSV-1 binds to galectin-3, GHSV-UL46 and 17 syn+ virus particles were subjected to galectin-3 affinity chromatography (Fig. 1). After removing unbound virus, the columns were sequentially eluted with sucrose, representing a specificity control for β-galactoside-dependent binding, and β-lactose, a competitive inhibitor of the carbohydrate recognition domain of galectin-3. Analysis of the eluates revealed that the two HSV-1 strains used in this study interacted with galectin-3 (Fig. 1a and b). As shown by densitometry, as much as 50% of GHSV-UL46 and 42% of 17 syn+ bound to galectin-3 beads in the affinity column. Twenty percent of the bound GHSV-UL46 was eluted with 0.5 M β-lactose, indicating that the virus can bind galectin-3 in a carbohydrate-dependent manner. Interestingly, most of the 17 syn+ virus remained bound to galectin-3 after β-lactose elution, suggesting that the mechanism of virus attachment to human galectin-3 is strain specific and may include sites different from the conventional carbohydrate recognition domain. GHSV-UL46 binding to rhGal-3-conjugated agarose beads was further confirmed by fluorescence microscopy (Fig. 1c).
Fig 1.
HSV-1 binds human galectin-3. (a) The HSV-1 strains GHSV-UL46 and 17 syn+ were applied to galectin-3 affinity columns (Input). After unbound virus was removed (FT), the columns were sequentially eluted with sucrose and β-lactose. As determined by Western blotting, as much as 50% of GHSV-UL46 bound to galectin-3 beads. Twenty percent of the bound GHSV-UL46 was eluted with 0.5 M β-lactose. (b) Similarly, 42% of the 17 syn+ strain bound to galectin-3 beads in the affinity column, but binding was primarily carbohydrate independent. Control experiments using unconjugated (Empty) beads demonstrated lack of nonspecific binding of the virus to the column. (c) Binding of GHSV-UL46 to galectin-3 beads was further confirmed by fluorescence microscopy. Representative images demonstrate binding of GFP-labeled GHSV-UL46 virus particles to galectin-3 beads (arrows). (d) No binding was observed between the replication-defective HSV-2 dl5 and dl29 mutant virus strains and the galectin-3 affinity columns. FT, flowthrough; IB, immunoblot; Lac, lactose; rGal-3, recombinant galectin-3; Suc, sucrose.
To determine whether the interaction of galectin-3 was specific to HSV-1, we performed additional pulldown assays with HSV-2, a distinct but closely related virus traditionally associated with genital disease (29, 30). Interestingly, the two replication-defective HSV-2 mutant virus strains used in this study, dl5 and dl29, did not bind to galectin-3 beads in affinity columns (Fig. 1d).
Knockdown of galectin-3 inhibits HSV-1 infection of human corneal keratinocytes.
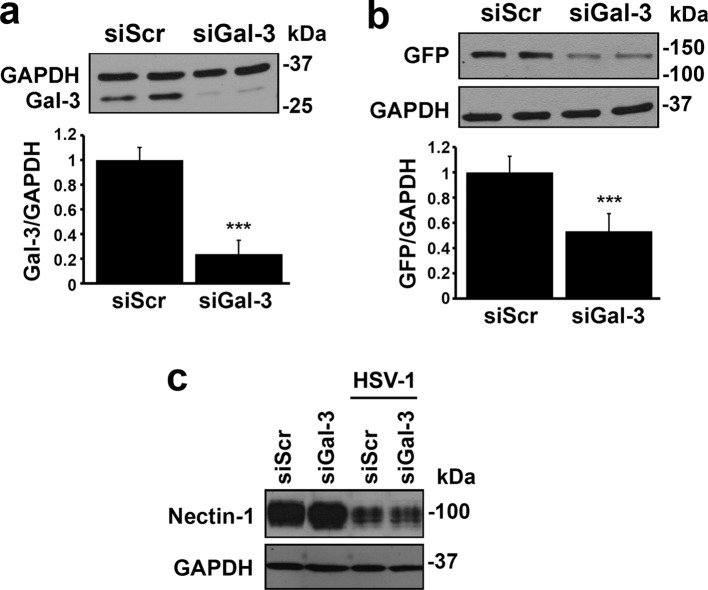
In view of the fact that HSV-1 binds human galectin-3, we next determined whether targeted abrogation of galectin-3 in host cells would inhibit the entry of GHSV-UL46. Targeting galectin-3 in multilayered cultures of corneal keratinocytes with siRNA resulted in 55 to 90% decreases in protein levels compared to treatment with scrambled siRNA (Fig. 2a). When virions were allowed to bind stratified cell cultures transfected with galectin-3 siRNA, the expression levels of viral GFP were 47% lower than in scrambled siRNA control cells (Fig. 2b), indicating that galectin-3 mediates HSV-1 infection in human corneal keratinocytes.
Fig 2.
Galectin-3 knockdown impairs HSV-1 infection in a 3D cell culture system of human corneal keratinocytes. (a) Corneal cells were transiently transfected twice with scrambled or galectin-3 siRNA. Analyses of whole-cell lysates 72 h after the final transfection revealed a 76% ± 11% galectin-3 protein reduction in cultures treated with galectin-3 siRNA compared to scrambled siRNA. A representative Western blot is shown at the top. (b) For viral infectivity assays, stratified cultures were incubated for 2 h with GFP-labeled GHSV-UL46 and analyzed 18 h postinfection by immunoblotting. Under these conditions, the expression levels of viral GFP were 47% ± 14% lower in cultures treated with galectin-3 siRNA than in those treated with scrambled siRNA control. A representative Western blot is shown at the top. (c) Transfection of corneal keratinocytes with galectin-3 siRNA did not affect the protein levels of nectin-1. Relative protein levels were determined by densitometry and were normalized to GAPDH. Experiments were performed in duplicate and repeated three times. ***, P < 0.001. siGal-3, galectin-3 siRNA; siScr, scrambled siRNA. The error bars indicate SD.
To determine whether galectin-3 assists in the infection event without affecting the rest of the HSV-1 entry process, we analyzed the protein levels of nectin-1. Nectin-1 is a major HSV-1 receptor in polarized human epithelial cells that becomes internalized and degraded after interaction with gD on the HSV-1 envelope (26, 31, 32). As shown in Fig. 2c, transfection of keratinocytes with galectin-3 siRNA did not affect nectin-1 protein levels compared to the scrambled control in either the absence or the presence of HSV-1 virions.
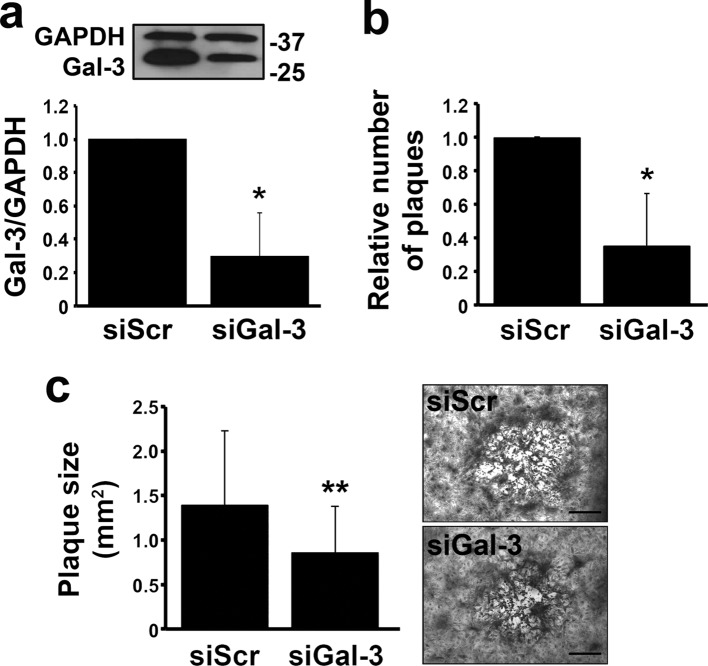
That galectin-3 mediates HSV-1 infection was also confirmed in plaque assays using monolayer cultures of human corneal keratinocytes. It is well established that, in these cells, HSV-1 has the ability to produce visible plaques that result in central clearing as the virus spreads (32). As shown in Fig. 3a, targeted abrogation of galectin-3 using siRNA led to an approximately 70% decrease in galectin-3 protein levels compared to the scrambled control. Infection of host cells after galectin-3 knockdown with serial dilutions of GHSV-UL46 led to a significant reduction in the number and average area of plaques (Fig. 3b and c).
Fig 3.
Galectin-3 knockdown reduces plaque formation in monolayer cultures of human corneal keratinocytes. (a) Confluent corneal cells were transiently transfected with scrambled or galectin-3 siRNA. The efficiency of galectin-3 knockdown in monolayer cultures was 70% ± 26%. A representative Western blot is shown at the top. (b) Infection of host cells with GHSV-UL46 after galectin-3 knockdown led to a 65% ± 31% reduction in the number of plaques normalized to the scrambled control. (c) The average area of viral plaques developed after galectin-3 knockdown was 38% ± 37% smaller than in control cells. Representative images for each condition are shown on the right. Experiments were performed in triplicate for each virus dilution. *, P < 0.05; **, P < 0.01. Bars = 0.5 mm. The error bars indicate SD.
Transmembrane mucins prevent HSV-1 infection and virus interaction with galectin-3.
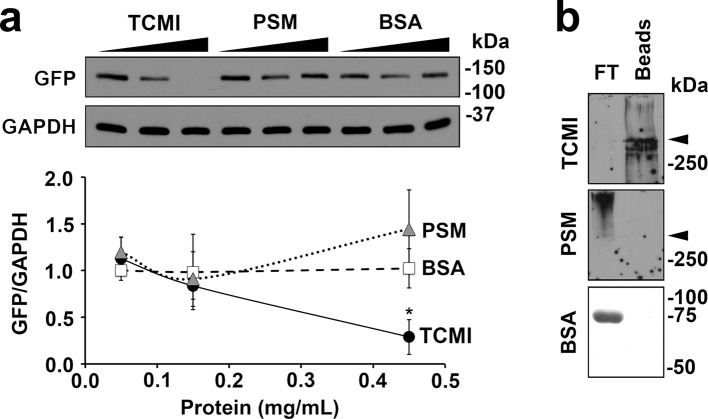
Previous studies have shown that steric hindrance is sufficient for neutralization of viral entry by targeting molecules to the vicinity of the virus and its receptor (33, 34). In the human cornea, transmembrane mucins localize to apical membranes of apical epithelial cells, where they closely associate with galectin-3 (13, 35). Thus, we considered the possibility that endogenous mucin could prevent HSV-1 infection by interacting with galectin-3. In subsequent experiments, we infected corneal keratinocytes with GHSV-UL46 in the presence of increasing concentrations of exogenous transmembrane mucin isolated from corneal keratinocytes. As shown in Fig. 4a, addition of exogenous corneal mucin isolates to the cell cultures significantly reduced HSV-1 infectivity in a concentration-dependent manner. As shown by peanut agglutinin staining, the transmembrane mucin isolates used in these experiments bound to galectin-3 in affinity assays (Fig. 4b), suggesting that transmembrane mucins in host cells regulate viral entry by interacting with galectin-3 on the cell surface. Incubation of cells with porcine stomach mucin and bovine serum albumin—two glycoprotein controls lacking affinity for human galectin-3—had no effect in preventing HSV-1 infection of human corneal keratinocytes.
Fig 4.
Exogenous transmembrane mucin efficiently prevents HSV-1 infection of human corneal keratinocytes. (a) Corneal keratinocytes were infected with GHSV-UL46 in the presence of increasing concentrations of transmembrane corneal mucin isolates (TCMI), porcine stomach mucin (PSM), and BSA. Addition of exogenous transmembrane mucin to corneal keratinocytes, but not stomach mucin or albumin, significantly reduced HSV-1 infectivity in a concentration-dependent manner. Relative protein levels were determined by densitometry and were normalized to GAPDH. Experiments were performed in triplicate for each dilution. A representative Western blot is shown at the top. (b) By affinity chromatography, transmembrane mucin bound to galectin-3 beads. In contrast, stomach mucin and albumin were detected in the flowthrough, indicating a lack of interaction with galectin-3. The arrowheads indicate the interface between the stacking and separating gels. *, P < 0.05.
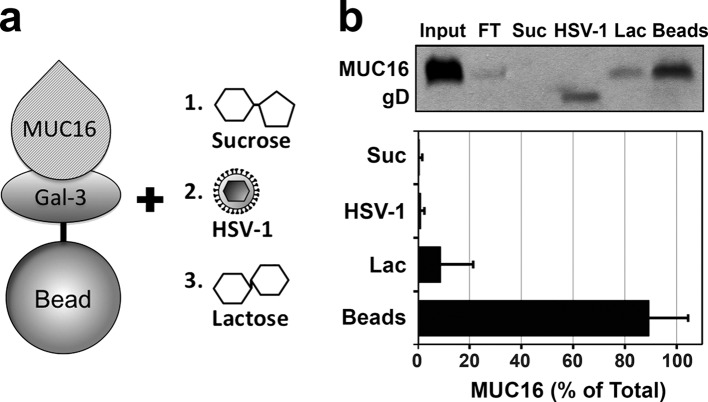
To further examine whether the interaction between galectin-3 and transmembrane mucins is responsible for inhibiting HSV-1 infectivity, we performed competition pulldown assays. Corneal mucin isolates containing endogenous MUC16 were incubated with galectin-3 beads, followed by sequential elution with sucrose, GHSV-UL46 virions, and β-lactose (Fig. 5a). Immunoblotting analysis showed that GHSV-UL46 could not bind to the galectin-3 affinity column in the presence of transmembrane mucins (Fig. 5b), indicating that transmembrane mucins from human corneal keratinocytes restrict the interaction between human galectin-3 and HSV-1 virions. In these experiments, MUC16 remained bound to galectin-3 beads after incubation with GHSV-UL46 but could be partially eluted with β-lactose, as shown previously (13). Overall, these results support the hypothesis that transmembrane mucins limit virus recognition of the epithelial cell surface by interacting with galectin-3, thereby blocking viral entry into host cells.
Fig 5.
Transmembrane mucins contribute to prevention of interactions between HSV-1 and human galectin-3. (a) General diagram of the competition pulldown assay. Transmembrane mucins were applied to a galectin-3 affinity column. After unbound mucin was removed, the column was sequentially incubated with sucrose (1), GHSV-UL46 virions (2), and β-lactose (3). The fractions were then analyzed by immunoblotting for MUC16 and gD protein content. (b) GHSV-UL46 virions failed to bind the galectin-3 column, as shown by the presence of gD in the HSV-1 fraction, suggesting that binding of transmembrane mucins to galectin-3 provides steric hindrance against virus attachment. MUC16 remained bound to galectin-3 beads after incubation with GHSV-UL46 but could be partially eluted with β-lactose, as described previously (13). The error bars indicate SD.
DISCUSSION
Cells throughout the whole body impose multiple barriers to prevent virus infection, yet viruses have evolved biologically to efficiently exploit fundamental cellular processes on host cells that allow virus entry and replication (17). In this study, we identified galectin-3 as a cell surface component exploited by herpesvirus 1 to infect corneal keratinocytes. More importantly, we show that transmembrane mucins, by acting as surface receptors of galectin-3, limit the interaction between herpesvirus 1 and the glycocalyx. This finding adds a novel mechanism for how transmembrane mucins, in addition to functioning as a physical matrix that excludes viral particles (9), provide a barrier function on epithelial surfaces.
Initial recruitment of viruses to susceptible cells is mediated through binding of viral surface components to attachment factors on the target cell surface. Many viruses, including herpesviruses, are known to use multiple cell surface receptors to collectively increase binding avidity but, of more consequence, allow tight sequential coordination of fusion and penetration events (17). The mechanism by which herpesvirus enters host cells is one of the more complex mechanisms studied to date. In humans, the most widely studied herpesvirus receptors are nectin-1, an adhesion protein implicated in the organization of adherens junctions and tight junctions in epithelial cells, and herpesvirus entry mediator, which belongs to the tumor necrosis factor receptor family (36, 37). In addition, it has been shown that 3-O-sulfated heparan sulfate also provides sites for the binding and initiation of HSV-1 entry (38). Interestingly, these various molecules bind the virus independently and do not act as coreceptors during entry (39). Here, we show that galectin-3, a lectin found on the apical glycocalyx of apical cells on corneal keratinocytes, provides similar functions essential to enhancement of infection efficiency. We show first that HSV-1 directly binds human galectin-3 in affinity assays (Fig. 1) and, second, that targeted disruption of galectin-3 impairs HSV-1 infectivity in human corneal keratinocytes (Fig. 2 and 3). Since galectin-3 is highly expressed at the apical surface of corneal epithelium, these results suggest that HSV-1 uses the lectin to increase binding avidity for host cells.
Within the past few years, it has become apparent that galectins bind pathogens, thereby functioning as regulatory factors in innate immunity (27). Several galectins have been shown to bind viruses, either to impair or to facilitate particle attachment and infection. Antiviral effects have been observed with galectin-1, a prototype lectin that specifically cross-links N-glycans in envelope glycoproteins of Nipah virus, causing aberrant oligomerization and blocking fusion of the virus with endothelial cells (40). Exogenous galectin-1, by modulating the immune response, also diminishes the severity of ocular lesions in a mouse model of HSV-1 keratitis (41). On the other hand, galectin-1 has also been shown to accelerate the binding kinetics of HIV-1 to susceptible cells and to facilitate virus replication (28, 42, 43). It is likely that structural differences in glycan composition, and/or their presentation mode, on envelope glycoproteins determine the relationship of individual viruses with galectins (27). In our experiments, we found that galectin-3, a chimera lectin that forms pentamers upon binding to multivalent ligands, facilitates herpesvirus 1 binding to and infection of corneal keratinocytes. Importantly, targeted abrogation of galectin-3 in host cells did not affect the levels of nectin-1 or its processing compared to controls, supporting previous data indicating that galectins assist initial virus binding events independently of the rest of the virus entry process (18).
It is now apparent that the presence of a highly glycosylated and abundant glycocalyx on the apical surface of polarized epithelium reduces the efficiency with which viruses infect host cells (7). In airway epithelium, the transmembrane mucin MUC1, among other tethered mucin types, provides a physical barrier that limits adenoviral vector interaction with the airway luminal surface (8, 44, 45). Electron and atomic force microscopy has shown that a finely textured glycocalyx with linear and rigid transmembrane mucins is responsible for the exclusion of large adenovirus particles (9). To test whether transmembrane mucins limit herpesvirus 1 infection, we added exogenous transmembrane mucin to our culture model of stratified human corneal keratinocytes. We found that transmembrane mucin—but not glycoprotein controls—with the ability to bind galectin-3 restricted herpesvirus 1 infection in a concentration-dependent manner (Fig. 4). Interestingly, herpesvirus 1 virions were unable to elute MUC16 from human galectin-3 beads in competition pulldown assays (Fig. 5), suggesting that transmembrane mucins block virus penetration by binding galectin-3 in the glycocalyx and providing steric hindrance. The fact that herpesvirus 1 was not able to elute mucins from the galectin-3 beads was not entirely surprising. It has been shown that, although individual interactions between viruses and their receptors are specific, they are often of low affinity (46).
Our model raises an obvious question with regard to the molecular events that trigger successful infection in the cornea. We have speculated that alteration in the glycocalyx after epithelial abrasion facilitates herpesvirus attachment to the cornea. This hypothesis is supported by corneal-wound-healing data indicating that pathogens, including adenoviruses, adhere to leading-edge cells migrating to cover the wound but not to apical cells of the stratified epithelium behind the leading edge (47, 48). Leading-edge cells are characterized by abnormal cell surface glycoprotein glycosylation (49, 50) and therefore are susceptible to alterations in the association between transmembrane mucins and galectin-3. Moreover, compared with normal, nonmigrating epithelia, migrating epithelia of healing corneas have significantly greater levels of galectin-3 (51), potentially increasing the availability of attachment sites for herpesvirus 1 into compromised corneas. In summary, our study identifies galectin-3 as a novel HSV-1 entry mediator in corneal keratinocytes and highlights the importance of the close association between transmembrane mucins and multivalent galectin-3 in limiting herpesvirus interactions with the glycocalyx. Modulating receptor interactions in the epithelial glycocalyx represents an interesting therapeutic strategy to decrease herpesvirus infectivity.
ACKNOWLEDGMENTS
This work was supported by a grant from the National Eye Institute of the National Institutes of Health (R01EY014847).
We thank Noorjahan Panjwani (Tufts University, Boston, MA) for providing galectin-3 agarose beads, Ilene Gipson (Schepens Eye Research Institute and Massachusetts Eye and Ear, Harvard Medical School Department of Ophthalmology, Boston, MA) for the telomerase-immortalized human corneal keratinocyte cell line, John Chen (Center for Systems Biology at Massachusetts General Hospital, Boston, MA) for the HSV-1 17 syn+ strain, and David Knipe (Harvard Medical School Department of Microbiology and Immunobiology, Boston, MA) for the replication-defective HSV-2 dl5 and dl29 mutant virus strains used in this study.
We declare no conflict of interest.
Footnotes
Published ahead of print 13 March 2013
REFERENCES
- 1. Xu F, Sternberg MR, Kottiri BJ, McQuillan GM, Lee FK, Nahmias AJ, Berman SM, Markowitz LE. 2006. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA 296:964–973 [DOI] [PubMed] [Google Scholar]
- 2. Liesegang TJ. 2001. Herpes simplex virus epidemiology and ocular importance. Cornea 20:1–13 [DOI] [PubMed] [Google Scholar]
- 3. Banerjee K, Rouse BT. 2007. Immunopathological aspects of HSV infection, p 642–655 In Arvin A, Campadelli-Fiume G, Mocarski E, Moore PS, Roizman B, Whitley R, Yamanishi K. (ed), Human herpesviruses: biology, therapy, and immunoprophylaxis. Cambridge University Press, New York, NY: [PubMed] [Google Scholar]
- 4. Kufe DW. 2009. Mucins in cancer: function, prognosis and therapy. Nat. Rev. Cancer. 9:874–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bafna S, Kaur S, Batra SK. 2010. Membrane-bound mucins: the mechanistic basis for alterations in the growth and survival of cancer cells. Oncogene 29:2893–2904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hilkens J, Ligtenberg MJ, Vos HL, Litvinov SV. 1992. Cell membrane-associated mucins and their adhesion-modulating property. Trends Biochem. Sci. 17:359–363 [DOI] [PubMed] [Google Scholar]
- 7. Pickles RJ. 2004. Physical and biological barriers to viral vector-mediated delivery of genes to the airway epithelium. Proc. Am. Thorac. Soc. 1:302–308 [DOI] [PubMed] [Google Scholar]
- 8. Stonebraker JR, Wagner D, Lefensty RW, Burns K, Gendler SJ, Bergelson JM, Boucher RC, O'Neal WK, Pickles RJ. 2004. Glycocalyx restricts adenoviral vector access to apical receptors expressed on respiratory epithelium in vitro and in vivo: role for tethered mucins as barriers to lumenal infection. J. Virol. 78:13755–13768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kesimer M, Ehre C, Burns KA, Davis CW, Sheehan JK, Pickles RJ. 2013. Molecular organization of the mucins and glycocalyx underlying mucus transport over mucosal surfaces of the airways. Mucosal Immunol. 6:379–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Senapati S, Das S, Batra SK. 2010. Mucin-interacting proteins: from function to therapeutics. Trends Biochem. Sci. 35:236–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bresalier RS, Byrd JC, Wang L, Raz A. 1996. Colon cancer mucin: a new ligand for the beta-galactoside-binding protein galectin-3. Cancer Res. 56:4354–4357 [PubMed] [Google Scholar]
- 12. Yu LG, Andrews N, Zhao Q, McKean D, Williams JF, Connor LJ, Gerasimenko OV, Hilkens J, Hirabayashi J, Kasai K, Rhodes JM. 2007. Galectin-3 interaction with Thomsen-Friedenreich disaccharide on cancer-associated MUC1 causes increased cancer cell endothelial adhesion. J. Biol. Chem. 282:773–781 [DOI] [PubMed] [Google Scholar]
- 13. Argueso P, Guzman-Aranguez A, Mantelli F, Cao Z, Ricciuto J, Panjwani N. 2009. Association of cell surface mucins with galectin-3 contributes to the ocular surface epithelial barrier. J. Biol. Chem. 284:23037–23045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Partridge EA, Le Roy C, Di Guglielmo GM, Pawling J, Cheung P, Granovsky M, Nabi IR, Wrana JL, Dennis JW. 2004. Regulation of cytokine receptors by Golgi N-glycan processing and endocytosis. Science 306:120–124 [DOI] [PubMed] [Google Scholar]
- 15. Lagana A, Goetz JG, Cheung P, Raz A, Dennis JW, Nabi IR. 2006. Galectin binding to Mgat5-modified N-glycans regulates fibronectin matrix remodeling in tumor cells. Mol. Cell. Biol. 26:3181–3193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nieminen J, Kuno A, Hirabayashi J, Sato S. 2007. Visualization of galectin-3 oligomerization on the surface of neutrophils and endothelial cells using fluorescence resonance energy transfer. J. Biol. Chem. 282:1374–1383 [DOI] [PubMed] [Google Scholar]
- 17. Grove J, Marsh M. 2011. The cell biology of receptor-mediated virus entry. J. Cell Biol. 195:1071–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sato S, Ouellet M, St-Pierre C, Tremblay MJ. 2012. Glycans, galectins, and HIV-1 infection. Ann. N. Y. Acad. Sci. 1253:133–148 [DOI] [PubMed] [Google Scholar]
- 19. Gipson IK, Spurr-Michaud S, Argueso P, Tisdale A, Ng TF, Russo CL. 2003. Mucin gene expression in immortalized human corneal-limbal and conjunctival epithelial cell lines. Invest. Ophthalmol. Vis. Sci. 44:2496–2506 [DOI] [PubMed] [Google Scholar]
- 20. Willard M. 2002. Rapid directional translocations in virus replication. J. Virol. 76:5220–5232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brown SM, Ritchie DA, Subak-Sharpe JH. 1973. Genetic studies with herpes simplex virus type 1. The isolation of temperature-sensitive mutants, their arrangement into complementation groups and recombination analysis leading to a linkage map. J. Gen. Virol. 18:329–346 [DOI] [PubMed] [Google Scholar]
- 22. Da Costa X, Kramer MF, Zhu J, Brockman MA, Knipe DM. 2000. Construction, phenotypic analysis, and immunogenicity of a UL5/UL29 double deletion mutant of herpes simplex virus 2. J. Virol. 74:7963–7971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Goins WF, Krisky DM, Wechuck JB, Huang S, Glorioso JC. 2008. Construction and production of recombinant herpes simplex virus vectors. Methods Mol. Biol. 433:97–113 [DOI] [PubMed] [Google Scholar]
- 24. Argueso P, Gipson IK. 2006. Quantitative analysis of mucins in mucosal secretions using indirect enzyme-linked immunosorbent assay. Methods Mol. Biol. 347:277–288 [DOI] [PubMed] [Google Scholar]
- 25. Bravo-Osuna I, Noiray M, Briand E, Woodward A, Argüeso P, Molina Martínez I, Herrero-Vanrell R, Ponchel G. 2012. Interfacial interaction between transmembrane ocular mucins and adhesive polymers and dendrimers analyzed by surface plasmon resonance. Pharm. Res. 29:2329–2340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Galen B, Cheshenko N, Tuyama A, Ramratnam B, Herold BC. 2006. Access to nectin favors herpes simplex virus infection at the apical surface of polarized human epithelial cells. J. Virol. 80:12209–12218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vasta GR. 2009. Roles of galectins in infection. Nat. Rev. Microbiol. 7:424–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. St-Pierre C, Manya H, Ouellet M, Clark GF, Endo T, Tremblay MJ, Sato S. 2011. Host-soluble galectin-1 promotes HIV-1 replication through a direct interaction with glycans of viral gp120 and host CD4. J. Virol. 85:11742–11751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chentoufi AA, Benmohamed L. 2012. Mucosal herpes immunity and immunopathology to ocular and genital herpes simplex virus infections. Clin. Dev. Immunol. 2012:149135 doi:10.1155/2012/149135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Serafini-Cessi F, Dall'Olio F, Malagolini N, Pereira L, Campadelli-Fiume G. 1988. Comparative study on O-linked oligosaccharides of glycoprotein D of herpes simplex virus types 1 and 2. J. Gen. Virol. 69:869–877 [DOI] [PubMed] [Google Scholar]
- 31. Stiles KM, Milne RS, Cohen GH, Eisenberg RJ, Krummenacher C. 2008. The herpes simplex virus receptor nectin-1 is down-regulated after trans-interaction with glycoprotein D. Virology 373:98–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shah A, Farooq AV, Tiwari V, Kim MJ, Shukla D. 2010. HSV-1 infection of human corneal epithelial cells: receptor-mediated entry and trends of re-infection. Mol. Vis. 16:2476–2486 [PMC free article] [PubMed] [Google Scholar]
- 33. Yang X, Lipchina I, Lifton M, Wang L, Sodroski J. 2007. Antibody binding in proximity to the receptor/glycoprotein complex leads to a basal level of virus neutralization. J. Virol. 81:8809–8813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chu JJ, Ng ML. 2004. Interaction of West Nile virus with alpha v beta 3 integrin mediates virus entry into cells. J. Biol. Chem. 279:54533–54541 [DOI] [PubMed] [Google Scholar]
- 35. Argueso P, Spurr-Michaud S, Russo CL, Tisdale A, Gipson IK. 2003. MUC16 mucin is expressed by the human ocular surface epithelia and carries the H185 carbohydrate epitope. Invest. Ophthalmol. Vis. Sci. 44:2487–2495 [DOI] [PubMed] [Google Scholar]
- 36. Montgomery RI, Warner MS, Lum BJ, Spear PG. 1996. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell 87:427–436 [DOI] [PubMed] [Google Scholar]
- 37. Geraghty RJ, Krummenacher C, Cohen GH, Eisenberg RJ, Spear PG. 1998. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science 280:1618–1620 [DOI] [PubMed] [Google Scholar]
- 38. Shukla D, Liu J, Blaiklock P, Shworak NW, Bai X, Esko JD, Cohen GH, Eisenberg RJ, Rosenberg RD, Spear PG. 1999. A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Cell 99:13–22 [DOI] [PubMed] [Google Scholar]
- 39. Heldwein EE, Krummenacher C. 2008. Entry of herpesviruses into mammalian cells. Cell. Mol. Life Sci. 65:1653–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Levroney EL, Aguilar HC, Fulcher JA, Kohatsu L, Pace KE, Pang M, Gurney KB, Baum LG, Lee B. 2005. Novel innate immune functions for galectin-1: galectin-1 inhibits cell fusion by Nipah virus envelope glycoproteins and augments dendritic cell secretion of proinflammatory cytokines. J. Immunol. 175:413–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rajasagi NK, Suryawanshi A, Sehrawat S, Reddy PB, Mulik S, Hirashima M, Rouse BT. 2012. Galectin-1 reduces the severity of herpes simplex virus-induced ocular immunopathological lesions. J. Immunol. 188:4631–4643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ouellet M, Mercier S, Pelletier I, Bounou S, Roy J, Hirabayashi J, Sato S, Tremblay MJ. 2005. Galectin-1 acts as a soluble host factor that promotes HIV-1 infectivity through stabilization of virus attachment to host cells. J. Immunol. 174:4120–4126 [DOI] [PubMed] [Google Scholar]
- 43. Mercier S, St-Pierre C, Pelletier I, Ouellet M, Tremblay MJ, Sato S. 2008. Galectin-1 promotes HIV-1 infectivity in macrophages through stabilization of viral adsorption. Virology 371:121–129 [DOI] [PubMed] [Google Scholar]
- 44. Arcasoy SM, Latoche J, Gondor M, Watkins SC, Henderson RA, Hughey R, Finn OJ, Pilewski JM. 1997. MUC1 and other sialoglycoconjugates inhibit adenovirus-mediated gene transfer to epithelial cells. Am. J. Respir. Cell Mol. Biol. 17:422–435 [DOI] [PubMed] [Google Scholar]
- 45. Nguyen Y, Procario MC, Ashley SL, O'Neal WK, Pickles RJ, Weinberg JB. 2011. Limited effects of Muc1 deficiency on mouse adenovirus type 1 respiratory infection. Virus Res. 160:351–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Marsh M, Helenius A. 2006. Virus entry: open sesame. Cell 124:729–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Danjo Y, Gipson IK. 2002. Specific transduction of the leading edge cells of migrating epithelia demonstrates that they are replaced during healing. Exp. Eye Res. 74:199–204 [DOI] [PubMed] [Google Scholar]
- 48. Spurr-Michaud SJ, Barza M, Gipson IK. 1988. An organ culture system for study of adherence of Pseudomonas aeruginosa to normal and wounded corneas. Invest. Ophthalmol. Vis. Sci. 29:379–386 [PubMed] [Google Scholar]
- 49. Gipson IK, Riddle CV, Kiorpes TC, Spurr SJ. 1983. Lectin binding to cell surfaces: comparisons between normal and migrating corneal epithelium. Dev. Biol. 96:337–345 [DOI] [PubMed] [Google Scholar]
- 50. McLaughlin BJ, Barlar EK, Donaldson DJ. 1986. Wheat germ agglutinin and concanavalin A binding during epithelial wound healing in the cornea. Curr. Eye Res. 5:601–609 [DOI] [PubMed] [Google Scholar]
- 51. Cao Z, Said N, Amin S, Wu HK, Bruce A, Garate M, Hsu DK, Kuwabara I, Liu FT, Panjwani N. 2002. Galectins-3 and -7, but not galectin-1, play a role in re-epithelialization of wounds. J. Biol. Chem. 277:42299–42305 [DOI] [PubMed] [Google Scholar]