Abstract
While development of an HIV vaccine that can induce neutralizing antibodies remains a priority, decades of research have proven that this is a daunting task. However, accumulating evidence suggests that antibodies with the capacity to harness innate immunity may provide some protection. While significant research has focused on the cytolytic properties of antibodies in acquisition and control, less is known about the role of additional effector functions. In this study, we investigated antibody-dependent phagocytosis of HIV immune complexes, and we observed significant differences in the ability of antibodies from infected subjects to mediate this critical effector function. We observed both quantitative differences in the capacity of antibodies to drive phagocytosis and qualitative differences in their FcγR usage profile. We demonstrate that antibodies from controllers and untreated progressors exhibit increased phagocytic activity, altered Fc domain glycosylation, and skewed interactions with FcγR2a and FcγR2b in both bulk plasma and HIV-specific IgG. While increased phagocytic activity may directly influence immune activation via clearance of inflammatory immune complexes, it is also plausible that Fc receptor usage patterns may regulate the immune response by modulating downstream signals following phagocytosis—driving passive degradation of internalized virus, release of immune modulating cytokines and chemokines, or priming of a more effective adaptive immune response.
INTRODUCTION
Antibodies are potent determinants of the humoral immune response and can act not only by direct neutralization of the pathogen but also via engagement of the cytotoxic Fc receptor (FcγR)-bearing cells of the innate immune system—providing a functional link between the innate and adaptive immune systems (1). The innate immune effector function of an antibody is determined by its constant, or Fc, domain, which has evolved to possess a large number of states with regard to potency. These states include the choice of antibody isotype and IgG subclass (2–5), as well as the precise glycan structure at a conserved glycosylation site at position Asn297 on the antibody heavy, or Fc, chain (6, 7), giving rise to remarkable combinatorial diversity.
Several recent reports have highlighted the possible importance of antibody Fc effector functions in HIV acquisition and progression (3, 5, 8–12), offering what may be a tractable handle for protection mediated by vaccination. While the profile of antibodies required for the induction of natural killer (NK) cell-mediated antibody-dependent cellular cytotoxicity (ADCC) has been elucidated, less is known about the humoral parameters associated with robust antibody-dependent cellular phagocytosis (ADCP). Critically, as a potent mechanism of antibody-mediated effector function, phagocytosis of immune complexes, opsonized virus, and infected host cells represents an important connection between the adaptive and innate immune systems, with potential roles both in priming of the adaptive immune response and in clearance of virus. Phagocytosis not only may rapidly remove virus or virally infected cells from the circulation but also could affect immune complex-induced inflammation, implicated in driving disease progression.
Importantly, there is evidence that disease susceptibility and severity in numerous autoimmune diseases and infectious diseases and responsiveness to monoclonal antibody therapy are impacted by antibody-driven phagocytosis (13–17). FcγR2a, the receptor implicated in phagocytosis, is expressed on cells capable of acting as professional phagocytes, including monocytes, macrophages, neutrophils, dendritic cells, and mast cells—making FcγR2a the most widely expressed FcγR (18). Interestingly, though 95% identical in its extracellular domain, FcγR2b has an intracellular ITIM motif and acts as an inhibitory receptor, but it has also been implicated in phagocytosis in the absence of cell activation (19, 20). Several lines of evidence support the importance of phagocytosis in HIV infection. First, FcγR2a polymorphisms have been found to correlate with disease progression (21) and susceptibility (22). Second, IgG2 subclass antibodies, in combination with the FcγR2a allele capable of interacting with IgG2, are associated with delayed progression (23). Lastly, progressive infection is associated with decreased expression of FcγR2a, correlating with reduced ability to phagocytose immune complexes (24).
Accumulating data from clinical and animal model studies suggest that there are significant differences in the ability of antibodies from HIV+ and vaccinated subjects to elicit the cytotoxic function of NK cells (ADCC) and complement (2, 5, 8, 25–27), which may have relevance to disease progression or infection. However, while HIV-specific antibodies are known to have the capacity to inhibit virus in the presence of phagocytes (28–30), less is known about the natural variability of this capacity to engage professional phagocytes and process immune complexes. Thus, we undertook a study to investigate whether differential Fc effector antibody functions extend to the induction of antibody-dependent phagocytosis, by investigating whether and how antibodies generated in individuals with differential control of infection exhibited an altered capacity to mediate this effector function. In this study, we paired biophysical measurements of antibody binding to FcγR2a and -2b with experimental measurement of immune complex phagocytosis and found that potentiated phagocytic activity is associated with antibodies that are able to preferentially interact with the activating FcγR2a over the inhibitory FcγR2b. Because significant clinical data suggest the involvement of phagocytic Fc receptors in antibody activity, understanding the interplay between these two receptors and the means by which receptor selectivity can be tuned is likely to be important for both recombinant therapeutics and vaccine design.
MATERIALS AND METHODS
Patient antibodies.
A total of 109 subjects were recruited for this study, including 20 healthy HIV-1-negative control subjects, 26 untreated viremic HIV-1-infected subjects with an average viral load of 5.6 × 104 copies of HIV-1 mRNA per ml of plasma (range, 7,890 to 127,000 copies per ml) and an average CD4 cell count of 504 cells per mm3 (range, 47 to 961 cells per mm3), 28 HIV-1-infected subjects receiving highly active antiretroviral therapy (HAART) with undetectable viral loads (<50 copies) for at least 6 months and an average CD4 cell count of 500 cells per mm3 (range, 39 to 1,150 cells per mm3), and 35 elite controllers able to spontaneously control viral replication below detectable limits (50 copies per ml of plasma) with an average CD4 cell count of 774 cells per mm3 (range, 495 to 1,024 cells per mm3). The study was approved by the Massachusetts General Hospital Institutional Review Board, and each subject gave written informed consent. Antibodies were separated from other serum proteins using Melon gel according to the manufacturer's instructions (Thermo Scientific).
THP-1 phagocytosis assay of HIV-specific antibodies.
The THP-1 phagocytosis assay was performed as described previously (4) using YU2 gp120. Phagocytic scores represent integrated mean fluorescence intensity (iMFI) values (frequency × MFI). Each antibody sample was tested over a range of concentrations (0.01 to 100 μg/ml). The concentration of antibody required for half-maximal phagocytosis (PC50) was determined utilizing Prism software.
ADCVI assay.
In a modification of previously published protocols (31), antibody-dependent cellular viral inhibition (ADCVI) was assayed as follows. CD4+ T cell targets were generated using purified peripheral blood mononuclear cells (PBMCs) from healthy control donors, by activating PBMCs with complete RPMI medium supplemented with 50 U/ml of IL-2 and 0.5 μg/ml of a bispecific CD3 CD8 antibody (Ab) (32). Following 3 days of culture, the CD4+ T cells were infected with JRCSF at a multiplicity of infection (MOI) of 0.1 for 4 h at 37°C, washed twice, and plated at 105 cells per well in a 48-well plate containing 100 μl of R10 per well. Three days later, blood was collected from the same donor for the generation of autologous monocytes and natural killer cells. Monocytes were purified following PBMC purification by Ficoll-Hypaque centrifugation using CD14+ magnetic beads (Miltenyi Biotech). NK cells were enriched directly from whole blood by negative selection using RosetteSep (Stem Cell Technologies). Effector cells were added to CD4+ T cells at a 10:1 ratio. Purified patient antibodies were then added to experimental wells at 50 μg/ml. Furthermore, a well containing medium alone and a well containing the neutralizing antibody B12 served as controls. The level of viral replication was then quantified by Gag p24 enzyme-linked immunosorbent assay (ELISA) (PerkinElmer), and the difference in p24 levels in wells containing CD4 cells alone and each experimental well was quantified and expressed as percent inhibition.
gp120, lectin, and FcγR ELISA analysis.
Binding titers of antibodies to YU2 gp120 (NIH AIDS reagents) were determined as described previously (33). Recombinant human mannose-binding protein (MBP; Sino Biological) was labeled with biotin (Pierce) and used to probe antibody characteristics by ELISA as previously described (34). ELISA determinations of antibody binding to FcγR were made by coating nickel functionalized plates (Qiagen) with His6-tagged FcγR extracellular domain (R&D Systems), as described previously (33).
Receptor blocking experiments.
Blocking antibodies to FcγR2a (Abcam), FcγR2b (clone 2B6 [35]), and FcγR3a (Sigma) were used according to the manufacturer's instructions. Cells were preincubated with blocking antibodies for at least 1 h prior to being mixed with opsonized beads and were analyzed as described above. Results are presented as the ratio of phagocytosis of FcγR blocked to untreated cells for each patient sample.
Competition phagocytosis assay.
For a subset of 9 individuals, bulk antibodies were biotinylated (Pierce) and individually used to coat to saturation 1-μm fluorescent neutravidin functionalized red and green fluorescent beads (Invitrogen). Excess soluble antibody was removed by washing, resulting in the generation of both red and green beads equally coated with antibody from each of the 9 subjects. These antibody-coated beads were then used to directly compare phagocytosis in competitive-uptake experiments. Pairwise competition experiments were conducted between all samples by combining equal numbers of red beads coated with antibody from a given subject with green beads coated with antibody from each other subject. As a control, all subjects were also competed against themselves to verify equivalent uptake of identical antibody samples. The mixed beads were incubated with monocytic THP-1 cells overnight. Uptake of beads of each color was determined by flow cytometry. An uptake ratio (calculated as red bead iMFI/green bead iMFI) was determined in order to assess differential uptake mediated by each antibody sample.
IgG istopying.
Antibody subclasses IgG1, -2, -3, and -4 were quantified using the Milliplex map immunoglobulin isoptying kit (Millipore) according to the manufacturer's instructions on a Bio-Plex 200 (Bio-Rad Laboratories).
FcγR Biacore analysis.
For Biacore experiments, research-grade CM5 plasmon surface resonance chips were coated with FcγR1, -2a, -2b, and -3a and an irrelevant protein as a negative control. After equilibration, the chip surface was activated with 30 μl of an equal-volume mixture of N-hydroxysuccinimide (NHS) (0.1 M in water) and EDC (0.1 M in water). Then individual cells were coated with 30 μl of a 40-μg/ml solution of the proteins of interest. Additional protein was injected to reach a minimum of a 1,000-relative-unit signal following injection. Residual NHS esters were deactivated by a 30-μl injection of 1 M ethanolamine, pH 8.5. In parallel, patient antibodies were diluted in phosphate-buffered saline (PBS) to 0.2 mg/ml in 96-well plates and loaded on a Biacore 3000, and binding was quantified as response units.
Statistical analysis.
Differences between subject groups were assessed by a two-tailed Mann-Whitney or unpaired t tests. Where appropriate, analyses of variance (ANOVA) with corrections for multiple tests were performed. All experimental data available were included in each analysis.
RESULTS
Phagocytic activity is enhanced in some subject groups.
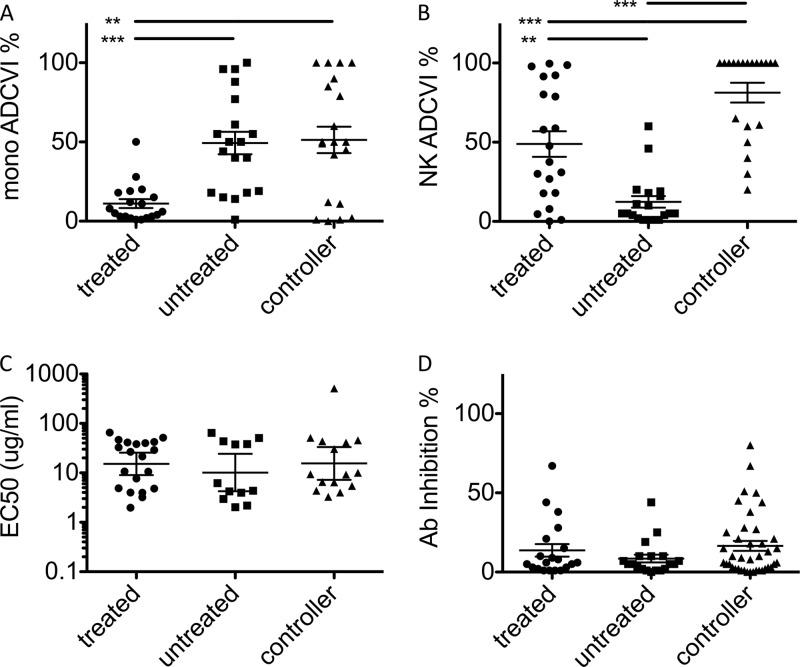
We tested whether antibodies from different patient groups exhibited differential effector functions in assays using primary effector cells and replicating virus. An antibody-dependent cellular viral inhibition (ADCVI) assay was conducted utilizing monocytes or natural killer (NK) cells as primary effector cells in the presence of autologous HIV-infected CD4+ T cells from HIV-negative donors following incubation with purified plasma IgG. When monocytes were used as effectors, antibodies from untreated subjects and controllers exhibited potentiated inhibition of viral replication relative to antibodies from treated subjects (Fig. 1A). In contrast, antibodies from treated subjects and controllers suppressed viral replication most profoundly in the presence of NK cells (Fig. 1B). These divergent effector function profiles could not be explained by differences in either titer or neutralization activity, which did not differ significantly among subject groups (Fig. 1C and D) and were not correlated with effector function. Collectively, these results indicate that specific features, such as IgG subclass and/or glycosylation state, rather than prevalence of HIV-specific antibodies may account for their enhanced activity.
Fig 1.
Differential effector function of antibodies from HIV-positive subjects. (A and B) ADCVI activities of antibodies from different subject groups in assays utilizing primary monocytes (A) or NK cells (B) as effectors. (C) Binding titers (EC50, in μg/ml) of anti-envelope antibodies. (D) Antibody neutralization as determined by viral inhibition in the absence of effector cells. **, P < 0.005; ***, P < 0.0005.
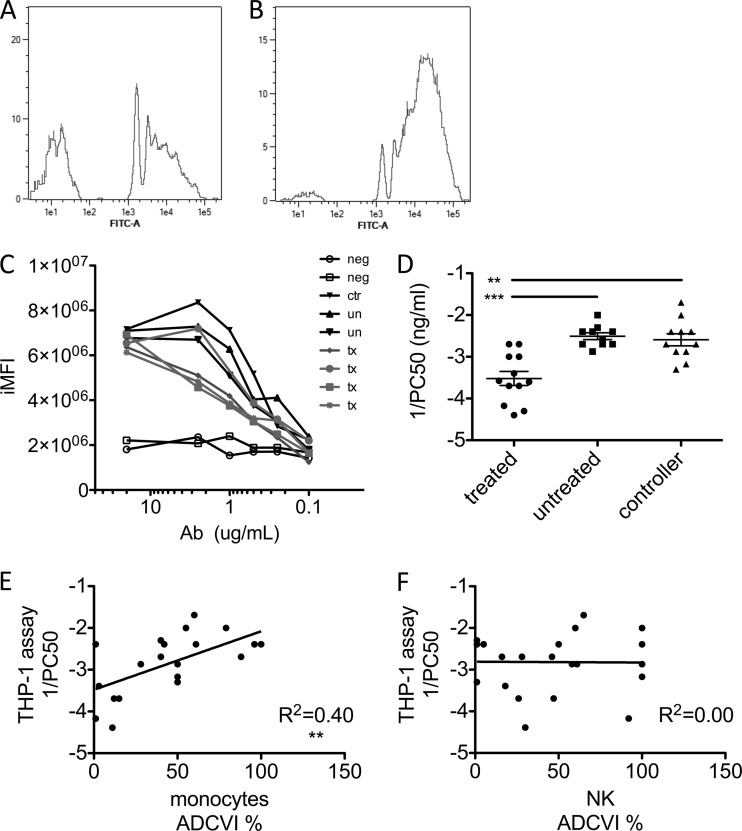
To gain deeper insights related to the observed differences in antibody-mediated monocyte antiviral suppressive activity, we applied a higher-throughput method, utilizing a flow cytometric assay that allows the quantitative analysis of HIV-specific antibody-mediated phagocytosis (4, 36). Fluorescent beads were functionalized with gp120 and incubated with various amounts of purified subject antibody in the presence of monocytic THP-1 cells. Significantly, only antibodies from infected subjects resulted in bead uptake, and despite exhibiting similar maximal levels of phagocytosis, there were dramatic differences in the concentration dependence of phagocytosis among subjects (Fig. 2A to C). Therefore, as a means to determine phagocytic potency, dose-response curves were used to calculate the concentration of antibody necessary to elicit half-maximal phagocytosis (PC50). Figure 2D presents a log plot of reciprocal PC50 for each of the HIV-infected subject groups, demonstrating elevated phagocytic activity of antibodies from spontaneous controllers and untreated progressors compared to subjects receiving antiretroviral therapy.
Fig 2.
Differential phagocytic function measured at high throughput. (A and B) Representative histograms of bead uptake by THP-1 cells for antibody samples with low (A) and high (B) phagocytosis activity. (C) Representative phagocytosis dose-response curves for HIV-negative subjects (neg), controllers (ctr), untreated subjects (un), and treated subjects (tx). Phagocytic activity is presented as iMFI. (D) Phagocytic potency of antibodies from HIV-infected subjects. Reciprocal log PC50s (1/concentration at which half-maximal phagocytosis was observed) for gp120-coated bead uptake by a monocytic cell line are shown. (E and F) Correlation of viral inhibition in primary monocytes (E) or NK cells (F) with phagocytic potential (1/PC50) determined by the THP-1 phagocytosis assay. **, P < 0.005; ***, P < 0.0005.
Importantly, despite the substitution of a cell line for primary cells and antigen-coated beads for virus, the high-throughput phagocytosis assay demonstrated the same pattern of activity among subject groups. Furthermore, good agreement was observed between assays (Fig. 2E), indicating that the bead-based phagocytosis assay may provide a meaningful metric of phagocytic processes relevant to HIV outgrowth and replication in primary cells. ADCVI assays conducted with NK cells (which express only FcγRIIIa) as effectors showed no correlation with THP-1 phagocytosis (Fig. 2F), indicating that in combination with differential expression of FcγR, specific Fc domain characteristics may have a strong impact on effector function. Thus, both the primary cell inhibition assay and the high-throughput phagocytic assay strongly suggested that both controllers and untreated chronic progressors possess HIV-specific antibodies with an enhanced capacity to trigger phagocytosis of immune complexes.
Differential reliance on FcγR2a and FcγR2b among subject groups.
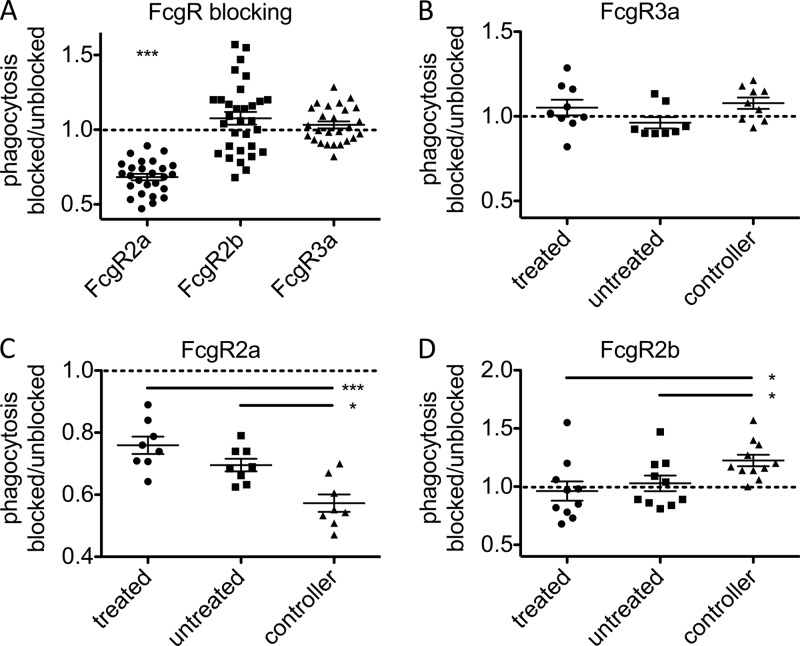
We next utilized FcγR-blocking antibodies to directly investigate the role of individual FcγRs in modulating phagocytosis driven by HIV-specific antibodies. Immune complex uptake was determined in the presence and absence of FcγR-blocking antibodies, and the ratio of phagocytosis observed for blocked to available receptor was determined for each sample. Figure 3A presents the results of blocking FcγR2a, FcγR2b, and FcγR3a. Across all subject groups, only FcγR2a blockade was found to significantly affect phagocytosis. Consistent with previous studies (37, 38), no effect was observed when FcγR3a, which is not implicated in phagocytosis and is expressed at low levels in THP-1 cells, was blocked (Fig. 3B). Blocking FcγR2a had a differential effect on the capacity of antibodies to induce phagocytosis in each class of subjects, with a profound decrease observed in phagocytosis for antibodies from controllers (Fig. 3C), indicating an increased reliance on this receptor in driving phagocytosis in this patient group. Interestingly, when the inhibitory FcγR2b was blocked, there was no effect on phagocytosis for antibodies from chronically infected patient samples, but a marked increase in phagocytosis was observed in antibodies from controllers (Fig. 3D). While factors modulating recognition of antibody Fc regions by FcγR2a and FcγR2b are not well characterized, these surprising data are consistent with the possibility that antibodies from these subject groups possess unique capacities to drive phagocytosis via differential interactions with FcγR2a and FcγR2b.
Fig 3.
Phagocytic dependence on specific FcγR. (A) Phagocytosis by cells pretreated with FcγR2a-, FcγR2b-, and FcγR3a-blocking antibodies or left untreated was determined. The ratio of bead uptake under blocked and unblocked conditions is presented. The effect of blocking FcγR3a (B), FcγR2a (C), and FcγR2b (D) was determined within each subject class, exposing differences between patient groups in reliance on these receptors. *, P < 0.05; ***, P < 0.0005.
The phagocytic activity of antibodies from controllers outcompetes antibodies from chronic progressors.
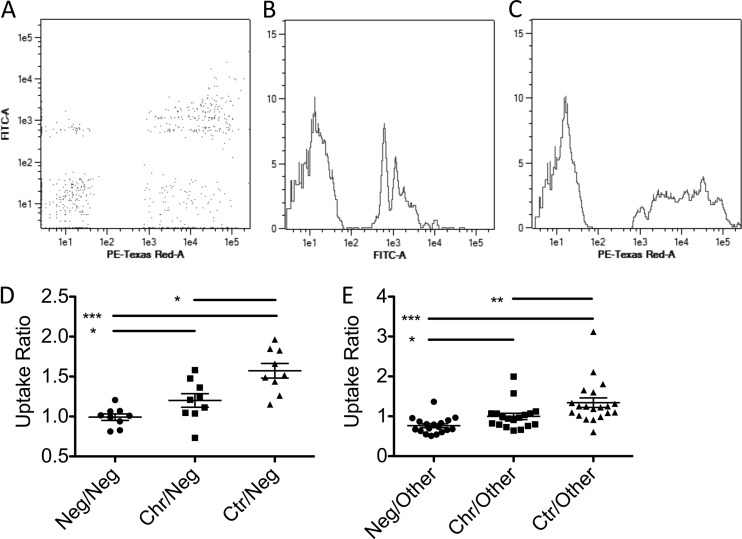
Given the surprising results of receptor blocking experiments, and because HIV infection is known to globally perturb the antibody compartment (39–41), we next attempted to determine whether the activity differences observed in the HIV-specific compartment were generalized to the bulk plasma IgG pool. Therefore, competition experiments were performed in which red or green fluorescent beads were opsonized with biotinylated bulk antibodies from controllers, treated chronic progressors, or HIV-negative patients. Red fluorescent antibody-coated beads from each subject were mixed with green fluorescent antibody-coated beads from each of the other subjects and were incubated with monocytic THP-1 cells overnight in a competitive assay of phagocytic potential. The ratio of uptake for red to green beads was calculated for each pairwise comparison, and importantly, when beads of both colors were opsonized with the same subject sample, equivalent uptakes of red and green beads were observed, resulting in uptake ratios equal to 1 (data not shown). Similarly, when uptake ratios were calculated for competition between members of the same class (e.g., HIV-negative subject A versus HIV-negative subject B), uptake ratios were more widely distributed, but also centered around 1 (data not shown).
In contrast, bulk plasma antibodies from HIV-infected patient populations drove elevated bead uptake compared to bulk plasma antibodies derived from healthy controls (Fig. 4A to D), indicating that antibodies from HIV-positive subjects possess an enhanced capacity to drive phagocytosis. Among HIV-infected subjects, bulk plasma antibodies from controllers induced significantly higher levels of bead uptake than did antibodies from all other subject groups (Fig. 4E). Together, these data suggest that global inflammatory cues associated with infection and viral replication may lead to global alterations in antibody composition or characteristics that enhance phagocytic activity.
Fig 4.
Plasma IgG exhibits differential phagocytosis activity among subject groups. (A to C) Representative flow cytometric dot plot (A) and histograms (B and C) of competitive uptake of green fluorescent beads (B) coated with bulk antibody from a low-activity subject relative to uptake of red fluorescent beads (C) opsonized with bulk antibody from a high-activity subject. (D and E) Beads opsonized with bulk antibody from negative subjects were competed against other HIV-negative subjects (Neg), chronically infected subjects (Chr), or controllers (Ctr) (D) or all other subjects (E). Data presented are ratios of phagocytosis of red to green beads. *, P < 0.05; **, P < 0.005; ***, P < 0.0005.
Global IgG features which may account for enhanced phagocytic activity.
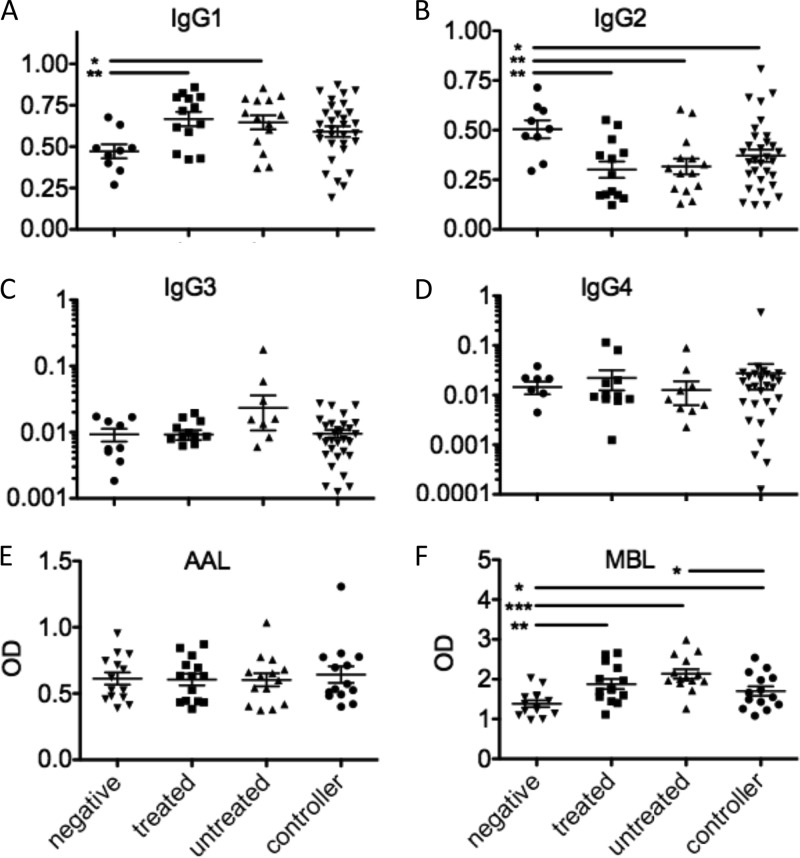
HIV infection is known to drive substantial IgG1 hypergammaglobulinemia and variance in bulk plasma antibody glycosylation (39, 40), offering tractable mechanisms that may underlie differences in phagocytic activity. Thus, to determine whether altered induction of any specific IgG subclass might account for differential phagocytic activity, we compared the global plasma distributions of IgG subclasses among subject groups. Increased IgG1 prevalence was observed in chronically infected subjects (P < 0.05), but not spontaneous controllers, compared to HIV-negative controls (Fig. 5A). The prevalence of IgG2, the second most abundant subclass, was found to decrease significantly in all HIV-infected subjects, including controllers (P < 0.05 for all comparisons) (Fig. 5B). No significant perturbations in the levels of IgG3 and IgG4, minor components of plasma IgG, were observed (Fig. 5C and D). Similarly, antibody binding to Aleutia aurantia lectin (AAL), a lectin which recognizes fucose, did not differ among subjects (Fig. 5E). Mannose-binding protein (MBP), a plasma lectin which functions in the complement cascade and recognizes terminal mannose residues, was enriched in all HIV-infected subjects, but at a reduced level in controllers (Fig. 5F). Thus, while skewing of both IgG subclass and glycosylation was apparent in bulk plasma IgG from all HIV-infected subject groups, the skewing observed was not consistent with the differences observed in phagocytic activity.
Fig 5.
Plasma antibody subclass and glycosylation. (A to D) Fraction of plasma IgG of the IgG1 (A), IgG2 (B), IgG3 (C), and IgG4 (D) subclasses. (E and F) Plasma IgG glycosylation as determined by ELISA for fucose (E) or terminal mannose (F). OD, optical density. *, P < 0.05; **, P < 0.005; ***, P < 0.0005.
Changes in Fc receptor binding are associated with differential antibody-mediated phagocytic activity.
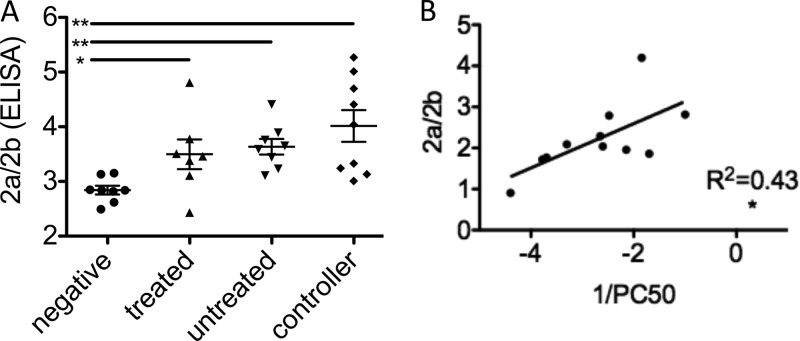
Thus, to determine whether specific changes occurred within the antibody pool among populations resulting in differing capacities to engage Fc receptors, we next assessed the ability of antibodies from different HIV+ subject populations to bind to FcγR, using biophysical and analytical experiments. Interestingly, HIV-infected patient populations exhibited elevated binding to FcγR2a compared to that for healthy controls by both ELISA and Biacore analysis (data not shown). Because the inflammatory activity of antibodies can be tempered by their ability to bind to the inhibitory FcγR2b receptor, also expressed on some phagocytes, some investigators have considered determination of a ratio of recognition by activating relative to inhibitory receptors the most accurate means of characterizing effector activity (42). Accordingly, ratios of FcγR2a to FcγR2b were evaluated, and they demonstrated that HIV-infected subjects exhibited preferential binding to FcγR2a over the inhibitory FcγR2b relative to healthy controls (Fig. 6A), with controllers exhibiting the highest activating potential. This trend toward increasing recognition by an activating receptor indicates a greater inflammatory potential, consistent with the results of phagocytic competition experiments, and may therefore account for the differences observed in antibody-mediated phagocytic activity between subject populations.
Fig 6.
Differential phagocytosis is associated with binding to FcγR2. (A) Ratio of binding to the activating FcγR2a relative to the inhibitory FcγR2b as determined by ELISA. (B) Correlation of log phagocytic potency (1/PC50) with binding to FcγR2a/FcγR2b (Biacore analysis). *, P < 0.05; **, P < 0.005.
FcγR2a/FcγR2b binding ratio predicts phagocytic activity.
Lastly, to determine whether FcγR2a/FcγR2b activity predicted the potency of antibody-mediated phagocytosis, the ratio of FcγR2a to FcγR2b binding was compared to the phagocytic potency for each sample tested. Figure 6B presents the positive correlation observed between the ratio of binding to receptor 2a to 2b and phagocytic potency (1/PC50). Significantly, 2a/2b ratios from both Biacore and ELISA measurements were correlated with phagocytic potency. Thus, these results are consistent with a mechanism whereby antibodies that preferentially bind to FcγR2a relative to FcγR2b have a superior ability to induce phagocytosis of immune complexes.
DISCUSSION
Fc-mediated antibody effector functions serve as a mechanism whereby antibodies can provide therapeutic benefit. In HIV, genetic evidence has been split as to the role of antibody engagement of innate immunity in protection from or after infection. High-affinity polymorphisms of FcγR3a, while protective in monoclonal antibody therapy of cancer, have been associated with HIV progression (43). Similarly, the allele of FcγR2a with improved recognition of IgG2 subclass antibodies has been found to be a risk factor in neonatal HIV transmission (22), whereas it has been associated with protection from progression in adults (21). Yet passive-transfer studies of both neutralizing monoclonal and vaccine-induced nonneutralizing antibodies have implicated antibody effector functions in protection (9, 44, 45). More recently, there has been speculation that the modest degree of protection observed in the RV144 trial may have been due to antibody effector mechanisms (46).
Here we show that antibodies from controllers exhibit enhanced humoral phagocytic potential and that this potentiation is related to the natural induction of antibodies with a propensity to bind the activating FcγR2a over its inhibitory FcγR2b counterpart. Given their high degree of sequence similarity, we were surprised to observe differential binding of antibodies to these receptors. Specificity to the activating or inhibitory receptor can be mediated by amino acid point mutations in the antibody Fc domain (47), and differences in binding to FcγR2a and FcγR2b among IgG subclasses have also been noted (1), supporting the possibility that these Fc receptors display some unique specificity for their antibody ligands. Receptor blocking experiments likewise demonstrated distinct mechanistic differences in FcγR usage from each subject group. These data strongly suggest that the immune system is able to tune the antibody effector profile to naturally produce antibodies with specific effector functions. While strong glycan-based modulations of interactions with FcγR3a have been described, glycan modifications that differentially modulate FcγR2a and FcγR2b binding have yet to be described. Careful dissection of the antibody profiles among subjects may provide new opportunities to define the specific glycan profiles that modulate binding to FcγR2a relative to FcγR2b, potentially providing critical information to drive the production of antibodies potentiated for this particular effector function.
Perhaps surprisingly, the phagocytic activity of antibodies from controllers was most similar to that of untreated subjects, in whom circulating virus, inflammation, and immune activation are high. Interestingly, while controllers durably maintain viral replication to undetectable levels in plasma, recent reports suggest that these individuals have elevated blood levels of microbial products, indicating that they may have residual viral replication within their gut (48). Residual replication may induce low-grade inflammation driving hypergammaglobulinemia, the production of antibodies with an inflammatory glycan, and the skewed interactions with FcγR2a and FcγR2b associated with potentiated capacity to induce phagocytosis observed in this study. While the subclass skewing in controllers was more subtle than in other HIV-positive subject classes, the presence of (i) high antibody titers, (ii) decreased plasma IgG2 levels and a trend toward elevated IgG1, and (iii) increased binding of plasma IgG to the complement protein MBP are marks of inflammation and stimulation even in the absence of detectable viral replication in this patient population.
While this study focused on antibody-driven phagocytosis, striking differences in viral suppression mediated by the same antibody samples were observed depending on the effector cell type utilized. Indeed, complex antibody functions do not necessarily correlate with titer or among different effector cell populations (49, 50). The observation that antibody titer does not predict effector function indicates that there are qualitative antibody features that can disparately affect function in different cellular assays; the “active” fraction of antibody may be only a component of the total antibody measured, and this fraction may vary among subjects. In this study, NK cell ADCVI activity differed significantly from monocyte ADCVI activity. Despite the fact that IgG1 and IgG3 antibodies are implicated as being important to both NK ADCC and phagocytosis, there are a number of differences between these effector cell types that may account for their differential engagement by antibody.
First, NK cells express only FcγR3a (51), and this receptor is sensitive to Fc domain fucosylation (52). Phagocytes can express FcγR3a but typically do so at lower levels, relying primarily on FcγR2a, which may also bind to a broader array of subclasses, but is insensitive to Fc domain fucosylation, for activity (18, 53). Thus, differences in IgG subclass distribution and glycosylation among or even within subclasses (particularly IgG1 and IgG3) within the broader pool of HIV-specific antibodies present in each patient population may lead to altered recruitment of innate immune cell subsets expressing different FcγRs, resulting in the differential antiviral clearance observed in this study. Consistent with the striking differences observed between effector cell types here, in a previous study IgG fucosylation was found to have opposing impacts on polymorphonuclear leukocyte (PMN) and mononuclear cell ADCC activity even in the context of a monoclonal antibody (50). Future in-depth analysis of glycosylation of HIV-specific antibodies may provide key insights into these divergent functional profiles.
Larger questions pertain to defining the humoral mechanism that may afford the greatest level of protection in HIV, and evidence as to the possible importance of phagocytosis in contributing to slower HIV disease progression has been accumulating (21, 23, 54). Moreover, often in the in vivo data in which ADCC or ADCVI has been implicated in protection, it has been difficult to separate whether distinct FcγR-based mechanisms, such as ADCC or phagocytosis or a combination of these and other effector activities, are involved in the protection observed. Studies aimed at defining the role of NK cells within the gut have demonstrated that these cells are found at relatively low frequencies, and a neutralizing antibody with potentiated NK effector function did not provide improved protection, suggesting that other Fc receptor-bearing innate immune cells may play a more central role in antiviral containment at this site (27, 55). In contrast, the gut and other mucosal membranes are abundantly lined with phagocytes. Thus, it is plausible that the activity difference observed in this in vitro study may have an impact in vivo. Furthermore, phagocytosis may be important not only in the rapid removal of inflammatory immune complexes or infectious particles but also in driving and regulating the adaptive immune response via phagocytic antigen-presenting cells (56, 57).
In this study, we have shown that antibodies from controllers and untreated chronic progressors exhibit increased phagocytic activity relative to antibodies from treated progressors. Beyond differences in phagocytic uptake, antibodies from controllers exhibited differential interactions with the activating FcγR2a and the inhibitory FcγR2b compared to chronic progressors, exhibiting a preference for the FcγR2a yet greater inhibition of phagocytosis driven by FcγR2b. Because the route of phagocytosis and receptors involved have been shown to alter downstream processing and cross-presentation of pathogens (58–60), patterns of receptor usage may dramatically impact the rate, downstream signaling, and outcome of immune complex clearance via this mechanism. Further elucidation of the role of these receptors in different cellular subsets will be critical to understanding their impact on HIV acquisition and progression. Continued research to define the properties that may provide specificity for FcγR2a over FcγR2b will be important for the design of potential monoclonal therapeutics for passive transfer as well as vaccines that can specifically induce these types of humoral immune responses. In HIV, however, defining specific features of the antibody Fc domain and effector mechanisms that may provide robust protection against or after infection remains a critical goal.
ACKNOWLEDGMENTS
These studies were supported by the Collaboration for AIDS Vaccine Discovery (43307): HIV Controllers: Implications for Vaccine Design and the Susan and Philip T. Ragon Foundation. M.E.A. was supported by a Harvard University Center for AIDS Research postdoctoral fellowship (HU CFAR NIH/NIAID fund 2P30AI060354-07).
M.E.A., D.J.I., and G.A. designed the research, analyzed data, and wrote the paper. M.E.A., A.-S.D., E.G.M., S.T., and A.F.L. performed research.
The authors have no conflicts of interest to declare.
Footnotes
Published ahead of print 6 March 2013
REFERENCES
- 1. Takai T. 2005. Fc receptors and their role in immune regulation and autoimmunity. J. Clin. Immunol. 25:1–18 [DOI] [PubMed] [Google Scholar]
- 2. Aasa-Chapman MM, Holuigue S, Aubin K, Wong M, Jones NA, Cornforth D, Pellegrino P, Newton P, Williams I, Borrow P, McKnight A. 2005. Detection of antibody-dependent complement-mediated inactivation of both autologous and heterologous virus in primary human immunodeficiency virus type 1 infection. J. Virol. 79:2823–2830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ackerman ME, Dugast AS, Alter G. 2012. Emerging concepts on the role of innate immunity in the prevention and control of HIV infection. Annu. Rev. Med. 63:113–130 [DOI] [PubMed] [Google Scholar]
- 4. Ackerman ME, Moldt B, Wyatt RT, Dugast AS, McAndrew E, Tsoukas S, Jost S, Berger CT, Sciaranghella G, Liu Q, Irvine DJ, Burton DR, Alter G. 2011. A robust, high-throughput assay to determine the phagocytic activity of clinical antibody samples. J. Immunol. Methods 366:8–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ahmad R, Sindhu ST, Toma E, Morisset R, Vincelette J, Menezes J, Ahmad A. 2001. Evidence for a correlation between antibody-dependent cellular cytotoxicity-mediating anti-HIV-1 antibodies and prognostic predictors of HIV infection. J. Clin. Immunol. 21:227–233 [DOI] [PubMed] [Google Scholar]
- 6. Burton DR, Dwek RA. 2006. Immunology. Sugar determines antibody activity. Science 313:627–628 [DOI] [PubMed] [Google Scholar]
- 7. Spiegelberg HL. 1974. Biological activities of immunoglobulins of different classes and subclasses. Adv. Immunol. 19:259–294 [DOI] [PubMed] [Google Scholar]
- 8. Forthal DN, Landucci G, Haubrich R, Keenan B, Kuppermann BD, Tilles JG, Kaplan J. 1999. Antibody-dependent cellular cytotoxicity independently predicts survival in severely immunocompromised human immunodeficiency virus-infected patients. J. Infect. Dis. 180:1338–1341 [DOI] [PubMed] [Google Scholar]
- 9. Hessell AJ, Hangartner L, Hunter M, Havenith CE, Beurskens FJ, Bakker JM, Lanigan CM, Landucci G, Forthal DN, Parren PW, Marx PA, Burton DR. 2007. Fc receptor but not complement binding is important in antibody protection against HIV. Nature 449:101–104 [DOI] [PubMed] [Google Scholar]
- 10. Sawyer LA, Katzenstein DA, Hendry RM, Boone EJ, Vujcic LK, Williams CC, Zeger SL, Saah AJ, Rinaldo CR, Jr, Phair JP, et al. 1990. Possible beneficial effects of neutralizing antibodies and antibody-dependent, cell-mediated cytotoxicity in human immunodeficiency virus infection. AIDS Res. Hum. Retroviruses 6:341–356 [DOI] [PubMed] [Google Scholar]
- 11. Tomaras GD, Yates NL, Liu P, Qin L, Fouda GG, Chavez LL, Decamp AC, Parks RJ, Ashley VC, Lucas JT, Cohen M, Eron J, Hicks CB, Liao HX, Self SG, Landucci G, Forthal DN, Weinhold KJ, Keele BF, Hahn BH, Greenberg ML, Morris L, Karim SS, Blattner WA, Montefiori DC, Shaw GM, Perelson AS, Haynes BF. 2008. Initial B-cell responses to transmitted human immunodeficiency virus type 1: virion-binding immunoglobulin M (IgM) and IgG antibodies followed by plasma anti-gp41 antibodies with ineffective control of initial viremia. J. Virol. 82:12449–12463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tyler DS, Lyerly HK, Weinhold KJ. 1989. Anti-HIV-1 ADCC. AIDS Res. Hum. Retroviruses 5:557–563 [DOI] [PubMed] [Google Scholar]
- 13. Bouglé A, Max A, Mongardon N, Grimaldi D, Pene F, Rousseau C, Chiche JD, Bedos JP, Vicaut E, Mira JP. 2012. Protective effects of FCGR2A polymorphism in invasive pneumococcal diseases. Chest 142:1474–1481 [DOI] [PubMed] [Google Scholar]
- 14. Cheung NK, Sowers R, Vickers AJ, Cheung IY, Kushner BH, Gorlick R. 2006. FCGR2A polymorphism is correlated with clinical outcome after immunotherapy of neuroblastoma with anti-GD2 antibody and granulocyte macrophage colony-stimulating factor. J. Clin. Oncol. 24:2885–2890 [DOI] [PubMed] [Google Scholar]
- 15. Smith KG, Clatworthy MR. 2010. FcgammaRIIB in autoimmunity and infection: evolutionary and therapeutic implications. Nat. Rev. Immunol. 10:328–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van der Pol WL, van den Berg LH, Scheepers RH, van der Bom JG, van Doorn PA, van Koningsveld R, van den Broek MC, Wokke JH, van de Winkel JG. 2000. IgG receptor IIa alleles determine susceptibility and severity of Guillain-Barre syndrome. Neurology 54:1661–1665 [DOI] [PubMed] [Google Scholar]
- 17. van Sorge NM, van der Pol WL, van de Winkel JG. 2003. FcgammaR polymorphisms: implications for function, disease susceptibility and immunotherapy. Tissue Antigens 61:189–202 [DOI] [PubMed] [Google Scholar]
- 18. Bruhns P. 2012. Properties of mouse and human IgG receptors and their contribution to disease models. Blood 119:5640–5649 [DOI] [PubMed] [Google Scholar]
- 19. Clynes R, Maizes JS, Guinamard R, Ono M, Takai T, Ravetch JV. 1999. Modulation of immune complex-induced inflammation in vivo by the coordinate expression of activation and inhibitory Fc receptors. J. Exp. Med. 189:179–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Samuelsson A, Towers TL, Ravetch JV. 2001. Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science 291:484–486 [DOI] [PubMed] [Google Scholar]
- 21. Forthal DN, Landucci G, Bream J, Jacobson LP, Phan TB, Montoya B. 2007. FcgammaRIIa genotype predicts progression of HIV infection. J. Immunol. 179:7916–7923 [DOI] [PubMed] [Google Scholar]
- 22. Brouwer KC, Lal RB, Mirel LB, Yang C, van Eijk AM, Ayisi J, Otieno J, Nahlen BL, Steketee R, Lal AA, Shi YP. 2004. Polymorphism of Fc receptor IIa for IgG in infants is associated with susceptibility to perinatal HIV-1 infection. AIDS 18:1187–1194 [DOI] [PubMed] [Google Scholar]
- 23. Ngo-Giang-Huong N, Candotti D, Goubar A, Autran B, Maynart M, Sicard D, Clauvel JP, Agut H, Costagliola D, Rouzioux C. 2001. HIV type 1-specific IgG2 antibodies: markers of helper T cell type 1 response and prognostic marker of long-term nonprogression. AIDS Res. Hum. Retroviruses 17:1435–1446 [DOI] [PubMed] [Google Scholar]
- 24. Dugast AS, Tonelli A, Berger CT, Ackerman ME, Sciaranghella G, Liu Q, Sips M, Toth I, Piechocka-Trocha A, Ghebremichael M, Alter G. 2011. Decreased Fc receptor expression on innate immune cells is associated with impaired antibody-mediated cellular phagocytic activity in chronically HIV-1 infected individuals. Virology 415:160–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huber M, Fischer M, Misselwitz B, Manrique A, Kuster H, Niederost B, Weber R, von Wyl V, Gunthard HF, Trkola A. 2006. Complement lysis activity in autologous plasma is associated with lower viral loads during the acute phase of HIV-1 infection. PLoS Med. 3:e441 doi:10.1371/journal.pmed.0030441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lambotte O, Ferrari G, Moog C, Yates NL, Liao HX, Parks RJ, Hicks CB, Owzar K, Tomaras GD, Montefiori DC, Haynes BF, Delfraissy JF. 2009. Heterogeneous neutralizing antibody and antibody-dependent cell cytotoxicity responses in HIV-1 elite controllers. AIDS 23:897–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moldt B, Shibata-Koyama M, Rakasz EG, Schultz N, Kanda Y, Dunlop DC, Finstad SL, Jin C, Landucci G, Alpert MD, Dugast AS, Parren PW, Nimmerjahn F, Evans DT, Alter G, Forthal DN, Schmitz JE, Iida S, Poignard P, Watkins DI, Hessell AJ, Burton DR. 2012. A nonfucosylated variant of the anti-HIV-1 MAb b12 has enhanced FcγRIIIa-mediated antiviral activity in vitro but does not improve protection against mucosal SHIV challenge in macaques. J. Virol. 86:6189–6196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Holl V, Hemmerter S, Burrer R, Schmidt S, Bohbot A, Aubertin AM, Moog C. 2004. Involvement of Fc gamma RI (CD64) in the mechanism of HIV-1 inhibition by polyclonal IgG purified from infected patients in cultured monocyte-derived macrophages. J. Immunol. 173:6274–6283 [DOI] [PubMed] [Google Scholar]
- 29. Holl V, Peressin M, Schmidt S, Decoville T, Zolla-Pazner S, Aubertin AM, Moog C. 2006. Efficient inhibition of HIV-1 replication in human immature monocyte-derived dendritic cells by purified anti-HIV-1 IgG without induction of maturation. Blood 107:4466–4474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tudor D, Bomsel M. 2011. The broadly neutralizing HIV-1 IgG 2F5 elicits gp41-specific antibody-dependent cell cytotoxicity in a FcgammaRI-dependent manner. AIDS 25:751–759 [DOI] [PubMed] [Google Scholar]
- 31. Florese RH, Van Rompay KK, Aldrich K, Forthal DN, Landucci G, Mahalanabis M, Haigwood N, Venzon D, Kalyanaraman VS, Marthas ML, Robert-Guroff M. 2006. Evaluation of passively transferred, nonneutralizing antibody-dependent cellular cytotoxicity-mediating IgG in protection of neonatal rhesus macaques against oral SIVmac251 challenge. J. Immunol. 177:4028–4036 [DOI] [PubMed] [Google Scholar]
- 32. Alter G, Martin MP, Teigen N, Carr WH, Suscovich TJ, Schneidewind A, Streeck H, Waring M, Meier A, Brander C, Lifson JD, Allen TM, Carrington M, Altfeld M. 2007. Differential natural killer cell-mediated inhibition of HIV-1 replication based on distinct KIR/HLA subtypes. J. Exp. Med. 204:3027–3036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moldt B, Schultz N, Dunlop DC, Alpert MD, Harvey JD, Evans DT, Poignard P, Hessell AJ, Burton DR. 2011. A panel of IgG1 b12 variants with selectively diminished or enhanced affinity for Fcgamma receptors to define the role of effector functions in protection against HIV. J. Virol. 85:10572–10581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Terai I, Kobayashi K, Vaerman JP, Mafune N. 2006. Degalactosylated and/or denatured IgA, but not native IgA in any form, bind to mannose-binding lectin. J. Immunol. 177:1737–1745 [DOI] [PubMed] [Google Scholar]
- 35. Veri MC, Gorlatov S, Li H, Burke S, Johnson S, Stavenhagen J, Stein KE, Bonvini E, Koenig S. 2007. Monoclonal antibodies capable of discriminating the human inhibitory Fcgamma-receptor IIB (CD32B) from the activating Fcgamma-receptor IIA (CD32A): biochemical, biological and functional characterization. Immunology 121:392–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McAndrew EG, Dugast AS, Licht AF, Eusebio JR, Alter G, Ackerman ME. 2011. Determining the phagocytic activity of clinical antibody samples. J. Vis. Exp. 2011:e3588 doi:10.3791/3588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cox D, Greenberg S. 2001. Phagocytic signaling strategies: Fc(gamma)receptor-mediated phagocytosis as a model system. Semin. Immunol. 13:339–345 [DOI] [PubMed] [Google Scholar]
- 38. Grage-Griebenow E, Flad HD, Ernst M, Bzowska M, Skrzeczynska J, Pryjma J. 2000. Human MO subsets as defined by expression of CD64 and CD16 differ in phagocytic activity and generation of oxygen intermediates. Immunobiology 202:42–50 [DOI] [PubMed] [Google Scholar]
- 39. Kekow J, Hobusch G, Gross WL. 1988. Predominance of the IgG1 subclass in the hypergammaglobulinemia observed in pre-AIDS and AIDS. Cancer Detect. Prev. 12:211–216 [PubMed] [Google Scholar]
- 40. Moore JS, Wu X, Kulhavy R, Tomana M, Novak J, Moldoveanu Z, Brown R, Goepfert PA, Mestecky J. 2005. Increased levels of galactose-deficient IgG in sera of HIV-1-infected individuals. AIDS 19:381–389 [DOI] [PubMed] [Google Scholar]
- 41. Ackerman M, et al. In press. [Google Scholar]
- 42. Nimmerjahn F, Ravetch JV. 2005. Divergent immunoglobulin G subclass activity through selective Fc receptor binding. Science 310:1510–1512 [DOI] [PubMed] [Google Scholar]
- 43. Poonia B, Kijak GH, Pauza CD. 2010. High affinity allele for the gene of FCGR3A is risk factor for HIV infection and progression. PLoS One 5:e15562 doi:10.1371/journal.pone.0015562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Florese RH, Demberg T, Xiao P, Kuller L, Larsen K, Summers LE, Venzon D, Cafaro A, Ensoli B, Robert-Guroff M. 2009. Contribution of nonneutralizing vaccine-elicited antibody activities to improved protective efficacy in rhesus macaques immunized with Tat/Env compared with multigenic vaccines. J. Immunol. 182:3718–3727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Van Rompay KK, Berardi CJ, Dillard-Telm S, Tarara RP, Canfield DR, Valverde CR, Montefiori DC, Cole KS, Montelaro RC, Miller CJ, Marthas ML. 1998. Passive immunization of newborn rhesus macaques prevents oral simian immunodeficiency virus infection. J. Infect. Dis. 177:1247–1259 [DOI] [PubMed] [Google Scholar]
- 46. Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birx DL, Chunsuttiwat S, Khamboonruang C, Thongcharoen P, Robb ML, Michael NL, Kunasol P, Kim JH. 2009. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 361:2209–2220 [DOI] [PubMed] [Google Scholar]
- 47. Richards JO, Karki S, Lazar GA, Chen H, Dang W, Desjarlais JR. 2008. Optimization of antibody binding to FcgammaRIIa enhances macrophage phagocytosis of tumor cells. Mol. Cancer Ther. 7:2517–2527 [DOI] [PubMed] [Google Scholar]
- 48. Hunt PW, Brenchley J, Sinclair E, McCune JM, Roland M, Page-Shafer K, Hsue P, Emu B, Krone M, Lampiris H, Douek D, Martin JN, Deeks SG. 2008. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J. Infect. Dis. 197:126–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Brown EP, Licht AF, Dugast AS, Choi I, Bailey-Kellogg C, Alter G, Ackerman ME. 2012. High-throughput, multiplexed IgG subclassing of antigen-specific antibodies from clinical samples. J. Immunol. Methods 386:117–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Peipp M, Lammerts van Bueren JJ, Schneider-Merck T, Bleeker WW, Dechant M, Beyer T, Repp R, van Berkel PH, Vink T, van de Winkel JG, Parren PW, Valerius T. 2008. Antibody fucosylation differentially impacts cytotoxicity mediated by NK and PMN effector cells. Blood 112:2390–2399 [DOI] [PubMed] [Google Scholar]
- 51. Anderson P, Caligiuri M, O'Brien C, Manley T, Ritz J, Schlossman SF. 1990. Fc gamma receptor type III (CD16) is included in the zeta NK receptor complex expressed by human natural killer cells. Proc. Natl. Acad. Sci. U. S. A. 87:2274–2278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shields RL, Lai J, Keck R, O'Connell LY, Hong K, Meng YG, Weikert SH, Presta LG. 2002. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J. Biol. Chem. 277:26733–26740 [DOI] [PubMed] [Google Scholar]
- 53. Shashidharamurthy R, Zhang F, Amano A, Kamat A, Panchanathan R, Ezekwudo D, Zhu C, Selvaraj P. 2009. Dynamics of the interaction of human IgG subtype immune complexes with cells expressing R and H allelic forms of a low-affinity Fc gamma receptor CD32A. J. Immunol. 183:8216–8224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. French MA, Tanaskovic S, Law MG, Lim A, Fernandez S, Ward LD, Kelleher AD, Emery S. 2010. Vaccine-induced IgG2 anti-HIV p24 is associated with control of HIV in patients with a ‘high-affinity’ FcgammaRIIa genotype. AIDS 24:1983–1990 [DOI] [PubMed] [Google Scholar]
- 55. Sips M, Sciaranghella G, Diefenbach T, Dugast AS, Berger CT, Liu Q, Kwon D, Ghebremichael M, Estes JD, Carrington M, Martin JN, Deeks SG, Hunt PW, Alter G. 2012. Altered distribution of mucosal NK cells during HIV infection. Mucosal Immunol. 5:30–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Igietseme JU, Eko FO, He Q, Black CM. 2004. Antibody regulation of T-cell immunity: implications for vaccine strategies against intracellular pathogens. Expert Rev. Vaccines 3:23–34 [DOI] [PubMed] [Google Scholar]
- 57. Ng CT, Jaworski JP, Jayaraman P, Sutton WF, Delio P, Kuller L, Anderson D, Landucci G, Richardson BA, Burton DR, Forthal DN, Haigwood NL. 2010. Passive neutralizing antibody controls SHIV viremia and enhances B cell responses in infant macaques. Nat. Med. 16:1117–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Geier H, Celli J. 2011. Phagocytic receptors dictate phagosomal escape and intracellular proliferation of Francisella tularensis. Infect. Immun. 79:2204–2214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Joller N, Weber SS, Muller AJ, Sporri R, Selchow P, Sander P, Hilbi H, Oxenius A. 2010. Antibodies protect against intracellular bacteria by Fc receptor-mediated lysosomal targeting. Proc. Natl. Acad. Sci. U. S. A. 107:20441–20446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Liu Y, Gao X, Masuda E, Redecha PB, Blank MC, Pricop L. 2006. Regulated expression of FcgammaR in human dendritic cells controls cross-presentation of antigen-antibody complexes. J. Immunol. 177:8440–8447 [DOI] [PubMed] [Google Scholar]