Abstract
H7 subtype influenza A viruses, responsible for numerous outbreaks in land-based poultry in Europe and the Americas, have caused over 100 cases of confirmed or presumed human infection over the last decade. The emergence of a highly pathogenic avian influenza H7N3 virus in poultry throughout the state of Jalisco, Mexico, resulting in two cases of human infection, prompted us to examine the virulence of this virus (A/Mexico/InDRE7218/2012 [MX/7218]) and related avian H7 subtype viruses in mouse and ferret models. Several high- and low-pathogenicity H7N3 and H7N9 viruses replicated efficiently in the respiratory tract of mice without prior adaptation following intranasal inoculation, but only MX/7218 virus caused lethal disease in this species. H7N3 and H7N9 viruses were also detected in the mouse eye following ocular inoculation. Virus from both H7N3 and H7N9 subtypes replicated efficiently in the upper and lower respiratory tracts of ferrets; however, only MX/7218 virus infection caused clinical signs and symptoms and was capable of transmission to naive ferrets in a direct-contact model. Similar to other highly pathogenic H7 viruses, MX/7218 replicated to high titers in human bronchial epithelial cells, yet it downregulated numerous genes related to NF-κB-mediated signaling transduction. These findings indicate that the recently isolated North American lineage H7 subtype virus associated with human conjunctivitis is capable of causing severe disease in mice and spreading to naive-contact ferrets, while concurrently retaining the ability to replicate within ocular tissue and allowing the eye to serve as a portal of entry.
INTRODUCTION
Avian influenza A (H7) viruses have been responsible for numerous zoonotic transmissions from poultry to humans since 2002 (1). The largest H7 outbreak in humans, resulting in over 80 cases with one fatality, occurred in The Netherlands in 2003 and was caused by a highly pathogenic avian influenza (HPAI) H7N7 virus that led to the culling of over 30 million birds (2, 3). While outbreaks of HPAI H7N3 viruses in poultry have previously occurred in Chile in 2002, British Columbia, Canada, in 2004, and Saskatchewan, Canada, in 2007 (4–7), a recent outbreak of HPAI H7N3 virus in Jalisco, Mexico, necessitating the culling of over 20 million birds, represents the largest documented epornitic of HPAI virus in North America to date (8, 9). Two virologically confirmed human cases were detected following exposure to infected poultry, with both cases presenting with conjunctivitis but without fever or respiratory symptoms (8). The scale of this poultry outbreak and its association with the first documented cases of human infection with an H7 virus in 5 years highlight the need for continued surveillance and study of this virus subtype.
North American lineage H7 avian influenza viruses generally exhibit reduced virulence in mammalian models compared with some equine H7N7 and Eurasian lineage H7 avian influenza viruses, which more closely resemble HPAI H5N1 viruses in their ability to cause systemic, lethal disease (10–13). However, several North American low-pathogenicity avian influenza (LPAI) H7N2 and HPAI H7N3 viruses are highly infectious in mice and capable of limited transmission among ferrets in direct contact (11, 14, 15). Unlike other virus subtypes, H7 influenza viruses of both lineages have predominantly been associated with ocular disease, typically, conjunctivitis, in humans (1, 3, 7). Most recently, an HPAI H7N3 virus isolated from a human conjunctivitis case during the 2004 poultry outbreaks in British Columbia, Canada, was shown to replicate efficiently in numerous human respiratory and ocular cell types (16, 17). However, it was unknown if other North American H7N3 and H7N9 viruses shared these properties.
H7N3 viruses have been the cause of all highly pathogenic avian H7 subtype influenza virus outbreaks in North America. However, the isolation of numerous LPAI H7N3 and H7N9 viruses in North America during the past decade underscores the potential for the generation of pathogenic variants from these LPAI precursors and the risk of human exposure (9, 18). To better understand the ability of North American H7N3 viruses to cause respiratory and ocular disease in mammalian species, we examined the pathogenesis, transmissibility, and ocular tropism of a virus isolated from one of the HPAI H7N3 human infections detected in Jalisco, Mexico, in 2012, A/Mexico/InDRE7218/12 (MX/7218). We found that this virus exhibited enhanced virulence in both mouse and ferret models compared with the virulence of several phylogenetically related LPAI H7N3 and H7N9 isolates and closely mirrored previously studied HPAI H7 human isolates in the capacity for efficient replication and subtype-specific activation of host responses in human respiratory cells.
MATERIALS AND METHODS
Viruses.
The influenza A viruses of the H7 subtype used in this study are shown in Table 1. Virus stocks were propagated in the allantoic cavity of 10-day-old embryonated hen's eggs at 37°C for 24 to 26 h (HPAI) or 35°C for 36 to 48 h (LPAI). Allantoic fluid pooled from multiple eggs was clarified by centrifugation and frozen in aliquots at −70°C. The 50% egg infectious dose (EID50) for each virus stock was calculated by the method of Reed and Muench following serial dilution in eggs (23). Viruses were additionally tested by standard plaque assay in Madin-Darby canine kidney (MDCK) cells (ATCC, Manassas, VA) for determination of the titer (in PFU/ml). All research with HPAI viruses was conducted under biosafety level 3 containment, including enhancements outlined in Biosafety in Microbiological and Biomedical Laboratories (24).
Table 1.
H7 viruses used in this study
| Virus | Name in this study | Subtype | IVPI phenotypea | Virus originb |
|---|---|---|---|---|
| A/chicken/Chile/180-54/02 | ck/Chile/180-54 | H7N3 | LPAI | Outbreak in broiler breeder flock |
| A/chicken/Chile/4322-2/02 | ck/Chile/4322-2 | H7N3 | HPAI | Outbreak in broiler breeder flock |
| A/red knot/NJ/1523470/06 | rk/NJ/06 | H7N3 | LPAI | Surveillance of waterfowl and shore birds |
| A/chicken/AR/10/08 | ck/AR/08 | H7N3 | LPAI | Surveillance of commercial poultry producer |
| A/guinea fowl/NE/17096-1/11 | gf/NE/11 | H7N9 | LPAI | Outbreak in backyard flock |
| A/goose/NE/17097-4/11 | gs/NE/11 | H7N9 | LPAI | Outbreak in backyard flock |
| A/Mexico/InDRE7218/12 | MX/7218 | H7N3 | HPAI | Human case of bilateral conjunctivitis |
Mouse experiments.
Female BALB/c mice (Jackson Laboratories) 6 to 8 weeks of age were anesthetized with 2,2,2-tribromoethanol in tert-amyl alcohol (Avertin; Sigma-Aldrich, St. Louis, MO). Mice were inoculated with 106 EID50 of virus by the intranasal (i.n.) route (50-μl volume) or the intraocular (i.o.) route (5-μl volume), in which the inoculum was dropped onto the corneal surface of the right eye and massaged in with the eyelids following corneal scarification as described previously (25). Five to seven mice per virus were monitored daily for 14 days postinoculation (p.i.) for morbidity, as measured by weight loss, and mortality for determination of a 50% lethal dose (LD50). Any mouse that lost greater than 25% of its preinfection body weight was euthanized. Replication and systemic spread of each virus were determined by harvesting the right eye, nose, lung, and brain of mice (n = 3) on days 3 and 6 p.i., and tissues were titrated in eggs as described previously (limits of detection, 101.5 EID50/ml i.n. tissues 100.8 EID50/ml i.o. tissues) (26).
Ferret experiments.
Male Fitch ferrets (Triple F Farms, Sayre, PA) 4 to 6 months of age and serologically negative by standard hemagglutination inhibition for currently circulating influenza viruses were used in this study. Ferrets were housed for the duration of each experiment in a Duo-Flo Bioclean mobile unit (Lab Products Incorporated, Seaford, DE). Ferrets (n = 3) were inoculated i.n. with 106 EID50 of each indicated virus and observed daily for clinical signs and symptoms of infection, and nasal washes were collected on the indicated days p.i. as previously described (27). A serologically naive ferret was placed in the same cage as an inoculated ferret at 24 h p.i. for assessment of virus transmission between ferrets in direct contact (28). An additional 3 ferrets inoculated with 106 EID50 of each virus were euthanized day 3 p.i. for the assessment of virus replication and systemic spread, as previously described (27).
Cell culture and viral replication.
The human bronchial epithelial cell line Calu-3 (ATCC) grown on membrane inserts was cultured as previously described; these polarized cells possess protease activity that can support human and avian influenza virus hemagglutinin cleavage without the addition of exogenous tosylsulfonyl phenylalanyl chloromethyl ketone (TPCK)-trypsin (29). Cells were grown to confluence in 6-well plates for 1 week. Virus was added to cells apically in serum-free medium at a multiplicity of infection (MOI) of 0.01 and incubated for 1 h before washing. Aliquots of culture supernatant taken p.i. were immediately frozen at −80°C until use. Medium with equal amounts of virus-free normal allantoic fluid (total volume, 10%) was used as a mock-infected control. Samples collected for replication kinetics were titrated for the presence of infectious virus by standard plaque assay in MDCK cells.
Real-time RT-PCR.
Isolation of RNA from clarified ferret lung tissue homogenates was performed with a QIAamp viral RNA kit (Qiagen, Valencia, CA). Semiquantitative reverse transcription-PCR (RT-PCR) was performed with primer sequences as described previously (30), with expression levels normalized to the level of expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and reported as the fold change compared with the level for mock-infected animals. Calu-3 cells were propagated in 12-well plates and infected with virus at an MOI of 2, as described above. Total RNA was extracted at 24 h p.i. RT2 Profiler PCR array analysis (SABiosciences, Frederick, MD) for the human NF-κB signaling pathway was performed in triplicate with total RNA extracted at 24 h p.i. and analyzed per the manufacturer's instruction.
Statistical analysis.
Statistical significance (P < 0.05) for murine and in vitro replication studies was determined by one-way analysis of variance with a Bonferroni posttest. Statistical significance for ferret studies and PCR array analysis was determined using Student's t test.
RESULTS
Pathogenicity of H7N3 and H7N9 influenza A viruses following intranasal inoculation in mice.
The BALB/c mouse model has been used extensively to study the pathogenesis of H7 influenza viruses administered by the i.n. route (11, 12, 20, 31). These studies have revealed that H7 viruses are highly infectious in mice without prior adaptation, with the capacity for systemic spread and lethal disease depending on the strain. We utilized this model to assess the virulence of the recently isolated HPAI H7N3 virus MX/7218, which was associated with human infection, and related LPAI and HPAI H7 subtype viruses with common phylogenetic ancestors (I. Lopez-Martinez, A. Balish, G. Barrera-Badillo, J. Jones, T. E. Nunez-Garcia, Y. Jang, R. Aparicio-Antonio, E. Azziz-Baumgartner, J. A. Belser, J. E. Ramirez-Gonzalez, J. C. Pedersen, J. Ortiz-Alcantara, E. Gonzalez-Duran, B. Shu, S. L. Emery, M. K. Poh, G. Reyes-Teran, J. A. Vasquez-Perez, S. Avila-Rios, T. Uyeki, S. Lindstrom, J. Villanueva, J. Tokars, C. Ruiz-Matus, J. F. Gonzalez-Roldan, B. Schmitt, A. Klimov, N. Cox, P. Kuri-Moralez, C. T. Davis, and J. A. Diaz-Quinonez, submitted for publication) (Table 1). The HPAI H7N3 virus MX/7218 was isolated from a patient with conjunctivitis (8). Isolates from Nebraska (LPAI H7N9) and Chile (LPAI and HPAI H7N3) were associated with outbreaks in domestic poultry flocks (6, 21, 33), whereas isolates from New Jersey and Arkansas (LPAI H7N3) were detected during surveillance activities (20, 22).
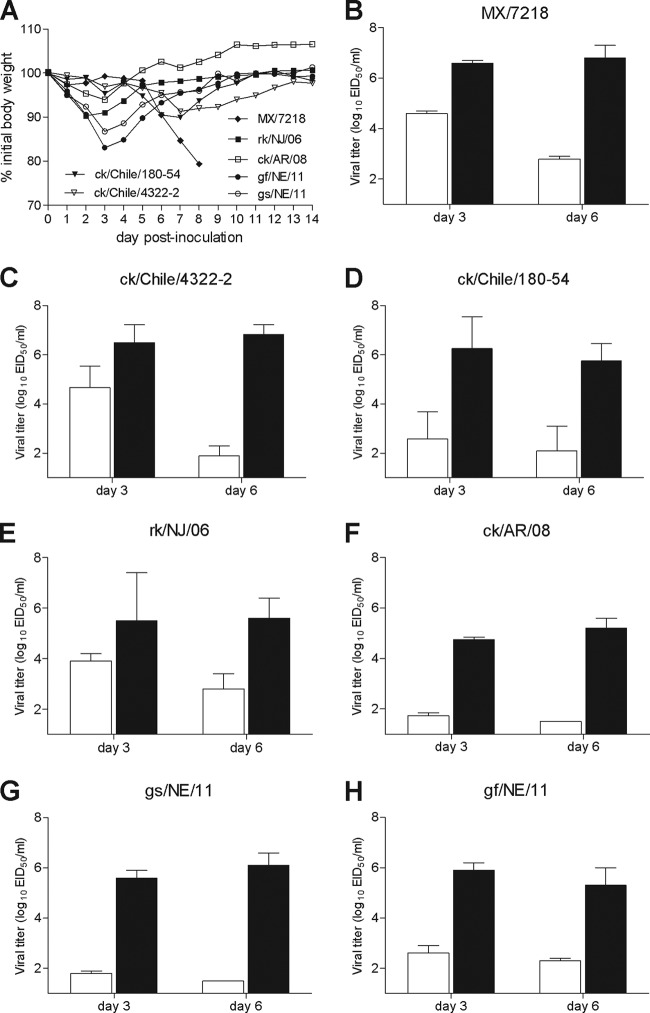
In agreement with previous studies which have reported lethality following inoculation with selected HPAI H7N3 viruses in mice (10, 12), MX/7218 virus-infected mice showed the greatest signs of illness following i.n. inoculation, resulting in >20% weight loss and an LD50 of 105.5 EID50. In contrast, the HPAI and LPAI H7N3 viruses from Chile in 2003 did not cause substantial morbidity and possessed an LD50 of >107 EID50 (Fig. 1A). Despite the disparity in morbidity between these viruses, the HPAI H7N3 viruses MX/7218 and ck/Chile/4322-2 replicated equally well in the lungs of mice, with titers of ≥106.5 EID50/ml persisting through day 6 p.i. (Fig. 1B and C). Following HPAI H7N3 virus inoculation (>104.5 EID50/ml), virus was also detected at the highest titer in the nose on day 3 p.i., with MX/7218-inoculated mice possessing at this time nose titers that were significantly higher than those of all other H7N3 and H7N9 viruses tested (P < 0.05). Despite the lethal disease following high-dose MX/7218 virus inoculation, systemic spread to the brain was not detected on day 6 p.i. (data not shown) and mice showed no signs of neurologic symptoms.
Fig 1.
Comparison of morbidity and viral replication following influenza A H7 influenza virus infection in mice. BALB/c mice were inoculated i.n. with 106 EID50/50 μl of each virus indicated. (A) Weight loss was monitored for 14 days following inoculation (5 to 7 mice/group). Weight loss in MX/7218-infected mice is representative of that for 6/7 mice which succumbed to infection at this challenge dose. (B to H) Nose (white bars) and lung (black bars) tissues were collected on days 3 and 6 p.i. with HPAI H7N3 viruses (B and C), LPAI H7N3 viruses (D to F), and LPAI H7N9 viruses (G and H). Tissue homogenates (3 mice/group) were titrated in eggs, and the results are expressed as the mean log10 EID50/ml plus standard deviation. The limit of virus detection was 101.5 EID50/ml.
Murine inoculation with LPAI H7N9 viruses from 2011 caused greater weight loss (13.3 to 17.0% on day 3 p.i.) than inoculation with LPAI H7N3 viruses from 2006 to 2008 (4.1 to 9.2% on days 2 to 3 p.i.), but all mice recovered by the end of the observation period. All LPAI viruses examined replicated efficiently in the murine lung following i.n. inoculation, with mean lung titers of ≥104.75 EID50/ml and ≥105.2 EID50/ml on days 3 and 6 p.i., respectively (Fig. 1D to H). In summary, while both HPAI H7N3 viruses MX/7218 and ck/Chile/4322-2 replicated to the highest titers in the lung, only the MX/7218 virus exhibited a lethal phenotype in mice at the doses tested. Mice inoculated with the LPAI H7N3 and H7N9 viruses exhibited reduced morbidity in this model, but the viruses nonetheless replicated efficiently in this species without the need for prior adaptation.
Pathogenicity of H7N3 and H7N9 influenza viruses following ocular inoculation in mice.
H7 virus infection in humans frequently results in ocular and not respiratory disease (1). We previously established a mouse model of ocular inoculation which corroborated that H7 subtype influenza viruses associated with human conjunctivitis during 2003 and 2004 were capable of efficient replication in both eye and respiratory tract tissues following inoculation by this route (25). To assess if this property has been maintained among recently isolated North American lineage H7 viruses, mice were inoculated by the intraocular (i.o.) route following corneal scarification with selected HPAI and LPAI H7 viruses (Table 2). Similar to prior H7 viruses associated with human disease, MX/7218 virus replicated efficiently in both eye and nose tissues following i.o. inoculation. The LPAI H7N9 virus gs/NE/11 was also capable of replication in both tissues, albeit at a reduced titer and frequency. In contrast, the LPAI H7N3 virus rk/NJ/06 was not capable of efficient replication following inoculation by this route. Unlike i.n. inoculation, mice inoculated by the ocular route did not exhibit significant morbidity or mortality following i.o. inoculation with any virus tested (data not shown). These findings closely mirror the pattern of virus isolation in mice following i.o. inoculation with other H7 viruses (25) and indicate that recently isolated H7 influenza viruses have maintained the ability to mount a productive infection following exposure to ocular tissue in vivo.
Table 2.
Replication of H7 influenza viruses following ocular inoculation in mice
| Tissue | Day p.i. | Virus titera |
||
|---|---|---|---|---|
| rk/NJ/06 | gs/NE/11 | MX/7218 | ||
| Eye | 3 | <0.8 | 2.5 (1/3) | 3.4 ± 1.2 (2/3) |
| Nose | 3 | <0.8 | <0.8 | 2.9 ± 0.5 (2/3) |
| Lung | 3 | <0.8 | <0.8 | <0.8 |
| Eye | 6 | <0.8 | 2.4 ± 1.0 | 3.4 ± 0.6 |
| Nose | 6 | <0.8 | 2.2 ± 0.4 | 3.3 ± 0.7 (2/3) |
| Lung | 6 | 1.5 (1/3) | <0.8 | 2.75 (1/3) |
Mean virus titers in mice inoculated with 106 EID50/5 μl of virus following corneal scarification. Virus titers are expressed as the mean log10 EID50/ml ± standard deviation among mice with positive virus detection (data are representative of all mice examined, unless denoted in parentheses, in which the number of mice with positive virus detection/total number of mice in the group are indicated). The limit of virus detection was 100.8 EID50/ml.
Pathogenicity and transmission of H7N3 and H7N9 viruses in ferrets.
Previous studies have shown that both LPAI and HPAI H7N3 viruses are capable of efficient replication throughout the respiratory tract of ferrets; however, H7N9 viruses have not been examined in this model (10, 14, 15, 34). Ferrets were inoculated i.n. with 106 EID50 and either observed for 14 days p.i. for clinical signs of infection or euthanized on day 3 p.i. for assessment of systemic spread. The HPAI H7N3 virus MX/7218 caused fever and moderate but transient weight loss, with all ferrets surviving the observation period (Table 3). In contrast, inoculation with the LPAI H7N9 virus gs/NE/11 did not cause clinical signs or symptoms of illness in this species. Respiratory signs, including sneezing and nasal discharge, were not observed following inoculation with either virus (data not shown). MX/7218 and gs/NE/11 viruses replicated efficiently in the nasal turbinates, trachea, and lungs of ferrets, and elevated mRNA levels of several proinflammatory cytokines and chemokines were detected in lung tissue from both H7 virus-infected ferrets on day 3 p.i. Both viruses were also detected at comparable titers in the olfactory bulb and brain; however, MX/7218 was detected at a higher frequency than gs/NE/11, which was detected in these tissues in only one of three ferrets (Table 3). Neither virus was detected in intestinal tissue on day 3 p.i., although MX/7218 virus was detected in rectal swabs on days 3 and 5 p.i. Virus was not detected in eye or conjunctival tissues on day 3 p.i. with either virus (data not shown). These data indicate that, despite disparate presentations of clinical signs and symptoms following virus inoculation, both MX/7218 and gs/NE/11 were capable of efficient, high-titer replication throughout the respiratory tract in ferrets.
Table 3.
Infection of ferrets with H7 influenza viruses
| Observation | Result for the following virus: |
|
|---|---|---|
| MX/7218 | gs/NE/11 | |
| Days 1–14 p.i. | ||
| Weight lossa | 7.8 (9–10) | NDf |
| Feverb | 2.9 (7) | ND |
| Virus in rectal swabc | 2/3 | 0/3 |
| Virus titer at day 3 p.i.d | ||
| Nasal turbinates | 7.2 ± 0.9 | 6.4 ± 0.1 |
| Trachea | 4.4 ± 2.2 (2/3) | 4.4 ± 0.2 |
| Lung | 5.0 ± 2.2 | 3.2 ± 1.2 (2/3) |
| Olfactory bulb | 2.8 ± 0.5 | 2.7 (1/3) |
| Brain | 2.3 ± 0.2 (2/3) | 2.2 (1/3) |
| Fold induction of cytokine and chemokine transcript in lungs at day 3 p.i.e | ||
| IFNα | 1.7 | 1.7 |
| IL-6 | 49.4 | 87.6 |
| IL-8 | 173.2 | 799.7 |
| IP-10 | 7.8 | 6.8 |
Percent mean maximum weight loss. Data in parentheses represent the day(s) p.i. on which the maximum weight loss was detected.
Mean maximum rise in body temperature in degrees centigrade (baseline body temperature, 38.0 to 38.2°C). Data in parentheses represents the day(s) p.i. on which the maximum rise in temperature was detected.
Number of ferrets with detectable virus in rectal swabs collected on days 3 and 5 p.i./total number of ferrets tested.
Virus titers are expressed as the mean log10 EID50/ml ± standard deviation among ferrets with positive virus detection (data are representative of all ferrets examined, unless denoted in parentheses, in which the number of mice with positive virus detection/total number of mice in the group are indicated). The limit of virus detection was 101.5 EID50/ml.
Cytokine and chemokine mRNA expression in lungs of ferrets (n = 3) was measured by RT-PCR and is presented as the mean fold change compared with the values in mock-infected animals. Replicate threshold cycle values for each sample were within 10% of the average.
ND, not detected.
Selected North American and Eurasian lineage H7 viruses have demonstrated the ability for limited transmission in a ferret direct-contact model (14, 15). To evaluate if recently isolated H7 viruses maintained this property, three ferrets were each inoculated i.n. with 106 EID50 of either the H7N3 or H7N9 virus and a naive ferret was cohoused with each inoculated ferret 24 h p.i. (Fig. 2). Criteria for efficient transmission included detection of virus in nasal washes and seroconversion of convalescent-phase sera (identified by a ≥4-fold rise in antibody titer from that in preinfection sera) from contact ferrets. Both MX/7218 and gs/NE/11 viruses replicated efficiently in the upper respiratory tract of inoculated ferrets; however, peak mean nasal wash virus titers were significantly higher following MX/7218 virus infection (106.0 EID50/ml at day 3 p.i.) than gs/NE/11 virus infection (105.0 EID50/ml at day 1 p.i.) (P < 0.05). MX/7218 virus transmitted to three of three ferrets when the ferrets were placed in direct contact, as determined by detection of infectious virus and seroconversion of all contact ferrets. In comparison, gs/NE/11 virus was not detected in nasal washes of contact ferrets and seroconversion was not observed. These findings demonstrate that the human isolate MX/7218 but not the genetically related LPAI virus gs/NE/11 is capable of transmission between ferrets in direct contact.
Fig 2.
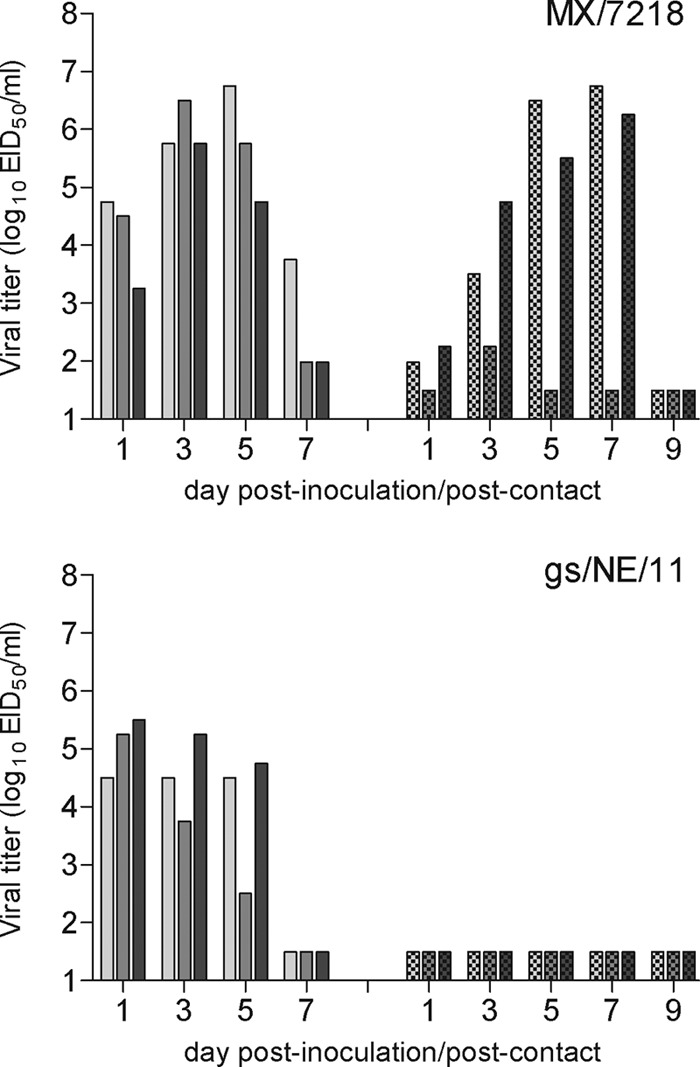
Transmissibility of H7N3 and H7N9 influenza viruses after direct contact. Three ferrets were inoculated with 106 EID50 of the H7N3 virus MX/7218 or the H7N9 virus gs/NE/11, and nasal washes were collected from each ferret on the indicated days p.i. (left set of bars) to assess viral replication. A naive ferret was placed in the same cage as each inoculated ferret at 24 h p.i., and nasal washes were collected from each contact ferret on the indicated days postcontact (right set of bars). The limit of virus detection was 101.5 EID50/ml.
Tropism of H7N3 and H7N9 viruses in human respiratory cells.
Previous studies have identified that epithelial and endothelial cells from multiple human organ systems support replication of H7 influenza viruses associated with disease in humans (16, 17, 35). To determine if the differences in virus pathogenicity observed in mouse and ferret models were indicative of different replicative abilities in human cells, we compared the ability of H7N3 and H7N9 viruses to replicate in polarized human bronchial epithelial cells (Calu-3) (Fig. 3). Among all viruses examined, the HPAI H7N3 virus MX/7218 replicated to the highest titer in Calu-3 cells, reaching titers of >107 PFU/ml by 36 h p.i. Furthermore, MX/7218 virus replicated to significantly higher titers than all other viruses examined over the 72-h time period (P < 0.05). ck/Chile/4322-2, ck/Chile/180-54, and rk/NJ/06 viruses replicated with comparable efficiency in Calu-3 cells, reaching higher titers than the LPAI virus ck/AR/08 and both H7N9 viruses at 24 to 36 h p.i. before reaching generally comparable titers by 72 h p.i. Despite replicating efficiently in the mouse and ferret respiratory tracts, the H7N9 viruses examined achieved significantly lower titers (<104 PFU/ml, P < 0.05) in Calu-3 cells than all HPAI H7N3 viruses at this time point. In summary, we found that the HPAI H7N3 human isolate MX/7218 replicated to higher titers in human bronchial epithelial cells than LPAI H7N9 viruses, which exhibited the lowest overall titers.
Fig 3.
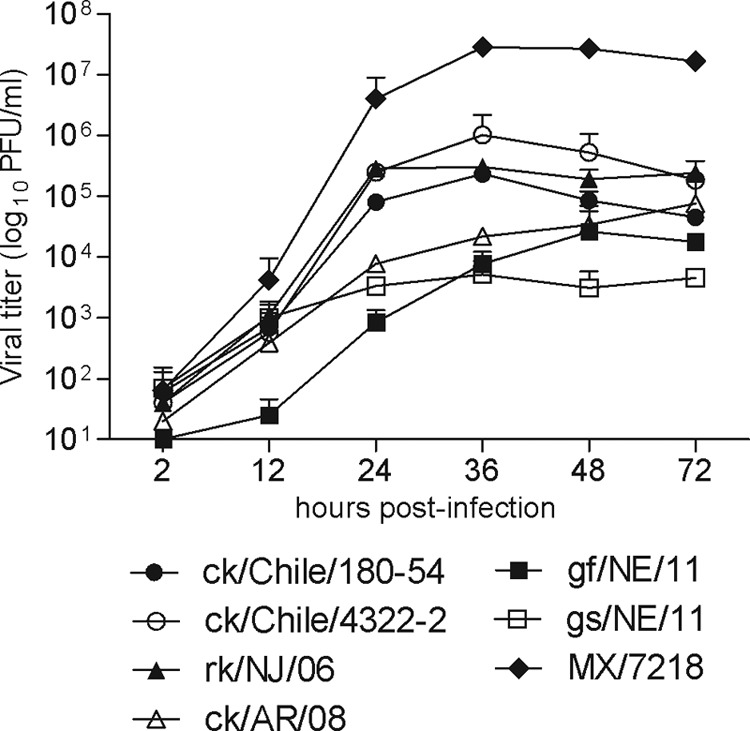
Replication kinetics of H7N3 and H7N9 viruses in human respiratory cells. Calu-3 cells were infected with the indicated viruses at an MOI of 0.01. Supernatants were removed at the indicated times p.i., and titers of infectious virus were determined by standard plaque assay. The limit of virus detection was 10 PFU/ml. The mean from duplicate independent cultures per virus plus standard deviation is shown.
Downregulation of NF-κB signaling in human respiratory cells following HPAI H7N3 virus infection.
We previously determined that, despite retaining the capacity for efficient replication, H7 influenza viruses elicit a weakened and delayed induction of proinflammatory cytokines and chemokines in human respiratory cells (16). Furthermore, compared with other virus subtypes capable of human infection, H7 viruses elicit a broad downregulation of genes related to the NF-κB signaling pathway in respiratory cells but not ocular cells following virus infection (17). We examined by real-time PCR array analysis a panel of genes related to NF-κB signaling in Calu-3 cells following infection with the human isolate MX/7218 to assess the ability of this HPAI H7N3 virus to elicit host responses. Genes related to the NF-κB signal transduction pathway that were significantly (P < 0.05) upregulated or downregulated (>3-fold) in Calu-3 cells at 24 h p.i. are shown in Fig. 4 as the fold change in expression compared with that in mock-infected cells. MX/7218 virus infection resulted in the significant upregulation of several genes, primarily cytokines such as beta 1 interferon (IFN-β1; notably associated with potent virus replication), IFN-γ, interleukin-6 (IL-6), colony-stimulating factor 2 (CSF2), lymphotoxin alpha (LTA), and tumor necrosis factor (TNF), all of which function as NF-κB-responsive genes (Fig. 4). However, despite the upregulation of these genes, the levels of many of them were approximately 4- to 18-fold lower than those typically observed following HPAI H5N1 virus infection in these cells (17). This is comparable to the findings for HPAI H7N7 virus A/Netherlands/219/03 (NL/219), which was previously shown to elicit reduced levels of these genes in Calu-3 cells (Fig. 4, gray bars). The only exception was IL-10, which was detected at higher levels following HPAI H7N3 and H7N7 virus infection than H5N1 and H1N1 virus subtype infection (17). Similar to the HPAI H7N7 virus NL/219, HPAI H7N3 virus infection in respiratory cells resulted in a broad downregulation of NF-κB-related genes, with several of these genes detected at levels lower than those detected after H5N1 virus infection (Fig. 4, gray bars). These results indicate that infection with the HPAI H7N3 virus MX/7218 elicits a host response comparable to that elicited by infection with other HPAI H7 viruses in respiratory cells, in accord with the tropism of this virus subtype.
Fig 4.
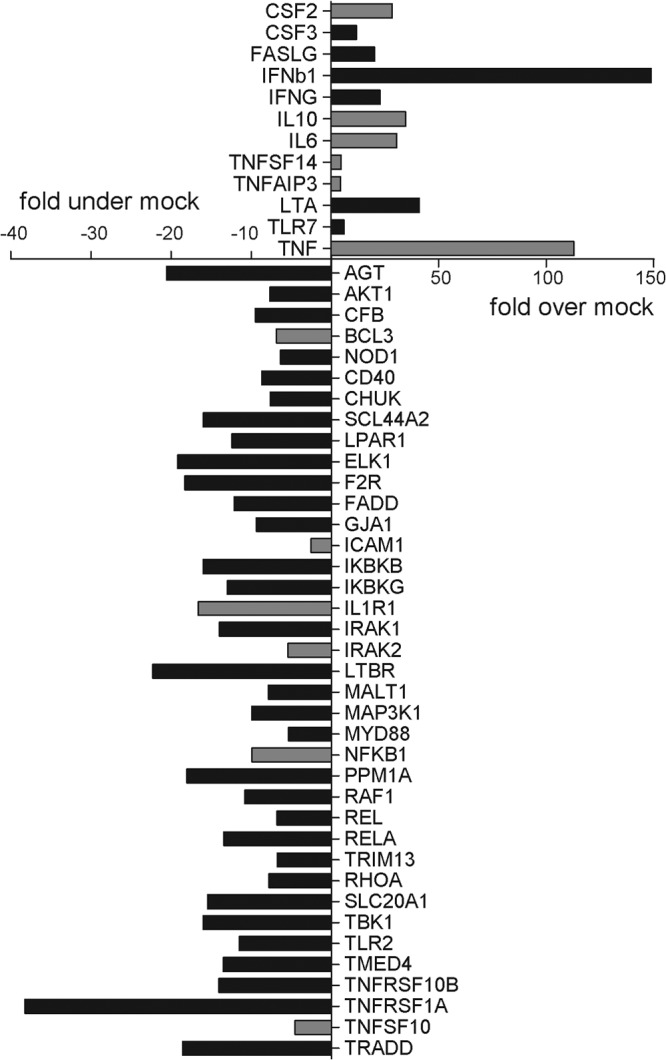
Fold regulation of selected genes related to NF-κB signaling in human respiratory cells following HPAI H7N3 virus infection. Calu-3 cells were infected with MX/7218 virus at an MOI of 2. Total RNA was isolated in triplicate from cells at 24 h p.i. and examined by real-time RT-PCR array analysis. Genes which exhibited a significant (P < 0.05) >3-fold change in expression over/under that in mock-infected cells are shown. Gray bars signify genes whose regulation is specific for the H7 subtype rather than other influenza viruses in this cell type (17).
DISCUSSION
Two conjunctivitis cases from the 2012 HPAI H7N3 virus outbreak in the state of Jalisco, Mexico, represent the first documented human infections with H7 viruses in recent years (1). However, LPAI and HPAI H7 viruses have routinely been detected in poultry and wild birds throughout North America and in several countries in Europe and Asia during the last decade (9, 33). Given the potential for human exposure in poultry workers or other risk groups that come into frequent contact with infected birds, it is critical to understand the public health threat posed by contemporary H7 viruses, especially those that have demonstrated a capacity for human infection. In this study, we used two mammalian models to compare the relative virulence of the human H7N3 isolate MX/7218 to that of several closely related HPAI and LPAI viruses isolated from various avian species. Despite generally similar replication profiles in respiratory tract tissues between all viruses tested, we observed enhanced virulence and transmission of MX/7218 virus in mouse and ferret models. Furthermore, MX/7218 virus replicated to the highest titer in human bronchial epithelial cells and following infection elicited a pattern of host responses typical of other HPAI H7 viruses.
Lethality has previously been reported among some HPAI H7N3 and H7N7 virus isolates following murine inoculation (10–12). Equine H7N7 viruses from North America were also found to be highly pathogenic in mice, possessing generally similar levels of lethality as MX/7218 virus (13). However, with the exception of an HPAI H7N7 virus associated with a fatal case from The Netherlands in 2003, HPAI H7 viruses do not maintain this high level of virulence in the ferret model. Detection of virus at modest titers in the ferret olfactory bulb and brain, as shown here with MX/7218 virus, has also been reported following infection with several LPAI and HPAI H7 strains (11, 34). The ability of gs/NE/11 virus to achieve high-titer replication throughout the respiratory tract while displaying a nonpathogenic phenotype is consistent with that of other LPAI H7 North American strains, which have caused only limited morbidity in this species. Detection of gs/NE/11 virus in the olfactory bulb and brain of one ferret at day 3 p.i. is likely a reflection of the high titers of virus replication present in the proximal nasal cavity and is in accord with the sporadic detection of seasonal influenza viruses in these tissues, despite maintaining a low-pathogenicity phenotype in this species (36). In this study, H7N3 and H7N9 virus-infected ferrets did not demonstrate frequent sneezing or nasal discharge, similar to prior studies with North American H7 lineage viruses, which were found to cause limited morbidity in this species (14). Levels of proinflammatory cytokine and chemokine transcripts in the lungs were generally comparable between both viruses tested and are in agreement with the viral loads detected in this tissue on day 3 p.i. (Table 3), demonstrating the ability of HPAI and LPAI H7 influenza viruses to elicit innate immune responses in the lower respiratory tract following intranasal inoculation (30, 37).
While multiple virus subtypes are capable of mounting a productive infection in the upper respiratory tract of ferrets following ocular inoculation, only selected H5 and H7 viruses possess this capacity in mice (25, 38–40). Peak titers of gs/NE/11 and MX/7218 viruses in ocular and respiratory tract tissues in mice inoculated by the ocular route were comparable in magnitude to those of H7 viruses previously examined in this model; the reduced morbidity caused by MX/7218 virus following i.o. inoculation compared with that following i.n. inoculation was also consistent with previous studies (25). In contrast, rk/NJ/06 virus did not efficiently use the eye as a portal of entry in mice; this virus was isolated during routine surveillance and was not associated with human infection or a poultry outbreak (Table 1) (20). Interestingly, MX/7218 virus was not detected in the eyes, conjunctiva, or conjunctival washes collected from i.n.-inoculated ferrets, unlike Eurasian HPAI H7N7 viruses, which have demonstrated the ability to replicate in these tissues following i.n. or i.o. inoculation (10, 38). Future research among viruses within the H7 subtype is needed to identify molecular properties associated with ocular tropism, as this subtype continues to cause ocular disease following human infection.
The transmission of MX/7218 virus to naive ferrets in the presence of direct contact is not the first time that an H7 subtype virus associated with ocular and not respiratory disease in humans has demonstrated this capacity. Ferrets inoculated with an HPAI H7N3 virus isolated from a human case in British Columbia, Canada, in 2004 transmitted low levels of virus (≤102 EID50/ml), which were detected in the nasal washes of two ferrets in direct contact with inoculated animals in the absence of seroconversion (14). An LPAI H7N3 mallard virus isolated in 2001 also transmitted to two of three ferrets (with positive virus isolation and seroconversion) (15). HPAI H7N7 and LPAI H7N2 viruses have further demonstrated the capacity for efficient transmission in the presence of direct contact following either i.n. or i.o. inoculation in ferrets (14, 38). Despite the capacity of these H7 subtype viruses to transmit to naive ferrets in the presence of direct contact, they are not transmissible by respiratory droplets (14); it is likely that MX/7218 virus shares this phenotype, though further study is required. These findings are similar to limited reports of direct-contact transmission of LPAI H9N2 viruses but in contrast to findings for wild-type HPAI H5N1 viruses, which have not demonstrated the ability for direct-contact transmission (28, 41). While the small sample size of the ferret transmission pairs used in this study precludes a rigorous statistical analysis of virus transmissibility (42), the detection of numerous H7N2, H7N3, and H7N7 strains, inclusive of both North American and Eurasian lineages, that are associated with human infection and possess a transmissible phenotype illustrates the pandemic potential and public health threat posed by this virus subtype. While the presence or absence of a multibasic hemagglutinin (HA) cleavage site is likely to influence mammalian replication capacity and, in turn, virus transmissibility, further examination of the receptor-binding properties of MX/7218 virus and molecular comparison of MX/7218 and gs/NE/11 viruses may provide further insight into these properties.
Human epithelial lung cells are permissive to multiple subtypes of influenza virus, with HPAI and LPAI H7 viruses demonstrating similar levels of infectivity in this cell type (16, 29). MX/7218 virus replicated the most efficiently in Calu-3 cells in this study, reaching peak mean titers in this cell type similar to those achieved by prior HPAI H7N3 and H7N7 viruses associated with human conjunctivitis; all of these viruses possess a glutamic acid at position 627 in PB2 (16). Despite the close HA gene similarity between the MX/7218 virus and the LPAI H7N9 viruses from 2011 (Lopez-Martinez et al., submitted), peak viral titers of H7N9 viruses were >3 log units lower than those of the human isolate, likely attributable to the presence of a multibasic cleavage site motif in MX/7218 virus. Interestingly, MX/7218 virus elicited a similar pattern of induction of NF-κB-related genes as HPAI H7N7 virus NL/219 in Calu-3 cells shown in a previous study, despite differences in their epidemiologic profiles and lineages, as well as different known molecular determinants of virulence between these two strains (17). The attenuation of host responses following HPAI H7 virus infection in multiple human cell types, in contrast to the hypercytokinemia often observed with H5N1 viruses, indicates that the suppression of early innate immune responses may represent an alternate mechanism to cause severe disease, as also demonstrated with the reconstructed 1918 virus (16, 17, 43–45). As many proinflammatory cytokines and chemokines are regulated by NF-κB, future studies examining the ability of H7 viruses to modulate NF-κB activation, potentially by the activity of the H7 NS1 protein, are warranted (46).
The emergence of HPAI viruses from LPAI virus precursors typically arises from the insertion or progressive accumulation of multiple basic amino acids at the HA cleavage site (47); to date, only H5 and H7 virus subtypes have demonstrated this capacity. However, viruses detected during the 2012 poultry outbreak in Mexico likely represent an example of an HPAI H7N3 virus emerging as a result of recombination at the HA0 cleavage site. Nonhomologous recombination between the HA and nucleoprotein (NP) genes in Chile in 2002, the HA and matrix protein (M) genes in British Columbia, Canada, in 2004, and the HA and an unidentified protein of eukaryotic origin in Saskatchewan, Canada, in 2007 has demonstrated the ability of H7 influenza viruses to acquire an HPAI phenotype by this mechanism (6, 48, 49). In vitro studies have further identified that possession of an insertion of 28S host rRNA or NP in the HA cleavage site in H7N3 and H7N7 viruses, respectively, is sufficient to confer increased pathogenicity (50, 51). The molecular mechanism of recombination in H7 viruses is not well understood (11, 12, 14); however, the enlarged exposed loop conferred by these insertional events is believed to permit increased accessibility of the HA0 cleavage site (5). Further research is needed to elucidate why the H7 virus subtype appears to be the most susceptible to this form of recombination.
Wild birds represent a reservoir of all influenza A viruses, and surveillance of wild aquatic birds in North America has identified the presence of both H7N3 and H7N9 viruses (52, 53). The frequent reassortment and rapid movement of H7 influenza viruses within this population necessitate continued surveillance of this virus subtype in both wild and domestic birds (54). The continued assessment of H7 viruses detected during routine surveillance, poultry outbreaks, and human infections is critical to both assess the pandemic potential of these strains in humans and characterize laboratory mammalian models suited to examine the efficacy and cross-reactivity of candidate H7 vaccines against viruses within this subtype (34, 55–57). Because recent H7 subtype viruses associated with poultry outbreaks and surveillance activities possess the ability to cause infection by the ocular route, the use of eye protection to reduce the potential for occupational exposure is prudent (58).
ACKNOWLEDGMENTS
We gratefully acknowledge Janice Pedersen and Beverly Schmitt for providing avian influenza virus isolates from the United States used for this study.
The findings and conclusions in this report are those of the authors and do not necessarily reflect the views of the funding agency (CDC).
Footnotes
Published ahead of print 13 March 2013
REFERENCES
- 1. Belser JA, Bridges CB, Katz JM, Tumpey TM. 2009. Past, present, and possible future human infection with influenza virus A subtype H7. Emerg. Infect. Dis. 15:859–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fouchier RA, Schneeberger PM, Rozendaal FW, Broekman JM, Kemink SA, Munster V, Kuiken T, Rimmelzwaan GF, Schutten M, Van Doornum GJ, Koch G, Bosman A, Koopmans M, Osterhaus AD. 2004. Avian influenza A virus (H7N7) associated with human conjunctivitis and a fatal case of acute respiratory distress syndrome. Proc. Natl. Acad. Sci. U. S. A. 101:1356–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Koopmans M, Wilbrink B, Conyn M, Natrop G, van der Nat H, Vennema H, Meijer A, van Steenbergen J, Fouchier R, Osterhaus A, Bosman A. 2004. Transmission of H7N7 avian influenza A virus to human beings during a large outbreak in commercial poultry farms in the Netherlands. Lancet 363:587–593 [DOI] [PubMed] [Google Scholar]
- 4. Berhane Y, Hisanaga T, Kehler H, Neufeld J, Manning L, Argue C, Handel K, Hooper-McGrevy K, Jonas M, Robinson J, Webster RG, Pasick J. 2009. Highly pathogenic avian influenza virus A (H7N3) in domestic poultry, Saskatchewan, Canada, 2007. Emerg. Infect. Dis. 15:1492–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hirst M, Astell CR, Griffith M, Coughlin SM, Moksa M, Zeng T, Smailus DE, Holt RA, Jones S, Marra MA, Petric M, Krajden M, Lawrence D, Mak A, Chow R, Skowronski DM, Tweed SA, Goh S, Brunham RC, Robinson J, Bowes V, Sojonky K, Byrne SK, Li Y, Kobasa D, Booth T, Paetzel M. 2004. Novel avian influenza H7N3 strain outbreak, British Columbia. Emerg. Infect. Dis. 10:2192–2195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Suarez DL, Senne DA, Banks J, Brown IH, Essen SC, Lee CW, Manvell RJ, Mathieu-Benson C, Moreno V, Pedersen JC, Panigrahy B, Rojas H, Spackman E, Alexander DJ. 2004. Recombination resulting in virulence shift in avian influenza outbreak, Chile. Emerg. Infect. Dis. 10:693–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tweed SA, Skowronski DM, David ST, Larder A, Petric M, Lees W, Li Y, Katz J, Krajden M, Tellier R, Halpert C, Hirst M, Astell C, Lawrence D, Mak A. 2004. Human illness from avian influenza H7N3, British Columbia. Emerg. Infect. Dis. 10:2196–2199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. CDC 2012. Notes from the field: highly pathogenic avian influenza A (H7N3) virus infection in two poultry workers—Jalisco, Mexico, July 2012. MMWR Morb. Mortal. Wkly. Rep. 61:726–727 [PubMed] [Google Scholar]
- 9. FAO 2012. Highly pathogenic avian influenza in Mexico (H7N3)—a significant threat to poultry production not to be underestimated. EMPRES WATCH 26. FAO, Rome, Italy [Google Scholar]
- 10. Aamir UB, Naeem K, Ahmed Z, Obert CA, Franks J, Krauss S, Seiler P, Webster RG. 2009. Zoonotic potential of highly pathogenic avian H7N3 influenza viruses from Pakistan. Virology 390:212–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Belser JA, Lu X, Maines TR, Smith C, Li Y, Donis RO, Katz JM, Tumpey TM. 2007. Pathogenesis of avian influenza (H7) virus infection in mice and ferrets: enhanced virulence of Eurasian H7N7 viruses isolated from humans. J. Virol. 81:11139–11147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Joseph T, McAuliffe J, Lu B, Jin H, Kemble G, Subbarao K. 2007. Evaluation of replication and pathogenicity of avian influenza A H7 subtype viruses in a mouse model. J. Virol. 81:10558–10566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kawaoka Y. 1991. Equine H7N7 influenza A viruses are highly pathogenic in mice without adaptation: potential use as an animal model. J. Virol. 65:3891–3894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Belser JA, Blixt O, Chen LM, Pappas C, Maines TR, Van Hoeven N, Donis R, Busch J, McBride R, Paulson JC, Katz JM, Tumpey TM. 2008. Contemporary North American influenza H7 viruses possess human receptor specificity: implications for virus transmissibility. Proc. Natl. Acad. Sci. U. S. A. 105:7558–7563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Song H, Wan H, Araya Y, Perez DR. 2009. Partial direct contact transmission in ferrets of a mallard H7N3 influenza virus with typical avian-like receptor specificity. Virol. J. 6:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Belser JA, Zeng H, Katz JM, Tumpey TM. 2011. Infection with highly pathogenic H7 influenza viruses results in an attenuated proinflammatory cytokine and chemokine response early after infection. J. Infect. Dis. 203:40–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Belser JA, Zeng H, Katz JM, Tumpey TM. 2011. Ocular tropism of influenza A viruses: identification of H7 subtype-specific host responses in human respiratory and ocular cells. J. Virol. 85:10117–10125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Krauss S, Webster RG. 2012. Predicting the next influenza virus. Science 337:644. [DOI] [PubMed] [Google Scholar]
- 19. World Organisation for Animal Health 2011. Avian influenza, Terrestrial Animal Health Code 2011, vol 2 World Organisation for Animal Health, Paris, France [Google Scholar]
- 20. Driskell EA, Jones CA, Stallknecht DE, Howerth EW, Tompkins SM. 2010. Avian influenza virus isolates from wild birds replicate and cause disease in a mouse model of infection. Virology 399:280–289 [DOI] [PubMed] [Google Scholar]
- 21. ProMED-mail 2011. Avian influenza (LPAI), poultry—USA (Nebraska). International Society for Infectious Diseases; http://www.promedmail.org/direct.php?id=20110420.1230 [Google Scholar]
- 22. ProMED-mail 2008. Avian influenza: USA (Arkansas), LPAI H7. International Society for Infectious Diseases; http://www.promedmail.org/direct.php?id=20080604.1782 [Google Scholar]
- 23. Reed LJ, Muench HA. 1938. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 27:493–497 [Google Scholar]
- 24. Chosewood LC, Wilson DE, Centers for Disease Control and Prevention (US), and National Institutes of Health (US) 2009. Biosafety in microbiological and biomedical laboratories, 5th ed U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institutes of Health, Washington, DC [Google Scholar]
- 25. Belser JA, Wadford DA, Xu J, Katz JM, Tumpey TM. 2009. Ocular infection of mice with influenza A (H7) viruses: a site of primary replication and spread to the respiratory tract. J. Virol. 83:7075–7084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lu X, Tumpey TM, Morken T, Zaki SR, Cox NJ, Katz JM. 1999. A mouse model for the evaluation of pathogenesis and immunity to influenza A (H5N1) viruses isolated from humans. J. Virol. 73:5903–5911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Maines TR, Lu XH, Erb SM, Edwards L, Guarner J, Greer PW, Nguyen DC, Szretter KJ, Chen LM, Thawatsupha P, Chittaganpitch M, Waicharoen S, Nguyen DT, Nguyen T, Nguyen HH, Kim JH, Hoang LT, Kang C, Phuong LS, Lim W, Zaki S, Donis RO, Cox NJ, Katz JM, Tumpey TM. 2005. Avian influenza (H5N1) viruses isolated from humans in Asia in 2004 exhibit increased virulence in mammals. J. Virol. 79:11788–11800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Maines TR, Chen LM, Matsuoka Y, Chen H, Rowe T, Ortin J, Falcon A, Nguyen TH, Mai le Q, Sedyaningsih ER, Harun S, Tumpey TM, Donis RO, Cox NJ, Subbarao K, Katz JM. 2006. Lack of transmission of H5N1 avian-human reassortant influenza viruses in a ferret model. Proc. Natl. Acad. Sci. U. S. A. 103:12121–12126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zeng H, Goldsmith C, Thawatsupha P, Chittaganpitch M, Waicharoen S, Zaki S, Tumpey TM, Katz JM. 2007. Highly pathogenic avian influenza H5N1 viruses elicit an attenuated type I interferon response in polarized human bronchial epithelial cells. J. Virol. 81:12439–12449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Maines TR, Belser JA, Gustin KM, van Hoeven N, Zeng H, Svitek N, von Messling V, Katz JM, Tumpey TM. 2012. Local innate immune responses and influenza virus transmission and virulence in ferrets. J. Infect. Dis. 205:474–485 [DOI] [PubMed] [Google Scholar]
- 31. de Wit E, Munster VJ, Spronken MI, Bestebroer TM, Baas C, Beyer WE, Rimmelzwaan GF, Osterhaus AD, Fouchier RA. 2005. Protection of mice against lethal infection with highly pathogenic H7N7 influenza A virus by using a recombinant low-pathogenicity vaccine strain. J. Virol. 79:12401–12407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Reference deleted.
- 33. Pasick J, Pedersen J, Hernandez MS. 2012. Avian influenza in North America, 2009-2011. Avian Dis. 56(4 Suppl):845–848 [DOI] [PubMed] [Google Scholar]
- 34. Joseph T, McAuliffe J, Lu B, Vogel L, Swayne D, Jin H, Kemble G, Subbarao K. 2008. A live attenuated cold-adapted influenza A H7N3 virus vaccine provides protection against homologous and heterologous H7 viruses in mice and ferrets. Virology 378:123–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zeng H, Pappas C, Belser JA, Houser KV, Zhong W, Wadford DA, Stevens T, Balczon R, Katz JM, Tumpey TM. 2012. Human pulmonary microvascular endothelial cells support productive replication of highly pathogenic avian influenza viruses: possible involvement in the pathogenesis of human H5N1 virus infection. J. Virol. 86:667–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zitzow LA, Rowe T, Morken T, Shieh WJ, Zaki S, Katz JM. 2002. Pathogenesis of avian influenza A (H5N1) viruses in ferrets. J. Virol. 76:4420–4429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Belser JA, Maines TR, Gustin KM, Katz JM, Tumpey TM. 2013. Kinetics of viral replication and induction of host responses in ferrets differs between ocular and intranasal routes of inoculation. Virology 438:56–60 [DOI] [PubMed] [Google Scholar]
- 38. Belser JA, Gustin KM, Maines TR, Pantin-Jackwood MJ, Katz JM, Tumpey TM. 2012. Influenza virus respiratory infection and transmission following ocular inoculation in ferrets. PLoS Pathog. 8:e1002569 doi:10.1371/journal.ppat.1002569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Belser JA, Wadford DA, Pappas C, Gustin KM, Maines TR, Pearce MB, Zeng H, Swayne DE, Pantin-Jackwood M, Katz JM, Tumpey TM. 2010. Pathogenesis of pandemic influenza A (H1N1) and triple-reassortant swine influenza A (H1) viruses in mice. J. Virol. 84:4194–4203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sun R, Luo J, Gao Y, He H. 2009. Different infection routes of avian influenza A (H5N1) virus in mice. Integr. Zool. 4:402–408 [DOI] [PubMed] [Google Scholar]
- 41. Wan H, Sorrell EM, Song H, Hossain MJ, Ramirez-Nieto G, Monne I, Stevens J, Cattoli G, Capua I, Chen LM, Donis RO, Busch J, Paulson JC, Brockwell C, Webby R, Blanco J, Al-Natour MQ, Perez DR. 2008. Replication and transmission of H9N2 influenza viruses in ferrets: evaluation of pandemic potential. PLoS One 3:e2923 doi:10.1371/journal.pone.0002923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nishiura H, Yen HL, Cowling BJ. 2013. Sample size considerations for one-to-one animal transmission studies of the influenza A viruses. PLoS One 8:e55358 doi:10.1371/journal.pone.0055358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Friesenhagen J, Boergeling Y, Hrincius E, Ludwig S, Roth J, Viemann D. 2012. Highly pathogenic avian influenza viruses inhibit effective immune responses of human blood-derived macrophages. J. Leukoc. Biol. 92:11–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kobasa D, Jones SM, Shinya K, Kash JC, Copps J, Ebihara H, Hatta Y, Kim JH, Halfmann P, Hatta M, Feldmann F, Alimonti JB, Fernando L, Li Y, Katze MG, Feldmann H, Kawaoka Y. 2007. Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus. Nature 445:319–323 [DOI] [PubMed] [Google Scholar]
- 45. Perrone LA, Plowden JK, Garcia-Sastre A, Katz JM, Tumpey TM. 2008. H5N1 and 1918 pandemic influenza virus infection results in early and excessive infiltration of macrophages and neutrophils in the lungs of mice. PLoS Pathog. 4:e1000115 doi:10.1371/journal.ppat.1000115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ludwig S, Planz O. 2008. Influenza viruses and the NF-kappaB signaling pathway—towards a novel concept of antiviral therapy. Biol. Chem. 389:1307–1312 [DOI] [PubMed] [Google Scholar]
- 47. Senne DA, Panigrahy B, Kawaoka Y, Pearson JE, Suss J, Lipkind M, Kida H, Webster RG. 1996. Survey of the hemagglutinin (HA) cleavage site sequence of H5 and H7 avian influenza viruses: amino acid sequence at the HA cleavage site as a marker of pathogenicity potential. Avian Dis. 40:425–437 [PubMed] [Google Scholar]
- 48. Pasick J, Berhane Y, Hisanaga T, Kehler H, Hooper-McGrevy K, Handel K, Neufeld J, Argue C, Leighton F. 2010. Diagnostic test results and pathology associated with the 2007 Canadian H7N3 highly pathogenic avian influenza outbreak. Avian Dis. 54:213–219 [DOI] [PubMed] [Google Scholar]
- 49. Pasick J, Handel K, Robinson J, Copps J, Ridd D, Hills K, Kehler H, Cottam-Birt C, Neufeld J, Berhane Y, Czub S. 2005. Intersegmental recombination between the haemagglutinin and matrix genes was responsible for the emergence of a highly pathogenic H7N3 avian influenza virus in British Columbia. J. Gen. Virol. 86:727–731 [DOI] [PubMed] [Google Scholar]
- 50. Khatchikian D, Orlich M, Rott R. 1989. Increased viral pathogenicity after insertion of a 28S ribosomal RNA sequence into the haemagglutinin gene of an influenza virus. Nature 340:156–157 [DOI] [PubMed] [Google Scholar]
- 51. Orlich M, Gottwald H, Rott R. 1994. Nonhomologous recombination between the hemagglutinin gene and the nucleoprotein gene of an influenza virus. Virology 204:462–465 [DOI] [PubMed] [Google Scholar]
- 52. Gonzalez-Reiche AS, Morales-Betoulle ME, Alvarez D, Betoulle JL, Muller ML, Sosa SM, Perez DR. 2012. Influenza a viruses from wild birds in Guatemala belong to the North American lineage. PLoS One 7:e32873 doi:10.1371/journal.pone.0032873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Krauss S, Obert CA, Franks J, Walker D, Jones K, Seiler P, Niles L, Pryor SP, Obenauer JC, Naeve CW, Widjaja L, Webby RJ, Webster RG. 2007. Influenza in migratory birds and evidence of limited intercontinental virus exchange. PLoS Pathog. 3:e167 doi:10.1371/journal.ppat.0030167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dugan VG, Dunham EJ, Jin G, Sheng ZM, Kaser E, Nolting JM, Alexander HL, Jr, Slemons RD, Taubenberger JK. 2011. Phylogenetic analysis of low pathogenicity H5N1 and H7N3 influenza A virus isolates recovered from sentinel, free flying, wild mallards at one study site during 2006. Virology 417:98–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cox RJ, Major D, Hauge S, Madhun AS, Brokstad KA, Kuhne M, Smith J, Vogel FR, Zambon M, Haaheim LR, Wood J. 2009. A cell-based H7N1 split influenza virion vaccine confers protection in mouse and ferret challenge models. Influenza Other Respir. Viruses 3:107–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jadhao SJ, Achenbach J, Swayne DE, Donis R, Cox N, Matsuoka Y. 2008. Development of Eurasian H7N7/PR8 high growth reassortant virus for clinical evaluation as an inactivated pandemic influenza vaccine. Vaccine 26:1742–1750 [DOI] [PubMed] [Google Scholar]
- 57. Pappas C, Matsuoka Y, Swayne DE, Donis RO. 2007. Development and evaluation of an influenza virus subtype H7N2 vaccine candidate for pandemic preparedness. Clin. Vaccine Immunol. 14:1425–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. CDC 2006. Interim guidance for protection of persons involved in U.S. avian influenza outbreak disease control and eradication activities. CDC, Atlanta, GA [Google Scholar]