Abstract
We previously performed a small interfering RNA (siRNA) screen and identified serum- and glucocorticoid-regulated kinase 1 (SGK1) as a host factor required for influenza A virus replication. However, the role of SGK1 in the influenza viral life cycle has never been examined. In this study, we demonstrate that SGK1 is required for optimal replication of influenza virus, using the SGK1 inhibitor GSK 650394 and SGK1-specific siRNAs. We also demonstrate that SGK1 is required for viral ribonucleoprotein nuclear export.
TEXT
Influenza A virus is an enveloped, negative-strand RNA virus that possesses eight RNA segments. It enters cells via receptor-mediated endocytosis. After internalization, the viral ribonucleoprotein complex (vRNP), composed of the viral RNA (vRNA), nucleoprotein (NP), and the polymerase proteins (PB1, PB2, PA), dissociates from the matrix protein (M1) and enters the nucleus, where vRNA replication and transcription occur (1). Newly synthesized vRNPs are exported from the nucleus through the chromosome region maintenance 1 protein (CRM1)-mediated pathway (2). Virus assembly is orchestrated by the M1 protein, which interacts with viral membrane proteins hemagglutinin (HA), neuraminidase (NA), and M2 ion channel protein and vRNP complexes at the plasma membrane (3, 4). Virion release from the cell surface is facilitated by the neuraminidase activity of NA (1).
The role of cellular factors in the life cycle of influenza virus is not completely understood. We previously performed a genome-wide small interfering RNA (siRNA) screen to identify host factors that are required for the replication of influenza A virus (5). One of the 295 host factors that we identified in this screen is serum- and glucocorticoid-regulated kinase 1 (SGK1), a serine/threonine kinase that is involved in a variety of processes, including cellular stress response, cell growth and survival, renal sodium excretion, insulin secretion, and neuronal excitability. SGK1 is ubiquitously expressed and is under the transcriptional control of a variety of stimuli, including cell shrinkage, glucocorticoids, mineralocorticoids, and DNA damage. The localization of SGK1 depends on the functional state of the cell. Exposure of cells to serum leads to entry of SGK1 into the nucleus, whereas glucocorticoids enhance its localization into the cytosol (reviewed in reference 6). SGK1 phosphorylates several enzymes, including the ubiquitin ligase Nedd4-2, SAPK/ERK kinase-1 (SEK1), inducible nitric oxide synthase (iNOS), glycogen synthase kinase 3 (GSK3), phosphomannomutase 2, and mitogen-activated protein kinase kinase kinase 3 (MEKK3) (7–12). SGK1 also regulates transcription factors, including nuclear factor kappa B (NF-κB), cyclic AMP response element binding protein (CREB), and forkhead box O3a (FoxO3a) (13–15). Although the function of SGK1 in cellular processes is well studied, its role in the life cycle of influenza virus has never been examined. Therefore, we sought to investigate the step(s) of the viral life cycle where SGK1 is involved. A better understanding of the role of host factors in the viral life cycle is important in discovering novel ways to combat the virus.
SGK1 is required for optimal replication of influenza virus.
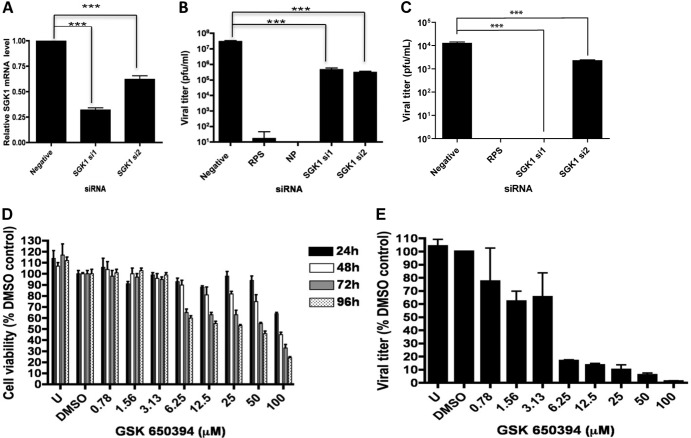
To determine whether SGK1 is important for replication of influenza A virus, we transfected each of two SGK1-specific siRNAs into a human lung adenocarcinoma cell line (A549), according to a previously published protocol (5). Briefly, A549 cells were transfected with SGK1 siRNA1 (GCGUUAGAGUGCCGCCUUAGA) or SGK1 siRNA2 (UACAGGCUUAUUUGUAAUGUA). At 48 h posttransfection, total RNA was prepared using TRIzol and cDNA was synthesized using the Superscript III first-strand synthesis system (Invitrogen). Real-time PCR was performed in a Roche LightCycler 480 II machine using previously published primers for SGK1 (16). As shown in Fig. 1A, the levels of SGK1 mRNA were reduced to 32% and 62% relative to the negative-control siRNA, for cells that were transfected with SGK1 siRNA1 and siRNA2, respectively. To determine whether knockdown of SGK1 inhibits replication of influenza virus, another set of SGK1 siRNA1- or siRNA2-transfected A549 cells were infected with influenza virus (A/WSN/33, here called WSN) at a multiplicity of infection (MOI) of 0.01 at 48 h posttransfection. Supernatants were harvested at 38 h postinfection (hpi), and a plaque assay was performed to quantify the amount of virus (Fig. 1B). As a positive control, we transfected cells with an siRNA specific to NP. As a transfection control, we used an siRNA against RPS27A which leads to cell death upon successful transfection. The amount of virus in the NP siRNA-transfected cells was below the limits of detection of the assay. Cells that were transfected with the negative-control siRNA had a viral titer of 2.9 × 107 PFU/ml. The amounts of virus in cells that were transfected with SGK1 siRNA1 and siRNA 2 were reduced to 4.6 × 105 PFU/ml and 3.0 × 105 PFU/ml, respectively (Fig. 1B). We also confirmed that the knockdown of SGK1 by siRNA inhibited the replication of an H3N2 influenza virus (A/Wisconsin/05) (Fig. 1C). These results indicate that SGK1 is required for optimal replication of influenza virus.
Fig 1.
SGK1 is required for optimal replication of influenza virus. (A) SGK1-specific siRNAs decrease SGK1 expression level. A549 cells were transfected with each of two SGK1-specific siRNAs or a negative-control siRNA from Qiagen. Total RNA was extracted from the cells at 48 h posttransfection, and quantitative reverse transcriptase (RT)-PCR was performed to determine the levels of SGK1. Statistical significance was determined using one-way analysis of variance (ANOVA) (P < 0.0001). (B and C) SGK1-specific siRNAs reduce influenza virus replication. A549 cells were transfected with each of two SGK1-specific siRNAs, a negative-control siRNA, RPS27A-specific siRNA, or an influenza virus nucleoprotein-specific siRNA. Forty-eight hours posttransfection, cells were infected with influenza virus H1N1 A/WSN/33 (B) or H3N2 A/Wisconsin/05 (C) at an MOI of 0.01 to allow multicycle replication. Supernatants were harvested at 38 to 40 h postinfection (hpi), and a plaque assay was performed. Statistical significance was determined using one-way ANOVA (P < 0.0001). (D) Effect of GSK 650394 on cell viability. A549 cells were treated with 2-fold serial dilutions of the SGK inhibitor GSK 650394 or solvent DMSO or left untreated (U). The amount of DMSO is the same for all the dilutions. Cell viability (expressed as a percentage of the DMSO control) was measured at 24, 48, 72, and 96 h posttreatment using the Promega CellTiter-Glo assay. (E) Effect of GSK 650394 on virus growth. GSK 650394-treated cells were infected with WSN at an MOI of 0.01. Supernatants were harvested 24 hpi, and a plaque assay was performed. The viral titer was expressed as a percentage of the DMSO control.
Next, we wanted to determine whether a compound that inhibits SGK1 can reduce viral replication. To this end, we tested GSK 650394, a commercially available compound that inhibits SGK1 and SGK2 with 50% inhibitory concentrations (IC50s) of 62 nM and 103 nM for SGK1 and SGK2, respectively (Tocris Bioscience, Ellisville, MO). First, we tested the effect of this compound on the viability of A549 cells by treating them with 2-fold serial dilutions (0.78 to 100 μM) of GSK 650394. At 24, 48, 72, and 96 h posttreatment, cell viability was measured using the CellTiter-Glo assay (Promega) that measures the amount of ATP in cells. As negative controls, cells were treated with the solvent dimethyl sulfoxide (DMSO) or left untreated. It is important to note that the amount of DMSO was kept the same for all dilutions of GSK 650394. The results were expressed as the percentage of viability relative to the DMSO control (Fig. 1D). The effect of GSK 650394 on cell viability was dose and time dependent. Cells had good viability (≥91% relative to the DMSO control) at 0.78 to 3.13 μM throughout the 96-hour period. At 6.25 to 50 μM, cell viability was good (≥75% relative to the DMSO control) up to 48 h. Cells that were treated with 100 μM the compound had poor viability (≤64% relative to the DMSO control). To determine whether GSK 650394 inhibits influenza virus replication, A549 cells were pretreated with the compound for 2 to 4 h and infected with WSN at an MOI of 0.01. After virus inoculation, cells were again incubated with medium containing GSK 650394. Supernatants were harvested 24 hpi, and a plaque assay was performed. The results were expressed relative to the DMSO control (Fig. 1E). Viral titers were reduced in a dose-dependent manner. The estimated IC50 for GSK 650394 is 3.59 μM, which results in a selectivity index (SI) of 34.84. There were several concentrations of the compound that reduced viral titers significantly without adversely affecting cell viability at 24 h posttreatment. For instance, at 50 μM, where the cell viability was 94%, the viral titer was reduced to 5.9% relative to the DMSO control. This demonstrates that the decrease in viral titer is not merely due to the cytotoxic effect of the compound. Although GSK 650394 also inhibits SGK2, it is unlikely that the reduction in viral titers caused by GSK 650394 is due to inhibition of SGK2 because SGK2-specific siRNAs did not inhibit the WSN-Renilla reporter virus in our siRNA screening (5). Taken together, our results confirm that SGK1 is required for optimal replication of influenza virus.
GSK 650394 does not inhibit influenza virus entry, polymerase activity, or expression of viral proteins.
Having shown that SGK1 is important for influenza virus replication, we next wanted to determine the step(s) of the virus life cycle where SGK1 is involved. First, we tested whether GSK 650394 inhibits entry of influenza virus using a previously published protocol (5). Briefly, A549 cells were treated with GSK 650394 (12.5, 25, and 50 μM) or solvent DMSO for 2 to 4 h prior to incubation with pseudoparticles bearing WSN-HA/NA proteins and encoding the Gaussia luciferase reporter gene. As a positive control, cells were also treated with diphyllin (2.1 μM), a compound previously shown to inhibit influenza virus entry (5). After 18 h, cells were washed several times and new medium was added. Gaussia luciferase activity was measured 24 h after the addition of medium. As shown in Fig. 2A, diphyllin reduced viral entry to 10% relative to the DMSO control, whereas GSK 650394 did not inhibit viral entry at any of the concentrations that were tested.
Fig 2.
GSK 650394 does not inhibit influenza virus entry, polymerase activity, or expression of viral proteins. (A) GSK 650394 does not inhibit entry of influenza virus. A549 cells were treated with GSK 650394 (12.5, 25, and 50 μM) or solvent DMSO. Diphyllin was used as a positive control. The cells were then incubated with pseudoparticles bearing influenza virus HA/NA proteins and encoding the Gaussia luciferase reporter gene. After 18 h, the cells were washed and medium was added. A luciferase assay was performed 24 h after addition of medium. (B) GSK 650394 does not inhibit the polymerase activity of influenza virus. A549 cells were treated with GSK 650394 (12.5, 25, and 50 μM) or solvent DMSO or A3 as a positive control. The cells were transfected with plasmids expressing influenza virus polymerase proteins (PB1, PB2, PA), NP, and an influenza virus-specific firefly luciferase reporter. A Renilla luciferase plasmid was cotransfected to normalize transfection efficiency. Four hours posttransfection, the medium was replaced with Dulbecco modified Eagle medium (DMEM) containing the above compounds. Twenty-four hours posttransfection, cells were lysed and luciferase activity was measured. (C) GSK 650394 does not affect expression of influenza viral proteins. A549 cells were treated with 2-fold dilutions of GSK 650394 (12.5, 25, or 50 μM) or solvent DMSO. Cells were infected with WSN at an MOI of 3. At 3, 5, and 7 h postinfection, cells were washed and lysed using RIPA buffer. Lysates were loaded onto an SDS-PAGE gel, and Western blotting was performed using CC11 (PB1), HT103 (NP), E10 (M1 and M2), and 9G9 (HA) monoclonal antibodies. An antibody against GAPDH was used as a loading control.
Next, we tested whether GSK 650394 inhibits the polymerase activity of influenza virus using a previously published mini-genome assay (17). Briefly, A549 cells were treated with GSK 650394 (12.5, 25, and 50 μM) or solvent DMSO. As a positive control, cells were also treated with A3 (20 μM), a compound previously shown to inhibit influenza virus polymerase function by acting on the pyrimidine synthesis pathway (17). The cells were transfected with plasmids expressing the influenza virus polymerase proteins (PB1, PB2, PA), NP, and an influenza virus-specific firefly luciferase reporter. A Renilla luciferase plasmid under the control of a simian virus 40 (SV40) promoter was also transfected to normalize transfection efficiency. As shown in Fig. 2B, A3 reduced the viral polymerase activity to 12% relative to the DMSO control, whereas GSK 650394 did not inhibit the viral polymerase activity at any of the concentrations that were tested.
To determine whether GSK 650394 inhibits the expression of influenza viral proteins, A549 cells were treated with GSK 650394 (12.5, 25, and 50 μM) or solvent DMSO. Two hours after treatment, cells were infected with WSN at an MOI of 3. After virus inoculation, the compound was added back to the cells. At 3, 5, and 7 hpi, cells were lysed with RIPA buffer. The lysates were denatured and loaded on a Bio-Rad 4 to 20% Mini-Protean TGX Precast gel. The proteins were transferred to a polyvinylidene difluoride (PVDF) membrane, and Western blotting was performed using monoclonal antibodies to influenza viral proteins. As shown in Fig. 2C, GSK 650394 did not inhibit the expression of PB1, NP, M1, M2, or HA proteins at any of the concentrations and time points that were tested.
GSK 650394 impairs the export of influenza vRNPs into the cytoplasm of A549 cells.
To determine whether SGK1 is involved in the trafficking of influenza vRNPs, we tested whether GSK 650394 inhibits trafficking of the vRNPs. A549 cells were pretreated with 2-fold serial dilutions of GSK 650394 (12.5, 25, and 50 μM) or solvent DMSO for 2 h. To synchronize infection, cells were prechilled for 10 min, infected with WSN virus at an MOI of 10 on ice for 20 min, and incubated at 37°C for 30 min. Cells were washed three times, and medium containing the compound was added back to the cells. At 5 and 7 hpi, cells were fixed, permeabilized, and stained with a monoclonal antibody against NP (HT103). The results for all 3 concentrations were similar, so only the results for the highest concentration (50 μM) are shown (Fig. 3). At 5 hpi, the vRNP localization was predominantly nuclear for both DMSO- and GSK 650394-treated cells (Fig. 3A). The percentages of nuclear accumulation for DMSO- and GSK 650394-treated cells were 90% ± 3% and 92% ± 3%, respectively (Fig. 3B). At 7 hpi, the vRNP localization was predominantly cytoplasmic for DMSO-treated cells. In contrast, the vRNP localization was predominantly nuclear for GSK 650394-treated cells (Fig. 3A). The percentages of nuclear accumulation for DMSO- and GSK 650394-treated cells were 12% ± 3% and 90% ± 3%, respectively (Fig. 3B). These results strongly indicate that GSK 650394 impairs the export of influenza vRNPs into the cytoplasm of A549 cells.
Fig 3.
GSK 650394 impairs the export of influenza vRNPs into the cytoplasm of A549 cells. (A) A549 cells were treated with 2-fold dilutions of GSK 650394 (12.5, 25, and 50 μM) or solvent DMSO. Only the 50 μM treatment is shown here. To synchronize infection, cells were prechilled for 10 min and infected with influenza virus (A/WSN/33) at an MOI of 10 on ice. At 5 and 7 h postinfection, cells were fixed, permeabilized, and stained with a monoclonal antibody against NP. 4′-6-Diamidino-2-phenylindole (DAPI) was used for nuclear staining. Results shown are representative images. White and red arrows indicate cells with NP cytoplasmic and nuclear accumulation, respectively. (B) Quantification of the percentage of NP nuclear signal at 5 hpi and 7 hpi. Results shown are the average of 3 replicates for the experiment in panel A. Approximately 80 to 100 cells were counted for each replicate. Statistical significance was determined using two-way ANOVA (P < 0.001).
SGK1-specific siRNAs impair the export of influenza vRNPs into the cytoplasm of A549 cells.
To further confirm that SGK1 is important for influenza vRNP trafficking, we tested whether SGK1-specific siRNAs would also inhibit this process. A549 cells were transfected with SGK1-specific siRNAs or negative-control siRNA. At 48 h posttransfection, cells were prechilled for 10 min, infected with WSN virus at an MOI of 10 on ice for 20 min, and incubated at 37°C for 30 min. At 5 and 7 hpi, cells were fixed, permeabilized, and stained with a monoclonal antibody against NP (HT103). At 5 hpi, the vRNP localization was predominantly nuclear for negative-control siRNA-, SGK1 siRNA1-, and SGK1 siRNA2-treated cells (Fig. 4A). The percentages of nuclear accumulation for negative-control siRNA-, SGK1 siRNA1-, and SGK1 siRNA2-treated cells were 94% ± 3%, 96 ± 0.4%, and 94% ± 2%, respectively (Fig. 4B). At 7 hpi, the vRNP localization was predominantly cytoplasmic for negative-control siRNA-treated cells (Fig. 4A). The percentage of nuclear accumulation for negative-control siRNA-treated cells was 12% ± 3%. In contrast, the percentages of nuclear accumulation for SGK1 siRNA1- and SGK1 siRNA2-treated cells were 75% ± 3% and 63% ± 5%, respectively (Fig. 4B). These results suggest that SGK1-specific siRNAs impair the export of influenza vRNPs into the cytoplasm of A549 cells.
Fig 4.
SGK1-specific siRNAs impair the export of influenza vRNPs into the cytoplasm of A549 cells. (A) A549 cells were transfected with each of two SGK1-specific siRNAs, each of two SGK2-specific siRNAs, or a negative-control siRNA. At 48 h posttransfection, cells were prechilled for 10 min and infected with influenza virus (A/WSN/33) at an MOI of 10 on ice. At 5 and 7 h postinfection, cells were fixed, permeabilized, and stained with a monoclonal antibody against NP. DAPI was used for nuclear staining. Results shown are representative images. (B) Quantification of the percentage of NP nuclear signal at 5 hpi and 7 hpi for SGK1-specific siRNAs and negative-control siRNA. Results shown are the average of 3 replicates. Approximately 80 to 100 cells were counted for each replicate. Statistical significance was determined using two-way ANOVA (P < 0.001).
We have shown in Fig. 3 that GSK 650394, a compound that inhibits SGK1 and SGK2, impairs influenza vRNP export. To rule out the possibility that SGK2 is involved in vRNP export, we also tested whether SGK2-specific siRNAs impair this process. As shown in Fig. 4A, the vRNP localizations were similar for negative-control siRNA, SGK2 siRNA1 (CAGGGCCAATGGGAACATCAA), and SGK2 siRNA2 (GUGCAUUCCUGGGAUUUUCTT) at 5 hpi and 7 hpi. Taken together, these results suggest that SGK1 but not SGK2 is involved in influenza vRNP export. This is consistent with our previous findings which indicated that SGK2 is not important for optimal influenza virus replication (5). Although SGK2 shares 80% identity with SGK1 in the catalytic domain, it differs from SGK1 in a number of respects. First, the SGK1 mRNA is widely expressed, whereas the SGK2 mRNA has a more restricted distribution, being highly expressed in kidney, liver, and pancreas and, at lower levels, in the brain. Unlike SGK1, the levels of SGK2 mRNA in fibroblasts are not induced by stimulation with serum. SGK2 is activated by 3-phosphoinositide-dependent protein kinase-1 (PDK1), albeit more slowly than SGK1. In contrast with SGK1, activation of SGK2 is suppressed only partially by inhibitors of phosphatidylinositol (PI) 3-kinase (18). At this time, it is not known whether these functional differences may explain the preference of influenza virus for SGK1.
GSK 650394 does not inhibit nuclear export of HIV Rev protein.
To determine whether GSK 650394 inhibits general nuclear export, we tested its activity against HIV Rev, using a well-described nuclear export assay (19). Briefly, A549 cells were treated with GSK 650394 (50 μM) or DMSO for 4 h prior to transfection with a plasmid encoding HIV Rev-green fluorescent protein (GFP) containing a mutated, nonfunctional nuclear export signal (NES) [pRev(1.4)-GFP] or the same plasmid with a functional NES reinserted upstream of GFP [pRev(1.4)-Rev NES-GFP]. The compound was added back to the cells at 5 h posttransfection. The cells were fixed and permeabilized at 24 h posttransfection. As a positive control, cells were treated with the nuclear export inhibitor leptomycin B (10 nM) or methanol at 18 h posttransfection. As shown in Fig. 5A, the nuclear export-defective Rev(1.4)-GFP was predominantly nuclear under all treatments. The percentages of nuclear accumulation in methanol-, leptomycin B-, DMSO-, and GSK 650394-treated cells were 96% ± 3%, 97% ± 2%, 96% ± 4%, and 94% ± 1%, respectively. Figures 5A and B show that Rev(1.4)-NES-GFP was predominantly cytoplasmic in methanol-treated cells (17% ± 16% nuclear accumulation). However, when cells were treated with leptomycin B, Rev(1.4)-NES-GFP was predominantly nuclear (75% ± 8% nuclear accumulation), consistent with previous findings (19). When cells were treated with DMSO or GSK 650394, Rev(1.4)-NES-GFP was predominantly cytoplasmic. The percentages of nuclear accumulation in DMSO- and GSK 650394-treated cells were 15% ± 6% and 21% ± 9%, respectively. These results show that GSK 650394 does not inhibit nuclear export of HIV Rev protein, indicating that this compound does not impair general nuclear export. Taken together with the results in Fig. 3, this suggests that GSK 650394 impairs influenza vRNP export in a specific manner.
Fig 5.
GSK 650394 does not inhibit nuclear export of HIV Rev protein. (A) A549 cells were treated with GSK 650394 (50 μM) or solvent DMSO. The cells were transfected with either a plasmid encoding HIV Rev-GFP containing a mutated, nonfunctional NES [pRev(1.4)-GFP] or the same plasmid with a functional NES reinserted upstream of GFP [pRev(1.4)-Rev NES-GFP]. As a positive control, cells were also treated with leptomycin B (10 nM) or solvent methanol at 18 h posttransfection. The cells were fixed, permeabilized and observed under a fluorescence microscope 24 h posttransfection. DAPI was used for nuclear staining. Approximately 70 to 80 cells were counted for each treatment. The average percentages of rev(1.4)-GFP or rev(1.4)-NES-GFP nuclear accumulation are shown. Statistical significance was determined using two-way ANOVA (P < 0.001). (B) Representative images of cells that were transfected with rev(1.4)-NES-GFP are shown. White and red arrows indicate cells with rev(1.4)-NES-GFP cytoplasmic and nuclear accumulation, respectively.
We also investigated the phosphorylation of SGK1 upon viral infection. For this purpose A549 cells were infected with WSN at an MOI of 3. After 8 h, cells were lysed as previously described. Western blotting was performed using a monoclonal antibody for phospho-S422 SGK1 (Abcam). As a positive control we used cells treated with dexamethasone (1 μM) for 8 h and insulin (300 nM) for 1 h (20). Infection with WSN virus led to partial phosphorylation of S422 in SGK1 (Fig. 6), confirming its role during the influenza A virus life cycle.
Fig 6.

Influenza virus infection activates the phosphorylation of SGK1. A549 cells were infected with influenza A/WSN/33 virus at an MOI of 3 for 8 h (WSN), or treated with dexamethasone (8 h) and insulin (1 h) (+), or left untreated (−). Cells were washed and lysed using RIPA buffer. Lysates were loaded onto an SDS-PAGE gel, and Western blots were performed using a polyclonal anti-influenza NS1, phospho-specific SGK1, and a monoclonal anti-histone 3 antibody as a loading control. An arrowhead indicates the band corresponding to pSGK1.
In summary, we have demonstrated that SGK1 is required for optimal replication of influenza A virus in A549 cells by using a compound that inhibits SGK1 (GSK 650394) and by siRNA knockdown of SGK1. GSK 650394 was originally developed as a compound that inhibits androgen-stimulated growth of the prostate cancer cell line LNCaP. In the absence of androgens, GSK 650394 was not toxic to LNCaP cells (21). However, GSK 650394 has not been tested in animal models of prostate cancer. Similarly, our results show that GSK 650394 inhibits viral replication at concentrations that are not toxic to cells. Thus, SGK1 inhibitors may serve as potential anti-influenza agents. Further studies need to be performed to design analogs of this compound to improve its pharmacokinetic properties for testing in animal models of influenza virus infection. It has already been shown that acetylsalicylic acid (an inhibitor of the cellular factor NF-κB) inhibits replication of the highly pathogenic avian influenza virus strain FPV (H7N7) without selection of resistant variants in MDCK cells (22). Therefore, the regulation of SGK1 might lead to the development of novel drugs to circumvent the problem of drug resistance. We have also demonstrated that the reduction in viral replication is due to impaired vRNP export. Previous studies have utilized several compounds to identify host factors that are involved in influenza vRNP export. The CRM1-specific export inhibitor leptomycin B was shown to inhibit influenza A vRNP export in MDCK cells (2). The MEK-specific inhibitor U0126 impaired influenza A vRNP nuclear export in MDCK cells (23). In this study, we have identified SGK1 as another host factor that is required for influenza vRNP export. We have demonstrated this using an SGK1 inhibitor and two SGK1-specific siRNAs. In contrast with leptomycin B, the SGK1 inhibitor GSK 650394 does not inhibit the general cellular nuclear export machinery, suggesting that SGK1 is involved in a distinct pathway that regulates influenza vRNP export. SGK1 is phosphorylated at position 422 upon viral infection; however, the specific phosphorylation target of SGK1 in the context of the viral life cycle needs to be investigated further. Several influenza virus proteins involved in vRNP export are known to be phosphorylated, including NP (24), PB1 (25), PA (26), M1 (27), and NS2 (28). Whether SGK1 phosphorylates any of these viral proteins or another host factor involved in the viral life cycle remains to be elucidated. Our study demonstrates a novel function of SGK1 in the life cycle of influenza A virus. This may be important in the development of new antiviral agents to circumvent the problem of drug resistance.
ACKNOWLEDGMENTS
We acknowledge Gene Tan, Mila Ortigoza, Hans-Heinrich Hoffmann, and Jenish Patel for helpful discussions. We thank Matthew Evans, J. S. Mymryk, and J. Torchia for generously providing reagents. All antibodies against influenza viral proteins were generated at the Icahn School of Medicine at Mount Sinai hybridoma facility, except for the PB1 antibody that was generated by Christopher Seibert at Icahn School of Medicine at Mount Sinai.
Partial support for this work was provided by National Institutes of Health grants HHSN26620070010C and U01 AI1074539. M. S. Miller is supported by a Canadian Institutes of Health Research Postdoctoral Fellowship.
Footnotes
Published ahead of print 13 March 2013
REFERENCES
- 1. Palese P, Shaw ML. 2007. Orthomyxoviridae: the viruses and their replication, p 1647–1689 In Knipe DM, Howley PM. (ed), Fields virology, fifth ed Lippincott Williams and Wilkins, Philadelphia, PA [Google Scholar]
- 2. Watanabe K, Takizawa N, Katoh M, Hoshida K, Kobayashi N, Nagata K. 2001. Inhibition of nuclear export of ribonucleoprotein complexes of influenza virus by leptomycin B. Virus Res. 77:31–42 [DOI] [PubMed] [Google Scholar]
- 3. Ali A, Avalos RT, Ponimaskin E, Nayak DP. 2000. Influenza virus assembly: effect of influenza virus glycoproteins on the membrane association of M1 protein. J. Virol. 74:8709–8719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ye Z, Liu T, Offringa DP, McInnis J, Levandowski RA. 1999. Association of influenza virus matrix protein with ribonucleoproteins. J. Virol. 73:7467–7473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Konig R, Stertz S, Zhou Y, Inoue A, Hoffmann HH, Bhattacharyya S, Alamares JG, Tscherne DM, Ortigoza MB, Liang Y, Gao Q, Andrews SE, Bandyopadhyay S, De Jesus P, Tu BP, Pache L, Shih C, Orth A, Bonamy G, Miraglia L, Ideker T, Garcia-Sastre A, Young JA, Palese P, Shaw ML, Chanda SK. 2010. Human host factors required for influenza virus replication. Nature 463:813–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lang F, Artunc F, Vallon V. 2009. The physiological impact of the serum and glucocorticoid-inducible kinase SGK1. Curr. Opin. Nephrol. Hypertens. 18:439–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chun J, Kwon T, Kim DJ, Park I, Chung G, Lee EJ, Hong SK, Chang SI, Kim HY, Kang SS. 2003. Inhibition of mitogen-activated kinase kinase kinase 3 activity through phosphorylation by the serum- and glucocorticoid-induced kinase 1. J. Biochem. 133:103–108 [DOI] [PubMed] [Google Scholar]
- 8. Debonneville C, Flores SY, Kamynina E, Plant PJ, Tauxe C, Thomas MA, Munster C, Chraibi A, Pratt JH, Horisberger JD, Pearce D, Loffing J, Staub O. 2001. Phosphorylation of Nedd4-2 by Sgk1 regulates epithelial Na(+) channel cell surface expression. EMBO J. 20:7052–7059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Helms MN, Yu L, Malik B, Kleinhenz DJ, Hart CM, Eaton DC. 2005. Role of SGK1 in nitric oxide inhibition of ENaC in Na+-transporting epithelia. Am. J. Physiol. Cell Physiol. 289:C717–C726 [DOI] [PubMed] [Google Scholar]
- 10. Kim MJ, Chae JS, Kim KJ, Hwang SG, Yoon KW, Kim EK, Yun HJ, Cho JH, Kim J, Kim BW, Kim HC, Kang SS, Lang F, Cho SG, Choi EJ. 2007. Negative regulation of SEK1 signaling by serum- and glucocorticoid-inducible protein kinase 1. EMBO J. 26:3075–3085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Menniti M, Iuliano R, Amato R, Boito R, Corea M, Le Pera I, Gulletta E, Fuiano G, Perrotti N. 2005. Serum and glucocorticoid-regulated kinase Sgk1 inhibits insulin-dependent activation of phosphomannomutase 2 in transfected COS-7 cells. Am. J. Physiol. Cell Physiol. 288:C148–C155 [DOI] [PubMed] [Google Scholar]
- 12. Wyatt AW, Hussain A, Amann K, Klingel K, Kandolf R, Artunc F, Grahammer F, Huang DY, Vallon V, Kuhl D, Lang F. 2006. DOCA-induced phosphorylation of glycogen synthase kinase 3beta. Cell. Physiol. Biochem. 17:137–144 [DOI] [PubMed] [Google Scholar]
- 13. David S, Kalb RG. 2005. Serum/glucocorticoid-inducible kinase can phosphorylate the cyclic AMP response element binding protein, CREB. FEBS Lett. 579:1534–1538 [DOI] [PubMed] [Google Scholar]
- 14. Dehner M, Hadjihannas M, Weiske J, Huber O, Behrens J. 2008. Wnt signaling inhibits Forkhead box O3a-induced transcription and apoptosis through up-regulation of serum- and glucocorticoid-inducible kinase 1. J. Biol. Chem. 283:19201–19210 [DOI] [PubMed] [Google Scholar]
- 15. Leroy V, De Seigneux S, Agassiz V, Hasler U, Rafestin-Oblin ME, Vinciguerra M, Martin PY, Feraille E. 2009. Aldosterone activates NF-kappaB in the collecting duct. J. Am. Soc. Nephrol. 20:131–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Abbruzzese C, Mattarocci S, Pizzuti L, Mileo AM, Visca P, Antoniani B, Alessandrini G, Facciolo F, Amato R, D'Antona L, Rinaldi M, Felsani A, Perrotti N, Paggi MG. 2012. Determination of SGK1 mRNA in non-small cell lung cancer samples underlines high expression in squamous cell carcinomas. J. Exp. Clin. Cancer Res. 31:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hoffmann HH, Kunz A, Simon VA, Palese P, Shaw ML. 2011. Broad-spectrum antiviral that interferes with de novo pyrimidine biosynthesis. Proc. Natl. Acad. Sci. U. S. A. 108:5777–5782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kobayashi T, Deak M, Morrice N, Cohen P. 1999. Characterization of the structure and regulation of two novel isoforms of serum- and glucocorticoid-induced protein kinase. Biochem. J. 344(Pt 1):189–197 [PMC free article] [PubMed] [Google Scholar]
- 19. Henderson BR, Eleftheriou A. 2000. A comparison of the activity, sequence specificity, and CRM1-dependence of different nuclear export signals. Exp. Cell Res. 256:213–224 [DOI] [PubMed] [Google Scholar]
- 20. Hall BA, Kim TY, Skor MN, Conzen SD. 2012. Serum and glucorticoid-regulated kinase 1 (SGK1) activation in breast cancer: requirement for mTORC1 activity associates with ER-alpha expression. Breast Cancer Res. Treat 135:469–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sherk AB, Frigo DE, Schnackenberg CG, Bray JD, Laping NJ, Trizna W, Hammond M, Patterson JR, Thompson SK, Kazmin D, Norris JD, McDonnell DP. 2008. Development of a small-molecule serum- and glucocorticoid-regulated kinase-1 antagonist and its evaluation as a prostate cancer therapeutic. Cancer Res. 68:7475–7483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mazur I, Wurzer WJ, Ehrhardt C, Pleschka S, Puthavathana P, Silberzahn T, Wolff T, Planz O, Ludwig S. 2007. Acetylsalicylic acid (ASA) blocks influenza virus propagation via its NF-kappaB-inhibiting activity. Cell Microbiol. 9:1683–1694 [DOI] [PubMed] [Google Scholar]
- 23. Pleschka S, Wolff T, Ehrhardt C, Hobom G, Planz O, Rapp UR, Ludwig S. 2001. Influenza virus propagation is impaired by inhibition of the Raf/MEK/ERK signalling cascade. Nat. Cell Biol. 3:301–305 [DOI] [PubMed] [Google Scholar]
- 24. Kistner O, Muller K, Scholtissek C. 1989. Differential phosphorylation of the nucleoprotein of influenza A viruses. J. Gen. Virol. 70(Pt 9):2421–2431 [DOI] [PubMed] [Google Scholar]
- 25. Mahmoudian S, Auerochs S, Grone M, Marschall M. 2009. Influenza A virus proteins PB1 and NS1 are subject to functionally important phosphorylation by protein kinase C. J. Gen. Virol. 90:1392–1397 [DOI] [PubMed] [Google Scholar]
- 26. Sanz-Ezquerro JJ, Fernandez Santaren J, Sierra T, Aragon T, Ortega J, Ortin J, Smith GL, Nieto A. 1998. The PA influenza virus polymerase subunit is a phosphorylated protein. J. Gen. Virol. 79(Pt 3):471–478 [DOI] [PubMed] [Google Scholar]
- 27. Gregoriades A, Christie T, Markarian K. 1984. The membrane (M1) protein of influenza virus occurs in two forms and is a phosphoprotein. J. Virol. 49:229–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Richardson JC, Akkina RK. 1991. NS2 protein of influenza virus is found in purified virus and phosphorylated in infected cells. Arch. Virol. 116:69–80 [DOI] [PubMed] [Google Scholar]