Abstract
Purpose
To identify longitudinal changes in fusional vergence ranges and their relationship to other clinical measures in young myopic subjects.
Methods
Measurements were collected annually for 10 years on 114 subjects from the University of Houston Correction of Myopia Evaluation Trial cohort. Subject age was 7 to 13 years at year 1 of follow-up. Measurements included refractive error, distance and near phoria, interpupillary distance (IPD), prism bar fusional vergence ranges, and nearpoint of convergence (NPC). Multilevel modeling was used to determine baseline and rate of change for fusional vergence ranges and the impact of phoria, IPD, and NPC on these measures.
Results
Year 1 mean distance base-out (BO) break was 20 prism diopters (pd) and decreased 5.6 pd over 10 years (p <0.001). Mean near BO break was 30 pd at year 1 and decreased 9.4 pd over 10 years (p < 0.001). Greater esophoria was significantly related to greater BO break (p < 0.02) and receded NPC was significantly related to lower magnitude BO break at near (p < 0.001). Distance IPD increased 3 mm over 10 years (p < 0.001) but was unrelated to the magnitude of the BO ranges (p > 0.2). Mean distance base-in (BI) break was 7 pd at year 1 and increased 0.5 pd in 10 years (p = 0.04). Mean near BI break was 13 pd at year 1 and did not significantly change. Mean distance phoria was 0.1 pd exophoria at year 1 and did not change, whereas near phoria was 2.4 pd esophoria at year 1 and became more exophoric (4 pd in 10 years, p < 0.001).
Conclusions
These results suggest that for myopic children convergence ranges decrease for both distance and near viewing during the school years as near phoria becomes more exophoric. These findings could have clinical implications given that compensating convergence ranges decrease as near phoria becomes more divergent.
Keywords: fusional vergence ranges, prism bar, children, longitudinal, binocular vision
Fusional vergence ranges are commonly measured in the clinical setting to provide information about a patient’s binocular status and ability to compensate for the natural resting position of their eyes (phoria). Reduced fusional vergence ranges, particularly in individuals with larger phorias, may be associated with symptoms of asthenopia or intermittent diplopia because of a poor ability to compensate for the phoria and maintain fusion.1 For these patients, prism lenses may be prescribed to decrease the demand on vergence, or vision therapy initiated to increase fusional vergence ranges, both with the aim of improving visual comfort and decreasing symptoms. Although it is generally accepted that fusional vergence ranges can be increased through vision therapy,2–4 it is not known if fusional vergence ranges change naturally with age irrespective of vision therapy.
A comparison of previous studies of step fusional vergence ranges in children and adults suggests differences in normative data between school children and young adults.5,6 On the basis of these studies, Scheiman and Wick7 recommend different expected normative fusional vergence ranges for adults than children, with adults having lower near convergence ranges [19 vs. 23 prism diopters (pd)], but slightly higher near divergence ranges (13 vs. 12 pd) than children aged 7 to 12 years. One concern with these predicted norms is that they are based on cross-sectional studies that were performed independently of each other by different examiners and have yet to be confirmed by a longitudinal study.
The Houston Correction of Myopia Evaluation Trial (COMET) Cohort provides longitudinal measurements for a large number of subjects that includes step fusional vergence ranges over a 10 year period from the early school years into young adulthood. In addition, longitudinal data of interpupillary distance (IPD), nearpoint of convergence (NPC), and phoria are available to identify relationships between these factors and vergence ability. The purpose of this study is to investigate the relationship of multiple clinical measurements of binocular vision and their potential change over time in the Houston COMET Cohort.
METHODS
This study was approved by the University of Houston Committee for the Protection of Human Subjects. Written parental consent and subject assent were obtained for all study participants. One hundred eighteen children were enrolled in the COMET study at the University of Houston College of Optometry and followed yearly for 10 years. COMET was a multicenter clinical trial, which evaluated the effectiveness of progressive addition lenses on slowing the progression of myopia in children over a 5 year period.8 After the clinical trial ended, the same cohort was consented and enrolled in the Collaborative Observational Study of Myopia in the COMET Cohort (COSMICC), which will continue until 2013. Major eligibility criteria for COMET included myopic refractive error of −1.25 to −4.50 D spherical equivalent in each eye and age of 6 to 11 years. Subjects were randomized to either single vision lenses or progressive addition lenses (Varilux Comfort with a +2.00 D addition [PALS]) for the first 5 years of the study (COMET). For the second 5 years of the study (COSMICC), in consultation with their study optometrist, subjects were permitted to select single vision lenses, progressive addition lenses, or contact lens correction regardless of their previous treatment assignment.
At each annual study visit, a standardized COMET protocol was used to collect a variety of study measurements for each subject. The annual measurements included in the present analysis are distance and near phoria, NPC, IPD, and subjective refraction, all of which were collected beginning the first year of follow-up after study enrollment (termed year 1). Individual sites were also given the ability to incorporate additional study measures to maintain consistency with the standard of care currently practiced at their academic institution for yearly eye examinations. At the Houston site, both distance and near prism bar fusional vergence ranges were such measures and were obtained annually beginning in year 1. All clinical measures were made by study optometrists and the IPD measured by a certified study optician using an Essilor pupilometer on the distance setting. During the course of 10 years, five different licensed optometrists participated in the testing.
At the beginning of each examination, NPC and prism bar fusional vergence ranges were measured while each subject wore their habitual refraction. For the first 5 years of the study, the habitual distance correction was placed in a trial frame to prevent unmasking the examiner as to the subject’s lens assignment (single vision vs. progressive addition lenses). For the second 5 years, examiners were no longer masked and the majority of the subjects wore single vision lenses or contact lenses for study measurements. Six subjects who still elected to wear PALS were typically tested in a trial frame with their habitual distance correction, or occasionally tested with their habitual correction viewing through the distance portion of the lenses for the distance and near measurements.
To measure NPC, subjects fixated a small, isolated picture (the parrot on the Children’s Fixation bar 7004001; Clement Clarke, Harlow, Essex, UK). The examiner identified the near distance at which the subject reported diplopia, or when the eyes lost fusion if the subject did not report diplopia. This procedure was repeated three times and the average measurement reported here.
Distance fusional vergence ranges were measured with an isolated letter above threshold acuity (typically 20/25), whereas near fusional vergence ranges were measured with an isolated picture on a nearpoint stick (Children’s Fixation bar 7004001; Clement Clarke, Harlow, Essex, UK). For both distance and near ranges, a horizontal prism bar was introduced over one eye and the prism power increased sequentially at a slow pace until the subject reported diplopia or the examiner observed the eyes to lose fusion (termed “break”). The examiner then paused at this prism value and asked the subject if the target stayed double, or if they could get it back together. If the subject was able to regain and maintain fusion at this point, the prism power was increased again until a sustainable break in fusion was observed and this value was then recorded as the break. The prism power was then decreased until the subject stated that the target was single or the examiner observed the eyes to regain fusion and the value recorded (termed “recovery”). Measurements of blur were not obtained during testing of fusional vergence ranges given that the subjects were young at the onset of the study and subjective reports of blur could not be confirmed with objective observations. The prism bar steps used were 1, 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, 25, 30, 35, and 40 pd. For subjects who did not lose fusion, break was recorded as >40 pd and no recovery value was recorded. The testing order for fusional vergence ranges was distance base-in (BI), distance base-out (BO), near BI, and near BO. These measurements were always performed at the beginning of the visit so as not to break the flow of the standardized COMET protocol, of which prism bar fusional vergence ranges was not a component.
Later in the examination, myopic refractive correction was determined using a standardized subjective refraction protocol. If the change in manifest refraction was ±0.50 D spherical equivalent different from the habitual refraction in either eye, an updated refraction was prescribed. If the difference was smaller, the decision to make a change in the subject’s prescription was at the discretion of the examiner. The examiner then placed the refraction to be prescribed in a trial frame for cover test measurements. This order of testing and use of the prescribed refraction for cover testing was dictated by the COMET study protocol, which was designed to ensure that subjects did not manifest strabismus through their prescribed refraction. Although this refraction would potentially be greater minus than the habitual refraction through which fusional vergence ranges and NPC had been tested, subjects were seen yearly, and thus the magnitude of increase in refraction was small (mean spherical equivalent change in spectacles ranged from −0.21 to −0.55 D per year over 10 years). Alternate cover test with prism neutralization was then performed to measure the phoria. If neutral was observed for a range of prism values, the midpoint of first neutral and reversal was documented for the measurement. This technique was used for all years of the study.
Data Analysis
Data were analyzed using the Proc Mixed analysis tool for multilevel modeling in SAS version 9.1.3 as described by Littell et al.9 This analysis fit a linear regression model to each individual subject’s longitudinal step vergence data to identify the intercept (value at year 1) and the slope (rate of change over time) for each testing condition (distance BI break and recovery, distance BO break and recovery, near BI break and recovery, and near BO break and recovery). The individual subject’s intercepts and slopes were then combined to identify the group’s mean year 1 values and mean rate of change in the break and recovery measurements. Individual predictive factors of fusional vergence ranges (distance and near phoria, NPC, IPD, refractive error, year 1 age, COMET treatment group, gender, and ethnicity) were tested to identify significant associations with year 1 measures of distance BO break, distance BI break, near BO break, and near BI break. The relationship between these factors and year 1 vergence recovery values was also analyzed. Clinically significant changes in the mean prism bar fusional vergence ranges, NPC, and phoria were defined according to the repeatability of these clinical measures between examiners and over time. Based on previous studies of test repeatability, a clinically significant change in mean prism bar fusional vergence ranges was defined as a difference >3 pd,10 mean change in NPC was a difference >1 cm,11 and mean change in phoria was a difference >2 pd.12
RESULTS
Analysis was performed on 114 subjects from the Houston COMET cohort. Of the original 118 enrolled, three subjects were eliminated from data analysis because they withdrew from the study during the first 3 years, and one subject was eliminated because of accommodative esotropia. None of the remaining 114 subjects included in analysis had strabismus. The group of 114 subjects included 52 males and 62 females and was ethnically diverse with 8 Asians, 19 Blacks, 29 whites, 51 Hispanics, and 7 individuals of mixed ethnicity. Because the data reported here were collected starting with the 12-month follow-up visit after COMET study enrollment, the year 1 age for this study is ~1 year greater than that of the subjects’ age at the initial COMET enrollment visit (year 1 age range 7 to 13 years, mean = 10.6 ± 1.4). Yearly examination completion was good with 90% of the subjects attending at least 90% of the visits. Mean refractive error of the group at year 1 was −2.45 ±0.95 D and −4.77 ±1.85 at year 10.
When analyzing the data for the subjects, any break value that was documented in the record as >40 pd (indicating the subject did not break fusion for the maximum prism value on the prism bar) was entered into the database as 45. This value was chosen because prism values traditionally increase in 5 pd steps beyond 20 pd, and thus 45 would be the next likely step tested if the prism bar had a greater range. This strategy may have slightly overestimated fusional vergence ranges for some subjects but could also possibly underestimate fusional vergence ranges for the same subjects. The number of measures entered as 45 was only 19 of 1076 (1.8%) for distance BO break and 76 of 1076 (7%) for near BO break.
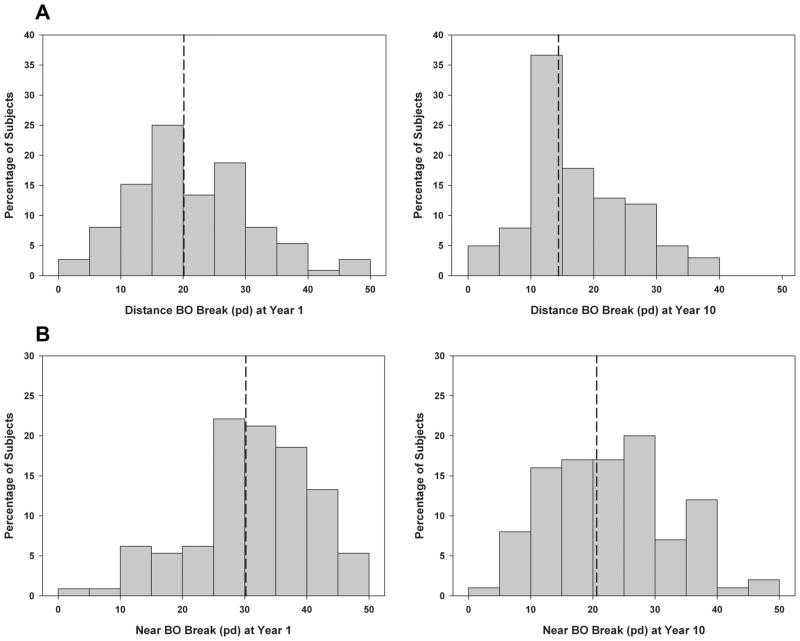
Table 1 shows mean year 1 values of study measures for all subjects combined and the total mean change in study measures over 10 years. For all subjects combined, distance BO break decreased 5.6 pd over 10 years and near BO break decreased 9.4 pd, both of which were statistically significant and clinically significant based on the fact that the size of the change was greater than the repeatability of prism bar fusional vergence measures10 (p <0.001; Fig. 1). Distance BI break increased 0.5 pd in 10 years, which was statistically significant (p = 0.04) but not clinically significant. Near BI break increased 0.4 pd over 10 years, which was not statistically significant (p = 0.9). The same statistically significant trends were observed for fusional vergence recovery values at both testing distances and for both prism directions as shown in Table 1. The mean distance phoria did not change over time (p = 0.6), but near phoria did change over time and became more exophoric as shown in Fig. 2 (4 pd in 10 years, p < 0.001). Distance IPD increased 3 mm over 10 years (p < 0.001), and there was no significant change in NPC over time (p = 0.3).
TABLE 1.
Changes in study measures over 10 years
| Distance findings | BO break | BO recovery | BI break | BI recovery | Phoria | IPD |
|---|---|---|---|---|---|---|
| Mean ± SD at year 1 | 20 ± 9 pd | 15 ± 6 pd | 7 ± 2 pd | 5 ± 2 pd | 0.1 ± 2.2 pd exo | 59 ± 3 mm |
| Change over 10 yrs | ↓ 5.6 pd | ↓ 4.6 pd | ↑ 0.5 pd | ↓ 0.4 pd | 0.01 pd more exo | ↑ 3 mm |
| p | <0.0001 | <0.0001 | 0.0413 | 0.1382 | 0.6117 | <0.0001 |
|
| ||||||
| Near findings | BO break | BO recovery | BI break | BI recovery | Phoria | NPC |
|
| ||||||
| Mean ± SD at year 1 | 30 ± 9 pd | 24 ± 7 pd | 13 ± 5 pd | 10 ± 4 pd | 2.4 ± 5.1 pd eso | 1 ± 2 cm |
| Change over 10 yrs | ↓ 9.4 pd | ↓ 8.3 pd | ↑ 0.04 pd | ↓ 0.09 pd | 4.1 pd more exo | ↑ 0.3 cm |
| p | <0.0001 | <0.0001 | 0.92 | 0.8027 | <0.0001 | 0.3147 |
BO, base-out; BI, base-in; pd, prism diopters; IPD, interpupillary distance; NPC, nearpoint of convergence.
FIGURE 1.
Distributions of distance BO break (A) and near BO break (B) at year 1 and the 10-year follow-up visit. Dotted lines indicate the group means, which significantly decreased by the 10-year follow-up visit.
FIGURE 2.
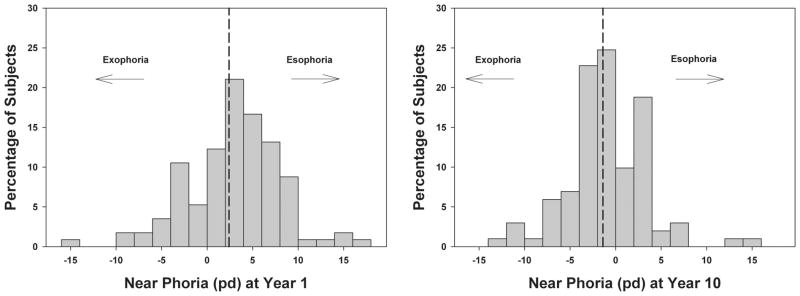
Distributions of near phoria at year 1 and the 10-year follow-up visit. Dotted lines indicate the group means, which became significantly more exophoric by the 10-year follow-up visit.
Seven measures (distance phoria, IPD, refractive error, year 1 age, COMET treatment group, gender, and ethnicity) were analyzed to determine their association with year 1 distance BO break and BI break measures. There was no significant relationship between distance vergence measures and IPD (p ≥ 0.11), refractive error (p ≥ 0.13), year 1 age (p ≥ 0.51), COMET treatment group (p ≥ 0.76), gender (p ≥ 0.10), or ethnicity (p ≥ 0.10). Distance phoria was significantly related to year 1 distance BO break measures (p = 0.012) with greater esophoria being associated with greater BO ranges (0.3 pd increase in BO range for every 1 pd greater esophoria), but there was no significant relationship between distance phoria and distance BI break measures (p = 0.25). The same significant relationships were observed between the tested measures and distance recovery values (data not shown).
Eight measures (near phoria, IPD, refractive error, NPC, year 1 age, COMET treatment group, gender, and ethnicity) were analyzed to determine their association with year 1 near BO break and BI break measures (Table 2). There was no significant association between near vergence measures and refractive error (p ≥ 0.052), year 1 age (p ≥ 0.71), gender (p ≥ 0.26), or ethnicity (p ≥ 0.10). There was also no significant relationship between distance IPD and near BO break measures (p =0.29), but there was a significant relationship between distance IPD and near BI break measures with greater IPD being associated with greater BI ranges (p = 0.006). There was not a significant relationship between COMET treatment group and near BO break measures (p = 0.91), but there was a significant relationship between treatment group and near BI break measures (p = 0.002) with the subjects randomized to single vision lenses having greater near BI break measures by an average of 1.4 pd. However, this effect was not clinically significant because 1.4 pd is less than the repeatability of prism bar fusional vergence testing.10 Near phoria was significantly related to year 1 near vergence measures (p = 0.004) with greater esophoria associated with greater BO ranges and smaller BI ranges. NPC was also significantly related to year 1 BO vergence measures (p = 0.003) with receded NPC corresponding to both lower magnitude BO (0.8 pd per 1 cm receded NPC) and BI (0.2 pd per 1 cm receded NPC) ranges. The same significant trends were observed between the tested measures and near recovery values (data not shown).
TABLE 2.
Predictive factors for year 1 near fusional vergence ranges
| Effect on year 1 near BO break measurement | p | Effect on year 1 near BI break measurement | p | |
|---|---|---|---|---|
| Near phoria | ↑ 0.2 pd for every pd of esophoria greater than the group mean | 0.004 | ↓ 0.2 pd for every pd of esophoria greater than the group mean | <0.001 |
| IPD | No effect | 0.29 | ↑ 0.2 pd for every mm of IPD greater than the group mean | 0.006 |
| NPC | ↓ 0.8 pd for every 1 cm recession of NPC from the group mean | <0.001 | ↓ 0.2 pd for every 1 cm recession of NPC from the group mean | 0.003 |
| Refractive error OD | No effect | 0.14 | No effect | 0.052 |
| Refractive error OS | No effect | 0.20 | No effect | 0.11 |
| Year 1 age | No effect | 0.74 | No effect | 0.98 |
| COMET treatment group | No effect | 0.91 | Subjects randomized to single vision lenses had a larger break by 1.4 pd | 0.002 |
| Gender | No effect | 0.26 | No effect | 0.72 |
| Ethnicity | No effect | 0.10 | No effect | 0.68 |
DISCUSSION
This study found significant decreases in convergence ranges at both distance and near over the 10 years that these young, myopic subjects were followed, but no significant change in divergence ranges. The findings of this study agreed well with the published trends in expected near fusional vergence range norms by Scheiman and Wick7 who predicted a decrease in near BO ranges from childhood to early adulthood, but no significant change in near BI ranges over this time period. However, the magnitude of change observed in BO ranges was greater in this study with mean fusional vergence ranges decreasing from 30 pd in childhood to ~21 pd in early adulthood vs. the change from 23 to 19 proposed by Scheiman and Wick.7 Near BI ranges were virtually identical between this study and the report by Scheiman and Wick7 at ~13 pd in both children and adults. A comparison of distance ranges is only possible for adults, as Scheiman and Wick did not report expected norms for distance fusional vergence ranges in children. When comparing distance ranges for adults, the BI break obtained in this study was identical to Scheiman and Wick, whereas the BO break was slightly larger in this study (14 vs. 11 pd).7
The mean year 1 values of this study also agreed well with a more recent study by Jimenez et al.13 of normative step fusional prism bar vergence ranges in a large population of elementary school children from Spain. Both distance and near BI and BO break measures reported by that study were within ± 3 pd of the values reported in this study, except for near BO break, which was much lower in Jimenez et al.13 at 18 vs. 30 pd reported in this study. However, the study by Jimenez et al. did not discuss whether subjects were given the opportunity to regain fusion on first observing a break, and thus it is possible that the near BO ranges observed in this study are greater because of subjects having the opportunity to regain fusion and continue beyond the first point at which a break occurred.
One limitation to the generalizability of the results from this study is that all the subjects were myopic. Although no relationship between year 1 ranges and the magnitude of myopia was observed, the data cannot identify whether or not year 1 fusional vergence ranges, or their rate of change differ in individuals with emmetropia or hyperopia. However, the study by Jimenez et al.13 included a large sample of elementary school children recruited directly from schools, and thus it is likely to have included children with a variety of refractive errors, including emmetropia and hyperopia. Thus given the good agreement between the findings of the Jimenez et al. study and the mean year 1 fusional vergence ranges from this study, it is not expected that the results of this study would differ greatly if subjects without myopia were included.
As stated above, the magnitude of myopia was not significantly related to year 1 fusional vergence ranges. Factors that were significantly related to year 1 fusional vergence ranges are distance and near phorias, with the observed trend in the same direction as would be clinically expected (i.e., greater esophoria is associated with greater BO ranges at both distance and near, but smaller BI ranges at near). Also in agreement with clinical expectation was the finding that receded NPC was significantly related to lower near BO ranges at approximately a 1 pd decrease in BO range for every 1 cm NPC recession. One unexpected finding was the significant association between distance IPD and near BI ranges. Although distance IPD was unrelated to all other measures, an increase in distance IPD was significantly related to an increase in near BI ranges. The effect was quite small and would amount to only a 2 pd increase in near BI ranges for an increase in IPD by 10 mm greater than the group mean and is thus likely not clinically significant given the repeatability of prism bar fusional vergence ranges.10 However, the direction of the observed trend may be logical given that individuals with a larger IPD would have a greater convergence demand for near viewing, and thus possibly a larger relative near BI range given their more converged starting point for testing.
One interesting association observed in this study is the finding that near phoria became more exophoric over 10 years of follow-up whereas near BO ranges decreased. Although a common clinical expectation is to observe greater near exophoria in patients as they become presbyopic, expectations about change in near phoria during childhood are less clear. Although our subjects were not reporting near symptoms, it does seem disadvantageous that the ability to compensate for exophoria decreases as the phoria becomes more divergent. If this trend continues during adulthood, it could eventually become more problematic as subjects lose the ability to accommodate and thus lose the benefit of accommodative convergence to compensate for their phoric posture. At this point, the demand on fusional vergence will be greater and the range of vergence ability less than that available during childhood. For subjects with higher degrees of exophoria, this trend toward even greater exophoria and loss of compensating fusional vergence ability could result in nearpoint symptoms.
CONCLUSIONS
The findings of this study suggest that convergence ranges decrease longitudinally for both distance and near viewing during the school years as near phoria becomes more exophoric. These findings could have clinical implications given that compensating convergence ranges decrease as near phoria becomes more divergent.
Acknowledgments
We thank Constance Crossnoe, OD, study optometrist, Ailene Kim, OD, study optometrist, Jane Gwiazda, PhD, COMET & COSMICC study chair, and COMET/COSMICC study participants and parents.
This work was supported by National Eye Institute, National Institute of Health, grant NEI EY11740.
References
- 1.Sheard C. Zones of ocular discomfort. Am J Optom. 1930;7:9–25. [Google Scholar]
- 2.Grisham JD. Visual therapy results for convergence insufficiency: a literature review. Am J Optom Physiol Opt. 1988;65:448–54. doi: 10.1097/00006324-198806000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Grisham JD, Bowman MC, Owyang LA, Chan CL. Vergence orthoptics: validity and persistence of the training effect. Optom Vis Sci. 1991;68:441–51. doi: 10.1097/00006324-199106000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Scheiman M, Rouse M, Kulp MT, Cotter S, Hertle R, Mitchell GL. Treatment of convergence insufficiency in childhood: a current perspective. Optom Vis Sci. 2009;86:420–8. doi: 10.1097/OPX.0b013e31819fa712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scheiman M, Herzberg H, Frantz K, Margolies M. A normative study of step vergence in elementary schoolchildren. J Am Optom Assoc. 1989;60:276–80. [PubMed] [Google Scholar]
- 6.Wesson MD. Normalization of prism bar vergences. Am J Optom Physiol Opt. 1982;59:628–34. doi: 10.1097/00006324-198208000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Scheiman M, Wick B. Clinical Management of Binocular Vision: Heterophoric, Accommodative, and Eye Movement Disorders. 2. Philadelphia, PA: Lippincott Williams & Wilkins; 2002. [Google Scholar]
- 8.Gwiazda J, Hyman L, Hussein M, Everett D, Norton TT, Kurtz D, Leske MC, Manny R, Marsh-Tootle W, Scheiman M. A randomized clinical trial of progressive addition lenses versus single vision lenses on the progression of myopia in children. Invest Ophthalmol Vis Sci. 2003;44:1492–500. doi: 10.1167/iovs.02-0816. [DOI] [PubMed] [Google Scholar]
- 9.Littell RD, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O. SAS for Mixed Models. 2. Cary, NC: SAS Institute; 2006. [Google Scholar]
- 10.Myung E, Choi J, Cotter SA, Rouse MW. Repeatability of prism bar vergences in elementary schoolchildren. Optom Vis Sci. 2006;83:E-abstract 060031. [Google Scholar]
- 11.Rouse MW, Borsting E, Deland PN. Reliability of binocular vision measurements used in the classification of convergence insufficiency. Optom Vis Sci. 2002;79:254–64. doi: 10.1097/00006324-200204000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Johns HA, Manny RE, Fern K, Hu YS. The intraexaminer and inter-examiner repeatability of the alternate cover test using different prism neutralization endpoints. Optom Vis Sci. 2004;81:939–46. [PubMed] [Google Scholar]
- 13.Jimenez R, Perez MA, Garcia JA, Gonzalez MD. Statistical normal values of visual parameters that characterize binocular function in children. Ophthalmic Physiol Opt. 2004;24:528–42. doi: 10.1111/j.1475-1313.2004.00234.x. [DOI] [PubMed] [Google Scholar]