Abstract
Recent neuropsychological investigations of apraxia have led to new hypotheses about the representational defects associated with imitation impairments in neurological patients. This fMRI experiment investigated the relation between imitation and the body schema in healthy subjects. Experimental conditions were derived from a factorial plan, and participants were asked to watch a human model performing bodily gestures and then to execute either an identical or a different action, with the same or different limbs. Brain areas activated when subjects imitated the model were traced to the representation of the action (main effect of performing an identical action regardless of limb), to the body schema (using the same limb regardless of action), or to both. The first set of analyses yielded a network associated with visual perception, indicating that action representation is primarily visuospatial not motor, while the second analysis highlighted regions involved in body schema including the inferior parietal cortex and the insula. It is suggested that imitation of simple body gestures requires both a visuospatial description of the observed model, sustained by visual perception areas in the right occipitotemporal and superior parietal cortices and a visuospatial description of one’s own body, supported by the left inferior parietal lobule. These results favor a model of praxis proposing that imitation deficits in left inferior parietal lobe patients with apraxia reflect primarily an impairment of the body schema, while deficits of praxis in right parietal patients are limited to gestures demanding in terms of visuospatial analysis.
Keywords: Neuroimaging, Imitation, Apraxia, Body schema, Parietal cortex
1. Introduction
The past two decades of research have expanded our knowledge of action imitation at both psychological and neural levels (see Meltzoff & Decety, 2003 for a recent review). There is ample of evidence in experimental psychology favoring a common coding between action and perception in humans (see Viviani, 2002 for a recent review). Neuroscience research has also demonstrated common neural mechanisms between executed and observed action at the neural level. Monkey’s ventral premotor area F5 contains mirror neurons that discharge both when a monkey executes a given goal-directed action and when it watches the same action performed by another individual (Fadiga, Fogassi, Gallese, & Rizzolatti, 2000). Similarly, neuroimaging experiments in humans have demonstrated the activation of a fronto-parietal neural network that is involved in the execution, observation and imagination of action (Decety & Chaminade, 2004).
An important aspect of the reappraisal of imitation thus requires expanding the knowledge of the brain basis of human imitation and its relationship with this common coding system (Rumiati & Bekkering, 2003; Wilson, 2001). Of particular interest is the representation of the body in this system (Gallagher & Meltzoff, 1996). Recent theories of imitation have dissected the imitative act into two components, the body part used and the action performed (Meltzoff & Moore, 1997). Does observation of biological motion and/or of human body parts provide a privileged stimulus for eliciting the activation of action representations? Both aspects have recently been investigated in psychophysics and neuroimaging experiments. Castiello, Lusher, Mari, Edwards, and Humphreys (2002) have explored the nature and specificity of motor priming by examining behavioral responses to actions produced by a robotic arm versus that produced by a human arm. They showed a priming advantage for the latter. A neuroimaging study (Perani et al., 2001) demonstrated that perception of real hand object-related actions but not virtual hand actions is associated with inferior parietal activation. It was also shown that biomechanically possible, but not impossible actions (Stevens, Fonlupt, Shiffrar, & Decety, 2000) activate left inferior parietal lobule, an essential component of the neural network involved in execution of action. To our knowledge, no investigation has compared in the same experiment attention to biological motion and attention to body part, though this distinction is critical to theories of imitation and plays an important role in imitative development.
Imitation is used as a classical test of ideomotor apraxia, a disturbance of verbally elicited voluntary movements, imitation of a model, or response to an object in the absence of motor deficits. Recently, fine-grained exploration of neuropsychological patients reported distinct impairments of imitation in relation with different lesion site (Haaland, Harrington, & Knight, 2000). The present experiment was aimed at the brain basis of imitation with an eye towards distinguishing between two models of praxis which recently emerged from the clinical studies of apraxic patients. We will present these models with an emphasis on the brain regions that are predicted to be involved in the imitation of meaningless and intransitive actions, used in the present experiment.
1.1. Neuropsychology
1.1.1. Apraxia as a disorder of the body schema
Goldenberg (1995, 2001) proposed that apraxia is not a deficit of the ability to translate an intention into a motor program, but rather of forming an intention in relation to a representation of the body, and is therefore related to an impairment of the body schema. Studies that investigated imitation of meaningless gestures on a manikin reported that apraxic patients were also impaired in this task, irrespective of the location of the lesion (Goldenberg, 1995; Goldenberg & Hagmann, 1997). Similarly, a study that required patients to select a match to a photograph of a target picture from a set of photographs showing postures performed by other individuals and in other orientations paralleled results of an imitation task (Goldenberg, 1999). Thus, apraxic patients show a general deficit in the representation of body part configurations and relationships.
Halsband et al. (2001) tested left and right parietal patients and found an increase of errors in imitation of gestures in the left-brain damaged group. Goldenberg also hypothesized a relationship between the lateralization of the lesion and the impairments in imitation of meaningless gestures. Patients with left-brain damage make more errors when imitating hands (Goldenberg, 1999) and feet (Goldenberg & Strauss, 2002) than with finger postures.2 The imitative capacities of both hemispheres were investigated in patients with callosotomy (Goldenberg, Laimgruber, & Hermsdorfer, 2001; Lausberg & Cruz, 2004). The imitation of hand–head and fingers configurations can be dissociated, and the authors reported that the former taps on left hemisphere resources while the latter taps on right hemisphere resources. Therefore Goldenberg’s model proposes that imitation of meaningless gestures requires a coding of gestures with reference to a classification of body parts and that the left inferior parietal lobe plays a crucial role in body part coding. A right hemisphere contribution is involved when gesture processing requires visuospatial discrimination. For example, demands on visuospatial processing of finger configurations are high because fingers are distinguished mainly by their spatial position. The numeral rank can be applied only after successful determination of this spatial position.
1.1.2. Dynamic and stored gestures representations
Another model of ideomotor apraxia was proposed by Buxbaum (2001). This model distinguishes two types of gesture representations. The ‘dynamic portion of gesture representation’ consists of representation of the body parts participating in a given action in a number of spatial reference frames. It is responsible for the imitation of meaningless actions using a direct mapping to transform an extrinsic code of the gesture (which reflects spatial relations between the body parts of the model) into an intrinsic code of the same relations in the imitator. This intrinsic code has been referred to as the body schema. The ‘stored aspect of gesture representation’ forms the core of the praxis system in that it stores gesture engrams, i.e., invariant and characteristic features of a given gesture. The two portions of gesture representation participate in the execution of action. For instance, while using a hammer, the stored aspect describes the canonical hand posture for holding and acting with a hammer, and the dynamic aspect adapts this archetypical gesture to the specifics of the actual hammer: finger aperture depending on size, strength depending on weight, etc.
In this model, lesions to the dynamic aspect would lead to dynamic apraxia, characterized by impairment in imitation of meaningless gestures with normal performance in meaningful gestures (visuoimitative apraxia). Lesions in the left superior parietal lobe, as in the case of patient BG (Buxbaum, Giovannetti, & Libon, 2000) would be responsible for such apraxia. Impairment of the stored aspect would lead to representational apraxia, characterized by impairments in the production of meaningful gestures, for example in the use of familiar objects for which the most efficient hand posture for manipulation is not available from the object structure, and must be extracted from memory (Buxbaum, Sirigu, Schwartz, & Klatzky, 2003). Left inferior parietal lesions would be responsible for representational apraxia.
1.2. Brain imaging studies of imitation
Several aspects of imitation have been investigated by neuroimaging experiments. A first series of experiments focused on the effect of the intention to imitate on the neural substrate involved in observation of action (Decety et al., 1997; Grezes, Costes, & Decety, 1999). These studies demonstrated a strong involvement of the superior and inferior parietal as well as premotor and dorsolateral prefrontal regions mainly in the right hemisphere, interpreted in relation to attention and action preparation in the absence of motor output.
A second line of research focused on simple and repetitive finger movement paradigms. Behavioral data with the same paradigm demonstrated an effect of visuomotor compatibility, i.e., the observation of a given action facilitates the execution of the same action and inhibits other actions (Brass, Bekkering, Wohlschlager, & Prinz, 2000). FMRI studies showed an increased signal in the left inferior frontal and right inferior parietal cortices associated with copying of finger movements (Iacoboni et al., 1999; Koski et al., 2002). Another study of imitation of finger configuration resulted in a bilateral involvement of the parietal cortices for meaningless configurations, and restricted to the left hemisphere for meaningful ones (Tanaka, Inui, Iwaki, Konishi, & Nakai, 2001).
Goldenberg and colleagues used PET measurements in a gesture discrimination task used to investigate the neural substrate of gesture representation (Hermsdorfer et al., 2001). They found hand gestures to be associated with highly lateralized left inferior parietal lobule activity, while finger gestures yielded more bilateral increases in the parietal and occipital lobes. Another neuroimaging study demonstrated that finding the match of a visually presented body parts posture in a set of photographs showing postures performed by other individuals and in other orientation activates Broca’s area for the matching of fingers but not hand gesture (Tanaka & Inui, 2002). This result fits with the existence of a dissociation between the matching of these two types of postures (Goldenberg, 1999). This also demonstrates a specific role of Broca’s area in finger movements as opposed to other body parts. In a study of object-directed or pantomime observation and imitation (Grezes, Armony, Rowe, & Passingham, 2003), Broca’s area involvement was found related to imitating or executing the object-directed actions. Thus, activity in Broca’s area can be associated with imitation (and more generally with action), in specific conditions in which a verbal component is at play (identifying a finger by its numeral, semantic knowledge about an object). These results are also compatible with Goldenberg’s hypothesis that the right hemisphere is involved in the imitation of finger postures because of the higher visuospatial discrimination needed to recognize single fingers.
Two previous neuroimaging studies of imitation of meaningless object-directed actions (Chaminade, Meltzoff, & Decety, 2002; Decety, Chaminade, Grezes, & Meltzoff, 2002) led to the identification of a brain network underlying imitation. This network includes the left superior temporal sulcus and inferior parietal lobule, as well as the right dorsolateral prefrontal cortex. This latter region is particularly important in encoding and maintaining in memory the goal of the model’s object-directed action. Taken together, we can say that the key regions for imitation, in particular when meaningless simple body actions are considered and finger configurations are excluded, involve the posterior parietal cortex in particular the left inferior parietal cortex, as well as the occipital cortex.
1.3. Goals of the study
While several neuroimaging studies have investigated the neural basis of imitation, none have tried to segregate the neural substrates of action representation and of body schema in imitation. One aspect of the body schema has been investigated using gesture discrimination tasks (Hermsdorfer et al., 2001; Tanaka & Inui, 2002); defects in such tasks do not exactly overlap with imitation defects in apraxic patients (Goldenberg, 1999). We designed the present experiment to investigate the neural correlates of action representation and of the body schema in imitation.
The current study of imitation used upper (hands) and lower (feet) limbs on the left and the right side of the body. Because a neuropsychological dissociation was found between imitation of fingers and hands gestures, no finger posture was used in the present experiment. A first-person perspective model was used, rather than having the model facing the subject to avoid left-right discrepancy between the model’s and the subjects’ actions, and mental transformation in recognizing the side of the body used by the model (Bonda, Petrides, Frey, & Evans, 1995; Koski et al., 2002). Meaningless gestures were used to avoid verbal contamination (Goldenberg & Strauss, 2002), but the repetition of a limited pool of gestures in the experiment does not rule out that these gestures were memorized during the training sessions prior to the fMRI experiment.
Experimental conditions were based on a factorial design. The factors were the limb used by the subjects, and the action they perform, which both could be the same or different from the model. On the basis of previous results, we expected segregation of the brain networks associated with the two components of the imitation of actions which did not involve objects (Chaminade & Decety, 2002). The first component was to reproduce the action that was presented irrespective of the limb used (for example, rotation of either the hand or the foot); the second was using the same limb irrespective of the action performed. Reproducing the action and using the same limb at the same time is full imitation.
Visual input and motor output were equated in all conditions and thus do not interfere with the effects of the experimental factors. Therefore, the present experimental paradigm allows us to investigate the neural network associated with imitation at the level of the action performed and the limb used. In addition to the left parietal lobule involved in imitation, we expect an increase of activity in cortical areas involved in perception of body parts in the occipitotemporal cortex (Downing, Jiang, Shuman, & Kanwisher, 2001). More importantly, it is predicted that areas involved in imitation would be found in one of the main effects of the experimental factors, namely the main effect of using the same limb (related to the body schema) and the main effect of performing an identical action (related to the action representation). Interestingly, the two models of praxis based on the study of apraxic patients lead to different predictions for the brain areas associated with the two main effects. According to Goldenberg’s model, gesture representation should activate the right posterior parietal cortex and limb representation should activate the left inferior parietal cortex. Under Buxbaum’s account limb representation should activate the left superior parietal cortex. Since a known gesture can be produced with different effectors (see e.g. Rijntjes et al., 1999), the engram for a given gesture should be represented in a limb independent manner in the stored portion of the gesture representation, thus in the left inferior parietal cortex.
2. Material and methods
2.1. Stimuli preparation
In this experiment, subjects watched video-clips depicting single hand or foot actions and were required to imitate the model or to execute another gesture, in four experimental conditions defined by a factorial plan (Table 1). The two factors were the limb used by the subjects (“the same” or “the other”) and the action they performed (“identical” or “different”). Two control conditions consisted of watching without acting, either the action stimuli or a blue cross on a black background (same duration as the experimental conditions).
Table 1.
Two factors were used in this experiment: (1) the action performed by the subject can either be identical to the model or a different one; (2) the limb used by the subject can either be the same or the other one
| Experimental condition |
Limb |
||
|---|---|---|---|
| The same | The other | ||
| Action | Identical | Identical action with the same limb | Identical action with the other limb |
| Different | Different action with the same limb | Different action with the other limb | |
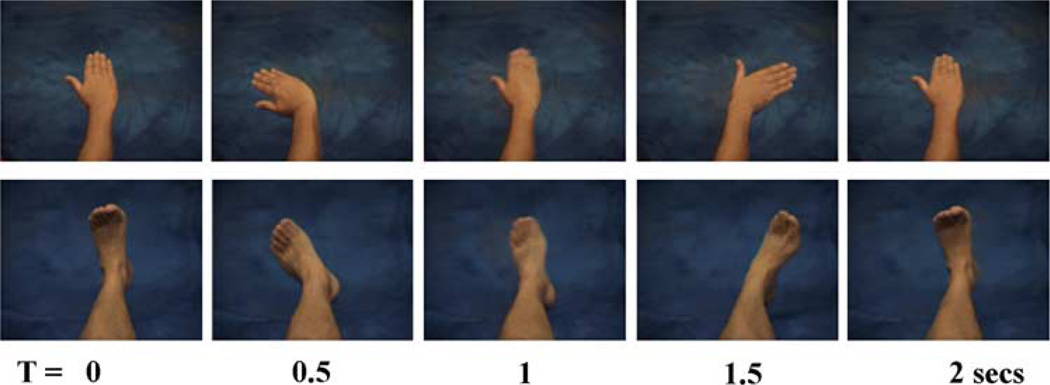
The stimuli were videotaped with a male actor lying on a blue background with the camera above his midline in order to obtain first-person visual perspective stimuli. The six actions were rotations, lateral and vertical movements, each being performed in either direction (clockwise versus counter clockwise, left versus right first, up versus down first respectively). The actors movements were guided by a metronome so that each video-clip, consisting of two successive similar actions, lasted 5 s (2 s for a first action, 1 s pause, 2 s for a second similar action), and avoided hesitations and shaking (see Fig. 1 for examples). Recordings were then edited using Final Cut Pro, each video-clip was cropped to last exactly 5 s. For each experimental block three video-clips showing actions performed with the same limb (selected randomly and counterbalanced between sessions), were assembled with the following template: a written reminder of the experimental condition for 2 s, and the three actions separated by 0.5 s., pauses. The duration of an experimental block was 18 s.
Fig. 1.
Snapshots from stimuli are used to depict two actions. Top: horizontal left–right horizontal movement with the right hand. Bottom: counterclockwise rotation with the left foot.
In the conditions defined by the factorial plan, subjects were asked to act during the second presentation of each of the three actions. A “different action” was freely chosen within the remaining five choices in the six possible actions of the experiment. In order to balance the limb used by the subjects in the different conditions, “the other limb” was defined as “the same limb on the other side of the body” in two of the four fMRI sessions, and “the other member (hand instead of foot and foot instead of hand) on the same side of the body” in the other two sessions.3 Each session started with a written reminder of the meaning of “the other limb” (i.e. “the other side” or “the other member”), and a written reminder at the beginning of each experimental condition explicitly stated “with the other side” or “with the other member”. The order of presentation of the two types of sessions was randomized between subjects.
Within each fMRI session, subjects were presented with each of the six conditions four times, one for each limb, leading to 24 scanning blocks per session. For example, in one session, they would be instructed four times “Do an identical action with the same limb”, and each time a different limb was presented so that the four limbs were used equally in the experimental conditions within each session. A session thus comprised 24 scanning blocks in a randomized order, for a total duration of 7 min 22 s.
2.2. Subjects training
Twelve right-handed participants (9 females and 3 males; mean age 21.2 years ± 4.1) with no history of neurological or psychiatric disease volunteered for the experiment, which was approved by the Ethics Committee of the University of Oregon. Participants received financial compensation for their participation.
At their arrival at the neuroimaging lab, subjects were briefly introduced to the requirements of the experiment, and were trained inside a mock scanner. Two full sessions were conducted to introduce the two situations, in which “the other limb” must be understood either as “the other side” or as “the other member”. An experimenter corrected subjects on-line to ensure they understood the instructions. Subjects made no mistakes during the second training session.
Then, participants were installed in the Siemens Allegra 3-T magnet. A pad was placed under the knees to allow free movement of the feet and cushions were placed under the arms to allow free movement of the hands. The subject’s head was firmly positioned with foam to minimize movement. Before each session subjects were orally reminded of the meaning of “the other limb”, also written at the beginning of the session’s video-clip. During the course of the experiment, an experimenter remained inside the magnet room to record the subjects’ responses.
2.3. Magnetic resonance imaging
Visual stimuli were projected on a screen located at the back of the magnet and viewed with a mirror located in front of the subjects’ eyes. Functional images were acquired using T2-weighted gradient echo, echoplanar imaging sequence, sensitive to blood oxygen level dependent (BOLD) contrast (repetition time 2000 ms, echo time 40 ms, flip angle of 90°, matrix 64 × 64, field of view 192 mm × 192 mm). The images consisted of 32 contiguous axial slices, with 4.5 mm thickness and 3 mm × 3 mm in plane resolution. To allow the equilibrium to reach its steady-state, two volumes corresponding to a 4 s delay were automatically discarded from the analysis. During each run 225 volumes were continuously acquired over a total duration of 450 s. High-resolution T1-weighted anatomical images were acquired (gradient-echo inversion-recovery sequence, repetition time 1.570 ms, echo time 3 ms, matrix 250 × 250 × 144, field of view 250 mm × 250 mm, slice thickness 1 mm).
2.4. Statistical analysis
All fMRI data were processed using the SPM2 software package (Wellcome Department of Cognitive Neurology, London). For each subject, the functional scans were realigned to correct for subjects motion, stereotactically normalized to the MNI space, and smoothed with a 6 mm full-width half-maximum Gaussian filter.
A first fixed level of analysis was computed subject-wise using the general linear model with hemodynamic response function modeled as a boxcar function which covers the three action presentations in each condition. In addition to the six experimental conditions, eight variables corresponding to the four different limbs observed and to the four different limbs acted with were introduced in the model to remove the effects of observing or acting with one of the four limbs used in the experiment. The main effect of each of these covariates was computed separately for each subject using F-tests of variance.
Three contrasts of interest were computed for each subject: imitating (“identical action with the same limb” versus “different action with the other limb”), performing an identical action (“identical action” versus “different action”) and using the same limb (“the same limb” versus “the other limb”).
Single-subjects first-level contrasts were introduced in second-level random-effect analysis to allow for population inference. Main effects were computed using one-sample t-test including 12 subjects for each of the contrasts reported earlier. The resulting set of voxel values for each contrast constituted an SPM map. The maps were then thresholded at P < 0.01 at the cluster level. Localization of cortical clusters of activity was performed with a human brain atlas (Duvernoy & Cabanis, 1991).
3. Results
3.1. Participant behavior
Subjects reported no specific difficulty during the experiment. Behavioral results were in accordance with their subjective reports since very few mistakes were observed (a total of 3 mistakes across all trials). These conditions were discarded from the analysis.
3.2. Main effect of the covariates
The 12 subjects were incorporated into the second-level random-effect analysis. The brain networks revealed by the covariate describing acting with both hands and both feet (Table 2) and by the covariate describing observing each of the two hands and feet (Table 3) were used to validate the analysis.
Table 2.
Effect of the covariables describing acting with one of the four limbs yielded strong increase of activity in the controlateral primary motor cortex and in the ipsilateral cerebellum
| Location | x | y | z | t values |
|---|---|---|---|---|
| Right hand | ||||
| (L) Primary motor cortex | −32 | −24 | 54 | 12.05 |
| (R) Cerebellum | 16 | −50 | −26 | 7.97 |
| Right foot | ||||
| (L) Primary motor cortex | −8 | −36 | 72 | 10.64 |
| (R) Cerebellum | 18 | −38 | −28 | 8.26 |
| Left hand | ||||
| (R) Primary motor cortex | 36 | −20 | 50 | 11.38 |
| (L) Cerebellum | −16 | −50 | −24 | 12.5 |
| Left foot | ||||
| (R) Primary motor cortex | 6 | −20 | 70 | 9.65 |
| (L) Cerebellum | −16 | −38 | −30 | 6.42 |
(P < 0.01 corrected at the cluster level)
Table 3.
Each of the covariables describing the observation of one of the four limbs yielded strong increase of activity in the right lateral occipital cortex
| Location | x | y | z | t values |
|---|---|---|---|---|
| Right hand | ||||
| (R) Occipitotemporal cortex | 42 | −68 | −6 | 6.64 |
| Right foot | ||||
| (R) Occipitotemporal cortex | 50 | −70 | −6 | 5.90* |
| Left hand | ||||
| (R) Occipitotemporal cortex | 48 | −68 | −2 | 7.65 |
| Left foot | ||||
| (R) Occipitotemporal cortex | 46 | −66 | −4 | 8.70 |
(P < 0.01 [*P < 0.02] corrected at the cluster level)
Results show that the action covariate mapped exactly the brain areas associated with acting with each given limb and side in the primary motor cortex (Lotze et al., 2000) and the cerebellum (Grodd, Hulsmann, Lotze, Wildgruber, & Erb, 2001). The observation covariate led to increased activity in the right lateral occipitotemporal cortex, with a lower threshold for the observation of the right foot, in a cluster spread over the motion-sensitive area MT/V5 (Dumoulin et al., 2000) and an area involved in the perception of body parts (extrastriate body area (EBA); Downing et al., 2001).
Since conditions were matched in terms of visual input (observed limb and gesture) and motor output (limb acted with), and the brain activity associated with the covariates describing the use and the observation of a given limb gave the expected results in the primary motor cortex and cerebellum, and in the lateral occipitotemporal cortex respectively, the following section describes the effects specific to the experimental factors.
3.3. Effect of the experimental factors
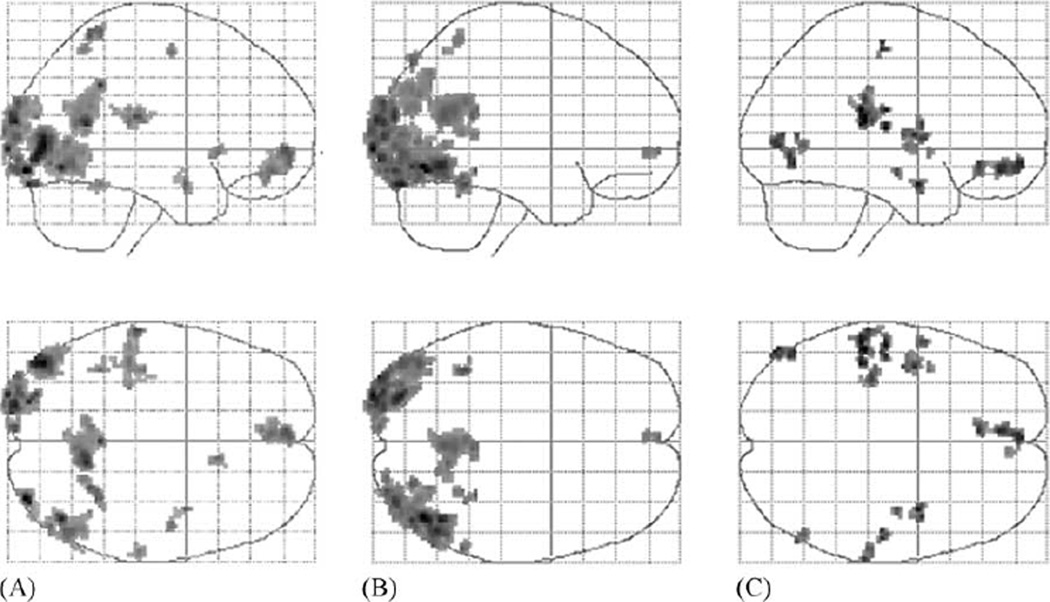
Brain areas associated with the contrasts of interest, “imitating”, are given in the first columns of Table 4. The next columns indicate the same regions that were found in the main effects of the two experimental factors, executing an “identical action”, and using “the same limb” (Fig. 2). Common to the three contrasts are the ventromedial prefrontal cortex and the occipitotemporal cortex bilaterally (Figs. 3 and 4). Areas found activated both in the contrast describing imitation and in the main effect of executing an identical action are related to visual perception (occipital cortex and fusiform gyrus). Additional clusters were found in the right intraparietal sulcus and the posterior cingulated cortex. Areas found activated both in the imitation contrasts and in the main effect of using the same limb correspond to higher-order association areas in the parietal lobes, as well as in the right insular cortex (Fig. 5) and right amygdala. Finally, activated clusters in the left insula and the right hippocampus found in the latter main effect, using “the same limb”, which were not present in the contrast of interest, “imitating”, are not reported in Table 4 but can be seen in Fig. 2.
Table 4.
Regions associated with contrast of interest “imitating”, grouped depending on their association with either the two (top) or only one of the main effects, executing an “identical gesture” and using the “same limb”
| Location | Imitating |
Identical action |
Same limb |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | t values | x | y | z | t values | x | y | z | t values | |
| Ventromedial prefrontal cortex | −6 | 56 | 0 | 5.93 | −2 | 60 | −2 | 5.04 | −2 | 56 | −8 | 6.21 |
| (R) Occipitotemporal cortex (EBA) | 52 | −68 | 2 | 5.14 | 54 | −68 | 2 | 4.59 | 54 | −68 | 4 | 5.12 |
| (L) Occipitotemporal cortex (MT) | −44 | −80 | 2 | 8.08 | −46 | −80 | −2 | 6.22 | −48 | −72 | −4 | 5.98 |
| (R) Intraparietal sulcus | 26 | −54 | 64 | 5.66 | 28 | −52 | 60 | 4.85 | ||||
| Posterior cingulate | 0 | −48 | 36 | 6.34 | 4 | −44 | 18 | 5.73 | ||||
| (R) Calcarin fissure | 12 | −56 | 14 | 7.12 | 12 | −58 | 16 | 6.07 | ||||
| (R) Occipitotemporal cortex (MT) | 44 | −70 | −10 | 7.15 | 42 | −72 | −10 | 10.76 | ||||
| (R) Inferior occipital gyrus | 32 | −90 | −12 | 8.66 | 34 | −88 | −12 | 7.14 | ||||
| (R) Fusiform gyrus | 30 | −42 | −14 | 4.64 | 30 | −42 | −12 | 5.46 | ||||
| (L) Fusiform gyrus | −40 | −48 | −20 | 4.8 | −40 | −48 | −20 | 6.31 | ||||
| (R) Precentral gyrus | 48 | −8 | 54 | 5.09 | 44 | −8 | 60 | 4.93 | ||||
| (R) Supramarginal gyrus | 62 | −26 | 20 | 4.82 | 60 | −28 | 22 | 5.64 | ||||
| (L) Supramarginal gyrus | −62 | −28 | 18 | 5.94 | −56 | −32 | 22 | 6.77 | ||||
| (L) Parietal operculum | −52 | −18 | 12 | 4.77 | −54 | −18 | 12 | 7.56 | ||||
| (R) Posterior insula | 40 | −2 | −2 | 4.63 | 42 | 2 | 6 | 5.73 | ||||
| (R) Amygdala | 38 | 0 | −20 | 5.28 | 36 | 2 | −24 | 4.24 | ||||
EBA: extrastriate body area (Downing et al., 2001); MT: motion-sensitive Middle Temporal area (P < 0.01 at the cluster level).
Fig. 2.
Top and lateral views of glass brains showing hemodynamic changes associated with: (A) imitating, (B) main effect of executing an identical action and (C) main effect of using the same limb.
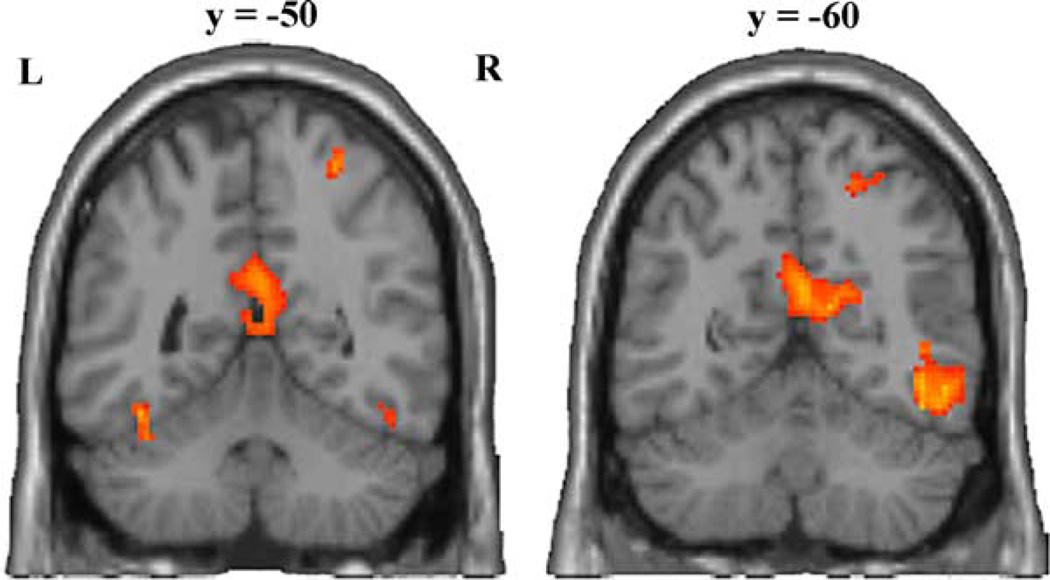
Fig. 3.
Two coronal sections of a representative brain showing activity associated with imitating. The anterior section (left) shows clusters of activity in the posterior cingulate, extending to the rostral end of the calcarin sulcus and bilaterally in the fusiform gyrus. The most posterior section shows clusters of activity in the calcarin sulcus and extrastriate body area in the right hemisphere. Both sections show activity in the right intraparietal sulcus.
Fig. 4.
Two sections of a representative brain showing activity associated with reproducing an identical action. The sagittal section (left) shows clusters of activity in the posterior cingulate, extending to the rostral end of the calcarin sulcus, and in the ventromedial cortex. The coronal section shows bilateral clusters of activity in area MT.
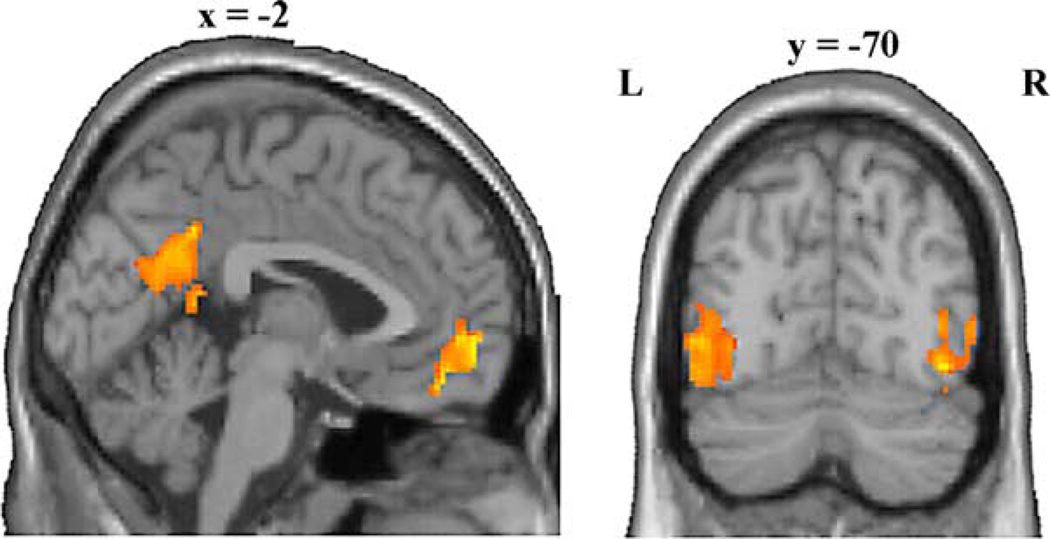
Fig. 5.
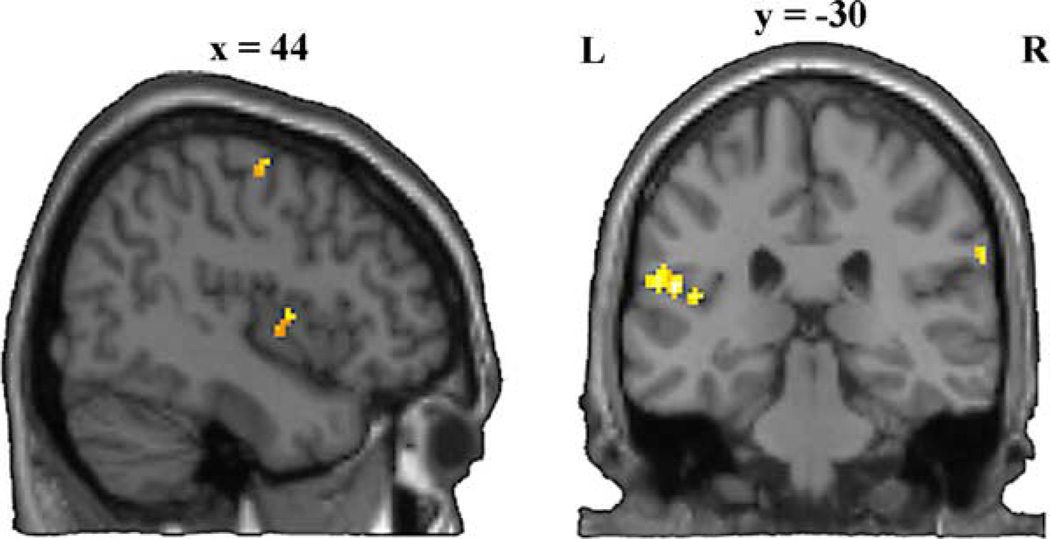
Two sections of a representative brain showing activity associated with using the same limb. The sagittal section (left) shows clusters of activity in the right posterior insula and precentral gyrus. The coronal section shows bilateral clusters of activity in the supramarginal gyrus as well as a left-lateralized cluster in the parietal operculum.
4. Discussion
The goal of this experiment was to identify the neural networks associated with using the same limb and of performing an identical action during imitation of meaningless intransitive hand and feet gestures, and to discuss these networks in relation to the models of imitation described in Section 1.
4.1. Imitation using the same limb to execute an identical action
4.1.1. Areas common to the two components
Three regions were found associated with the contrast describing imitation as well as the two main effects. Bilateral activity in the occipitotemporal cortex (Fig. 3) corresponds to a region specialized in the visual analysis of body part (Downing et al., 2001; Peigneux et al., 2000). The coordinates in the left hemisphere correspond to the location of area MT (Dumoulin et al., 2000), specifically involved in the analysis of visual motion. At first blush, this may seem surprising, since stimuli were matched in terms of the body parts and the movement observed, and that activity in the same regions in the right hemisphere was found in all covariates describing the observation of a given limb (Table 3). But this result makes more sense when considering an effect of attention on the activity of these regions. All the contrasts given in Table 4 share the cognitive component that subjects were required to reproduce the whole or parts of the presented gesture. The increase of activity in the occipitotemporal areas can be interpreted as the neural correlate of an increased attention to the modeled gesture, irrespective of which element, the limb or the action, must be reproduced.
The finding of the ventromedial prefrontal cortex was not predicted. This region is found activated in a number of tasks including the suppression of irrelevant memory traces (Schnider, Treyer, & Buck, 2000), motor learning (Jackson, Lafleur, Malouin, Richards, & Doyon, 2003) or decision-making (Bechara, Damasio, & Damasio, 2000). A common feature of these tasks has been described as the “felt rightness” of the current behavior (Moscovitch & Winocur, 2002), whether it is related to memory, action or other aspects of behavior. This monitoring of the rightness of the current behavior could account for the activity of this region in all situations in which participants’ behavior was constrained by the word “same”, namely using the same limb and/or performing the same action, compared to situations in which their behavior was defined by “other”. Indeed, participants could directly compute the correctness of their response by comparing the relevant feature(s) of the gesture presented in the video stimulus (either the limb used and/or the action performed) to the similar feature(s) in the gesture they perform. Such a direct comparison was not possible in the “other” conditions. In addition, we speculate that subjects’ knowledge that their behavior was coded on-line by an experimenter who was present in the scanning room could have emphasized their desire to accomplish the task as accurately as possible. The ventromedial prefrontal activity could reflect such a process (Rosenthal, 1964).
4.1.2. Areas specific to executing an identical action
If the neural representation of meaningless action is similar to that of meaningful actions, we should find similar activated areas to the ones found in an investigation of the limb independent brain network for representing gestures described (Rijntjes et al., 1999). Additionally, repetition of a limited number of actions could have yielded a memorization of these actions. In the experiment by Rijntjes et al. (1999), subjects were requested to execute their own signature with either their index finger or their big toe. Areas involved in both tasks were found in the anterior premotor cortex in control of the dominant hand and in the intraparietal sulcus. This result led the authors to suggest that the representation of an over trained action (i.e., the signature) in the secondary sensorimotor cortices can be accessed by other extremities performing the same action.
In the present experiment we note an absence of activation in the left premotor cortex, one right-hemisphere intraparietal cluster, and massive occipital involvement when performing an identical gesture. The signature gesture is an over-trained movement, and is related to extensive self experience, while the gestures used in our experiment were novel and meaningless for the subjects. Observation of meaningful gestures chiefly activates a left hemisphere frontal network, while meaningless gestures activate the right occipito-parietal pathway (Decety et al., 1997). In addition, signatures were retrieved from memory with eyes closed, while our study used visual stimuli to present the to-be-performed gestures. The visuomotor transformation involved in imitating requires an increase of visual attention (Decety et al., 2002; Hermsdorfer et al., 2001) demonstrated by the involvement of medial and lateral occipital regions. In the present experiment, reproducing the gesture was more demanding in terms of visual attention than using the same limb.
The fusiform gyrus, found activated in both hemispheres when subjects were required to reproduce a meaningless gesture, is also related with visual aspects of the task. One possible hypothesis could be that they imagined the presence of a face in the absence of actual presentation (Grossman & Blake, 2002; O’Craven & Kanwisher, 2000), but the perspectives used in the stimuli (see Fig. 1) make this hypothesis unlikely. Since the fusiform area is acknowledged to be involved in visual expertise (Tarr & Gauthier, 2000), such as, but not restricted to faces (Kanwisher, 2000), another hypothesis is that subjects became experts at discriminating between the different actions. Indeed actions intentionally avoided further references to the body other than the part used and could therefore only be described in term of spatial features. They could be categorized along two axes: their type (rotation, horizontal and vertical translation) and their direction. But the training period was too short for subjects to develop full expertise. Finally activity of the fusiform gyrus has also been reported to be sensitive to the perception of meaningful hand movements (Grezes, Costes & Decety, 1998) and motions of simple shapes (Castelli, Happe, Frith, & Frith, 2000). Since the gestures of the present experiment could be learned throughout the fMRI experiment, a plausible interpretation is that this region is associated with the recognition of the six gestures, and is therefore activated when participants reproduced the same gesture, which required the recognition of the two aspects of the gestures (type and direction) but not when they performed mismatching gesture, which could be accomplish by paying attention to only one aspect of the gesture.
The actions we used were mainly distinguishable by their spatial properties. The right intraparietal sulcus activity, associated with the representation of the signature gesture (Rijntjes et al., 1999) and the imitation of finger movements (Hermsdorfer et al., 2001; Tanaka & Inui, 2002) contributes to the visual guidance of an action, especially for its spatial attributes (Chaminade & Decety, 2002). In addition, the posterior cingulate cortex, also found in the main effect of reproducing an identical action, is involved in evaluative functions for spatial orientation (Berthoz, 1997). The focus of activity in this region can be ascribed to monitoring the spatial features of the stimuli (Vogt, Finch, & Olson, 1992).
In conclusion the coding of the action for reproduction was not primarily associated with regions involved in shared motor representation as we expected, but with regions involved in visual perception (right area MT), analysis (the posterior cingulate cortex) and expertise (the fusiform gyrus).
4.1.3. Areas specific to using the same limb
The main effect of choosing the same limb reveals the brain networks associated with: (a) recognizing a limb shown in a visual stimuli, preparing and (b) executing an action with this limb irrespective of the relation (identical or different) between the presented and executed action. The bottom of Table 4 indicates that activity was found bilaterally in the inferior parietal lobule and in the right inferior insular cortex and amygdala. An additional left insular activity was specifically associated with the main effect of using the same limb. The finding of a cluster of activity in the amygdala, a region involved in emotional aspects of social cognition (Adolphs, 2003), during imitation and when using the same limb, reinforces the idea of a strong relation between imitation and the body schema in social cognition.
The insular cortex is involved in higher somatic integration, in relation with both somatic, autonomic and limbic systems (Flynn, Benson, & Ardila, 1999) and involved in body representation (Berlucchi & Aglioti, 1997). It is related with mental rotation of limbs in space (Bonda et al., 1995) and the feeling of a discrepancy between the visual feed-back of an action and the executed action (Farrer et al., 2003). Though these aspects were not present in our paradigm, it is notable that activity of the insula is related to the situations of comparing different orientations of a limb (either two observed orientations in Bonda et al., 1995 or one observed and one felt in Farrer et al., 2003). Our results suggest that attention to limbs recruits this region in a variety of tasks.
Lesions of the left inferior parietal cortex are generally associated with Wernicke’s aphasia and ideomotor apraxia, the latter being often accompanied with faulty imitation of meaningless gestures. Previous neuroimaging investigations found this region to be involved in imitation of hands compared to fingers gestures (Tanaka & Inui, 2002), and also in imitation of object-related actions compared to execution of different actions (Decety et al., 2002). However, this region was not found in neuroimaging experiments based on copying finger movements (e.g. Iacoboni et al., 1999; Koski, Iacoboni, Dubeau, Woods, & Mazziotta, 2003). In monkeys, neurons showing mirror properties comparable to those described in the premotor area F5 have also been found in the posterior parietal cortex (Gallese, Fadiga, Fogassi, & Rizzolatti, 2002), and were hypothesized to be involved in action representation. In humans, the activity of the inferior parietal lobule in association with action execution is consistently lateralized to the left hemisphere (Grezes & Decety, 2001; Rushworth, Nixon, & Passingham, 1997). Altogether, these arguments are in line with an important role of the left inferior parietal cortex in relation to action imitation.
The bilateral activity in the supramarginal gyrus was not predicted. Several proposals can be put forward to explain this result. First, though the left hemisphere is dominant for the action control, it has been demonstrated that the right homologous region also displays a sensorimotor role (Mattingley, Husain, Rorden, Kennard, & Driver, 1998). It is thus possible that the two hemispheres contribute to selecting the appropriate limb given a visual input. For example, there could be a stronger left hemisphere involvement in selecting the type of limb, and of the right hemisphere in selecting the side of the limb. This proposal is also compatible with the proposal that the right hemisphere would be more strongly involved in visuospatial tasks, since deciding which side of the body used, even when hands and feet stimuli are presented in a first-person perspective, requires localizing elements of the limb, such as the big toe or the thumb. This interpretation parallels the finding that the right parietal cortex is involved in a gesture discrimination task with finger- compared to hand-gestures (Hermsdorfer et al., 2001), and that right brain damage patients are impaired in imitating finger postures, which relies on a precise visuospatial analysis. Alternatively one can speculate about a lateralization similar to the primary motor and sensory cortices, the left inferior parietal involved in the selection of the right limbs and vice versa.
Interestingly, the parietal operculum activity was restricted to the left hemisphere with a high statistical score. It is the only parietal area found in the left hemisphere, in the region which lesion is often associated with ideomotor apraxia. The left parietal operculum is a secondary somatosensory region that shows decreased activity for self-tickling compared to external tickling (Blakemore, Wolpert, & Frith, 1998). It has been proposed that this region compares actual sensory feed-back to the sensory feed-back expected given the initial motor command, and would thus participate in building ‘internal models’ for action (Blakemore & Frith, 2003). We previously argued that imitating an action could involve inverse modeling to map the consequences of an action, in this case its observation, to their motor preparation (Chaminade et al., 2002). Activity in this region was recently found during perception of gestures (Grezes et al., 2003), and it was proposed that it could host multimodal, visual and somatosensory, representations of actions related to motor representations. Present results are in favor of this interpretation, in the sense that this secondary somatosensory cortex is involved in imitating a meaningless gesture and thus plays a role in relating visual as well as motor aspects of a gesture.
5. Models of praxis
The previous description of the results did not refer to the models of apraxia described in the introduction, which will now be discussed on their own. We will argue that the present results favor the predictions drawn from Goldenberg’s model.
5.1. Right intraparietal sulcus and action representation
In the Goldenberg model, the right hemisphere, particularly in the intraparietal sulcus (Hermsdorfer et al., 2001), encodes the visuospatial components of a to-be-reproduced gesture. For instance it is more activated for the reproduction of finger postures than imitation of hand–head configurations. In contrast, in the Buxbaum model, the left superior parietal cortex transforms an extrinsic code of the spatial relations between the body parts of the model into an intrinsic code of the same relations in the imitator.
In the present experiment a right superior parietal activity is found in the main effect of reproducing the action independently of the limb used. The actions used consisted of simple movements of the limb in space. The same main effect, i.e., reproducing the action, also yields activation in a neural network involved in the analysis of the visual components of the presented action (including the calcarin fissure, lateral occipital cortex and in particular the right MT area, and the fusiform gyrus in both hemispheres).
In addition this activity is restricted to the right hemisphere, as postulated by Goldenberg’s model, and no similar activity was found in the left hemisphere, neither in the main effect of reproducing the same action or of using the same limb. This result is in agreement with the neuropsychological finding that the right hemisphere is specifically involved in the perceptual discrimination of visuospatial components in imitative behavior, particularly prominent when spatial features are necessary to the identification of the action to reproduce.
Thus in this experiment, the actions used as substrates for imitation were categorized in terms of their visuospatial, and not motoric features; and this representation relies on the superior parietal cortex in the right hemisphere, and not the inferior parietal cortex of the left hemisphere. Thus the present results favor the hypothesis that the superior parietal cortex deals with visuospatial analysis of gestures, in accordance with Goldenberg’s model of praxis.
5.2. Left inferior parietal cortex and body schema
Three results thus emerge from this study in relation to the function of the inferior parietal cortex in imitation. First, the parietal cortex is primarily involved in using the same limb, and this experimental factor was used to explore the neural structures involved in the body schema. Second, the parietal cortex has been found by various neuroimaging studies to be associated with imitation (Decety & Chaminade, 2004). Third, the parietal operculum activity is in part lateralized in the left hemisphere, as was expected on the basis of the left hemisphere dominance for action and imitation.
Indeed, irrespective of which limb and which gesture was presented, and irrespective of which gesture was performed, the task of using the same limb activates the supramarginal gyrus bilaterally and the parietal operculum in the left hemisphere. Focusing on the left hemisphere, dominant in motor control and whose lesion produces the most severe forms of ideomotor apraxia, our results favor the hypothesis that the left parietal operculum plays a role in associating three types of representations of acting limbs: visual (observation of the model’s limb), somatosensory (selection of the appropriate limb) and possibly motor (preparation of an action with that limb) (see also Grezes et al., 2003). It is thus a fundamental component of imitation related to the coding of body parts.
In addition using the same limb activated the insula involved in body schema (Flynn et al., 1999). This is in line with the Goldenberg proposal of the function of the left inferior parietal cortex in praxis. His hypothesis is that ideomotor apraxia, caused by left parietal lesions in particular in the inferior parietal cortex, are primarily defects of the representation of the body. In contrast Buxbaum’s model proposes that it would store engrams of known gestures. If we consider that gestures were learned in the course of the experiment, we would expect executing an identical action to activate the left inferior parietal lobule.
Our results thus favor the hypothesis that during imitation of intransitive and meaningless actions, the inferior parietal cortex, and in particular the left parietal operculum, is involved in coding the representation of the body.
5.3. Different body schema?
Our results strongly imply that the left inferior parietal cortex is involved in the coding of body parts, thus the body schema, in imitation. Lesions to the left parietal lobe can cause other defects such as asomatognosia, the inability to identify body parts, on oneself and on another person. Asomatognosia is particularly interesting for the present discussion because it is an impairment of the body schema (Buxbaum & Coslett, 2001). Indeed, asomatognosia was recently found to relate to apraxic patients’ deficits in the imitation of meaningless action (Schwoebel, Buxbaum, & Coslett, 2004). In addition, a dissociation between autotopagnosia, the inability to point to on one’s body parts, and heterotopagnosia, the inability to point to someone else’ body parts, has recently been found (Felician, Ceccaldi, Didic, Thinus-Blanc, & Poncet, 2003), and these two functions could rely on different parietal regions in the left hemisphere.
Finally, it cannot be ruled out that the representation of gestures, in the superior parietal cortex, use dynamic representations of body movements, another component of the body schema. The body schema has indeed been extensively studied during the last decade, and it appears that different and independent schemata exist (Buxbaum & Coslett, 2001; Sirigu, Grafman, Bressler, & Sunderland, 1991). Until recently, the body schema has been understood as a system of visuospatial structural descriptions of the subject’s and other people’s bodies, and a dynamic body schema, involved in the coding of the location of the body parts during the execution of movements, has been overlooked. It has been argued that the superior parietal lobule could be involved in this latter aspect of the coding of body parts (Wolpert, Goodbody, & Husain, 1998).
Thus it remains possible that Buxbaum’s model views this dynamic aspect of the body schema in association with the execution of movement, and that the superior parietal lobule involvement in the reproduction of gestures relies on this aspect. Nevertheless our results strongly suggest that this system, if it exists, is not involved in the coding of body parts per se.
6. Conclusion
The results of the current neuroimaging study fit together nicely with recent studies of apraxic patients’ disorders in imitation and related behavior. Goldenberg (2001) assigned to the left inferior parietal cortex a crucial role in coding the relationships between body parts that could explain deficits of imitation in apraxic patients. In accordance with this prediction, we suggest that imitation is primarily related to body schema, in the inferior parietal gyrus bilaterally with a specific involvement of the parietal operculum in the left hemisphere. In addition the coding of the actions used in this experiment involved visual perception, analysis and expertise mainly associated with areas in the right hemisphere. This makes sense because imitation, taken from a cognitive view-point, involves important aspects of visual attention necessary to encode a gesture within a spatial, and not a motor, frame of reference.
On the basis of the present results, we propose that the brain network involved in imitation of meaningless intransitive limb gestures is first associated with an increased visual attention (independent of which aspect of the model’s action is considered) as demonstrated by an increased bilateral occipitotemporal activity. In a second step, two aspects of the gesture would be dissociated by the activity of the parietal, temporal and insular cortices. A visuospatial description of the body would be sustained by the left inferior parietal lobule as well as insular cortices, while a visuospatial description of the observed gesture would be sustained by visual perception areas in the superior parietal and ventral temporal cortices. Although these would in a third step converge to cortices involved in the preparation of action to perform imitation, we found no evidence of a modulation of motor-related cortices in imitation independent of the limb and the action investigated.
Highly relevant for the understanding of apraxia, these results support the proposal that apraxic patients with damage in the left inferior parietal cortex show primarily show a disorder of one’s own body representation. These findings about the neural bases of body actions also potentially shed light on the mechanisms and brain bases of infant imitation (Meltzoff & Decety, 2003).
Acknowledgements
We thank Craig Harris, Mark Dow and Scott B. Watrous for their help in preparing and running this study. The fMRI experiments were conducted at the Lewis Center for Neuroimaging, University of Oregon, Eugene. This research was funded in part by a grant from NIH (HD22514) and through a generous gift from the Talaris Research Institute and the Apex Foundation, the family foundation of Bruce and Jolene McCaw.
Footnotes
This finding of similar impairments in hands and feet imitation in patients was paramount in deciding to collapse conditions with the four different limbs in the present experiment.
We consider that “identification” of the limb is the same in all conditions, whether they require using the “same” or the “other” limb. Our study investigates the neural network associated with “using the same limb”.
References
- Adolphs R. Is the human amygdala specialized for processing social information? Annals of the New York Academy of Science. 2003;985:326–340. doi: 10.1111/j.1749-6632.2003.tb07091.x. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cerebral Cortex. 2000;10(3):295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Berlucchi G, Aglioti S. The body in the brain: Neural bases of corporeal awareness. Trends in Neuroscience. 1997;20(12):560–564. doi: 10.1016/s0166-2236(97)01136-3. [DOI] [PubMed] [Google Scholar]
- Berthoz A. Parietal and hippocampal contribution to topokinetic and topographic memory. Philosophical Transactions of the Royal Society of London B Biological Sciences. 1997;352(1360):1437–1448. doi: 10.1098/rstb.1997.0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ, Frith C. Self-awareness and action. Current Opinion in Neurobiology. 2003;13(2):219–224. doi: 10.1016/s0959-4388(03)00043-6. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Wolpert DM, Frith CD. Central cancellation of self-produced tickle sensation. Nature Neuroscience. 1998;1(7):635–640. doi: 10.1038/2870. [DOI] [PubMed] [Google Scholar]
- Bonda E, Petrides M, Frey S, Evans A. Neural correlates of mental transformations of the body-in-space. Proceedings of the National Academy of Science USA. 1995;92(24):11180–11184. doi: 10.1073/pnas.92.24.11180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass M, Bekkering H, Wohlschlager A, Prinz W. Compatibility between observed and executed finger movements: Comparing symbolic, spatial, and imitative cues. Brain and Cognition. 2000;44(2):124–143. doi: 10.1006/brcg.2000.1225. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ. Ideomotor apraxia: A call to action. Neurocase. 2001;7(6):445–458. doi: 10.1093/neucas/7.6.445. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Coslett HB. Specialised structural descriptions for human body parts: Evidence from autotopagnosia. Cognitive Neuropsychology. 2001;18(4):289–306. doi: 10.1080/02643290126172. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Giovannetti T, Libon D. The role of the dynamic body schema in praxis: Evidence from primary progressive apraxia. Brain and Cognition. 2000;44(2):166–191. doi: 10.1006/brcg.2000.1227. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Sirigu A, Schwartz MF, Klatzky R. Cognitive representations of hand posture in ideomotor apraxia. Neuropsychologia. 2003;41(8):1091–1113. doi: 10.1016/s0028-3932(02)00314-7. [DOI] [PubMed] [Google Scholar]
- Castelli F, Happe F, Frith U, Frith C. Movement and mind: A functional imaging study of perception and interpretation of complex intentional movement patterns. Neuroimage. 2000;12(3):314–325. doi: 10.1006/nimg.2000.0612. [DOI] [PubMed] [Google Scholar]
- Castiello U, Lusher D, Mari M, Edwards M, Humphreys G. Observing a human or a robotic hand grasping an object: Differential motor priming effects. In: Prinz W, Hommel B, editors. Common mechanisms in perception and action: Attention and performance. Vol. XIX. Oxford: Oxford University Press; 2002. pp. 315–333. [Google Scholar]
- Chaminade T, Decety J. Leader or follower? Involvement of the inferior parietal lobule in agency. Neuroreport. 2002;13(15):1975–1978. doi: 10.1097/00001756-200210280-00029. [DOI] [PubMed] [Google Scholar]
- Chaminade T, Meltzoff AN, Decety J. Does the end justify the means? A PET exploration of the mechanisms involved in human imitation. Neuroimage. 2002;15(2):318–328. doi: 10.1006/nimg.2001.0981. [DOI] [PubMed] [Google Scholar]
- Decety J, Chaminade T. When the self represents the other: A new cognitive neuroscience view of psychological identification. Consciousness and Cognition. 2004;12:577–596. doi: 10.1016/s1053-8100(03)00076-x. [DOI] [PubMed] [Google Scholar]
- Decety J, Chaminade T, Grezes J, Meltzoff AN. A PET exploration of the neural mechanisms involved in reciprocal imitation. Neuroimage. 2002;15(1):265–272. doi: 10.1006/nimg.2001.0938. [DOI] [PubMed] [Google Scholar]
- Decety J, Grezes J, Costes N, Perani D, Jeannerod M, Procyk E, et al. Brain activity during observation of actions. Influence of action content and subject’s strategy. Brain. 1997;120(Pt 10):1763–1777. doi: 10.1093/brain/120.10.1763. [DOI] [PubMed] [Google Scholar]
- Downing PE, Jiang Y, Shuman M, Kanwisher N. A cortical area selective for visual processing of the human body. Science. 2001;293(5539):2470–2473. doi: 10.1126/science.1063414. [DOI] [PubMed] [Google Scholar]
- Dumoulin SO, Bittar RG, Kabani NJ, Baker CL, Jr, Le Goualher G, Bruce Pike G. A new anatomical landmark for reliable identification of human area V5/MT: A quantitative analysis of sulcal patterning. Cerebral Cortex. 2000;10(5):454–463. doi: 10.1093/cercor/10.5.454. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM, Cabanis EA. Human brain: Surface, three-dimensional sectional anatomy with MRI & vascularization. New York: Springer Verlag; 1991. [Google Scholar]
- Fadiga L, Fogassi L, Gallese V, Rizzolatti G. Visuomotor neurons: Ambiguity of the discharge or ‘motor’ perception? International Journal of Psychophysiology. 2000;35(2–3):165–177. doi: 10.1016/s0167-8760(99)00051-3. [DOI] [PubMed] [Google Scholar]
- Farrer C, Franck N, Georgieff N, Frith CD, Decety J, Jeannerod M. Modulating the experience of agency: A positron emission tomography study. Neuroimage. 2003;18(2):324–333. doi: 10.1016/s1053-8119(02)00041-1. [DOI] [PubMed] [Google Scholar]
- Felician O, Ceccaldi M, Didic M, Thinus-Blanc C, Poncet M. Pointing to body parts: A double dissociation study. Neuropsychologia. 2003;41(10):1307–1316. doi: 10.1016/s0028-3932(03)00046-0. [DOI] [PubMed] [Google Scholar]
- Flynn F, Benson D, Ardila A. Anatomy of the insula functional and clinical correlates. Aphasiology. 1999;13(1):55–78. [Google Scholar]
- Gallagher S, Meltzoff AN. The earliest sense of self and others: Merleau-Ponty and recent developmental studies. Philosophical Psychology. 1996;9:211–233. doi: 10.1080/09515089608573181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallese V, Fadiga L, Fogassi L, Rizzolatti G. Action representation and the inferior parietal lobule. In: Prinz W, Hommel B, editors. Common mechanisms in perception and action: Attention and performance. Vol. XIX. Oxford: Oxford University Press; 2002. pp. 247–266. [Google Scholar]
- Goldenberg G. Imitating gestures and manipulating a mannikin—the representation of the human body in ideomotor apraxia. Neuropsychologia. 1995;33(1):63–72. doi: 10.1016/0028-3932(94)00104-w. [DOI] [PubMed] [Google Scholar]
- Goldenberg G. Matching and imitation of hand and finger postures in patients with damage in the left or right hemispheres. Neuropsychologia. 1999;37(5):559–566. doi: 10.1016/s0028-3932(98)00111-0. [DOI] [PubMed] [Google Scholar]
- Goldenberg G. Imitation and matching of hand and finger postures. Neuroimage. 2001;14(1 Pt 2):S132–S136. doi: 10.1006/nimg.2001.0820. [DOI] [PubMed] [Google Scholar]
- Goldenberg G, Hagmann S. The meaning of meaningless gestures: A study of visuo-imitative apraxia. Neuropsychologia. 1997;35(3):333–341. doi: 10.1016/s0028-3932(96)00085-1. [DOI] [PubMed] [Google Scholar]
- Goldenberg G, Laimgruber K, Hermsdorfer J. Imitation of gestures by disconnected hemispheres. Neuropsychologia. 2001;39(13):1432–1443. doi: 10.1016/s0028-3932(01)00062-8. [DOI] [PubMed] [Google Scholar]
- Goldenberg G, Strauss S. Hemisphere asymmetries for imitation of novel gestures. Neurology. 2002;59(6):893–897. doi: 10.1212/wnl.59.6.893. [DOI] [PubMed] [Google Scholar]
- Grezes J, Armony JL, Rowe J, Passingham RE. Activations related to “mirror” and “canonical” neurones in the human brain: An fMRI study. Neuroimage. 2003;18(4):928–937. doi: 10.1016/s1053-8119(03)00042-9. [DOI] [PubMed] [Google Scholar]
- Grezes J, Costes N, Decety J. The effects of learning and intention on the neural network involved in the perception of meaningless actions. Brain. 1999;122(Pt 10):1875–1887. doi: 10.1093/brain/122.10.1875. [DOI] [PubMed] [Google Scholar]
- Grezes J, Costes N, Decety J. Top down effect of strategy on the perception of human biological motion: A PET investigation. Cognitive Neuropsychology. 1998;15(6):553–582. doi: 10.1080/026432998381023. [DOI] [PubMed] [Google Scholar]
- Grezes J, Decety J. Functional anatomy of execution, mental simulation, observation, and verb generation of actions: A metaanalysis. Human Brain Mapping. 2001;12(1):1–19. doi: 10.1002/1097-0193(200101)12:1<1::AID-HBM10>3.0.CO;2-V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodd W, Hulsmann E, Lotze M, Wildgruber D, Erb M. Sensorimotor mapping of the human cerebellum: fMRI evidence of somatotopic organization. Human Brain Mapping. 2001;13(2):55–73. doi: 10.1002/hbm.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman ED, Blake R. Brain areas active during visual perception of biological motion. Neuron. 2002;35(6):1167–1175. doi: 10.1016/s0896-6273(02)00897-8. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Harrington DL, Knight RT. Neural representations of skilled movement. Brain. 2000;123(Pt 11):2306–2313. doi: 10.1093/brain/123.11.2306. [DOI] [PubMed] [Google Scholar]
- Halsband U, Schmitt J, Weyers M, Binkofski F, Grutzner G, Freund HJ. Recognition and imitation of pantomimed motor acts after unilateral parietal and premotor lesions: A perspective on apraxia. Neuropsychologia. 2001;39(2):200–216. doi: 10.1016/s0028-3932(00)00088-9. [DOI] [PubMed] [Google Scholar]
- Hermsdorfer J, Goldenberg G, Wachsmuth C, Conrad B, Ceballos- Baumann AO, Bartenstein P, et al. Cortical correlates of gesture processing: Clues to the cerebral mechanisms underlying apraxia during the imitation of meaningless gestures. Neuroimage. 2001;14(1 Pt 1):149–161. doi: 10.1006/nimg.2001.0796. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Woods RP, Brass M, Bekkering H, Mazziotta JC, Rizzolatti G. Cortical mechanisms of human imitation. Science. 1999;286(5449):2526–2528. doi: 10.1126/science.286.5449.2526. [DOI] [PubMed] [Google Scholar]
- Jackson PL, Lafleur MF, Malouin F, Richards CL, Doyon J. Functional cerebral reorganization following motor sequence learning through mental practice with motor imagery. Neuroimage. 2003;20(2):1171–1180. doi: 10.1016/S1053-8119(03)00369-0. [DOI] [PubMed] [Google Scholar]
- Kanwisher N. Domain specificity in face perception. Nature Neuroscience. 2000;3(8):759–763. doi: 10.1038/77664. [DOI] [PubMed] [Google Scholar]
- Koski L, Iacoboni M, Dubeau MC, Woods RP, Mazziotta JC. Modulation of cortical activity during different imitative behaviors. Journal of Neurophysiology. 2003;89(1):460–471. doi: 10.1152/jn.00248.2002. [DOI] [PubMed] [Google Scholar]
- Koski L, Wohlschlager A, Bekkering H, Woods RP, Dubeau MC, Mazziotta JC, et al. Modulation of motor and premotor activity during imitation of target-directed actions. Cerebral Cortex. 2002;12(8):847–855. doi: 10.1093/cercor/12.8.847. [DOI] [PubMed] [Google Scholar]
- Lausberg H, Cruz RF. Hemispheric specialisation for imitation of hand–head positions and finger configurations: A controlled study in patients with complete callosotomy. Neuropsychologia. 2004;42(3):320–334. doi: 10.1016/j.neuropsychologia.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Lotze M, Erb M, Flor H, Huelsmann E, Godde B, Grodd W. fMRI evaluation of somatotopic representation in human primary motor cortex. Neuroimage. 2000;11(5 Pt 1):473–481. doi: 10.1006/nimg.2000.0556. [DOI] [PubMed] [Google Scholar]
- Mattingley JB, Husain M, Rorden C, Kennard C, Driver J. Motor role of human inferior parietal lobe revealed in unilateral neglect patients. Nature. 1998;392(6672):179–182. doi: 10.1038/32413. [DOI] [PubMed] [Google Scholar]
- Meltzoff AN, Decety J. What imitation tells us about social cognition: A rapprochement between developmental psychology and cognitive neuroscience. Philosophical Transactions of the Royal Society of London B Biological Sciences. 2003;358(1431):491–500. doi: 10.1098/rstb.2002.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzoff AN, Moore MK. Explaining facial imitation: A theoretical model. Early Development and Parenting. 1997;6:179–192. doi: 10.1002/(SICI)1099-0917(199709/12)6:3/4<179::AID-EDP157>3.0.CO;2-R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovitch M, Winocur G. The frontal cortex and working memory. In: Stuss DT, Knight RT, editors. Principles of frontal lobe function. New York: Oxford University Press; 2002. pp. 188–208. [Google Scholar]
- O’Craven KM, Kanwisher N. Mental imagery of faces and places activates corresponding stimulus-specific brain regions. Journal of Cognitive Neuroscience. 2000;12(6):1013–1023. doi: 10.1162/08989290051137549. [DOI] [PubMed] [Google Scholar]
- Peigneux P, Salmon E, van der Linden M, Garraux G, Aerts J, Delfiore G, et al. The role of lateral occipitotemporal junction and area MT/V5 in the visual analysis of upper-limb postures. Neuroimage. 2000;11(6 Pt 1):644–655. doi: 10.1006/nimg.2000.0578. [DOI] [PubMed] [Google Scholar]
- Perani D, Fazio F, Borghese NA, Tettamanti M, Ferrari S, Decety J, et al. Different brain correlates for watching real and virtual hand actions. Neuroimage. 2001;14(3):749–758. doi: 10.1006/nimg.2001.0872. [DOI] [PubMed] [Google Scholar]
- Rijntjes M, Dettmers C, Buchel C, Kiebel S, Frackowiak RS, Weiller C. A blueprint for movement: Functional and anatomical representations in the human motor system. Journal of Neuroscience. 1999;19(18):8043–8048. doi: 10.1523/JNEUROSCI.19-18-08043.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R. The effect of the experimenter on the results of psychological research. Progress in Experimental Personality Research. 1964;72:79–114. [PubMed] [Google Scholar]
- Rumiati RI, Bekkering H. To imitate or not to imitate? How the brain can do it, that is the question! Brain and Cognition. 2003;53(3):479–482. doi: 10.1016/s0278-2626(03)00208-2. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Nixon PD, Passingham RE. Parietal cortex and movementIMovement selection and reaching. Experimental Brain Research. 1997;117(2):292–310. doi: 10.1007/s002210050224. [DOI] [PubMed] [Google Scholar]
- Schnider A, Treyer V, Buck A. Selection of currently relevant memories by the human posterior medial orbitofrontal cortex. Journal of Neuroscience. 2000;20(15):5880–5884. doi: 10.1523/JNEUROSCI.20-15-05880.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwoebel J, Buxbaum LJ, Coslett HB. Representations of the human body in the production and imitation of complex movements. Cognitive Neuropsychology. 2004;21:285–298. doi: 10.1080/02643290342000348. [DOI] [PubMed] [Google Scholar]
- Sirigu A, Grafman J, Bressler K, Sunderland T. Multiple representations contribute to body knowledge processing. Evidence from a case of autotopagnosia. Brain. 1991;114(Pt 1B):629–642. doi: 10.1093/brain/114.1.629. [DOI] [PubMed] [Google Scholar]
- Stevens JA, Fonlupt P, Shiffrar M, Decety J. New aspects of motion perception: Selective neural encoding of apparent human movements. Neuroreport. 2000;11(1):109–115. doi: 10.1097/00001756-200001170-00022. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Inui T. Cortical involvement for action imitation of hand/arm postures versus finger configurations: An fMRI study. Neuroreport. 2002;13(13):1599–1602. doi: 10.1097/00001756-200209160-00005. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Inui T, Iwaki S, Konishi J, Nakai T. Neural substrates involved in imitating finger configurations: An fMRI study. Neuroreport. 2001;12(6):1171–1174. doi: 10.1097/00001756-200105080-00024. [DOI] [PubMed] [Google Scholar]
- Tarr MJ, Gauthier I. FFA: A flexible fusiform area for subordinate-level visual processing automatized by expertise. Nature Neuroscience. 2000;3(8):764–769. doi: 10.1038/77666. [DOI] [PubMed] [Google Scholar]
- Viviani P. Motor competence in the perception of dynamic events: A tutorial. In: Prinz W, Hommel B, editors. Attention and performance XIX: Common mechanisms in perception and action. Oxford: Oxford University Press; 2002. pp. 406–442. [Google Scholar]
- Vogt BA, Finch DM, Olson CR. Functional heterogeneity in cingulate cortex: The anterior executive and posterior evaluative regions. Cerebral Cortex. 1992;2:435–443. doi: 10.1093/cercor/2.6.435-a. [DOI] [PubMed] [Google Scholar]
- Wilson M. Perceiving imitatible stimuli: Consequences of isomorphism between input and output. Psychology Bulletin. 2001;127(4):543–553. doi: 10.1037/0033-2909.127.4.543. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Goodbody SJ, Husain M. Maintaining internal representations: The role of the human superior parietal lobe. Nature Neuroscience. 1998;1(6):529–533. doi: 10.1038/2245. [DOI] [PubMed] [Google Scholar]