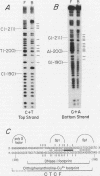
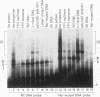
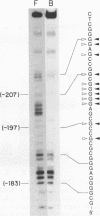
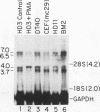
Abstract
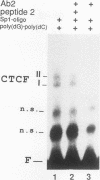
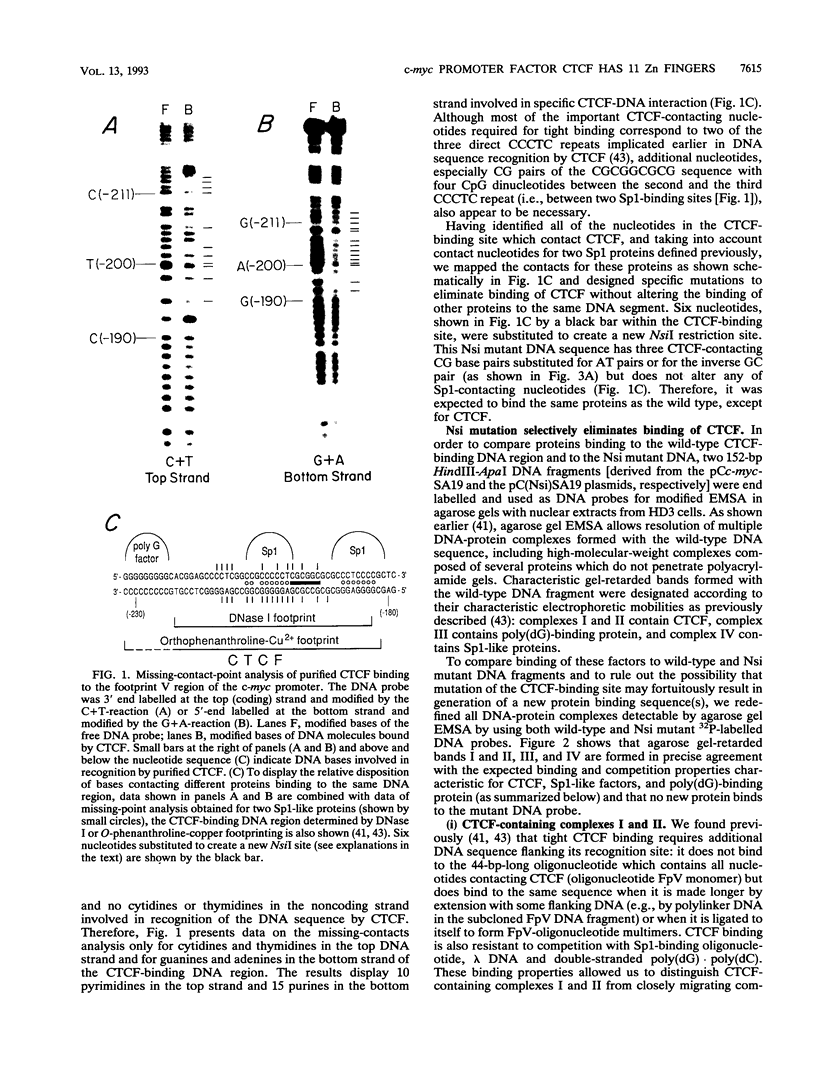
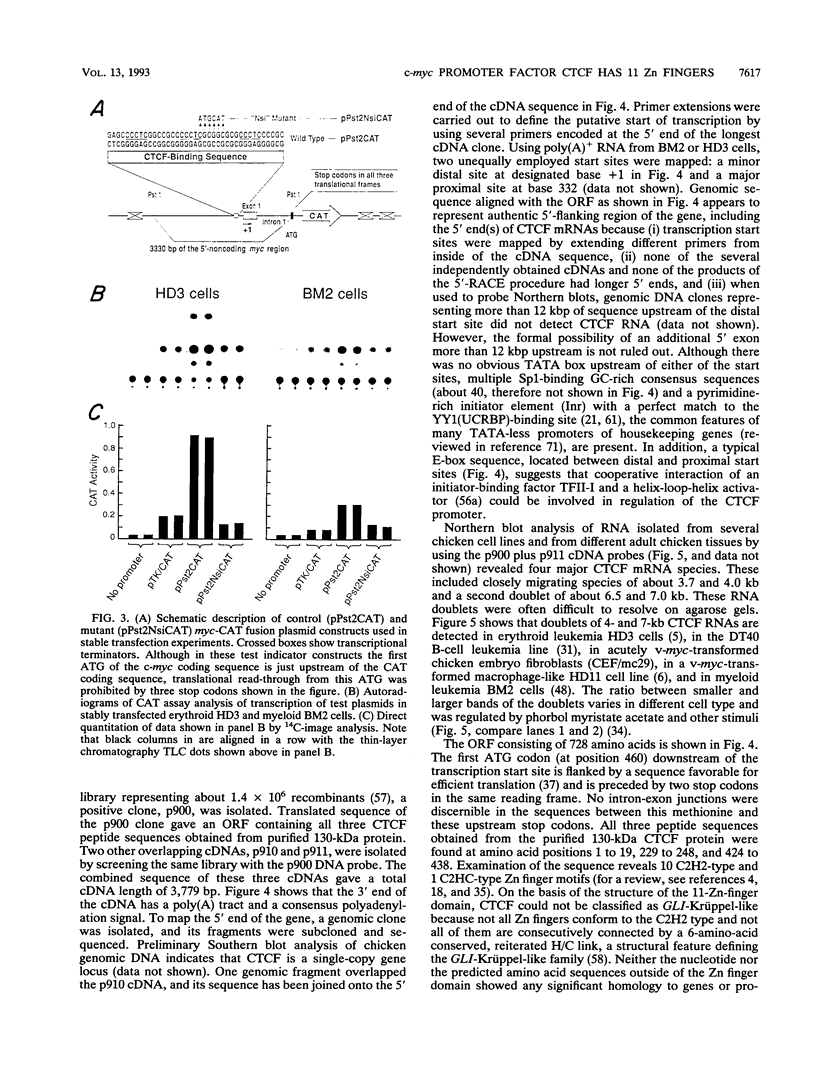
A novel sequence-specific DNA-binding protein, CTCF, which interacts with the chicken c-myc gene promoter, has been identified and partially characterized (V. V. Lobanenkov, R. H. Nicolas, V. V. Adler, H. Paterson, E. M. Klenova, A. V. Polotskaja, and G. H. Goodwin, Oncogene 5:1743-1753, 1990). In order to test directly whether binding of CTCF to one specific DNA region of the c-myc promoter is important for chicken c-myc transcription, we have determined which nucleotides within this GC-rich region are responsible for recognition of overlapping sites by CTCF and Sp1-like proteins. Using missing-contact analysis of all four nucleotides in both DNA strands and homogeneous CTCF protein purified by sequence-specific chromatography, we have identified three sets of nucleotides which contact either CTCF or two Sp1-like proteins binding within the same DNA region. Specific mutations of 3 of 15 purines required for CTCF binding were designed to eliminate binding of CTCF without altering the binding of other proteins. Electrophoretic mobility shift assay of nuclear extracts showed that the mutant DNA sequence did not bind CTCF but did bind two Sp1-like proteins. When introduced into a 3.3-kbp-long 5'-flanking noncoding c-myc sequence fused to a reporter CAT gene, the same mutation of the CTCF binding site resulted in 10- and 3-fold reductions, respectively, of transcription in two different (erythroid and myeloid) stably transfected chicken cell lines. Isolation and analysis of the CTCF cDNA encoding an 82-kDa form of CTCF protein shows that DNA-binding domain of CTCF is composed of 11 Zn fingers: 10 are of C2H2 class, and 1 is of C2HC class. CTCF was found to be abundant and conserved in cells of vertebrate species. We detected six major nuclear forms of CTCF protein differentially expressed in different chicken cell lines and tissues. We conclude that isoforms of 11-Zn-finger factor CTCF which are present in chicken hematopoietic HD3 and BM2 cells can act as a positive regulator of the chicken c-myc gene transcription. Possible functions of other CTCF forms are discussed.
Full text
PDF




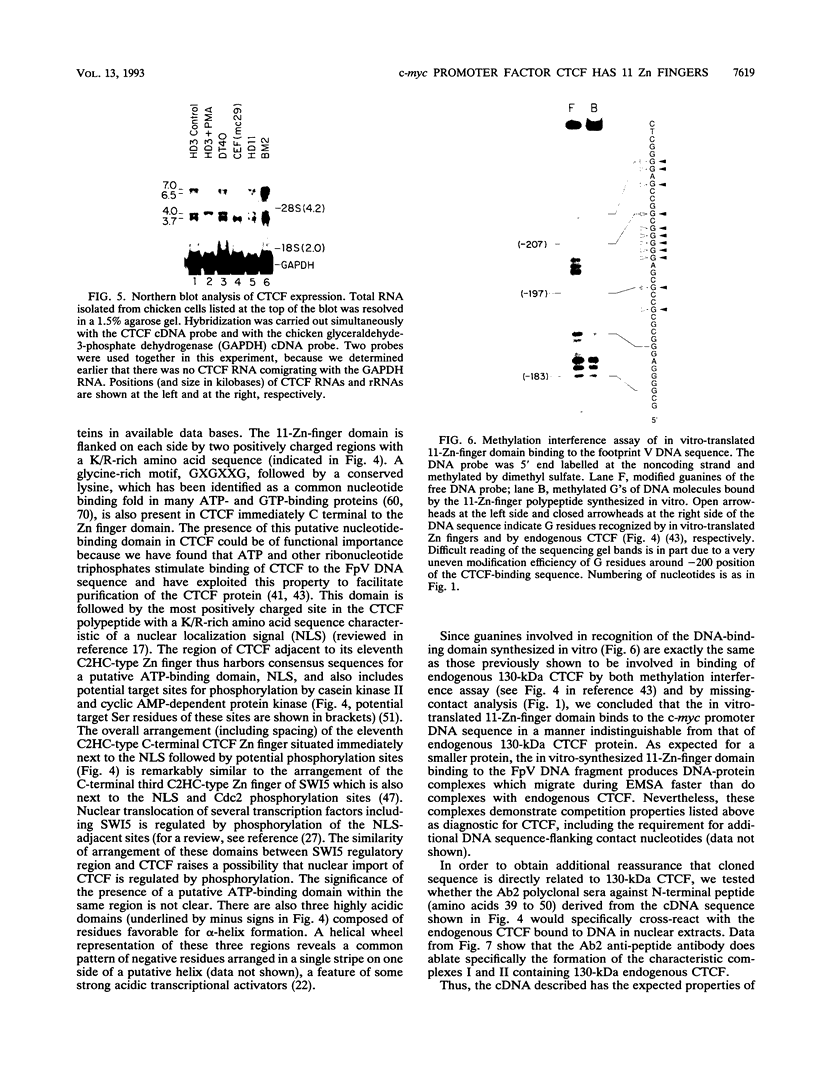
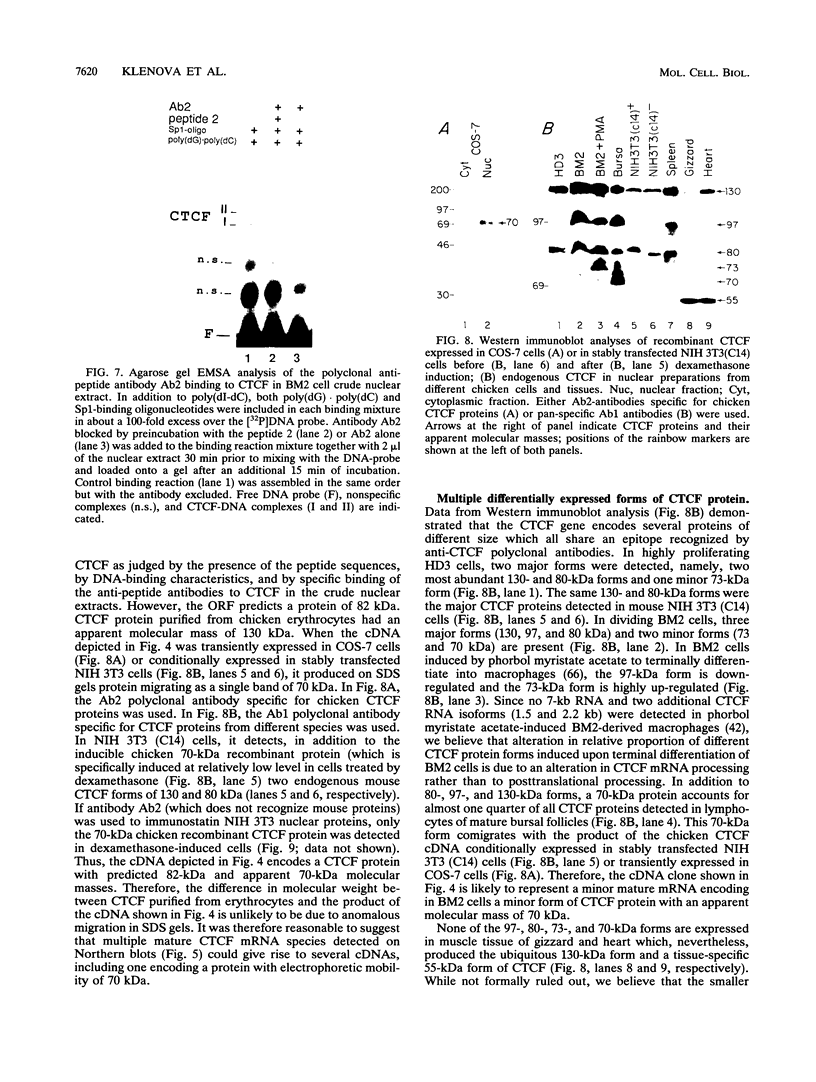
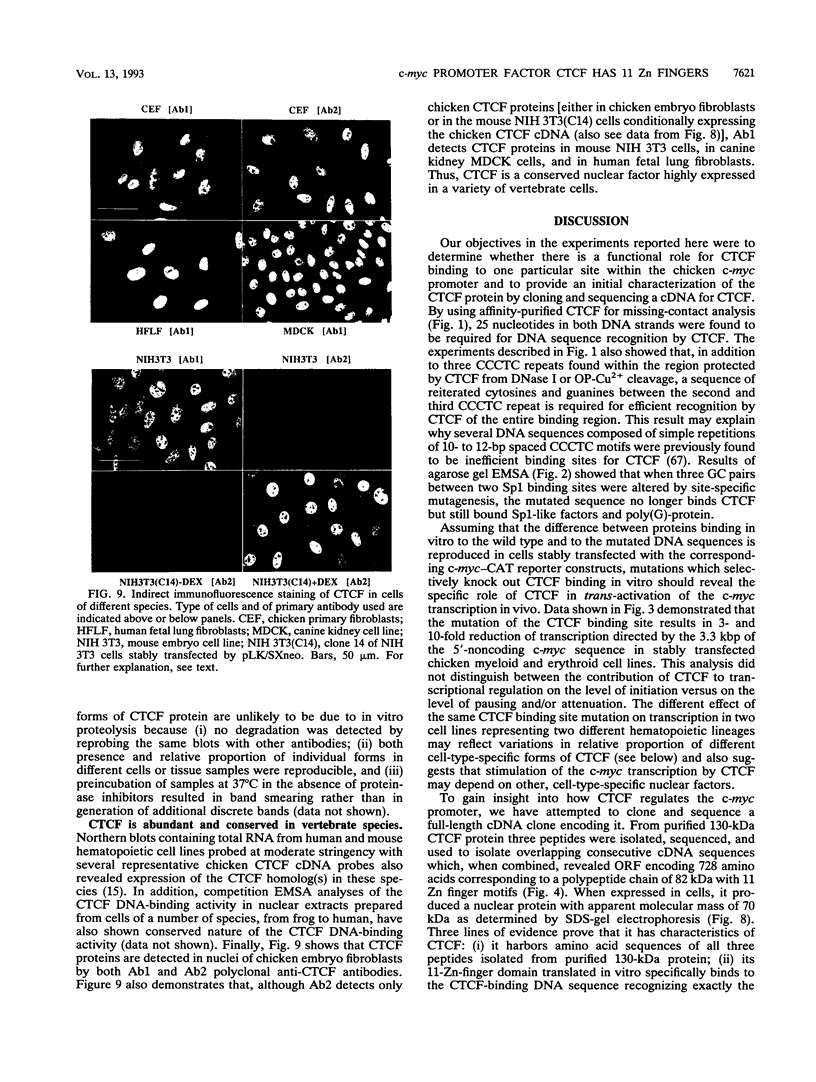



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson P., Paterson H. F., Hall A. Intracellular localization of the P21rho proteins. J Cell Biol. 1992 Nov;119(3):617–627. doi: 10.1083/jcb.119.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askew D. S., Ashmun R. A., Simmons B. C., Cleveland J. L. Constitutive c-myc expression in an IL-3-dependent myeloid cell line suppresses cell cycle arrest and accelerates apoptosis. Oncogene. 1991 Oct;6(10):1915–1922. [PubMed] [Google Scholar]
- Basu A., Park K., Atchison M. L., Carter R. S., Avadhani N. G. Identification of a transcriptional initiator element in the cytochrome c oxidase subunit Vb promoter which binds to transcription factors NF-E1 (YY-1, delta) and Sp1. J Biol Chem. 1993 Feb 25;268(6):4188–4196. [PubMed] [Google Scholar]
- Berg J. M. Zinc fingers and other metal-binding domains. Elements for interactions between macromolecules. J Biol Chem. 1990 Apr 25;265(12):6513–6516. [PubMed] [Google Scholar]
- Beug H., Doederlein G., Freudenstein C., Graf T. Erythroblast cell lines transformed by a temperature-sensitive mutant of avian erythroblastosis virus: a model system to study erythroid differentiation in vitro. J Cell Physiol Suppl. 1982;1:195–207. doi: 10.1002/jcp.1041130427. [DOI] [PubMed] [Google Scholar]
- Bickmore W. A., Oghene K., Little M. H., Seawright A., van Heyningen V., Hastie N. D. Modulation of DNA binding specificity by alternative splicing of the Wilms tumor wt1 gene transcript. Science. 1992 Jul 10;257(5067):235–237. doi: 10.1126/science.1321494. [DOI] [PubMed] [Google Scholar]
- Bird A. P. CpG-rich islands and the function of DNA methylation. Nature. 1986 May 15;321(6067):209–213. doi: 10.1038/321209a0. [DOI] [PubMed] [Google Scholar]
- Blackwood E. M., Eisenman R. N. Max: a helix-loop-helix zipper protein that forms a sequence-specific DNA-binding complex with Myc. Science. 1991 Mar 8;251(4998):1211–1217. doi: 10.1126/science.2006410. [DOI] [PubMed] [Google Scholar]
- Bordereaux D., Fichelson S., Tambourin P., Gisselbrecht S. Alternative splicing of the Evi-1 zinc finger gene generates mRNAs which differ by the number of zinc finger motifs. Oncogene. 1990 Jun;5(6):925–927. [PubMed] [Google Scholar]
- Bossone S. A., Asselin C., Patel A. J., Marcu K. B. MAZ, a zinc finger protein, binds to c-MYC and C2 gene sequences regulating transcriptional initiation and termination. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7452–7456. doi: 10.1073/pnas.89.16.7452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunelle A., Schleif R. F. Missing contact probing of DNA-protein interactions. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6673–6676. doi: 10.1073/pnas.84.19.6673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cole M. D. The myc oncogene: its role in transformation and differentiation. Annu Rev Genet. 1986;20:361–384. doi: 10.1146/annurev.ge.20.120186.002045. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingwall C., Laskey R. A. Nuclear targeting sequences--a consensus? Trends Biochem Sci. 1991 Dec;16(12):478–481. doi: 10.1016/0968-0004(91)90184-w. [DOI] [PubMed] [Google Scholar]
- Evan G. I., Wyllie A. H., Gilbert C. S., Littlewood T. D., Land H., Brooks M., Waters C. M., Penn L. Z., Hancock D. C. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992 Apr 3;69(1):119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- Felgner P. L., Gadek T. R., Holm M., Roman R., Chan H. W., Wenz M., Northrop J. P., Ringold G. M., Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan J. R., Becker K. G., Ennist D. L., Gleason S. L., Driggers P. H., Levi B. Z., Appella E., Ozato K. Cloning of a negative transcription factor that binds to the upstream conserved region of Moloney murine leukemia virus. Mol Cell Biol. 1992 Jan;12(1):38–44. doi: 10.1128/mcb.12.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giniger E., Ptashne M. Transcription in yeast activated by a putative amphipathic alpha helix linked to a DNA binding unit. Nature. 1987 Dec 17;330(6149):670–672. doi: 10.1038/330670a0. [DOI] [PubMed] [Google Scholar]
- Helin K., Lees J. A., Vidal M., Dyson N., Harlow E., Fattaey A. A cDNA encoding a pRB-binding protein with properties of the transcription factor E2F. Cell. 1992 Jul 24;70(2):337–350. doi: 10.1016/0092-8674(92)90107-n. [DOI] [PubMed] [Google Scholar]
- Hirt R. P., Poulain-Godefroy O., Billotte J., Kraehenbuhl J. P., Fasel N. Highly inducible synthesis of heterologous proteins in epithelial cells carrying a glucocorticoid-responsive vector. Gene. 1992 Feb 15;111(2):199–206. doi: 10.1016/0378-1119(92)90687-k. [DOI] [PubMed] [Google Scholar]
- Hsu T., Gogos J. A., Kirsh S. A., Kafatos F. C. Multiple zinc finger forms resulting from developmentally regulated alternative splicing of a transcription factor gene. Science. 1992 Sep 25;257(5078):1946–1950. doi: 10.1126/science.1411512. [DOI] [PubMed] [Google Scholar]
- Hunter T., Karin M. The regulation of transcription by phosphorylation. Cell. 1992 Aug 7;70(3):375–387. doi: 10.1016/0092-8674(92)90162-6. [DOI] [PubMed] [Google Scholar]
- Imataka H., Sogawa K., Yasumoto K., Kikuchi Y., Sasano K., Kobayashi A., Hayami M., Fujii-Kuriyama Y. Two regulatory proteins that bind to the basic transcription element (BTE), a GC box sequence in the promoter region of the rat P-4501A1 gene. EMBO J. 1992 Oct;11(10):3663–3671. doi: 10.1002/j.1460-2075.1992.tb05451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadonaga J. T., Courey A. J., Ladika J., Tjian R. Distinct regions of Sp1 modulate DNA binding and transcriptional activation. Science. 1988 Dec 16;242(4885):1566–1570. doi: 10.1126/science.3059495. [DOI] [PubMed] [Google Scholar]
- Kadonaga J. T. Purification of sequence-specific binding proteins by DNA affinity chromatography. Methods Enzymol. 1991;208:10–23. doi: 10.1016/0076-6879(91)08004-2. [DOI] [PubMed] [Google Scholar]
- Kim S., Humphries E. H., Tjoelker L., Carlson L., Thompson C. B. Ongoing diversification of the rearranged immunoglobulin light-chain gene in a bursal lymphoma cell line. Mol Cell Biol. 1990 Jun;10(6):3224–3231. doi: 10.1128/mcb.10.6.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsley C., Winoto A. Cloning of GT box-binding proteins: a novel Sp1 multigene family regulating T-cell receptor gene expression. Mol Cell Biol. 1992 Oct;12(10):4251–4261. doi: 10.1128/mcb.12.10.4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolluri R., Kinniburgh A. J. Full length cDNA sequence encoding a nuclease-sensitive element DNA binding protein. Nucleic Acids Res. 1991 Sep 11;19(17):4771–4771. doi: 10.1093/nar/19.17.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretzner L., Blackwood E. M., Eisenman R. N. Myc and Max proteins possess distinct transcriptional activities. Nature. 1992 Oct 1;359(6394):426–429. doi: 10.1038/359426a0. [DOI] [PubMed] [Google Scholar]
- Lachmann P. J., Strangeways L., Vyakarnam A., Evan G. Raising antibodies by coupling peptides to PPD and immunizing BCG-sensitized animals. Ciba Found Symp. 1986;119:25–57. doi: 10.1002/9780470513286.ch3. [DOI] [PubMed] [Google Scholar]
- Letovsky J., Dynan W. S. Measurement of the binding of transcription factor Sp1 to a single GC box recognition sequence. Nucleic Acids Res. 1989 Apr 11;17(7):2639–2653. doi: 10.1093/nar/17.7.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobanenkov V. V., Nicolas R. H., Adler V. V., Paterson H., Klenova E. M., Polotskaja A. V., Goodwin G. H. A novel sequence-specific DNA binding protein which interacts with three regularly spaced direct repeats of the CCCTC-motif in the 5'-flanking sequence of the chicken c-myc gene. Oncogene. 1990 Dec;5(12):1743–1753. [PubMed] [Google Scholar]
- Lobanenkov V. V., Nicolas R. H., Plumb M. A., Wright C. A., Goodwin G. H. Sequence-specific DNA-binding proteins which interact with (G + C)-rich sequences flanking the chicken c-myc gene. Eur J Biochem. 1986 Aug 15;159(1):181–188. doi: 10.1111/j.1432-1033.1986.tb09850.x. [DOI] [PubMed] [Google Scholar]
- Marcu K. B., Bossone S. A., Patel A. J. myc function and regulation. Annu Rev Biochem. 1992;61:809–860. doi: 10.1146/annurev.bi.61.070192.004113. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Moll T., Tebb G., Surana U., Robitsch H., Nasmyth K. The role of phosphorylation and the CDC28 protein kinase in cell cycle-regulated nuclear import of the S. cerevisiae transcription factor SWI5. Cell. 1991 Aug 23;66(4):743–758. doi: 10.1016/0092-8674(91)90118-i. [DOI] [PubMed] [Google Scholar]
- Neiman P. E., Thomas S. J., Loring G. Induction of apoptosis during normal and neoplastic B-cell development in the bursa of Fabricius. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5857–5861. doi: 10.1073/pnas.88.13.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson R. B., Kemp B. E. Protein kinase phosphorylation site sequences and consensus specificity motifs: tabulations. Methods Enzymol. 1991;200:62–81. doi: 10.1016/0076-6879(91)00127-i. [DOI] [PubMed] [Google Scholar]
- Plumb M. A., Lobanenkov V. V., Nicolas R. H., Wright C. A., Zavou S., Goodwin G. H. Characterisation of chicken erythroid nuclear proteins which bind to the nuclease hypersensitive regions upstream of the beta A- and beta H-globin genes. Nucleic Acids Res. 1986 Oct 10;14(19):7675–7693. doi: 10.1093/nar/14.19.7675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyrc J. J., Moberg K. H., Hall D. J. Isolation of a novel cDNA encoding a zinc-finger protein that binds to two sites within the c-myc promoter. Biochemistry. 1992 Apr 28;31(16):4102–4110. doi: 10.1021/bi00131a029. [DOI] [PubMed] [Google Scholar]
- Ray R., Miller D. M. Cloning and characterization of a human c-myc promoter-binding protein. Mol Cell Biol. 1991 Apr;11(4):2154–2161. doi: 10.1128/mcb.11.4.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read D., Manley J. L. Alternatively spliced transcripts of the Drosophila tramtrack gene encode zinc finger proteins with distinct DNA binding specificities. EMBO J. 1992 Mar;11(3):1035–1044. doi: 10.1002/j.1460-2075.1992.tb05142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman D. G., Toledano M. B., Leonard W. J. Sp1 represses IL-2 receptor alpha chain gene expression. New Biol. 1990 Jul;2(7):642–647. [PubMed] [Google Scholar]
- Roy A. L., Meisterernst M., Pognonec P., Roeder R. G. Cooperative interaction of an initiator-binding transcription initiation factor and the helix-loop-helix activator USF. Nature. 1991 Nov 21;354(6350):245–248. doi: 10.1038/354245a0. [DOI] [PubMed] [Google Scholar]
- Rupp R. A., Kruse U., Multhaup G., Göbel U., Beyreuther K., Sippel A. E. Chicken NFI/TGGCA proteins are encoded by at least three independent genes: NFI-A, NFI-B and NFI-C with homologues in mammalian genomes. Nucleic Acids Res. 1990 May 11;18(9):2607–2616. doi: 10.1093/nar/18.9.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruppert J. M., Kinzler K. W., Wong A. J., Bigner S. H., Kao F. T., Law M. L., Seuanez H. N., O'Brien S. J., Vogelstein B. The GLI-Kruppel family of human genes. Mol Cell Biol. 1988 Aug;8(8):3104–3113. doi: 10.1128/mcb.8.8.3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste M., Sibbald P. R., Wittinghofer A. The P-loop--a common motif in ATP- and GTP-binding proteins. Trends Biochem Sci. 1990 Nov;15(11):430–434. doi: 10.1016/0968-0004(90)90281-f. [DOI] [PubMed] [Google Scholar]
- Seto E., Shi Y., Shenk T. YY1 is an initiator sequence-binding protein that directs and activates transcription in vitro. Nature. 1991 Nov 21;354(6350):241–245. doi: 10.1038/354241a0. [DOI] [PubMed] [Google Scholar]
- Spencer C. A., Groudine M. Control of c-myc regulation in normal and neoplastic cells. Adv Cancer Res. 1991;56:1–48. doi: 10.1016/s0065-230x(08)60476-5. [DOI] [PubMed] [Google Scholar]
- Spencer C. A., Groudine M. Transcription elongation and eukaryotic gene regulation. Oncogene. 1990 Jun;5(6):777–785. [PubMed] [Google Scholar]
- St-Arnaud R., Moir J. M. Wnt-1-inducing factor-1: a novel G/C box-binding transcription factor regulating the expression of Wnt-1 during neuroectodermal differentiation. Mol Cell Biol. 1993 Mar;13(3):1590–1598. doi: 10.1128/mcb.13.3.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stappert J., Wirsching J., Kemler R. A PCR method for introducing mutations into cloned DNA by joining an internal primer to a tagged flanking primer. Nucleic Acids Res. 1992 Feb 11;20(3):624–624. doi: 10.1093/nar/20.3.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symonds G., Klempnauer K. H., Evan G. I., Bishop J. M. Induced differentiation of avian myeloblastosis virus-transformed myeloblasts: phenotypic alteration without altered expression of the viral oncogene. Mol Cell Biol. 1984 Dec;4(12):2587–2593. doi: 10.1128/mcb.4.12.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tevosian S. G., Gromova E. S., Adler V. V., Lobanenkov V. V. Reguliatornyi belkovyi faktor CTCF vzaimodeistvuet s uchastkom promotora onkogena c-myc kur, sposobnym k perekhody v nekanonicheskuiu konformatsiiu. Mol Biol (Mosk) 1991 Jul-Aug;25(4):1013–1023. [PubMed] [Google Scholar]
- Thalmeier K., Synovzik H., Mertz R., Winnacker E. L., Lipp M. Nuclear factor E2F mediates basic transcription and trans-activation by E1a of the human MYC promoter. Genes Dev. 1989 Apr;3(4):527–536. doi: 10.1101/gad.3.4.527. [DOI] [PubMed] [Google Scholar]
- Tsang S. S., Yin X., Guzzo-Arkuran C., Jones V. S., Davison A. J. Loss of resolution in gel electrophoresis of RNA: a problem associated with the presence of formaldehyde gradients. Biotechniques. 1993 Mar;14(3):380–381. [PubMed] [Google Scholar]
- Walker J. E., Saraste M., Runswick M. J., Gay N. J. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1(8):945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis L., Reinberg D. Transcription by RNA polymerase II: initiator-directed formation of transcription-competent complexes. FASEB J. 1992 Nov;6(14):3300–3309. doi: 10.1096/fasebj.6.14.1426767. [DOI] [PubMed] [Google Scholar]
- el-Baradi T., Pieler T. Zinc finger proteins: what we know and what we would like to know. Mech Dev. 1991 Nov;35(3):155–169. doi: 10.1016/0925-4773(91)90015-x. [DOI] [PubMed] [Google Scholar]