Abstract
Iron overload is a risk factor for diabetes. The link between iron and diabetes was first recognized in pathologic conditions—hereditary hemochromatosis and thalassemia—but high levels of dietary iron also impart diabetes risk. Iron plays a direct and causal role in diabetes pathogenesis mediated both by β-cell failure and insulin resistance. Iron is also a factor in the regulation of metabolism in most tissues involved in fuel homeostasis, with the adipocyte in particular serving an iron-sensing role. The underlying molecular mechanisms mediating these effects are numerous and incompletely understood, but include oxidant stress and modulation of adipokines and intracellular signal transduction pathways.
Type 2 diabetes is a common and ever-increasing worldwide health problem. Although well described in terms of its hallmarks of insulin resistance and β-cell failure, the proximal cause(s) of type 2 diabetes and the mechanisms underlying its genetic predisposition remain largely unknown. Plausible cases have been made for the primacy of abnormalities in insulin signaling, insulin secretion, activation of stress pathways, mitochondrial dysfunction, hepatic fuel homeostasis, and CNS regulation (reviewed in (Hotamisligil, 2003; Kahn, 1998; Kahn, 2003; Lowell and Shulman, 2005)). It is well accepted that the most reliable predictor for the disease is obesity, therefore much attention has also been paid to the contribution of nutrients and nutrient sensing pathways in situations of chronic caloric excess. Most of the interest in the role of nutrients in diabetes is centered on macronutrients, but a micronutrient, iron, is also closely associated with diabetes risk in a number of hereditary syndromes as well as in common forms of type 2 diabetes. Iron deficiency is also associated with obesity. In this review we will briefly summarize the control of iron homeostasis at the levels of the organism and the cell, and then review the evidence that excess iron is associated with increased diabetes risk, that this relationship is causal, and that excess iron even within the “normal” range has important detrimental effects on insulin secretion, insulin sensitivity, adipokine levels, and metabolic flexibility. Finally, we will consider the molecular mechanisms for these relationships.
Iron homeostasis
Iron plays an essential role as a cofactor for fuel oxidation and electron transport, but it also has the potential to cause oxidative damage if not carefully regulated, chaperoned, and, when in excess, sequestered. Thus, extensive mechanisms to control the uptake and fate of iron have evolved. The connections between iron and metabolism are well established, particularly in lower organisms. Iron entry into cells increases when needed for fuel oxidation, and conversely, the metabolic fate of glucose and ethanol are dependent on the availability of iron. In Saccharomyces cerevisiae both glucose exhaustion and iron limitation trigger iron uptake signaled by Snf1 kinase, the yeast orthologue of AMP-dependent kinase (AMPK) (Haurie et al., 2003). The SWI/SNF chromatin-remodeling complex also controls the induction of iron transport genes in S. pombe (Monahan et al., 2008). Thus, in the shift from fermentative to respiratory glucose metabolism, iron uptake is stimulated to allow metallation of the enzymes and electron carriers necessary for oxidative metabolism.
The regulation of iron metabolism has been extensively reviewed (Andrews and Schmidt, 2007; De Domenico et al., 2008; Ganz, 2011; Hentze et al., 2010) and will be briefly summarized here. This is a current summary of mechanisms and pathways that are still being explored; many details and controversies are not presented because of space constraints. Most iron in mammalian organisms is recycled at a rate of 20–25 mg/day through the erythroid pool as macrophages endocytose senescent erythrocytes. Roughly 5–10% of that amount per day is taken up through the intestine. Mammals do not have the capacity to secrete excess iron in a regulated fashion. In equilibrium, losses through sloughing of the intestinal epithelium, death of other cells, and biliary excretion balance intestinal uptake, but when uptake exceeds loss, excess iron is sequestered intracellularly.
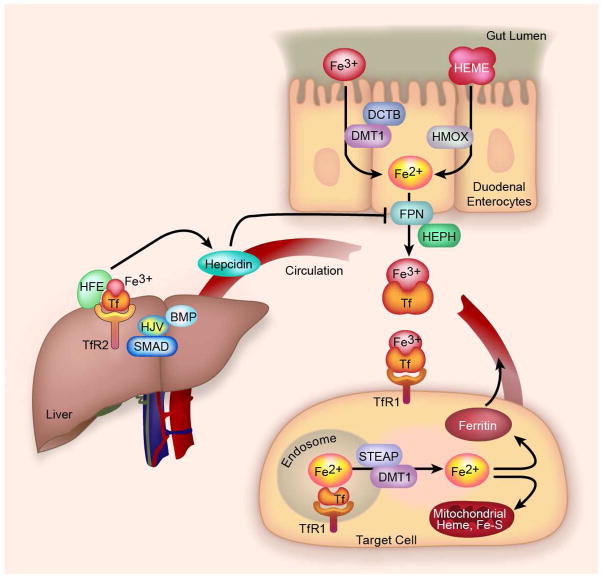
Because disposal of excess iron is usually a slow process in humans, uptake of iron from the intestinal is highly regulated (Fig. 1). In the duodenum, ferric (Fe3+) iron is first reduced to ferrous iron (Fe2+) by the ferrireductase duodenal cytochrome b (DCTB). Ferrous ions enter the cell through the divalent metal-ion transporter 1 (DMT1 or SLC11A2). Iron exits the enterocyte through the only known iron export channel, ferroportin (FPN or SLC40A1). The iron is oxidized to Fe3+ by hephaestin (HEPH) whereupon it binds to transferrin in the circulation. Transferrin bound iron can then be taken into cells by transferrin receptors (TfR), in most cells TfR1. A soluble form of the transferrin receptor bound to transferrin also exists, and its level in serum is a sensitive indicator of functional iron deficiency (Beguin, 2003).
Fig. 1. Overview of iron trafficking.
Intestinal free ferric (Fe3+) iron is reduced to Fe2+ by DCTB and enters the cell through the divalent metal-ion transporter 1 (DMT1) and possibly other carriers. Dietary heme is directly absorbed and iron is released by heme oxygenase (HMOX). Iron exits the enterocyte through the iron export channel ferroportin (FPN). After oxidization by hephaestin (HEPH) iron binds to transferrin (Tf) in the bloodstream which binds to transferrin receptors (TfR)-1 and -2 on the surface of target cells. In most cells, after endocytosis of TfR1 and acidification of the endosome, iron is released, reduced by STEAP, and enters the cytosol through DMT1 where it is used (e.g. for heme or Fe-S-cluster synthesis in the mitochondrion) or if in excess, sequestered by ferritin. Ferritin secreted into the blood serves as a marker for tissue iron stores. In the liver, Tf binds TfR 2 and the protein HFE, and in concert with signaling via GPI-anchored protein hemojuvelin (HJV), bone morphogenic proteins (BMP) and the SMAD signal transduction pathway, production of hepcidin is signaled. Hepcidin induces internalization and degradation of FPN, thus completing a negative feedback regulatory loop.
TfR1 mediates iron uptake in most cells. Upon endocytosis the endosome is acidified and ferric iron is released from transferrin, reduced by the STEAP family of ferrireductases, and enters the cytosol through DMT1, although this may not be the exclusive transporter. Non-transferrin-bound iron can also enter the cells directly through DMT1 and other transporters such as Zip14 (Liuzzi et al., 2006; Mackenzie et al., 2006). The latter mechanisms likely play a major role only when transferrin approaches saturation, that is, in conditions of iron overload. Most of the iron is used in the mitochondrion for heme and iron-sulfur cluster synthesis, although its trafficking to the mitochondrion for such utilization is incompletely understood. Cytosolic iron levels are autoregulated through binding to Iron Regulatory Proteins (IRP). Excess iron releases IRPs from the iron responsive element (IRE) on the 3′-UTR of the TFR1 mRNA and the 5′-UTR of the ferritin mRNA, as well as on the UTRs of mRNAs of several other iron-regulated proteins. This results in decreased stability of TfR mRNA, decreasing further iron uptake, and increased translation of ferritin, sequestering iron inside the cell. Part of the increased ferritin that is translated is secreted, largely iron-free, and serves as a marker of tissue iron stores.
Transferrin-bound iron also interacts with the hepatocyte TfR2 and the protein HFE on the surface of hepatocytes (D’Alessio et al., 2012). Through a signaling process that is still incompletely understood but that also requires hemojuvelin (HJV), bone morphogenic protein (BMP)-6 (Andriopoulos et al., 2009; Meynard et al., 2009), and the SMAD (human homolog of Drosophila mad) pathway (Wang et al., 2005), the production of hepcidin is stimulated. The involvement of TfR2, HJV, HFE, and hepcidin in human iron homeostasis is demonstrated by human mutations in all that result in iron overload. Hepcidin, a 25 amino acid peptide, enters the systemic circulation and induces internalization and degradation of intestinal epithelial ferroportin, thus acting as a negative feedback regulator of iron absorption (Nemeth et al., 2004b). Hepcidin also regulates efflux of iron from other cells that express high levels of ferroportin including macrophages. Although iron egress from the enterocyte is the major control point for entry of iron into the body, DMT1 is also regulated by iron- and possibly hepcidin-dependent mechanisms and by the hypoxia-inducible transcription factor HIF-2α (Mastrogiannaki et al., 2009; Shah et al., 2009). Dietary heme is also directly absorbed by the enterocyte through less defined pathways (Shayeghi et al., 2005). Release of iron from heme is accomplished by heme oxygenase (HMOX).
An important fact to consider in evaluating the effects of iron on metabolism is the very wide range of “normal” serum ferritin in humans, 30–300 ng/mL in men and 15–200 ng/mL in women (Fleming et al., 2001; Nelson et al., 1978). The levels of very few human blood constituents have such a 10-fold normal variation, suggesting the possibility that “normal” may not be ideal. Despite the extensive regulation of iron uptake, it is possible with dietary iron excess to achieve levels of tissue iron higher than are necessary to maintain normal erythropoiesis and metabolic function. Commercial “normal” rodent chows vary by a factor greater than ten in iron content. More important than the absolute levels of iron is its bioavailability, but when all factors are considered, many normal chows deliver significantly higher amounts of iron than are consumed by mice living in the wild or are necessary to maintain normal breeding and blood hemoglobin concentrations. The same is true of the diets of many humans, particularly in affluent Western cultures. Thus, the results to be presented below suggest that within the boundaries of tissue iron levels defined by overt iron deficiency and pathologic overload, the broad range of “normal” iron may, in fact, include levels that confer health risks of which we are currently unaware.
Iron homeostatic pathways are tightly linked to inflammatory stressors. Inflammation causes significant up regulation of hepcidin, largely through IL-6, and also results in large increases in serum ferritin levels (reviewed in (Ganz and Nemeth, 2009)). The fact that inflammation results in the suppression of intestinal iron uptake has been hypothesized to be related to the beneficial effect of sequestering iron from invading microbes. It may also be important in elucidating the link of iron to diabetes that in turn is linked to inflammation (Hotamisligil, 2006). The relationships among diabetes, inflammation, and ferritin, therefore, could be complex, with ferritin either reflecting excess iron stores that cause diabetes, or reflecting inflammation that causes diabetes, or both. Furthermore, if iron causes diabetes, one of the mechanisms could be through its ability to cause oxidant stress that may be linked to inflammation. We will first consider the evidence that suggests that iron overload is sufficient to cause diabetes.
Diabetes associated with iron overload in pathologic states: β-cell failure and insulin sensitivity
The first and clearest evidence for a relation between iron and human diabetes came from clinical observations of individuals with pathologic iron overload. These included cases of hereditary hemochromatosis (HH) (Buysschaert et al., 1997; Moirand et al., 1997) as well as transfusional iron overload (Dmochowski et al., 1993; Merkel et al., 1988). The best-studied example of the latter is beta thalassemia major, although diabetes is also a complication of other conditions requiring frequent or long-term transfusion such as bone marrow transplantation (Baker et al., 2007). Some rare causes of diabetes such as Friedreich ataxia are also associated with disturbances of iron balance and with mutations in proteins regulating iron metabolism (Radisky et al., 1999). Below we describe the phenotypes of these iron overload conditions.
Hereditary hemochromatosis
HH is transmitted as an autosomal recessive trait and occurs in approximately five per thousand Caucasians of Northern European descent (Pietrangelo, 2010). Most patients with hemochromatosis are homozygous for a mutation in the HFE gene resulting in the C282Y substitution in the HFE protein (Feder et al., 1996). Mutations in TfR2, HJV, and hepcidin are rarer causes of hemochromatosis (Pietrangelo, 2010). Normal HFE is partially required for iron stimulation of hepcidin (Nemeth et al., 2004a), and in the absence of HFE protein hepcidin expression is reduced and iron absorption is inappropriately high for a given body iron load (Nemeth et al., 2004b). The high prevalence of HFE mutations argues that it might have served an adaptive function, preventing a restriction on iron uptake in populations evolving in locations without consistent access to dietary iron (Toomajian et al., 2003). HH was originally described as a triad of diabetes, cirrhosis, and skin pigmentation. In relatively small clinical studies not well controlled for selection bias, the prevalence of diabetes in hemochromatosis had been found to be 7–40% (Buysschaert et al., 1997; Moirand et al., 1997). With identification of the genetic cause of HH, however, it was possible to perform more unbiased screens for diabetes prevalence. Recent studies show the prevalence of diabetes to be 13–22% and impaired glucose tolerance 18–30% (Hatunic et al., 2010a; McClain et al., 2006). Of note, HH is largely a disease of individuals of Northern European descent, wherein the background prevalence rate of diabetes is only ~5–10%. Other studies have not found increased diabetes prevalence in C282Y homozygotes (Beutler et al., 2002), although in that case comparing homozygotes to a control population that was of mixed racial/ethnic descent might have skewed the baseline diabetes prevalence.
The pathophysiology of diabetes associated with HH is controversial, with evidence suggesting that both insulin deficiency and insulin resistance are contributing factors (Hramiak et al., 1997; Mendler et al., 1999). Some of this work is difficult to interpret because subjects with established diabetes are studied, in which case the attendant hyperglycemia may itself have resulted in insulin resistance and insulin secretory abnormalities (Rossetti et al., 1990). In the author’s study of this question, when only HH subjects with prediabetes are considered, those subjects differ significantly from controls only in terms of insulin secretory capacity and in fact had a trend toward increased insulin sensitivity (McClain et al., 2006). The subjects with overt diabetes exhibit insulin resistance, but the great majority of those individuals (80%) are also obese. One interpretation of these data is that HH itself is diabetogenic mainly because of decreased insulin secretion, and diabetes usually results when insulin resistance from an independent mechanism such as obesity intervenes. Individuals with HH cannot respond with increased insulin secretion because of primary pathology in the β-cells, and therefore are highly prone to develop diabetes when insulin resistant (McClain et al., 2006). Consistent with this hypothesis, insulin secretory abnormalities but not insulin sensitivity improve when persons with HH undergo phlebotomy (Abraham et al., 2006; Hatunic et al., 2010b).
Study of mouse models of HH, namely mice with targeted deletion of Hfe or replacement of deleted wild type Hfe with a gene encoding the C282Y mutant, have contributed to our understanding of the mechanisms involved in diabetes associated with HH (Cooksey et al., 2004). Hfe−/− mice exhibit decreased insulin secretory capacity secondary to oxidative stress, decreased glucose-stimulated insulin secretion, and increased β-cell apoptosis. These mice have increased insulin sensitivity, however, and do not develop diabetes, supporting the hypothesis that insulin resistance is a causal but secondary factor in HH diabetes. Study of these mice has provided further mechanistic insights. For example, the observed oxidative stress in islets and other tissues is not only caused directly by the generation of free radicals from iron reacting with hydrogen peroxide (Fenton chemistry). In addition, iron interferes with the trafficking of other transition metals. Mitochondrial uptake of manganese (Mn2+) is inhibited, resulting in decreased metallation and activity of superoxide dismutase 2 (SOD2), and much of the oxidant damage can be ameliorated by Mn supplementation(Jouihan HA, 2008). Thus, the effect of excess iron on the levels and trafficking of other metals is an important but understudied area.
Beta thalassemia major and transfusional iron overload
Thalassemia is a group of disorders characterized by deficient production of the β-globin subunit of hemoglobin (Weatherall, 1998). Patients with thalassemia become iron overloaded because of the numerous transfusions required to maintain adequate erythrocyte levels as well as from increased iron absorption (Weatherall, 1998). A single unit of blood contains up to 100 times the amount of iron that enters the circulation daily through the gut. The prevalence of diabetes in patients with thalassemia is 6–14% (Borgna-Pignatti et al., 2004; Vogiatzi et al., 2009). Several studies have shown that insulin resistance and insulin deficiency mark both the prediabetic state and diabetes in thalassemia (Dmochowski et al., 1993; Merkel et al., 1988; Messina et al., 2002). Insulin secretory defects, however, may appear earlier than insulin resistance (Jaruratanasirikul et al., 2008). The existence of insulin sensitivity in HH but insulin resistance in transfusional iron overload is likely explained by the different tissues in which the excess iron accumulates in situations of low hepcidin (HH) compared to high hepcidin (thalassemia patients with iron overload). The causal role of iron in the pathogenesis of diabetes is suggested by the declining rates of diabetes since the more aggressive and widespread use of iron chelation therapy (Borgna-Pignatti et al., 1998; Gamberini et al., 2008).
Transfusions for other conditions are also associated with increased diabetes risk. A relatively young cohort of survivors of pediatric bone marrow transplantation, for example, had a 5% prevalence of type 2 diabetes (Hoffmeister et al., 2004). Another study revealed a prevalence of diabetes three times that of sibling controls (Baker et al., 2007). Interpretation of these studies is complicated, however, by the additional effects of chemotherapeutic agents such as glucocorticoids and asparaginase on insulin production and action.
Effects of iron in nonpathologic states: Diabetes and dietary iron
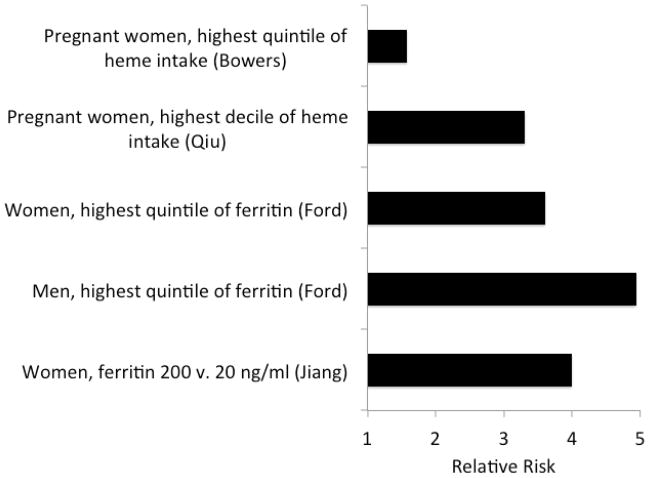
Increased iron stores are also associated with the development of typical type 2 diabetes [reviewed in (Fernandez-Real et al., 2002a)]. For example, in 9,486 U.S. adults studied as part of the National Health and Nutrition Education Survey (NHANES), the odds ratios for newly diagnosed diabetes in those with elevated serum ferritin levels are 4.94 for men and 3.61 for women (Ford and Cogswell, 1999). This iron-associated risk approaches the relative risk engendered by obesity (Kriska et al., 2003). Similar relationships between iron and diabetes risk, insulin resistance, or both have emerged from studies of other populations including Europeans and African-Americans (Jiang et al., 2004; Tuomainen et al., 1997; Wilson et al., 2003), and from other conditions including gestational diabetes (Afkhami-Ardekani and Rashidi, 2009) and prediabetes (Sharifi et al., 2008). The strong relationship between ferritin and diabetes risk, across sexes and in different types of diabetes (type 2 and gestational), even across the normal range of ferritin, is evident in Fig. 2 (Jiang et al., 2004). Recent data from the NHANES population also demonstrate that high ferritin approximately doubles the risk for metabolic syndrome after accounting for age, race, alcohol, smoking, and inflammatory state as assessed by C-reactive protein (CRP) levels (Jehn et al., 2004). High ferritin is also positively correlated with central adiposity (Gillum, 2001; Iwasaki et al., 2005), hepatic steatohepatitis (Dongiovanni et al., 2011), and cardiovascular disease (Iwasaki et al., 2005; Qi et al., 2007).
Fig. 2. Relative risk of diabetes as a function of ferritin or dietary iron intake in four studies.
Plotted are the relative risks from four studies (Bowers et al., 2011) (Qiu et al., 2011) (Ford and Cogswell, 1999) (Jiang et al., 2004), with the comparator groups listed on the y-axis.
Type 2 diabetes is a disease marked by chronic inflammation (Hotamisligil, 2006), and ferritin increases with inflammation. The question therefore arises whether high iron, whose best biomarker is high ferritin, causes diabetes or diabetes causes high ferritin. Several lines of evidence support the former causality. In the study of metabolic syndrome quoted above, for example, independent markers of inflammatory stress such as CRP did not account for the association of ferritin with diabetes (Jehn et al., 2004). Other studies have also concluded that the diabetes risk associated with high iron is not accounted for by HH or inflammation but rather is related to dietary iron overload (Fleming et al., 2001; Fleming et al., 2002). Two recent studies of gestational diabetes have observed that the increased risk is associated in particular with dietary heme iron, which is more efficiently absorbed than non-heme iron (Bowers et al., 2011; Qiu et al., 2011). The best evidence for the causality of iron, however, is those studies in which reversal of diabetes occurs with iron reduction (below).
Differences between the diabetes phenotype of hemochromatosis and dietary or transfusional iron overload: Role of adiponectin
An important dichotomy is evident when considering the phenotype of individuals with hemochromatosis compared to those with typical type 2 diabetes. Namely, in both humans and in the mouse models, pre-diabetic HH is associated with lower insulin levels but enhanced insulin sensitivity (McClain et al., 2006). The typical phenotype of subjects type 2 diabetes and those with dietary and transfusional iron overload, however, is one of insulin resistance (Fernandez-Real et al., 2002a).
Mouse models (Cooksey et al., 2004) and humans with HH exhibit significantly higher levels of adiponectin (Gabrielsen et al., 2012). Adiponectin is an adipokine, a secreted, hormone-like protein produced by adipocytes and causally linked to insulin sensitivity (Kubota et al., 2002). In contrast to HH, mice with dietary iron overload have lower adiponectin levels than mice on lower iron chows (Gabrielsen et al., 2012). This finding is explained by the high ferroportin expression in adipocytes (Gabrielsen et al., 2012). In the limited tissues expressing high levels of ferroportin such as macrophages, decreased hepcidin in HH results in increased ferroportin expression, iron export, and, therefore, decreased iron levels despite total body iron overload (Brink et al., 1976; Cairo et al., 1997; Knutson et al., 2005).
In dietary iron overload, by contrast, hepcidin levels rise and iron accumulates in cells that express ferroportin, including adipocytes, because ferroportin is down regulated Thus, in healthy non-HH populations, there is a negative relationship between serum ferritin and adiponectin (Fargnoli et al., 2008; Forouhi et al., 2007; Gabrielsen et al., 2012; Mojiminiyi et al., 2008). A recent large study has also demonstrated that not only is ferritin inversely associated with adiponectin, but that both of these markers are associated with adipocyte insulin resistance as defined by elevated serum nonesterified fatty acid levels despite elevated insulin (Wlazlo et al., 2012). Inflammation increases ferritin and decreases adiponectin production (Subauste and Burant, 2007), and adipose tissue is inflamed in diabetes (Trayhurn and Wood, 2005), but this does not fully account for the ferritin-adiponectin relationship. We have determined, for example, that normal-range serum ferritin levels are strongly and inversely associated with adiponectin independently of inflammation as assessed by serum levels of CRP, IL-6, or TNFα (Gabrielsen et al., 2012). These observations were made in large and independent cohorts of subjects with type 2 diabetes and of obese subjects with diabetes compared to equally obese individuals without metabolic syndrome. Ferritin predicts adiponectin levels significantly better than body mass index. The relationship of ferritin to adiponectin is weaker in females, likely because the range of ferritin values is significantly narrower and lower in females than in males. The data suggest that ferritin may be one determinant of the higher adiponectin levels seen in females (Arita et al., 1999) and provide a potential contributing reason for the decreased incidence of diabetes in women prior to menopause (Szmuilowicz et al., 2009).
The use by adipocytes of iron levels to regulate adiponectin suggests an endocrine role for adipocytes in coordinating organism-wide metabolic responses to iron availability, as they do for responses to overall macronutrient status. There is other evidence for crosstalk between iron and adipocyte metabolism. Insulin treatment, for example, increases iron uptake by increasing cell surface expression of TfR1 in adipocytes (Davis et al., 1986; Tanner and Lienhard, 1987). Iron induces lipolysis in cultured adipocytes and modulates the lipolytic response to norepinephrine (Rumberger et al., 2004; Yamagishi et al., 2000). Adipocytes express not only common regulators of iron homeostasis such as ferritin and iron regulatory proteins (Festa et al., 2000), but also iron-related proteins with restricted tissue expression (TfR2, HFE, hepcidin). (Bekri et al., 2006; Farahani et al., 2004). In addition, expression of high levels of ferroportin in adipocytes allows them to serve an iron sensing function with greater dynamic range and sensitivity, as reflected by the blunting of adiponectin response to dietary iron in animals with targeted deletion of the ferroportin gene in adipocytes (Gabrielsen et al., 2012).
Reduction of iron as a treatment for diabetes
The previous data lead to the prediction that decreasing iron stores in dietary iron excess will be doubly beneficial to diabetes risk, increasing both insulin secretion, as in the studies of HH, and insulin sensitivity, through mechanisms that include increasing adiponectin. The β-cell will have similar response and susceptibility to iron overload in both HH and dietary overload because of low or absent ferroportin (Hudson et al., 2010). In addition, β-cells may be especially sensitive to iron because of high expression of DMT1 (Koch et al., 2003), which is needed for import of zinc for secretory packaging, but can also transport free serum iron whose levels will increase as transferrin approaches higher levels of iron saturation. Beneficial effects of phlebotomy have been demonstrated in a number of animal models of type 2 diabetes. Otsuka Long-Evans Tokushima Fatty (OLETF) rats that are phlebotomized or fed an iron deficient diet exhibit lower hemoglobin A1c levels than controls (Minamiyama et al., 2010). Similar results are seen in the leptin-deficient Ob/Ob model of type 2 diabetes wherein low iron diets or iron chelators result in significant protection from diabetes that is related both to increased insulin secretion and sensitivity (Cooksey et al., 2010). In the latter study, the effects of lowering iron are long lasting and reversible, and importantly are seen with levels of iron restriction that did not result in iron-deficiency anemia. Supporting these observations, iron-deficient veal calves and rats are more insulin sensitive than iron sufficient controls and exhibit increased glucose utilization (Borel et al., 1993; Henderson et al., 1986; Hostettler-Allen et al., 1993).
Somewhat surprisingly, human data on the effects of iron depletion in common type 2 diabetes are scant. In relatively small and short-term studies of non-HH subjects either with or without known type 2 diabetes, phlebotomy improves insulin sensitivity, insulin secretion, and glycemia (Facchini, 1998; Fernandez-Real et al., 2002b). Blood donors also exhibit increased insulin sensitivity and secretion, although there may be selection bias in these studies in terms of the population that donates blood (Fernandez-Real et al., 2005). The study on the relation of iron to adiponectin, summarized in the previous section, also included a proof-of-concept study of a very small sample of prediabetic humans in which phlebotomy was performed to bring the individuals from the highest quartile of normal ferritin down to the lowest quartile(Gabrielsen et al., 2012). This intervention improved adiponectin, the area under the glucose curve, and the insulin disposition index, which is the product of insulin secretion and insulin sensitivity and a useful predictor of diabetes risk.
Phlebotomy also improves other factors associated with diabetes and the metabolic syndrome including hypertriglyceridemia (Bofill et al., 1994), vascular reactivity (Fernandez-Real et al., 2002c), and markers of non-alcoholic steatohepatitis (NASH) (Facchini et al., 2002). More recently, phlebotomy of 550–800 mL of blood (1–1.5 Units) from individuals with metabolic syndrome was shown to decrease blood pressure, fasting glucose, HbA1c, and the ratio of LDL to HDL cholesterol when measured 6 weeks after the first phlebotomy (Houschyar et al., 2012). There was a trend toward an increase in insulin sensitivity that was not significant (p=0.28). As a cautionary note, however, the individuals in the NASH study gained weight with blood donation, and the OLETF rats on the iron deficient diet had higher blood triglyceride and cholesterol levels (Minamiyama et al., 2010). Both the weight gain and increased blood lipids are consistent with the effects of iron on fat metabolism and energy expenditure, and the phenotypes of those with overt iron deficiency, to be described below. Larger and longer-term studies are required in humans to address both the beneficial and potential adverse effects of iron depletion, their dose-responsiveness, and their longevity.
Iron, obesity and lipid metabolism
Most investigations into the effects of iron on metabolism have focused on glucose metabolism because of the important epidemiologic connections between pathologic iron overload and diabetes. These connections have been evident since the early descriptions of hemochromatosis and its subsequent link to tissue iron overload in the 19th century. There is evidence, however, that iron also affects lipid metabolism, adipocyte biology, and obesity. This is not surprising, given the effects of iron on adiponectin, the requirement for iron in the oxidation of lipids, and the potential additive effects of oxidative stress from lipid metabolism and excess iron.
There is a greater prevalence of iron deficiency in obese (39%) and overweight (12%) children and adolescents than normal weight children whose prevalence of iron deficiency is only 4% (Pinhas-Hamiel et al., 2003). The association of iron deficiency with obesity has been confirmed in other populations that include children and adults of both sexes (Cepeda-Lopez et al., 2011; Yanoff et al., 2007). The immediate question that arises is one of causality: The observations might simply be coincidental, for example the diets of many obese individuals may be enriched in iron-poor foods. Plausible cases for causality, in turn, can be made in both directions: Normal or high iron stores may be needed to support higher rates of fatty acid oxidation so that iron deficient individuals are less able to mobilize and use high fat, or conversely, the inflammatory nature of obesity might trigger increased hepcidin levels that limit dietary iron absorption. Support for the latter hypothesis comes from an study that observed a 2-fold increase in the prevalence of iron deficiency in obese Mexican women and children compared to lean controls (Cepeda-Lopez et al., 2011). In this study it was not dietary iron content but the inflammatory marker CRP, which was higher in the obese group, that was the best predictor of iron deficiency. Another study found BMI to be associated with low serum iron but high ferritin, consistent with low-grade inflammation in obesity causing iron sequestration, perhaps mediated by hepcidin(Ausk and Ioannou, 2008). Other studies have found patterns both of primary iron deficiency and of inflammation-mediated iron sequestration in obese adults (Yanoff et al., 2007). Thus, the iron phenotype of obese individuals is likely heterogeneous.
Animal studies demonstrate that iron restriction can be a primary and independent cause of adiposity. Namely, rats fed an iron deficient diet have increased fat mass, although there is a compensating loss of lean mass such that obesity per se does not develop (McClung et al., 2008). More recently, adipocyte-restricted over expression of the protein mitoNEET, which sequesters iron sulfur clusters, was shown to lower adipocyte heme and mitochondrial iron and result in extreme obesity in Ob/Ob mice (Kusminski et al., 2012). The obesity, however, is well tolerated, and the mice remain insulin-sensitive and nondiabetic. The phenotype is reminiscent of the “healthy obese” without metabolic syndrome who have lower ferritin levels that those with metabolic syndrome (Gabrielsen et al., 2012). Other studies have revealed further layers of complexity in the relationship between iron and obesity, for example an obesogenic high fat diet can cause decreased iron absorption in the gut, mediated by dysregulation of the oxidoreductases DCTB and hephaestin (Sonnweber et al., 2012).
The possible mechanisms underlying weight regulation by iron are therefore likely to be multifactorial. Iron is required for lipolysis in cultured adipocytes (Rumberger et al., 2004), and this may contribute to the observations that rats fed an iron-deficient diet (Stangl and Kirchgessner, 1998) and iron-deficient women (Ozdemir et al., 2007) have lower plasma levels of triglycerides. Another contributing factor to lower triglycerides is that high levels of iron augment fatty acid oxidation as was shown in the HH mouse model (Hfe−/−) (Huang et al., 2011). On normal chow, although Hfe−/− mice exhibit increased glucose uptake in skeletal muscle, glucose oxidation is decreased and the ratio of fatty acid to glucose oxidation is increased. When put onto a high fat diet, the Hfe−/− mice exhibit increased fatty acid oxidation, are hypermetabolic, and are protected from obesity. In addition to promoting lipid oxidation, high dietary iron also stimulates lipogenesis (Baquer et al., 1982), so the net effects of iron on lipid metabolism are complex.
In addition to direct modulation of lipid metabolism pathways, the finding that iron regulates adiponectin secretion suggests the possibility that iron availability might also be used to coordinate metabolism by regulating other hormones important to fuel and energy homeostasis. Indeed, levels of the anorexigenic hormone leptin are low in thalassemia patients (Chaliasos et al., 2010), although this would be expected to contribute to greater caloric intake, not leanness, in iron-overloaded individuals. The effects of iron on adiponectin also cannot be simply reconciled with the actions of iron to increase fatty acid oxidation rates, since adiponectin promotes fatty acid oxidation but is decreased by iron. The decrease in adiponectin could be seen as a mechanism to compensate for the fact that iron can stimulate fatty acid oxidation directly (see below), thus protecting the organism from additive oxidative stressors. Clearly much more basic knowledge on the effects and mechanism of action of iron on these diverse pathways in multiple tissues is needed before we will understand the complex phenomena. Finally, an understanding of why both low and high iron are associated with obesity is needed: Is there a modal dose-response curve for some of these effects, and are they related to independent and distinct mechanisms at the two ends of the spectrum of tissue iron levels?
Potential molecular mechanisms for iron regulation of glucose metabolism
The mechanisms underlying the effects of altering tissue iron on metabolism are just beginning to be understood. Given the many effects in multiple tissues already described, and the involvement of iron and heme in processes as diverse as glucose and fat oxidation, hypoxia sensing (Semenza, 2012), CO and NO sensing, transcriptional regulation (Yin et al., 2007), generation of reactive oxygen species, and regulation of hormone levels, the effects of iron are likely to be protean. Furthermore, the effects will have dose thresholds that will differ across the range from iron deficiency, through the broad range of normal, to iron excess.
Reactive Oxygen Species (ROS)
Iron is capable of generating hydroxyl radicals from peroxide and can also inhibit antioxidant defenses such as SOD2 (Jouihan HA, 2008), although it is worth noting that iron deficiency is also associated with increased ROS (Walter et al., 2002). High iron has been linked to oxidative damage to DNA, lipids, and proteins that in turn has been implicated in cardiovascular disease, diabetes, atherosclerosis, and neurological degeneration as seen in Alzheimer’s (Jomova and Valko, 2011). In the progression of diabetes ROS can cause both β-cell failure and insulin resistance. β-cells are particularly sensitive to ROS because of low expression of antioxidants such as catalase and SOD2, overexpression of which have been shown to increase β-cell viability (Azevedo-Martins et al., 2003; Lenzen et al., 1996). ROS can cause β-cell dysfunction by multiple mechanisms including decreased insulin gene expression secondary to decreased expression of transcription factors necessary for β-cell differentiation, maintenance, and insulin gene transcription (Kaneto et al., 2010). ROS have also been reported to directly affect circulating human insulin by hydroxylation of phenylalanine residues that results in lower affinity binding to the insulin receptor (Montes-Cortes et al., 2010). Finally, ROS can induce insulin resistance through multiple mechanisms, for example activation of FOXO1 which prevents down regulation of gluconeogenesis even in the presence of insulin signaling (Ponugoti et al., 2012).
The number of other potential targets of ROS that could contribute to diabetes is immense. It should be kept in mind that low levels of ROS also act as physiologic and normal cell signaling molecules that mediate cellular differentiation, survival, and metabolism (Ray et al., 2012). In mammals, for example, ROS generated by the electron transport complex III is released into the intermembrane space of mitochondria where it can escape to the cytosol and activate PPARγ, C/EBPα, and adipocyte differentiation (Tormos et al., 2011). Other ROS signaling targets include MAPK and PI3K pathways wherein oxidation of cysteines in protein phosphatases results in activation (Ray et al., 2012). Thus, ROS generated by intermediate levels of iron may play a role in normal metabolic regulation, once again emphasizing the theme that the transition between signaling normal physiologic responses and pathophysiologic ones may be a subtle one and that the effects of iron are likely to be highly context-dependent. Finally, it should be mentioned that iron is also capable of generating reactive nitrogen species that are also capable of triggering modification of macromolecules (Nappi and Vass, 1998).
Hypoxia inducible factors
Hypoxia inducible factors (HIF)-1 and -2 regulate cellular response to low oxygen by up regulating transcription of a diverse set of proteins involved in angiogenesis, erythropoiesis, and glycolytic flux (reviewed in (Semenza, 2012)). HIFs also regulate iron metabolism, and under conditions of low iron HIF-2 up regulates DMT-1 and DCYTB, while HIF-1 up regulates DMT-1 and decreases ferritin (Romney et al., 2011; Shah et al., 2009). Conversely, cellular iron levels regulate HIF protein levels through control of prolyl hydroxylase (PHD) activity (Semenza, 2012). PHDs require iron and oxygen to hydroxylate HIF subunits to target them for degradation by von Hippel-Lindau (VHL) E3 ubiquitin ligase. Under conditions of low iron or low oxygen PHDs are inactive and HIF is stabilized. As a result, β-cells treated with an iron chelator increase Hif1α protein, while iron treatment decreases Hif1α (Cheng et al., 2010). Decreased Hif1α results in down regulation of its targets including the glucose transporters Glut1 and Glut2, which in turn results in impaired glucose sensing and glucose-stimulated insulin secretion (Cheng et al., 2010). Mice with targeted deletion of Vhl in β-cells develop basal hypoglycemia with increased insulin. When challenged with glucose, however, they have impaired glucose tolerance due to impaired glucose stimulated insulin secretion (Zehetner et al., 2008). The latter effect is explained by increased Hif-activated glucose uptake and glycolysis stimulating higher levels of ATP production and insulin secretion in the basal state, whereas with rising glucose there is less glucose oxidation resulting in less ATP and insulin secretion than in normal cells.
HIFs are also known to regulate energy utilization in muscle, liver, and fat by stimulating glycolysis and cellular glucose uptake to allow for a shift to glycolytic metabolism. Specifically, Hif-1α up regulates glucose transporters GLUT1 and GLUT3, and increases expression of hexokinase 1 and 2 (Aragones et al., 2009). Hypoxia impacts insulin signaling in adipocytes by reducing the level of insulin receptor phosphorylation (Regazzetti et al., 2009). HIF also plays a role of increasing the inflammatory state of adipose tissue and increasing fibrosis through a HIF target enzyme lysyl oxidase (Halberg et al., 2009). Targeted disruption Hif-1α or Hif-1β in adipose tissue improves insulin signaling and decreases fat mass even when the mice are challenged with a high fat diet (Jiang et al., 2011). The integration of these and other effects of hypoxia on metabolism is seen in a disease of chronic pseudohypoxia, namely Chuvash polycythemia. Patients with Chuvash polycythemia are homozygous for a mutation in VHL that prevents HIF degradation, allowing it to be constitutively active(Ang et al., 2002). These patients have decreased circulating glucose, increased Glut1, and decreased hemoglobin A1c (McClain DA, 2012).
AMP-activated protein kinase (AMPK)
The AMPK pathway controls energy balance by sensing cellular energy status through its activation by an elevated ratio of AMP to ATP (Hardie, 2011). A large variety of hormonal signals and metabolic stresses such as glucose deprivation, ischemia, hypoxia, oxidative stress, and hyperosmotic stress activate AMPK, although not all of these work through altering the AMP:ATP ratio (Kahn et al., 2005). Activated AMPK stimulates glucose uptake and fatty acid oxidation in tissues and suppresses gluconeogenesis in liver (Long and Zierath, 2006). These are pathways that play important roles in the pathogenesis and treatment of diabetes and are also pathways that are affected by iron. Close co-regulation of iron levels and metabolic parameters such as fuel preference is conserved from yeast to mammals and in yeast this co-regulation is mediated by the orthologue of AMPK, Snf1 (Haurie et al., 2003; Shakoury-Elizeh et al., 2010)
As described above, Hfe−/− mice demonstrate increased adiponectin concentrations. One of the principal targets mediating adiponectin action, AMPK, is activated in Hfe−/− mice (Huang et al., 2007), but iron can also activate AMPK independently of adiponectin. Although the mechanism for this activation is not completely understood, plausible candidates include: (1) Alterations in AMP/ATP ratios caused by iron-induced mitochondrial dysfunction (Jouihan HA, 2008), or; (2) Stimulation of the upstream activating kinase of AMPK, LKB1, by SIRT1-mediated deacetylation (Hou et al., 2008; Lan et al., 2008). SIRT1 is itself activated by oxidant stress and/or NAD (Alcendor et al., 2007; Brunet et al., 2004). These results serve further to illustrate the complexity of the association of iron and diabetes risk in that the effects of AMPK activation by iron on glucose disposal, gluconeogenesis, and lipid oxidation are generally antidiabetic. Thus, the integration of the pleiotropic effects of iron on diabetes risk in multiple tissues is not yet fully understood.
Iron responsive elements
IRPs maintain cellular iron homeostasis through iron regulated binding to IREs [Reviewed in (Anderson et al., 2012)]. IRP1 is known to have dual functions, both as an iron sensor and as a cytosolic aconitase when bound to iron sulfur clusters. In the mitochondrion aconitase functions in the TCA cycle where it catalyzes the reversible isomerization of citrate and isocitrate, but in the cytosol the production of citrate generates a substrate for ATP-citrate lyase. Cytosolic aconitase is also important in reducing NADP+ to NADPH, a cofactor for enzymes involved in synthesis of glutathione, lipids, and cholesterol (Minard and McAlister-Henn, 2005). Mitochondrial aconitase is sensitive to iron because it contains four iron sulfur clusters and is down regulated by IRPs in low iron conditions (Eisenstein and Ross, 2003). Thus, IRPs not only control cellular iron metabolism but IRP-dependent regulation also has far reaching metabolic effects through control of mitochondrial and cytosolic citrate levels. Abnormal IRP1 activity is seen in patients with Friedreich ataxia (Condo et al., 2010; Lobmayr et al., 2005), a human degenerative neuromuscular disease caused by a mutation in a mitochondrial protein, frataxin, which is thought to be an iron chaperone or storage protein (Babcock et al., 1997). In these patients IRP1 aconitase activity is decreased, while IRP binding to IREs is increased. Interestingly, Friedreich ataxia is associated with increased prevalence of type 2 diabetes.
Epigenetic effects of iron
The Dutch Famine Birth Cohort Study’s finding of increased prevalence of type 2 diabetes and metabolic syndrome in adults prenatally exposed to extreme caloric restriction has prompted investigations on the epigenetic impact of maternal nutrition on the metabolic state of offspring (Ravelli et al., 1998). Maternal iron intake impacts the metabolic programing of offspring, although this relationship may be complex, with factors such as the period of altered iron status and the direction of change (high or low iron) affecting the phenotype. In rats, prenatal iron deficiency increases susceptibility to high fat diet-induced obesity, glucose intolerance, and hypertension (Bourque et al., 2012). When not challenged with the high fat diet, however, offspring of rat dams with dietary iron restriction have improved glucose tolerance and lower serum triglycerides although they also demonstrate higher systolic blood pressure (Lewis et al., 2001). Thus, the effects of iron on metabolic programming may be diet-dependent, consistent with its effects on mediators such as AMPK. Human studies have also largely focused on maternal iron deficiency, not iron excess, and not in the context of diabetes risk. Anemia during the third trimester of pregnancy lowers systolic pressure in 7-year-old offspring (Brion et al., 2008). In contrast, iron supplementation in the first trimester was associated with increased blood pressure in offspring (Belfort et al., 2008). Collectively these results indicate that dietary iron may be a factor in signaling the fed verse fasted state of an organism, which could signal epigenetic modifications to initiate an altered metabolic state that is compliant with nutritional availability. More research is needed in this field to assess the role of prenatal iron exposure in adult glucose homeostasis.
There are several possible mechanisms for iron control of epigenetic modification including regulation of the sirtuin (Sirt) family of histone deacetylases, the Jumonji C (JmjC)-domain-containing histone demethylases, or both (reviewed in (Houtkooper et al., 2012; Takeuchi et al., 2006)). Sirts couple lysine deacetylation with NAD hydrolysis. Because NAD is limiting for this reaction, it is one pathway through which metabolism can directly affect epigenetics. NAD can be recycled from nicotinamide or synthesized de novo through a pathway rate-limited by nicotinamide phosphoribosyltransferase (NAMPT also known as visfatin). There is a positive association between circulating levels of NAMPT and levels of ferritin and prohepcidin (Fernandez-Real et al., 2007), although the physiologic importance of circulating NAMPT is not fully understood, and prohepcidin levels poorly reflect those of mature hepcidin. Histone demethylation also affects gene expression in a manner dependent on iron and the metabolic state of the cell. The JmjC-domain family of demethylases removes mono- or dimethyl groups through iron- and α-ketoglutarate-dependent oxidation reactions. The importance of iron in this reaction is highlighted by treatment of endothelial cells by desferoxamine, an iron chelator, which decreases demethylase activity in Jmj-domain-containing protein 6 to a similar level as protein knockdown (Boeckel et al., 2011). Interestingly, deletion of JmjC-domain-containing histone demethylase 2 (JHDM2a−/−) in mice leads to obesity hyperlipidemia, hyperinsulinemia, and hyperleptinemia (Inagaki et al., 2009).
The mechanisms outlined in this section do not represent an exhaustive catalogue of all the pathways and mechanisms by which iron may contribute to diabetes risk. For example, one possible mediator of the effects of iron for which evidence is emerging is heme, which is involved in several regulatory nodes. For example:
PGC-1α, a master transcriptional regulator of metabolic programs also regulates heme synthesis (Handschin et al., 2005; Yin et al., 2007);
The regulation of circadian metabolic rhythm, disruption of which is a risk factor for diabetes, is dependent on several heme-containing proteins (Handschin et al., 2005; Yin et al., 2007);
Heme and iron play a role in microRNA processing through regulation of Drosha/Pasha and Dicer microRNA processing complexes (Faller et al., 2007); Li, 2012 #1941}.
Iron is also linked to diabetes through mitoNEET, an iron-sulfur cluster protein and postulated target of the antidiabetic thiazolidinediones (Colca et al., 2004; Wiley et al., 2007). Thiazolidinediones prevent mitochondrial iron accumulation, suggesting that they may act, in part, through regulation of cellular iron stores (Zuris et al., 2012; Zuris et al., 2011). Finally, iron is essential for the multitude of pathways that require heme or iron sulfur clusters for activity including the electron transport chain, TCA cycle, and DNA repair (Rouault and Tong, 2009). These diverse cellular processes illustrate that the relationship between iron and metabolism is complex and more research is needed to further establish how iron overload or depletion may affect these pathways.
Summary
Epidemiologic observations in humans and experimental studies in animal models have established a clear association between tissue iron stores and diabetes risk. A subset of these studies suggests that the relation is causal, that is, high iron is sufficient to cause diabetes. At the same time, it is clear that iron has a multiplicity of effects in many tissues that can be either pro- or anti-diabetic at the ends of the spectrum that runs from iron deficiency to iron excess. In the beta cell, for example, excess iron is toxic, but there is clearly also a minimum level required for full metallation of the proteins needed for glucose oxidation and glucose sensing. Likewise, although iron overload is associated with diabetes risk, iron deficiency is associated with another major risk factor for diabetes, obesity. The phenotypes of iron excess and obesity are certainly not mutually exclusive, however, and it may be in fact that the combination of obesity and iron overload is particularly prone to causing diabetes through its resulting combination of insulin deficiency and insulin resistance. It is likely also the case that the full expression of these effects depends on environmental and genetic factors. For example, high iron-induced augmentation of fat oxidation may be protective of obesity in individuals consuming diets with modest fat content but may cause excessive oxidant stress and concomitantly greater diabetes risk in individuals consuming a high fat diet. Clearly much work is needed to address many unanswered questions such as:
Can long lasting protection or cure of diabetes be afforded to individuals with high iron, and if so, what are optimal levels of tissue iron and how may they be assessed?
What is the effect of the full range of “normal” iron concentrations on diverse metabolic processes in tissues including liver, fat, muscle, pancreas, gut, and brain?
What are the molecular mechanisms for these effects and can they be targeted by approaches other than changing global iron levels?
How might this knowledge be relevant to other diseases with important components of both metabolism and redox signaling/stress including cancer and neurodegenerative diseases?
Do the known polymorphisms in genes regulating iron uptake, antioxidant defenses, and related regulatory pathways modify diabetes risk in humans?
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham D, Rogers J, Gault P, Kushner J, McClain D. Increased insulin secretory capacity but decreased insulin sensitivity after correction of iron overload by phlebotomy in hereditary haemochromatosis. Diabetologia. 2006;49:2546–2551. doi: 10.1007/s00125-006-0445-7. [DOI] [PubMed] [Google Scholar]
- Afkhami-Ardekani M, Rashidi M. Iron status in women with and without gestational diabetes mellitus. J Diabetes Complications. 2009;23:194–198. doi: 10.1016/j.jdiacomp.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Alcendor RR, Gao S, Zhai P, Zablocki D, Holle E, Yu X, Tian B, Wagner T, Vatner SF, Sadoshima J. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res. 2007;100:1512–1521. doi: 10.1161/01.RES.0000267723.65696.4a. [DOI] [PubMed] [Google Scholar]
- Anderson CP, Shen M, Eisenstein RS, Leibold EA. Mammalian iron metabolism and its control by iron regulatory proteins. Biochim Biophys Acta. 2012 doi: 10.1016/j.bbamcr.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews NC, Schmidt PJ. Iron homeostasis. Annual review of physiology. 2007;69:69–85. doi: 10.1146/annurev.physiol.69.031905.164337. [DOI] [PubMed] [Google Scholar]
- Andriopoulos B, Jr, Corradini E, Xia Y, Faasse SA, Chen S, Grgurevic L, Knutson MD, Pietrangelo A, Vukicevic S, Lin HY, Babitt JL. BMP6 is a key endogenous regulator of hepcidin expression and iron metabolism. Nat Genet. 2009;41:482–487. doi: 10.1038/ng.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang SO, Chen H, Hirota K, Gordeuk VR, Jelinek J, Guan Y, Liu E, Sergueeva AI, Miasnikova GY, Mole D, Maxwell PH, Stockton DW, Semenza GL, Prchal JT. Disruption of oxygen homeostasis underlies congenital Chuvash polycythemia. Nat Genet. 2002;32:614–621. doi: 10.1038/ng1019. [DOI] [PubMed] [Google Scholar]
- Aragones J, Fraisl P, Baes M, Carmeliet P. Oxygen sensors at the crossroad of metabolism. Cell Metab. 2009;9:11–22. doi: 10.1016/j.cmet.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- Ausk KJ, Ioannou GN. Is obesity associated with anemia of chronic disease? A population-based study. Obesity (Silver Spring) 2008;16:2356–2361. doi: 10.1038/oby.2008.353. [DOI] [PubMed] [Google Scholar]
- Azevedo-Martins AK, Lortz S, Lenzen S, Curi R, Eizirik DL, Tiedge M. Improvement of the mitochondrial antioxidant defense status prevents cytokine-induced nuclear factor-kappaB activation in insulin-producing cells. Diabetes. 2003;52:93–101. doi: 10.2337/diabetes.52.1.93. [DOI] [PubMed] [Google Scholar]
- Babcock M, de Silva D, Oaks R, Davis-Kaplan S, Jiralerspong S, Montermini L, Pandolfo M, Kaplan J. Regulation of mitochondrial iron accumulation by Yfh1p, a putative homolog of frataxin. Science. 1997;276:1709–1712. doi: 10.1126/science.276.5319.1709. [DOI] [PubMed] [Google Scholar]
- Baker KS, Ness KK, Steinberger J, Carter A, Francisco L, Burns LJ, Sklar C, Forman S, Weisdorf D, Gurney JG, Bhatia S. Diabetes, hypertension, and cardiovascular events in survivors of hematopoietic cell transplantation: a report from the bone marrow transplantation survivor study. Blood. 2007;109:1765–1772. doi: 10.1182/blood-2006-05-022335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baquer NZ, Hothersall JS, Sochor M, McLean P. Bio-inorganic regulation of pathways of carbohydrate and lipid metabolism. 1. Effect of iron and manganese on the enzyme profile of pathways of carbohydrate metabolism in adipose tissue during development. Enzyme. 1982;27:61–68. doi: 10.1159/000459027. [DOI] [PubMed] [Google Scholar]
- Beguin Y. Soluble transferrin receptor for the evaluation of erythropoiesis and iron status. Clinica chimica acta; international journal of clinical chemistry. 2003;329:9–22. doi: 10.1016/s0009-8981(03)00005-6. [DOI] [PubMed] [Google Scholar]
- Bekri S, Gual P, Anty R, Luciani N, Dahman M, Ramesh B, Iannelli A, Staccini-Myx A, Casanova D, Ben Amor I, Saint-Paul MC, Huet PM, Sadoul JL, Gugenheim J, Srai SKS, Tran A, Le Marchand-Brustel Y. Increased Adipose Tissue Expression of Hepcidin in Severe Obesity Is Independent From Diabetes and NASH. Gastroenterology. 2006;131:788–796. doi: 10.1053/j.gastro.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Belfort MB, Rifas-Shiman SL, Rich-Edwards JW, Kleinman KP, Oken E, Gillman MW. Maternal iron intake and iron status during pregnancy and child blood pressure at age 3 years. International journal of epidemiology. 2008;37:301–308. doi: 10.1093/ije/dyn002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler E, Felitti VJ, Koziol JA, Ho NJ, Gelbart T. Penetrance of 845G--> A (C282Y) HFE hereditary haemochromatosis mutation in the USA. Lancet. 2002;359:211–218. doi: 10.1016/S0140-6736(02)07447-0. [DOI] [PubMed] [Google Scholar]
- Boeckel JN, Guarani V, Koyanagi M, Roexe T, Lengeling A, Schermuly RT, Gellert P, Braun T, Zeiher A, Dimmeler S. Jumonji domain-containing protein 6 (Jmjd6) is required for angiogenic sprouting and regulates splicing of VEGF-receptor 1. Proc Natl Acad Sci U S A. 2011;108:3276–3281. doi: 10.1073/pnas.1008098108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bofill C, Joven J, Bages J, Vilella E, Sans T, Cavalle P, Miralles R, Llobet J, Camps J. Response to repeated phlebotomies in patients with non-insulin-dependent diabetes mellitus. Metabolism. 1994;43:614–620. doi: 10.1016/0026-0495(94)90204-6. [DOI] [PubMed] [Google Scholar]
- Borel MJ, Beard JL, Farrell PA. Hepatic glucose production and insulin sensitivity and responsiveness in iron-deficient anemic rats. Am J Physiol. 1993;264:E380–390. doi: 10.1152/ajpendo.1993.264.3.E380. [DOI] [PubMed] [Google Scholar]
- Borgna-Pignatti C, Rugolotto S, De Stefano P, Piga A, Di Gregorio F, Gamberini MR, Sabato V, Melevendi C, Cappellini MD, Verlato G. Survival and disease complications in thalassemia major. Ann N Y Acad Sci. 1998;850:227–231. doi: 10.1111/j.1749-6632.1998.tb10479.x. [DOI] [PubMed] [Google Scholar]
- Borgna-Pignatti C, Rugolotto S, De Stefano P, Zhao H, Cappellini MD, Del Vecchio GC, Romeo MA, Forni GL, Gamberini MR, Ghilardi R, Piga A, Cnaan A. Survival and complications in patients with thalassemia major treated with transfusion and deferoxamine. Haematologica. 2004;89:1187–1193. [PubMed] [Google Scholar]
- Bourque SL, Komolova M, McCabe K, Adams MA, Nakatsu K. Perinatal iron deficiency combined with a high-fat diet causes obesity and cardiovascular dysregulation. Endocrinology. 2012;153:1174–1182. doi: 10.1210/en.2011-1700. [DOI] [PubMed] [Google Scholar]
- Bowers K, Yeung E, Williams MA, Qi L, Tobias DK, Hu FB, Zhang C. A prospective study of prepregnancy dietary iron intake and risk for gestational diabetes mellitus. Diabetes Care. 2011;34:1557–1563. doi: 10.2337/dc11-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brink B, Disler P, Lynch S, Jacobs P, Charlton R, Bothwell T. Patterns of iron storage in dietary iron overload and idiopathic hemochromatosis. J Lab Clin Med. 1976;88:725–731. [PubMed] [Google Scholar]
- Brion MJ, Leary SD, Smith GD, McArdle HJ, Ness AR. Maternal anemia, iron intake in pregnancy, and offspring blood pressure in the Avon Longitudinal Study of Parents and Children. Am J Clin Nutr. 2008;88:1126–1133. doi: 10.1093/ajcn/88.4.1126. [DOI] [PubMed] [Google Scholar]
- Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- Buysschaert M, Paris I, Selvais P, Hermans MP. Clinical aspects of diabetes secondary to idiopathic haemochromatosis in French-speaking Belgium. Diabetes Metab. 1997;23:308–313. [PubMed] [Google Scholar]
- Cairo G, Recalcati S, Montosi G, Castrusini E, Conte D, Pietrangelo A. Inappropriately High Iron Regulatory Protein Activity in Monocytes of Patients With Genetic Hemochromatosis. Blood. 1997;89:2546–2553. [PubMed] [Google Scholar]
- Cepeda-Lopez AC, Osendarp SJ, Melse-Boonstra A, Aeberli I, Gonzalez-Salazar F, Feskens E, Villalpando S, Zimmermann MB. Sharply higher rates of iron deficiency in obese Mexican women and children are predicted by obesity-related inflammation rather than by differences in dietary iron intake. Am J Clin Nutr. 2011;93:975–983. doi: 10.3945/ajcn.110.005439. [DOI] [PubMed] [Google Scholar]
- Chaliasos N, Challa A, Hatzimichael E, Koutsouka F, Bourantas DK, Vlahos AP, Siamopoulou A, Bourantas KL, Makis A. Serum adipocytokine and vascular inflammation marker levels in Beta-thalassaemia major patients. Acta Haematol. 2010;124:191–196. doi: 10.1159/000320274. [DOI] [PubMed] [Google Scholar]
- Cheng K, Ho K, Stokes R, Scott C, Lau SM, Hawthorne WJ, O’Connell PJ, Loudovaris T, Kay TW, Kulkarni RN, Okada T, Wang XL, Yim SH, Shah Y, Grey ST, Biankin AV, Kench JG, Laybutt DR, Gonzalez FJ, Kahn CR, Gunton JE. Hypoxia-inducible factor-1alpha regulates beta cell function in mouse and human islets. J Clin Invest. 2010;120:2171–2183. doi: 10.1172/JCI35846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colca JR, McDonald WG, Waldon DJ, Leone JW, Lull JM, Bannow CA, Lund ET, Mathews WR. Identification of a novel mitochondrial protein (“mitoNEET”) cross-linked specifically by a thiazolidinedione photoprobe. Am J Physiol Endocrinol Metab. 2004;286:E252–260. doi: 10.1152/ajpendo.00424.2003. [DOI] [PubMed] [Google Scholar]
- Condo I, Malisan F, Guccini I, Serio D, Rufini A, Testi R. Molecular control of the cytosolic aconitase/IRP1 switch by extramitochondrial frataxin. Hum Mol Genet. 2010;19:1221–1229. doi: 10.1093/hmg/ddp592. [DOI] [PubMed] [Google Scholar]
- Cooksey RC, Jones D, Gabrielsen S, Huang J, Simcox JA, Luo B, Soesanto Y, Rienhoff H, Abel ED, McClain DA. Dietary iron restriction or iron chelation protects from diabetes and loss of beta-cell function in the obese (ob/ob lep−/−) mouse. Am J Physiol Endocrinol Metab. 2010;298:E1236–1243. doi: 10.1152/ajpendo.00022.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooksey RC, Jouihan HA, Ajioka RS, Hazel MW, Jones DL, Kushner JP, McClain DA. Oxidative stress, beta-cell apoptosis, and decreased insulin secretory capacity in mouse models of hemochromatosis. Endocrinology. 2004;145:5305–5312. doi: 10.1210/en.2004-0392. [DOI] [PubMed] [Google Scholar]
- D’Alessio F, Hentze MW, Muckenthaler MU. The hemochromatosis proteins HFE, TfR2, and HJV form a membrane-associated protein complex for hepcidin regulation. J Hepatol. 2012 doi: 10.1016/j.jhep.2012.06.015. [DOI] [PubMed] [Google Scholar]
- Davis RJ, Corvera S, Czech MP. Insulin stimulates cellular iron uptake and causes the redistribution of intracellular transferrin receptors to the plasma membrane. J Biol Chem. 1986;261:8708–8711. [PubMed] [Google Scholar]
- De Domenico I, McVey Ward D, Kaplan J. Regulation of iron acquisition and storage: consequences for iron-linked disorders. Nature reviews. Molecular cell biology. 2008;9:72–81. doi: 10.1038/nrm2295. [DOI] [PubMed] [Google Scholar]
- Dmochowski K, Finegood DT, Francombe W, Tyler B, Zinman B. Factors determining glucose tolerance in patients with thalassemia major. J Clin Endocrinol Metab. 1993;77:478–483. doi: 10.1210/jcem.77.2.8345055. [DOI] [PubMed] [Google Scholar]
- Dongiovanni P, Fracanzani AL, Fargion S, Valenti L. Iron in fatty liver and in the metabolic syndrome: a promising therapeutic target. J Hepatol. 2011;55:920–932. doi: 10.1016/j.jhep.2011.05.008. [DOI] [PubMed] [Google Scholar]
- Eisenstein RS, Ross KL. Novel roles for iron regulatory proteins in the adaptive response to iron deficiency. J Nutr. 2003;133:1510S–1516S. doi: 10.1093/jn/133.5.1510S. [DOI] [PubMed] [Google Scholar]
- Facchini FS. Effect of phlebotomy on plasma glucose and insulin concentrations. Diabetes Care. 1998;21:2190. [PubMed] [Google Scholar]
- Facchini FS, Hua NW, Stoohs RA. Effect of iron depletion in carbohydrate-intolerant patients with clinical evidence of nonalcoholic fatty liver disease. Gastroenterology. 2002;122:931–939. doi: 10.1053/gast.2002.32403. [DOI] [PubMed] [Google Scholar]
- Faller M, Matsunaga M, Yin S, Loo JA, Guo F. Heme is involved in microRNA processing. Nat Struct Mol Biol. 2007;14:23–29. doi: 10.1038/nsmb1182. [DOI] [PubMed] [Google Scholar]
- Farahani P, Chiu S, Bowlus CL, Boffelli D, Lee E, Fisler JS, Krauss RM, Warden CH. Obesity in BSB Mice Is Correlated with Expression of Genes for Iron Homeostasis and Leptin[ast][ast] Obesity. 2004;12:191–204. doi: 10.1038/oby.2004.26. [DOI] [PubMed] [Google Scholar]
- Fargnoli JL, Fung TT, Olenczuk DM, Chamberland JP, Hu FB, Mantzoros CS. Adherence to healthy eating patterns is associated with higher circulating total and high-molecular-weight adiponectin and lower resistin concentrations in women from the Nurses’ Health Study. Am J Clin Nutr. 2008;88:1213–1224. doi: 10.3945/ajcn.2008.26480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder JN, Gnirke A, Thomas W, Tsuchihashi Z, Ruddy DA, Basava A, Dormishian F, Domingo R, Jr, Ellis MC, Fullan A, Hinton LM, Jones NL, Kimmel BE, Kronmal GS, Lauer P, Lee VK, Loeb DB, Mapa FA, McClelland E, Meyer NC, Mintier GA, Moeller N, Moore T, Morikang E, Wolff RK, et al. A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Nat Genet. 1996;13:399–408. doi: 10.1038/ng0896-399. [DOI] [PubMed] [Google Scholar]
- Fernandez-Real JM, Lopez-Bermejo A, Ricart W. Cross-talk between iron metabolism and diabetes. Diabetes. 2002a;51:2348–2354. doi: 10.2337/diabetes.51.8.2348. [DOI] [PubMed] [Google Scholar]
- Fernandez-Real JM, Lopez-Bermejo A, Ricart W. Iron stores, blood donation, and insulin sensitivity and secretion. Clin Chem. 2005;51:1201–1205. doi: 10.1373/clinchem.2004.046847. [DOI] [PubMed] [Google Scholar]
- Fernandez-Real JM, Moreno JM, Chico B, Lopez-Bermejo A, Ricart W. Circulating visfatin is associated with parameters of iron metabolism in subjects with altered glucose tolerance. Diabetes Care. 2007;30:616–621. doi: 10.2337/dc06-1581. [DOI] [PubMed] [Google Scholar]
- Fernandez-Real JM, Penarroja G, Castro A, Garcia-Bragado F, Hernandez-Aguado I, Ricart W. Blood letting in high-ferritin type 2 diabetes: effects on insulin sensitivity and beta-cell function. Diabetes. 2002b;51:1000–1004. doi: 10.2337/diabetes.51.4.1000. [DOI] [PubMed] [Google Scholar]
- Fernandez-Real JM, Penarroja G, Castro A, Garcia-Bragado F, Lopez-Bermejo A, Ricart W. Blood letting in high-ferritin type 2 diabetes: effects on vascular reactivity. Diabetes Care. 2002c;25:2249–2255. doi: 10.2337/diacare.25.12.2249. [DOI] [PubMed] [Google Scholar]
- Festa M, Ricciardelli G, Mele G, Pietropaolo C, Ruffo A, Colonna A. Overexpression of H ferritin and up-regulation of iron regulatory protein genes during differentiation of 3T3-L1 pre-adipocytes. J Biol Chem. 2000;275:36708–36712. doi: 10.1074/jbc.M004988200. [DOI] [PubMed] [Google Scholar]
- Fleming DJ, Jacques PF, Tucker KL, Massaro JM, D’Agostino RB, Sr, Wilson PW, Wood RJ. Iron status of the free-living, elderly Framingham Heart Study cohort: an iron-replete population with a high prevalence of elevated iron stores. Am J Clin Nutr. 2001;73:638–646. doi: 10.1093/ajcn/73.3.638. [DOI] [PubMed] [Google Scholar]
- Fleming DJ, Tucker KL, Jacques PF, Dallal GE, Wilson PW, Wood RJ. Dietary factors associated with the risk of high iron stores in the elderly Framingham Heart Study cohort. Am J Clin Nutr. 2002;76:1375–1384. doi: 10.1093/ajcn/76.6.1375. [DOI] [PubMed] [Google Scholar]
- Ford ES, Cogswell ME. Diabetes and serum ferritin concentration among U.S. adults. Diabetes Care. 1999;22:1978–1983. doi: 10.2337/diacare.22.12.1978. [DOI] [PubMed] [Google Scholar]
- Forouhi NG, Harding AH, Allison M, Sandhu MS, Welch A, Luben R, Bingham S, Khaw KT, Wareham NJ. Elevated serum ferritin levels predict new-onset type 2 diabetes: results from the EPIC-Norfolk prospective study. Diabetologia. 2007;50:949–956. doi: 10.1007/s00125-007-0604-5. [DOI] [PubMed] [Google Scholar]
- Gabrielsen JS, Gao Y, Simcox JA, Huang J, Thorup D, Jones D, Cooksey RC, Gabrielsen D, Adams TD, Hunt SC, Hopkins PN, Cefalu WT, McClain DA. Adipocyte iron regulates adiponectin and insulin sensitivity. J Clin Invest. 2012;122:3529–3540. doi: 10.1172/JCI44421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamberini MR, De Sanctis V, Gilli G. Hypogonadism, diabetes mellitus, hypothyroidism, hypoparathyroidism: incidence and prevalence related to iron overload and chelation therapy in patients with thalassaemia major followed from 1980 to 2007 in the Ferrara Centre. Pediatr Endocrinol Rev. 2008;6(Suppl 1):158–169. [PubMed] [Google Scholar]
- Ganz T. Hepcidin and iron regulation, 10 years later. Blood. 2011;117:4425–4433. doi: 10.1182/blood-2011-01-258467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz T, Nemeth E. Iron sequestration and anemia of inflammation. Semin Hematol. 2009;46:387–393. doi: 10.1053/j.seminhematol.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillum RF. Association of serum ferritin and indices of body fat distribution and obesity in Mexican American men--the Third National Health and Nutrition Examination Survey. Int J Obes Relat Metab Disord. 2001;25:639–645. doi: 10.1038/sj.ijo.0801561. [DOI] [PubMed] [Google Scholar]
- Halberg N, Khan T, Trujillo ME, Wernstedt-Asterholm I, Attie AD, Sherwani S, Wang ZV, Landskroner-Eiger S, Dineen S, Magalang UJ, Brekken RA, Scherer PE. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol. 2009;29:4467–4483. doi: 10.1128/MCB.00192-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handschin C, Lin J, Rhee J, Peyer AK, Chin S, Wu PH, Meyer UA, Spiegelman BM. Nutritional regulation of hepatic heme biosynthesis and porphyria through PGC-1alpha. Cell. 2005;122:505–515. doi: 10.1016/j.cell.2005.06.040. [DOI] [PubMed] [Google Scholar]
- Hardie DG. AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function. Genes Dev. 2011;25:1895–1908. doi: 10.1101/gad.17420111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatunic M, Finucane FM, Brennan AM, Norris S, Pacini G, Nolan JJ. Effect of iron overload on glucose metabolism in patients with hereditary hemochromatosis. Metabolism. 2010a;59:380–384. doi: 10.1016/j.metabol.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Hatunic M, Finucane FM, Norris S, Pacini G, Nolan JJ. Glucose metabolism after normalization of markers of iron overload by venesection in subjects with hereditary hemochromatosis. Metabolism. 2010b;59:1811–1815. doi: 10.1016/j.metabol.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Haurie V, Boucherie H, Sagliocco F. The Snf1 protein kinase controls the induction of genes of the iron uptake pathway at the diauxic shift in Saccharomyces cerevisiae. J Biol Chem. 2003;278:45391–45396. doi: 10.1074/jbc.M307447200. [DOI] [PubMed] [Google Scholar]
- Henderson SA, Dallman PR, Brooks GA. Glucose turnover and oxidation are increased in the iron-deficient anemic rat. Am J Physiol. 1986;250:E414–421. doi: 10.1152/ajpendo.1986.250.4.E414. [DOI] [PubMed] [Google Scholar]
- Hentze MW, Muckenthaler MU, Galy B, Camaschella C. Two to tango: regulation of Mammalian iron metabolism. Cell. 2010;142:24–38. doi: 10.1016/j.cell.2010.06.028. [DOI] [PubMed] [Google Scholar]
- Hoffmeister PA, Storer BE, Sanders JE. Diabetes mellitus in long-term survivors of pediatric hematopoietic cell transplantation. J Pediatr Hematol Oncol. 2004;26:81–90. doi: 10.1097/00043426-200402000-00003. [DOI] [PubMed] [Google Scholar]
- Hostettler-Allen R, Tappy L, Blum JW. Enhanced insulin-dependent glucose utilization in iron-deficient veal calves. J Nutr. 1993;123:1656–1667. doi: 10.1093/jn/123.10.1656. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS. Inflammatory pathways and insulin action. Int J Obes Relat Metab Disord. 2003;27(Suppl 3):S53–55. doi: 10.1038/sj.ijo.0802502. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- Hou X, Xu S, Maitland-Toolan KA, Sato K, Jiang B, Ido Y, Lan F, Walsh K, Wierzbicki M, Verbeuren TJ, Cohen RA, Zang M. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J Biol Chem. 2008;283:20015–20026. doi: 10.1074/jbc.M802187200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houschyar KS, Ludtke R, Dobos GJ, Kalus U, Brocker-Preuss M, Rampp T, Brinkhaus B, Michalsen A. Effects of phlebotomy-induced reduction of body iron stores on metabolic syndrome: Results from a randomized clinical trial. BMC medicine. 2012;10:54. doi: 10.1186/1741-7015-10-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtkooper RH, Pirinen E, Auwerx J. Sirtuins as regulators of metabolism and healthspan. Nature reviews. Molecular cell biology. 2012;13:225–238. doi: 10.1038/nrm3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hramiak IM, Finegood DT, Adams PC. Factors affecting glucose tolerance in hereditary hemochromatosis. Clin Invest Med. 1997;20:110–118. [PubMed] [Google Scholar]
- Huang J, Gabrielsen JS, Cooksey RC, Luo B, Boros LG, Jones DL, Jouihan HA, Soesanto Y, Knecht L, Hazel MW, Kushner JP, McClain DA. Increased glucose disposal and AMP-dependent kinase signaling in a mouse model of hemochromatosis. J Biol Chem. 2007;282:37501–37507. doi: 10.1074/jbc.M703625200. [DOI] [PubMed] [Google Scholar]
- Huang J, Jones D, Luo B, Sanderson M, Soto J, Abel ED, Cooksey RC, McClain DA. Iron overload and diabetes risk: a shift from glucose to Fatty Acid oxidation and increased hepatic glucose production in a mouse model of hereditary hemochromatosis. Diabetes. 2011;60:80–87. doi: 10.2337/db10-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson DM, Curtis SB, Smith VC, Griffiths TA, Wong AY, Scudamore CH, Buchan AM, MacGillivray RT. Human hephaestin expression is not limited to enterocytes of the gastrointestinal tract but is also found in the antrum, the enteric nervous system, and pancreatic {beta}-cells. American journal of physiology. Gastrointestinal and liver physiology. 2010;298:G425–432. doi: 10.1152/ajpgi.00453.2009. [DOI] [PubMed] [Google Scholar]
- Inagaki T, Tachibana M, Magoori K, Kudo H, Tanaka T, Okamura M, Naito M, Kodama T, Shinkai Y, Sakai J. Obesity and metabolic syndrome in histone demethylase JHDM2a-deficient mice. Genes to cells: devoted to molecular & cellular mechanisms. 2009;14:991–1001. doi: 10.1111/j.1365-2443.2009.01326.x. [DOI] [PubMed] [Google Scholar]
- Iwasaki T, Nakajima A, Yoneda M, Yamada Y, Mukasa K, Fujita K, Fujisawa N, Wada K, Terauchi Y. Serum ferritin is associated with visceral fat area and subcutaneous fat area. Diabetes Care. 2005;28:2486–2491. doi: 10.2337/diacare.28.10.2486. [DOI] [PubMed] [Google Scholar]
- Jaruratanasirikul S, Chareonmuang R, Wongcharnchailert M, Laosombat V, Sangsupavanich P, Leetanaporn K. Prevalence of impaired glucose metabolism in beta-thalassemic children receiving hypertransfusions with a suboptimal dosage of iron-chelating therapy. Eur J Pediatr. 2008;167:873–876. doi: 10.1007/s00431-007-0602-0. [DOI] [PubMed] [Google Scholar]
- Jehn M, Clark JM, Guallar E. Serum ferritin and risk of the metabolic syndrome in U.S. adults. Diabetes Care. 2004;27:2422–2428. doi: 10.2337/diacare.27.10.2422. [DOI] [PubMed] [Google Scholar]
- Jiang C, Qu A, Matsubara T, Chanturiya T, Jou W, Gavrilova O, Shah YM, Gonzalez FJ. Disruption of hypoxia-inducible factor 1 in adipocytes improves insulin sensitivity and decreases adiposity in high-fat diet-fed mice. Diabetes. 2011;60:2484–2495. doi: 10.2337/db11-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang R, Manson JE, Meigs JB, Ma J, Rifai N, Hu FB. Body iron stores in relation to risk of type 2 diabetes in apparently healthy women. Jama. 2004;291:711–717. doi: 10.1001/jama.291.6.711. [DOI] [PubMed] [Google Scholar]
- Jomova K, Valko M. Advances in metal-induced oxidative stress and human disease. Toxicology. 2011;283:65–87. doi: 10.1016/j.tox.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Jouihan HACP, Cooksey RC, Hoagland EA, Boudina S, Abel ED, Winge DR, McClain DA. Iron-mediated inhibition of mitochondrial manganese uptake mediates mitochondrial dysfunction in a mouse model of hemochromatosis. Mol Med. 2008;14:98–108. doi: 10.2119/2007-00114.Jouihan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn BB. Type 2 diabetes: when insulin secretion fails to compensate for insulin resistance. Cell. 1998;92:593–596. doi: 10.1016/s0092-8674(00)81125-3. [DOI] [PubMed] [Google Scholar]
- Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of Type 2 diabetes. Diabetologia. 2003;46:3–19. doi: 10.1007/s00125-002-1009-0. [DOI] [PubMed] [Google Scholar]
- Kaneto H, Katakami N, Matsuhisa M, Matsuoka TA. Role of reactive oxygen species in the progression of type 2 diabetes and atherosclerosis. Mediators of inflammation. 2010;2010:453892. doi: 10.1155/2010/453892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson MD, Oukka M, Koss LM, Aydemir F, Wessling-Resnick M. Iron release from macrophages after erythrophagocytosis is up-regulated by ferroportin 1 overexpression and down-regulated by hepcidin. Proc Natl Acad Sci U S A. 2005;102:1324–1328. doi: 10.1073/pnas.0409409102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch RO, Zoller H, Theuri I, Obrist P, Egg G, Strohmayer W, Vogel W, Weiss G. Distribution of DMT 1 within the human glandular system. Histology and histopathology. 2003;18:1095–1101. doi: 10.14670/HH-18.1095. [DOI] [PubMed] [Google Scholar]
- Kriska AM, Saremi A, Hanson RL, Bennett PH, Kobes S, Williams DE, Knowler WC. Physical activity, obesity, and the incidence of type 2 diabetes in a high-risk population. Am J Epidemiol. 2003;158:669–675. doi: 10.1093/aje/kwg191. [DOI] [PubMed] [Google Scholar]
- Kubota N, Terauchi Y, Yamauchi T, Kubota T, Moroi M, Matsui J, Eto K, Yamashita T, Kamon J, Satoh H, Yano W, Froguel P, Nagai R, Kimura S, Kadowaki T, Noda T. Disruption of Adiponectin Causes Insulin Resistance and Neointimal Formation. J Biol Chem. 2002;277:25863–25866. doi: 10.1074/jbc.C200251200. [DOI] [PubMed] [Google Scholar]
- Kusminski CM, Holland WL, Sun K, Park J, Spurgin SB, Lin Y, Askew GR, Simcox JA, McClain DA, Li C, Scherer PE. MitoNEET-driven alterations in adipocyte mitochondrial activity reveal a crucial adaptive process that preserves insulin sensitivity in obesity. Nat Med. 2012 doi: 10.1038/nm.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan F, Cacicedo JM, Ruderman N, Ido Y. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible role in AMP-activated protein kinase activation. J Biol Chem. 2008;283:27628–27635. doi: 10.1074/jbc.M805711200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzen S, Drinkgern J, Tiedge M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free radical biology & medicine. 1996;20:463–466. doi: 10.1016/0891-5849(96)02051-5. [DOI] [PubMed] [Google Scholar]
- Lewis RM, Petry CJ, Ozanne SE, Hales CN. Effects of maternal iron restriction in the rat on blood pressure, glucose tolerance, and serum lipids in the 3-month-old offspring. Metabolism. 2001;50:562–567. doi: 10.1053/meta.2001.22516. [DOI] [PubMed] [Google Scholar]
- Liuzzi JP, Aydemir F, Nam H, Knutson MD, Cousins RJ. Zip14 (Slc39a14) mediates non-transferrin-bound iron uptake into cells. Proc Natl Acad Sci U S A. 2006;103:13612–13617. doi: 10.1073/pnas.0606424103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobmayr L, Brooks DG, Wilson RB. Increased IRP1 activity in Friedreich ataxia. Gene. 2005;354:157–161. doi: 10.1016/j.gene.2005.04.040. [DOI] [PubMed] [Google Scholar]
- Long YC, Zierath JR. AMP-activated protein kinase signaling in metabolic regulation. J Clin Invest. 2006;116:1776–1783. doi: 10.1172/JCI29044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005;307:384–387. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
- Mackenzie B, Ujwal ML, Chang MH, Romero MF, Hediger MA. Divalent metal-ion transporter DMT1 mediates both H+-coupled Fe2+ transport and uncoupled fluxes. Pflugers Archiv: European journal of physiology. 2006;451:544–558. doi: 10.1007/s00424-005-1494-3. [DOI] [PubMed] [Google Scholar]
- Mastrogiannaki M, Matak P, Keith B, Simon MC, Vaulont S, Peyssonnaux C. HIF-2alpha, but not HIF-1alpha, promotes iron absorption in mice. J Clin Invest. 2009;119:1159–1166. doi: 10.1172/JCI38499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain D, Abraham D, Rogers J, Brady R, Gault P, Ajioka R, Kushner J. High prevalence of abnormal glucose homeostasis secondary to decreased insulin secretion in individuals with hereditary haemochromatosis. Diabetologia. 2006;49:1661–1669. doi: 10.1007/s00125-006-0200-0. [DOI] [PubMed] [Google Scholar]
- McClain DA, AK, Nouraie M, Salomon-Andonie J, Niu X, Miasnikova G, Polyakova LA, Sergueeva A, Okhotin DJ, Cherqaoui R, Okhotin D, Cox JE, Swierczek S, Song J, Simon MC, Huang J, Simcox JA, Yoon D, Prchal JT, Gordeuk VR. Decreased serum glucose and glycosylated hemoglobin levels in patients with Chuvash polycythemia: a role for HIF in glucose metabolism. J Mol Med. 2012 doi: 10.1007/s00109-012-0961-5. epub Sept 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung JP, Andersen NE, Tarr TN, Stahl CH, Young AJ. Physical activity prevents augmented body fat accretion in moderately iron-deficient rats. J Nutr. 2008;138:1293–1297. doi: 10.1093/jn/138.7.1293. [DOI] [PubMed] [Google Scholar]
- Mendler MH, Turlin B, Moirand R, Jouanolle AM, Sapey T, Guyader D, Le Gall JY, Brissot P, David V, Deugnier Y. Insulin resistance-associated hepatic iron overload. Gastroenterology. 1999;117:1155–1163. doi: 10.1016/s0016-5085(99)70401-4. [DOI] [PubMed] [Google Scholar]
- Merkel PA, Simonson DC, Amiel SA, Plewe G, Sherwin RS, Pearson HA, Tamborlane WV. Insulin resistance and hyperinsulinemia in patients with thalassemia major treated by hypertransfusion. N Engl J Med. 1988;318:809–814. doi: 10.1056/NEJM198803313181303. [DOI] [PubMed] [Google Scholar]
- Messina MF, Lombardo F, Meo A, Miceli M, Wasniewska M, Valenzise M, Ruggeri C, Arrigo T, De Luca F. Three-year prospective evaluation of glucose tolerance, beta-cell function and peripheral insulin sensitivity in non-diabetic patients with thalassemia major. Journal of endocrinological investigation. 2002;25:497–501. doi: 10.1007/BF03345490. [DOI] [PubMed] [Google Scholar]
- Meynard D, Kautz L, Darnaud V, Canonne-Hergaux F, Coppin H, Roth MP. Lack of the bone morphogenetic protein BMP6 induces massive iron overload. Nat Genet. 2009;41:478–481. doi: 10.1038/ng.320. [DOI] [PubMed] [Google Scholar]
- Minamiyama Y, Takemura S, Kodai S, Shinkawa H, Tsukioka T, Ichikawa H, Naito Y, Yoshikawa T, Okada S. Iron restriction improves type 2 diabetes mellitus in Otsuka Long-Evans Tokushima fatty rats. Am J Physiol Endocrinol Metab. 2010;298:E1140–1149. doi: 10.1152/ajpendo.00620.2009. [DOI] [PubMed] [Google Scholar]
- Minard KI, McAlister-Henn L. Sources of NADPH in yeast vary with carbon source. J Biol Chem. 2005;280:39890–39896. doi: 10.1074/jbc.M509461200. [DOI] [PubMed] [Google Scholar]
- Moirand R, Adams PC, Bicheler V, Brissot P, Deugnier Y. Clinical features of genetic hemochromatosis in women compared with men. Ann Intern Med. 1997;127:105–110. doi: 10.7326/0003-4819-127-2-199707150-00002. [DOI] [PubMed] [Google Scholar]
- Mojiminiyi OA, Marouf R, Abdella NA. Body iron stores in relation to the metabolic syndrome, glycemic control and complications in female patients with type 2 diabetes. Nutrition, Metabolism and Cardiovascular Diseases. 2008;18:559–566. doi: 10.1016/j.numecd.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Monahan BJ, Villen J, Marguerat S, Bahler J, Gygi SP, Winston F. Fission yeast SWI/SNF and RSC complexes show compositional and functional differences from budding yeast. Nat Struct Mol Biol. 2008;15:873–880. doi: 10.1038/nsmb.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montes-Cortes DH, Hicks JJ, Ceballos-Reyes GM, Garcia-Sanchez JR, Medina-Navarro R, Olivares-Corichi IM. Chemical and functional changes of human insulin by in vitro incubation with blood from diabetic patients in oxidative stress. Metabolism. 2010;59:935–942. doi: 10.1016/j.metabol.2009.10.013. [DOI] [PubMed] [Google Scholar]
- Nappi AJ, Vass E. Hydroxyl radical formation resulting from the interaction of nitric oxide and hydrogen peroxide. Biochim Biophys Acta. 1998;1380:55–63. doi: 10.1016/s0304-4165(97)00125-6. [DOI] [PubMed] [Google Scholar]
- Nelson R, Chawla M, Connolly P, LaPorte J. Ferritin as an index of bone marrow iron stores. South Med J. 1978;71:1482–1484. doi: 10.1097/00007611-197812000-00011. [DOI] [PubMed] [Google Scholar]
- Nemeth E, Roetto A, Garozzo G, Ganz T, Camaschella C. Hepcidin is decreased in TFR2-Hemochromatosis. Blood. 2004a;105:1803–1806. doi: 10.1182/blood-2004-08-3042. [DOI] [PubMed] [Google Scholar]
- Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004b;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- Ozdemir A, Sevinc C, Selamet U, Turkmen F. The relationship between iron deficiency anemia and lipid metabolism in premenopausal women. Am J Med Sci. 2007;334:331–333. doi: 10.1097/MAJ.0b013e318145b107. [DOI] [PubMed] [Google Scholar]
- Pietrangelo A. Hereditary hemochromatosis: pathogenesis, diagnosis, and treatment. Gastroenterology. 2010;139:393–408. 408 e391–392. doi: 10.1053/j.gastro.2010.06.013. [DOI] [PubMed] [Google Scholar]
- Pinhas-Hamiel O, Newfield RS, Koren I, Agmon A, Lilos P, Phillip M. Greater prevalence of iron deficiency in overweight and obese children and adolescents. Int J Obes Relat Metab Disord. 2003;27:416–418. doi: 10.1038/sj.ijo.0802224. [DOI] [PubMed] [Google Scholar]
- Ponugoti B, Dong G, Graves DT. Role of forkhead transcription factors in diabetes-induced oxidative stress. Experimental diabetes research. 2012;2012:939751. doi: 10.1155/2012/939751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi L, van Dam RM, Rexrode K, Hu FB. Heme iron from diet as a risk factor for coronary heart disease in women with type 2 diabetes. Diabetes Care. 2007;30:101–106. doi: 10.2337/dc06-1686. [DOI] [PubMed] [Google Scholar]
- Qiu C, Zhang C, Gelaye B, Enquobahrie DA, Frederick IO, Williams MA. Gestational diabetes mellitus in relation to maternal dietary heme iron and nonheme iron intake. Diabetes Care. 2011;34:1564–1569. doi: 10.2337/dc11-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radisky DC, Babcock MC, Kaplan J. The yeast frataxin homologue mediates mitochondrial iron efflux. Evidence for a mitochondrial iron cycle. J Biol Chem. 1999;274:4497–4499. doi: 10.1074/jbc.274.8.4497. [DOI] [PubMed] [Google Scholar]
- Ravelli AC, van der Meulen JH, Michels RP, Osmond C, Barker DJ, Hales CN, Bleker OP. Glucose tolerance in adults after prenatal exposure to famine. Lancet. 1998;351:173–177. doi: 10.1016/s0140-6736(97)07244-9. [DOI] [PubMed] [Google Scholar]
- Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cellular signalling. 2012;24:981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regazzetti C, Peraldi P, Gremeaux T, Najem-Lendom R, Ben-Sahra I, Cormont M, Bost F, Le Marchand-Brustel Y, Tanti JF, Giorgetti-Peraldi S. Hypoxia decreases insulin signaling pathways in adipocytes. Diabetes. 2009;58:95–103. doi: 10.2337/db08-0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romney SJ, Newman BS, Thacker C, Leibold EA. HIF-1 regulates iron homeostasis in Caenorhabditis elegans by activation and inhibition of genes involved in iron uptake and storage. PLoS genetics. 2011;7:e1002394. doi: 10.1371/journal.pgen.1002394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti L, Giaccari A, DeFronzo RA. Glucose toxicity. Diabetes Care. 1990;13:610–630. doi: 10.2337/diacare.13.6.610. [DOI] [PubMed] [Google Scholar]
- Rouault TA, Tong WH. Tangled up in red: intertwining of the heme and iron-sulfur cluster biogenesis pathways. Cell Metab. 2009;10:80–81. doi: 10.1016/j.cmet.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Rumberger JM, Peters T, Jr, Burrington C, Green A. Transferrin and iron contribute to the lipolytic effect of serum in isolated adipocytes. Diabetes. 2004;53:2535–2541. doi: 10.2337/diabetes.53.10.2535. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah YM, Matsubara T, Ito S, Yim SH, Gonzalez FJ. Intestinal hypoxia-inducible transcription factors are essential for iron absorption following iron deficiency. Cell Metab. 2009;9:152–164. doi: 10.1016/j.cmet.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakoury-Elizeh M, Protchenko O, Berger A, Cox J, Gable K, Dunn TM, Prinz WA, Bard M, Philpott CC. Metabolic response to iron deficiency in Saccharomyces cerevisiae. J Biol Chem. 2010;285:14823–14833. doi: 10.1074/jbc.M109.091710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifi F, Nasab NM, Zadeh HJ. Elevated serum ferritin concentrations in prediabetic subjects. Diab Vasc Dis Res. 2008;5:15–18. doi: 10.3132/dvdr.2008.003. [DOI] [PubMed] [Google Scholar]
- Shayeghi M, Latunde-Dada GO, Oakhill JS, Laftah AH, Takeuchi K, Halliday N, Khan Y, Warley A, McCann FE, Hider RC, Frazer DM, Anderson GJ, Vulpe CD, Simpson RJ, McKie AT. Identification of an intestinal heme transporter. Cell. 2005;122:789–801. doi: 10.1016/j.cell.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Sonnweber T, Ress C, Nairz M, Theurl I, Schroll A, Murphy AT, Wroblewski V, Witcher DR, Moser P, Ebenbichler CF, Kaser S, Weiss G. High-fat diet causes iron deficiency via hepcidin-independent reduction of duodenal iron absorption. J Nutr Biochem. 2012 doi: 10.1016/j.jnutbio.2011.10.013. [DOI] [PubMed] [Google Scholar]
- Stangl GI, Kirchgessner M. Different degrees of moderate iron deficiency modulate lipid metabolism of rats. Lipids. 1998;33:889–895. doi: 10.1007/s11745-998-0285-8. [DOI] [PubMed] [Google Scholar]
- Subauste AR, Burant CF. Role of FoxO1 in FFA-induced oxidative stress in adipocytes. Am J Physiol Endocrinol Metab. 2007;293:E159–164. doi: 10.1152/ajpendo.00629.2006. [DOI] [PubMed] [Google Scholar]
- Szmuilowicz ED, Stuenkel CA, Seely EW. Influence of menopause on diabetes and diabetes risk. Nat Rev Endocrinol. 2009;5:553–558. doi: 10.1038/nrendo.2009.166. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Watanabe Y, Takano-Shimizu T, Kondo S. Roles of jumonji and jumonji family genes in chromatin regulation and development. Developmental dynamics: an official publication of the American Association of Anatomists. 2006;235:2449–2459. doi: 10.1002/dvdy.20851. [DOI] [PubMed] [Google Scholar]
- Tanner LI, Lienhard GE. Insulin elicits a redistribution of transferrin receptors in 3T3-L1 adipocytes through an increase in the rate constant for receptor externalization. J Biol Chem. 1987;262:8975–8980. [PubMed] [Google Scholar]
- Toomajian C, Ajioka RS, Jorde LB, Kushner JP, Kreitman M. A method for detecting recent selection in the human genome from allele age estimates. Genetics. 2003;165:287–297. doi: 10.1093/genetics/165.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tormos KV, Anso E, Hamanaka RB, Eisenbart J, Joseph J, Kalyanaraman B, Chandel NS. Mitochondrial complex III ROS regulate adipocyte differentiation. Cell Metab. 2011;14:537–544. doi: 10.1016/j.cmet.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trayhurn P, Wood IS. Signalling role of adipose tissue: adipokines and inflammation in obesity. Biochem Soc Trans. 2005;33:1078–1081. doi: 10.1042/BST0331078. [DOI] [PubMed] [Google Scholar]
- Tuomainen TP, Nyyssonen K, Salonen R, Tervahauta A, Korpela H, Lakka T, Kaplan GA, Salonen JT. Body iron stores are associated with serum insulin and blood glucose concentrations. Population study in 1,013 eastern Finnish men. Diabetes Care. 1997;20:426–428. doi: 10.2337/diacare.20.3.426. [DOI] [PubMed] [Google Scholar]
- Vogiatzi MG, Macklin EA, Trachtenberg FL, Fung EB, Cheung AM, Vichinsky E, Olivieri N, Kirby M, Kwiatkowski JL, Cunningham M, Holm IA, Fleisher M, Grady RW, Peterson CM, Giardina PJ. Differences in the prevalence of growth, endocrine and vitamin D abnormalities among the various thalassaemia syndromes in North America. Br J Haematol. 2009;146:546–556. doi: 10.1111/j.1365-2141.2009.07793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter PB, Knutson MD, Paler-Martinez A, Lee S, Xu Y, Viteri FE, Ames BN. Iron deficiency and iron excess damage mitochondria and mitochondrial DNA in rats. Proc Natl Acad Sci U S A. 2002;99:2264–2269. doi: 10.1073/pnas.261708798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RH, Li C, Xu X, Zheng Y, Xiao C, Zerfas P, Cooperman S, Eckhaus M, Rouault T, Mishra L, Deng CX. A role of SMAD4 in iron metabolism through the positive regulation of hepcidin expression. Cell Metab. 2005;2:399–409. doi: 10.1016/j.cmet.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Weatherall DJ. Pathophysiology of thalassaemia. Bailliere’s clinical haematology. 1998;11:127–146. doi: 10.1016/s0950-3536(98)80072-3. [DOI] [PubMed] [Google Scholar]
- Wiley SE, Murphy AN, Ross SA, van der Geer P, Dixon JE. MitoNEET is an iron-containing outer mitochondrial membrane protein that regulates oxidative capacity. Proc Natl Acad Sci U S A. 2007;104:5318–5323. doi: 10.1073/pnas.0701078104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JG, Lindquist JH, Grambow SC, Crook ED, Maher JF. Potential role of increased iron stores in diabetes. Am J Med Sci. 2003;325:332–339. doi: 10.1097/00000441-200306000-00004. [DOI] [PubMed] [Google Scholar]
- Wlazlo N, van Greevenbroek MM, Ferreira I, Jansen EH, Feskens EJ, van der Kallen CJ, Schalkwijk CG, Bravenboer B, Stehouwer CD. Iron Metabolism Is Associated With Adipocyte Insulin Resistance and Plasma Adiponectin: The Cohort on Diabetes and Atherosclerosis Maastricht (CODAM) study. Diabetes Care. 2012 doi: 10.2337/dc12-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi H, Okazaki H, Shimizu M, Izawa T, Komabayashi T. Relationships among serum triacylglycerol, fat pad weight, and lipolysis in iron-deficient rats. The Journal of Nutritional Biochemistry. 2000;11:455–460. doi: 10.1016/s0955-2863(00)00113-3. [DOI] [PubMed] [Google Scholar]
- Yanoff LB, Menzie CM, Denkinger B, Sebring NG, McHugh T, Remaley AT, Yanovski JA. Inflammation and iron deficiency in the hypoferremia of obesity. Int J Obes (Lond) 2007;31:1412–1419. doi: 10.1038/sj.ijo.0803625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L, Wu N, Curtin JC, Qatanani M, Szwergold NR, Reid RA, Waitt GM, Parks DJ, Pearce KH, Wisely GB, Lazar MA. Rev-erbalpha, a heme sensor that coordinates metabolic and circadian pathways. Science. 2007;318:1786–1789. doi: 10.1126/science.1150179. [DOI] [PubMed] [Google Scholar]
- Zehetner J, Danzer C, Collins S, Eckhardt K, Gerber PA, Ballschmieter P, Galvanovskis J, Shimomura K, Ashcroft FM, Thorens B, Rorsman P, Krek W. PVHL is a regulator of glucose metabolism and insulin secretion in pancreatic beta cells. Genes Dev. 2008;22:3135–3146. doi: 10.1101/gad.496908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuris JA, Ali SS, Yeh H, Nguyen TA, Nechushtai R, Paddock ML, Jennings PA. NADPH inhibits [2Fe-2S] cluster protein transfer from diabetes drug target MitoNEET to an apo-acceptor protein. J Biol Chem. 2012;287:11649–11655. doi: 10.1074/jbc.M111.319731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuris JA, Harir Y, Conlan AR, Shvartsman M, Michaeli D, Tamir S, Paddock ML, Onuchic JN, Mittler R, Cabantchik ZI, Jennings PA, Nechushtai R. Facile transfer of [2Fe-2S] clusters from the diabetes drug target mitoNEET to an apo-acceptor protein. Proc Natl Acad Sci U S A. 2011;108:13047–13052. doi: 10.1073/pnas.1109986108. [DOI] [PMC free article] [PubMed] [Google Scholar]