Abstract
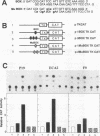
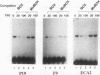
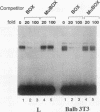
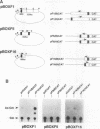
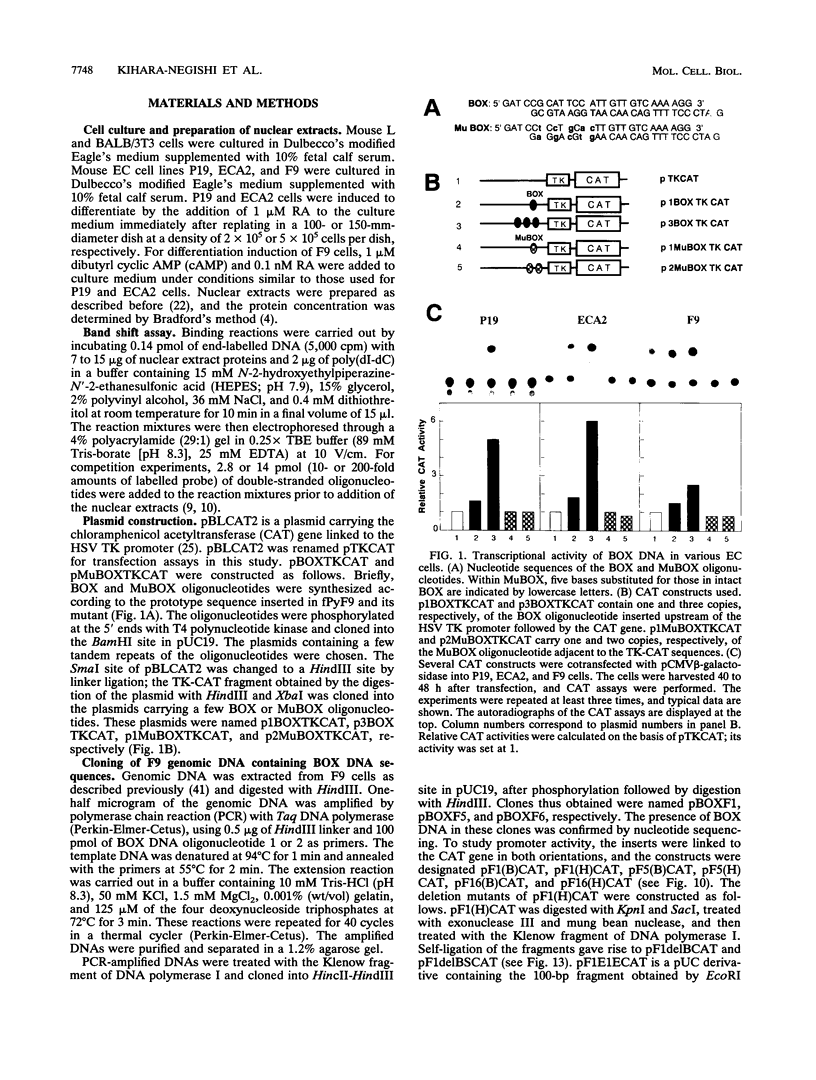
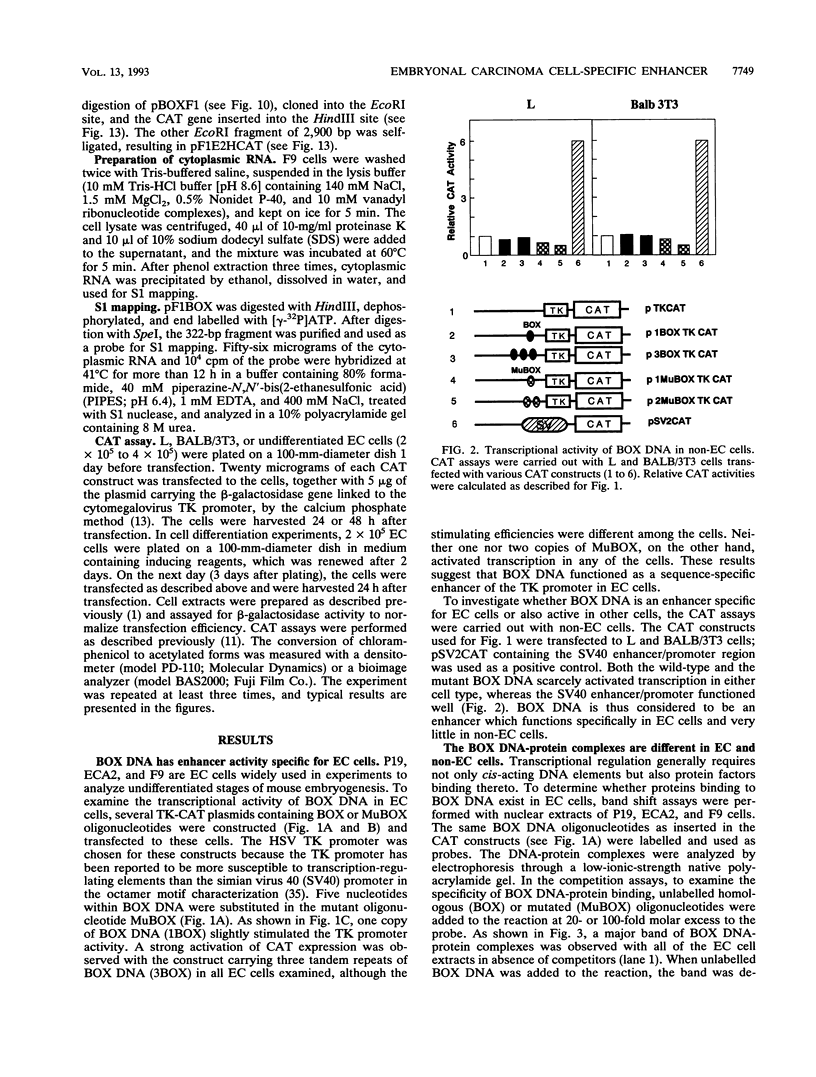
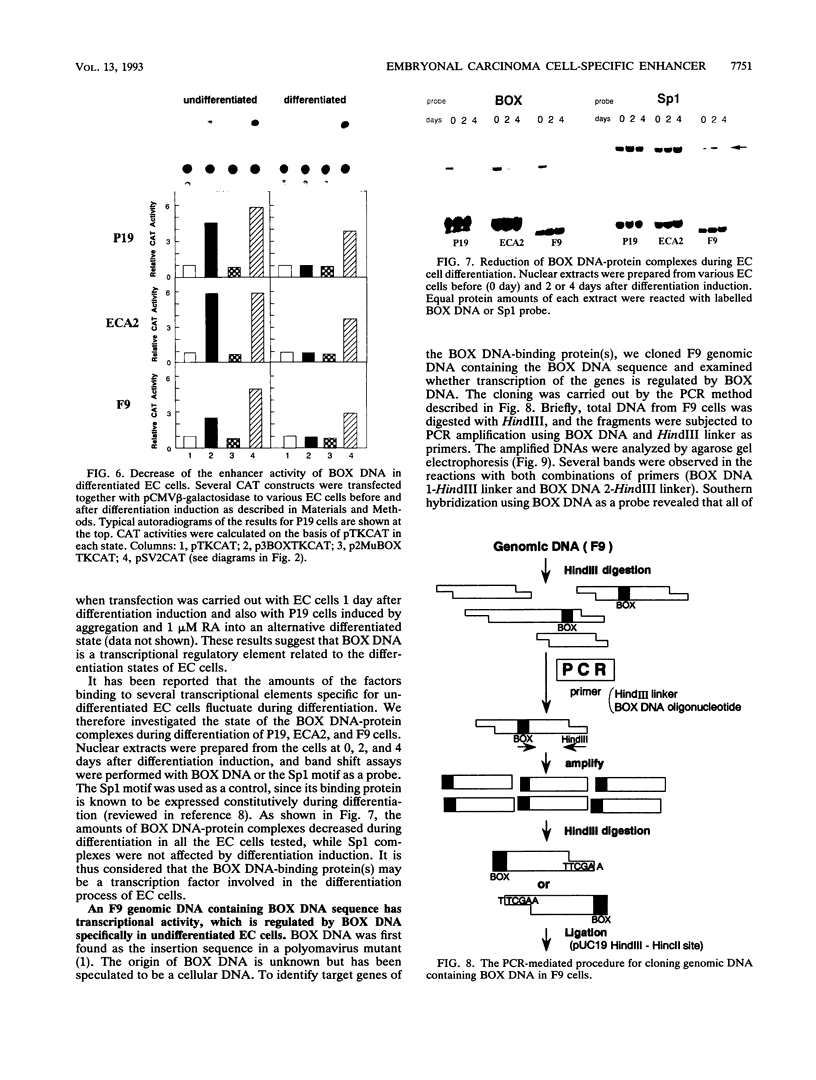
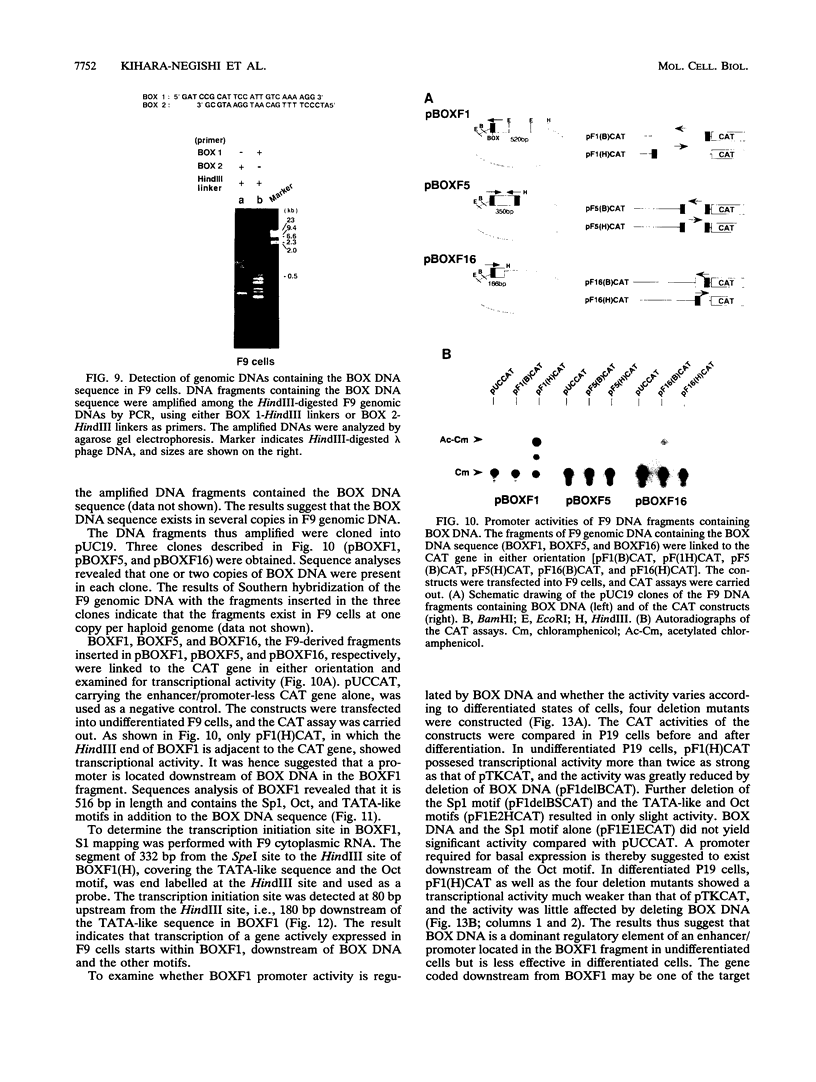
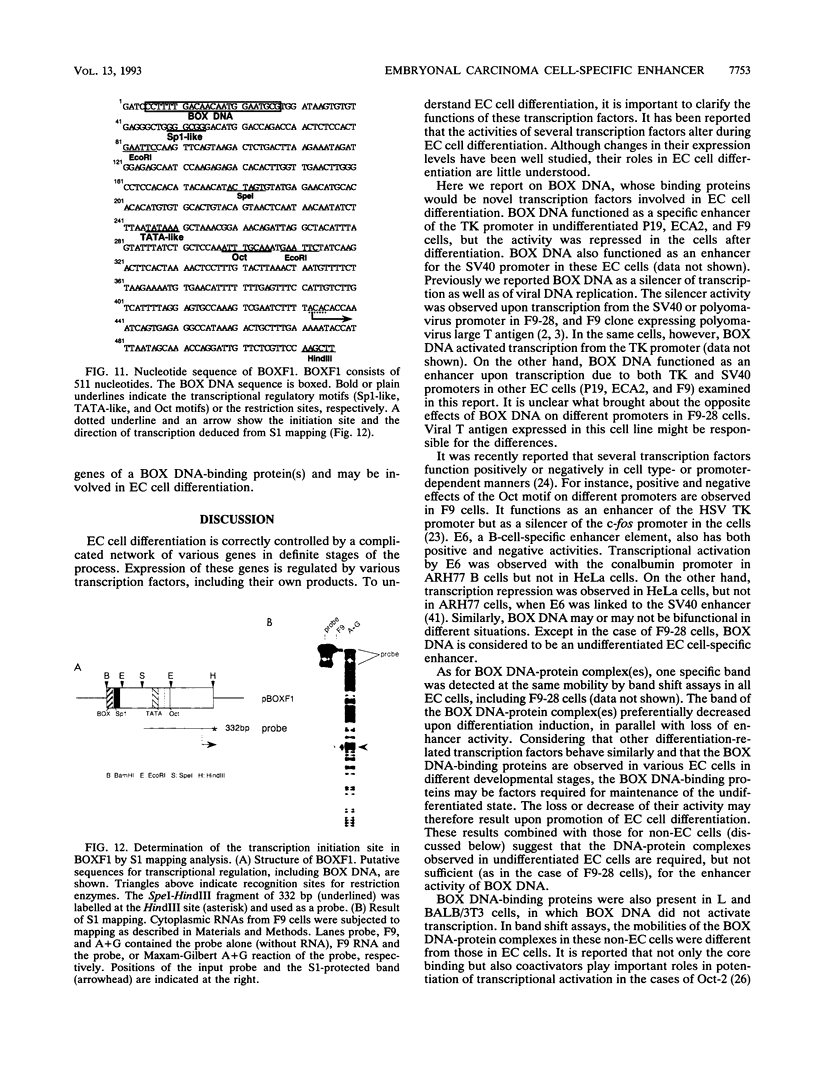
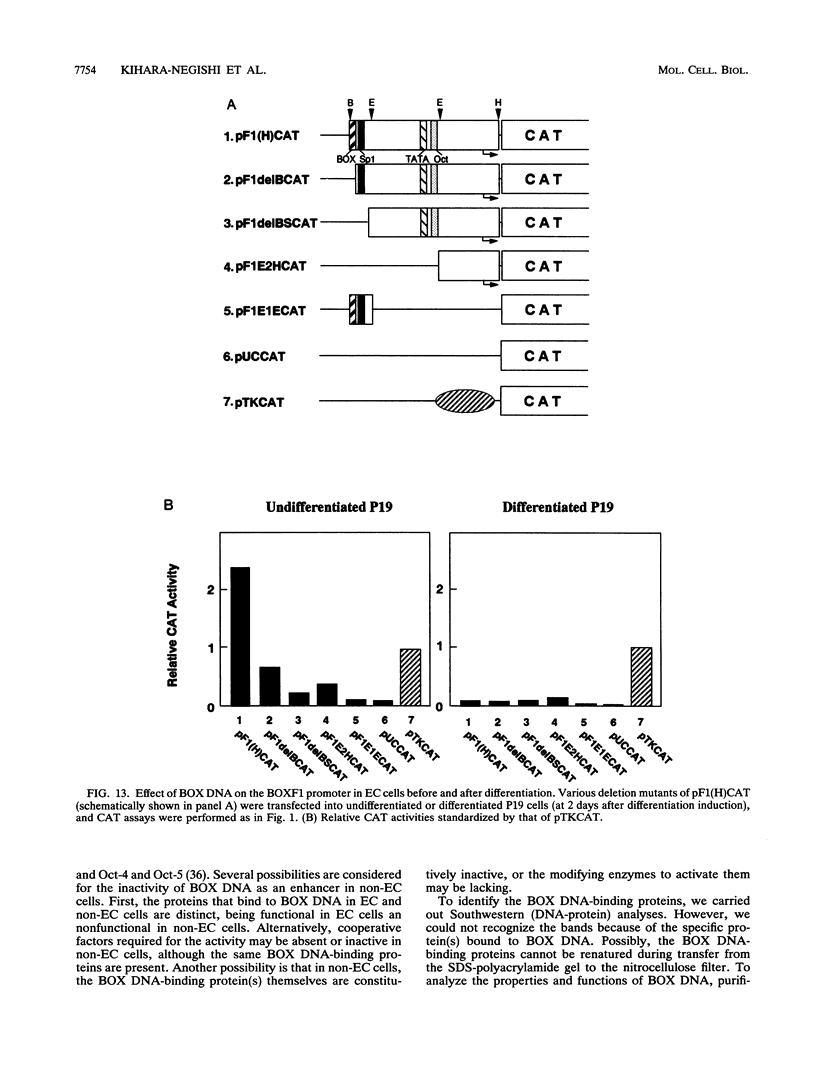
BOX DNA was previously isolated from the DNA sequence inserted in the enhancer B domain of mutant polyomavirus (fPyF9) DNA. We also reported that BOX DNA functioned negatively on DNA replication and transcription of another polyomavirus mutant (PyhrN2) in F9-28 cells, a subclone of mouse F9 embryonal carcinoma (EC) cells expressing the polyomavirus large T antigen. In this study, we demonstrate that BOX DNA enhances transcription from the thymidine kinase (TK) promoter in various EC cells. One or three copies of BOX DNA, linked to the bacterial chloramphenicol acetyltransferase gene under the control of the herpes simplex virus TK promoter, activated promoter activity in F9, P19, and ECA2 cells. Band shift assays using BOX DNA as a probe revealed that specific binding proteins were present in all EC cells examined; the patterns of BOX DNA-protein complexes were the same among them. A mutation introduced within BOX DNA abolished enhancer activity as well as the formation of specific DNA-protein complexes. In non-EC cells, including L and BALB/3T3 cells, the enhancer activity of BOX DNA on the TK promoter was not observed, although binding proteins specific to the sequence exist. In band shift assays, the patterns of the DNA-protein complexes of either L or BALB/3T3 cells were different from those of EC cells. Furthermore, the enhancer activity of BOX DNA decreased upon differentiation induction in all EC cells examined, of different origins and distinct differentiation ability. In parallel with the loss of enhancer activity, the binding proteins specific for BOX DNA decreased in these cells. Moreover, we cloned a genomic DNA of F9, termed BOXF1, containing BOX DNA sequence approximately 400 bp upstream from the RNA start site of the gene. BOXF1, containing a TATA-like motif and the binding elements for Sp1 and Oct in addition to BOX DNA, possessed promoter activity deduced by a BOXF1-chloramphenicol acetyltransferase construct. Deletion analyses of the construct revealed that the transcription of BOXF1 gene is regulated by BOX DNA, preferentially in undifferentiated EC cells versus differentiated cells. Hence, BOX DNA is probably a novel transcriptional element related to EC cell differentiation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ariizumi K., Ariga H. New class of polyomavirus mutant that can persist as free copies in F9 embryonal carcinoma cells. Mol Cell Biol. 1986 Nov;6(11):3920–3927. doi: 10.1128/mcb.6.11.3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariizumi K., Takahashi H., Nakamura M., Ariga H. Effect of silencer on polyomavirus DNA replication. Mol Cell Biol. 1989 Sep;9(9):4026–4031. doi: 10.1128/mcb.9.9.4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariizumi K., Takahashi H., Nakamura M., Ariga H. Negative transcriptional regulatory element that functions in embryonal carcinoma cells. Mol Cell Biol. 1989 Sep;9(9):4032–4037. doi: 10.1128/mcb.9.9.4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Breier G., Bućan M., Francke U., Colberg-Poley A. M., Gruss P. Sequential expression of murine homeo box genes during F9 EC cell differentiation. EMBO J. 1986 Sep;5(9):2209–2215. doi: 10.1002/j.1460-2075.1986.tb04486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colberg-Poley A. M., Voss S. D., Chowdhury K., Gruss P. Structural analysis of murine genes containing homoeo box sequences and their expression in embryonal carcinoma cells. 1985 Apr 25-May 1Nature. 314(6013):713–718. doi: 10.1038/314713a0. [DOI] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner M. M., Revzin A. A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions: application to components of the Escherichia coli lactose operon regulatory system. Nucleic Acids Res. 1981 Jul 10;9(13):3047–3060. doi: 10.1093/nar/9.13.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Rigby P. W., Lane D. P. Negative regulation of viral enhancers in undifferentiated embryonic stem cells. Cell. 1985 Sep;42(2):519–526. doi: 10.1016/0092-8674(85)90109-6. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hosler B. A., LaRosa G. J., Grippo J. F., Gudas L. J. Expression of REX-1, a gene containing zinc finger motifs, is rapidly reduced by retinoic acid in F9 teratocarcinoma cells. Mol Cell Biol. 1989 Dec;9(12):5623–5629. doi: 10.1128/mcb.9.12.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones-Villeneuve E. M., McBurney M. W., Rogers K. A., Kalnins V. I. Retinoic acid induces embryonal carcinoma cells to differentiate into neurons and glial cells. J Cell Biol. 1982 Aug;94(2):253–262. doi: 10.1083/jcb.94.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones-Villeneuve E. M., Rudnicki M. A., Harris J. F., McBurney M. W. Retinoic acid-induced neural differentiation of embryonal carcinoma cells. Mol Cell Biol. 1983 Dec;3(12):2271–2279. doi: 10.1128/mcb.3.12.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T., Takahashi N., Muramatsu M. The regulation of the murine Hox-2.5 gene expression during cell differentiation. Nucleic Acids Res. 1992 Nov 11;20(21):5729–5735. doi: 10.1093/nar/20.21.5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruithof E. K., Cousin E. Plasminogen activator inhibitor 2. Isolation and characterization of the promoter region of the gene. Biochem Biophys Res Commun. 1988 Oct 14;156(1):383–388. doi: 10.1016/s0006-291x(88)80852-0. [DOI] [PubMed] [Google Scholar]
- La Thangue N. B., Rigby P. W. An adenovirus E1A-like transcription factor is regulated during the differentiation of murine embryonal carcinoma stem cells. Cell. 1987 May 22;49(4):507–513. doi: 10.1016/0092-8674(87)90453-3. [DOI] [PubMed] [Google Scholar]
- La Thangue N. B., Thimmappaya B., Rigby P. W. The embryonal carcinoma stem cell Ela-like activity involves a differentiation-regulated transcription factor. Nucleic Acids Res. 1990 May 25;18(10):2929–2938. doi: 10.1093/nar/18.10.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRosa G. J., Gudas L. J. An early effect of retinoic acid: cloning of an mRNA (Era-1) exhibiting rapid and protein synthesis-independent induction during teratocarcinoma stem cell differentiation. Proc Natl Acad Sci U S A. 1988 Jan;85(2):329–333. doi: 10.1073/pnas.85.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. A., Bindereif A., Green M. R. A small-scale procedure for preparation of nuclear extracts that support efficient transcription and pre-mRNA splicing. Gene Anal Tech. 1988 Mar-Apr;5(2):22–31. doi: 10.1016/0735-0651(88)90023-4. [DOI] [PubMed] [Google Scholar]
- Lenardo M. J., Staudt L., Robbins P., Kuang A., Mulligan R. C., Baltimore D. Repression of the IgH enhancer in teratocarcinoma cells associated with a novel octamer factor. Science. 1989 Jan 27;243(4890):544–546. doi: 10.1126/science.2536195. [DOI] [PubMed] [Google Scholar]
- Levine M., Manley J. L. Transcriptional repression of eukaryotic promoters. Cell. 1989 Nov 3;59(3):405–408. doi: 10.1016/0092-8674(89)90024-x. [DOI] [PubMed] [Google Scholar]
- Luckow B., Schütz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res. 1987 Jul 10;15(13):5490–5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y., Fujii H., Gerster T., Roeder R. G. A novel B cell-derived coactivator potentiates the activation of immunoglobulin promoters by octamer-binding transcription factors. Cell. 1992 Oct 16;71(2):231–241. doi: 10.1016/0092-8674(92)90352-d. [DOI] [PubMed] [Google Scholar]
- Lüscher B., Mitchell P. J., Williams T., Tjian R. Regulation of transcription factor AP-2 by the morphogen retinoic acid and by second messengers. Genes Dev. 1989 Oct;3(10):1507–1517. doi: 10.1101/gad.3.10.1507. [DOI] [PubMed] [Google Scholar]
- Martin G. R. Teratocarcinomas and mammalian embryogenesis. Science. 1980 Aug 15;209(4458):768–776. doi: 10.1126/science.6250214. [DOI] [PubMed] [Google Scholar]
- McBurney M. W., Reuhl K. R., Ally A. I., Nasipuri S., Bell J. C., Craig J. Differentiation and maturation of embryonal carcinoma-derived neurons in cell culture. J Neurosci. 1988 Mar;8(3):1063–1073. doi: 10.1523/JNEUROSCI.08-03-01063.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer D., Graus A., Kraay R., Langeveld A., Mulder M. P., Grosveld G. The octamer binding factor Oct6: cDNA cloning and expression in early embryonic cells. Nucleic Acids Res. 1990 Dec 25;18(24):7357–7365. doi: 10.1093/nar/18.24.7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy S. P., Garbern J., Odenwald W. F., Lazzarini R. A., Linney E. Differential expression of the homeobox gene Hox-1.3 in F9 embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5587–5591. doi: 10.1073/pnas.85.15.5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K., Okazawa H., Okuda A., Sakai M., Muramatsu M., Hamada H. A novel octamer binding transcription factor is differentially expressed in mouse embryonic cells. Cell. 1990 Feb 9;60(3):461–472. doi: 10.1016/0092-8674(90)90597-8. [DOI] [PubMed] [Google Scholar]
- Rudnicki M. A., Sawtell N. M., Reuhl K. R., Berg R., Craig J. C., Jardine K., Lessard J. L., McBurney M. W. Smooth muscle actin expression during P19 embryonal carcinoma differentiation in cell culture. J Cell Physiol. 1990 Jan;142(1):89–98. doi: 10.1002/jcp.1041420112. [DOI] [PubMed] [Google Scholar]
- Schöler H. R., Balling R., Hatzopoulos A. K., Suzuki N., Gruss P. Octamer binding proteins confer transcriptional activity in early mouse embryogenesis. EMBO J. 1989 Sep;8(9):2551–2557. doi: 10.1002/j.1460-2075.1989.tb08393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöler H. R., Ciesiolka T., Gruss P. A nexus between Oct-4 and E1A: implications for gene regulation in embryonic stem cells. Cell. 1991 Jul 26;66(2):291–304. doi: 10.1016/0092-8674(91)90619-a. [DOI] [PubMed] [Google Scholar]
- Simeone A., Acampora D., Arcioni L., Andrews P. W., Boncinelli E., Mavilio F. Sequential activation of HOX2 homeobox genes by retinoic acid in human embryonal carcinoma cells. Nature. 1990 Aug 23;346(6286):763–766. doi: 10.1038/346763a0. [DOI] [PubMed] [Google Scholar]
- Strickland S., Mahdavi V. The induction of differentiation in teratocarcinoma stem cells by retinoic acid. Cell. 1978 Oct;15(2):393–403. doi: 10.1016/0092-8674(78)90008-9. [DOI] [PubMed] [Google Scholar]
- Suzuki N., Rohdewohld H., Neuman T., Gruss P., Schöler H. R. Oct-6: a POU transcription factor expressed in embryonal stem cells and in the developing brain. EMBO J. 1990 Nov;9(11):3723–3732. doi: 10.1002/j.1460-2075.1990.tb07585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukiyama T., Niwa O., Yokoro K. Analysis of the binding proteins and activity of the long terminal repeat of Moloney murine leukemia virus during differentiation of mouse embryonal carcinoma cells. J Virol. 1991 Jun;65(6):2979–2986. doi: 10.1128/jvi.65.6.2979-2986.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Oketani M., Watanabe T. Positive and negative regulation of immunoglobulin gene expression by a novel B-cell-specific enhancer element. Mol Cell Biol. 1991 Jan;11(1):75–83. doi: 10.1128/mcb.11.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin J. H., Cowie A., Lachance P., Hassell J. A. Molecular cloning and characterization of PEA3, a new member of the Ets oncogene family that is differentially expressed in mouse embryonic cells. Genes Dev. 1992 Mar;6(3):481–496. doi: 10.1101/gad.6.3.481. [DOI] [PubMed] [Google Scholar]
- de Groot R. P., Schoorlemmer J., van Genesen S. T., Kruijer W. Differential expression of jun and fos genes during differentiation of mouse P19 embryonal carcinoma cells. Nucleic Acids Res. 1990 Jun 11;18(11):3195–3202. doi: 10.1093/nar/18.11.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]