Abstract
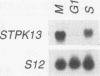
We determined the nucleotide sequence of a mouse and a human cDNA, which we designate STPK13, that encodes an apparent protein kinase related to that encoded by the Drosophila melanogaster polo gene and the Saccharomyces cerevisiae CDC5 gene. The polo and CDC5 gene products are required for normal mitosis. The STPK13 mRNA is regulated during terminal erythrodifferentiation and during the cell cycle. Within the precommitment period of murine erythroleukemia cell terminal differentiation, most of the poly(A) tail is lost from the STPK13 mRNA, but the body of the mRNA remains unchanged in abundance; this poly(A) loss does not occur in mutant erythroleukemia cells that fail to commit to terminal differentiation. During the cell cycle, the abundance of the body of the STPK13 mRNA fluctuates. The mRNA is present in growing but not in nongrowing cells. It reaches a maximum abundance during G2/M phase, is absent or present at only low levels during G1 phase, and begins to reaccumulate at approximately the middle of S phase. The cell cycle-associated accumulation and loss of the STPK13 mRNA could cause a similar fluctuation in abundance of its encoded protein kinase, thereby providing a maximum amount during M phase, when the kinase is thought to function, and little or none at other times of the cell cycle. Posttranscriptional regulation must be responsible for the cell cycle-associated fluctuations because transcription rates are relatively constant during different times of the cell cycle when there are large differences in mRNA abundance.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachvarova R. F. A maternal tail of poly(A): the long and the short of it. Cell. 1992 Jun 12;69(6):895–897. doi: 10.1016/0092-8674(92)90606-d. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clay F. J., McEwen S. J., Bertoncello I., Wilks A. F., Dunn A. R. Identification and cloning of a protein kinase-encoding mouse gene, Plk, related to the polo gene of Drosophila. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):4882–4886. doi: 10.1073/pnas.90.11.4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton B., Glover D. M. A conserved mitotic kinase active at late anaphase-telophase in syncytial Drosophila embryos. Nature. 1993 Jun 17;363(6430):637–640. doi: 10.1038/363637a0. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Gusella J., Geller R., Clarke B., Weeks V., Housman D. Commitment to erythroid differentiation by friend erythroleukemia cells: a stochastic analysis. Cell. 1976 Oct;9(2):221–229. doi: 10.1016/0092-8674(76)90113-6. [DOI] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M. Protein kinase catalytic domain sequence database: identification of conserved features of primary structure and classification of family members. Methods Enzymol. 1991;200:38–62. doi: 10.1016/0076-6879(91)00126-h. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H. Sequential function of gene products relative to DNA synthesis in the yeast cell cycle. J Mol Biol. 1976 Jul 15;104(4):803–817. doi: 10.1016/0022-2836(76)90183-2. [DOI] [PubMed] [Google Scholar]
- Huarte J., Stutz A., O'Connell M. L., Gubler P., Belin D., Darrow A. L., Strickland S., Vassalli J. D. Transient translational silencing by reversible mRNA deadenylation. Cell. 1992 Jun 12;69(6):1021–1030. doi: 10.1016/0092-8674(92)90620-r. [DOI] [PubMed] [Google Scholar]
- Hunter T. A thousand and one protein kinases. Cell. 1987 Sep 11;50(6):823–829. doi: 10.1016/0092-8674(87)90509-5. [DOI] [PubMed] [Google Scholar]
- Jackson R. J., Standart N. Do the poly(A) tail and 3' untranslated region control mRNA translation? Cell. 1990 Jul 13;62(1):15–24. doi: 10.1016/0092-8674(90)90235-7. [DOI] [PubMed] [Google Scholar]
- Jacobs T. Control of the cell cycle. Dev Biol. 1992 Sep;153(1):1–15. doi: 10.1016/0012-1606(92)90087-w. [DOI] [PubMed] [Google Scholar]
- Kitada K., Johnson A. L., Johnston L. H., Sugino A. A multicopy suppressor gene of the Saccharomyces cerevisiae G1 cell cycle mutant gene dbf4 encodes a protein kinase and is identified as CDC5. Mol Cell Biol. 1993 Jul;13(7):4445–4457. doi: 10.1128/mcb.13.7.4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. An analysis of vertebrate mRNA sequences: intimations of translational control. J Cell Biol. 1991 Nov;115(4):887–903. doi: 10.1083/jcb.115.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llamazares S., Moreira A., Tavares A., Girdham C., Spruce B. A., Gonzalez C., Karess R. E., Glover D. M., Sunkel C. E. polo encodes a protein kinase homolog required for mitosis in Drosophila. Genes Dev. 1991 Dec;5(12A):2153–2165. doi: 10.1101/gad.5.12a.2153. [DOI] [PubMed] [Google Scholar]
- Norbury C., Nurse P. Animal cell cycles and their control. Annu Rev Biochem. 1992;61:441–470. doi: 10.1146/annurev.bi.61.070192.002301. [DOI] [PubMed] [Google Scholar]
- Nüsse M., Egner H. J. Can nocodazole, an inhibitor of microtubule formation, be used to synchronize mammalian cells? Accumulation of cells in mitosis studied by two parametric flow cytometry using acridine orange and by DNA distribution analysis. Cell Tissue Kinet. 1984 Jan;17(1):13–23. [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrali-Noy G., Spadari S., Miller-Faurès A., Miller A. O., Kruppa J., Koch G. Synchronization of HeLa cell cultures by inhibition of DNA polymerase alpha with aphidicolin. Nucleic Acids Res. 1980 Jan 25;8(2):377–387. doi: 10.1093/nar/8.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelech S. L., Sanghera J. S. Mitogen-activated protein kinases: versatile transducers for cell signaling. Trends Biochem Sci. 1992 Jun;17(6):233–238. doi: 10.1016/s0968-0004(00)80005-5. [DOI] [PubMed] [Google Scholar]
- Skowronski J., Singer M. F. Expression of a cytoplasmic LINE-1 transcript is regulated in a human teratocarcinoma cell line. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6050–6054. doi: 10.1073/pnas.82.18.6050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunkel C. E., Glover D. M. polo, a mitotic mutant of Drosophila displaying abnormal spindle poles. J Cell Sci. 1988 Jan;89(Pt 1):25–38. doi: 10.1242/jcs.89.1.25. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Shiraishi T., Sasaki H., Oishi M. Inhibitors for protein-tyrosine kinases, ST638 and genistein: induce differentiation of mouse erythroleukemia cells in a synergistic manner. Exp Cell Res. 1989 Aug;183(2):335–342. doi: 10.1016/0014-4827(89)90394-7. [DOI] [PubMed] [Google Scholar]
- Wilks A. F. Two putative protein-tyrosine kinases identified by application of the polymerase chain reaction. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1603–1607. doi: 10.1073/pnas.86.5.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]