Abstract
Evidence of antibody isotype/subtype switching may provide prognostic value regarding the state of immune responses to therapeutic proteins, e.g. anti-factor VIII (FVIII) antibodies that develop in many hemophilia A patients, clinically termed “inhibitors”. A sensitive, high- information-content surface plasmon resonance (SPR) assay has been developed to quantify IgG subtype distributions and the domain specificity of anti-drug antibodies. Plasma samples from 22 subjects with an allo- or auto-immune reaction to FVIII were analyzed. Pre-analytical treatment protocols were developed to minimize non-specific binding and specific matrix interference due to von Willebrand factor-FVIII interactions. The dynamic range for IgG quantification was 0.2–5 µg/ml (∼1–33 nM), allowing characterization of inhibitor-positive samples. Subtype-specific monoclonal antibodies were used to quantify the IgG subtype distribution of FVIII-specific antibodies. Most samples obtained from multiply-infused inhibitor subjects contained IgG4 antibodies. Several distinct phenotypes were assigned based on the IgG subtype distribution: IgG1, IgG4, IgG1 & IgG4, and IgG1, IgG2 & IgG4. An IgG1-only response was found in mild/moderate HA subjects during early FVIII infusions, and analysis of serial samples followed antibody class switching as several subjects’ immune responses developed. Competition studies utilizing a recombinant FVIII-C2 domain indicated 40–80% of FVIII-specific antibodies in most samples were directed against this domain.
Introduction
The development of anti-FVIII allo-antibodies (“inhibitors”) occurs in a significant proportion of congenital Hemophilia A (HA) patients receiving exogenous FVIII, thereby rendering protein replacement therapy ineffective [1]. Additionally, anti-FVIII auto-antibody responses, though rare, can also occur, primarily in the elderly, postpartum or following traumatic injury. Allo antibodies develop as an anti-drug antibody response to FVIII infusions used to treat HA, and earlier detection and characterization of these responses may be useful to clinicians, e.g. as they tailor FVIII infusion schedules or consider immunosuppression regimes based on the perceived risk of a given patient developing a higher-titer response. In contrast, FVIII autoantibodies are virtually always diagnosed after they have reached a high titer, as testing is carried out after a non-hemophilic patient presents with unexplained bleeding and/or bruising. Clinical diagnosis of inhibitors is based on the Bethesda assay, a functional measurement of the inhibition of FVIII-mediated clotting of normal human plasma by antibodies in test plasma [2], [3]. An inhibitor titer of 1 Bethesda Unit (BU)/ml inhibits FVIII activity in normal pooled plasma by 50%. Non-inhibitory anti-FVIII antibodies are not detected by the Bethesda assay and quantification of inhibitors becomes unreliable when responses are <1 BU/ml; alternative assays are required to accurately quantify low-titer anti-FVIII antibodies. Although inhibitory antibodies are the primary concern when attempting to restore hemostatic function, both inhibitory and non-inhibitory antibodies provide information about the immunological state of a patient. A number of sensitive immunoassays have been developed to allow the screening of clinical samples for total (inhibitory+non-inhibitory) anti-FVIII antibodies and to provide complementary information to the Bethesda assay [4]–[9].
Early stages of alloimmune responses to FVIII include stimulation of helper T cells, which secrete cytokines leading to production of anti-FVIII antibodies by plasma cells, antibody class switching, affinity maturation, and generation of antibodies recognizing specific epitopes on the FVIII surface [10]. The complexity of these responses, for example the immunoglobulin isotypes and subtypes involved, the number of epitopes recognized, the clonality (polyclonal, oligoclonal, monoclonal) of the response, and the antibody affinities, provides important information as to the phenotypes of developing immune responses. Detailed characterization of the early stages of anti-drug antibody responses may provide information needed to design new clinical assays and may also indicate mechanisms leading to high-titer inhibitors versus immune tolerance (defined operationally for HA patients as having either no anti-FVIII antibodies or a low-titer response that does not seriously compromise hemostasis).
Comprehensive characterization of complex anti-FVIII antibody responses can be time- and resource intensive and numerous technical challenges, including inadequate sensitivity, exist. Surface Plasmon Resonance (SPR) offers a detection platform that is versatile, robust, and amenable to complex, multiplexed measurements of plasma samples. The relative speed with which SPR sensorgrams can be generated and analyzed also makes this technique suitable for medium- to high-throughput analysis of multiple samples. This report describes the use of an SPR assay to define phenotypes of allo- and autoimmune antibody responses based on antigen-specific IgG subclass distribution and epitope (FVIII domain) specificity. Plasma samples were collected from 18 HA and four acquired HA (autoimmune) patients with developing or persistent immune responses. Serial samples were collected from one young HA subject as he received initial FVIII infusions, and from one mild HA subject and two autoimmune HA subjects beginning with their initial inhibitor diagnosis. Although correlation of phenotypes with clinical outcomes is not definitive due to the small set of ADA-positive samples analyzed herein, the current study lays groundwork for analyzing plasma/serum samples from larger studies, including prospective studies. The stability and sensitivity of the SPR assay platform is demonstrated, and specific measurements containing clinically relevant information are identified, e.g. the quantitative distribution of antigen-specific IgG subtypes and the domain specificity of human anti-FVIII antibodies, specifically the fraction directed against the FVIII-C2 versus other domains.
Materials and Methods
Ethics Statement
This study was approved by the Seattle Children's Hospital IRB (SCH IRB#13018). Written informed consent was obtained from all adult subjects and from the next of kin, caretakers, or guardians on the behalf of the minors/children participants involved in this study, according to the principles expressed in the Declaration of Helsinki.
Reagents
Expired Recombinatetm (Baxter) was reconstituted as directed and used without further manipulation as the source of full-length human FVIII. Amino-terminally His10-tagged FVIII-C2 domain was produced as a soluble cytoplasmic protein in E. coli OrigamiB(DE3)pLysS (EMD Chemicals, Gibbstown, NJ). Caprylic acid, carboxy methyl dextran, 99.5% L-arginine and other reagents were from Sigma (St. Louis, MO). CM5 sensor chips, 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide HCl (EDC), N-hydroxysuccinimide (NHS), ethanolamine, HBS-P+ buffer (10 mM HEPES, 150 mM NaCl, 0.05% (v/v) surfactant P20, pH 7.4) and sodium acetate pH 5.0 were from GE Healthcare Life Sciences (Piscataway, NJ).
Antibodies
Mouse anti-human FVIII-A1 domain specific mAb (clone GMA-8004) was generously provided by Green Mountain Antibodies. An additional FVIII-C2 domain specific antibody (ESH4) was from American Diagnostica (Stamford, CT). Monoclonal anti-huIgG1 (clone HP6188) was obtained from Fitzgerald Industries International (Acton, MA). Anti-huIgG2 (clone HP6002), anti huIgG3 (clones HP6050 and HP6047), and anti-huIgG4 (clone HP6023) were from Southern Biotech (Birmingham, AL). Anti-huIgA (clone 8203) and anti-huIgM (clone 7408) were from Medix Biochemica (Finland). The human anti-FVIII-C2 mAb B02C11, both the IgG4 and Fab forms, were generously provided by Dr. M. Jacquemin [11]. Antibody concentrations were measured using a nominal extinction coefficient of ε280 nm,0.1% = 1.38.
Plasma Samples
Blood samples from subjects with HA and with autoimmune responses to FVIII (acquired HA) were collected as part of a cross-sectional study (NIH 1RC2HL101851) or were obtained from a Repository maintained by the Pratt laboratory. Plasma samples from subjects with and without a recently measured inhibitor titer in BU/ml were characterized using the SPR assay. Two types of samples were studied: sodium citrate anti-coagulated plasma (citrated plasma) and diluted heparin-anticoagulated plasma retained following isolation of peripheral blood mononuclear cells (“Ficoll plasma”).
Pre-analytical Treatment
Pre-analytical treatment of plasma samples was performed using caprylic acid (CA) to precipitate non-IgG proteins and other interfering substances, including von Willebrand factor and hence baseline circulating FVIII (“CA treated plasma”). Citrated plasma samples (100–500 µL) were treated by mixing 1 part plasma with 2 parts 40 mM sodium acetate pH 4.0 and adding CA to a final concentration of 2.5% v/v (158 mM). Following 60 min incubation at room temperature with occasional mixing, samples were centrifuged for 5 minutes at 16,000×g to pellet the precipitate and filtered using a 0.2 µm Spin-X filter (Corning). The transparent filtrate was neutralized by adding 1 part to 9 parts 800 mM HEPES pH 8.0, 4 M NaCl and 5% carboxy methyl dextran. Ficoll plasma (typically 2–6 fold diluted) was treated similarly, however initial acidification was performed by adding 1 part to 9 parts 400 mM sodium acetate pH 4.0 to minimize further dilution. Nominal pre-analytical dilution factors were calculated for each sample.
SPR Method
SPR measurements were carried out using a Biacore T-100 instrument (GE Healthcare Life Sciences) with binding measurements taken at 25°C. Murine anti-FVIII-A1 (GMA-8004) capture antibody was immobilized covalently onto a CM5 sensor chip from a 100 µg/ml solution in 10 mM sodium acetate pH 5.0 using a mixture of 0.4 M EDC and 0.1 M NHS. After immobilizing the capture antibody, the remaining active sites on the sensor chip were blocked by treatment with 1 M ethanolamine. A final immobilization signal of 9000 RU was targeted.
Binding experiments were performed in HBS-P+ containing 5 mM CaCl2 (HBS-P+/Ca2+). All injection and binding steps were performed at a slow flow rate (5 µl/min) to minimize FVIII, test plasma and secondary mAb consumption. FVIII (2000–3500 RU) was captured on the GMA-8004 antibody surface by injecting undiluted drug product for 300–600 sec. The dissociation of FVIII from this mAb was slow enough that the effect on RU signals measured at the report points was negligible (Figure S1). CA-treated plasma samples were injected for 300 sec followed by sequential 120 sec injections of 25–50 µg/ml secondary (isotype-specific) mAbs. Regeneration of the capture surface was achieved with three 20 sec injections of 2 M arginine pH 3.0 at 30 µl/min. To confirm that CA treatment did not alter the anti-FVIII IgG content of the test plasma, independent samples of untreated inhibitor negative HA plasma containing 1 µg/ml B02C11 (human IgG4) were prepared, CA treated, and the RU signals were compared.
To measure the fraction of the antibody response specific for the FVIII-C2 domain, paired plasma samples from four inhibitor subjects were tested by SPR with and without the addition of increasing concentrations of recombinant FVIII-C2 protein (the CA-treated plasma samples were added to either FVIII-C2 or the same volume of PBS as a negative control). Plasma samples were diluted first if necessary to bring the total anti-FVIII IgG titer below 5 µg/ml (∼33 nM). Samples were vortexed, centrifuged, and the supernatants stored at 4°C until analysis by SPR.
Data Analysis
The SPR experiments were carried out under saturation binding conditions for the secondary mAbs to determine the maximum signal from each secondary mAb. This should correspond to stoichiometric binding of the secondary mAbs to the primary IgGs from plasma. Since the nominal molecular weights of human plasma anti-FVIII IgG and mouse anti-human IgG mAbs are comparable (∼150 kDa), the binding signal (RU) for both primary (binding of human anti-FVIII antibodies to the captured FVIII) and secondary (binding of subtype-specific mouse mAbs to human IgG captured from plasma) events should be directly comparable. Quantitative measurements (report points) of FVIII capture level, primary human IgG binding level, and secondary mAb binding levels were recorded 30 sec after the end of each sequential injection step.
Singly referenced binding curves were recorded as the signal from an active flow cell (with captured FVIII) minus the signal from a reference flow cell (without FVIII). Each assay sequence contained mAb B02C11 calibrators (0, 0.2, 1.0, 2.0, and 5.0 µg/ml prepared using CA-treated inhibitor negative HA plasma). Since the FVIII capture level declined slowly over the course of each sequence of samples (due to gradual degradation of the capture mAb following multiple regeneration cycles) and subsequent binding of plasma Abs and secondary mAbs scaled with the FVIII capture level, all binding signals were first normalized to a nominal capture level of 3000 RU FVIII. Calibrators and test samples were typically tested in blocks of 5 injections that were bracketed by a complete injection cycle in which assay buffer was substituted for the test sample. The average binding signals for the bracketing buffer injections were subtracted from the test sample signals to correct for minor signal variations due to incomplete regeneration and/or sensor degradation. Binding signals were converted from RU to µg/ml IgG using the secondary binding levels for the B02C11 calibrators. The ratios of the total cumulative secondary mAb binding signal to the primary human antibody binding signal were also calculated.
Results
Assay Performance
Acceptable assay performance was typically achieved for 100–150 cycles with a single sensor chip. FVIII capture capacity declined slowly, but this was not typically a limitation. A more significant limitation was a progressive increase in non-sample-specific secondary antibody binding signal, necessitating the frequent inclusion of bracketing injections of buffer before and after the injection of plasma samples. Therefore, subtraction of reference RU values sometimes caused apparent negative referenced binding signals for samples with very low measured RU binding signals, e.g. the % anti-IgG2 signals from several plasma samples ( Tables 1 – 3 ). If the response (in RUs) of bracketing buffer injections was reproducible, sample signals were corrected by subtracting the mean signals from the bracketing buffer injections. If not, samples were retested using a new sensor chip.
Table 1. Antibody subtypes and estimated titers by SPR.
| Subject | IgG1+2+3+4 (RU)a/polyclonal IgG (RU) | % IgG1 | % IgG2 | % IgG3 | %IgG4 | Total anti-FVIII IgG from SPR (µg/ml) |
| Predominantly IgG1 Response | ||||||
| 17A (n = 2) b | 1.11(.02)c | 95%(2%) | 0%(1%) | −1%(0%) | 5%(1%) | 3.11(0.62) |
| 17A+FVIII-C2 | NDd | ND | ND | ND | ND | <0.2 |
| N-008 | 0.98 | 104% | −6% | 1% | 1% | 5.45 |
| N-008+ FVIII-C2 | 1.01 | 106% | −10% | 3% | 0% | 2.4 |
| L-006-001 | 0.87 | 92% | 4% | 4% | 0% | 11.85 |
| L-006-001+ FVIII-C2 | 0.83 | 88% | 7% | 5% | −1% | 4.89 |
| Predominantly IgG4 Response | ||||||
| F-014 (n = 2) | 1.26(.07) | 16%(1%) | −1%(0%) | 0%(1%) | 85%(1%) | 2.67(0.53) |
| F-014+ FVIII-C2(n = 2) | 1.49(.13) | 14%(1%) | −17%(0%) | 2%(2%) | 101%(3%) | 1.09(0.18) |
| B-002 | 1.24 | 8% | 7% | −1% | 86% | 2.42 |
| B-002+ FVIII-C2 | 1.26 | 7% | 7% | −1% | 86% | 2.38 |
| A-002 | 1.16 | 4% | 5% | −1% | 92% | 4.38 |
| A-002+ FVIII-C2 | 1.24 | 1% | 7% | −1% | 93% | 2.57 |
| Mixed IgG Subtype Response | ||||||
| G-004 | 1.13 | 43% | −4% | −1% | 62% | 9.1 |
| G-004+ FVIII-C2 | 1.25 | 42% | −10% | −1% | 69% | 5.11 |
| C-010 | 0.81 | 80% | −3% | −2% | 25% | 1.59 |
| C-010+ FVIII-C2 | 0.82 | 55% | −3% | −2% | 50% | 0.9 |
| D-006 (n = 3) | 2.04(.4) | 45%(3%) | −6%(7%) | 1%(1%) | 61%(3%) | 1.53(0.41) |
| D-006+ FVIII-C2 | ND | ND | ND | ND | ND | <0.2 |
| L-025 | 1.12 | 72% | 1% | −1% | 28% | 3.56 |
| L-025+ FVIII-C2 | 1.15 | 64% | 3% | −1% | 34% | 2.17 |
| P-011 | 1.18 | 38% | 1% | −1% | 61% | 18.29 |
| P-011+ FVIII-C2 | 1.2 | 29% | −1% | −1% | 73% | 11.28 |
| P-001 | 0.98 | 23% | 3% | 0% | 75% | 22.58 |
| P-001+ FVIII-C2 | 1.02 | 34% | 4% | −1% | 63% | 3.97 |
| F-006 | 1.01 | 31% | 8% | −2% | 62% | 24.94 |
| F-006+ FVIII-C2 | 1.19 | 42% | 6% | −2% | 54% | 6.46 |
| A-008 | 1.09 | 41% | 11% | −1% | 49% | 3.78 |
| A-008+ FVIII-C2 | 1.12 | 36% | 11% | −1% | 54% | 2.02 |
| F-025 (n = 2) | 2.19(.51) | 30%(1%) | 22%(4%) | −1%(0%) | 50%(3%) | 0.90(0.16) |
| F-025+ FVIII-C2 | ND | ND | ND | ND | ND | <0.2 |
| C-019 | 4.07 | 61% | −9% | −1% | 49% | 1.62 |
| C-019+ FVIII-C2 | 1.25 | 63% | −8% | −3% | 48% | 1.52 |
| C-028 | 0.94 | 19% | 14% | −1% | 68% | 8.17 |
| C-028+ FVIII-C2 | 0.74 | 16% | 18% | −2% | 69% | 4.18 |
| Primary binding to FVIII signal (in RU) does not match summed IgG1+IgG2+IgG3+IgG4 signal (in RU) | ||||||
| H-001 | 0.22 | 56% | 29% | −4% | 19% | 2.54 |
| H-001+ FVIII-C2 | 0.15 | 56% | 34% | −5% | 15% | 0.96 |
| Autoimmune Subjects | ||||||
| Q-011-001 | 0.96 | 79% | 4% | −1% | 18% | 34.25 |
| Q-011-001+ FVIII-C2 | 0.98 | 79% | 4% | −1% | 18% | 33.19 |
| Q-012-001 (n = 4) | 1.02(.04) | 6%(1%) | 2%(4%) | −1%(0%) | 94%(5%) | 6.40(3.66) |
| Q-012-001+ FVIII-C2 | 1.23 | 2% | −4% | −1% | 103% | 2.78 |
| Q-033 (n = 2) | 0.75(.06) | 82%(1%) | 6%(1%) | −1%(0%) | 13%(0%) | 23.97 |
| Q-033+ FVIII-C2 | 0.85 | 89% | 2% | −1% | 10% | 11.39 |
| Q-016 (n = 2) | 0.96(.01) | 23%(1%) | 8%(1%) | −1%(0%) | 70%(3%) | 26.88 |
| Q-016+ FVIII-C2 (n = 2) | 1.02(.03) | 23%(1%) | 5%(2%) | −1%(0%) | 72%(3%) | 11.19(1.54) |
The ratios indicate the agreement between the summed SPR signals from the binding of secondary detection antibodies specific for IgG1, IgG2, IgG3 and IgG4 (numerator) to the initial SPR signal generated by the anti-FVIII antibodies in plasma that bound to the immobilized FVIII (denominator).
Multiple measurements (n) were made when sufficient plasma was available.
Standard deviations are reported for these experiments in parentheses.
ND = Not Determined because the low total IgG titer made estimates of ratios and %Ig subtypes unreliable.
Table 3. Clinical data for subjects.
| Subject | Age | HA Severity | Baseline FVIII | Peak Titer(BU/ml)a | Inhibitor Treatment History | Hemophilia Genotype(if known) |
| Predominantly IgG1 Response | ||||||
| 17A | 24 | mild | 6–14% | 250 | ITI failedb | A2201P |
| N-008 | 2 | moderate | 3% | 11 | no ITI | 14–21 delc |
| L-006 | 2 | moderate | 1% | 87 | ITI initiated | R2304C |
| Predominantly IgG4 Response | ||||||
| F-014 | 19 | severe | <1% | 32 | ITI partly successful | int-22 invd |
| B-002 | 20 | severe | <1% | 667 | ITI failed | 9–11 dele |
| A-002 | 14 | severe | <1% | 256 | ITI failed | not inversionf |
| Mixed IgG Subtype Response | ||||||
| G-004 | 16 | severe | <1% | 1000+ | no ITI | int-22 inv |
| C-010 | 27 | severe | <1% | 80 | ITI partly successful | not inversion |
| D-006 | 10 | severe | <1% | 496 | ITI failed | not inversion |
| L-025 | 35 | severe | <1% | 191 | no ITI | not inversion |
| P-011 | 8 | severe | <1% | 1084.4 | ITI failed | int-22 inv |
| P-001 | 12 | severe | <1% | 308.7 | ITI failed | int-22 inv |
| F-006 | 27 | severe | <0.25% | 44 | no ITI | int-22 inv |
| A-008 | 31 | severe | <1% | 86 | ITI successful | int-22 inv |
| F-025 | 21 | severe | <1% | 43.8 | ITI failed | int-22 inv |
| C-019 | 60 | severe | <1% | 336 | ITI failed | int-22 inv |
| C-028 | 2 | severe | <1% | 96 | ITI failed | not inversion |
| Secondary and primary SPR binding signals (in RU) do not match | ||||||
| H-001 | 50 | severe | <1% | 742 | no ITI | int-22 inv |
| Autoimmune subjects | ||||||
| Q-011 | 77 | autoimmune | normal | 6 | prednisone | autoimmune |
| Q-012 | 77 | autoimmune | normal | 2 | prednisone | autoimmune |
| Q-033 | 79 | autoimmune | normal | 39 | prednisone | autoimmune |
| Q-016 | 62 | autoimmune | normal | 20 | prednisone | autoimmune |
BU/ml = Bethesda Units/milliliter;
ITI = Immune Tolerance Induction;
14–21del = exons 14–21 deleted;
int-22 inv = intron 22 inversion;
9–11del = exons 9–11 deleted;
not inversion = not an intron-22 or intron-1 inversion mutation.
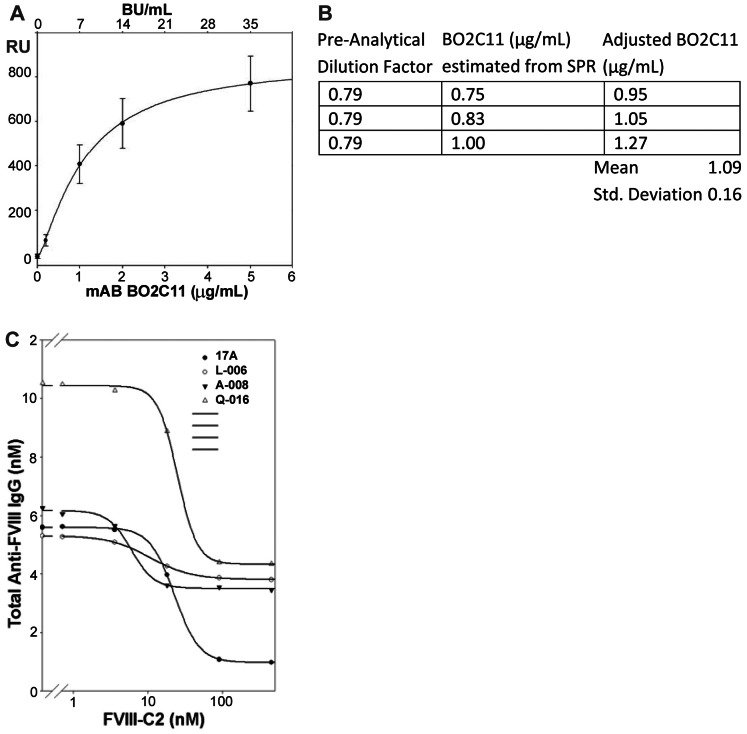
The use of affinity-captured FVIII antigen placed limits on the dynamic range of quantitative measurements. Although normalized calibration curves using the patient-derived inhibitory antibody BO2C11 were highly reproducible across multiple days and sensors ( Figure 1A ) the dynamic range for the SPR assay was narrow, with a range of quantification from 0.2 µg/ml (∼1 nM) to 5 µg/ml (∼33 nM). Below 0.2 µg/ml, signal to noise ratios were too low to obtain reliable information. BO2C11 binds to FVIII with an apparent dissociation constant KD ∼ 2×10−11 mol L−1 and inhibits its pro-coagulant activity with a specific activity of ∼7,000 BU/mg [11]; these spike-recovery assays indicated the lower limit for detection of this unusually high-affinity neutralizing antibody by SPR was 0.2 µg/ml ∼1.4 BU/ml. Above 5 µg/ml, accurate concentration measurements could not be obtained due to saturation of the affinity-captured FVIII, but the IgG subtype distribution could still be measured. The spike-recovery experiment in which 1.0 µg/ml (∼7 nM) B02C11 was added to plasma from a HA subject that contained no FVIII or anti-FVIII antibody, and then measured before and after CA treatment, demonstrated a recovery of 109±16% ( Figure 1B ). As expected for B02C11 (human IgG4), this response was IgG4–restricted and the ratio of secondary (anti huIgG1+ anti huIgG2+ anti huIgG3+ anti-huIgG4 signals) to primary (human anti-FVIII antibodies from the test plasma) binding RU signals was close to stoichiometric (94±10%). In addition to satisfactory recovery of B02C11 following CA treatment, the behavior of independently treated and tested samples from a given subject, including both citrated plasma and Ficoll-treated heparin-anticoagulated plasma, was reproducible when assayed using different sensors and with different sample preparations. Once treated with CA, the samples remained stable for several weeks at 4°C. SPR of four plasma samples incubated with different concentrations of FVIII-C2 showed that in all cases, the competitive response (recombinant FVIII-C2 displacing FVIII-C2-specific antibodies) was saturated by >100 nM FVIII-C2 ( Figure 1C ). Figure 2 depicts a representative binding curve for a plasma sample having a complex antibody phenotype with injection steps and report points annotated.
Figure 1. Characterization of plasma samples by SPR.
A) Representative BO2C11 calibration curve obtained from 8 independent SPR runs in which this mAb was added to a FVIII- and inhibitor-negative plasma sample that was pretreated with CA. The plasma used in these experiments showed no evidence of anti-FVIII antibodies when tested by SPR using the FVIII-capture format (not shown). The final added BO2C11 concentrations are shown below the x-axis and the FVIII inhibitor titers indicated in Bethesda units (BU)/ml are based on the specific activity of BO2C11 = 7,000 BU/mg [11]. B) Spike recovery of independent samples in which 1 µg/ml B02C11 was added to a FVIII- and inhibitor-negative plasma sample that was subsequently diluted and treated with CA and then analyzed by SPR. The measured RU values were converted to concentrations in µg/ml (central column) based on calibration curves generated for BO2C11 as shown in Figure 1A. The adjusted BO2C11 concentration (third column) is the measured BO2C11 concentration corrected for the 0.79 preanalytical dilution factor C) Titration inhibition curves showing addition of increasing concentrations of recombinant FVIII-C2 to CA-treated plasma from 4 inhibitor-positive subjects. The sample from subject Q-016 was diluted first in order to bring the total anti-FVIII antibody titer below 5 µg/mL (33 nM). The FVIII-C2-specific antibody fraction was saturated above 100 nM FVIII-C2 in all 4 samples.
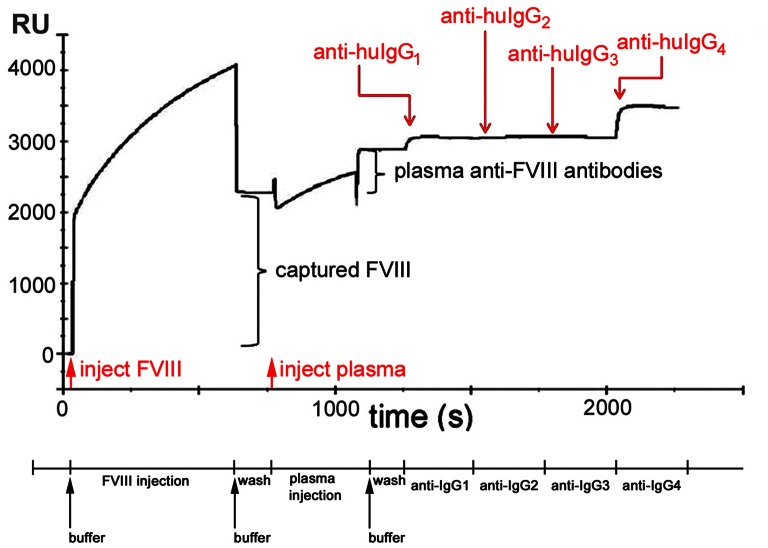
Figure 2. Representative binding curve (sensorgram) characterizing anti-FVIII antibodies in a human plasma sample.
The sensorgram depicts the injection and capture of FVIII, the injection of test plasma and capture of human anti-FVIII antibodies, and sequential 120 sec injections and binding of mouse anti-human IgG1, anti-huIgG2, anti-huIgG3, and anti-huIgG4. The sequence of sample injections and wash steps is indicated below the x-axis, while the sensorgram shows the sequential contributions to the signal in RU due to (1) capture of injected FVIII (2277 RU); (2) attachment of antibodies from plasma to captured FVIII (839 RU); (3) attachment of anti-IgG1 secondary detection mAb to anti-FVIII antibodies that are subclass IgG1 (191 RU); (4) attachment of anti-IgG2 secondary mAb to anti-FVIII antibodies that are subclass IgG2 (75 RU); (5) (negligible) attachment of anti-IgG3 secondary mAb to anti-FVIII antibodies that are subclass IgG3 (−9 RU); (6) attachment of anti-IgG4 secondary mAb to anti-FVIII antibodies that are subclass IgG4 (558 RU). The baseline signal is set to 0 RU for the sensor surface with the immobilized capture antibody GMA-8004. Red arrows indicate injection points for samples and black arrows indicate injections points for buffer to initiate the intermediate wash steps. Note that a mismatch in the refractive index between the injected solution and the assay buffer results in a transient upward baseline shift in signal (during the injection of the FVIII in formulation buffer) or a transient downward shift (during the injection of the CA treated plasma sample). Since the secondary detection antibodies were diluted into assay buffer, such transient baseline shifts are less evident following the later injections. In order to accurately measure the binding signals following injections of FVIII, plasma antibodies, and detection antibodies, baseline measurements were taken 10 seconds prior to each injection, and the binding level measurements (report points) were taken 30 seconds after the end of each injection, when the sensor was again exposed to assay buffer and the refractive index shift was resolved. These report points were used to obtain the quantitative results summarized in Tables 1&2.
HA Phenotypes
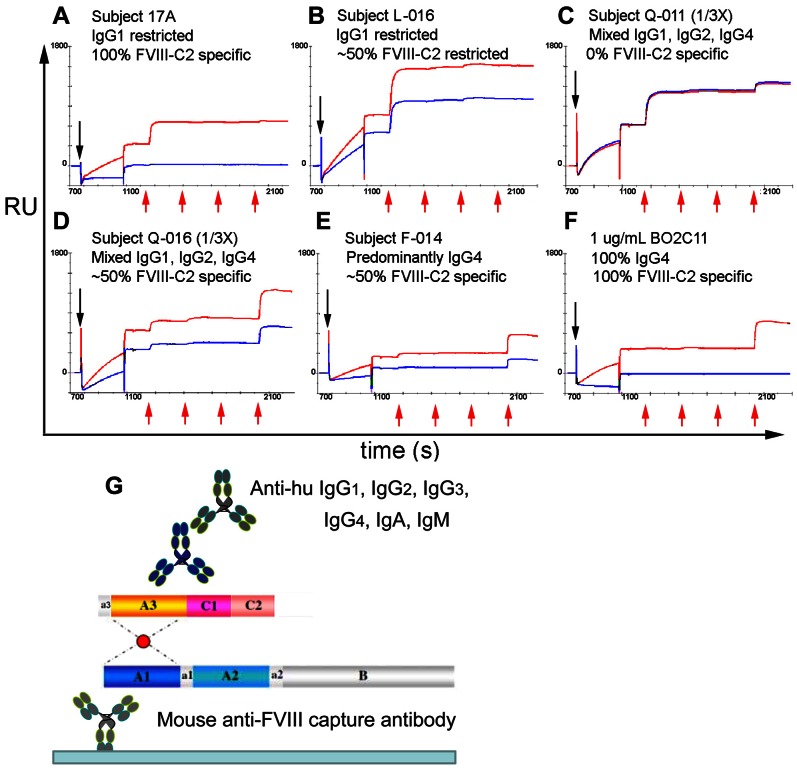
Representative binding curves illustrating the range of phenotypic responses are shown in Figure 3 . Each panel shows binding curves obtained in the presence and absence of excess (1 µM) FVIII-C2. Quantitative measurements (percent of the response derived from each human IgG subtype, total anti-FVIII IgG concentration (µg/ml), and the ratio of secondary to primary binding signal in %) obtained from the binding curves are tabulated in Tables 1 & 2 . The total anti-FVIII IgG concentrations were corrected for pre analytical dilution factors and thus reflect the concentrations in undiluted plasma. Likewise values <0.2 µg/ml (the lower limit of quantification) are reported based on the assay dynamic range corrected for the sample dilution factor. The % IgG subtype values were not considered reliable when the total anti-FVIII IgG of a diluted or undiluted sample was <0.2 µg/ml so they are not reported in the tables. Almost every permutation (IgG subtype distribution, proportion of FVIII-C2 specific antibodies, and anti-FVIII IgG concentration) of phenotypic response was observed. Two subjects (B-002 and Q-011) demonstrated a complete lack of competition with FVIII-C2, whereas anti-FVIII antibodies in one sample from subject 17A were completely specific for FVIII-C2. However, the most common response was a mixed IgG subtype distribution with 40–80% FVIII-C2 specificity. For both the cross-sectional, single time point samples and the serial samples, no significant divergence between the total IgG subtype distribution and the FVIII-C2-specific IgG subtype distribution was observed. Three HA subjects (17A, N-008 and L-006) exhibited a predominantly IgG1 restricted response. Another three HA subjects (F-014, B-002 and A-002) exhibited predominantly IgG4-restricted responses, however detectable levels of other IgG subtypes were also observed. Samples from the four autoimmune HA subjects (Q-011, Q-012, Q-016 and Q-033) all exhibited complex mixtures of IgG1, IgG2 and IgG4 in addition to high total anti-FVIII IgG concentrations. In addition to testing with IgG subtype-specific secondary antibodies, the samples were screened with anti-IgA and anti-IgM secondary antibodies (not shown). No samples in this study exhibited an IgA or IgM response.
Figure 3. Binding curves from matched plasma samples with and without the addition of saturating (1 µM) recombinant FVIII-C2.
(A–F) Black arrows pointing downwards indicate injection of CA treated plasma and red arrows indicate injections of anti-huIgG1, anti-huIgG2, anti-huIgG3 and anti-huIgG4. Injection of FVIII and its capture on mAb GMA-8004 (0–700 s) are not shown. (G) The Biosensor assay format is shown schematically.
Table 2. Antibody subtypes and estimated titers by SPR.
| Subject | FVIII-C2 competition assay | Time since inhibitor diagnosis | IgG1+2+3+4 (RU)a/polyclonal IgG (RU) | % IgG1 | % IgG2 | % IgG3 | %IgG4 | Total anti-FVIII IgG (µg/ml) |
| Q-011 | day1 | 0.96 | 79% | 4% | −1% | 18% | 34.25 | |
| day4 | 0.96 | 71% | 9% | −1% | 22% | 27.43 | ||
| 4 wk | 1.2 | 78% | 5% | −1% | 18% | 22.49 | ||
| 6 wk (n = 3)b | 1.09(.30)c | 69%(4%) | 10%(1%) | −2%(1%) | 23%(2%) | 6.02(3.79) | ||
| 8 wk (n = 3) | 1.17(.74) | 72%(7%) | 10%(2%) | −1%(3%) | 19%(3%) | 1.36(0.95) | ||
| 22 wk (n = 2) | 0.97(.05) | 89%(7%) | 4%(2%) | −2%(2%) | 9%(4%) | 0.94(0.11) | ||
| 32 wk (n = 2) | 1.04(.03) | 83%(1%) | 2%(0%) | −1%(0%) | 15%(1%) | 1.58(0.35) | ||
| Q-012 | day1 (n = 4) | 1.02(.04) | 6%(1%) | 2%(4%) | −1%(0%) | 94%(5%) | 6.40(3.66) | |
| day7 | 1 | 6% | 5% | −1% | 90% | >23.50 | ||
| day9 (n = 3) | 0.99(.06) | 6%(0%) | 5%(1%) | 1%(1%) | 90%(0%) | 8.90(4.14) | ||
| day13 (n = 2) | 0.99(.11) | 7%(0%) | 4%(0%) | 0%(1%) | 89%(1%) | 6.72(1.97) | ||
| 26 wk | NDd | ND | ND | ND | ND | <0.2 | ||
| 34 wk | ND | ND | ND | ND | ND | <0.2 | ||
| 42 wk | ND | ND | ND | ND | ND | <0.2 | ||
| 17A | (−) FVIII-C2 | 1 wk | 1 | 101% | 0% | −1% | −1% | 31.39 |
| (+) FVIII-C2 | 0.7 | 102% | 0% | −1% | −1% | 9.11 | ||
| (−) FVIII-C2 | 3 wk | 1.05 | 101% | 1% | −1% | 0% | 23.54 | |
| (+) FVIII-C2 | 0.74 | 104% | −1% | −3% | −1% | 1.42 | ||
| (−) FVIII-C2 | 51 wk | 1.04(.01) | 99%(0%) | 1%(0%) | (1%)(0%) | 1%(0%) | 5.49(0.09) | |
| (+) FVIII-C2 | ND | ND | ND | ND | ND | <0.2 | ||
| (−) FVIII-C2 | 5 yrs | 1.11(.02) | 95%(2%) | 0%(1%) | −1%(0%) | 5%(1%) | 3.11(0.62) | |
| (+) FVIII-C2 | ND | ND | ND | ND | ND | <0.2 | ||
| L-006 | 1 wk | 0.87 | 92% | 4% | 4% | 0% | 11.85 | |
| 5 wk (n = 2) | 0.98(.01) | 91%(2%) | 2%(4%) | 4%(0%) | 3%(2%) | 13.13(1.46) | ||
| 9 wk | 0.98 | 85% | 6% | 2% | 7% | 11.97 | ||
| 21 wk | ND | ND | ND | ND | ND | <1.09 | ||
| 35 wk | ND | ND | ND | ND | ND | <0.99 |
The ratios indicate the agreement between the summed SPR signals from the binding of secondary detection antibodies specific for IgG1, IgG2, IgG3 and IgG4 (numerator) to the initial SPR signal generated by the anti-FVIII antibodies in plasma that bound to the immobilized FVIII (denominator).
Multiple measurements (n) were made when sufficient plasma was available.
Standard deviations are reported for these experiments in parentheses.
ND = Not Determined because the low total IgG titer made estimates of ratios and %Ig subtypes unreliable.
Serial samples were obtained from two of the autoimmune HA subjects (Q-011 and Q-012) and from two congenital HA subjects (L-006 and 17A) following initial presentation with an inhibitor. For samples from subjects Q-011 and Q-012 ( Table 2 ), a progressive decrease in total anti-FVIII IgG concentrations was observed, with levels becoming undetectable six months after inhibitor diagnosis for Q-012, at which time the FVIII activity of the plasma was 36%. For the serial samples from moderate HA subject L-006, trace levels of IgG3 were observed in the earliest sample, and trace IgG4 was found in samples obtained one and two months later. Rituximab therapy subsequently resulted in a predictable decrease in his anti-FVIII IgG concentrations. For mild HA subject 17A, the response was IgG1-restricted throughout the first year following initial inhibitor detection, but a low-titer sample obtained 5 years later, during which time he received several additional FVIII infusions following a traumatic injury, showed partial IgG4 character. SPR measurements of a sample obtained from this subject 1–3 weeks after initial inhibitor detection indicated that ∼30% of the anti-FVIII antibodies bound to the FVIII C2 domain (estimated from RUs measured in the presence of saturating FVIII-C2 protein, Table 2 ) and samples obtained later in the course of this immune response exhibited nearly complete specificity for the C2 domain.
Discussion
Inhibitor formation is a serious complication in the management of HA patients and more than thirty years of research has provided insight into humoral anti-FVIII immune responses, which often include IgG4 immunoglobulins [7], [12], [13]. The IgG4 subtype is frequently associated with chronic exposure to protein antigens [14]. Previous studies have demonstrated that anti-FVIII antibodies target multiple domains in FVIII [15]–[20]. Both the IgG subtype distribution and the complexity of the epitope distribution have been reported to be immunologically important, but they are not routinely measured [13], [17], [21], [22]. The present study describes a new methodology that allows quantification of anti-FVIII IgG isotype/subtype distributions and their gross domain specificity. (Future studies will examine specificity of IgGs for other domains besides FVIII-C2). The assay format is suitable for measurement of small (50–100 µL) volumes (e.g. residual samples from clinical assays) and for medium-throughput analysis of multiple samples. The ability to quantify the proportion of anti-FVIII IgGs with particular subtypes or domain specificities allows precise measurement of dynamic changes in both developing and resolving inhibitor responses.
Assay Performance
The dynamic range of the SPR assay was from 0.2–5 µg/ml anti-FVIII antibody, corresponding to ∼1.4–35 BU/ml for the high-affinity neutralizing monoclonal antibody BO2C11 ( Figure 1A ). Subject Q-012 had an initial inhibitor titer of 2 BU/ml and the corresponding anti-FVIII antibody titer by SPR was ∼6.4 µg/ml ( Table 2 ), indicating that a polyclonal inhibitor titer of ∼0.3 BU/ml could be detected. Thus the usable range of the SPR-based assay is similar to that of the Bethesda assay in detecting inhibitor responses, but the former can also be used to detect and characterize non-neutralizing antibody responses to FVIII.
The use of plasma and serum samples can introduce significant matrix interference into immunoassays due to competitive or nonspecific binding of proteins or other components. The SPR format is particularly sensitive to non-specific binding since the mass of bound protein (or other components in plasma or buffer) is measured, whether or not the interaction is specific. The SPR format, in which samples are tested sequentially rather than in parallel, also requires that an active sensor surface be regenerated between tests. Several factors contribute to the reproducibility and accuracy of the measurements reported herein: the use of CA-treatment as a pre-analytical step, the use of affinity-captured FVIII, and the use of monoclonal secondary antibodies to detect specific IgG subtypes.
Since inhibitory anti-FVIII antibodies may compete with vWF [23] for binding to FVIII [24], vWF was removed from test samples. Caprylic acid proved to be an effective pre-clearance step yielding samples with no residual vWF (Figure S2) and very low non-specific binding [25]. Like any pre analytical treatment, CA treatment may potentially alter the distribution of antibody populations in test samples [26]. The quantitative recovery of B02C11 from CA-treated samples and the highly reproducible behavior of test plasmas treated independently at different times suggest that CA treatment did not alter the antibody profiles. More exhaustive spike-recovery experiments were not possible due to the lack of additional purified FVIII-specific human antibodies. FVIII is a labile protein, so covalent immobilization followed by repeated assays in which it is exposed to plasma would not be feasible. The use of affinity-captured FVIII as the antigen, although it limits the dynamic range of quantitative measurements, allows a fresh antigen surface to be used for each testing cycle. The capture format allows FVIII to be immobilized directly from a solution while it is still in formulation buffer, alleviating potential problems with antigen instability due to dilution and buffer exchange. Additionally, it allows the assay to be performed with different sources of FVIII without having to optimize immobilization conditions individually. The Biacore T100 and similar instruments have multiple flow cells that can be simultaneously exposed to test samples, so this assay format could easily be adapted to carry out multiple parallel measurements, e.g. to simultaneously test antibody responses to different FVIII products.
Although a variety of domain-specific FVIII mouse mAbs are available, capture of FVIII via different domains was not compared rigorously, primarily due to the lack of a well-characterized polyclonal pooled control plasma sample. FVIII-A1 specificity has not been reported for neutralizing anti-FVIII antibodies, therefore an anti-A1 domain monoclonal antibody (GMA-8004) was chosen to capture FVIII from plasma. Low pH, high concentration arginine is an effective eluant for the affinity purification of antibodies [27], and it proved to be an effective regeneration solution for this capture antibody.
Since the nominal molecular weights of human and mouse IgG are similar, the use of monoclonal secondary antibodies with a defined 1∶1 binding stoichiometry provides additional information that is not obtainable with polyclonal secondary antibodies. For most test samples, the total cumulative binding signal from the secondary mAbs corresponded closely (80–120%) to the primary binding signal ( Tables 1 & 2 ). This provided confidence that the measured IgG subtype distribution accurately reflected the FVIII-specific antibody response in the test plasma. The sample from subject H-001 was a notable exception (Figure S3). This sample exhibited a strong, partial FVIII-C2 specific response with a complex, mixed IgG1+IgG2+IgG4 profile. However, the cumulative secondary mAb signal accounted for only approximately 20% of the primary signal in RUs. The cause of this discrepancy (i.e. a non-IgG plasma component bound to captured FVIII) is unknown. All of the subtype-specific detection mAbs used in this study have been reported to be acceptable for detection of human IgG subtypes by ELISA [13], [28]–[30]. For H-001, an additional IgG3-specific secondary antibody (clone HP6047) also failed to detect a measurable IgG3 component to this anti-FVIII immune response. Although no samples from this study exhibited a strong IgG3 response, serial samples from L-006 reproducibly showed trace IgG3 content.
Both the quantity of monoclonal antibody GMA-8004 that can be covalently linked to the sensor and the relatively low concentration of the FVIII drug product used as the antigen source limit the ability to capture large quantities of FVIII and achieve mass transport limited conditions that are necessary to obtain accurate concentration measurements. Consequently there was some variability in the calibration curves, especially at the higher B02C11 concentrations. This necessitated the inclusion of a calibration curve for every sequence of samples tested ( Figure 1A ). Satisfactory and reproducible assay responses were obtained when test samples were diluted to 0.2–5 µg/ml (1.3–33 nM) total anti-FVIII antibody.
This was a preliminary study and suitable control samples with well defined polyclonal distributions of human anti-FVIII antibodies were not available to formally assess recovery after CA treatment, accuracy, and precision. However, a number of samples were tested multiple times over the course of assay development and routine sample testing. The results from these tests provide a measure of the reproducibility of this assay format. When multiple tests were performed, the number of tests (n = #) and the standard deviation of each measurement (#) are indicated in parentheses in Tables 1 and 2.
HA Phenotypes
The Concerted Action on Neutralizing Antibodies in severe hemophilia A (CANAL) study reported that inhibitor development generally occurs after a median of 14 exposure days [31]. The present study enrolled HA subjects age 2 and above. Therefore, it was not surprising that class switching had already occurred in almost all of the inhibitor subjects. Table 3 summarizes demographic and HA-related clinical information regarding the inhibitor-positive subjects. Consistent with previous studies [12], [13], [21], their immune responses to FVIII were dominated by IgG1 and IgG4, with minor IgG2 and/or IgG3 components observed in higher-titer, more complex responses. The most notable responses from this panel of samples were for subjects 17A (mild HA, infused multiple times), N-008 (moderately severe HA, inhibitor detected after his 9th FVIII infusion, sample obtained 2 months later), and L-006 (severe HA, serial samples obtained following initial inhibitor detection after 11 FVIII infusions), who all demonstrated IgG1-restricted responses. Several other mild/moderate HA subjects had no anti-FVIII antibodies detectable by SPR. The IgG1-restricted responses may simply reflect limited exposure to FVIII, as mild/moderate HA patients generally receive FVIII infusions only to treat severe bleeding or during surgery.
At the other end of the spectrum, the autoimmune HA subjects exhibited a complex response with respect to the IgG subtype distribution, total anti-FVIII IgG concentration and apparent ratio of anti-FVIII IgG to inhibitory antibodies (BU/ml). The complexity of these responses suggests they were flare-ups of previous sub-clinical autoimmune responses to FVIII. Prescott et al (1997), measuring only inhibitory antibodies, observed that the autoimmune responses were less complex than alloimmune HA responses [17]. However, they noted that although inhibitors primarily targeted only a few FVIII domains, their specificity for additional FVIII domains was indicated by immunoprecipitation experiments, suggesting that non-inhibitory antibodies were also present. The present study also evaluated the fractions of antibodies specific for the FVIII-C2 domain versus those specific for other FVIII domains in the autoimmune subjects. Samples from a larger number of subjects, and utilizing other FVIII fragments or hybrid/mutant FVIII proteins for competition assays, will be required to further characterize and quantify the epitope specificity of anti-FVIII antibodies by SPR.
In general, solution phase competition experiments with matched samples yield unequivocal, easily interpretable results. This was observed in the present study, measuring the FVIII-C2 specific IgG response, and in earlier studies that identified the domain specificity of inhibitory antibodies using the Bethesda assay [17]. Alternative approaches, based on competition binding to immobilized FVIII between mAbs with known specificity and anti-FVIII antibodies in plasma [32]–[34], use of hybrid porcine/human FVIII proteins [35], [36], FVIII mutagenesis [36], phage display [37] FVIII peptide-binding assays [38]–[40] and Luminex technology [41] have also been described.
As noted above, competition studies with FVIII-C2 protein consistently demonstrated that specificity for this domain was not linked to specific IgG subtypes. This result is consistent with the observations of Kessel et al. [37] and also with the concept that IgG class switching occurs after epitope specificity has been determined. Some measurement of the clonality of FVIII-specific antibodies would be a valuable metric to gauge the complexity of inhibitor responses. However, both IgG class switching and the prevalence of IgG4 in the samples complicate the definition of clonality. The desired information may actually be the clonality of FVIII-specific precursor B-cells prior to class switching. It is important to note that in IgG4-dominated responses, ELISAs using anti-kappa and anti-lambda chain secondary antibodies to address clonality of the responses may be misleading since circulating IgG4 molecules are functionally “bi-clonal” due to exchange of half-IgG4 molecules with other (non FVIII-specific) IgG4 antibodies [42]–[44].
Conclusions
The SPR method described herein is an easily adaptable assay format with which to characterize anti-FVIII antibody responses. The assay sensitivity is satisfactory to characterize most inhibitors detectable using the Bethesda assay, as well as samples containing anti-FVIII antibodies (neutralizing+non-neutralizing) with concentrations >0.2 µg/ml. Several observations were notable: As has already been reported, the IgG4 subtype was commonly observed, typically in mixed subtype responses. However, three HA subjects with inhibitor responses (2 emerging, 1 chronic) demonstrated IgG1-restricted responses. Also, most subjects exhibited partial FVIII-C2 specificity. Autoimmune subjects exhibited complex responses involving multiple IgG subtypes, multiple domain specificities, high total anti-FVIII antibody concentrations, and an apparently high ratio of total to inhibitory anti FVIII IgG. The present study analyzed plasma samples from 22 inhibitor subjects, including serial samples from two HA subjects with a recently diagnosed inhibitor and two acquired HA subjects following initial detection of their inhibitor. Future studies analyzing a larger set of plasma samples will compare the anti-FVIII total antibody and antibody-subtype titers estimated from SPR sensorgrams with titers derived from quantitative ELISA assays [13]. Such larger studies will also establish the relative sensitivity of SPR, ELISA and Bethesda assays in detecting and characterizing anti-FVIII antibody responses. The SPR platform described herein is a promising approach to carry out future prospective studies of FVIII inhibitors and other anti-drug antibody responses. Because of the small plasma volumes required and the quick assay turnaround time, this method is especially suitable for batch analysis of multiple samples, e.g. central laboratory characterization of antibody responses to FVIII or other clinically important antigens.
Supporting Information
Binding kinetics of FVIII captured on the anti-FVIII-A1 domain antibody GMA-8004. A. MAb GMA-8004 was immobilized on a CM5 chip as described in Methods. Recombinate was then injected and the binding kinetics were measured at flow rates 5 µl/min and 30 µl/min. X-offset and y-offset were performed using the Biacore software to match the end of the association phase for the 5 µl/min and 30 µl/min curves. B. Magnified view of the dissociation over 30 min, which was ∼10 RU at 5 µl/min (compared to the initial binding signal of 3215 RU) vs. ∼5 RU at 30 µl/min (compared to the initial binding signal of 865 RU). At both flow rates the total dissociation over 30 min was <1% of the initial signal in RUs. Note that the capture times were not adjusted to yield matching capture levels at the different flow rates so the amount of captured FVIII is lower at the lower flow rate.
(TIF)
ELISA assays showing VWF in serially diluted Untreated and CA-treated plasma and serum samples. No VWF was detected in the CA-treated samples.
(TIF)
Binding curves for subject H-001 obtained in the presence and absence of excess (1 µM) FVIII-C2. Quantitative measurements (percent of the response derived from each human IgG subtype, total anti-FVIII IgG concentration (µg/ml), and the ratio of secondary to primary binding signal in %) obtained from the binding curves are tabulated in Table 1.
(TIF)
Subjects and samples.
(DOC)
A detailed description of FVIII dissociation kinetics from capture antibody GMA-8004 is provided and the preanalytical treatment of plasma to remove vWF is described.
(DOC)
Acknowledgments
We thank Colette Norby-Slycord and Suni Allen for study coordination, Rebecca LeFavor and Amjad Hussain for technical assistance, Dr. Phuong-Cac Nguyen for protein production, Dr. Jason Schuman (GE Healthcare Life Sciences) for helpful discussions, and Drs. Marc Jacquemin (Katholieke University of Lueven, Belgium) and Bill Church (Green Mountain Antibodies, Burlington, VT) for donations of monoclonal antibodies. We thank the PATH Study Steering & Oversight Committee (Drs. Lusher, Kessler and Mann), Publications Committee (Drs. Key, Kempton and Thompson) and Community Advisory Board (Dr. Louis Aledort, Dr. Yvette Latchman, Dr. William Hobbs III and Ms. Toni Allen-Ellingson) and Prof. Eri Srivatsan for oversight of the PATH study, and we thank administrators at all participating sites. PATH study investigators are:
1. Biogen-Idec Hemophilia, Waltham, MA – Dr. Glenn F. Pierce and Dr. Robert T. Peters
2. Department of Biochemistry, University of Vermont, Burlington, VT – Dr. Kenneth G. Mann and Dr. Saulius S. Butenas
3. Department of Genetics, Texas Biomedical Research Institute, San Antonio, TX – Dr. Shelley A. Cole, Dr. Laura Almasy, Dr. John Blangero, Dr. Joanne Curran and Dr. Mel Carless
4. Department of Pathology and Laboratory Medicine, Immunogenetics Center, University of California, Los Angeles, CA – Dr. Rajalingam Raja and Dr. Elaine Reed
5. Department of Pathology and Molecular Medicine, Queen’s University, Kingston, ON, Canada – Dr. David Lillicrap and Dr. Natalia Rydz
6. Emory University, Atlanta, GA – Dr. Christine L. Kempton
7. Georgia Health Sciences University, Augusta, GA – Dr. Afshin Ameri and Dr. Kavita Natrajan
8. Georgetown University, Washington, DC – Dr. Craig M. Kessler
9. Histonis, Inc., Atlanta, GA – Dr. Kevin R. Viel
10. King Edward VIII Hospital, Durban, KwaZulu Natal, S. Africa – Dr. Rajendra Thejpal, Dr. Nadine Rapiti and Dr. Yasmin Goga
11. Mount Sinai University, New York, NY – Dr. Christopher E. Walsh
12. Northwestern University, Chicago, IL – Dr. Alexis A. Thompson
13. Tulane University, New Orleans, LA – Dr. Rebecca Kruse-Jarres and Dr. Cindy A. Leissinger
14. Palmetto Health, Columbia, SC – Dr. Kevin P. McRedmond and Dr. Janice S. Withycombe
15. Puget Sound Blood Center Research Institute – Dr. Kathleen P. Pratt, Dr. Neil S. Josephson, Dr. Barbara A. Konkle, Mr. Douglas C. Bolgiano, MS
16. Seattle Children’s Hospital, Seattle, WA – Dr. Dana S. Matthews
17. University of Mississippi Medical Center, Jackson, MS – Dr. Rathi V. Iyer
18. University of Alabama, Birmingham, AL – Dr. Raymond G. Watts
19. University of North Carolina, Chapel Hill, NC – Dr. Nigel S. Key
20. University of Southern California, Los Angeles, CA – Dr. Carol K. Kasper
21. University of the Witwatersrand, Johannesburg, S. Africa – Dr. Johnny Mahlangu, Dr. Amanda Krause and Dr. Rosemary Schwyzer
22. University of the Free State, Bloemfontein, Free State, S. Africa – Dr. Marius Coetzee and Dr. David Stones
23. Virginia Commonwealth University, Richmond, CA – Dr. John C. Barrett and Dr. Erica J. Martin
24. Wayne State University, Detroit, MI – Dr. Jeanne M. Lusher and Dr. Meera B. Chitlur.
Funding Statement
Major support for this work was from NHLBI 1RC2 HL101851: "Mechanisms of Race-Based Differences in Factor VIII Immunogenicity in Hemophilia A" (PIs TE Howard and KP Pratt). Minor support for this work was from: 1. Unrestricted research support was received under a CSL Behring Foundation Hemophilia grant: "Do Non-Synonymous Single Nucleotide Polymorphisms in Factor VIII Lead to T-Cell Responses and Subsequent Inhibitor Development in Black Hemophilia A Patients?" (PI: KP Pratt), funder: CSL Behring Foundation for Research and Advancement of Patient Health, http://www.cslbehringfoundation.com/. 2. Unrestricted research support was received from a Bayer Healthcare LLC grant: “Repository for Human HLA-restricted T-Cell Clones" (PI: KP Pratt), funder: Bayer HealthCare LLC, http://grants-contributions.bayerweb.com/en/home/index.php, 800 Dwight Way, Berkeley, California 94710. These funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Ehrenforth S, Kreuz W, Scharrer I, Linde R, Funk M, et al. (1992) Incidence of development of factor VIII and factor IX inhibitors in haemophiliacs. Lancet 339: 594–598. [DOI] [PubMed] [Google Scholar]
- 2. Kasper CK, Aledort L, Aronson D, Counts R, Edson JR, et al. (1975) Proceedings: A more uniform measurement of factor VIII inhibitors. Thromb Diath Haemorrh 34: 612. [PubMed] [Google Scholar]
- 3. Verbruggen B, Novakova I, Wessels H, Boezeman J, van den Berg M, et al. (1995) The Nijmegen modification of the Bethesda assay for factor VIII:C inhibitors: improved specificity and reliability. Thromb Haemost 73: 247–251. [PubMed] [Google Scholar]
- 4. Sahud MA, Pratt KP, Zhukov O, Qu K, Thompson AR (2007) ELISA system for detection of immune responses to FVIII: a study of 246 samples and correlation with the Bethesda assay. Haemophilia 13: 317–322. [DOI] [PubMed] [Google Scholar]
- 5. Zakarija A, Harris S, Rademaker AW, Brewer J, Krudysz-Amblo J, et al. (2011) Alloantibodies to factor VIII in haemophilia. Haemophilia 17: 636–640. [DOI] [PubMed] [Google Scholar]
- 6. Krudysz-Amblo J, Parhami-Seren B, Butenas S, Brummel-Ziedens KE, Gomperts ED, et al. (2009) Quantitation of anti-factor VIII antibodies in human plasma. Blood 113: 2587–2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Towfighi F, Gharagozlou S, Sharifian RA, Kazemnejad A, Esmailzadeh K, et al. (2005) Comparative measurement of anti factor VIII antibody by Bethesda assay and ELISA reveals restricted isotype profile and epitope specificity. Acta Haematol 114: 84–90. [DOI] [PubMed] [Google Scholar]
- 8. Shetty S, Ghosh K, Mohanty D (2003) An ELISA assay for the detection of factor VIII antibodies - comparison with the conventional Bethesda assay in a large cohort of haemophilia samples. Acta Haematol 109: 18–22. [DOI] [PubMed] [Google Scholar]
- 9. Irigoyen MB, Primiani L, Felippo M, Candela M, Bianco RP, et al. (2011) A flow cytometry evaluation of anti-FVIII antibodies: correlation with ELISA and Bethesda assay. Haemophilia 17: 267–274. [DOI] [PubMed] [Google Scholar]
- 10. Pratt KP, Thompson AR (2009) B-Cell and T-Cell epitopes in anti-factor VIII immune responses. Clin Rev Allergy Immunol 37: 80–95. [DOI] [PubMed] [Google Scholar]
- 11. Jacquemin MG, Desqueper BG, Benhida A, Vander Elst L, Hoylaerts MF, et al. (1998) Mechanism and kinetics of factor VIII inactivation: study with an IgG4 monoclonal antibody derived from a hemophilia A patient with inhibitor. Blood 92: 496–506. [PubMed] [Google Scholar]
- 12. Fulcher CA, de Graaf Mahoney S, Zimmerman TS (1987) FVIII inhibitor IgG subclass and FVIII polypeptide specificity determined by immunoblotting. Blood 69: 1475–1480. [PubMed] [Google Scholar]
- 13. van Helden PM, van den Berg HM, Gouw SC, Kaijen PH, Zuurveld MG, et al. (2008) IgG subclasses of anti-FVIII antibodies during immune tolerance induction in patients with hemophilia A. Br J Haematol. 142: 644–652. [DOI] [PubMed] [Google Scholar]
- 14. Aalberse RC, van der Gaag R, van Leeuwen J (1983) Serologic aspects of IgG4 antibodies. I. Prolonged immunization results in an IgG4-restricted response. J Immunol 130: 722–726. [PubMed] [Google Scholar]
- 15. Scandella D, Mattingly M, de Graaf S, Fulcher CA (1989) Localization of epitopes for human factor VIII inhibitor antibodies by immunoblotting and antibody neutralization. Blood 74: 1618–1626. [PubMed] [Google Scholar]
- 16. Fulcher CA, Lechner K, de Graaf Mahoney S (1988) Immunoblot analysis shows changes in factor VIII inhibitor chain specificity in factor VIII inhibitor patients over time. Blood 72: 1348–1356. [PubMed] [Google Scholar]
- 17. Prescott R, Nakai H, Saenko EL, Scharrer I, Nilsson IM, et al. (1997) The inhibitor antibody response is more complex in hemophilia A patients than in most nonhemophiliacs with factor VIII autoantibodies. Recombinate and Kogenate Study Groups. Blood 89: 3663–3671. [PubMed] [Google Scholar]
- 18. Scandella D, Gilbert GE, Shima M, Nakai H, Eagleson C, et al. (1995) Some factor VIII inhibitor antibodies recognize a common epitope corresponding to C2 domain amino acids 2248 through 2312, which overlap a phospholipid-binding site. Blood 86: 1811–1819. [PubMed] [Google Scholar]
- 19. Barrow RT, Healey JF, Gailani D, Scandella D, Lollar P (2000) Reduction of the antigenicity of factor VIII toward complex inhibitory antibody plasmas using multiply-substituted hybrid human/porcine factor VIII molecules. Blood 95(2): 564–8. [PubMed] [Google Scholar]
- 20.Paschal RD, Meeks SL, Neff AT. Development of factor VIII inhibitors in two patients with moderate haemophilia A. Haemophilia. 2013 in press. [DOI] [PubMed]
- 21. Reding MT, Lei S, Lei H, Green D, Gill J, et al. (2002) Distribution of Th1- and Th2-induced anti-factor VIII IgG subclasses in congenital and acquired hemophilia patients. Thromb Haemost 88: 568–575. [PubMed] [Google Scholar]
- 22. Sánchez-Cuenca JM, Carmona E, Villanueva MJ, Aznar JA (1990) Immunological characterization of factor VIII inhibitors by a sensitive micro-ELISA method. Thromb Res 57: 897–908. [DOI] [PubMed] [Google Scholar]
- 23. Lollar P, Hill-Eubanks DC, Parker CG (1988) Association of the factor VIII light chain with von Willebrand factor. J Biol Chem 263: 10451–10455. [PubMed] [Google Scholar]
- 24. Ling M, Duncan EM, Rodgers SE, Street M, Lloyd JV (2001) Classification of the kinetics of factor VIII inhibitors in haemophilia A: plasma dilution studies are more discriminatory than time-course studies. Br J Haematol 114: 861–867. [DOI] [PubMed] [Google Scholar]
- 25. Parkkinen J, Rahola A, von Bonsdorff L, Tölö H, Törmä E (2006) A modified caprylic acid method for manufacturing immunoglobulin G from human plasma with high yield and efficient virus clearance. Vox Sang 90: 97–104. [DOI] [PubMed] [Google Scholar]
- 26. Bergmann-Leitner ES, Mease RM, Duncan EH, Khan F, Waitumbi J, et al. (2008) Evaluation of immunoglobulin purification methods and their impact on quality and yield of antigen-specific antibodies. Malar J 7: 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ejima D, Yumioka R, Tsumoto K, Arakawa T (2005) Effective elution of antibodies by arginine and arginine derivatives in affinity column chromatography. Anal Biochem. 345: 250–257. [DOI] [PubMed] [Google Scholar]
- 28. Gibbs E, Oger J (2008) A biosensor-based characterization of the affinity maturation of the immune response against interferon-beta and correlations with neutralizing antibodies in treated multiple sclerosis patients. J Interferon Cytokine Res 28: 713–723. [DOI] [PubMed] [Google Scholar]
- 29. Narita M, Yamada S, Matsuzono Y, Itakura O, Togashi T, et al. (1997) Measles virus-specific immunoglobulin G subclass response in serum and cerebrospinal fluid. Clin Diagn Virol 8: 233–239. [DOI] [PubMed] [Google Scholar]
- 30. Hamilton RG, Morrison SL (1993) Epitope mapping of human immunoglobulin-specific murine monoclonal antibodies with domain-switched, deleted and point-mutated chimeric antibodies. J Immunol Methods 158: 107–122. [DOI] [PubMed] [Google Scholar]
- 31. Gouw SC, van der Bom JG, Auerswald G, Ettinghausen CE, Tedgard U, et al. (2007) Recombinant versus plasma-derived FVIII products and the development of inhibitors in previously untreated patients with severe hemophilia A: the CANAL cohort study. Blood 109: 4693–4697. [DOI] [PubMed] [Google Scholar]
- 32. Gilles JG, Arnout J, Vermylen J, Saint-Remy JM (1993) Anti-factor VIII antibodies of hemophiliac patients are frequently directed towards nonfunctional determinants and do not exhibit isotypic restriction. Blood 82: 2452–2461. [PubMed] [Google Scholar]
- 33. Meeks SL, Healey JF, Parker ET, Barrow RT, Lollar P (2008) Nonclassical anti-C2 domain antibodies are present in patients with factor VIII inhibitors. Blood 112: 1151–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Meeks SL, Healey JF, Parker ET, Barrow RT, Lollar P (2007) Antihuman factor VIII C2 domain antibodies in hemophilia A mice recognize a functionally complex continuous spectrum of epitopes dominated by inhibitors of factor VIII activation. Blood 110: 4234–4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lollar P (1997) Analysis of factor VIII inhibitors using hybrid human/porcine factor VIII. Thromb Haemostas 78: 647–651. [PubMed] [Google Scholar]
- 36. Barrow RT, Healey JF, Gailani D, Scandella D, Lollar P (2000) Reduction of the antigenicity of factor VIII toward complex inhibitory antibody plasmas using multiply-substituted hybrid human/porcine factor VIII molecules. Blood 95: 564–568. [PubMed] [Google Scholar]
- 37. Kessel C, Königs C, Linde R, Excuriola-Ettinghausen C, Stoll H, et al. (2008) Humoral immune responsiveness to a defined epitope on factor VIII before and after B cell ablation with rituximab. Mol Immunol 46: 8–15. [DOI] [PubMed] [Google Scholar]
- 38. Raut S, Villard S, Grailly S, Gilles JG, Granier C, et al. (2003) Anti-heavy-chain monoclonal antibodies directed to the acidic regions of the factor VIII molecule inhibit the binding of factor VIII to phospholipids and von Willebrand factor. Thromb Haemost 90: 385–397. [DOI] [PubMed] [Google Scholar]
- 39. Kopecky EM, Greinstetter S, Pabinger I, Buchacher A, Römisch J, et al. (2006) Mapping of FVIII inhibitor epitopes using cellulose-bound synthetic peptide arrays. J Immunol Methods. 308: 90–100. [DOI] [PubMed] [Google Scholar]
- 40. Albert T, Egler C, Jakuschev S, Schuldenzucker U, Schmitt A, et al. (2008) The B-cell epitope of the monoclonal anti-factor VIII antibody ESH8 characterized by peptide array analysis. Thromb Haemost 99: 634–637. [DOI] [PubMed] [Google Scholar]
- 41. Lavigne-Lissalde G, Tarrade C, Lapalud P, Chtourou S, Schved JF, et al. (2008) Simultaneous detection and epitope mapping of anti-factor VIII antibodies. Thromb Haemost 99: 1090–1096. [DOI] [PubMed] [Google Scholar]
- 42. van der Neut Kolfschoten M, Schuurman J, Losen M, Bleeker WK, Martinez-Martinez P, et al. (2007) Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science 317: 1554–1557. [DOI] [PubMed] [Google Scholar]
- 43. Lewis KB, Meengs B, Bondensgaard K, Chin L, Hughes SD, et al. (2009) Comparison of the ability of wild type and stabilized human IgG4 to undergo Fab arm exchange with endogenous IgG4 in vitro and in vivo . Mol Immunol 46: 3488–3494. [DOI] [PubMed] [Google Scholar]
- 44. Labrijn AF, Buijsse AO, van den Bremer ET, Verwilligen AY, Bleeker WK, et al. (2009) Therapeutic IgG4 antibodies engage in Fab-arm exchange with endogenous human IgG4 in vivo . Nat Biotechnol 27: 767–771. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Binding kinetics of FVIII captured on the anti-FVIII-A1 domain antibody GMA-8004. A. MAb GMA-8004 was immobilized on a CM5 chip as described in Methods. Recombinate was then injected and the binding kinetics were measured at flow rates 5 µl/min and 30 µl/min. X-offset and y-offset were performed using the Biacore software to match the end of the association phase for the 5 µl/min and 30 µl/min curves. B. Magnified view of the dissociation over 30 min, which was ∼10 RU at 5 µl/min (compared to the initial binding signal of 3215 RU) vs. ∼5 RU at 30 µl/min (compared to the initial binding signal of 865 RU). At both flow rates the total dissociation over 30 min was <1% of the initial signal in RUs. Note that the capture times were not adjusted to yield matching capture levels at the different flow rates so the amount of captured FVIII is lower at the lower flow rate.
(TIF)
ELISA assays showing VWF in serially diluted Untreated and CA-treated plasma and serum samples. No VWF was detected in the CA-treated samples.
(TIF)
Binding curves for subject H-001 obtained in the presence and absence of excess (1 µM) FVIII-C2. Quantitative measurements (percent of the response derived from each human IgG subtype, total anti-FVIII IgG concentration (µg/ml), and the ratio of secondary to primary binding signal in %) obtained from the binding curves are tabulated in Table 1.
(TIF)
Subjects and samples.
(DOC)
A detailed description of FVIII dissociation kinetics from capture antibody GMA-8004 is provided and the preanalytical treatment of plasma to remove vWF is described.
(DOC)