Prologue
By separating the strands in duplex DNA, processing DNA structures, and remodeling nucleoprotein complexes, DNA helicases are indispensable for different facets of DNA metabolism. Here, we review recent research on helicases involved in homologous recombination, one of the two main pathways that eliminate double-stranded breaks in DNA. We focus on the RecQ helicases and the Hef-like helicases, which belong to the SF2 helicase superfamily, as well as the SF1 family member Srs2. Mutations in RecQ and Hef-like helicases cause several human syndromes that predispose patients to cancer, highlighting the importance of these enzymes in genome maintenance and mutation avoidance.
DNA Double-strand Break (DSB) Repair Pathways
DSBs are extremely toxic because of their propensity to induce genome alterations and rearrangements, including deletions and translocations. DSBs can be eliminated either by nonhomologous DNA end joining (NHEJ) or homologous recombination (HR). During NHEJ, the broken ends are first aligned, processed to reveal microhomology between them, and then ligated (Lieber, 2010). In HR, which is mechanistically much more complex, a homologous DNA sequence, located within either the sister chromatid or the chromosome homolog, is used as the template to direct the repair reaction (Mimitou and Symington, 2009; San Filippo et al., 2008). Herein, we will focus on the roles of DNA helicases in HR mediation and regulation.
Homologous Repair Pathways
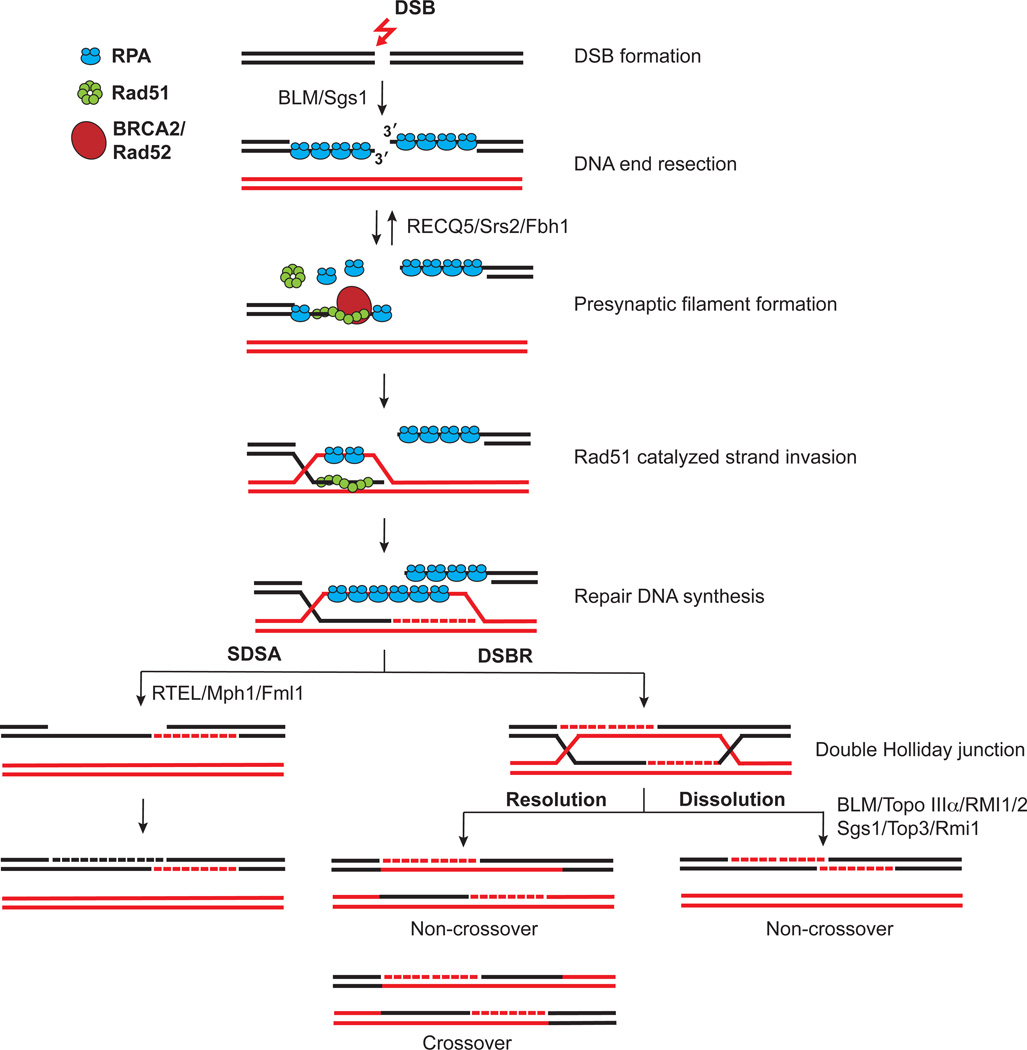
HR-mediated DSB repair is initiated by the nucleolytic degradation of the 5’ strands of the break ends, referred to as DNA end resection. The nucleases Mre11, Exo1, and Dna2 participate in this resection process (Gravel et al., 2008; Mimitou and Symington, 2008; Zhu et al., 2008). The 3’ tails derived from resection (Figure 1) are engaged by the Rad51 recombinase, which catalyzes the search for a homologous duplex sequence and the invasion of the sequence to form a DNA joint called the displacement loop, or D-loop (San Filippo et al., 2008). DNA synthesis is initiated from the 3’ end of the invading strand. At this stage, the D-loop structure can be disassembled via the action of a specialized helicase, thus allowing the newly synthesized DNA to anneal with the other 3’ ssDNA tail derived from end resection. This recombination path, termed synthesis-dependent strand annealing (SDSA), yields only noncrossover products (Figure 1). Alternatively, as in the DNA double-strand break (DSBR) repair pathway, the displaced strand in the D-loop structure pairs with the other resected end, forming a DNA intermediate termed the double Holliday junction (dHJ), which is processed endonucleolytically by one of several resolvases (Figure 1) (Schwartz and Heyer, 2011). This type of resolvase-mediated dHJ resolution has the probability of generating chromosome arm crossovers in the recombinants made. As will be discussed at length later, the dHJ can also be resolved by the combined action of a specialized DNA helicase and topoisomerase in a process termed dHJ dissolution to form noncrossover recombinants exclusively (Figure 1). As alluded to above and expounded upon below, helicases play a critical catalytic or regulatory role in nearly every step of the HR reaction, highlighting their importance in genome maintenance.
Figure 1. DNA break repair by either the Synthesis-Dependent Strand Annealing (SDSA) pathway or the Double Strand Break Repair (DSBR) pathway.
Double strand breaks induced by DNA damage are first resected to produce 3’ DNA tails, which become coated by the ssDNA binding protein RPA. Recombination mediator proteins, such as Rad52 in yeast and BRCA2 in humans, promote the exchange of RPA by a helical filament of Rad51. DNA synthesis occurs after strand invasion catalyzed by the Rad51-ssDNA nucleoprotein filament. The resulting DNA joint can be processed through either the SDSA pathway or the DSBR pathway. In the former, the invading strand is ejected by Mph1/FANCM/Fml1 and then becomes annealed with the other ssDNA end. The SDSA pathway generates exclusively noncrossover recombinants. In the DSBR pathway, the second DSB end is captured to yield a double Holliday junction (dHJ), which is either resolved by a HJ resolvase to generate crossover or noncrossover products, or it can be dissolved by the concerted action of the Sgs1-Top3-Rmi1 complex in yeast and BLM-Topo IIIa-RMI1/2 complex in humans to generate noncrossover products exclusively.
Multifaceted Roles of RecQ Helicases in HR
a. General biochemical properties of the RecQ helicases
The RecQ family of helicases are named after the founding member, the product of the E. coli recQ+ gene, which prevents replication fork demise and suppresses illegitimate recombination in bacteria (Hanada et al., 1997). While five such helicases exist in mammals (BLM, WRN, RECQ1, RECQ4, and RECQ5), Sgs1 is the sole ortholog in S. cerevisiae. The RecQ helicases share a common domain structure, including a conserved central region of 350–400 residues that harbors the seven classical helicase motifs. Besides this helicase domain, most RecQ helicases also contain the RecQ C-terminal (RQC) domain, believed to mediate protein-protein interactions. The RNase D C-terminal (HRDC) domain (~80 amino acids), which has been implicated in the recognition of various DNA structures, is found in Sgs1, BLM, and WRN but absent in RECQ1, RECQ4, and RECQ5. All RecQ helicases examined to date translocate on ssDNA with a 3′→5′ polarity.
b. S. cerevisiae Sgs1
(i) Genetic characteristics
The SGS1 gene was isolated as a suppressor of the slow growth phenotype of mutants of the TOP3 gene, which encodes a type IA toposiomerase (Gangloff et al., 1994). Sgs1 protein forms a stable complex with Top3 and another protein called Rmi1 (Chang et al., 2005; Mullen et al., 2005). Mutations in SGS1 also lead to hypersensitivity to different DNA damaging agents, such as ultraviolet (UV) light and methylmethane sulfonate (MMS), and also to replicative stress caused by exposure to hydroxyurea (HU) (Ii and Brill, 2005; Onoda et al., 2000). These phenotypes indicate a role of Sgs1 protein in DNA repair and are suggestive of an involvement in replication fork maintenance. Detailed analysis of HR efficiency and pathway choice in mutants has revealed a pro-recombination role of Sgs1 and also a role in the suppression of crossover formation (Gangloff et al., 2000; Ira et al., 2003; Wu and Hickson, 2003). Remarkably, recent genetic and biochemical studies have revealed that Sgs1 also functions in DSB end resection (Gravel et al., 2008; Mimitou and Symington, 2008; Zhu et al., 2008).
(ii) Role of Sgs1 in DNA end resection
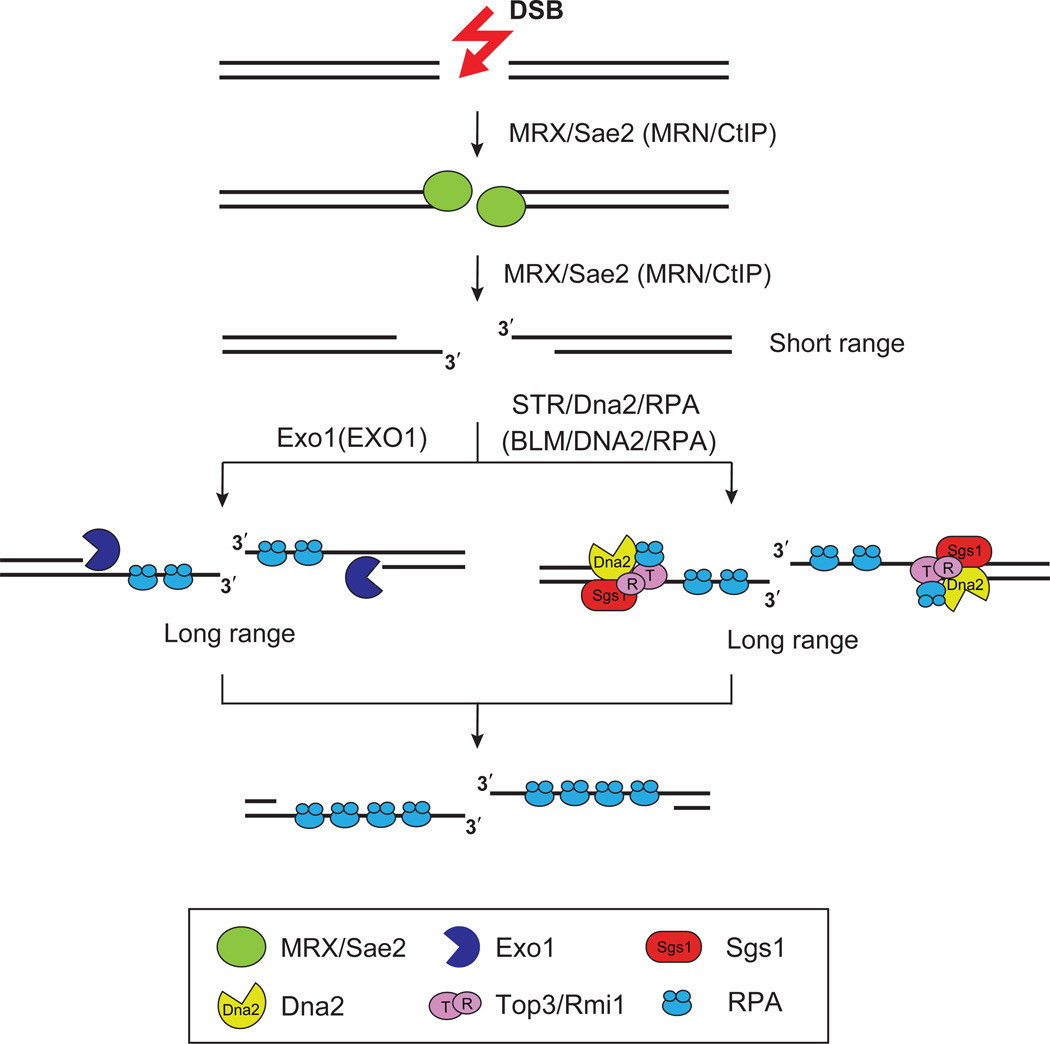
Studies on the genetic requirements and control of DNA end resection are typically conducted in mutants of RAD51, which codes for the recombinase enzyme responsible for DNA strand invasion, so as to uncouple DNA resection from the subsequent steps of HR (San Filippo et al., 2008; Sugawara and Haber, 2006). The Mre11-Rad50-Xrs2 (MRX) complex has long been associated with resection in S. cerevisiae. However, the observation that resection is not abolished in MRX mutants indicated that additional proteins are involved (Krogh and Symington, 2004). Recent studies by three groups in S. cerevisiae and human cells revealed a multiplicity of resection pathways. Deletion of either SGS1 or EXO1, the latter being a 5’-3’ exonuclease, slows the rate of ssDNA formation several kilobases away from a DSB, but little long-range resection occurs in the sgs1∆ exo1∆ double mutant (Gravel et al., 2008; Mimitou and Symington, 2008; Zhu et al., 2008). Interestingly, the double retains the ability to resect DNA closer to the break site, and further analysis showed that the short-range resection depends on the MRX complex and its companion protein Sae2 (Mimitou and Symington, 2008; Zhu et al., 2008). Based on these results, a model was proposed in which the MRX/Sae2 ensemble initiates DSB processing at the break, then parallel pathways involving either Sgs1 or Exo1 conduct extensive resection independently.
The nuclease that functions together with Sgs1 is Dna2 (Zhu et al., 2008), which contains both 5’ flap endonuclease and 5’→3’ helicase activities and functions in Okazaki fragment processing (Bae and Seo, 2000; Budd et al., 1995). Interestingly, while the helicase activity of Sgs1 is essential for end resection, that of Dna2 is dispensable (Mimitou and Symington, 2008; Zhu et al., 2008). Two groups have reconstituted the Sgs1-Dna2 pathway using purified MRX complex, Sgs1-Top3-Rmi1 complex, Dna2 and the heterotrimeric ssDNA binding protein RPA (Cejka et al., 2010a; Niu et al., 2010). The results from these studies demonstrate that DNA strand separation during resection is mediated by Sgs1, in a manner that is enhanced by the Top3-Rmi1 and MRX complexes (Cejka et al., 2010a; Niu et al., 2010). In congruence with genetic observations, although the Dna2 nuclease activity is critical for resection, the Mre11 nuclease activity is dispensable (Mimitou and Symington, 2008; Niu et al., 2010). Interestingly, the topoisomerase activity of Top3, although crucial for the suppression of crossover recombination, is not needed for resection either in cells or in the reconstituted system (Niu et al., 2010). Importantly, these studies have unveiled a multifaceted role of RPA. Aside from stimulating Sgs1-mediated DNA unwinding, RPA regulates the Dna2 nuclease activity by promoting 5’ end cleavage while attenuating 3’ cleavage, to help impose the 5’ resection polarity observed (Cejka et al., 2010a; Niu et al., 2010).
(iii) Suppression of mitotic crossover formation by the Sgs1-Top3-Rmi1 complex
A dramatic increase in crossover recombination is a hallmark phenotype of sgs1∆ strains, which stems from altered processing of the dHJ recombination intermediate. Even though crossover events are important for orchestrating the segregation of chromosome homologs in the first cell division during meiosis, they can also lead to chromosomal translocations. By suppressing mitotic crossovers, Sgs1 helps minimize gross chromosome rearrangements and the loss of heterozygosity during DNA repair by HR. Importantly, top3 and rmi1 mutants also exhibit elevated HR-associated chromosome crossovers, and genetic analyses have provided evidence for epistasis among these mutants and the sgs1 mutant (Mullen et al., 2005). Insights into the anti-crossover activity of the Sgs1-Top3-Rmi1 complex have originated from studies on its human equivalent, the BLM-Topo IIIa-Rmi1-Rmi2 complex (see Section C below) (Chu and Hickson, 2009; Wu and Hickson, 2003). Specifically, the BLM associated complex and subsequently the Sgs1 protein ensemble have been shown to possess the ability to dissolve the dHJ intermediate into products that are exclusively noncrossover in nature (Cejka and Kowalczykowski, 2010; Raynard et al., 2006; Singh et al., 2008; Wu et al., 2006).
(iv) Role of Sgs1 in meiotic HR regulation
Unlike in mitotic cells, HR during meiosis is geared towards the production of crossover products between non-sister homologous chromatids, to ensure the proper segregation of homolog pairs in the first division. A series of protein factors including Zip1, Zip2, Zip3, Zip4, Mer3, Msh4 and Msh5, constrain the activity of Sgs1 to promote crossover formation (Borner et al., 2004; Jessop et al., 2006). It should be noted that Sgs1 does fulfill important HR regulatory roles during meiosis, by ensuring the proper distribution of crossovers and suppressing the formation of complex DNA joint molecules involving multiple chromatids (Oh et al., 2007). It remains to be established whether these meiotic functions of Sgs1 are mediated via dHJ dissolution.
(v) Sgs1 and homeologous recombination
Occasionally, recombination can occur between moderately divergent DNA sequences, a process known as “homeologous recombination”. Homeologous recombination is actively suppressed via a Sgs1-dependent mechanism. Herein, Sgs1 functions in conjunction with the DNA mismatch repair proteins (mainly the MutSa complex harboring Msh2 and Msh6 proteins) in discriminating against non-identical DNA sequences during HR (Evans et al., 2000). Thus, deletion of SGS1 leads to defects in rejecting heteroduplexes containing divergent sequences (Sugawara et al., 2004). A more recent study has found that when both SGS1 and MPH1, which also codes for a DNA helicase discussed in detail below, are mutated, the resulting strain is further compromised in the ability to discriminate homeology during HR repair (Tay et al., 2010). These results suggest that both Sgs1 and Mph1, in an independent fashion, reject heteroduplex intermediates that harbor homeologous sequences.
c. Bloom syndrome and the BLM helicase
(i) Bloom syndrome and cellular phenotypes
BLM is the human RecQ helicase most closely related to Sgs1 in structure and function. Mutations in BLM lead to the rare disease Bloom syndrome (BS). Patients exhibit an abnormally small stature and are highly cancer-prone, with a mean age onset of 24 years (German et al., 2007). The cancers in BS patients are not restricted to any particular organ or type, indicative of a housekeeping role of BLM in genome maintenance.
Chromosomal aberrations, including chromatid gaps, breaks, and rearrangements, occur frequently in BS (Chaganti et al., 1974). BS cells exhibit an abnormality in HR regulation, manifested as a striking increase in sister chromatid exchanges (SCEs) (Chaganti et al., 1974). Ablation of the BLM gene is lethal in the embryonic stage, but heterozygous animals can be obtained (Chester et al., 1998; Goss et al., 2002). These BLM+/- animals do not show increased cancer predisposition unless crossed with mice carrying a mutation in the Apc tumor suppressor gene (Goss et al., 2002).
(ii) Biochemical properties of the BLM helicase activity
BLM unwinds a variety of DNA structures including double-stranded DNA with a 3’ overhang, forked structures, bubbles, G quadruplexes, and recombination intermediates such as D-loops and Holliday junctions (Bachrati et al., 2006; Mohaghegh et al., 2001; Orren et al., 2002; Popuri et al., 2008; Sharma et al., 2005; Sun et al., 1998). BLM can also branch migrate Holliday junctions (Constantinou et al., 2000; Karow et al., 2000; LeRoy et al., 2005).
(iii) BLM-Topo IIIα-Rmi1-Rmi2 complex and its dHJ dissolution activity
BLM is stably associated with Topo IIIa, Rmi1, and Rmi2 in a complex analogous to yeast Sgs1-Top3-Rmi1. Topo IIIα is the ortholog of S. cerevisiae Top3, but Rmi2 is not present in S. cerevisiae (Chang et al., 2005; Singh et al., 2008; Xu et al., 2008; Yin et al., 2005). Importantly, BLM in conjunction with Topo IIIa can dissolve the dHJ in a manner that yields exclusively noncrossover recombinants (Wu and Hickson, 2003). In this dHJ dissolution reaction, BLM catalyzes the convergent branch migration of the two Holliday junctions, to process the dHJ into a hemicatenane structure that is resolved by the topoisomerase activity of Topo IIIa (Wu and Hickson, 2003). The Rmi1-Rmi2 complex greatly stimulates the dHJ activity of the BLM-Topo IIIa pair (Raynard et al., 2006; Singh et al., 2008; Wu and Hickson, 2003; Xu et al., 2008), and this functional synergy requires the physical interaction between Rmi1 and Topo IIIα as revealed by examining mutations in Rmi1 that ablate its interaction with Topo IIIa (Bussen et al., 2007; Raynard et al., 2008; Yang et al., 2010). As alluded to earlier, the S. cerevisiae Sgs1-Top3-Rmi1 complex also possesses a robust dHJ dissolvase activity (Cejka et al., 2010b; Niu et al., 2010).
(iv) Multifaceted role of BLM in DNA end resection
Like Sgs1, BLM cooperates with human DNA2 to promote long-range resection (Figure 2) (Gravel et al., 2008; Nimonkar et al., 2011). As in the case of Sgs1-Dna2, the efficiency of resection mediated by the BLM-DNA2 pair is enhanced by the MRE11-RAD50-NBS1 (MRN) complex, which is the equivalent of S. cerevisiae MRX complex (Nimonkar et al., 2011). RPA exerts functions in the reconstituted human resection system similar to what have been reported for S. cerevisiae RPA, namely, by enhancing BLM-mediated DNA unwinding and imposing a preference for 5’ flap cutting by DNA2 (Cejka et al., 2010a; Nimonkar et al., 2011; Niu et al., 2010). However, the role of Topo IIIa-Rmi1-Rmi2 in end resection has not yet been examined. Interestingly, BLM also interacts with and enhances the activity of hEXO1 (Nimonkar et al., 2008). Thus, in addition to being an integral component of the BLM-DNA2 resection path, BLM may also function in hEXO1-dependent resection.
Figure 2. Multiplicity of DNA end resection means.
A limited amount of 5’ resection is catalyzed by the MRX-Sae2 complex in yeast or the MRN-CtIP complex in humans. Two parallel pathways are responsible for long range resection, with one being mediated by Exo1 and the other by the Sgs1 or BLM-associated protein ensemble. Abbreviations: MRX, Mre11-Rad50-Xrs2; MRN, MRE11-RAD50-NBS1; STR, Sgs1-Top3-Rmi1.
d. RECQ5 helicase and its role on HR regulation
(i) Genetic characterization
RECQ5 has not been linked to a human disease, but its ablation in mice results in cancer susceptibility (Hu et al., 2007). RECQ5-deficient cells exhibit elevated SCEs and HR events, and are prone to gross chromosomal rearrangements upon genotoxic stress (Hu et al., 2005; Hu et al., 2007). Depletion of BLM on top of RECQ5 deletion exacerbates the SCE phenotype, indicating that the two proteins function in parallel pathways to regulate HR (Hu et al., 2005).
(ii) Anti-recombinase activity of RECQ5
To gain insights into its HR regulatory function, RECQ5 protein was purified and examined in conjunction with the RAD51 recombinase. RECQ5 was found to physically interact with RAD51 and inhibits the recombinase activity of RAD51 (Hu et al., 2007). The RAD51 inhibitory activity of RECQ5 is potentiated by RPA. Extensive biochemical analysis and electron microscopy have shown that RECQ5 displaces RAD51 from ssDNA, in a reaction that is linked to ATP hydrolysis by the former. RPA enhances this anti-recombinase attribute of RECQ5 by preventing the renucleation of RAD51 onto ssDNA (Hu et al., 2007). Mutants of RECQ5 impaired for RAD51 interaction have been generated, and characterization of these mutants have furnished evidence that physical interaction with RAD51 as being indispensable for the anti-recombinase function (Schwendener et al., 2010). Together, these findings implicate RECQ5 as a tumor suppressor that acts by preventing inappropriate HR events via the disruption of RAD51-ssDNA nucleoprotein filaments.
(iii) Prevention of transcription-induced genome instability
RECQ5 also associates with RNA polymerase II in a complex distinct from that harbors Rad51. In this context, RECQ5 seems to be important for minimizing transcription-associated genome instability (Islam et al., 2010; Kanagaraj et al., 2010; Li et al., 2011). Thus, loss of RECQ5 leads to the accumulation of spontaneous DSBs during DNA replication that is linked to RNA Pol II transcription (Li et al., 2011). While the specific role of RECQ5 is not yet known in this case, a reasonable assumption is that the helicase activity of RECQ5 unwinds DNA structures or clears DNA of proteins when the transcription and replication machineries collide.
The Hef family of helicases
a. Conservation and biochemical properties of Hef
Several conserved DNA helicases/translocases related to archael Hef (Helicase-associated endonuclease for fork-structured DNA) have been shown to play an important role in homologous recombination regulation. These proteins are members of the SF2 helicase superfamily. S. cerevisiae and humans contain one member of this family, Mph1 and FANCM, respectively. The latter is associated with complementation group M of the cancer-prone syndrome Fanconi anemia (FA) (Meetei et al., 2005). The Hef protein from Pyrococcus furiosus contains a conserved helicase domain and a C-terminal endonuclease domain that is structurally related to that found in the nucleases Mus81 and XPF (Nishino et al., 2003). The crystal structure of the helicase and endonuclease domains of Hef has been solved (Nishino et al., 2005). Hef is able to process DNA fork and four-way junction structures to produce splayed duplexes (Komori et al., 2004). The Hef nuclease, which homodimerizes as revealed in the crystal structure, preferentially cleaves replication fork structures in a manner that is stimulated by the helicase activity (Komori et al., 2004). Taken together, these results suggest a role of Hef in processing injured replication forks.
b. Yeast Mph1 and its HR regulatory role
(i) Genetic characteristics
Yeast MPH1 gene was identified in a screen for mutants that exhibit a mutator phenotype (Scheller et al., 2000). Cells lacking Mph1 are hypersensitive to genotoxic chemicals such as MMS, EMS, 4-NQO and camptothecin (Scheller et al., 2000). Interestingly, the mph1 spontaneous mutator phenotype depends on REV3, which encodes the catalytic subunit of the translesion synthesis DNA polymerase ζ (Schurer et al., 2004). Further studies have shown that the mph1 mutation is epistatic to the HR mutants rad51, rad52 and rad55 in terms of spontaneous mutation rates and DNA damage sensitivity (Schurer et al., 2004). These results suggest that Mph1 functions with HR proteins to promote the error-free bypass of DNA lesions. Additional evidence supporting this premise comes from recent studies on the S. cerevisiae Smc5/6 complex, one of the three conserved structural maintenance of chromosomes (SMC) complexes. Mutations in SMC5 or SMC6 render yeast cells hypersensitive to agents that interfere with DNA replication, such as HU or MMS. Upon treatment with HU or MMS, accumulation of Rad51-dependent X-shaped DNA intermediates occurs in the smc5 or smc6 mutant (Choi et al., 2010). Importantly, deletion of MPH1 or inactivation of the helicase activity of Mph1 protein largely eliminates X-shaped intermediates in smc5 or smc6 mutants and suppresses the sensitivity of the mutant cells to HU or MMS (Chavez et al., 2011; Choi et al., 2010). On the other hand, overexpression of MPH1 further exacerbates some of the phenotypes observed in smc5/6 mutants (Chen et al., 2009). These results suggest that Mph1 functions in HR-mediated replication fork repair, but the activity of Mph1 must be restrained by the Smc5/6 complex to prevent the generation of toxic DNA intermediates.
In a study that examined mutations capable of intensifying the HR regulatory phenotype of srs2∆ cells, MPH1 was discovered as a suppressor of spontaneous unequal sister chromatid exchanges and DNA double-strand break-induced chromosome crossovers (Ira et al., 2003). Importantly, Mph1 protein functions in crossover control via a novel mechanism independent of the Srs2 and Sgs1 helicases, which is consistent with the biochemical finding that Mph1 dissociates Rad51-catalyzed D-loops to promote DSB repair via SDSA (See below) (Prakash et al., 2009).
(ii)Biochemical properties of the Mph1 helicase activity
While archael Hef contains helicase and endonuclease domains, Mph1 has only the helicase domain. Purified Mph1 exhibits a ssDNA-dependent ATPase activity and a 3’-5’ helicase activity that is enhanced by the single-strand DNA binding protein RPA (Prakash et al., 2005). Recently, our laboratory and others have demonstrated that Mph1 possesses a robust branch migration activity on replication fork and four-way junction structures and can mediate extensive branch migration of a sigma structure that mimics a DNA replication fork (Kang et al., 2009; Zheng et al., 2011). The biochemical results suggest a role of Mph1 in processing replication fork structures, similar to Hef. This premise is further supported by the recent finding that cleavage of 5’-flap structures by the Fen1 nuclease, which is needed for Okazaki fragment processing and maturation, is stimulated by Mph1 (Kang et al., 2009).
(iii) D-loop dissociative activity of Mph1
Since genetic studies have implicated MPH1 in the suppression of crossovers, purified Mph1 was examined for activities relevant for this HR regulatory activity. The protein binds D-loop structures and is particularly adept at unwinding these structures. Importantly, Mph1 dissociates Rad51-made D-loops with high efficiency (Prakash et al., 2009). D-loop dissociation is linked to ATP hydrolysis by Mph1, as the D209N mutation in the helicase motif II of Mph1 that abolishes its ATPase activity is inactive in this regard (Prakash et al., 2009). Dissociation of D-loops by Mph1 can explain how it suppresses crossover formation by promoting the SDSA pathway of HR, which only leads to a noncrossover outcome (Figure 1). The S. pombe ortholog, Fml1, appears to function similarly (Sun et al., 2008). We note that this HR regulatory function of Mph1 distinguishes it in mechanism from Sgs1 and Srs2, which suppress crossover formation by dHJ dissolution (see above) and displacing the Rad51 recombinase from DNA (see below), respectively.
c. FANCM and its genetic and biochemical properties
As mentioned above, the protein FANCM is the likely human ortholog of Mph1 and Hef (Meetei et al., 2005). It is part of the large multi-subunit FA complex, which is necessary for the efficient repair of interstrand crosslinks (Kee and D'Andrea, 2010). Like Mph1, FANCM lacks nuclease activity. While FANCM does not possess a classical helicase activity, it can, as in the case of Mph1, utilize its DNA-dependent ATPase activity to translocate along DNA and to promote the migration of Holliday junctions and replication fork reversal (Gari et al., 2008a; Gari et al., 2008b; Meetei et al., 2005). Importantly, FANCM can also efficiently disrupt protein-free D-loops, although it remains to be established whether it can do so within the context of a homologous pairing reaction mediated by Rad51 (Gari et al., 2008b)). As expected, the FANCM-K117R mutant that is defective in ATPase activity, is unable to process the Holliday junction or replication fork and is similarly defective in D-loop dissociation (Gari et al., 2008b)). Importantly, FANCM deficient human and chicken DT40 cells exhibit elevated SCEs, but the phenotype is not as severe as that caused by BLM depletion (Rosado et al., 2009; Xue et al., 2008). Simultaneous ablation of FANCM and BLM does not further increase SCE levels beyond BLM alone, consistent with a role of FANCM in preventing some SCEs in a BLM-dependent manner (Rosado et al., 2009). It seems reasonable to propose that FANCM favors noncrossover formation during HR via its D-loop dissociative activity.
The Srs2 helicase and its HR regulatory function
(i) SRS2 identification and genetic characteristics
A mutant allele of SRS2 was identified as a suppressor of the sensitivity of rad6 and rad18 mutants, defective in post-replicative DNA repair, to ultraviolet light induced DNA damage (Lawrence and Christensen, 1979). The suppression depends on HR, and srs2 mutants show a hyper-recombination phenotype (Nguyen and Livingston, 1997; Palladino and Klein, 1992). Taken together, the above genetic observations suggest that Srs2 functions to restrain HR in cells, and an enhanced ability of srs2 cells to conduct HR can override the DNA repair deficiency of a rad6 or rad18 mutation. Mutations in SRS2 also cause severe synthetic grow defects with mutations in genes that encode factors involved in recombination intermediate processing, such as SGS1 and RAD54 (Lee et al., 1999; Palladino and Klein, 1992). This and other phenotypes of srs2 mutants can be suppressed by mutating RAD51 (Gangloff et al., 2000). Interestingly, SRS2 also attenuates crossover formation by promoting the use of the SDSA pathway, and this occurs independently of SGS1 and MPH1 (Ira et al., 2003). These genetic results reinforce the idea of Srs2 acting as a negative regulator of HR and a promoter of DNA break repair by SDSA.
(ii) Anti-recombinase activity of Srs2
The mechanism by which Srs2 negatively regulates Rad51-mediated HR was revealed in biochemical studies. Purified Srs2 displays ssDNA-dependent ATPase and 3’-5’ helicase activities and physically interacts with Rad51 (Krejci et al., 2003; Van Komen et al., 2003). Importantly, the addition of a catalytic quantity of Srs2 to Rad51-mediated homologous DNA pairing reactions strongly inhibits these reactions. Further analysis showed that Srs2 acts by dislodging Rad51 from ssDNA, and that this attribute of Srs2 is enhanced by RPA, which, by occupying ssDNA sites made available as a result of Rad51 eviction, minimizes the reassembly of the Rad51-ssDNA complex (Krejci et al., 2003; Veaute et al., 2003). These findings thus provide evidence that Srs2 attenuates HR via dismantling the Rad51-ssDNA complex. Examination of mutant Srs2 proteins that are impaired for complex formation with Rad51 revealed that the efficiency of the anti-recombinase function is dependent on interaction with the later (Antony et al., 2009; Colavito et al., 2009). It has been proposed that interaction of Srs2 with Rad51 triggers ATP hydrolysis within the Rad51 protein filament assembled on ssDNA, causing Rad51 to dissociate from the DNA (Antony et al., 2009).
Two HR mediators, namely, Rad52 protein and Rad55-Rad57 heterodimer, capable of enhancing the assembly or stability of the Rad51-ssDNA nucleoprotein complex have been described (San Filippo et al., 2008). Rad52 can antagonize the Srs2 anti-recombinase activity by reloading Rad51 onto RPA-coated ssDNA (Burgess et al., 2009), while the Rad55-Rad57 complex attenuates the activity of Srs2 via a direct interaction with it (Liu et al., 2011).
(iii) Anti-recombinase function of Srs2 at the DNA replication fork
Studies conducted in S. cerevisiae have unveiled distinct means of lesion bypass during DNA replication, and the choice of DNA damage tolerance pathway is dependent on the ubiquitination or SUMOylation of the DNA polymerase clamp PCNA (Moldovan et al., 2007). SUMOylation of PCNA also occurs in the absence of exogenously induced DNA damage during S phase. Genetic analysis has shown that SUMO-modified PCNA helps recruit Srs2 to the DNA replication fork, where it inhibits HR by disrupting Rad51 protein filament assembled on ssDNA stemming from fork stalling (Moldovan et al., 2007; Papouli et al., 2005; Pfander et al., 2005). Srs2 interacts directly with the SUMO-modified form of PCNA, via SIM and PIP motifs located in the C-terminal region of Srs2 that specifically recognize SUMO and PCNA, respectively (Armstrong et al., 2012; Papouli et al., 2005; Pfander et al., 2005). These findings suggest that SUMO-modified PCNA recruits Srs2 to prevent undesirable HR events from occurring during DNA replication. The high-resolution structure of the C-terminal region of Srs2 bound to SUMO-PCNA has been solved recently (Armstrong et al., 2012).
RTEL and its D-loop dissociative properties
To search for the Srs2 equivalent in higher organisms, a genetic screen for synthetic lethality with the SGS1 ortholog HIM-6 was performed in C. elegans, which led to the identification of RTEL-1, a helicase (Barber et al., 2008). RTEL-1 is orthologous to the previously identified murine Rtel protein shown to be a regulator of telomere length (Ding et al., 2004). rtel-1 him-6 double mutant worms show a drastic increase in RAD51 foci, indicating an accumulation of recombination intermediates (Barber et al., 2008). Loss of rtel-1 alone causes sensitivity to DNA damaging agents and elevated crossover recombination in meiosis (Barber et al., 2008; Youds et al., 2010). siRNA against human RTEL also leads to increased recombination (Barber et al., 2008). In vitro, RTEL can disrupt RAD51-mediated D-loops in a manner that is dependent on RPA, but it cannot disassemble RAD51-ssDNA nucleoprotein filaments, unlike Srs2 and RECQ5 (Barber et al., 2008; Youds et al., 2010). Thus, RTEL appears to employ the same mechanism as S. cerevisiae Mph1 in the regulation of HR (Prakash et al., 2009).
Epilogue
Concerted efforts in model organisms such as S. cerevisiae and C. elegans, and companion studies in humans and other vertebrate species have helped identify three distinct means of HR regulation, each of which is mediated by a DNA helicase. These include the disassembly of the RAD51-ssDNA nucleoprotein filament by Srs2/RECQ5, D-loop dissociation by Mph1/RTEL, and dHJ dissolution by the BLM and Sgs1 protein ensembles. These mechanisms function in parallel to ensure that the conservative SDSA mechanism is used predominantly during HR in mitotic cells.
Aside from the known HR regulators as reviewed above, there are tantalizing clues that additional players are involved in HR regulation. Recent evidence suggests that Fbh1, a SF1 helicase that also possesses a ubiquitin E3 ligase activity, may act as an anti-recombinase similar to S0rs2 and RECQ5 (Lorenz et al., 2009; Osman et al., 2005). The other RecQ family members WRN and RECQ1 may also influence HR (LeRoy et al., 2005; Opresko et al., 2009; Sharma et al., 2005). Further investigation will be needed to clarify the HR role of these helicases.
References
- Antony E, Tomko EJ, Xiao Q, Krejci L, Lohman TM, Ellenberger T. Srs2 disassembles Rad51 filaments by a protein-protein interaction triggering ATP turnover and dissociation of Rad51 from DNA. Mol Cell. 2009;35:105–115. doi: 10.1016/j.molcel.2009.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong AA, Mohideen F, Lima CD. Recognition of SUMO-modified PCNA requires tandem receptor motifs in Srs2. Nature. 2012;483:59–63. doi: 10.1038/nature10883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachrati CZ, Borts RH, Hickson ID. Mobile D-loops are a preferred substrate for the Bloom's syndrome helicase. Nucleic Acids Res. 2006;34:2269–2279. doi: 10.1093/nar/gkl258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae SH, Seo YS. Characterization of the enzymatic properties of the yeast dna2 Helicase/endonuclease suggests a new model for Okazaki fragment processing. J Biol Chem. 2000;275:38022–38031. doi: 10.1074/jbc.M006513200. [DOI] [PubMed] [Google Scholar]
- Barber LJ, Youds JL, Ward JD, McIlwraith MJ, O'Neil NJ, Petalcorin MI, Martin JS, Collis SJ, Cantor SB, Auclair M, et al. RTEL1 maintains genomic stability by suppressing homologous recombination. Cell. 2008;135:261–271. doi: 10.1016/j.cell.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borner GV, Kleckner N, Hunter N. Crossover/noncrossover differentiation, synaptonemal complex formation, and regulatory surveillance at the leptotene/zygotene transition of meiosis. Cell. 2004;117:29–45. doi: 10.1016/s0092-8674(04)00292-2. [DOI] [PubMed] [Google Scholar]
- Budd ME, Choe WC, Campbell JL. DNA2 encodes a DNA helicase essential for replication of eukaryotic chromosomes. J Biol Chem. 1995;270:26766–26769. doi: 10.1074/jbc.270.45.26766. [DOI] [PubMed] [Google Scholar]
- Burgess RC, Lisby M, Altmannova V, Krejci L, Sung P, Rothstein R. Localization of recombination proteins and Srs2 reveals anti-recombinase function in vivo. J Cell Biol. 2009;185:969–981. doi: 10.1083/jcb.200810055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussen W, Raynard S, Busygina V, Singh AK, Sung P. Holliday junction processing activity of the BLM-Topo IIIalpha-BLAP75 complex. J Biol Chem. 2007;282:31484–31492. doi: 10.1074/jbc.M706116200. [DOI] [PubMed] [Google Scholar]
- Cejka P, Cannavo E, Polaczek P, Masuda-Sasa T, Pokharel S, Campbell JL, Kowalczykowski SC. DNA end resection by Dna2-Sgs1-RPA and its stimulation by Top3-Rmi1 and Mre11-Rad50-Xrs2. Nature. 2010a;467:112–116. doi: 10.1038/nature09355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cejka P, Kowalczykowski SC. The full-length Saccharomyces cerevisiae Sgs1 protein is a vigorous DNA helicase that preferentially unwinds holliday junctions. J Biol Chem. 2010;285:8290–8301. doi: 10.1074/jbc.M109.083196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cejka P, Plank JL, Bachrati CZ, Hickson ID, Kowalczykowski SC. Rmi1 stimulates decatenation of double Holliday junctions during dissolution by Sgs1-Top3. Nat Struct Mol Biol. 2010b;17:1377–1382. doi: 10.1038/nsmb.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaganti RS, Schonberg S, German J. A manyfold increase in sister chromatid exchanges in Bloom's syndrome lymphocytes. Proc Natl Acad Sci U S A. 1974;71:4508–4512. doi: 10.1073/pnas.71.11.4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang M, Bellaoui M, Zhang C, Desai R, Morozov P, Delgado-Cruzata L, Rothstein R, Freyer GA, Boone C, Brown GW. RMI1/NCE4, a suppressor of genome instability, encodes a member of the RecQ helicase/Topo III complex. EMBO J. 2005;24:2024–2033. doi: 10.1038/sj.emboj.7600684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez A, Agrawal V, Johnson FB. Homologous recombination-dependent rescue of deficiency in the structural maintenance of chromosomes (Smc) 5/6 complex. J Biol Chem. 2011;286:5119–5125. doi: 10.1074/jbc.M110.201608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YH, Choi K, Szakal B, Arenz J, Duan X, Ye H, Branzei D, Zhao X. Interplay between the Smc5/6 complex and the Mph1 helicase in recombinational repair. Proc Natl Acad Sci U S A. 2009;106:21252–21257. doi: 10.1073/pnas.0908258106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester N, Kuo F, Kozak C, O'Hara CD, Leder P. Stage-specific apoptosis, developmental delay, and embryonic lethality in mice homozygous for a targeted disruption in the murine Bloom's syndrome gene. Genes Dev. 1998;12:3382–3393. doi: 10.1101/gad.12.21.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K, Szakal B, Chen YH, Branzei D, Zhao X. The Smc5/6 complex and Esc2 influence multiple replication-associated recombination processes in Saccharomyces cerevisiae. Mol Biol Cell. 2010;21:2306–2314. doi: 10.1091/mbc.E10-01-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu WK, Hickson ID. RecQ helicases: multifunctional genome caretakers. Nat Rev Cancer. 2009;9:644–654. doi: 10.1038/nrc2682. [DOI] [PubMed] [Google Scholar]
- Colavito S, Macris-Kiss M, Seong C, Gleeson O, Greene EC, Klein HL, Krejci L, Sung P. Functional significance of the Rad51-Srs2 complex in Rad51 presynaptic filament disruption. Nucleic Acids Res. 2009;37:6754–6764. doi: 10.1093/nar/gkp748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinou A, Tarsounas M, Karow JK, Brosh RM, Bohr VA, Hickson ID, West SC. Werner's syndrome protein (WRN) migrates Holliday junctions and co-localizes with RPA upon replication arrest. EMBO Rep. 2000;1:80–84. doi: 10.1093/embo-reports/kvd004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding H, Schertzer M, Wu X, Gertsenstein M, Selig S, Kammori M, Pourvali R, Poon S, Vulto I, Chavez E, et al. Regulation of murine telomere length by Rtel: an essential gene encoding a helicase-like protein. Cell. 2004;117:873–886. doi: 10.1016/j.cell.2004.05.026. [DOI] [PubMed] [Google Scholar]
- Evans E, Sugawara N, Haber JE, Alani E. The Saccharomyces cerevisiae Msh2 mismatch repair protein localizes to recombination intermediates in vivo. Mol Cell. 2000;5:789–799. doi: 10.1016/s1097-2765(00)80319-6. [DOI] [PubMed] [Google Scholar]
- Gangloff S, McDonald JP, Bendixen C, Arthur L, Rothstein R. The yeast type I topoisomerase Top3 interacts with Sgs1, a DNA helicase homolog: a potential eukaryotic reverse gyrase. Mol Cell Biol. 1994;14:8391–8398. doi: 10.1128/mcb.14.12.8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangloff S, Soustelle C, Fabre F. Homologous recombination is responsible for cell death in the absence of the Sgs1 and Srs2 helicases. Nat Genet. 2000;25:192–194. doi: 10.1038/76055. [DOI] [PubMed] [Google Scholar]
- Gari K, Decaillet C, Delannoy M, Wu L, Constantinou A. Remodeling of DNA replication structures by the branch point translocase FANCM. Proc Natl Acad Sci U S A. 2008a;105:16107–16112. doi: 10.1073/pnas.0804777105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gari K, Decaillet C, Stasiak AZ, Stasiak A, Constantinou A. The Fanconi anemia protein FANCM can promote branch migration of Holliday junctions and replication forks. Mol Cell. 2008b;29:141–148. doi: 10.1016/j.molcel.2007.11.032. [DOI] [PubMed] [Google Scholar]
- German J, Sanz MM, Ciocci S, Ye TZ, Ellis NA. Syndrome-causing mutations of the BLM gene in persons in the Bloom's Syndrome Registry. Hum Mutat. 2007;28:743–753. doi: 10.1002/humu.20501. [DOI] [PubMed] [Google Scholar]
- Goss KH, Risinger MA, Kordich JJ, Sanz MM, Straughen JE, Slovek LE, Capobianco AJ, German J, Boivin GP, Groden J. Enhanced tumor formation in mice heterozygous for Blm mutation. Science. 2002;297:2051–2053. doi: 10.1126/science.1074340. [DOI] [PubMed] [Google Scholar]
- Gravel S, Chapman JR, Magill C, Jackson SP. DNA helicases Sgs1 and BLM promote DNA double-strand break resection. Genes Dev. 2008;22:2767–2772. doi: 10.1101/gad.503108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada K, Ukita T, Kohno Y, Saito K, Kato J, Ikeda H. RecQ DNA helicase is a suppressor of illegitimate recombination in Escherichia coli. Proc Natl Acad Sci U S A. 1997;94:3860–3865. doi: 10.1073/pnas.94.8.3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Lu X, Barnes E, Yan M, Lou H, Luo G. Recql5 and Blm RecQ DNA helicases have nonredundant roles in suppressing crossovers. Mol Cell Biol. 2005;25:3431–3442. doi: 10.1128/MCB.25.9.3431-3442.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Raynard S, Sehorn MG, Lu X, Bussen W, Zheng L, Stark JM, Barnes EL, Chi P, Janscak P, et al. RECQL5/Recql5 helicase regulates homologous recombination and suppresses tumor formation via disruption of Rad51 presynaptic filaments. Genes Dev. 2007;21:3073–3084. doi: 10.1101/gad.1609107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ii M, Brill SJ. Roles of SGS1, MUS81, and RAD51 in the repair of lagging-strand replication defects in Saccharomyces cerevisiae. Curr Genet. 2005;48:213–225. doi: 10.1007/s00294-005-0014-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ira G, Malkova A, Liberi G, Foiani M, Haber JE. Srs2 and Sgs1-Top3 suppress crossovers during double-strand break repair in yeast. Cell. 2003;115:401–411. doi: 10.1016/s0092-8674(03)00886-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MN, Fox D, 3rd, Guo R, Enomoto T, Wang W. RecQL5 promotes genome stabilization through two parallel mechanisms--interacting with RNA polymerase II and acting as a helicase. Mol Cell Biol. 2010;30:2460–2472. doi: 10.1128/MCB.01583-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessop L, Rockmill B, Roeder GS, Lichten M. Meiotic chromosome synapsis-promoting proteins antagonize the anti-crossover activity of sgs1. PLoS Genet. 2006;2:e155. doi: 10.1371/journal.pgen.0020155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanagaraj R, Huehn D, MacKellar A, Menigatti M, Zheng L, Urban V, Shevelev I, Greenleaf AL, Janscak P. RECQ5 helicase associates with the C-terminal repeat domain of RNA polymerase II during productive elongation phase of transcription. Nucleic Acids Res. 2010;38:8131–8140. doi: 10.1093/nar/gkq697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang YH, Kang MJ, Kim JH, Lee CH, Cho IT, Hurwitz J, Seo YS. The MPH1 gene of Saccharomyces cerevisiae functions in Okazaki fragment processing. J Biol Chem. 2009;284:10376–10386. doi: 10.1074/jbc.M808894200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karow JK, Constantinou A, Li JL, West SC, Hickson ID. The Bloom's syndrome gene product promotes branch migration of holliday junctions. Proc Natl Acad Sci U S A. 2000;97:6504–6508. doi: 10.1073/pnas.100448097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee Y, D'Andrea AD. Expanded roles of the Fanconi anemia pathway in preserving genomic stability. Genes Dev. 2010;24:1680–1694. doi: 10.1101/gad.1955310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori K, Hidaka M, Horiuchi T, Fujikane R, Shinagawa H, Ishino Y. Cooperation of the N-terminal Helicase and C-terminal endonuclease activities of Archaeal Hef protein in processing stalled replication forks. J Biol Chem. 2004;279:53175–53185. doi: 10.1074/jbc.M409243200. [DOI] [PubMed] [Google Scholar]
- Krejci L, Van Komen S, Li Y, Villemain J, Reddy MS, Klein H, Ellenberger T, Sung P. DNA helicase Srs2 disrupts the Rad51 presynaptic filament. Nature. 2003;423:305–309. doi: 10.1038/nature01577. [DOI] [PubMed] [Google Scholar]
- Krogh BO, Symington LS. Recombination proteins in yeast. Annu Rev Genet. 2004;38:233–271. doi: 10.1146/annurev.genet.38.072902.091500. [DOI] [PubMed] [Google Scholar]
- Lawrence CW, Christensen RB. Metabolic suppressors of trimethoprim and ultraviolet light sensitivities of Saccharomyces cerevisiae rad6 mutants. J Bacteriol. 1979;139:866–876. doi: 10.1128/jb.139.3.866-876.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SK, Johnson RE, Yu SL, Prakash L, Prakash S. Requirement of yeast SGS1 and SRS2 genes for replication and transcription. Science. 1999;286:2339–2342. doi: 10.1126/science.286.5448.2339. [DOI] [PubMed] [Google Scholar]
- LeRoy G, Carroll R, Kyin S, Seki M, Cole MD. Identification of RecQL1 as a Holliday junction processing enzyme in human cell lines. Nucleic Acids Res. 2005;33:6251–6257. doi: 10.1093/nar/gki929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Xu X, Liu Y. The SET2-RPB1 interaction domain of human RECQ5 is important for transcription-associated genome stability. Mol Cell Biol. 2011;31:2090–2099. doi: 10.1128/MCB.01137-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber MR. The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway. Annu Rev Biochem. 2010;79:181–211. doi: 10.1146/annurev.biochem.052308.093131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Renault L, Veaute X, Fabre F, Stahlberg H, Heyer WD. Rad51 paralogues Rad55-Rad57 balance the antirecombinase Srs2 in Rad51 filament formation. Nature. 2011;479:245–248. doi: 10.1038/nature10522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz A, Osman F, Folkyte V, Sofueva S, Whitby MC. Fbh1 limits Rad51-dependent recombination at blocked replication forks. Mol Cell Biol. 2009;29:4742–4756. doi: 10.1128/MCB.00471-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meetei AR, Medhurst AL, Ling C, Xue Y, Singh TR, Bier P, Steltenpool J, Stone S, Dokal I, Mathew CG, et al. A human ortholog of archaeal DNA repair protein Hef is defective in Fanconi anemia complementation group M. Nat Genet. 2005;37:958–963. doi: 10.1038/ng1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimitou EP, Symington LS. Sae2, Exo1 and Sgs1 collaborate in DNA double-strand break processing. Nature. 2008;455:770–774. doi: 10.1038/nature07312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimitou EP, Symington LS. Nucleases and helicases take center stage in homologous recombination. Trends Biochem Sci. 2009;34:264–272. doi: 10.1016/j.tibs.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Mohaghegh P, Karow JK, Brosh RM, Jr, Bohr VA, Hickson ID. The Bloom's and Werner's syndrome proteins are DNA structure-specific helicases. Nucleic Acids Res. 2001;29:2843–2849. doi: 10.1093/nar/29.13.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldovan GL, Pfander B, Jentsch S. PCNA, the maestro of the replication fork. Cell. 2007;129:665–679. doi: 10.1016/j.cell.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Mullen JR, Nallaseth FS, Lan YQ, Slagle CE, Brill SJ. Yeast Rmi1/Nce4 controls genome stability as a subunit of the Sgs1-Top3 complex. Mol Cell Biol. 2005;25:4476–4487. doi: 10.1128/MCB.25.11.4476-4487.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen MM, Livingston DM. The effect of a suppressed rad52 mutation on the suppression of rad6 by srs2. Yeast. 1997;13:1059–1064. doi: 10.1002/(SICI)1097-0061(19970915)13:11<1059::AID-YEA165>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Nimonkar AV, Genschel J, Kinoshita E, Polaczek P, Campbell JL, Wyman C, Modrich P, Kowalczykowski SC. BLM-DNA2-RPA-MRN and EXO1-BLM-RPA-MRN constitute two DNA end resection machineries for human DNA break repair. Genes Dev. 2011;25:350–362. doi: 10.1101/gad.2003811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimonkar AV, Ozsoy AZ, Genschel J, Modrich P, Kowalczykowski SC. Human exonuclease 1 and BLM helicase interact to resect DNA and initiate DNA repair. Proc Natl Acad Sci U S A. 2008;105:16906–16911. doi: 10.1073/pnas.0809380105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino T, Komori K, Ishino Y, Morikawa K. X-ray and biochemical anatomy of an archaeal XPF/Rad1/Mus81 family nuclease: similarity between its endonuclease domain and restriction enzymes. Structure. 2003;11:445–457. doi: 10.1016/s0969-2126(03)00046-7. [DOI] [PubMed] [Google Scholar]
- Nishino T, Komori K, Tsuchiya D, Ishino Y, Morikawa K. Crystal structure and functional implications of Pyrococcus furiosus hef helicase domain involved in branched DNA processing. Structure. 2005;13:143–153. doi: 10.1016/j.str.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Niu H, Chung WH, Zhu Z, Kwon Y, Zhao W, Chi P, Prakash R, Seong C, Liu D, Lu L, et al. Mechanism of the ATP-dependent DNA end-resection machinery from Saccharomyces cerevisiae. Nature. 2010;467:108–111. doi: 10.1038/nature09318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SD, Lao JP, Hwang PY, Taylor AF, Smith GR, Hunter N. BLM ortholog, Sgs1, prevents aberrant crossing-over by suppressing formation of multichromatid joint molecules. Cell. 2007;130:259–272. doi: 10.1016/j.cell.2007.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoda F, Seki M, Miyajima A, Enomoto T. Elevation of sister chromatid exchange in Saccharomyces cerevisiae sgs1 disruptants and the relevance of the disruptants as a system to evaluate mutations in Bloom's syndrome gene. Mutat Res. 2000;459:203–209. doi: 10.1016/s0921-8777(99)00071-3. [DOI] [PubMed] [Google Scholar]
- Opresko PL, Sowd G, Wang H. The Werner syndrome helicase/exonuclease processes mobile D-loops through branch migration and degradation. PLoS One. 2009;4:e4825. doi: 10.1371/journal.pone.0004825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orren DK, Theodore S, Machwe A. The Werner syndrome helicase/exonuclease (WRN) disrupts and degrades D-loops in vitro. Biochemistry. 2002;41:13483–13488. doi: 10.1021/bi0266986. [DOI] [PubMed] [Google Scholar]
- Osman F, Dixon J, Barr AR, Whitby MC. The F-Box DNA helicase Fbh1 prevents Rhp51-dependent recombination without mediator proteins. Mol Cell Biol. 2005;25:8084–8096. doi: 10.1128/MCB.25.18.8084-8096.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palladino F, Klein HL. Analysis of mitotic and meiotic defects in Saccharomyces cerevisiae SRS2 DNA helicase mutants. Genetics. 1992;132:23–37. doi: 10.1093/genetics/132.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papouli E, Chen S, Davies AA, Huttner D, Krejci L, Sung P, Ulrich HD. Crosstalk between SUMO and ubiquitin on PCNA is mediated by recruitment of the helicase Srs2p. Mol Cell. 2005;19:123–133. doi: 10.1016/j.molcel.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Pfander B, Moldovan GL, Sacher M, Hoege C, Jentsch S. SUMO-modified PCNA recruits Srs2 to prevent recombination during S phase. Nature. 2005;436:428–433. doi: 10.1038/nature03665. [DOI] [PubMed] [Google Scholar]
- Popuri V, Bachrati CZ, Muzzolini L, Mosedale G, Costantini S, Giacomini E, Hickson ID, Vindigni A. The Human RecQ helicases, BLM and RECQ1, display distinct DNA substrate specificities. J Biol Chem. 2008;283:17766–17776. doi: 10.1074/jbc.M709749200. [DOI] [PubMed] [Google Scholar]
- Prakash R, Krejci L, Van Komen S, Anke Schurer K, Kramer W, Sung P. Saccharomyces cerevisiae MPH1 gene, required for homologous recombination-mediated mutation avoidance, encodes a 3' to 5' DNA helicase. J Biol Chem. 2005;280:7854–7860. doi: 10.1074/jbc.M413898200. [DOI] [PubMed] [Google Scholar]
- Prakash R, Satory D, Dray E, Papusha A, Scheller J, Kramer W, Krejci L, Klein H, Haber JE, Sung P, et al. Yeast Mph1 helicase dissociates Rad51-made D-loops: implications for crossover control in mitotic recombination. Genes Dev. 2009;23:67–79. doi: 10.1101/gad.1737809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynard S, Bussen W, Sung P. A double Holliday junction dissolvasome comprising BLM, topoisomerase IIIalpha, and BLAP75. J Biol Chem. 2006;281:13861–13864. doi: 10.1074/jbc.C600051200. [DOI] [PubMed] [Google Scholar]
- Raynard S, Zhao W, Bussen W, Lu L, Ding YY, Busygina V, Meetei AR, Sung P. Functional role of BLAP75 in BLM-topoisomerase IIIalpha-dependent holliday junction processing. J Biol Chem. 2008;283:15701–15708. doi: 10.1074/jbc.M802127200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosado IV, Niedzwiedz W, Alpi AF, Patel KJ. The Walker B motif in avian FANCM is required to limit sister chromatid exchanges but is dispensable for DNA crosslink repair. Nucleic Acids Res. 2009;37:4360–4370. doi: 10.1093/nar/gkp365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Filippo J, Sung P, Klein H. Mechanism of eukaryotic homologous recombination. Annu Rev Biochem. 2008;77:229–257. doi: 10.1146/annurev.biochem.77.061306.125255. [DOI] [PubMed] [Google Scholar]
- Scheller J, Schurer A, Rudolph C, Hettwer S, Kramer W. MPH1, a yeast gene encoding a DEAH protein, plays a role in protection of the genome from spontaneous and chemically induced damage. Genetics. 2000;155:1069–1081. doi: 10.1093/genetics/155.3.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurer KA, Rudolph C, Ulrich HD, Kramer W. Yeast MPH1 gene functions in an error-free DNA damage bypass pathway that requires genes from Homologous recombination, but not from postreplicative repair. Genetics. 2004;166:1673–1686. doi: 10.1534/genetics.166.4.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz EK, Heyer WD. Processing of joint molecule intermediates by structure-selective endonucleases during homologous recombination in eukaryotes. Chromosoma. 2011;120:109–127. doi: 10.1007/s00412-010-0304-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwendener S, Raynard S, Paliwal S, Cheng A, Kanagaraj R, Shevelev I, Stark JM, Sung P, Janscak P. Physical interaction of RECQ5 helicase with RAD51 facilitates its anti-recombinase activity. J Biol Chem. 2010;285:15739–15745. doi: 10.1074/jbc.M110.110478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Sommers JA, Choudhary S, Faulkner JK, Cui S, Andreoli L, Muzzolini L, Vindigni A, Brosh RM., Jr Biochemical analysis of the DNA unwinding and strand annealing activities catalyzed by human RECQ1. J Biol Chem. 2005;280:28072–28084. doi: 10.1074/jbc.M500264200. [DOI] [PubMed] [Google Scholar]
- Singh TR, Ali AM, Busygina V, Raynard S, Fan Q, Du CH, Andreassen PR, Sung P, Meetei AR. BLAP18/RMI2, a novel OB-fold-containing protein, is an essential component of the Bloom helicase-double Holliday junction dissolvasome. Genes Dev. 2008;22:2856–2868. doi: 10.1101/gad.1725108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara N, Goldfarb T, Studamire B, Alani E, Haber JE. Heteroduplex rejection during single-strand annealing requires Sgs1 helicase and mismatch repair proteins Msh2 and Msh6 but not Pms1. Proc Natl Acad Sci U S A. 2004;101:9315–9320. doi: 10.1073/pnas.0305749101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara N, Haber JE. Repair of DNA double strand breaks: in vivo biochemistry. Methods Enzymol. 2006;408:416–429. doi: 10.1016/S0076-6879(06)08026-8. [DOI] [PubMed] [Google Scholar]
- Sun H, Karow JK, Hickson ID, Maizels N. The Bloom's syndrome helicase unwinds G4 DNA. J Biol Chem. 1998;273:27587–27592. doi: 10.1074/jbc.273.42.27587. [DOI] [PubMed] [Google Scholar]
- Sun W, Nandi S, Osman F, Ahn JS, Jakovleska J, Lorenz A, Whitby MC. The FANCM ortholog Fml1 promotes recombination at stalled replication forks and limits crossing over during DNA double-strand break repair. Mol Cell. 2008;32:118–128. doi: 10.1016/j.molcel.2008.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay YD, Sidebotham JM, Wu L. Mph1 requires mismatch repair-independent and -dependent functions of MutSalpha to regulate crossover formation during homologous recombination repair. Nucleic Acids Res. 2010;38:1889–1901. doi: 10.1093/nar/gkp1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Komen S, Reddy MS, Krejci L, Klein H, Sung P. ATPase and DNA helicase activities of the Saccharomyces cerevisiae anti-recombinase Srs2. J Biol Chem. 2003;278:44331–44337. doi: 10.1074/jbc.M307256200. [DOI] [PubMed] [Google Scholar]
- Veaute X, Jeusset J, Soustelle C, Kowalczykowski SC, Le Cam E, Fabre F. The Srs2 helicase prevents recombination by disrupting Rad51 nucleoprotein filaments. Nature. 2003;423:309–312. doi: 10.1038/nature01585. [DOI] [PubMed] [Google Scholar]
- Wu L, Bachrati CZ, Ou J, Xu C, Yin J, Chang M, Wang W, Li L, Brown GW, Hickson ID. BLAP75/RMI1 promotes the BLM-dependent dissolution of homologous recombination intermediates. Proc Natl Acad Sci U S A. 2006;103:4068–4073. doi: 10.1073/pnas.0508295103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Hickson ID. The Bloom's syndrome helicase suppresses crossing over during homologous recombination. Nature. 2003;426:870–874. doi: 10.1038/nature02253. [DOI] [PubMed] [Google Scholar]
- Xu D, Guo R, Sobeck A, Bachrati CZ, Yang J, Enomoto T, Brown GW, Hoatlin ME, Hickson ID, Wang W. RMI, a new OB-fold complex essential for Bloom syndrome protein to maintain genome stability. Genes Dev. 2008;22:2843–2855. doi: 10.1101/gad.1708608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y, Li Y, Guo R, Ling C, Wang W. FANCM of the Fanconi anemia core complex is required for both monoubiquitination and DNA repair. Hum Mol Genet. 2008;17:1641–1652. doi: 10.1093/hmg/ddn054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Bachrati CZ, Ou J, Hickson ID, Brown GW. Human topoisomerase IIIalpha is a single-stranded DNA decatenase that is stimulated by BLM and RMI1. J Biol Chem. 2010;285:21426–21436. doi: 10.1074/jbc.M110.123216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J, Sobeck A, Xu C, Meetei AR, Hoatlin M, Li L, Wang W. BLAP75, an essential component of Bloom's syndrome protein complexes that maintain genome integrity. EMBO J. 2005;24:1465–1476. doi: 10.1038/sj.emboj.7600622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youds JL, Mets DG, McIlwraith MJ, Martin JS, Ward JD, NJ ON, Rose AM, West SC, Meyer BJ, Boulton SJ. RTEL-1 enforces meiotic crossover interference and homeostasis. Science. 2010;327:1254–1258. doi: 10.1126/science.1183112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng XF, Prakash R, Saro D, Longerich S, Niu H, Sung P. Processing of DNA structures via DNA unwinding and branch migration by the S. cerevisiae Mph1 protein. DNA Repair (Amst) 2011;10:1034–1043. doi: 10.1016/j.dnarep.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Chung WH, Shim EY, Lee SE, Ira G. Sgs1 helicase and two nucleases Dna2 and Exo1 resect DNA double-strand break ends. Cell. 2008;134:981–994. doi: 10.1016/j.cell.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]