Abstract
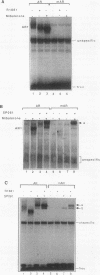
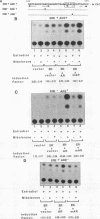
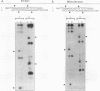
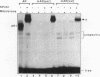
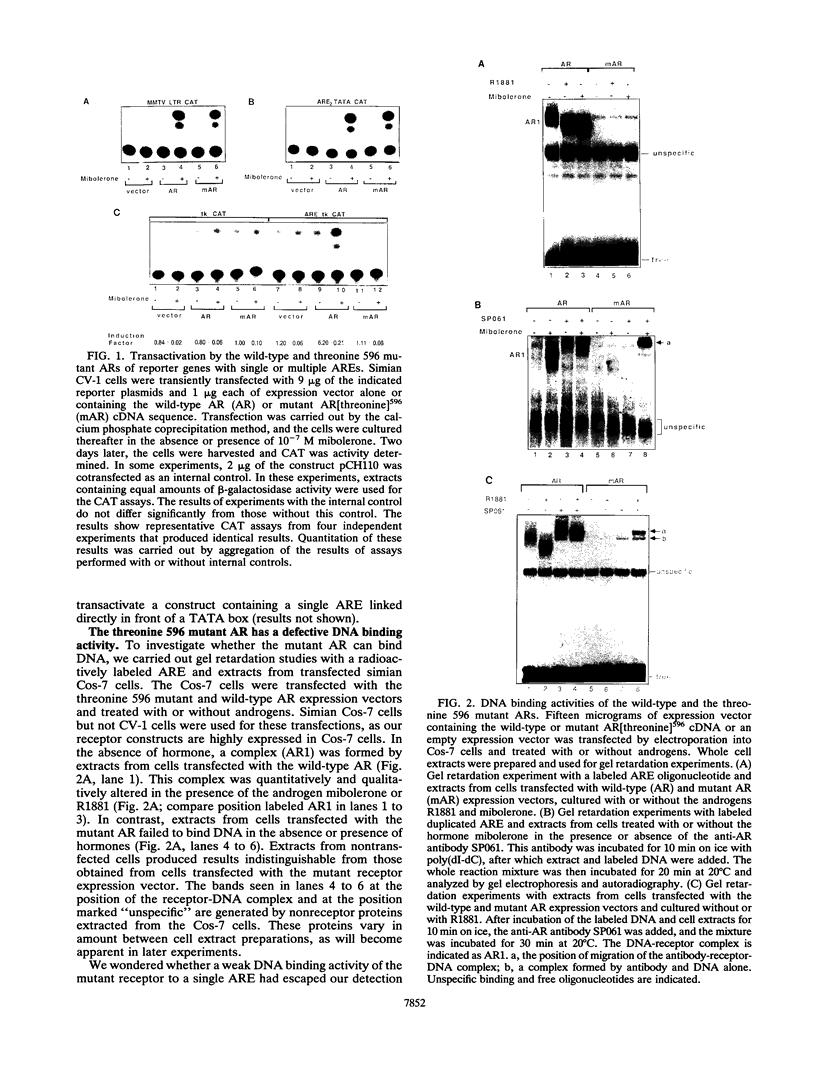
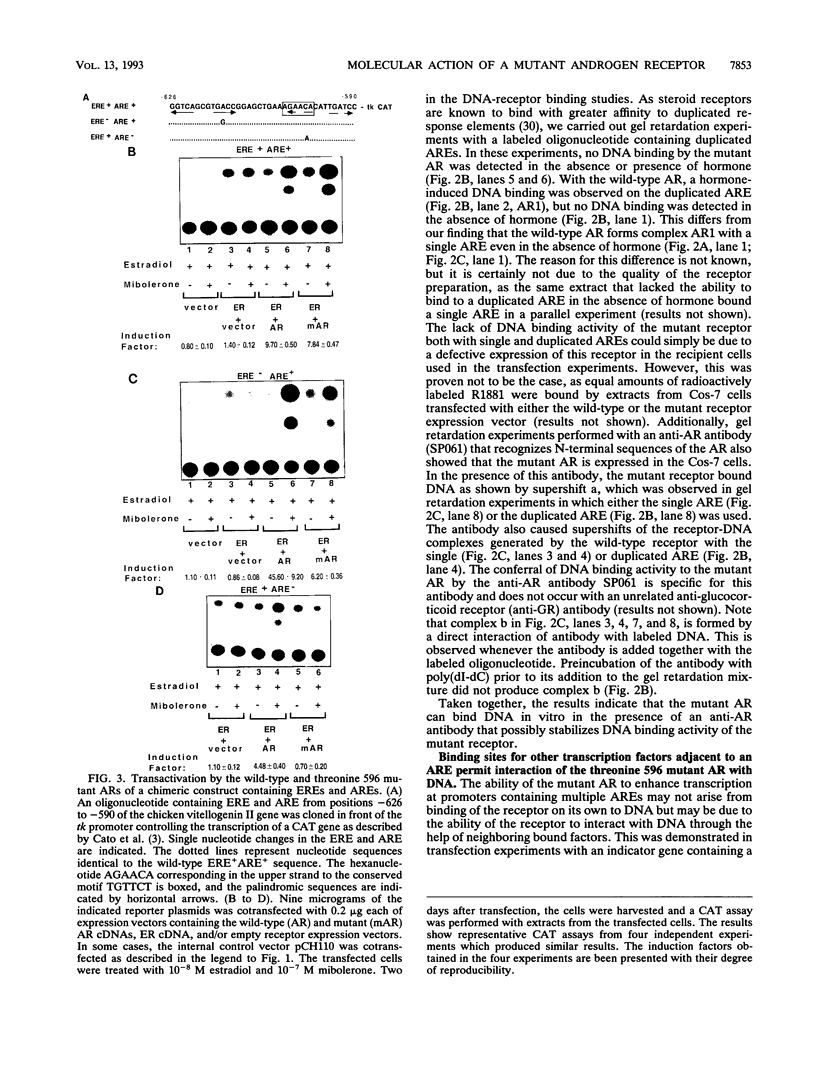
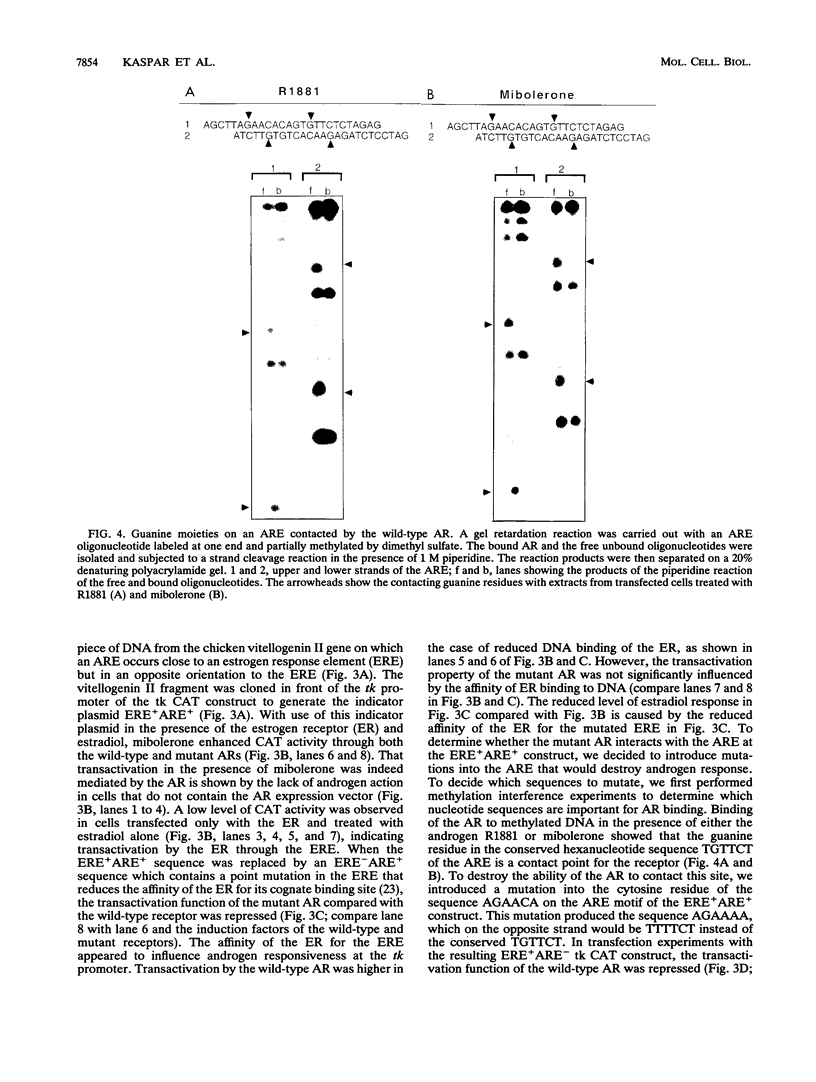
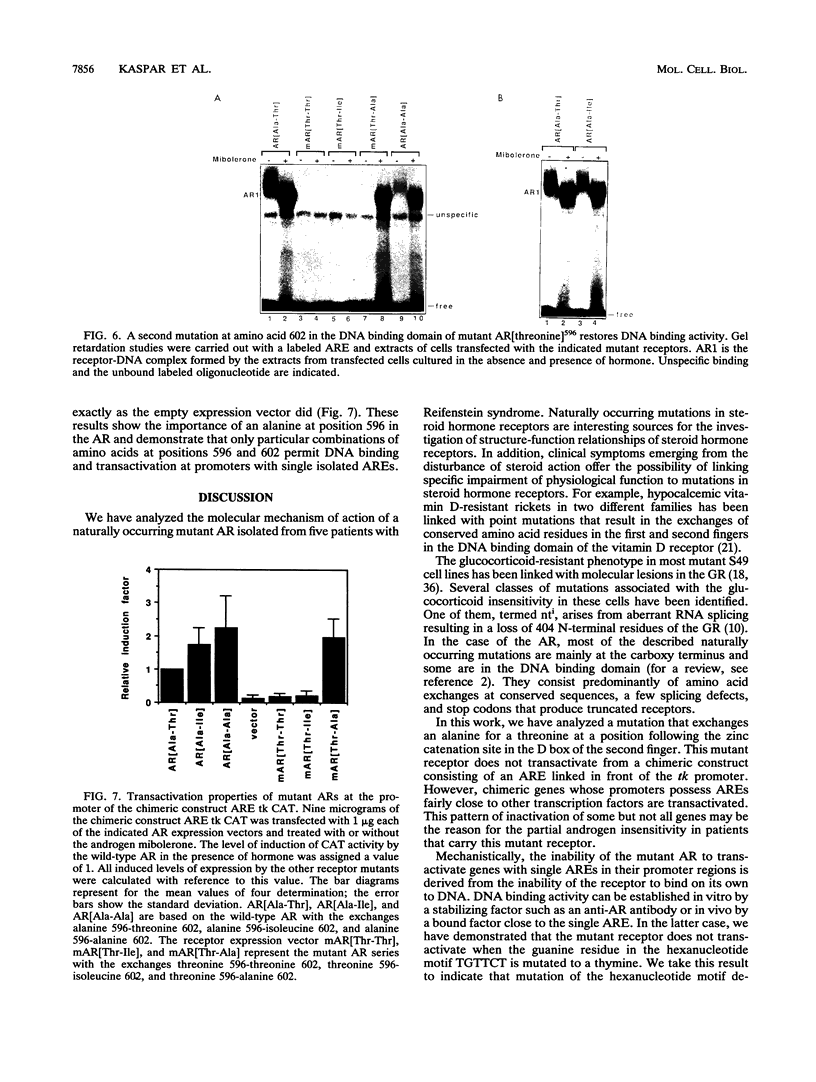
Reifenstein syndrome is an eponymic term that describes partial androgen-insensitive disorders. Androgen receptor isolated from five patients with this syndrome contains a specific mutation in the DNA binding domain of the receptor. This mutation converts an alanine to a threonine at position 596 next to the zinc catenation site at the second finger. The threonine 596 mutant receptor mediated normal androgen response at promoters with closely positioned multiple regulatory elements for the androgen receptor and other transcription factors. Promoters with single isolated androgen response elements were not transactivated by the mutant receptor. In in vitro receptor-DNA binding studies, interaction with DNA by the mutant receptor was achieved only in the presence of an anti-androgen receptor antibody. Exchanging alanine 596 in the wild-type androgen receptor with serine or valine produced mutants with properties indistinguishable from those of the naturally occurring threonine 596 mutant receptor. These results indicate that an alanine residue at position 596 contributes important structural and functional activities to the androgen receptor. In the androgen receptor from the patients with Reifenstein syndrome, in which this alanine is converted to a threonine, wild-type receptor properties can be restored by exchanging an additional threonine at position 602 to an alanine. An alanine residue at position 596 or 602 in the DNA binding domain of the androgen receptor is therefore important for the full function of this receptor. In all steroid receptors that bind the core sequence AGAACANNNTGTTCT, an alanine residue is also present at a position equivalent to alanine 596 in the androgen receptor.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beato M. Gene regulation by steroid hormones. Cell. 1989 Feb 10;56(3):335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Cato A. C., Heitlinger E., Ponta H., Klein-Hitpass L., Ryffel G. U., Bailly A., Rauch C., Milgrom E. Estrogen and progesterone receptor-binding sites on the chicken vitellogenin II gene: synergism of steroid hormone action. Mol Cell Biol. 1988 Dec;8(12):5323–5330. doi: 10.1128/mcb.8.12.5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cato A. C., Ponta H., Herrlich P. Regulation of gene expression by steroid hormones. Prog Nucleic Acid Res Mol Biol. 1992;43:1–36. doi: 10.1016/s0079-6603(08)61042-9. [DOI] [PubMed] [Google Scholar]
- Cato A. C., Skroch P., Weinmann J., Butkeraitis P., Ponta H. DNA sequences outside the receptor-binding sites differently modulate the responsiveness of the mouse mammary tumour virus promoter to various steroid hormones. EMBO J. 1988 May;7(5):1403–1410. doi: 10.1002/j.1460-2075.1988.tb02957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. S., Kokontis J., Liao S. T. Structural analysis of complementary DNA and amino acid sequences of human and rat androgen receptors. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7211–7215. doi: 10.1073/pnas.85.19.7211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley M., Ludwig M., Stowell K. M., De Vos P., Olek K., Brownlee G. G. Recovery from hemophilia B Leyden: an androgen-responsive element in the factor IX promoter. Science. 1992 Jul 17;257(5068):377–379. doi: 10.1126/science.1631558. [DOI] [PubMed] [Google Scholar]
- Dahlman-Wright K., Siltala-Roos H., Carlstedt-Duke J., Gustafsson J. A. Protein-protein interactions facilitate DNA binding by the glucocorticoid receptor DNA-binding domain. J Biol Chem. 1990 Aug 15;265(23):14030–14035. [PubMed] [Google Scholar]
- Dahlman-Wright K., Wright A., Gustafsson J. A., Carlstedt-Duke J. Interaction of the glucocorticoid receptor DNA-binding domain with DNA as a dimer is mediated by a short segment of five amino acids. J Biol Chem. 1991 Feb 15;266(5):3107–3112. [PubMed] [Google Scholar]
- Dieken E. S., Meese E. U., Miesfeld R. L. nti glucocorticoid receptor transcripts lack sequences encoding the amino-terminal transcriptional modulatory domain. Mol Cell Biol. 1990 Sep;10(9):4574–4581. doi: 10.1128/mcb.10.9.4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drouin J., Sun Y. L., Chamberland M., Gauthier Y., De Léan A., Nemer M., Schmidt T. J. Novel glucocorticoid receptor complex with DNA element of the hormone-repressed POMC gene. EMBO J. 1993 Jan;12(1):145–156. doi: 10.1002/j.1460-2075.1993.tb05640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eul J., Meyer M. E., Tora L., Bocquel M. T., Quirin-Stricker C., Chambon P., Gronemeyer H. Expression of active hormone and DNA-binding domains of the chicken progesterone receptor in E. coli. EMBO J. 1989 Jan;8(1):83–90. doi: 10.1002/j.1460-2075.1989.tb03351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawell S. E., Lees J. A., White R., Parker M. G. Characterization and colocalization of steroid binding and dimerization activities in the mouse estrogen receptor. Cell. 1990 Mar 23;60(6):953–962. doi: 10.1016/0092-8674(90)90343-d. [DOI] [PubMed] [Google Scholar]
- Fawell S. E., White R., Hoare S., Sydenham M., Page M., Parker M. G. Inhibition of estrogen receptor-DNA binding by the "pure" antiestrogen ICI 164,384 appears to be mediated by impaired receptor dimerization. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6883–6887. doi: 10.1073/pnas.87.17.6883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman B. M., Samuels H. H. Interactions among a subfamily of nuclear hormone receptors: the regulatory zipper model. Mol Endocrinol. 1990 Sep;4(9):1293–1301. doi: 10.1210/mend-4-9-1293. [DOI] [PubMed] [Google Scholar]
- Freedman L. P., Luisi B. F., Korszun Z. R., Basavappa R., Sigler P. B., Yamamoto K. R. The function and structure of the metal coordination sites within the glucocorticoid receptor DNA binding domain. Nature. 1988 Aug 11;334(6182):543–546. doi: 10.1038/334543a0. [DOI] [PubMed] [Google Scholar]
- Gehring U., Tomkins G. M. A new mechanism for steroid unresponsiveness: loss of nuclear binding activity of a steroid hormone receptor. Cell. 1974 Nov;3(3):301–306. doi: 10.1016/0092-8674(74)90145-7. [DOI] [PubMed] [Google Scholar]
- Griffin J. E. Androgen resistance--the clinical and molecular spectrum. N Engl J Med. 1992 Feb 27;326(9):611–618. doi: 10.1056/NEJM199202273260906. [DOI] [PubMed] [Google Scholar]
- Hughes M. R., Malloy P. J., Kieback D. G., Kesterson R. A., Pike J. W., Feldman D., O'Malley B. W. Point mutations in the human vitamin D receptor gene associated with hypocalcemic rickets. Science. 1988 Dec 23;242(4886):1702–1705. doi: 10.1126/science.2849209. [DOI] [PubMed] [Google Scholar]
- Issemann I., Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990 Oct 18;347(6294):645–650. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- Klein-Hitpass L., Ryffel G. U., Heitlinger E., Cato A. C. A 13 bp palindrome is a functional estrogen responsive element and interacts specifically with estrogen receptor. Nucleic Acids Res. 1988 Jan 25;16(2):647–663. doi: 10.1093/nar/16.2.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein-Hitpass L., Tsai S. Y., Weigel N. L., Allan G. F., Riley D., Rodriguez R., Schrader W. T., Tsai M. J., O'Malley B. W. The progesterone receptor stimulates cell-free transcription by enhancing the formation of a stable preinitiation complex. Cell. 1990 Jan 26;60(2):247–257. doi: 10.1016/0092-8674(90)90740-6. [DOI] [PubMed] [Google Scholar]
- Klocker H., Kaspar F., Eberle J., Uberreiter S., Radmayr C., Bartsch G. Point mutation in the DNA binding domain of the androgen receptor in two families with Reifenstein syndrome. Am J Hum Genet. 1992 Jun;50(6):1318–1327. [PMC free article] [PubMed] [Google Scholar]
- Luisi B. F., Xu W. X., Otwinowski Z., Freedman L. P., Yamamoto K. R., Sigler P. B. Crystallographic analysis of the interaction of the glucocorticoid receptor with DNA. Nature. 1991 Aug 8;352(6335):497–505. doi: 10.1038/352497a0. [DOI] [PubMed] [Google Scholar]
- Mink S., Härtig E., Jennewein P., Doppler W., Cato A. C. A mammary cell-specific enhancer in mouse mammary tumor virus DNA is composed of multiple regulatory elements including binding sites for CTF/NFI and a novel transcription factor, mammary cell-activating factor. Mol Cell Biol. 1992 Nov;12(11):4906–4918. doi: 10.1128/mcb.12.11.4906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlodzik M., Hiromi Y., Weber U., Goodman C. S., Rubin G. M. The Drosophila seven-up gene, a member of the steroid receptor gene superfamily, controls photoreceptor cell fates. Cell. 1990 Jan 26;60(2):211–224. doi: 10.1016/0092-8674(90)90737-y. [DOI] [PubMed] [Google Scholar]
- Nauber U., Pankratz M. J., Kienlin A., Seifert E., Klemm U., Jäckle H. Abdominal segmentation of the Drosophila embryo requires a hormone receptor-like protein encoded by the gap gene knirps. Nature. 1988 Dec 1;336(6198):489–492. doi: 10.1038/336489a0. [DOI] [PubMed] [Google Scholar]
- Schmid W., Strähle U., Schütz G., Schmitt J., Stunnenberg H. Glucocorticoid receptor binds cooperatively to adjacent recognition sites. EMBO J. 1989 Aug;8(8):2257–2263. doi: 10.1002/j.1460-2075.1989.tb08350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J. A., Marschke K. B., Ho K. C., Perry S. T., Wilson E. M., French F. S. Response elements of the androgen-regulated C3 gene. J Biol Chem. 1992 Mar 5;267(7):4456–4466. [PubMed] [Google Scholar]
- Tsai S. Y., Carlstedt-Duke J., Weigel N. L., Dahlman K., Gustafsson J. A., Tsai M. J., O'Malley B. W. Molecular interactions of steroid hormone receptor with its enhancer element: evidence for receptor dimer formation. Cell. 1988 Oct 21;55(2):361–369. doi: 10.1016/0092-8674(88)90059-1. [DOI] [PubMed] [Google Scholar]
- Umesono K., Evans R. M. Determinants of target gene specificity for steroid/thyroid hormone receptors. Cell. 1989 Jun 30;57(7):1139–1146. doi: 10.1016/0092-8674(89)90051-2. [DOI] [PubMed] [Google Scholar]
- Wang L. H., Tsai S. Y., Cook R. G., Beattie W. G., Tsai M. J., O'Malley B. W. COUP transcription factor is a member of the steroid receptor superfamily. Nature. 1989 Jul 13;340(6229):163–166. doi: 10.1038/340163a0. [DOI] [PubMed] [Google Scholar]
- Wright A. P., Gustafsson J. A. Mechanism of synergistic transcriptional transactivation by the human glucocorticoid receptor. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8283–8287. doi: 10.1073/pnas.88.19.8283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K. R., Gehring U., Stampfer M. R., Sibley C. H. Genetic approaches to steroid hormone action. Recent Prog Horm Res. 1976;32:3–32. doi: 10.1016/b978-0-12-571132-6.50008-7. [DOI] [PubMed] [Google Scholar]