Abstract
Caribbean coral reefs have been transformed in the past few decades with the demise of reef-building corals, and sponges are now the dominant habitat-forming organisms on most reefs. Competing hypotheses propose that sponge communities are controlled primarily by predatory fishes (top-down) or by the availability of picoplankton to suspension-feeding sponges (bottom-up). We tested these hypotheses on Conch Reef, off Key Largo, Florida, by placing sponges inside and outside predator-excluding cages at sites with less and more planktonic food availability (15 m vs. 30 m depth). There was no evidence of a bottom-up effect on the growth of any of 5 sponge species, and 2 of 5 species grew more when caged at the shallow site with lower food abundance. There was, however, a strong effect of predation by fishes on sponge species that lacked chemical defenses. Sponges with chemical defenses grew slower than undefended species, demonstrating a resource trade-off between growth and the production of secondary metabolites. Surveys of the benthic community on Conch Reef similarly did not support a bottom-up effect, with higher sponge cover at the shallower depth. We conclude that the structure of sponge communities on Caribbean coral reefs is primarily top-down, and predict that removal of sponge predators by overfishing will shift communities toward faster-growing, undefended species that better compete for space with threatened reef-building corals.
Introduction
Food chain dynamics is considered a central theory in ecology [1], and proposes that populations of organisms that make up communities are controlled by processes that are bottom-up (nutrients, food) or top-down (predation). The relative effect of these two processes has been the subject of considerable debate [2], but in most cases, a greater understanding of the complexity of an ecosystem reveals that both are important [3], [4], [5], [6], [7]. Ecosystem functioning has gained renewed interest [8], [9] as regulatory agencies increasingly adopt ecosystem-based management strategies, particularly for marine systems [10], [11], [12].
Coral reefs of the Caribbean region have undergone a marked transformation as reef-building corals have declined due to multiple stressors including disease, temperature extremes, storm damage, and the loss of key herbivores [13]. Macroalgae now cover the greatest surface area on many reefs [14], and while arborescent gorgonian corals are often visually dominant [15], sponges have become a primary component of Caribbean coral reef ecosystems [16]. In addition to their large biomass on shallow-water reefs, sponges dominate light-limited reef interstices, caves and mesophotic reefs [17], but are also found in grassbed, hardbottom, and mangrove habitats [18]. Moreover, recent evidence indicates that sponge populations on Caribbean reefs are increasing [16].
Marine sponges are primarily suspension-feeding organisms that derive their food from picoplankton, the size category that includes bacteria and prochlorophytes. Two recent studies concluded that the availability of picoplanktonic food was of principal importance in structuring sponge communities on Caribbean coral reefs [19], [20], concluding that “most of the variability in their distribution and abundance, from reef to reef, and with depth, can be explained principally by bottom-up processes” ([19], p. 286). The first study compared 3 tube sponge species at different reef sites and found that tubes were longer and elongated faster at greater depth, which correlated with higher abundances of food, mostly prochlorophytes and heterotrophic bacteria, on deeper reefs [19]. In a companion study, the tube sponge Callyspongia vaginalis was transplanted to shallow and deep sites (12 m and 25 m) on Conch Reef, off Key Largo, Florida, and exhibited greater growth at greater depth, again correlated with higher picoplankton abundances on deeper reefs [20]. These data were corroborated by in situ measurements of sponge respirometry and pumping rates at deep and shallow sites, which were combined with flow cytometry measurements of food availability to construct energetic budgets for sponges at each depth that indicated a greater scope for growth for sponges at the deep site where food availability was higher [20].
Research on the chemical ecology of Caribbean reef sponges supports an alternative hypothesis that predatory fishes (mostly angelfishes and parrotfishes) play an important role in controlling the structure of sponge communities through differential predation on chemically undefended sponges [21], [22], [23]. Many sponge species on reefs produce distasteful secondary metabolites in their tissues that deter feeding by fish predators [24], [25], [26], [27], [28], while other species persist despite their lack of a chemical defense. When pieces of branching sponges in these two categories were attached to the reef inside and outside of predator-excluding cages, defended sponges grew slower and were unaffected by predation, while undefended sponges grew faster inside, but were grazed by predators outside, resulting in a slower accumulation of biomass [29]. Not only have these caging experiments provided evidence of a resource trade-off between the production of chemical defenses and rapid growth, they also suggest that sponge communities are composed of slow-growing species that are chemically defended from predation and faster-growing species that are not, with the relative abundance of each type on the reef determined by predation pressure (i.e. top-down control).
We tested the relative importance of bottom-up and top-down processes on sponge growth by performing predator-exclusion experiments at both shallow (15 m) and deep (30 m) sites on Conch Reef, off Key Largo, Florida. The site of the undersea research station Aquarius for two decades [30], Conch Reef is one of the best studied coral reefs in the world [31]. Deep-water internal waves break over Conch Reef, bringing picoplankton-rich water to deeper portions of the reef for greater periods of time than shallower portions [32]. The physical oceanography of this reef is very well described [33], and it was the location of previous studies that concluded that bottom-up effects played a predominant role in sponge growth [19], [20], but these studies did not consider the impact of predation. We combined our test of the relative importance of bottom-up and top-down processes with a comparison of the growth of both chemically defended and undefended sponge species to further assess the importance of resource trade-offs between chemical defenses and growth among Caribbean sponges.
Materials and Methods
Caging Experiments
All experiments were conducted along a depth gradient running west to east ∼100 m south of the Aquarius undersea research laboratory on Conch Reef, off Key Largo, Florida (24°57′00″N, 80°27′13″W). This is the same site and depth profile as previous surveys and experiments that concluded that food abundance (picoplankton concentration) was the primary factor structuring sponge communities on Caribbean reefs [19], [20].
The first experiment was conducted with 4 branching sponge species for 287 days (18 Aug 2010–1 Jun 2011). Branching sponges were chosen for the first set of experiments because sponges of this morphology are adapted to breakage and reattachment (fragmentation) as a form of asexual reproduction when exposed to high water flow from storm events or currents, so they easily survive and grow after manipulation, including brief removal from water for determining wet mass in the lab [29], [34]. We collected, tagged and weighed pieces of two chemically undefended species (Callyspongia armigera, Iotrochota birotulata) and two chemically defended species (Amphimedon compressa, Aplysina cauliformis) [24]. The tissues of Amphimedon compressa contain predator-deterring pyridinium alkaloids, primarily amphitoxin [35], while Aplysina cauliformis produces distasteful brominated tyrosine derivatives, primarily fistularin-3 [36]. A second experiment using the same methods was performed with Callyspongia vaginalis, the same species of tube sponge used in previous studies demonstrating bottom-up effects [19], [20] for a period of 328 days (18 June 2011–11 May 2012).
Experimental methods were the same as previously used over 9 years to compare growth rates of chemically defended and undefended sponges of different species at shallower depths on nearby reefs [29]. Sponge pieces used in experiments were collected from 10–30 m depth at nearby reefs (Conch Wall, N. Dry Rocks Reef) that were outside research-only areas where collections were prohibited. Sponge pieces were cut with a scalpel to a length of ∼10 cm (∼50 g wet mass), brought into the laboratory in clean, aerated seawater, wet mass determined on an electronic scale, a unique tag on a zip-tie was attached to each piece, and all pieces returned to the field within 4 h. Sponge pieces were distributed haphazardly on plastic plates that were affixed to 0.3×0.6 m plastic mesh (2.5 cm2 holes) bases that were anchored to the limestone reef with galvanized nails topped with 5 cm washers. On each mesh base, two sponge pieces on plastic plates were attached with zip-ties, one ∼15 cm from each short edge of the rectangular base, along the center axis. One of these two sponges was haphazardly chosen to be covered with a cube-shaped mesh cage top, 0.3 m on each side. At each depth location (15 m and 30 m), 20 sponge pieces of each species were placed both inside and outside of cages. Biofouling on cages was minimal during the experimental period, and was similar across sites. At the end of the experiment, sponges were carefully removed from plastic plates and mesh bottoms with their individual tags intact and brought back to the laboratory (often in individual zipped bags), where they were re-weighed. Many of the labor-intensive portions of this experiment, particularly deploying the mesh bases and cages, were performed using saturation diving from the undersea habitat Aquarius.
There has been considerable debate in the marine ecology literature about technical issues involved in caging experiments [37]. As previously discussed [29], we did not include cage controls in these experiments (a third treatment in which cages have one or more sides left open) because the results of past experiments showed they were unnecessary (no difference in the growth of defended species inside and outside of cages). Moreover, while cages may have some effect of altering flow around suspension-feeding sponges that could reduce feeding capability, this effect would be in the opposite direction from the outcome expected for undefended sponge species (caged sponges will not be grazed and should grow more); therefore, enhanced growth of caged versus uncaged sponges would be a conservative result.
Hydrographic Data
Temperature, current speed and current direction data were collected near continuously at 20 m depth on Conch Reef for 22 Aug 2010–1 Jun 2011 using an InterOcean S4 current meter. Additionally, temperature data were collected at 15 m and 30 m for 18 June 2011–11 May 2012 using Onset HOBO Water Temp Pro loggers. Data were collected every minute over five-minute intervals every 10 minutes.
Surveys of Benthos and Fishes
Surveys of the benthic community and spongivorous fish abundance were carried out at 15 m and 30 m on Conch Reef, Florida. At each depth, five 20-m line transects were laid in a single file, with each 20 m line separated by a 5 m gap. The reef bottom was sampled using five evenly spaced 1×1 m2 quadrats per transect line, with the benthos under 25 points within each quadrat recorded, for a total of 625 points per depth. The benthic categories recorded were sponge, hard corals, gorgonians and macroalgae. Spongivorous fishes were counted along the same 5 transect lines as the benthic surveys using the Reef Check survey method (see [38] for description of method and list of known spongivorous fish species).
Statistical Analyses
Sponge growth data were transformed prior to statistical analyses, as in a previous study [29], but without the time component because the duration of the experiment for the 4 branching sponge species was the same. Growth index (final wet mass/initial wet mass) was compared between caging treatments and depth using a 2-way ANOVA for each branching sponge species in the program JMP 7.0 (SAS Institute). With the exception of Amphimedon compressa, growth indices for the other 3 branching species were log transformed to reduce heteroscedasticity in sample variance. For the tube sponge Callyspongia vaginalis, the growth index was compared between depths for the uncaged sponges only (cages were lost at the 15 m site following winter storms), and the caging effect was only analyzed for the sponges at 30 m. Growth indices were log transformed and compared in separate Student’s t-tests for depth and caging. The proportional cover of hard coral, sponges, and macroalgae was calculated for each 20 m transect, arc-sine transformed and compared between 15 and 30 m using the t-test. Abundances of angelfishes and parrotfishes within 500 m3, the volume surveyed along each 20 m transect, were also compared between 15 m and 30 m using the t-test.
Sponge collections, caging experiments, and surveys of fishes and benthos were carried out under a permit from the Florida Keys National Marine Sanctuary (FKNMS-2009-126-A1), with the caging experiments and surveys conducted within the Conch Reef Special Protected Area.
Results
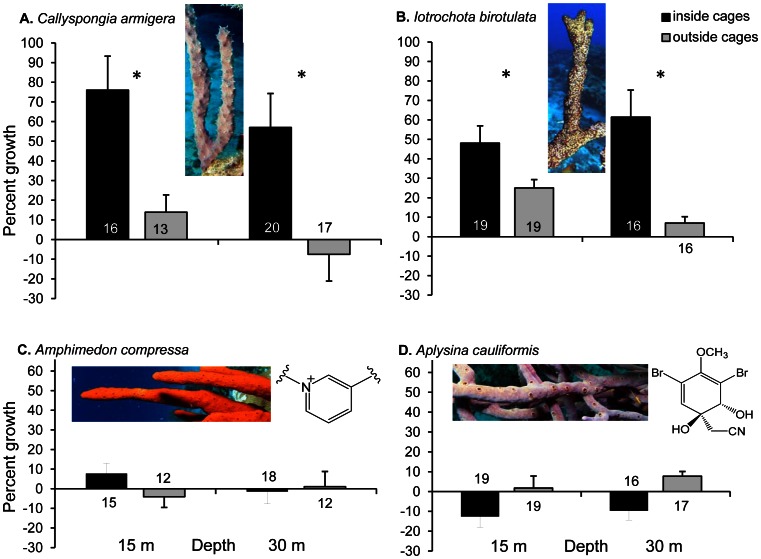
Regardless of depth, the two chemically undefended branching sponge species grew significantly more inside cages that excluded predatory fishes, with no significant interactions between depth and caging (Fig. 1, Callyspongia armigera: F = 19.94, df = 1, p<0.0001, Iotrochota birotulata: F = 10.80, df = 1, p = 0.0016). Bite marks were observed on many of the pieces of chemically undefended species outside of cages, despite rapid rates of healing for these species [39]. Greater growth was observed for Callyspongia armigera at 15 m vs. 30 m (F = 4.97, df = 1, p = 0.0294), and growth was similar between depths for the other 3 branching sponge species. Chemically defended sponge species grew relatively little compared to undefended species (<10% vs. ∼45–75% inside cages). Growth was not significantly different whether defended sponges were caged or exposed to predators (Fig. 1).
Figure 1. Percentage growth of branching sponge pieces (change in wet mass) 287 days after attachment inside and outside of predator-excluding cages at 15 and 30 m depth on Conch Reef, Florida.
A: Callyspongia armigera and B: Iotrochota birotulata lack chemical defenses, while C: Amphimedon compressa and D: Aplysina cauliformis contain alkaloids that deter fish predators, represented by a portion of the chemical structure of amphitoxin for the former and aeroplysinin-1 for the latter. Surviving number of 20 replicates is shown for each bar, error bars are standard error. Statistical analyses were performed on transformed data (growth index). An asterisk indicates a significant difference in growth inside vs. outside cages (p<0.01).
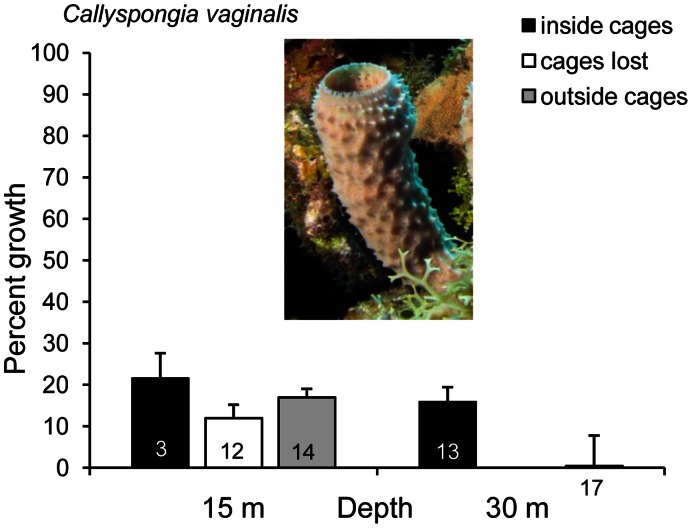
During the second iteration of the experiment using the tube sponge Callyspongia vaginalis, winter storms removed all but a few cages at the shallow site. Despite the loss of cages, growth of the remaining sponge tubes was greater at the shallow than at the deep site for uncaged sponge tubes (Fig. 2, 1-tailed Student’s t-test, t = −2.20, df = 29, p = 0.0180), and the effect of caging at the deep site was significant, with virtually no sponge growth outside of cages (Fig. 2, t = −1.72, df = 28, p = 0.0486). Again, bite marks were observed on many of the pieces of C. vaginalis outside of cages.
Figure 2. Percentage growth of the gray tube sponge Callyspongia vaginalis (change in wet mass) 358 days after placement inside and outside of predator-excluding cages at 15 m and 30 m depth on Conch Reef, Florida.
Winter storms removed all but 3 cages at the 15 m site; growth of sponges that had been caged by lost cages is shown separately. Surviving number of 20 replicates is shown for each bar, error bars are standard error. Statistical analyses were performed on transformed data (growth index).
Data from temperature sensors on Conch Reef showed similar hydrography to previous years, including a data set from 2000–2005 that was recently published [40]. These measurements confirmed the periodic arrival of cold-water nutrient transport events that increase in magnitude and duration with increasing depth, providing higher concentrations of picoplankton to deeper sites, all of which is well described for Conch Reef [20], [32], [33], [40]. Additionally, analysis of water samples taken at both depths in December 2011 using flow cytometry confirmed that there was an ∼1.5 fold enhancement of picoplankton (see [20], Fig. 2) at 30 m compared to 15 m (McMurray, unpublished data), validating previous studies that have described this persistent hydrographic feature at Conch Reef using chlorophyll signatures [32], [33].
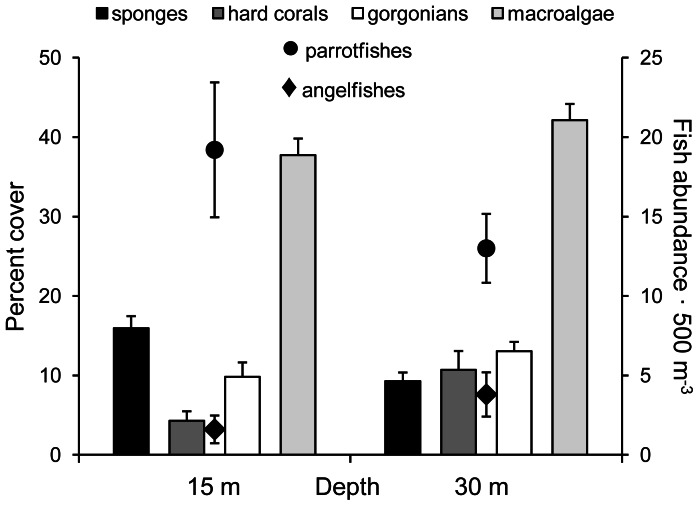
Surveys of benthic organisms and of sponge-eating fishes at depths of 15 m and 30 m on Conch Reef revealed overall higher sponge cover at the shallower depth (Fig. 3). Abundances of angelfishes and parrotfishes were not significantly different at shallow and deep sites (Fig. 3, t-test, p>0.05). Sponge cover was greater at the shallower site (16.0% vs 9.3%; t-test, t = −3.61, df = 8, p = 0.0034), with about the same cover of chemically defended sponges at each site (62.5% vs. 57.6%) and the remaining cover at each site split between chemically undefended and variably defended species. Cover of reef-building corals was greater at the deeper depth (Fig. 3, t-test, t = 2.41, df = 8, p = 0.0212), while gorgonian and macroalgal cover was not significantly different between 15 m and 30 m (Fig. 3).
Figure 3. Percentage benthic cover of sponges, reef-building corals, gorgonian corals and macroalgae and abundance of the dominant sponge-eating fishes at 15 m and 30 m depth on Conch Reef, Florida.
Benthic cover is shown in bars (y-axis on left), fish abundance in dots and diamonds (y-axis on right). Error bars are standard error.
Discussion
The results of our experiments do not support bottom-up control of sponge communities on Conch Reef, a site where higher concentrations of picoplanktonic food at deeper sites is well established, because sponges in predator-excluding cages at the deeper site either exhibited no difference in growth (3 species) or grew less (2 species) than caged sponges at the shallower site. The absence of a bottom-up effect on sponge growth at Conch Reef was surprising, given that most ecosystems that have been studied show evidence of both top-down and bottom-up processes in structuring communities [5], [7], and because the two previous studies of sponges at the same research location concluded that bottom-up effects were dominant [19], [20]. The first of these looked at differences in the length and elongation of tube sponges as a function of depth, but these methods are problematic because sponges grow in many dimensions besides elongation, including wall thickening and the addition of new tubes [41]. Possible explanations for longer tube sponges at greater depth include less of an impact of storm-related currents at depth, which tend to rip sponges off the substratum in shallower water and result in older, longer tubes at greater depth, and differences in tube thickness and colony formation (number of tubes) because of flow differences in shallow and deep water [42]. Our own observations confirm a conspicuous removal of non-recumbent sponges by storm surge during the second iteration of the experiment that resulted in the loss of cages at the shallow site (Fig. 2), with many branching and tube-shaped sponges unattached and dying in the sand channels between reefs.
The second study that concluded that bottom-up effects primarily influenced sponge growth [20] did so on the basis of growth experiments with a single species of tube sponge, Callyspongia vaginalis, which is a primary food item of sponge-eating fishes [21], [43]. In the first iteration of the experiment designed for the present study, we used the closely related branching sponge, Callyspongia armigera, which, like C. vaginalis, is chemically undefended [24], and observed significantly greater growth of this species at the shallower depth (Fig. 1A). We repeated the experiment using C. vaginalis a year later, but winter storms removed all but a few cages at the shallow site. Nevertheless, growth of the remaining sponge tubes was greater at shallow than at deep sites for uncaged sponge tubes (Fig. 2), and the effect of caging at the deep site was significant, with virtually no sponge growth outside of cages due to predation. Taken together, these results are also contrary to bottom-up control of sponge growth and support the hypothesis that top-down effects structure the sponge community. The enhanced growth of C. vaginalis at the deep site previously observed by Trussell et al. [20] may have been due to lower levels of predation at that site over the period of their experiment, considering that no exclosures were used to prevent sponge predation. In previous caging experiments, we demonstrated that the tube sponge C. vaginalis grows more slowly than the branching C. armigera, but that the former produces many more larvae than the latter, evidence of an additional resource trade-off between growth and larval production [34].
Other recent studies, also at Conch Reef, have not supported bottom-up control of sponge growth. There was no significant difference in the growth of the dominant habitat-forming organism, the giant barrel sponge Xestospongia muta, at 15 m, 20 m, and 30 m depths using digital image analysis of 104 tagged sponges over 4.5 years [41]. Further, density of X. muta was greater in plots at 15 m and 20 m depth than at 30 m [16]. Also contrary to the bottom-up hypothesis, data from our surveys of benthic organisms and of sponge-eating fishes at 15 m and 30 m revealed overall higher sponge cover at the shallower depth (Fig. 3). Abundances of angelfishes and parrotfishes were not significantly different at shallow and deep sites (Fig. 3), indicating that despite similar levels of predation on a mixed population of chemically defended and undefended sponges, sponge cover was greater at the shallower site, where food availability was lower than at the deep site. This modest difference is probably due to a disproportionate effect of sponge consumption by large angelfishes, which were more abundant at the 30 m site (Fig. 3) and, unlike parrotfishes, primarily eat sponge tissue [43], [44]. Alternatively (or in addition), higher sponge cover at 15 m could be due to greater light levels at shallower depths, as some sponge species have photosynthetic microbial symbionts that enhance sponge growth [45]. The only phototrophic sponge used in the present study was Aplysina cauliformis, which exhibited negative growth inside cages at both 15 m and 30 m, although this effect was not significant when compared to the minimal growth of sponges outside of cages for this slow-growing, chemically defended species (Fig. 1D). Interestingly, this species grew much faster in similar experiments conducted at 7 m [29], suggesting that the high light levels found on very shallow reefs may promote the growth of some phototrophic species [46].
Overall, experimental and distributional data suggest that the growth of sponges is not limited by food availability based on experiments and surveys at Conch Reef, where the parameters regarding picoplankton distribution and supply as a function of depth is well characterized. But are these data generally applicable for coral reefs across the Caribbean? For several reasons, we believe they are. First, Conch Reef is unusual in its topography and hydrodynamics in having the “plankton pump” of internal waves bringing greater levels of picophytoplankton from deeper water up the steep reef slope to oligotrophic shallow water [32]. Most Caribbean reefs have a more gradual slope and lack the same level of hydrodynamic forcing; hence, if a bottom-up effect on sponge growth is likely to be demonstrated for any coral reef in the Caribbean, it should be evident at Conch Reef, but it is not. In fact, identical sponge growth experiments conducted on a shallow reef (7 m) more typical of the Caribbean with a very gradual reef slope yielded similar or higher rates of sponge growth for all 4 of the branching species used in the present study [29]. Second, sponge community composition is remarkably homogeneous on reefs across the Caribbean [22]; specifically, sponge diversity and abundance at Conch Reef is similar to many reefs across the Caribbean (Loh and Pawlik, unpublished data forthcoming). Third, the diversity of sponge-eating fishes is also similar on reefs across the Caribbean, although the abundance of these predators varies as a function of human fishing activities, resulting in a range from highly protected (Bonaire, Cayman Brac, Exuma Cays) to heavily fished (Jamaica, Martinique), with Conch Reef falling between them (Loh and Pawlik, unpublished data forthcoming). Therefore, the combination of region-wide similarities in sponge ecology with the lack of evidence for food limitation from manipulative experiments at sites that span the hydrographic diversity of reefs in the region provide a compelling argument against bottom-up effects having an important role in structuring sponge communities on Caribbean coral reefs.
Although the results of the present study support the conclusion that top-down effects primarily structure sponge communities on Caribbean coral reefs, there are certainly other factors, particularly abiotic ones, that affect recruitment, growth, and sponge community development. Just as temperature extremes are known to limit sponge distributions in mangrove habitats [18], [47], temperature fluctuations can completely alter reef sponge communities [48]. High water flow events, such as those generated by storm surge, may depopulate non-recumbent sponges (as observed during the second iteration of the experiments reported here) or may generally prevent some species of sponges from recruiting, as on the windward side of many islands and atolls. More broadly, ocean currents could restrict the dispersal of sponge recruits, although this is not an important factor for the well-mixed Caribbean region [49]. Sponges require water flow to suspension-feed, and may grow faster when exposed to higher flow. One recent study demonstrated that pieces of Amphimedon compressa and Iotrochota birotulata (two of the same species used herein) grew faster when suspended higher in the water column above the reef, as when these branching species grow upward or attach onto other sponges or gorgonian corals [50]. Interestingly, this study reported faster growth for A. compressa than I. birotulata, but did not incorporate a caging component to remove the effect of predation on either species.
Although sponge cover was greater at the shallower site on Conch Reef, the reverse was true for the cover of reef-building corals (Fig. 3). This pattern is opposite that expected for hard corals, which rely on photosynthetic symbionts for growth, and may reflect higher rates of corallivory by parrotfishes or more frequent heat stress events at shallower depths, but may also represent enhanced competition between sponges and corals at shallower sites. Macroalgal cover might also be expected to decrease with depth, but there was no difference between sites in our surveys (Fig. 3).
The sponge community on Caribbean coral reefs provides a remarkably simple system for testing ecological theory on foodweb dynamics and resource allocation, particularly when compared to more commonly studied terrestrial plant-herbivore communities that are complicated by variations in abiotic factors such as light, rainfall, nutrients, and soil chemistry, as well as the heterogeneity resulting from limited dispersal and allopatric speciation [22]. The present study demonstrates that, rather than a complex or context-dependent combination of bottom-up and top-down effects, predation is the primary determinant of sponge community structure on Caribbean coral reefs. As a clear example of alternative resource allocation, chemically defended sponges are largely unaffected by predation, but heal wounds [39], grow [29], and recruit [51] at slower rates than chemically undefended sponge species, which are not protected by structural defenses [52], [53], and are subject to fish grazing. While branching sponge species are preferred subjects for manipulative experiments, recumbent and encrusting species often dominate substratum coverage, and this morphological group is also made up of both chemically defended and undefended species [21], [24]. Sponges are often competitively dominant over reef-building corals, and one of the most common Caribbean sponges, Mycale laevis, a chemically undefended, recumbent species, will smother adjacent corals on reefs where sponge-eating fishes have been removed by overfishing [38], [54]. We predict that overfished reefs that lack spongivores will become dominated by faster-growing undefended sponge species, which better compete for space with reef-building corals. This has important implications for fisheries management across the Caribbean, as some coral species are already listed as “critically endangered” on the IUCN Red List, with 4 reef-building Caribbean species on the top ten list for extinction risk [55]. Sponge populations are already increasing on Caribbean reefs [16], [56], and as the impacts of climate change and ocean acidification further disrupt marine communities [57], it seems likely that reef-building corals and some macroalgae will suffer greater harm than sponges, which do not form limestone skeletons [58]; hence, Caribbean reefs of the future are likely to become increasingly dominated by sponges.
Acknowledgments
We thank Inga Conti-Jerpe, Lindsey Deignan, John Hanmer, Micah Marty, Andy Miller, and the staff of Aquarius Reef Base for assistance in performing this research.
Funding Statement
This work was supported by a grant from the National Science Foundation’s Biological Oceanography Program (1029515) and funding from the National Oceanic and Atmospheric Administration’s Aquarius Reef Base and Coral Reef Conservation Programs. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Fretwell SD (1987) Food-chain dynamics - The central theory of ecology. Oikos 50: 291–301. [Google Scholar]
- 2. Hunter MD, Price PW (1992) Playing chutes and ladders - Heterogeneity and the relative roles of bottom-up and top-down forces in natural communities. Ecology 73: 724–732. [Google Scholar]
- 3. Menge BA (2000) Top-down and bottom-up community regulation in marine rocky intertidal habitats. J Exp Mar Biol Ecol 250: 257–289. [DOI] [PubMed] [Google Scholar]
- 4. Freidenburg TL, Menge BA, Halpin PM, Webster M, Sutton-Grier A (2007) Cross-scale variation in top-down and bottom-up control of algal abundance. J Exp Mar Biol Ecol 347: 8–29. [Google Scholar]
- 5. Ainley DG, Hyrenbach KD (2010) Top-down and bottom-up factors affecting seabird population trends in the California current system (1985–2006). Prog Oceanogr 84: 242–254. [Google Scholar]
- 6. Madrigal J, Kelt DA, Meserve PL, Gutierrez JR, Squeo FA (2011) Bottom-up control of consumers leads to top-down indirect facilitation of invasive annual herbs in semiarid Chile. Ecology 92: 282–288. [DOI] [PubMed] [Google Scholar]
- 7. Denyer JL, Hartley SE, John EA (2010) Both bottom-up and top-down processes contribute to plant diversity maintenance in an edaphically heterogeneous ecosystem. J Ecol 98: 498–508. [Google Scholar]
- 8. Naeem S, Duffy JE, Zavaleta E (2012) The functions of biological diversity in an age of extinction. Science 336: 1401–1406. [DOI] [PubMed] [Google Scholar]
- 9. Mougi A, Kondoh M (2012) Diversity of interaction types and ecological community stability. Science 337: 349–351. [DOI] [PubMed] [Google Scholar]
- 10. Ruckelshaus M, Klinger T, Knowlton N, Demaster DR (2008) Marine ecosystem-based management in practice: Scientific, and governance challenges. Bioscience 58: 53–63. [Google Scholar]
- 11. Levin SA, Lubchenco J (2008) Resilience, robustness, and marine ecosystem-based management. Bioscience 58: 27–32. [Google Scholar]
- 12. Marasco RJ, Goodman D, Grimes CB, Lawson PW, Punt AE, et al. (2007) Ecosystem-based fisheries management: some practical suggestions. Can J Fish Aquat Sci 64: 928–939. [Google Scholar]
- 13. Mumby PJ, Hastings A, Edwards HJ (2007) Thresholds and the resilience of Caribbean coral reefs. Nature 450: 98–101. [DOI] [PubMed] [Google Scholar]
- 14. Mumby PJ (2009) Phase shifts and the stability of macroalgal communities on Caribbean coral reefs. Coral Reefs 28: 761–773. [Google Scholar]
- 15. Chiappone M, Sullivan KM (1994) Ecological structure and dynamics of nearshore hard-bottom communities in the Florida Keys. Bull Mar Sci 54: 747–756. [Google Scholar]
- 16. McMurray SE, Henkel TP, Pawlik JR (2010) Demographics of increasing populations of the giant barrel sponge Xestospongia muta in the Florida Keys. Ecology 91: 560–570. [DOI] [PubMed] [Google Scholar]
- 17. Lesser MP, Slattery M, Leichter JJ (2009) Ecology of mesophotic coral reefs. J Exp Mar Biol Ecol 375: 1–8. [Google Scholar]
- 18. Nagelkerken I, Blaber SJM, Bouillon S, Green P, Haywood M, et al. (2008) The habitat function of mangroves for terrestrial and marine fauna: A review. Aquat Bot 89: 155–185. [Google Scholar]
- 19. Lesser MP (2006) Benthic-pelagic coupling on coral reefs: Feeding and growth of Caribbean sponges. J Exp Mar Biol Ecol 328: 277–288. [Google Scholar]
- 20. Trussell GC, Lesser MP, Patterson MR, Genovese SJ (2006) Depth-specific differences in growth of the reef sponge Callyspongia vaginalis: role of bottom-up effects. Mar Ecol-Prog Ser 323: 149–158. [Google Scholar]
- 21. Pawlik JR (1997) Fish predation on Caribbean reef sponges: An emerging perspective of chemical defenses. Proceedings of the 8th International Coral Reef Symposium 2: 1255–1258. [Google Scholar]
- 22. Pawlik JR (2011) The chemical ecology of sponges on Caribbean reefs: Natural products shape natural systems. Bioscience 61: 888–898. [Google Scholar]
- 23. Pawlik JR (1998) Coral reef sponges: Do predatory fishes affect their distribution? Limnol Oceanogr 43: 1396–1399. [Google Scholar]
- 24. Pawlik JR, Chanas B, Toonen RJ, Fenical W (1995) Defenses of Caribbean sponges against predatory reef fish.1. Chemical deterrency. Mar Ecol-Prog Ser 127: 183–194. [Google Scholar]
- 25. Wilson DM, Puyana M, Fenical W, Pawlik JR (1999) Chemical defense of the Caribbean reef sponge Axinella corrugata against predatory fishes. J Chem Ecol 25: 2811–2823. [Google Scholar]
- 26. Kubanek J, Pawlik JR, Eve TM, Fenical W (2000) Triterpene glycosides defend the Caribbean reef sponge Erylus formosus from predatory fishes. Mar Ecol-Prog Ser 207: 69–77. [Google Scholar]
- 27. Assmann M, Lichte E, Pawlik JR, Kock M (2000) Chemical defenses of the Caribbean sponges Agelas wiedenmayeri and Agelas conifera . Mar Ecol-Prog Ser 207: 255–262. [Google Scholar]
- 28. Pawlik JR, McFall G, Zea S (2002) Does the odor from sponges of the genus Ircinia protect them from fish predators? J Chem Ecol 28: 1103–1115. [DOI] [PubMed] [Google Scholar]
- 29. Leong W, Pawlik JR (2010) Evidence of a resource trade-off between growth and chemical defenses among Caribbean coral reef sponges. Mar Ecol-Prog Ser 406: 71–78. [Google Scholar]
- 30. Miller SL, Cooper C (2000) The Aquarius underwater laboratory: America’s “inner space” station. Mar Technol Soc J 34: 69–74. [Google Scholar]
- 31. Stokes MD, Leichter JJ, Wing S, Frew R (2011) Temperature variability and algal isotopic heterogeneity on a Floridian coral reef. Marine Ecology-an Evolutionary Perspective 32: 364–379. [Google Scholar]
- 32. Leichter JJ, Shellenbarger G, Genovese SJ, Wing SR (1998) Breaking internal waves on a Florida (USA) coral reef: a plankton pump at work? Mar Ecol-Prog Ser 166: 83–97. [Google Scholar]
- 33. Leichter JJ, Deane GB, Stokes MD (2005) Spatial and temporal variability of internal wave forcing on a coral reef. J Phys Oceanogr 35: 1945–1962. [Google Scholar]
- 34. Leong W, Pawlik JR (2010) Fragments or propagules? Reproductive tradeoffs among Callyspongia spp. from Florida coral reefs. Oikos 119: 1417–1422. [Google Scholar]
- 35. Albrizio S, Ciminiello P, Fattorusso E, Magno S, Pawlik JR (1995) Amphitoxin, a new high molecular weight antifeedant pyridinium salt from the Caribbean sponge Amphimedon compressa . J Nat Prod 58: 647–652. [DOI] [PubMed] [Google Scholar]
- 36.Puyana M (2001) Chemical Ecology of Caribbean sponges of the genus Aplysina. La Jolla: UCSD PhD Thesis. 214 p.
- 37. Hall SJ, Raffaelli D, Turrell WR (1990) Predator-caging experiments in marine systems: A re-examination of their value. Am Nat 136: 657–672. [Google Scholar]
- 38. Loh TL, Pawlik JR (2009) Bitten down to size: Fish predation determines growth form of the Caribbean coral reef sponge Mycale laevis . J Exp Mar Biol Ecol 374: 45–50. [Google Scholar]
- 39. Walters KD, Pawlik JR (2005) Is there a trade-off between wound-healing and chemical defenses among Caribbean reef sponges? Integr Comp Biol 45: 352–358. [DOI] [PubMed] [Google Scholar]
- 40. McMurray SE, Blum JE, Leichter JJ, Pawlik JR (2011) Bleaching of the giant barrel sponge Xestospongia muta in the Florida Keys. Limnol Oceanogr 56: 2243–2250. [Google Scholar]
- 41. McMurray SE, Blum JE, Pawlik JR (2008) Redwood of the reef: growth and age of the giant barrel sponge Xestospongia muta in the Florida Keys. Mar Biol 155: 159–171. [Google Scholar]
- 42. Kaandorp JA (1999) Morphological analysis of growth forms of branching marine sessile organisms along environmental gradients. Mar Biol 134: 295–306. [Google Scholar]
- 43. Randall JE, Hartman WD (1968) Sponge-feeding fishes of the West Indies. Mar Biol 1: 216–225. [Google Scholar]
- 44. Dunlap M, Pawlik JR (1996) Video monitored predation by Caribbean reef fishes on an array of mangrove and reef sponges. Mar Biol 126: 117–123. [Google Scholar]
- 45. Freeman CJ, Thacker RW (2011) Complex interactions between marine sponges and their symbiotic microbial communities. Limnol Oceanogr 56: 1577–1586. [Google Scholar]
- 46. Erwin PM, Thacker RW (2008) Phototrophic nutrition and symbiont diversity of two Caribbean sponge-cyanobacteria symbioses. Mar Ecol Prog Ser 362: 139–147. [Google Scholar]
- 47. Pawlik JR, McMurray SE, Henkel TP (2007) Abiotic factors control sponge ecology in Florida mangroves. Mar Ecol-Prog Ser 339: 93–98. [Google Scholar]
- 48. Colella MA, Ruzicka RR, Kidney JA, Morrison JM, Brinkhuis VB (2012) Cold-water event of January 2010 results in catastrophic benthic mortality on patch reefs in the Florida Keys. Coral Reefs 31: 621–632. [Google Scholar]
- 49. Lopez-Legentil S, Pawlik JR (2009) Genetic structure of the Caribbean giant barrel sponge Xestospongia muta using the I3-M11 partition of COI. Coral Reefs 28: 157–165. [Google Scholar]
- 50.McLean EL, Lasker HR (2012) Height matters: position above the substratum influences the growth of two demosponge species. Mar Ecol. In press.
- 51. Pawlik JR, Henkel TP, McMurray SE, Lopez-Legentil S, Loh TL, et al. (2008) Patterns of sponge recruitment and growth on a shipwreck corroborate chemical defense resource trade-off. Mar Ecol-Prog Ser 368: 137–143. [Google Scholar]
- 52. Chanas B, Pawlik JR (1995) Defenses of Caribbean sponges against predatory reef fish. 2. Spicules, tissue toughness, and nutritional quality. Mar Ecol-Prog Ser 127: 195–211. [Google Scholar]
- 53. Chanas B, Pawlik JR (1996) Does the skeleton of a sponge provide a defense against predatory reef fish? Oecologia 107: 225–231. [DOI] [PubMed] [Google Scholar]
- 54. Loh TL, Pawlik JR (2012) Friend or foe? No evidence that association with the sponge Mycale laevis provides a benefit to corals of the genus Montastraea. . Mar Ecol Prog Ser 465: 111–117. [Google Scholar]
- 55. Huang DW (2012) Threatened reef corals of the World. PLoS ONE 7: e34459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Maliao RJ, Turingan RG, Lin J (2008) Phase-shift in coral reef communities in the Florida Keys National Marine Sanctuary (FKNMS), USA. Mar Biol 154: 841–853. [Google Scholar]
- 57. Pandolfi JM, Connolly SR, Marshall DJ, Cohen AL (2011) Projecting coral reef futures under global warming and ocean acidification. Science 333: 418–422. [DOI] [PubMed] [Google Scholar]
- 58. Duckworth AR, West L, Vansach T, Stubler A, Hardt M (2012) Effects of water temperature and pH on growth and metabolite biosynthesis of coral reef sponges. Mar Ecol Prog Ser 462: 67–77. [Google Scholar]