Abstract
Coronins are a highly conserved family of actin binding proteins that regulate actin-dependent processes such as cell motility and endocytosis. We found that treatment of human pulmonary artery endothelial cells (HPAECs) with the bioactive lipid, sphingosine-1-phosphate (S1P) rapidly stimulates coronin 1B translocation to lamellipodia at the cell leading edge, which is required for S1P-induced chemotaxis. Further, S1P-induced chemotaxis of HPAECs was attenuated by pretreatment with small interfering RNA (siRNA) targeting coronin 1B (∼36%), PLD2 (∼45%) or Rac1 (∼50%) compared to scrambled siRNA controls. Down regulation PLD2 expression by siRNA also attenuated S1P-induced coronin 1B translocation to the leading edge of the cell periphery while PLD1 silencing had no effect. Also, S1P-induced coronin 1B redistribution to cell periphery and chemotaxis was attenuated by inhibition of Rac1 and over-expression of dominant negative PKC δ, ε and ζ isoforms in HPAECs. These results demonstrate that S1P activation of PLD2, PKC and Rac1 is part of the signaling cascade that regulates coronin 1B translocation to the cell periphery and the ensuing cell chemotaxis.
Introduction
Sphingosine-1-phospahte (S1P) is a bioactive sphingophospholipid that has been shown to enhance endothelial chemotaxis during wound healing [1]. Coronin is one of the actin-regulatory proteins present at the leading edge of migrating cells [2] and has been shown to enhance cofilin-mediated actin de-polymerization [3], [4] and inhibit Arp2/3-mediated actin nucleation [5]. The idea that coronin is a critical protein for efficient cell migration is supported by the literature which reports on the presence of coronin at the leading edge of migrating cells [2], [6], [7], its co-localization with other actin-regulating proteins at sites of rapid actin turnover [8], [9] and the impaired migration of coronin mutant cells [10], [11]. However, the detailed mechanisms of coronin-mediated cell motility are still unclear.
The leading edge, or lamellipodia, of migrating cells exhibits a unique type of actin dynamics characterized by the fast “treadmilling” of actin filaments [12] where F-actin filaments are depolymerized at their pointed ends to liberate G-actin monomers that are recycled to extend F-actin filaments at their barbed end. Rapid actin disassembly is an important aspect of lamellipodia actin dynamics as it replenishes the G-actin monomers necessary for extending F-actin filaments. Compromise of actin depolymerization has been shown in cell models to reduce migration rates. Cofilin is the major actin-regulating protein involved in actin depolymerization by facilitating the removal of ADP-bound G-monomers from the pointed ends of F-actin filaments [13], [14]. However, in the presence of G-actin monomers, cofilin is unable to depolymerize actin without coronin [3]. Although coronin has been identified as a critical cofactor for cofilin, signaling pathways regulating cofilin dephosphorylation by SSH1 and coronin relocalization to leading edges of cells are currently not well defined.
Recently, the role of phospholipase D (PLD) in cell migration has been demonstrated [15], [16], [17]. PLD isoforms 1 & 2 hydrolyze phosphatidylcholine to phosphatidic acid (PA), which is a second messenger and involved in membrane trafficking [18], actin cytoskeleton remodeling [19], [20] and cell survival [21]. Over-expression of catalytically inactive PLD2 in normal endothelial [15] and cancer cells [22] inhibited cell migration, suggesting a role for PLD in regulation of cell motility. The signaling pathways downstream of PLD leading to cell migration have not been clearly defined; however, PA can directly activate PKC ζ [23], and PKC isoforms have been shown to be involved in cell migration in various cell types [15], [24], [25].
We and others have demonstrated that S1P activates PLD in endothelial and other cell types [26]; however, the potential role of PLD in S1P-induced chemotaxis in endothelial cells is not well defined. In the present paper, we investigated the role of coronin 1B and PLD signaling in S1P-induced endothelial cell chemotaxis. Treatment of human pulmonary artery endothelial cells (HPAECs) with S1P rapidly induced coronin 1B localization to lamellipodia and enhanced chemotaxis. Silencing coronin 1B with small interfering RNA (siRNA) attenuated S1P-induced HPAEC chemotaxis. Further, PLD2, PKC δ, ε and ζ and Rac1 signal transduction regulated S1P-mediated coronin 1B localization to lamellipodia and chemotaxis.
Results
Expression and Localization of Coronin 1B in Human Endothelial Cells
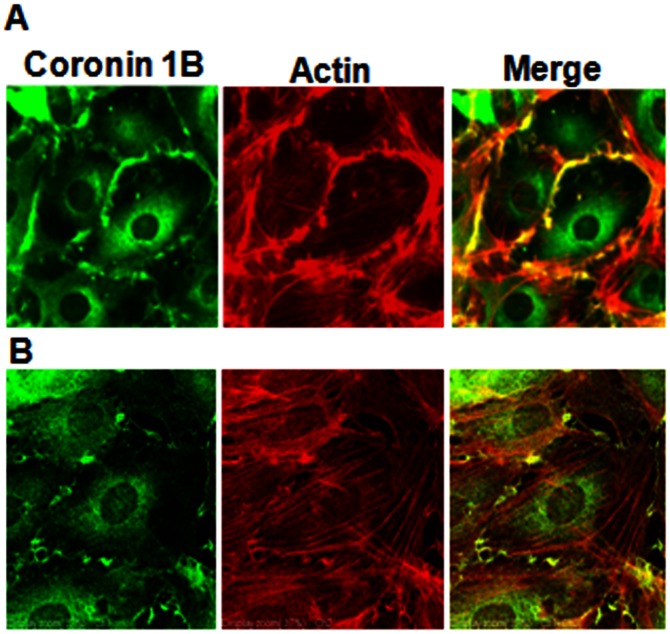
Coronin 1B mRNA and protein are highly expressed in human pulmonary artery, umbilical vein, aortic and lung microvascular endothelial cells ( Figure 1 A & B). Under normal growth conditions, as evidenced by immunocytochemistry, coronin 1B co-localizes with F-actin in a ∼2 µM thick region at the leading edge of the cell periphery ( Figure 2 ). This is presumably the fast “tread-milling” region of F-actin polymerization that has been well-characterized for cell lamellipodia. Furthermore, a significant fraction of coronin is also diffusely distributed within the cell cytoplasm, but this population of coronin does not co-localize with F-actin or cortactin. Upon serum starvation, coronin redistributes from the cell periphery and is distributed only within the cell cytosol ( Figure 3 ).
Figure 1. Expression of coronin 1B in human endothelial cells.
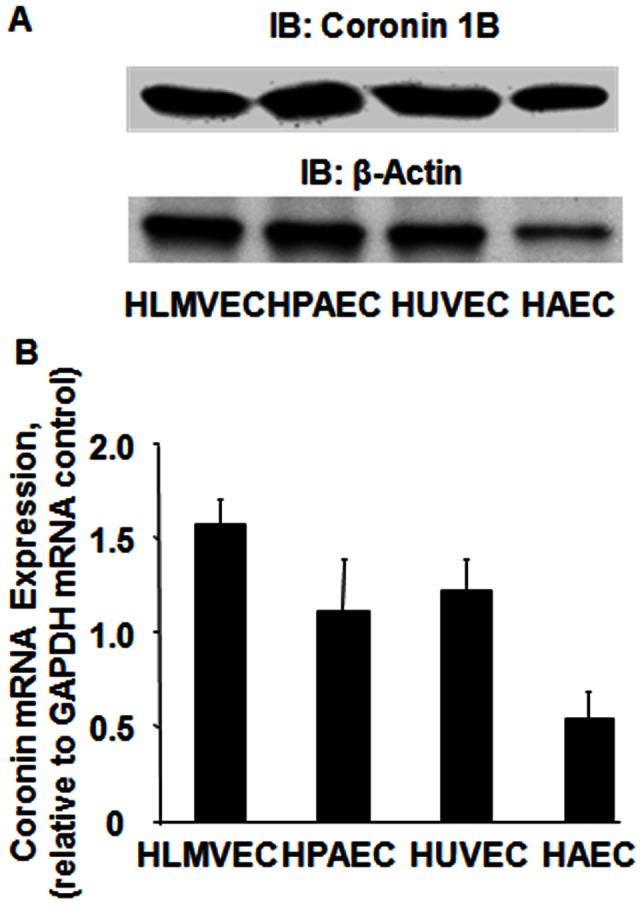
Human lung microvascular, pulmonary artery, umbilical vein and aortic ECs grown to ∼90% confluence in 60 mm dishes were lysed in cell lysis buffer. Cell lysates (20–40 µg of protein) were subjected to 10% SDS-PAGE, transferred to PVDF membrane and probed with anti-coronin 1B and β-actin antibodies as described under Materials and Methods. (A), Shown is a representative Western blot depicting coronin 1B protein expression in different human ECs. (B), In parallel experiments total RNA was isolated from various human ECs and were analyzed for mRNA expression of Coronin 1B by quantitative real time RT-PCR. The values are mean ± S.E.M for three independent experiments each g002performed in triplicate and normalized to GAPDH mRNA expression.
Figure 2. Coronin 1B localization in human lung endothelial cells.
HPAECs grown to ∼90% confluence on slide chambers were fixed, permeabilized and localization of Coronin 1B, actin and co-localization of Coronin 1B with actin was visualized by immunocytochemistry as described in Materials and Methods. Shown are representative immunofluorescence images from several independent experiments as measured by regular (A) immunofluorescence and (B) confocal microscopy.
Figure 3. Effect of starvation and serum supplementation on Coronin 1B localization in human lung endothelial cells.
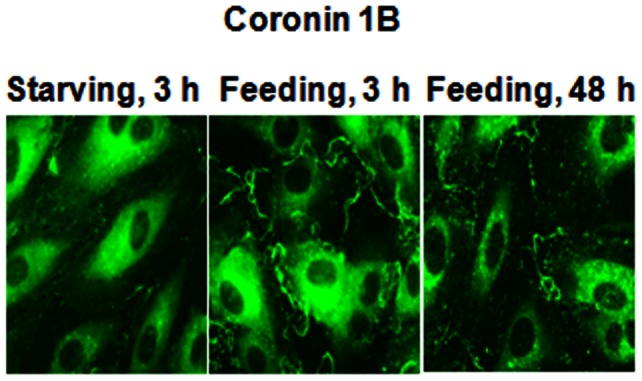
HPAECs grown on slide chambers (∼90% confluence) were incubated in EBM-2 medium containing either 0.1% serum for 3 h or in EBM-2 medium containing 5% serum for 3 h and 48 h. Cells were fixed, permeabilized and Coronin 1B localization was visualized by immunocytochemistry as described in Materials and Methods. Shown are representative immunofluorescence images from several independent experiments.
S1P Stimulates Accumulation of Coronin 1B and Cortactin to Lamellipodia
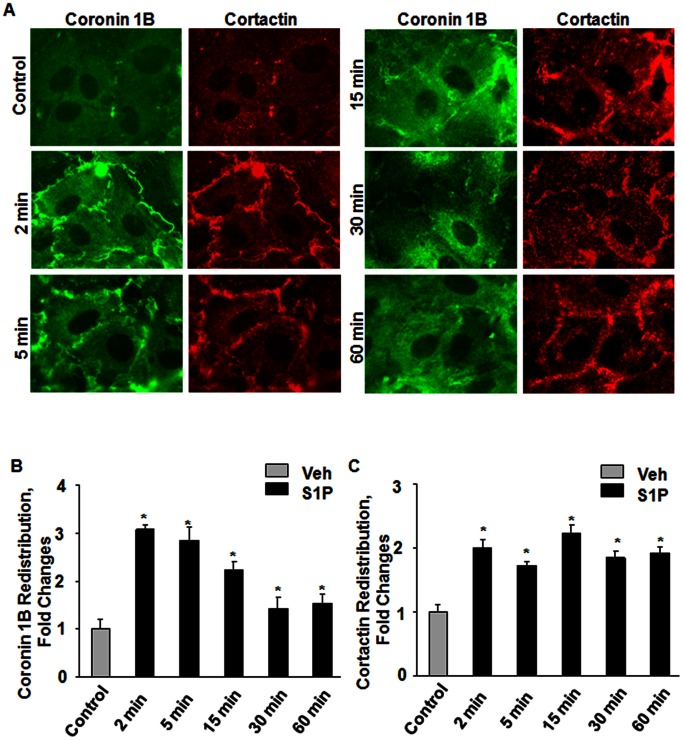
S1P is a potent angiogenic factor present in plasma at nM to µM levels [27], [28] and an activator of endothelial signal transduction [29], [30], [31]. Stimulation of HPAECs with S1P (1 µM) resulted in a rapid (2–30 min) and transient accumulation of coronin 1B in membrane ruffles ( Figure 4 A) at the leading edge of the lamellipodia ( Figure 4 B). In addition to coronin 1B, S1P also stimulated redistribution of cortactin to lamellipodia ( Figure 4 A and C).
Figure 4. S1P stimulates redistribution of coronin 1B and cortactin to lamellipodia in human lung endothelial cells.
HPAECs grown on slide chambers (∼90% confluence) were stimulated with 1 µM S1P for different time interval (2, 5, 15, 30 and 60 min) as indicated. Redistribution of Coronin 1B (B) and Cortactin (C) was visualized by immunocytochemistry and quantified by ImageJ software as described in Materials and Methods. Shown are representative immunofluorescence images from several independent experiments.
S1P Stimulated HPAEC Chemotaxis is Coronin 1B-depedent
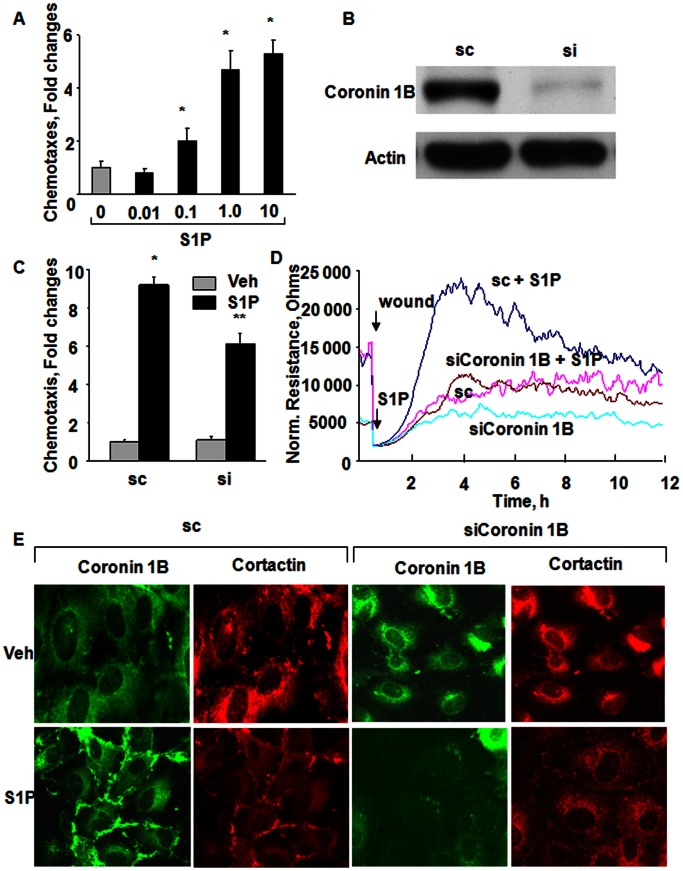
Having established that S1P stimulates redistribution of coronin1B to cell periphery, next we investigated the role of coronin1B in endothelial cell (EC) chemotaxis. Treatment of HPAECs with S1P induced cell chemotaxis in a Boyden chamber-based trans-well assay with increased cell transmigration observed at S1P concentrations of 0.1 µM and reaching a plateau at 1 µM ( Figure 5 A). Down-regulation of coronin 1B expression by coronin 1B small interfering RNA (siRNA) (50 nM, 72 h) knocked down >85% of coronin 1B expression ( Figure 5 B) and inhibited S1P-induced endothelial chemotaxis (∼36%) and cell migration (∼85%) in a wound healing assay ( Figure 5 C and D). This divergent result between chemotaxis and migration may be explained by inherent differences in the time frame of exposure and presentation of S1P to endothelial cells. In the chemotaxis assay, endothelial cells were allowed to migrate through a membrane filter for 6 h against a S1P gradient while in the migration assay, cells were allowed to migrate for 12–16 h in the presence of S1P that was added on to top of the cells. Further, S1P stimulated redistribution of coronin 1B and cortactin to lamellipodia compared to scrambled siRNA (Coronin 1B: = Control, 1±0.2, S1P = 3.7±0.1; Cortactin: Control, 1±0.4, S1P, 3.2±0.1); however, coronin 1B siRNA did not affect S1P-induced cortactin translocation to the lamellipodia (Control = 1±0.4; S1P = 3.2±0.1; siRNA = 1.5±0.2; siRNA+S1P = 2.0±0.1) ( Figure 5 E). These results suggest a role for coronin 1B in S1P mediated chemotaxis of lung endothelial cells.
Figure 5. Coronin 1B siRNA attenuates S1P-induced chemotaxis, wound healing and lamellipodia localization of cortactin in HPAECs.
(A), HPAECs grown on transwell inserts were stimulated with different S1P concentration (0.01, 0.1, 1 and 10 µM) for 15 min and chemotaxis was estimated by a Boyden chamber-based trans-well assay as described in Materials and Methods. The values are mean±SEM of three independent experiments. *, p<0.05 compared to cells without S1P. (B) HPAECs were transfected with scrambled (sc) or siRNA for Coronin 1B (50 ng/ml, 72 h), and cell lysates (20 µg of protein) were subjected to 10% SDS-PAGE and probed with Coronin 1B and actin antibodies as indicated. (C) HPAECs grown to 50% confluence in 100-mm dishes were transfected with sc (sc) or Coronin 1B siRNA (50 ng/ml) for 72 h. The cells were trypsinazied and plated on to transwell inserts and S1P-induced chemotaxis was determines as described in (A). The values are mean±SEM of three independent experiments in triplicate. *, p<0.05 compared cells without S1P; **, p<0.001 compared to scrambled siRNA transfected cells plus S1P. (D), HPAECs transfected with scrambled (sc) or Coronin 1B siRNA (50 nM, 72 h) were wounded on the gold electrodes as described under Materials and Methods. Measurement of transendothelial electrical resistance (TER) using an electrical cell substrate impedance-sensing system (ECIS) for 12 h after wounding the cells on the gold electrode and exposure to 1.0 µM S1P was carried out. Shown is a tracing from three independent experiments in triplicate. (E), HPAECs transfected with scrambled (sc) or Coronin 1B siRNA (50 nM, 72 h) were seeded on slide chambers for 24 h prior to stimulation with 1 µM S1P for 15 min. Cells were fixed and Coronin 1B and Cortactin redistribution to cell periphery was visualized by immunocytochemistry as described in Materials and Methods. Shown is a representative immunofluorescence image taken using an X 60 oil objective as described under Materials and Methods.
PLD2, but not PLD1, Regulates S1P-induced Coronin 1B Translocation to Cell Periphery and Chemotaxis of HPAECs
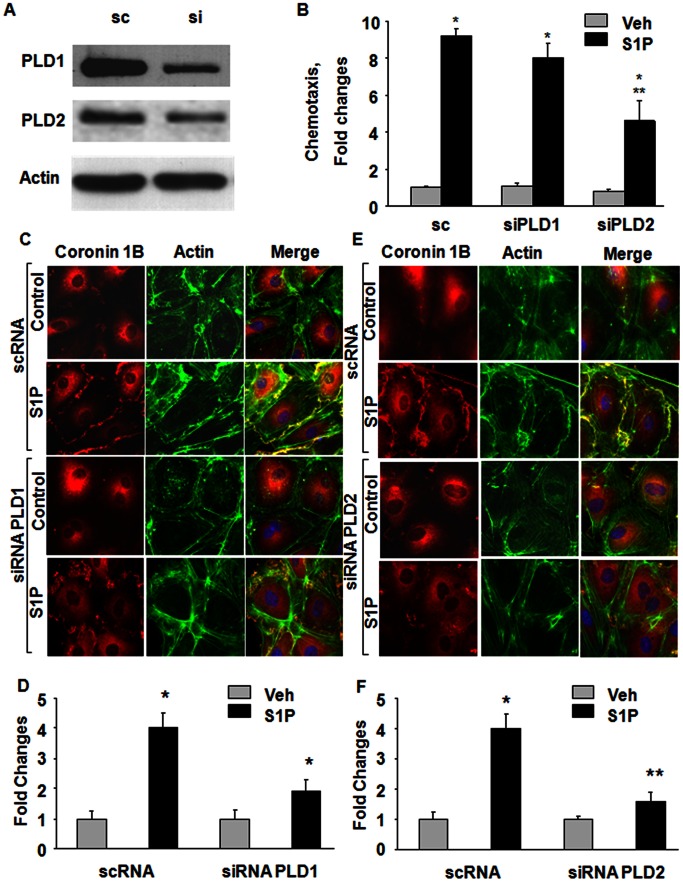
We have earlier demonstrated that S1P activates PLD1 and PLD2 in human bronchial epithelial cells [32], [33] and human lung ECs [15]. To determine the role of PLD1 and PLD2 in S1P-mediated coronin 1B translocation to cell periphery, and chemotaxis, HPAECs were transfected with scrambled, PLD1 or PLD2 siRNA (50 nM, 48 h) prior to S1P (1 µM) treatment. In scrambled siRNA treated cells, S1P stimulated [32P]PBt accumulation, an index of PLD activation [32], [33] ∼4 fold (vehicle, 1089±124 dpm; S1P, 7504±234 dpm). Downregulation of PLD1 (PBt formed: PLD1 siRNA, 965±158 dpm; PLD1 siRNA+S1P, 3168±198 dpm) or PLD2 (PBt formed: PLD2 siRNA, 690±176 dpm; S1P, 2166±122 dpm) with siRNA partially attenuated S1P-induced [32P]PBt formation without altering basal activity. In cells transfected with PLD1 or PLD2 siRNA, the efficacy of knocking down the protein was ∼80% compared to scrambled siRNA treated cells ( Figure 6 A). Downregulation of PLD2, but not PLD1, by siRNA attenuated both S1P-induced endothelial chemotaxis (∼45%) ( Figure 6 B). Knockdown of PLD2, but not PLD1, with siRNA blocked S1P-induced coronin 1B and actin translocation to lamellipodia ( Figure 6 C–F). These results suggest a role for PLD2, but not PLD1, in S1P-induced translocation of coronin 1B to cell periphery and chemotaxis.
Figure 6. Role of PLD2 in S1P-induced chemotaxis, Coronin 1B and actin lamellipodia localization in HPAECs.
HPAECs (∼50% confluence) were transfected with scrambled (sc), PLD1 or PLD2 siRNA (50 ng/ml) for 72 h. (A) Cell lysates (20–40 µg of protein) were subjected to 10% SDS-PAGE, Western blotted and probed with PLD1 and PLD2 antibodies as indicated; (B) chemotaxis of scrambled (sc) or siRNA transfected cells to S1P (1 µM) for 15 min was carried out in a Boyden chamber-based trans-well assay as described under Materials and Methods. Values are mean±SEM of three independent experiments in triplicate. *, p<0.01 compared cells without S1P; **, p<0.005 compared to scrambled siRNA transfected cells plus S1P; HPAECs transfected with sc, PLD1 (C) or PLD2 (E) siRNA in 100-mm dishes as described under (A) were trypsinazied and seeded onto slide chambers prior to stimulation with S1P (1 µM) for 15 min. Cells were washed, fixed, permeabilized, and probed with anti-Coronin 1B and AlexaFluor Phalloidin antibodies, and redistribution of Coronin 1B and actin due to downregulation of PLD1 (D) or PLD2 (F) was examined by immunofluorescence microscopy using a 60 X oil objective and quantified by ImageJ software as described under “Experimental Procedures”. Shown is an immunofluorescence micrograph from three independent experiments.
Role of Rac1 in S1P-induced Coronin 1B Distribution to Cell Periphery and Chemotaxis of HPAECs
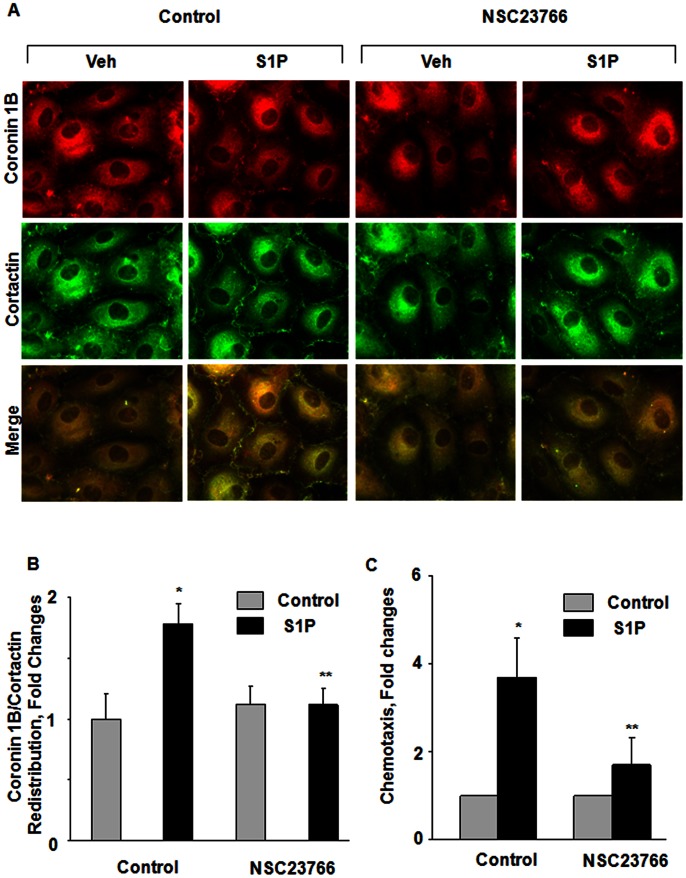
We have previously reported that PLD2 regulates S1P-induced HPAEC migration via Rac1 [15]. The role of Rac1 in S1P-induced chemotaxis and coronin 1B translocation to cell periphery is unclear; therefore, we determined whether Rac1 is involved S1P-induced chemotaxis and coronin 1B localization to lamellipodia. S1P (1 µM) stimulated the translocation of Rac1 to lamellipodia ( Figure 7 A and B), which was blocked by NSC23766, an inhibitor of Rac1 [34], [35]. Further, S1P-induced coronin 1B translocation to cell periphery and chemotaxis was also attenuated by Rac1 inhibitor NCS23766 ( Figure 7 A, B and C). In addition to coronin 1B, the Rac1 inhibitor also blocked S1P-mediated translocation of cortactin to cell plasma membrane ( Figure 7 A and B). These results suggest a role for Rac1 in S1P-induced redistribution of coronin 1B and cortactin to cell periphery as well as chemotaxis of HPAECs.
Figure 7. Role of Rac1 in S1P-induced chemotaxis and redistribution of coronin 1B and cortactin to lamellipodia in human lung endothelial cells.
(A), HPAECs (∼90% confluence) grown on chamber slides were pretreated for 30 min with NSC23766 (50 µM), a Rac1 inhibitor, prior to stimulation with S1P (1 µM) for 15 min. Cells were washed, fixed, permeabilized, probed with antibodies, and redistribution of Coronin 1B and Cortactin was examined by immunofluorescence microscopy using a 60 X oil objective and quantified by ImageJ software (B) as described under Materials and Methods. Shown is an immunofluorescence micrograph from three independent experiments. (C), In parallel experiments the effect of NSC23766 on chemotaxis was determined by a Boyden chamber-based trans-well assay as described in Materials and Methods. Values are mean±SEM of three independent experiments. *, p<0.05 compared cells without S1P; **, p<0.005 compared to cells stimulated with S1P in the absence of NSC23766.
Role of PLD2 in S1P-induced Rac1 Activation
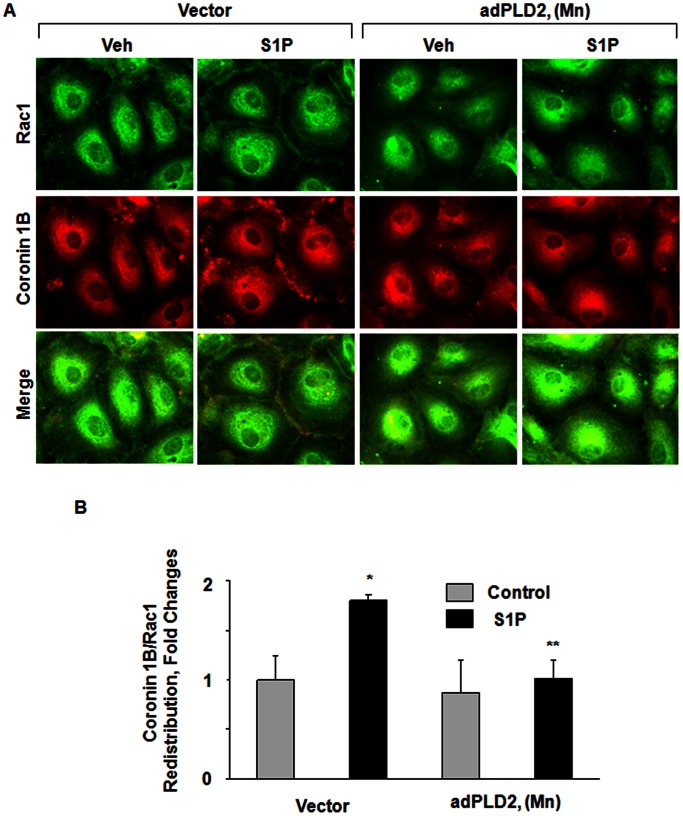
Having demonstrated a role for PLD2 and Rac1 in S1P-induced coronin 1B translocation to lamellipodia and chemotaxis, next we investigated the role of PLD2 in S1P-induced Rac1 activation. HPAECs grown on glass cover slips were infected with vector control or adenoviral mPLD2 mutant K758R (5 MOI, 24 h). Over-expression of mPLD2 mutant in HPAECs attenuated S1P-induced translocation of Rac1 to lamellipodia and decreased S1P-induced association of Rac1 with coronin 1B ( Figure 8 A and B). These results further establish that S1P-induced coronin 1B translocation to lamellipodia is through PLD2-Rac1 signaling cascade.
Figure 8. PLD2 mutant attenuates S1P-induced lamellipodial localization of Rac1 and Coronin 1B in human lung endothelial cells.
HPAECs (∼50% confluence) grown on slide chambers were infected with vector-control or adenoviral mPLD2 K758R mutant (5 MOI) for 24 h, prior to stimulation with 1 µM S1P for 15 min. Cells were washed, fixed, permeabilized, probed with antibodies, and redistribution of Rac1 and Coronin 1B was examined by immunofluorescence microscopy using a 60 X oil objective and quantified by ImageJ software (B) as described under Materials and Methods. Shown are representative immunofluorescence micrographs from three independent experiments.
Role of PKC Isoforms on S1P Mediated Chemotaxis of HPAECs
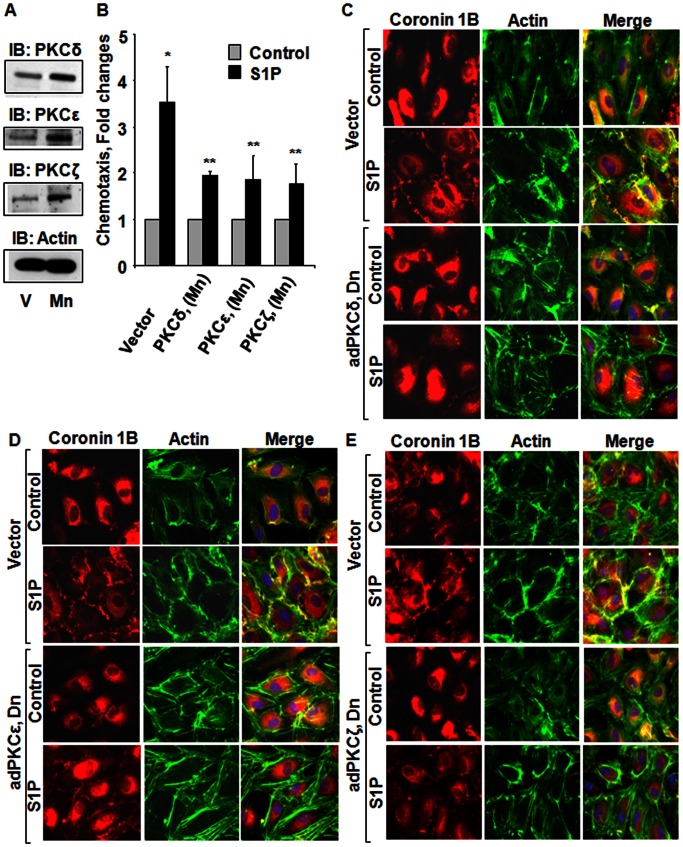
After establishing a role for PLD2 and Rac1 in S1P-mediated coronin 1B redistribution to cell periphery and chemotaxis of HPAECs, next we determined the role of PKC δ, ε, and ζ isoforms in S1P-induced coronin 1B translocation to cell periphery and chemotaxis. Challenge of HPAECs with S1P (1 µM) activated PKC δ, ε, and ζ isoforms [15]. To investigate the role of PKC δ, ε, and ζ isoforms on S1P-stimulated coronin 1B translocation and chemotaxis, HPAECs were infected with adenoviral vectors encoding for dominant negative (dn) δ, ε, and ζ (5 MOI) for 24 h, which resulted in over-expression of each of the isoform protein (∼3–5 fold) ( Figure 9 A). Over-expression of dn PKC δ, ε, and ζ isoforms significantly reduced S1P-induced chemotaxis ( Figure 9 B) and redistribution of coronin 1B and actin to cell periphery ( Figure 9 C, D and E) compared to vector-infected cells and co-localization of actin and coronin 1B was quantified (Coronin 1B and Actin Merged: Vector = 1±0.2; Vector+S1P = 4.0±0.1; adPKCδ (Dn) = 1±0.1; adPKCδ (Dn)+S1P = 1.7±0.1; adPKCε (Dn) = 1±0.1; adPKCε (Dn)+S1P = 1.3±0.2; adPKCζ (Dn) = 1±0.2; adPKCζ (Dn)+S1P = 1.9±0.2). These results establish that S1P-induced chemotaxis and coronin 1B translocation are dependent on PKC δ, ε, and ζ isoforms.
Figure 9. Role of PKC δ, ε, and ζ isoforms on S1P mediated chemotaxis and lamellipodial localization of Coronin 1B in human lung endothelial cells.
HPAECs grown on slide chambers or 35-mm dishes (∼70% confluence) were infected with empty vector or adenoviral vectors encoding PKC dominant negative (dn) δ, ε, and ζ isoforms (5 MOI) in complete EGM-2 medium for 24 h. (A), Cell lysates (20 µg of protein) were subjected to 10% SDS-PAGE, Western blotting and probed with anti-PKC δ, ε, ζ and actin antibodies. In parallel experiments, the effect of dn PKC δ, ε and ζ isoforms on chemotaxis (B) and lamellipodial localization of coronin 1B and actin (C, D and E) was examined as described in Materials and Methods. Values are mean±SEM of three independent experiments. *, p<0.01 compared cells without S1P; **, p<0.005 compared to cells infected with empty vector and stimulated with S1P.
Discussion
Directional migration or chemotaxis of endothelial cells plays a fundamental role in many physiological and pathological processes such as embryonic development, wound healing, tissue remodeling, angiogenesis, and tumor metastasis [36]. Chemotaxis of ECs is mediated by growth factors, chemokines, extracellular matrix-derived molecules and bioactive lipids such as platelet activating factor, and S1P [37], [38]. S1P mediated migration of human ECs requires activation of αvβ3 and PKC-ε dependent activation of PLD2 and subsequently a PLD2→PKC-ζ→Rac1 signaling cascade [15]. The data presented here reveal that coronin 1B, an actin binding protein, regulates S1P-induced EC chemotaxis. Furthermore, we show that S1P-induced translocation of coronin 1B to lamellipodia and chemotaxis is regulated by PKC δ, ε, and ζ isoforms, PLD2 and Rac1 signaling cascade. In addition, it is well established that S1P mobilizes sequestered calcium by activating G protein-coupled receptors via the PLC → PIP2 → IP3 pathway, which induces a transient calcium release from the endoplasmic reticulum followed by activation of store-operated calcium entry resulting in Ca2+-influx from extracellular media [39]. Thus, S1P-induced intracellular calcium changes result in cytoskeletal remodeling, enhanced chemotaxis, motility, vascular maturation and angiogenesis in ECs [1], [29], [30].
Chemotaxis depends upon a cell’s coordinated management of its actin cytoskeleton and is thought to occur at the leading edge of plasma membrane of the cell and the cell then pulling itself toward this leading edge [12], [40], [41] in response to gradient-dependent extracellular stimuli such as S1P. There is considerable evidence that S1P mediated activation of Rho GTPase family including Rho, Rac and Cdc42 are involved in cytoskeletal reorganization and cell migration [38], [42], [43]. Our results show that S1P treatment leads to increased localization of Rac1 and coronin 1B at the leading edge and blocking Rac1 attenuated S1P-induced coronin 1B reorganization at the leading edge and chemotaxis. S1P mediates its action via S1P1–5 G-protein coupled receptors and S1P signaling via S1P1 stimulates chemotaxis of ECs [44], [45]. In contrast to ECs, S1P mediates chemotaxis of fibroblasts through S1P1,3 or S1P2. In human lung fibroblasts, S1P-mediated chemotaxis was through S1P2 [46]; however, S1P dependent chemotaxis in human primary dermal fibroblast was dependent on S1P1,3 [47] . In contrast to stimulation of chemotaxis/migration of ECs [15], [48], [49] , keratinocytes [50], glioma cells [51] and fibroblast [46], [47], S1P inhibited migration of breast cancer cells [38] and melanoma and fibrosarcoma cells [52]. This bimodal regulation of chemotaxis/migration by S1P may be due to expression of stimulatory or inhibitory S1P receptors on different cell types, concentrations of S1P used, and coupling to varying down-stream signaling molecules.
S1P stimulates PLD isoforms 1 & 2 in human lung ECs [15], [26] and both the isoforms catalyze the hydrolysis of phosphatidylcholine and other phospholipids to PA. PA acts intracellularly as a second messenger [53] and PA generated via PLD signal transduction has been shown to be involved in membrane trafficking [54], [55], actin cytoskeleton remodeling [56], NADPH oxidase activation [39], [57], [58], cytokine secretion [33] and endothelial barrier function [59], [60]. Further, several studies suggest a role for PLD1 and PLD2 in cell motility. Over-expression of catalytically inactive PLD2 inhibited migration of ECs [15], fibroblasts [61] and cancer cells [16], [62] suggesting a role for PLD2/PA signaling in regulation of cell motility. Consistent with these studies, S1P-induced migration of lung ECs was attenuated by over-expression of PLD2 (K758R), but not PLD1 (K898R), mutant in HPAECs [63]. Further, S1P-induced cell motility was dependent on intracellular S1P generation as blocking SphK1 attenuated the cell migration mediated by exogenous S1P (64).
At present, the potential explanation for the differential participation of PLD1 and PLD2 is unclear but could be due to differences in the sub-cellular localization of PLD1 and PLD2 in mammalian cells. In mammalian cells, PLD1 is localized in the cytosol, Golgi membranes, nucleus and plasma membrane while PLD2 is primarily localized in the plasma membrane [64], [65], [66]. It is unclear how PA generated by PLD2 activation regulates coronin 1B [67]. Previously, we have shown that PLD regulates Rac1 via PKC-ζ and migration of HPAECs to S1P [15]; however, mechanism(s) of PA-dependent activation of PKC-ζ is yet to be fully defined. PKC-ζ can be activated by acidic lipids including PA [68] but it is unclear if PKC-ζ has any domain structure for PA binding. Several studies have demonstrated an important role for RhoA family of GTPases, Rho, Rac, and Cdc42 in regulating cell migration in response to agonists [67], [69], [70]. It is well recognized that actin polymerization leading to the formation of stress fibers is RhoA-dependent [71], [72], a process that is partly regulated by the PLD/PA signaling axis [56], [59]. Interestingly, PLD2 has guanine nucleotide-exchange factor (GEF) activity for Rho and regulates actin stress fibers in a manner independent of its lipase activity [73], [74]. Additionally, PA activates phosphatidylinositol-4-phosphate 5 kinase (PI4P5K) [75], [76], [77] to generate phosphatidylinositol-4,5-bisphosphate (PIP2), an activator of actin cytoskeleton and of interactions between actin and actin-binding proteins such as vinculin and filamin [78], [79]. However, the role of PLD2-generated PA in activation of Coronin 1B via PI4P5K is not known.
Current evidence supports that Coronin 1B disassembles Arp2/3 containing actin filament branches by inducing Arp2/3 dissociation and alters the branch angle [80]. Phosphorylation of Coronin 1B at ser-2 by PKC regulates its interaction with Arp2/3 complex and reduces phorbol ester-induced motility of fibroblast [81] . However, in vascular smooth muscle cells, phosphorylation of coronin 1B at ser-2 was essential for PDGF-induced migration [82]. Although PDGF-induced phosphorylation of Coronin 1B reduced its interaction with Arp2/3 complex, an important step in inducing cell migration, the differential effect of Coronin 1B phosphorylation on cell motility mediated by phorbol ester in fibroblast and PDGF in vascular smooth muscle cells remains controversial. In lung ECs, S1P stimulated tyrosine phosphorylation of Coronin 1B while phosphorylation at ser-2 was comparatively less to tyrosine phosphorylation (V. Natarajan, unpublished results); however the role of tyrosine phosphorylation of Coronin 1B in chemotaxis remains to be established.
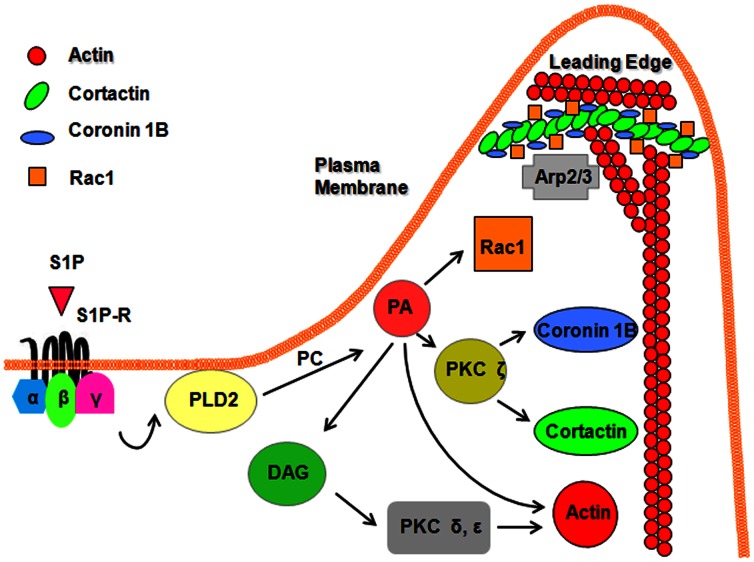
In conclusion, the present studies demonstrate that translocation of Coronin 1B to cell periphery participates in S1P-induced chemotaxis of HPAECs. Further, our results suggest that S1P-induced phosphorylation of Coronin 1B and redistribution to cell periphery is regulated by PLD2, Rac1 and PKC isoforms ε and ζ. Taken together, these observations provide new insights into role of Coronin 1B in S1P-induced regulation of chemotaxis in lung ECs ( Fig. 10 ).
Figure 10. Proposed signaling mechanisms involved in S1P-induced lamellipodial localization of Coronin 1B, Cortactin and chemotaxis of human lung endothelial cells.
S1P binding to G-protein coupled S1P1-5 receptors activates PLD2 via PKC δ and ε and activation of PLD2 results in hydrolysis of membrane associated phosphatidylcholine (PC) to phosphatidic acid (PA) and phospho-choline. PA can be converted to DAG by PA-phosphatases or can activate PKC ζ via of phosphatidylinositol-4-phosphate-5kinase activation. Activation of PKC ζ results in redistribution of Coronin 1B and Cortactin to cell periphery and localization in lamellipodia of endothelial cells. PA can directly bind to and activate Rac1 and formation of actin stress fibers. Additionally, PLD2 has guanine nucleotide-exchange factor (GEF) activity for Rho and can regulate actin stress fibers in a manner independent of its lipase activity. S1P-induced activation of PKC δ/ε → PLD2/PA → PKC ζ → Rac1 signaling cascade facilitates recruitment of Coronin 1B, Cortactin and Actin to lamellipodia and chemotaxis of endothelial cells.
Materials and Methods
Materials
Sphingosine-1-phosphate (S1P) was obtained from Avanti Polar Lipids (Alabaster, AL, USA). Scrambled siRNA and target siRNA for PLD1, PLD2 and Coronin1B, and antibodies for cortactin, PKC δ, ε, and ζ were obtained from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). Anti-coronin1B antibody was kind gift from Dr. James Bear (UNC, Chapel Hill, NC), and anti-Rac1 antibody was from BD Biosciences Pharmingen (San Jose, CA, USA). Internal and N-terminal antibodies for PLD1 and PLD2 were purchased from BioSource International Inc. (Camarillo, CA, USA), and anti-PLD2 antibody was kindly provided by Dr. Sylvain Bourgoin (Quebec, PQ, Canada). NSC23766 was from Calbiochem (San Diego, CA, USA). Transwell inserts were from Becton Dickinson Lab ware (Franklin Lakes, NJ, USA), and slide chambers were from Millipore (Bedford, MA, USA). Lysis buffer was purchased from Cell Signaling Technology Inc. (Danvers, MA, USA). Precast Tris-Glycine PAAG (Invitrogen-Molecular Probes, Eugene, OR, USA). Protease inhibitor cocktail tablets (EDTA-free Complete) were from Roche Diagnostics (Indianapolis, IN, USA). Aprotinin and phosphatase inhibitor cocktail 1, actin antibody were from Sigma-Aldrich (St. Louis, MO, USA).
Cell Culture
HPAECs were purchased from Lonza (San Diego, CA, USA), cultured in complete endothelial growth medium (EGM)-2 medium [15]. Cells (passage number 4–6) plated in 35-mm, 100-mm dishes or slide chambers were used for all the experiments.
Endothelial Cell Chemotaxis
HPAECs were cultured to ∼90% confluence, starved in EBM-2 medium containing 0.1% FBS for 1–3 h. Starved cells were suspended in 0.25% Trypsin-EDTA, neutralized with trypsin neutralizing solution, pelleted by centrifugation (500×g, 5 min), resuspended in EBM-2 media (0.1% serum), and counted using hemocytometer. 200 µl of cell suspension, containing 2×103 cells was added to the top well of a 24-mm diameter, 8.0 µM pore size Transwell insert and 600 µl of starvation media was added to the bottom chamber. Cells were allowed to attach for 1 h on the insert, S1P was added to the bottom chamber to a final concentration of 1 µM, and cells were allowed to migrate for 6 h. Cells that did not migrate were removed from the top portion of the insert using a cotton swab and migrated cells at the bottom side of the insert were fixed submerging the insert in 3.7% paraformaldehyde. The transwell membrane was removed and sealed using Vectashield containing DAPI. Cells were visualized under UV fluorescence using a Hamamatsu digital camera connected to the Nikon Eclipse TE2000-S microscope with ×10 objective and MetaVue software (Universal Imaging Corp., PA, USA). The effect of S1P and other agents on cell chemotaxis was quantified by manual counting of DAPI-stained nuclei of cells that have migrated across the Transwell membrane.
Electrical Cell Substrate Impedance Sensing (ECIS) Assay
HPAECs were cultured in 8-well ECIS electrode arrays (8W1E, Applied Biophysics, NY, USA) [83] to ∼95% confluence and starved in the EBM-2 medium with 0.1% BSA for 1–3 h. An elevated field (3 V at 40,000 Hz for 10 sec) was applied to wound the cells on the electrode and S1P was immediately added and endothelial wound healing was monitored for 12 h by measuring the transendothelial electrical resistance.
Infection of HPAECs with Adenoviral Vectors
cDNA for wild type and catalytically inactive mutants of PLD1, PLD2, and dominant negative (dn) PKC δ, ε, and ζ were sub-cloned into the pShuttle-CMV vector [15], [33]. The recombinant plasmid was linearized and transfected into HEK293 cells to generate replication-defective adenovirus. Generation of purified virus [1010plaque-forming units (p.f.u.)/ml] was carried out by the University of Iowa Gene Transfer Vector Core. Purified adenovirus (1–10 M.O.I) in complete EGM-2 medium was added to HPAECs grown to ∼80% confluence in 6-well plates, slide chambers, 60- or 100-mm dishes. After 24 h, the virus-containing medium was replaced with complete EGM-2 medium. Vector control or infected cells were subjected to scratch and wound healing ECIS assays and immunoprecipitates or cell lysates from parallel experiments were analyzed by Western blotting.
Western Blot Analysis
HPAECs were cultured in 6-well plates or 60-mm dishes to ∼95% confluence and starved for 3 h in EBM-2 medium containing 0.1% FBS. Cells were stimulated with S1P (100–1000 nM) for 5–60 min, washed with PBS and lysed with 100–300 µl lysis buffer containing 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 µg/ml leupeptin, 1 µg/ml aprotinin and protease inhibitors, EDTA-free complete tablets (Roche Applied Science, Indianapolis, IN). Cell lysates were cleared by centrifugation at 10,000×g for 10 min, and boiled with the Laemmli sample buffer for 5 min. Cell lysates (20–30 µg protein) were separated on 10% or 4–20% SDS-PAGE, transferred to PVDF membranes, blocked in TBST containing 5% BSA prior to incubation with primary antibody (1∶1000 dilution) overnight. After blocking, washing and incubation with appropriate secondary antibody (1∶2000 dilution), blots were developed using an ECL chemiluminescence kit. Western blots were scanned by densitometry and integrated density of pixels in identified areas was quantified using Image Quant version 5.2 software (Molecular Dynamics).
Immunofluorescence and Confocal Microscopy
HPAECs grown on chamber slides were starved for 3 h in EBM-2 containing 0.1% FBS prior to treatment with S1P (100–1000 nM) for 5–60 min. Cells were fixed in 3.7% para-formaldehyde in PBS for 10 min, washed three times with PBS, permeabilized for 4 min in 3.7% paraformaldehyde containing 0.25% Triton X-100, blocked with 2% BSA in TBST, incubated for 1 h with appropriate primary antibody (1∶200 dilution), washed with TBST, and stained for 1 h with secondary antibody (1∶200 dilution) in TBST containing 2% BSA. Cells were examined using a Nikon Eclipse TE2000-S immunofluorescence microscope and a Hamamatsu digital camera with ×60 oil immersion objective and Meta Vue software (Universal Imaging Corp., PA, USA). Coronin 1B and actin redistribution to lamellipodia was also investigated using Zeiss 510 Meta laser scanning microscope. Quantification of lamellipodia was performed as described earlier [39]. Briefly, for each image, background signal was subtracted by drawing a region of interest around the cell periphery of individual cells. All areas outside the cell were cleared to best visualize the leading edges including cell periphery and the fluorescence intensity within the entire cell was quantified by MBF ImageJ bundle (Tony Collins, McMaster University, http://www.macbiophotonics.ca/imageJ/and Wayne Rasband, NIH, http://rsb.info.nih.gov/ij/).
Transfection Procedures
HPAECs grown to ∼50% confluence in 6-well plates or chamber slides were transfected with Gene Silencer® (Gene Therapy System, Inc. San Diego, CA, USA) transfecting agent containing scrambled siRNA (50–100 nM) or siRNA for target proteins (50–100 nM) in serum-free EBM-2 medium according to manufacturer’s recommendation. To optimize conditions for efficient transfection, HPAECs were transfected with Fl-Luciferase GL2 Duplex siRNA (Target Sequence: 5′-CGTACGCGGAATACTTCGA-3′, Dharmacon, CO, USA) as a positive control. After 3 h transfection, 1 ml of fresh complete EGM-2 medium containing 10% FBS was added, cells were cultured for additional 72 h, and analyzed for mRNA level by real time PCR or protein expression by Western blotting.
PLD Activation in Intact Lung Endothelial Cells
HPAECs were labeled with [32P] orthophosphate (5 µCi/ml) in phosphate-free medium containing 2% FBS for 18–24 h. Cells were washed in minimal essential medium without serum and challenged either with vehicle or S1P (1 µM) for 30 min in the presence of 0.05% 1-butanol or tertiary butanol. The incubations were terminated by addition of 1ml of methanol-concentrated HCl (100∶1/vol/vol), followed by extraction of lipids [26]. [32P]PBt formed as a result of PLD activation and transphosphatidylation reaction, an index of in vivo PLD stimulation [84], was separated by TLC on 1% potassium oxalate-impregnated silica gel H plates using the upper phase of ethyl acetate-2,2,4-trimethyl pentane-glacial acetic acid-water (65∶10∶15∶50 vol/vol/vol/vol) as the developing solvent system [26]. Unlabeled PBt was added as a carrier during the lipid separation by TLC and was visualized under iodine vapors. Radioactivity associated with PBt was quantified by liquid scintillation counting, and data are expressed as dpm normalized to 106 counts in total lipid extract.
Statistical Analysis
Analysis of variance and Student-Newman-Keul’s test were used to compare means of two or more different treatment groups. The level of significance was set to p<0.05 unless otherwise stated. Results are expressed as mean ± S.E. M.
Funding Statement
This work was supported by National Institutes of Health grant P01 HL 98050 to VN. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
References
- 1. English D, Welch Z, Kovala AT, Harvey K, Volpert OV, et al. (2000) Sphingosine 1-phosphate released from platelets during clotting accounts for the potent endothelial cell chemotactic activity of blood serum and provides a novel link between hemostasis and angiogenesis. Faseb J 14: 2255–2265. [DOI] [PubMed] [Google Scholar]
- 2. Mishima M, Nishida E (1999) Coronin localizes to leading edges and is involved in cell spreading and lamellipodium extension in vertebrate cells. J Cell Sci 112 (Pt 17): 2833–2842. [DOI] [PubMed] [Google Scholar]
- 3. Brieher WM, Kueh HY, Ballif BA, Mitchison TJ (2006) Rapid actin monomer-insensitive depolymerization of Listeria actin comet tails by cofilin, coronin, and Aip1. J Cell Biol 175: 315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kueh HY, Charras GT, Mitchison TJ, Brieher WM (2008) Actin disassembly by cofilin, coronin, and Aip1 occurs in bursts and is inhibited by barbed-end cappers. J Cell Biol 182: 341–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Humphries CL, Balcer HI, D’Agostino JL, Winsor B, Drubin DG, et al. (2002) Direct regulation of Arp2/3 complex activity and function by the actin binding protein coronin. J Cell Biol 159: 993–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. de Hostos EL, Bradtke B, Lottspeich F, Guggenheim R, Gerisch G (1991) Coronin, an actin binding protein of Dictyostelium discoideum localized to cell surface projections, has sequence similarities to G protein beta subunits. Embo J 10: 4097–4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gerisch G, Albrecht R, Heizer C, Hodgkinson S, Maniak M (1995) Chemoattractant-controlled accumulation of coronin at the leading edge of Dictyostelium cells monitored using a green fluorescent protein-coronin fusion protein. Curr Biol 5: 1280–1285. [DOI] [PubMed] [Google Scholar]
- 8. Clarke M, Muller-Taubenberger A, Anderson KI, Engel U, Gerisch G (2006) Mechanically induced actin-mediated rocketing of phagosomes. Mol Biol Cell 17: 4866–4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maniak M, Rauchenberger R, Albrecht R, Murphy J, Gerisch G (1995) Coronin involved in phagocytosis: dynamics of particle-induced relocalization visualized by a green fluorescent protein Tag. Cell 83: 915–924. [DOI] [PubMed] [Google Scholar]
- 10. de Hostos EL, Rehfuess C, Bradtke B, Waddell DR, Albrecht R, et al. (1993) Dictyostelium mutants lacking the cytoskeletal protein coronin are defective in cytokinesis and cell motility. J Cell Biol 120: 163–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Foger N, Rangell L, Danilenko DM, Chan AC (2006) Requirement for coronin 1 in T lymphocyte trafficking and cellular homeostasis. Science 313: 839–842. [DOI] [PubMed] [Google Scholar]
- 12. Lauffenburger DA, Horwitz AF (1996) Cell migration: a physically integrated molecular process. Cell 84: 359–369. [DOI] [PubMed] [Google Scholar]
- 13. Hotulainen P, Paunola E, Vartiainen MK, Lappalainen P (2005) Actin-depolymerizing factor and cofilin-1 play overlapping roles in promoting rapid F-actin depolymerization in mammalian nonmuscle cells. Mol Biol Cell 16: 649–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pfaendtner J, De La Cruz EM, Voth GA (2010) Actin filament remodeling by actin depolymerization factor/cofilin. Proc Natl Acad Sci U S A 107: 7299–7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gorshkova I, He D, Berdyshev E, Usatuyk P, Burns M, et al. (2008) Protein kinase C-epsilon regulates sphingosine 1-phosphate-mediated migration of human lung endothelial cells through activation of phospholipase D2, protein kinase C-zeta, and Rac1. J Biol Chem 283: 11794–11806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zheng Y, Rodrik V, Toschi A, Shi M, Hui L, et al. (2006) Phospholipase D couples survival and migration signals in stress response of human cancer cells. J Biol Chem 281: 15862–15868. [DOI] [PubMed] [Google Scholar]
- 17. Knapek K, Frondorf K, Post J, Short S, Cox D, et al. (2010) The molecular basis of phospholipase D2-induced chemotaxis: elucidation of differential pathways in macrophages and fibroblasts. Mol Cell Biol 30: 4492–4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morris AJ (2007) Regulation of phospholipase D activity, membrane targeting and intracellular trafficking by phosphoinositides. Biochem Soc Symp: 247–257. [DOI] [PubMed]
- 19. Kam Y, Exton JH (2001) Phospholipase D activity is required for actin stress fiber formation in fibroblasts. Mol Cell Biol 21: 4055–4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Porcelli AM, Ghelli A, Hrelia S, Rugolo M (2002) Phospholipase D stimulation is required for sphingosine-1-phosphate activation of actin stress fibre assembly in human airway epithelial cells. Cell Signal 14: 75–81. [DOI] [PubMed] [Google Scholar]
- 21. Wu M, Wang Q, Luo JY, Jiang B, Li XY, et al. (2010) Activation of phospholipase D involved in both injury and survival in A549 alveolar epithelial cells exposed to H2O2. Toxicol Lett 196: 168–174. [DOI] [PubMed] [Google Scholar]
- 22. Snider AJ, Zhang Z, Xie Y, Meier KE (2010) Epidermal growth factor increases lysophosphatidic acid production in human ovarian cancer cells: roles for phospholipase D2 and receptor transactivation. Am J Physiol Cell Physiol 298: C163–170. [DOI] [PubMed] [Google Scholar]
- 23. Limatola C, Schaap D, Moolenaar WH, van Blitterswijk WJ (1994) Phosphatidic acid activation of protein kinase C-zeta overexpressed in COS cells: comparison with other protein kinase C isotypes and other acidic lipids. Biochem J 304 (Pt 3): 1001–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Harrington EO, Loffler J, Nelson PR, Kent KC, Simons M, et al. (1997) Enhancement of migration by protein kinase Calpha and inhibition of proliferation and cell cycle progression by protein kinase Cdelta in capillary endothelial cells. J Biol Chem 272: 7390–7397. [DOI] [PubMed] [Google Scholar]
- 25. Oubaha M, Lin MI, Margaron Y, Filion D, Price EN, et al. (2012) Formation of a PKCzeta/beta-catenin complex in endothelial cells promotes angiopoietin-1-induced collective directional migration and angiogenic sprouting. Blood 120: 3371–3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Natarajan V, Jayaram HN, Scribner WM, Garcia JG (1994) Activation of endothelial cell phospholipase D by sphingosine and sphingosine-1-phosphate. Am J Respir Cell Mol Biol 11: 221–229. [DOI] [PubMed] [Google Scholar]
- 27. Hammad SM, Pierce JS, Soodavar F, Smith KJ, Al Gadban MM, et al. (2010) Blood sphingolipidomics in healthy humans: impact of sample collection methodology. J Lipid Res 51: 3074–3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Venkataraman K, Lee YM, Michaud J, Thangada S, Ai Y, et al. (2008) Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ Res 102: 669–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Argraves KM, Wilkerson BA, Argraves WS (2010) Sphingosine-1-phosphate signaling in vasculogenesis and angiogenesis. World J Biol Chem 1: 291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Takuwa Y, Okamoto Y, Yoshioka K, Takuwa N (2008) Sphingosine-1-phosphate signaling and biological activities in the cardiovascular system. Biochim Biophys Acta 1781: 483–488. [DOI] [PubMed] [Google Scholar]
- 31. Sun X, Singleton PA, Letsiou E, Zhao J, Belvitch P, et al. (2012) Sphingosine-1-phosphate receptor-3 is a novel biomarker in acute lung injury. Am J Respir Cell Mol Biol 47: 628–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cummings RJ, Parinandi NL, Zaiman A, Wang L, Usatyuk PV, et al. (2002) Phospholipase D activation by sphingosine 1-phosphate regulates interleukin-8 secretion in human bronchial epithelial cells. J Biol Chem 277: 30227–30235. [DOI] [PubMed] [Google Scholar]
- 33. Wang L, Cummings R, Usatyuk P, Morris A, Irani K, et al. (2002) Involvement of phospholipases D1 and D2 in sphingosine 1-phosphate-induced ERK (extracellular-signal-regulated kinase) activation and interleukin-8 secretion in human bronchial epithelial cells. Biochem J 367: 751–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Desire L, Bourdin J, Loiseau N, Peillon H, Picard V, et al. (2005) RAC1 inhibition targets amyloid precursor protein processing by gamma-secretase and decreases Abeta production in vitro and in vivo. J Biol Chem 280: 37516–37525. [DOI] [PubMed] [Google Scholar]
- 35. Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y (2004) Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc Natl Acad Sci U S A 101: 7618–7623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Iglesias PA, Devreotes PN (2008) Navigating through models of chemotaxis. Curr Opin Cell Biol 20: 35–40. [DOI] [PubMed] [Google Scholar]
- 37. Estrada R, Zeng Q, Lu H, Sarojini H, Lee JF, et al. (2008) Up-regulating sphingosine 1-phosphate receptor-2 signaling impairs chemotactic, wound-healing, and morphogenetic responses in senescent endothelial cells. J Biol Chem 283: 30363–30375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang L, Lee JF, Lin CY, Lee MJ (2008) Rho GTPases mediated integrin alpha v beta 3 activation in sphingosine-1-phosphate stimulated chemotaxis of endothelial cells. Histochem Cell Biol 129: 579–588. [DOI] [PubMed] [Google Scholar]
- 39. Usatyuk PV, Gorshkova IA, He D, Zhao Y, Kalari SK, et al. (2009) Phospholipase D-mediated activation of IQGAP1 through Rac1 regulates hyperoxia-induced p47phox translocation and reactive oxygen species generation in lung endothelial cells. J Biol Chem 284: 15339–15352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tilney LG, Bonder EM, DeRosier DJ (1981) Actin filaments elongate from their membrane-associated ends. J Cell Biol 90: 485–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Min J, Defea K beta-arrestin-dependent actin reorganization: bringing the right players together at the leading edge. Mol Pharmacol 80: 760–768. [DOI] [PubMed] [Google Scholar]
- 42. Li Z, Paik JH, Wang Z, Hla T, Wu D (2005) Role of guanine nucleotide exchange factor P-Rex-2b in sphingosine 1-phosphate-induced Rac1 activation and cell migration in endothelial cells. Prostaglandins Other Lipid Mediat 76: 95–104. [DOI] [PubMed] [Google Scholar]
- 43. Koh E, Clair T, Hermansen R, Bandle RW, Schiffmann E, et al. (2007) Sphingosine-1-phosphate initiates rapid retraction of pseudopodia by localized RhoA activation. Cell Signal 19: 1328–1338. [DOI] [PubMed] [Google Scholar]
- 44. Lee JF, Zeng Q, Ozaki H, Wang L, Hand AR, et al. (2006) Dual roles of tight junction-associated protein, zonula occludens-1, in sphingosine 1-phosphate-mediated endothelial chemotaxis and barrier integrity. J Biol Chem 281: 29190–29200. [DOI] [PubMed] [Google Scholar]
- 45. Paik JH, Chae S, Lee MJ, Thangada S, Hla T (2001) Sphingosine 1-phosphate-induced endothelial cell migration requires the expression of EDG-1 and EDG-3 receptors and Rho-dependent activation of alpha vbeta3- and beta1-containing integrins. J Biol Chem 276: 11830–11837. [DOI] [PubMed] [Google Scholar]
- 46. Hashimoto M, Wang X, Mao L, Kobayashi T, Kawasaki S, et al. (2008) Sphingosine 1-phosphate potentiates human lung fibroblast chemotaxis through the S1P2 receptor. Am J Respir Cell Mol Biol 39: 356–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gil PR, Japtok L, Kleuser B (2010) Sphingosine 1-phosphate mediates chemotaxis of human primary fibroblasts via the S1P-receptor subtypes S1P(1) and S1P(3) and Smad-signalling. Cytoskeleton (Hoboken) 67: 773–783. [DOI] [PubMed] [Google Scholar]
- 48. Lee MJ, Thangada S, Claffey KP, Ancellin N, Liu CH, et al. (1999) Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell 99: 301–312. [DOI] [PubMed] [Google Scholar]
- 49. Okamoto H, Yatomi Y, Ohmori T, Satoh K, Matsumoto Y, et al. (2000) Sphingosine 1-phosphate stimulates G(i)- and Rho-mediated vascular endothelial cell spreading and migration. Thromb Res 99: 259–265. [DOI] [PubMed] [Google Scholar]
- 50. Sauer B, Vogler R, von Wenckstern H, Fujii M, Anzano MB, et al. (2004) Involvement of Smad signaling in sphingosine 1-phosphate-mediated biological responses of keratinocytes. J Biol Chem 279: 38471–38479. [DOI] [PubMed] [Google Scholar]
- 51. Van BrocklynJR, Young N, Roof R (2003) Sphingosine-1-phosphate stimulates motility and invasiveness of human glioblastoma multiforme cells. Cancer Lett 199: 53–60. [DOI] [PubMed] [Google Scholar]
- 52. Sadahira Y, Ruan F, Hakomori S, Igarashi Y (1992) Sphingosine 1-phosphate, a specific endogenous signaling molecule controlling cell motility and tumor cell invasiveness. Proc Natl Acad Sci U S A 89: 9686–9690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cummings R, Parinandi N, Wang L, Usatyuk P, Natarajan V (2002) Phospholipase D/phosphatidic acid signal transduction: role and physiological significance in lung. Mol Cell Biochem 234–235: 99–109. [PubMed] [Google Scholar]
- 54. Liscovitch M (1996) Phospholipase D: role in signal transduction and membrane traffic. J Lipid Mediat Cell Signal 14: 215–221. [DOI] [PubMed] [Google Scholar]
- 55. Hashimoto Y, Okiyoneda T, Harada K, Ueno K, Sugahara T, et al. (2008) Phosphatidic acid metabolism regulates the intracellular trafficking and retrotranslocation of CFTR. Biochim Biophys Acta 1783: 153–162. [DOI] [PubMed] [Google Scholar]
- 56. Komati H, Naro F, Mebarek S, De Arcangelis V, Adamo S, et al. (2005) Phospholipase D is involved in myogenic differentiation through remodeling of actin cytoskeleton. Mol Biol Cell 16: 1232–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Erickson RW, Langel-Peveri P, Traynor-Kaplan AE, Heyworth PG, Curnutte JT (1999) Activation of human neutrophil NADPH oxidase by phosphatidic acid or diacylglycerol in a cell-free system. Activity of diacylglycerol is dependent on its conversion to phosphatidic acid. J Biol Chem 274: 22243–22250. [DOI] [PubMed] [Google Scholar]
- 58. Regier DS, Greene DG, Sergeant S, Jesaitis AJ, McPhail LC (2000) Phosphorylation of p22phox is mediated by phospholipase D-dependent and -independent mechanisms. Correlation of NADPH oxidase activity and p22phox phosphorylation. J Biol Chem 275: 28406–28412. [DOI] [PubMed] [Google Scholar]
- 59. Zeiller C, Mebarek S, Jaafar R, Pirola L, Lagarde M, et al. (2009) Phospholipase D2 regulates endothelial permeability through cytoskeleton reorganization and occludin downregulation. Biochim Biophys Acta 1793: 1236–1249. [DOI] [PubMed] [Google Scholar]
- 60.Usatyuk PV KS, Parinandi NL, Natarajan V (2012) Phospholipase D Signaling Mediates Reactive Oxygen Species-Induced Lung Endothelial Barrier Dysfunction. Pulm Circulation In Press. [DOI] [PMC free article] [PubMed]
- 61. Pilquil C, Dewald J, Cherney A, Gorshkova I, Tigyi G, et al. (2006) Lipid phosphate phosphatase-1 regulates lysophosphatidate-induced fibroblast migration by controlling phospholipase D2-dependent phosphatidate generation. J Biol Chem 281: 38418–38429. [DOI] [PubMed] [Google Scholar]
- 62. Chen Q, Hongu T, Sato T, Zhang Y, Ali W, et al. (2012) Key roles for the lipid signaling enzyme phospholipase d1 in the tumor microenvironment during tumor angiogenesis and metastasis. Sci Signal 5: ra79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Berdyshev EV, Gorshkova I, Usatyuk P, Kalari S, Zhao Y, et al. (2011) Intracellular S1P generation is essential for S1P-induced motility of human lung endothelial cells: role of sphingosine kinase 1 and S1P lyase. PLoS One 6: e16571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Jenkins GM, Frohman MA (2005) Phospholipase D: a lipid centric review. Cell Mol Life Sci 62: 2305–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Freyberg Z, Sweeney D, Siddhanta A, Bourgoin S, Frohman M, et al. (2001) Intracellular localization of phospholipase D1 in mammalian cells. Mol Biol Cell 12: 943–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Du G, Huang P, Liang BT, Frohman MA (2004) Phospholipase D2 localizes to the plasma membrane and regulates angiotensin II receptor endocytosis. Mol Biol Cell 15: 1024–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Spiering D, Hodgson L (2011) Dynamics of the Rho-family small GTPases in actin regulation and motility. Cell Adh Migr 5: 170–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hirai T, Chida K (2003) Protein kinase Czeta (PKCzeta): activation mechanisms and cellular functions. J Biochem 133: 1–7. [DOI] [PubMed] [Google Scholar]
- 69. Williams MJ (2012) Rho GTPases central regulators of cell migration. Small GTPases 3: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Parri M, Chiarugi P (2010) Rac and Rho GTPases in cancer cell motility control. Cell Commun Signal 8: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ruiz-Loredo AY, Lopez E, Lopez-Colome AM (2011) Thrombin promotes actin stress fiber formation in RPE through Rho/ROCK-mediated MLC phosphorylation. J Cell Physiol 226: 414–423. [DOI] [PubMed] [Google Scholar]
- 72. Amiri A, Noei F, Feroz T, Lee JM (2007) Geldanamycin anisimycins activate Rho and stimulate Rho- and ROCK-dependent actin stress fiber formation. Mol Cancer Res 5: 933–942. [DOI] [PubMed] [Google Scholar]
- 73. Mahankali M, Peng HJ, Henkels KM, Dinauer MC, Gomez-Cambronero J (2011) Phospholipase D2 (PLD2) is a guanine nucleotide exchange factor (GEF) for the GTPase Rac2. Proc Natl Acad Sci U S A 108: 19617–19622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Jeon H, Kwak D, Noh J, Lee MN, Lee CS, et al. (2011) Phospholipase D2 induces stress fiber formation through mediating nucleotide exchange for RhoA. Cell Signal 23: 1320–1326. [DOI] [PubMed] [Google Scholar]
- 75. Jones DR, Sanjuan MA, Merida I (2000) Type Ialpha phosphatidylinositol 4-phosphate 5-kinase is a putative target for increased intracellular phosphatidic acid. FEBS Lett 476: 160–165. [DOI] [PubMed] [Google Scholar]
- 76. Jarquin-Pardo M, Fitzpatrick A, Galiano FJ, First EA, Davis JN (2007) Phosphatidic acid regulates the affinity of the murine phosphatidylinositol 4-phosphate 5-kinase-Ibeta for phosphatidylinositol-4-phosphate. J Cell Biochem 100: 112–128. [DOI] [PubMed] [Google Scholar]
- 77. Cockcroft S (2009) Phosphatidic acid regulation of phosphatidylinositol 4-phosphate 5-kinases. Biochim Biophys Acta 1791: 905–912. [DOI] [PubMed] [Google Scholar]
- 78. Hilpela P, Vartiainen MK, Lappalainen P (2004) Regulation of the actin cytoskeleton by PI(4,5)P2 and PI(3,4,5)P3. Curr Top Microbiol Immunol 282: 117–163. [DOI] [PubMed] [Google Scholar]
- 79. Mao YS, Yin HL (2007) Regulation of the actin cytoskeleton by phosphatidylinositol 4-phosphate 5 kinases. Pflugers Arch 455: 5–18. [DOI] [PubMed] [Google Scholar]
- 80. Cai L, Makhov AM, Schafer DA, Bear JE (2008) Coronin 1B antagonizes cortactin and remodels Arp2/3-containing actin branches in lamellipodia. Cell 134: 828–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Cai L, Holoweckyj N, Schaller MD, Bear JE (2005) Phosphorylation of coronin 1B by protein kinase C regulates interaction with Arp2/3 and cell motility. J Biol Chem 280: 31913–31923. [DOI] [PubMed] [Google Scholar]
- 82. Williams HC, San Martin A, Adamo CM, Seidel-Rogol B, Pounkova L, et al. (2012) Role of coronin 1B in PDGF-induced migration of vascular smooth muscle cells. Circ Res 111: 56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wegener J, Keese CR, Giaever I (2000) Electric cell-substrate impedance sensing (ECIS) as a noninvasive means to monitor the kinetics of cell spreading to artificial surfaces. Exp Cell Res 259: 158–166. [DOI] [PubMed] [Google Scholar]
- 84. Natarajan V, Scribner WM, Vepa S (1996) Regulation of phospholipase D by tyrosine kinases. Chem Phys Lipids 80: 103–116. [DOI] [PubMed] [Google Scholar]