Abstract
Objective
To determine the effect of physical activity on knee osteoarthritis (OA) development in persons without knee injury and according to knee alignment
Design
We combined data from MOST and OAI, studies of persons with or at high risk of OA. Subjects had long limb and repeated posteroanterior knee radiographs and completed the physical activity survey for the elderly (PASE). We studied persons without radiographic OA and excluded knees with major injury and without long limb films. We followed subjects 30 months (in MOST) and 48 months (in OAI) for one of two incident outcomes: 1. symptomatic tibiofemoral OA (radiographic OA and knee pain), or 2. tibiofemoral narrowing. ‘Active’ persons were those with PASE score in the highest quartile by gender. We examined risk of OA in active group using logistic regression adjusting for age, gender, BMI, WOMAC pain score, Kellgren and Lawrence grade (0 or 1), and study of origin. We also analyzed knees from malaligned and neutrally aligned limbs.
Results
The combined sample comprised 2073 subjects (3542 knees) with mean age 61 years. The cumulative incidence of symptomatic tibiofemoral OA was 1.12% in the active group vs. 1.82% in the others (OR among active group 0.6, 95% CI 0.3, 1.3). Joint space narrowing occurred in 3.41% of knees in the active group vs. 4.04% in the others (OR among active group 0.9 (95% CI 0.5, 1.5)). Results did not differ by alignment status.
Conclusions
Physical activity in the highest quartile did not affect the risk of developing OA.
Keywords: physical activity, knee osteoarthritis, alignment, radiography
INTRODUCTION
The relation of physical activity to the development of knee osteoarthritis (OA) is an important clinical and public health issue. Persons interested in preventing knee OA want to know if physical activity either puts them at risk or protects them from disease and health agencies worried about the epidemic of knee OA look toward increased physical activity as a possible approach to prevent disease. Lastly those with early disease may seek out physical activity regimens in the hope that activity would prevent them from developing more advanced and more frequently, symptomatic disease.
Unfortunately, literature examining the relationship of physical activity to OA is conflicting at best. For example, some studies suggest that those who are most active are at increased risk of developing knee OA1,2,3. Others show the opposite effect: that those who are most active are at a significantly decreased risk of developing OA4,5,6. Lastly, there are studies that show no significant association between physical activity and the development of knee OA7,8,9.
How do we make sense of these conflicting studies and arrive at a valid estimate of the risk posed by physical activity? A meta-analysis could evaluate the net effect of all these studies but there is such heterogeneity in the results of these studies that a meta-analysis might not provide insight.
Biases could account for some of the findings. For example, persons with early painful disease, who are therefore predisposed to later/progressive disease, may limit their activity, making it falsely appear that the lower activity level predisposed them to develop/progress OA when it was in fact the existence of early disease. Secondly, it is well known that major knee injury predisposes to later knee OA and Sutton et al.8 have suggested that sports activity is associated with later OA only because of its association with major knee injury. Thus, failing to account for major knee injury may reveal a spurious association of activity with knee OA.
Particular study design biases may also contribute to our failure to reveal the underlying association of physical activity with OA. In recent work, we have described how collider bias10 has limited the ability to detect risk factors for progressive disease. For example, in large scale studies, obesity has increased the risk of incident knee OA but not of progressive disease11. Any studies of physical activity and its relation to progression of disease would be hampered by the presence of collider bias which would make it difficult to detect any effects of physical activity on disease especially if those in the study already had established disease.
Lastly, malalignment is a major risk factor for both incident12 and progressive knee OA13. Malalignment may increase the focal load conferred by activity so that, in the context of malalignment, any activity may be more likely to be injurious. Thus, the relation of physical activity to knee OA incidence may be complicated by whether the knee joint that is experiencing increased loads from physical activity is malaligned.
Thus, there are many potential biases and study design concerns, any of which could threaten the validity of any detected association between physical activity and OA. To best reveal the relationship between physical activity levels and the development of OA, a study should adjust for the effects of knee pain and exclude those with a history of substantial knee injury, a major risk factor for OA. Because all knees with prevalent disease have risk factors for disease, evaluating risk factors for progression among OA knees is challenging because one is evaluating one risk factor for progression among knees all of which have risk factors for progression, so called collider bias10. To avoid collider bias a study should focus on the development of early disease. To examine effects of physical activity, it would be better to look both at structural outcomes (radiographic disease) and symptomatic outcomes as the effects of physical activity on these outcomes may be different.
The Osteoarthritis Initiative (OAI) and Multicenter Osteoarthritis (MOST) studies together offer a unique opportunity to carry out these focused analyses. They are both very large cohort studies of persons at high risk of knee OA. Both studies are large enough that limiting analyses to subjects most likely to provide valid information on physical activity effects still leaves enough subjects at risk of OA that the effect of activity on disease incidence can be assessed. Furthermore, both used the same tools to evaluate disease and to assess physical activity using a well-validated, widely used activity questionnaire. Lastly, both have similarly assessed alignment information that permits an evaluation of whether the effects of physical activity differ by alignment status.
We examined the relationship of physical activity to knee OA using data from both these studies.
METHODS
Data was drawn from two cohort studies, MOST and OAI.
MOST Study
The MOST cohort includes persons with or at high risk of knee OA recruited from the communities of Birmingham, Alabama and Iowa City, Iowa. The goal of the study was to evaluate risk factors for incidence and progression of knee OA. 3,026 subjects aged 50–79 at baseline were recruited and studied at baseline and 30 months. At each visit, weight and height were measured and PA and lateral weight bearing radiographs obtained. Long limb radiographs were acquired in all MOST subjects at the baseline visit as described elsewhere12. Mechanical alignment (also known as HKA) was measured to the nearest 0.1°on these x-rays with high inter-reader reproducibility (ICC = 0.98) by readers trained by Dr. Derek Cooke14. The Physical Activity Scale for the Elderly (PASE)15, a well validated survey, was administered to all subjects at baseline. PASE comprises measures of self-reported occupational, household, and leisure activities during a one-week period.
Osteoarthritis Initiative (OAI)
The OAI is a longitudinal cohort study of risk factors for incidence and progression of OA. 4796 subjects with or at high risk of knee OA were recruited from four sites, Columbus, Ohio, Providence, Rhode Island, Baltimore, Maryland and Pittsburgh, Pennsylvania. Eligibility for OAI was similar to that of MOST with a few exceptions: in OAI, the risk factors permitting eligibility to the study were broader and the age range extended to as young as age 45. Assessments were similar to those in MOST except that they were done annually during four years of follow-up. The other relevant difference between OAI and MOST is that in OAI, long limb radiographs using the same protocol as in MOST, were acquired at the 12 month visit in most subjects, but if time did not permit, these x-rays were acquired for some but not all subjects at later visits. In OAI, knee radiographs were read and adjudicated by the same team as in MOST using the same protocol. The same rule for designating the presence of radiographic OA was used. Also, long limb x-rays were measured using the same protocols and personnel as in MOST.
Definition of Variables
For examination of both MOST and OAI data and based on past studies examining malalignment, we defined malalignment as mechanical axis of 2 degrees or more in either varus or valgus direction on a long limb x-ray. Neutral alignment was defined as anything less than 2 degrees varus or valgus.
In MOST and OAI, subjects obtained posteroanterior weight bearing knee radiographs using a Synaflexer frame (Synarc, San Francisco, CA) to create a fixed standardized knee position. This protocol has been shown to provide reproducible estimates of joint space and to provide consistency in terms of the image of the knee over time16,17. X-ray readings for both studies were carried out centrally at Boston University by a team of three readers (PA, BS, DTF). For each subject, all of their x-rays were read together. Each of two readers (PA, BS) read all x-rays from all subjects. If there was a disagreement as to whether the knee at any time point had radiographic OA (Kellgren & Lawrence Grade 2 or greater) or if between time points, there was disagreement as to whether there was a worsening of disease (defined either as an increase in Kellgren and Lawrence grade or as an increase in joint space narrowing grade), the reading was adjudicated by a panel of three experienced readers including the two who read the films and one other (DTF). A consensus reading was arrived at when at least two of three readers agreed. Because of the large change required in joint space width to progress a whole integer in score (e.g. from OARSI grade 0–1, 1–2 or 2–3), we created a partial grade narrowing scoring system that allowed us to characterize change in joint space width when that change was clear cut but did not reach an integer change threshold (for details, see18). For example, if a baseline knee had a medial joint space score of 1 and medial narrowing had clearly progressed in a subsequent image but the subsequent narrowing did not reach the threshold for grade 2 narrowing according to the OARSI Atlas19, then we gave that subsequent knee a partial grade (e.g., 1.5) between 1 and 2. In previous work18 we have validated these partial grades by showing that they corresponded to risk factors for progression, such as malalignment, or measures of worsening, such as cartilage damage. We defined medial or lateral progression on the x-ray as present when there was at least a partial grade change in its joint space from the knee x-ray acquired at the time of the long limb x-ray to the later knee x-ray. Agreement was high when the same knee films were sent repeatedly by the OAI coordinating center (for medial joint space grade, weighted kappa = 0.75, p<.0001 and for lateral grade, weighted kappa = 0.86, p <.0001).
Knees Eligible for this Study
All subjects in both studies were asked about “any history of knee injury sufficient to limit your ability to walk for at least 2 days.” Because of the likelihood that previously injured knees would be at high risk of OA with activity, we removed knees that were reported as having sustained a prior injury. To reduce the possibility of collider bias, we excluded knees whose radiographs showed OA at baseline (Kellgren and Lawrence grade 2 or greater). Those with TKR’s at baseline or during follow-up were also removed as they could not reach our endpoints. Because of our interest in examining whether the effect of physical activity differed across strata of malalignment, we limited analyses to subjects who had acquired long limb films as part of their participation in MOST and OAI.
Analysis Approach
Since our goal was to avoid analyzing the decrease in physical activity which may be a consequence of knee pain, and yet we wanted to evaluate effects of physical activity even in those with some knee pain, we adjusted for the severity of knee pain in our analyses by adding the knee specific WOMAC pain score as a covariate. Additional analyses in which we excluded all those with non-zero WOMAC scores yielded the same findings, albeit with fewer outcomes and wider confidence limits.
We tested two outcomes, one a structural outcome and the other a symptom-based one. For the structural outcome, we used any increase (narrowing) of the knee joint on the x-ray in either medial or lateral joint using the semiquantitative central readings. For the symptom outcome, we used the new onset of symptomatic knee OA defined as the new combination of frequent knee pain and radiographic OA (Kellgren and Lawrence grade >=2) in knees that were Kellgren and Lawrence grade 0 or 1 at baseline. We defined the baseline for this study as the exam at which long limb films were acquired (at baseline for MOST, for the majority of subjects, at 12 months for OAI but varied). We then followed subjects for OA outcomes—for 30 months for MOST and 48 months for OAI.
To examine the relation of PASE score with OA outcomes, we used logistic regression analyses in which other independent variables were age, sex, BMI, WOMAC pain score, baseline Kellgren and Lawrence (KL) grade (0 or 1) and in analyses that combined data from both studies, study of origin (MOST or OAI). We carried out analyses of PASE scores using sex specific quartiles. To adjust for the correlation between knees, we used generalized estimating equations.
RESULTS
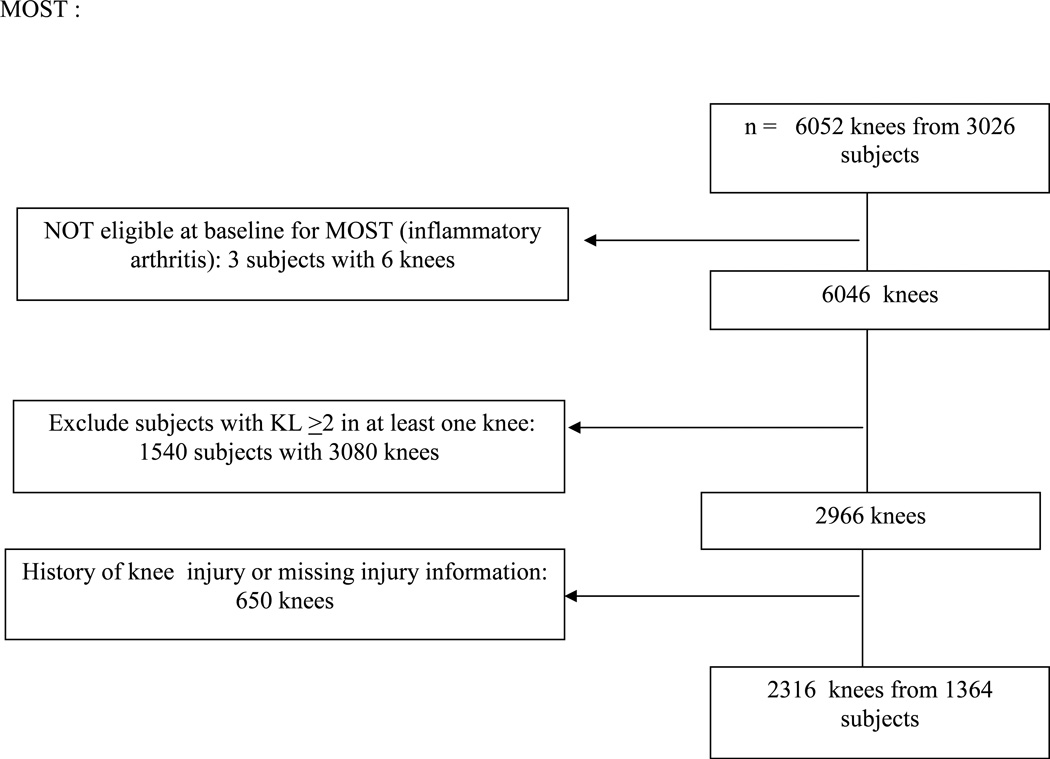
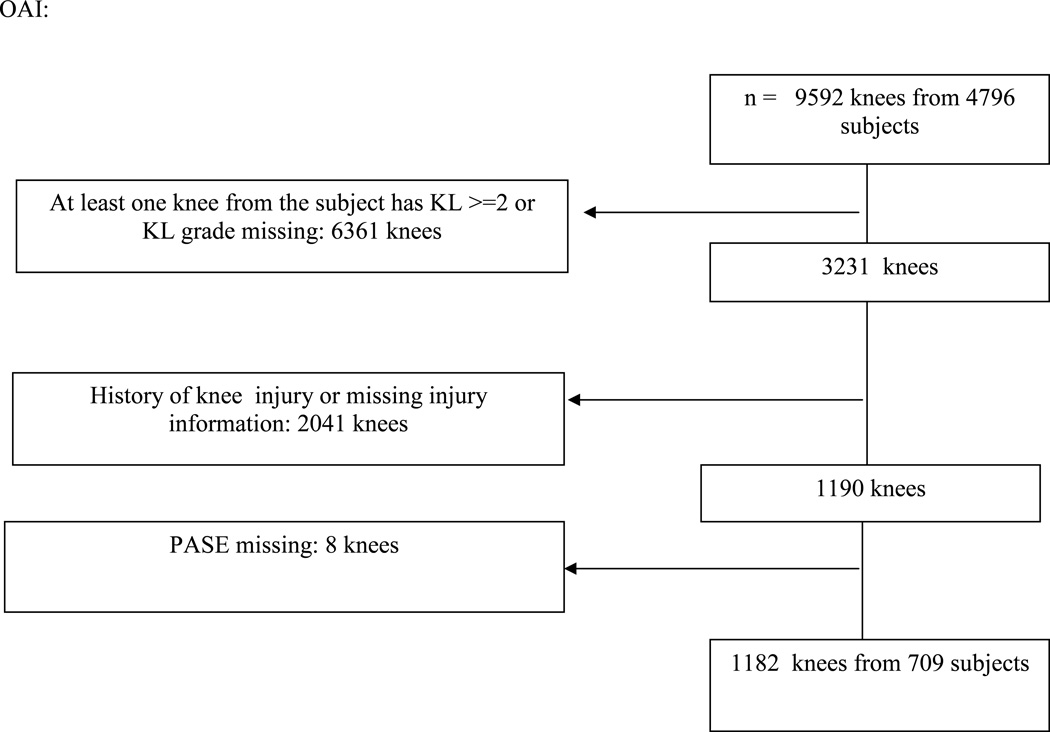
In the MOST study, 1364 subjects with 2360 knees met our inclusion criteria and in the Osteoarthritis Initiative (OAI), 709 subjects (1182 knees) did so (see figure 1). All subjects did not contribute two knees since some knees had a history of major knee injury. The mean age in both groups was a little over 61 years and most of the subjects were female (see table 1). The mean body mass index was slightly higher in MOST than in OAI (mean 29.3 for the subjects in MOST in this analysis vs. 27.4 for OAI subjects). Most of the knees to be tracked started out with Kellgren and Lawrence grade 0 disease. Roughly half of these knees were not painful at all at baseline in that they had WOMAC scores of 0. A little less than 50% were neutrally aligned. When we examined the PASE scores of the people whose knees were involved in this study, we found that the highest quartile scores tended to be lower on average in women (>208.1) than in men (>248), and we took the highest quartile on a sex-specific basis for our subsequent analyses.
Figure 1.
Subjects in this analysis
Table 1.
Description of sample from MOST and OAI in this study at the time of the long limb examinations (knees with KL 2 or greater or with a history of injury were excluded)
| MOST and OAI (N=2073 subjects, 3498 knees)* |
MOST (1364 subjects, 2316 knees) |
OAI 709 subjects, 1182 knees) |
|
|---|---|---|---|
| Age at baseline ± s.d. | 61.2 (8.4) | 61.0 (7.9) | 61.7 (9.4) |
| Sex (% female) | 1206 (58.2) | 810 (59.4) | 396 (55.9) |
| BMI (mean ± s.d.) | 28.7 (4.9) | 29.3 (5.0) | 27.4 (4.6) |
| Percentage of knees with KL 0 | 2620 (74.9) | 1791 (77.3) | 829 (70.1) |
| with KL 1 | 878 (25.1) | 525 (22.7) | 353 (29.9) |
| Percentage of knees with all WOMAC pain with score of 0 | 1693 (48.8) | 1013 (44.3) | 680 (57.9) |
| Percentage of limbs with neutral alignment | 1565 (45.1) | 980 (42.6) | 585 (50.0) |
The proportion of those in the highest quartile of physical activity and their likelihood of developing incident tibiofemoral symptomatic knee osteoarthritis is shown in table 2. Only 1.8% of those in the lowest three quartiles developed this outcome vs. 1.12% in those in the upper quartile. This translated into a slight protective effect of high physical activity (adjusted odds ratio, 0.6), a finding which did not reach significance (p=.18). When we examined joint space loss, defined as an increase in semiquantitative measurement of joint space over time (see table 3), we found that the proportion of knees with joint space loss in the lowest 3 quartiles of the PASE score (the least active 75%) was 4.04% of knees. This compared to 3.41% of knees in the persons that were most active. This translated into an adjusted odds ratio for joint space loss of 0.9 (95% CI, 0.5, 1.5).
Table 2.
The relation of high PASE score with the development of incident tibiofemoral symptomatic knee OA in MOST and OAI knees
| Sex-specific PASE quartile |
N of knees |
N (%) of Increase of incident TF ROA |
Crude OR | Adjusted OR* | p-value |
|---|---|---|---|---|---|
| Men and Women | |||||
| Lower 75% | 2435 | 44(1.8) | 1.0 | 1.0 | |
| High (upper 25%) | 807 | 9(1.1) | 0.6(0.3,1.4) | 0.6(0.3,1.3) | 0.18 |
adjusting for age, sex, BMI, WOMAC pain and KL grade and study of origin.
Table 3.
The relation of high PASE score with joint space loss in MOST and OAI
| Sex-specific PASE quartile |
N of knees |
N (%) of Increase of JSN |
Crude OR | Adjusted OR* |
p- value |
|---|---|---|---|---|---|
| Men and Women | |||||
| Lower 75% | 2374 | 96 (4.0) | 1.0 | 1.0 | |
| High (upper 25%) | 792 | 27 (3.4) | 0.8 (0.5, 1.3) | 0.9 (0.5, 1.5) | 0.71 |
adjusting for age, sex, BMI, WOMAC pain and KL grade and study of origin.
Additional analyses in which we examined the incidence of OA outcomes across all PASE quartiles showed no relation of PASE scores with any of these outcomes either by sex or combining genders (data not shown).
We then examined whether the effect of physical activity was different in those whose knees showed malalignment vs. those without malalignment. After examining data to confirm that the effects in varus and valgus knees were similar, we combined the data on knees that were malaligned in either direction. Among malaligned knees, we found that rates of incident tibiofemoral symptomatic OA (see Table 4) were similar in those with high levels of physical activity vs. those in the 75% most sedentary group. Specifically, 1.97% of knees in the more sedentary group developed incident symptomatic knee osteoarthritis vs. 1.61% of knees in the more active group (adjusted odds ratio, 0.9; 95% confidence interval 0.3 2.4). When we looked at those in the neutrally aligned group, the more sedentary group had a cumulative incidence rate of 1.64% vs. 0.55% in the active group, which translated into an adjusted odds ratio of 0.3 suggesting a possible protective effect of activity in those with neutral alignment. The confidence bounds however were wide (0.1, 1.2) in part because of the small number of incident cases in neutrally aligned knees, and results did not reach statistical significance (p=.09). Among those with malalignment, high levels of physical activity did not confer any increased or decreased risk of worsening joint space loss compared to lower activity levels (see Table 4). Specifically, the adjusted odds ratio for high levels of physical activity was 0.9 (95% CI 0.5, 1.8). Similarly for men and women whose limbs were neutrally aligned, physical activity conferred neither an increase nor a decrease in risk of joint space loss (adjusted odds ratio, 1.0; 95% CI 0.4, 2.3).
Table 4.
The relation of high PASE score with knee outcomes by whether the limb was malaligned
| Sex-specific PASE quartile |
N of knees |
N (%) of Incident TF SxOA |
Crude OR | Adjusted OR* | p-value |
|---|---|---|---|---|---|
| INCIDENT TIBIOFEMORAL SYMPTOMATIC KNEE OA | |||||
| Men and Women, varus <=−2.0 or valgus >=2.0 degree | |||||
| Lower 75% | 1323 | 26 (2.0) | 1.0 | 1.0 | |
| High (upper 25%) | 434 | 7 (1.6) | 0.8 (0.3,2.1) | 0.9 (0.3,2.4) | 0.83 |
| Men and Women, Neutral −1.99 to 1.99 degree | |||||
| Lower 75% | 1096 | 18 (1.6) | 1.0 | 1.0 | |
| High (upper 25%) | 363 | 2 (0.5) | 0.3 (0.1, 1.4) | 0.3 (0.1, 1.2) | 0.09 |
| JOINT SPACE LOSS | |||||
| Men and Women, varus <=−2.0 or valgus >=2.0 degree | |||||
| Lower 75% | 1296 | 59 (4.5) | 1.0 | 1.0 | |
| High (upper 25%) | 427 | 15 (3.5) | 0.8 (0.4, 1.4) | 0.9 (0.5, 1.8) | 0.84 |
| Men and Women, Neutral −1.99 to 1.99 degree | |||||
| Lower 75% | 1062 | 36 (3.4) | 1.0 | 1.0 | |
| High (upper 25%) | 355 | 12 (3.3) | 1 (0.5, 2) | 1 (0.4, 2.3) | 0.99 |
adjusting for age, sex, BMI, WOMAC pain and KL grade and study of origin.
DISCUSSION
In two large studies with longitudinal follow-up of persons at high risk of OA, we did not find any association between high levels of physical activity and the development of symptomatic OA, nor was physical activity associated with worsening of the radiograph in early disease as evidenced by joint space loss. Our intention in this set of analyses was to circumvent biases from previous studies which might have made it impossible for previous investigators to detect an association of physical activity with OA. We combined data from two of the largest cohorts ever studied at risk of OA, both of them with careful and standardized follow-up and failed to find any suggestive relationship with the development of OA.
It is unclear why our findings differ from those of other studies, although a number of studies (cited above) also have reported no association of physical activity with OA. A recent magnetic resonance imaging (MRI) -based study suggested that physical activity as assessed by accelerometry was associated with an increased risk of OA in initially unaffected persons20, but a similar study done by members of the same investigative group and reported several years earlier reported opposite findings, that physical activity prevented MRI findings of OA21.
One explanation for these conflicting studies may be that different types of physical activities pose different risks of knee injury or of protection against disease. For example, in one of the studies in which there was a reported association between high levels of physical activity and OA2, the physical activity being studied was running and those found to have a high risk of OA ran more than 20 miles per week. Certainly this is well beyond the level of physical activity carried out by most of our subjects. In one of the studies suggesting that physical activity protected against the development of severe knee OA4, only recreational activity was assessed and it was evaluated over the lifetime rather than just at one time point. In general, that exercise did not consist of running so that it might have been less than was studied by Cheng et al. above. Other reasons for disparate findings include the use of different OA outcomes; some studies used self-reported OA, others used knee replacement and yet others used MRI or radiographic outcomes and it is possible that the effects of physical activity differ by outcome studied.
It is also conceivable that survey instruments may inaccurately reflect the actual physical activity carried out. Indeed, a recent study suggests that accelerometry may more accurately reflect daily activities than do survey instruments, although surveys are better at getting at longer term activity levels and are the only way of assessing relatively uncommon activities and their effects on disease. We suspect that the main reason for the great difference between studies is because of the different types and intensities of physical activity that have been evaluated in these studies and because of the time frame of physical activity during a lifespan.
We studied the upper quartile of physical activity among our subjects, and PASE scores in our cohorts corresponded roughly to those previously published for those in the age range of interest (50’s – 70’s). The median scores on PASE for women in our highest quartile were roughly 250 and roughly 300 for men. Scoring the PASE is complicated but a person working 40 hours a week in a job involving sitting or standing with some walking, who also walks outside the home 1–2 hours a day occasionally, who may seldom golf and has done light housework or lawn work in the past 7 days would achieve a score in this high range15. The most common combined activities accounting for high PASE scores tend to involve walking, lawn work or yard care, light or heavy housework, and a job involving standing or walking. In the age range of subjects we studied, high scores are unlikely to be related to extensive sports participation.
There are a number of important limitations to our study. First, in the future such studies may need to be done using accelerometry to provide accurate assessments of at least short-term activity. Second, it may be easier to detect effects on knee structure by carrying out MRIs although doing so in a large number of subjects is obviously expensive. Third, our follow-up may not be long enough to detect effects of activity on disease. Fourth, our results may not generalize to persons who are different from those in OAI and MOST. For example, physical activity may have different and measurable effects on OA in those in the general population not at high risk of OA. Lastly, even though we studied two very large cohorts with lots of persons at risk, we were ultimately limited by small numbers of incident events, especially for symptomatic OA, and this constrained our ability to say anything definitive about effects of physical activity on this particular outcome. We did not have enough cases to examine effects of physical activity in limbs with moderate to severe malalignment, although we note that this degree of malalignment is distinctly uncommon in persons without knee OA, as it usually develops as a consequence of OA. For the structural outcomes, there were a sufficient number of events to say that physical activity had little if any effect.
In summary, in a combined sample of two very large cohorts at high risk of OA, we were unable to find any relation of high levels of community-based physical activity with the development of OA either by radiograph or by symptoms.
Acknowledgements
We are grateful to the participants and staff of both the MOST and OAI studies and to Anne Plunkett for technical assistance.
Supported by NIH Grants: U01-AG18820, U01 AG18832; U01 AG18947; U01 AG19069, P60 AR47785, R01-AR051568 and R01 HD43500. The OAI is a public-private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Merck Research Laboratories; Novartis Pharmaceuticals Corporation, GlaxoSmithKline; and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health.
Role of the funding source: the funding sources played no role in data analysis or write up of this paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributions
All persons designated as authors should qualify for authorship, and all those who qualify should be listed as authors.
Each author should have participated sufficiently in the work to take public responsibility for appropriate parts of the content.
- the conception and design of the study, or acquisition of data, or analysis and interpretation of data
- drafting the article or revising it critically for important intellectual content
- final approval of the version to be submitted
Each manuscript should be accompanied by a declaration of each author's contributions relating to sections (1), (2) and (3) above. This declaration should also name one or more authors (including email addresses) who take responsibility for the integrity of the work as a whole, from inception to finished article. These declarations will be included in the published manuscript.
- Conception and design: DTF,
- Analysis and interpretation of the data: JN, TY
- Drafting of the article: DTF
- Critical revision of the article for important intellectual content: LS, MCN
- Final approval of the article: JT, CEL, PA, BS, AG, JG
- Provision of study materials or patients: JL, CEL
- Statistical expertise: JN, TY
- Obtaining of funding: DTF
- Administrative, technical, or logistic support: JG, JL, CEL
- Collection and assembly of data: PA, BS, AG
Competing interests
At the end of the manuscript text, under a subheading "Competing interest statement" every author must disclose any financial and personal relationships with other people or organizations that could potentially and inappropriately influence (bias) their work and conclusions. Examples of potential conflicts of interest include employment, consultancies, stock ownership, honoraria, paid expert testimony, patent applications/registrations, and research grants or other funding.
The existence of competing interests is common and often inevitable. Competing interests are not inherently unethical, but not declaring them is.
Contributor Information
David T. Felson, Email: dfelson@bu.edu.
Jingbo Niu, Email: niujp@bu.edu.
Tianzhong Yang, Email: tianzhongwin@gmail.com.
James Torner, Email: james-torner@uiowa.edu.
C. Elizabeth Lewis, Email: clewis@dopm.uab.edu.
Piran Aliabadi, Email: paliabadi@partners.org.
Burton Sack, Email: bsack@bu.edu.
Leena Sharma, Email: L-Sharma@northwestern.edu.
Ali Guermazi, Email: guermazi@bu.edu.
Joyce Goggins, Email: jgoggins@bu.edu.
Michael C. Nevitt, Email: MNevitt@psg.ucsf.edu.
References
- 1.McAlindon TE, Wilson PW, Aliabadi P, Weissman B, Felson DT. Level of physical activity and the risk of radiographic and symptomatic knee osteoarthritis in the elderly: the Framingham study. American Journal of Medicine. 1999;106:151–157. doi: 10.1016/s0002-9343(98)00413-6. [DOI] [PubMed] [Google Scholar]
- 2.Cheng Y, Macera CA, Davis DR, Ainsworth BE, Troped PJ, Blair SN. Physical activity and self-reported, physician-diagnosed osteoarthritis: is physical activity a risk factor? Journal of Clinical Epidemiology. 2000;53:315–322. doi: 10.1016/s0895-4356(99)00168-7. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Simpson JA, Wluka AE, Teichtahl AJ, English DR, Giles GG, et al. Is physical activity a risk factor for primary knee or hip replacement due to osteoarthritis? A prospective cohort study. Journal of Rheumatology. 2011;38:350–357. doi: 10.3899/jrheum.091138. [DOI] [PubMed] [Google Scholar]
- 4.Manninen P, Riihimaki H, Heliovaara M, Suomalainen O. Physical exercise and risk of severe knee osteoarthritis requiring arthroplasty. Rheumatology (Oxford) 2001;40:432–437. doi: 10.1093/rheumatology/40.4.432. [DOI] [PubMed] [Google Scholar]
- 5.White JA, Wright V, Hudson AM. Relationships between habitual physical activity and osteoarthrosis in ageing women. Public Health. 1993;107:459–470. doi: 10.1016/s0033-3506(05)80172-6. [DOI] [PubMed] [Google Scholar]
- 6.Rogers LQ, Macera CA, Hootman JM, Ainsworth BE, Blairi SN. The association between joint stress from physical activity and self-reported osteoarthritis: an analysis of the Cooper Clinic data. Osteoarthritis and Cartilage. 2002;10:617–622. doi: 10.1053/joca.2002.0802. [DOI] [PubMed] [Google Scholar]
- 7.Felson DT, Niu J, Clancy M, Sack B, Aliabadi P, Zhang Y. Effect of recreational physical activities on the development of knee osteoarthritis in older adults of different weights: the Framingham Study. Arthritis and Rheumatism. 2007;57:6–12. doi: 10.1002/art.22464. [DOI] [PubMed] [Google Scholar]
- 8.Sutton AJ, Muir KR, Mockett S, Fentem P. A case-control study to investigate the relation between low and moderate levels of physical activity and osteoarthritis of the knee using data collected as part of the Allied Dunbar National Fitness Survey. Annals of the Rheumatic Diseases. 2001;60:756–764. doi: 10.1136/ard.60.8.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hootman JM, Macera CA, Helmick CG, Blair SN. Influence of physical activity-related joint stress on the risk of self-reported hip/knee osteoarthritis: a new method to quantify physical activity. Preventive Medicine. 2003;36:636–644. doi: 10.1016/s0091-7435(03)00018-5. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Niu J, Felson DT, Choi HK, Nevitt M, Neogi T. Methodologic challenges in studying risk factors for progression of knee osteoarthritis. Arthritis Care Res (Hoboken) 2010;62:1527–1532. doi: 10.1002/acr.20287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niu J, Zhang YQ, Torner J, Nevitt M, Lewis CE, Aliabadi P, et al. Is obesity a risk factor for progressive radiographic knee osteoarthritis? Arthritis Rheum. 2009;61:329–335. doi: 10.1002/art.24337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma L, Song J, Dunlop D, Felson D, Lewis CE, Segal N, et al. Varus and valgus alignment and incident and progressive knee osteoarthritis. Annals of the Rheumatic Diseases. 2010;69:1940–1945. doi: 10.1136/ard.2010.129742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma L, Song J, Felson DT, Cahue S, Shamiyeh E, Dunlop DD. The role of knee alignment in disease progression and functional decline in knee osteoarthritis. JAMA. 2001;286:188–195. doi: 10.1001/jama.286.2.188. [DOI] [PubMed] [Google Scholar]
- 14.Sled EA, Sheehy LM, Felson DT, Costigan PA, Lam M, Cooke TD. Reliability of lower limb alignment measures using an established landmark-based method with a customized computer software program. Rheumatol Int. 2011;31:71–77. doi: 10.1007/s00296-009-1236-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. Journal of Clinical Epidemiology. 1993;46:153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 16.Nevitt MC, Peterfy C, Guermazi A, Felson DT, Duryea J, Woodworth T, et al. Longitudinal performance evaluation and validation of fixed-flexion radiography of the knee for detection of joint space loss. Arthritis and Rheumatism. 2007;56:1512–1520. doi: 10.1002/art.22557. [DOI] [PubMed] [Google Scholar]
- 17.Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12:177–190. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Felson DT, Nevitt MC, Yang M, Clancy M, Niu J, Torner J, et al. A new approach yields high rates of radiographic progression in knee osteoarthritis. J Rheumatol. 2008;35:2047–2054. [PMC free article] [PubMed] [Google Scholar]
- 19.Altman RD, Gold GE. Atlas of individual radiographic features in osteoarthritis, revised. Osteoarthritis Cartilage. 2007;15(Suppl A):A1–A56. doi: 10.1016/j.joca.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 20.Dore DA, Winzenberg TM, Ding C, Otahal P, Pelletier JP, Martel-Pelletier J, et al. The association between objectively measured physical activity and knee structural change using MRI. Annals of the Rheumatic Diseases. 2012 doi: 10.1136/annrheumdis-2012-201691. [DOI] [PubMed] [Google Scholar]
- 21.Racunica TL, Teichtahl AJ, Wang Y, Wluka AE, English DR, Giles GG, et al. Effect of physical activity on articular knee joint structures in community-based adults. Arthritis and Rheumatism. 2007;57:1261–1268. doi: 10.1002/art.22990. [DOI] [PubMed] [Google Scholar]