Abstract
The neuropeptide galanin has been shown to alter the rewarding properties of morphine. To identify potential cellular mechanisms that might be involved in the ability of galanin to modulate opiate reward, we measured excitatory post-synaptic potentials (EPSPs) using both field and whole-cell recordings from striatal brain slices extracted from wild type mice and mice lacking specific galanin receptor (GalR) subtypes. We found that galanin decreases the amplitude of EPSPs in both the dorsal striatum and nucleus accumbens. We then performed recordings in slices from knockout mice lacking either the GalR1 or GalR2 gene and found that the ability of galanin to decrease EPSP amplitude was absent from both mouse lines, suggesting that both receptor subtypes are required for this effect. In order to determine whether behavioral responses to opiates were dependent on the same receptor subtypes, we tested GalR1 and GalR2 mice for morphine conditioned place preference (CPP). Morphine CPP was significantly attenuated in both GalR1 and GalR2 knockout mice. These data suggest that mesolimbic excitatory signaling is significantly modulated by galanin in a GalR1- and GalR2-dependent manner and that morphine CPP is dependent on the same receptor subtypes.
Keywords: Morphine, conditioned place preference, galanin, mice, nucleus accumbens, patch clamp, extracellular recording, addiction
Introduction
Opioids are extremely effective analgesics and are the most widely prescribed class of drugs in the United States (Kuehn, 2007). Unfortunately, the actions of these drugs on the brain reward circuitry (Matthews and German, 1984; Shippenberg and Elmer, 1998) confers a substantial risk for abuse (Compton and Volkow, 2006). It is therefore critical to understand the endogenous mechanisms that modulate morphine reward in order to identify novel pathways to combat the development of opiate addiction.
The neuropeptide galanin interacts with morphine in several behavioral paradigms. Galanin and morphine can both alleviate pain (Wiesenfeld-Hallin et al., 1990; Hobson et al., 2006; Hulse et al., 2011), but in combination, produce significantly greater antinociception than would be explained by an additive effect (Wiesenfeld-Hallin et al., 1990; Przewłocka et al., 1995; Zhang et al., 2000; Hua et al., 2004; Sun and Yu, 2005). In contrast, galanin can interfere with the rewarding properties of morphine. For example, in a conditioned place preference (CPP) paradigm, intracerebroventricular infusion of galanin decreased CPP to a threshold dose (Zachariou et al., 1999). Similarly, mice lacking the galanin peptide showed morphine CPP at a lower dose than their wild-type littermates (Hawes et al., 2008), indicating a potential role for galanin in morphine reward processing.
Morphine CPP requires activity of the mesocorticolimbic system (Koob, 1992; Carlezon and Wise, 1996). Both the dorsal striatum (DS) and nucleus accumbens (NAc) are critical for the integration of dopaminergic signaling of the midbrain with glutamatergic inputs from the prefrontal cortex, hippocampus, and amygdala (Koob, 1992; Chao and Nestler, 2004; Carlezon and Thomas, 2009; Stuber et al., 2012). The medium spiny neuron (MSN) outputs from these areas influence drug taking and habit formation (Koob and Volkow, 2010). Galanin signals through three receptor subtypes, GalR1, GalR2 and GalR3, all of which can couple to Gi signaling cascades (Smith et al., 1997, 1998; Branchek et al., 2000; Lang et al., 2007). So far, inhibitory effects of galanin have been characterized in the dorsal raphe, locus coeruleus, hypothalamus, and hippocampus (Xu et al., 2005; Picciotto et al., 2010). Despite reports that galanin can alter dopamine dynamics (Tsuda et al., 1998; Ericson and Ahlenius, 1999), a direct effect of galanin on excitability of neurons in the mesocorticolimbic system has never been shown.
In order to identify physiological mechanisms that could underlie the interplay between galanin signaling and morphine reward, we recorded excitatory post-synaptic field potentials (fEPSPs) in the DS and in the NAc before and after bath application of galanin. We also recorded the effect of galanin on EPSPs from individual MSNs in GalR1 and GalR2 knockout (KO) mice and their wild type (WT) siblings. Finally, to determine whether the same GalR subtypes were involved in effects of galanin on mesolimbic physiology and morphine reward, we tested GalR1 or GalR2 KO mice and their WT siblings in a morphine CPP paradigm. These experiments revealed a novel interaction between the morphine and galanin systems that provides a mechanistic basis for the role of individual GalR subtypes in modulation of the mesolimbic system.
Materials and Methods
Animals
All mice were housed under standard laboratory conditions (21±2°C, lights on 7am–7pm), in Sealsafe Plus individually ventilated cages (Tecniplast, Buguggiate, Italy) and given ad libitum access to chow (Harlan Teklad #2018) and water. For electrophysiological recordings in wild type mice, C57BL/6J litters were obtained from Jackson Laboratories (Bar Harbor, ME, USA) and housed together with the dam until weaning at P28. Mice with an inactivating mutation in exon 1 of the GalR1 gene were generated as described previously (Tsuda et al., 1998; Ericson and Ahlenius, 1999) and backcrossed onto a C57Bl/6J background for at least 10 generations. Mice with an early termination mutation in the intron of the GalR2 gene were generated as described previously (Hobson et al., 2006) and backcrossed onto a C57Bl/6J background for at least 10 generations. Heterozygous mating pairs of GalR1 +/− or GalR2 +/− mice were used to generate GalR1WT and GalR1KO or GalR2WT and GalR2KO littermates, respectively, for behavioral testing. In some physiology experiments, GalR1 or GalR2 KO mice were generated from homozygous breeding pairs. Adult mice were housed together (2–5 per cage) and habituated to the colony for at least one week before behavioral testing. Both male and female mice were tested in behavioral studies. Male mice were used for field recording studies in wild type mice. In patch clamp studies, recordings were performed in both slices from male and female WT, GalR1KO and GalR2KO mice. There was no significant difference between males and females in these recordings, so the data from both sexes were combined for statistical analysis. All animal procedures were approved by the Yale Animal Care and Use Committee and conducted in compliance with the Guidelines laid down by the National Institutes of Health regarding the care and use of animals for experimental procedures.
Drugs
Morphine HCl (NIDA drug supply program) was dissolved in 0.9% saline, and delivered subcutaneously at a volume of 0.01 mL/g. Galanin (1–29) (rat-mouse) (Tocris, Bristol, UK) and galnon (Tocris) were dissolved in artificial cerebrospinal fluid (ACSF) to a stock concentration of 1 mM. Picrotoxin (PTX) (Sigma-Aldrich, St. Louis, MO, USA) and CGP55845 (Tocris) were dissolved in DMSO to respective stock concentrations of 100 mM and 50 mM.
Slice Preparation
Acute striatal slices were prepared from mice aged P19–P33. Mice were anesthetized with isoflurane and decapitated, and the brain was rapidly dissected into ice-cold cutting solution containing, in mM: 200 sucrose, 2.5 KCl, 1.3 MgSO4, 2.5 CaCl2, 1 NaH2PO4, 26 NaHCO3,11 dextrose for field recordings or 25 NaHCO3, 1.25 NaH2PO4, 25 glucose, 2.5 KCl, 7.0 MgCl, 110 cholineCl, 11.6 ascorbate, 3.1 pyruvate, 0.5 CaCl2 for patch clamp recordings. Coronal or parasagittal slices were cut to a thickness of 300 μm using a Leica VT1000S Vibratome and then transferred to oxygenated ACSF, containing, in mM: 127 NaCl, 25 NaHCO3, 1.25 NaH2PO4, 25 Glucose, 2.5 KCl, 1 MgCl, 2 CaCl. Slices were incubated at 37°C for 30 min before recovering at room temp for an additional 30 min.
For recordings, slices were submerged in a recording chamber and continuously superfused with room temperature ACSF, which was constantly bubbled with 95% O2-5% CO2. Responses were collected for a total duration of 40–50 minutes for fEPSPs and 20–30 minutes for EPSPs. Recording ACSF contained 100 μM picrotoxin to block GABAA receptors and 2 μM CGP55845 to block GABAB receptors. Galanin was bath applied at a concentration of 100 nM or 1 μM, and galnon was bath applied at a concentration of 1 μM.
Field Potential Recordings
fEPSP recordings were performed in the striatum to measure responses evoked by stimulation of excitatory cortical afferents in the corpus collosum (for experiments in the DS) or within the NAc (for experiments in NAc) using bipolar tungsten electrodes (FHC, Bowdoinham, ME, USA). Test stimuli were applied at a low frequency to elicit an fEPSP amplitude of 30–50% of maximum. Synaptic responses were recorded extracellularly using glass microelectrodes (0.5–1MΩ) filled with 2M NaCl, placed 200–600 μm ventral to the stimulation point. were amplified using a DP-301 differential amplifier (Warner Instrument Corportation, Hamden, CT, USA) and digitized at 10 kHz for analysis with pClamp 9.0 software (Axon Instruments, Union City, CA, USA). fEPSPs demonstrated a wave shape characteristic of cortico-striatal activation. These waves consist of two negative potentials, N1 and N2, which have been associated with direct and synaptic activation, respectively (Lovinger et al., 1993; Sergeeva et al., 2003). fEPSPs were quantified using the initial slope of N2 as calculated between points at about 10% and 30% of the negative potential in order to isolate initial synaptic input. To be sure that effects of galanin were not due to run-down, we also tested the effects of the small molecule, galanin receptor agonist galnon (Saar et al., 2002). To control for wash-in of galanin, statistical analyses were performed on the last 5 evoked responses of the baseline and galanin epochs for each experiment. All data were analyzed using repeated measures ANOVA. No significant effect of, or interaction with, time was detected, so responses were averaged for two-tailed, paired sample t-tests and the level of significance was set at p < 0.05.
Whole-cell Current Clamp Recordings
Single cell EPSPs were evoked by stimulation of excitatory cortical afferents using theta glass stimulating electrodes. Stimuli were applied to evoke responses between 5–10 mV. Whole-cell recordings were obtained from MSNs identified with video-IR/DIC. Glass recording electrodes (2–4 MΩ) were filled with internal solution containing (in mM): 135 KMeSO3, 10 HEPES, 4 MgCl2, 4 Na2ATP, 0.4 NaGTP, and 10 Na2CreatinePO4, adjusted to pH 7.4 with KOH. Data were collected with a Molecular Devices Multiclamp 700B and digitized at 10 kHz. To control for wash-in of galanin, statistical analyses were performed on the maximum amplitude of the last 5 evoked responses of the baseline and galanin epochs for each experiment. All data were analyzed using repeated measures ANOVA. No significant effect of, or interaction with, time was detected, so responses were averaged for two-tailed, paired sample t-tests and the level of significance was set at p < 0.05.
Conditioned Place Preference
Mice (aged 3–5 months) were habituated to handling once a day for 3 days prior to testing. The CPP paradigm was carried out exactly as has been described previously (Narasimhaiah et al., 2009; Neugebauer et al., 2011) using modified three-chamber boxes (ENV-256C Med Associates, Inc, St. Albans, VT, USA) with a central gray chamber and two black conditioning chambers distinguishable by their respective grid and bar floors. Movement within and among chambers was quantified by photocell beam breaks and time spent in each chamber was recorded with MED-PC IV software. Day 1 of of testing consisted of a midday pre-test (beginning at approximately 11:00 AM) during which mice were placed in the central chamber and allowed to freely explore all chambers for 15 min. Animals that spent greater than 70% of the test period in one chamber were excluded from the experiment (n=3 of 142). On Days 2–4, mice received conditioning sessions. During morning sessions (beginning at approximately 9:00 AM), mice were given a subcutaneous injection of 0.9% saline (0.01 mL/g) immediately before confinement in the saline-paired chamber for 30 min. For afternoon sessions (beginning at approximately 1:00 PM), the mice were given a subcutaneous injection of drug (0, 3, or 5 mg/kg morphine) immediately before confinement for 30 min in the drug-paired chamber. Mice within treatment and genotype groups were counterbalanced for bar/grid floor drug chamber pairing. On Day 5, a post-test was carried out at a time intermediate between the conditioning sessions to avoid any associations with time of saline or drug administration, in which the mice were once again placed in the central chamber and allowed to freely explore for 30 min. Data are expressed as a difference score, which has been calculated by subtracting the post-test time spent in the saline-paired chamber from the post-test time spent in the drug-paired chamber. This difference score was then corrected by the average of the difference score from saline-treated mice. Because of the large number of animals in the behavioral studies, multiple cohorts were bred and tested at 3–5 months of age. Each cohort included all genotypes and morphine doses, and to control for variability in preference ratios over time, each cohort was normalized to its own control group to allow pooling of the data. Data from GalR1 and GalR2 experiments were analyzed using two-way ANOVA with genotype and treatment as factors.
Results
Field Potential Recordings
To determine the effect of galanin on excitatory signaling in the striatum, we recorded fEPSPs in both the DS and NAc. The initial fEPSP slope was significantly decreased by bath application of galanin (100 nM) in the DS. Repeated measures ANOVA did not reveal a significant effect of time or a significant interaction of time and treatment (F(4,32) = 1.626, p = 0.1917 and F(4,32) = 0.2684, p = 0.8961), but there was a significant effect of galanin treatment (F(1,8) = 7.255, p = 0.0273), which reduced the slope to 66% of baseline (n = 5 slices from 5 male mice; t4 = 6.88, p = 0.0011; Fig. 1). In NAc shell, repeated measures ANOVA again did not reveal a significant effect of time or a significant interaction of time and treatment (F(4,40) = 0.4151, p = 0.7967 and F(4,40) = 0.5301, p = 0.7143), but did show a significant effect of galanin treatment (F(1,10) = 32.88, p = 0.0002), which reduced the slope to 55% of baseline (n = 6 slices from 6 male mice; t5 = 2.62, p = 0.0234; Fig. 2).
Fig. 1.
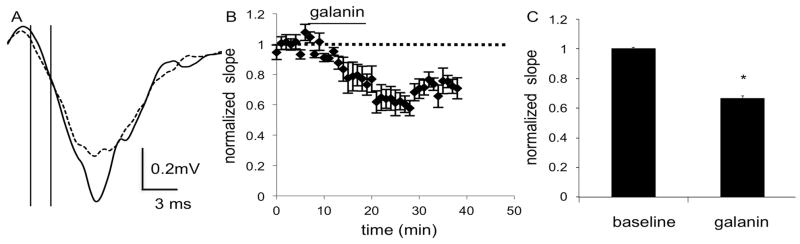
Field recordings in the DS of C57BL/6J mice show a decrease in fEPSPs after application of galanin (100 nM), as seen in A) the average of 4 consecutive traces during the baseline (solid line) and galanin (dashed line) epochs of a representative experiment. The initial slope was calculated using the points at which the vertical lines intersect with the trace. B) The combined normalized amplitudes (n = 6 slices, 6 mice (m)) before and after galanin application and C) the mean of the averaged last five responses of baseline and galanin epochs. Data are expressed as mean ± SEM. *, p<0.05
Fig. 2.
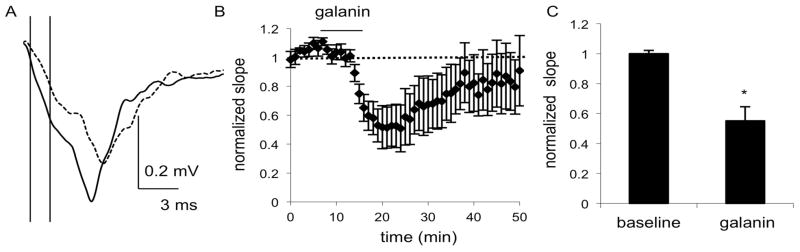
Field recordings in the NAc of C57BL/6J mice show a decrease in fEPSPs after application of galanin (100 nM), as seen in A) the average of 4 consecutive traces during the baseline (solid line) and galanin (dashed line) epochs of a representative experiment. The initial slope was calculated using the points at which the vertical lines intersect with the trace. B) The combined normalized amplitudes (n = 6 slices, 6 mice (m)) before and after galanin application and C) the mean of the averaged last five responses of baseline and galanin epochs. Data are expressed as mean ± SEM. *, p<0.05
The ability of galnon to reduce the slope of the fEPSP in the DS and NAc, as well as the wash out of this effect, suggests that the ability of galanin to decrease the fEPSP is unlikely to be due to run-down in the slice. For the DS, time and interaction of time and treatment were not significant (F(4,40) = 1.194, p = 0.0960 and F(4,40) = 2.121, p = 0.3284; Fig. 3), but there was a main effect of treatment (F(10,40) = 49.49, p < 0.0001). Slope was reduced to 48% of baseline. For the NAc, time and interaction of time and treatment were also not significant (F(4,40) = 2.169, p = 0.0900 and F(4,40) = 1.358, p = 0.2656), but there was again a main effect of treatment (F(10,40) = 22.51, p < 0.0001; Fig. 4). Slope was reduced to 67% of baseline.
Fig. 3.
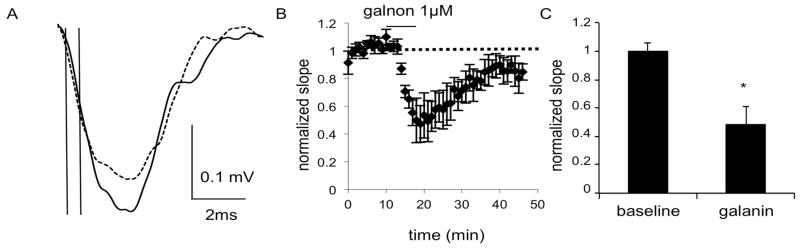
Field recordings in the DS of C57BL/6J mice show a decrease in fEPSPs after application of galnon (1 μM), as seen in A) the average of 4 consecutive traces during the baseline (solid line) and galnon (dashed line) epochs of a representative experiment. The initial slope was calculated using the points at which the vertical lines intersect with the trace. B) The combined normalized amplitudes (6 slices from 6 male mice) before and after galnon application and C) the mean of the averaged last five responses of baseline and galnon epochs. Data are expressed as mean ± SEM. *, p<0.05
Fig. 4.
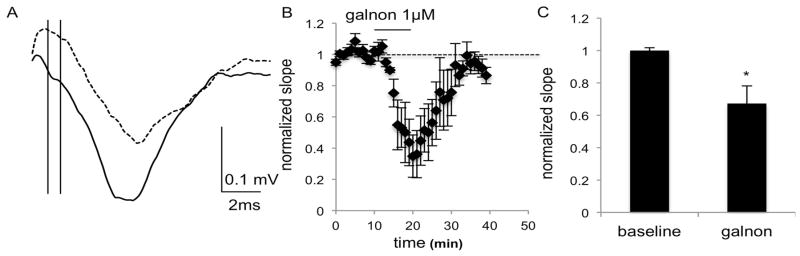
Field recordings in the NAc of C57BL/6J mice show a decrease in fEPSPs after application of galnon (1 μM), as seen in A) the average of 4 consecutive traces during the baseline (solid line) and galnon (dashed line) epochs of a representative experiment. The initial slope was calculated using the points at which the vertical lines intersect with the trace. B) The combined normalized amplitudes (6 slices from 6 male mice) before and after galnon application and C) the mean of the averaged last five responses of baseline and galnon epochs. Data are expressed as mean ± SEM. *, p<0.05
Whole-Cell Current-Clamp Recordings
To determine whether the inhibitory actions of galanin identified in field potential recordings were mediated by changes in synaptic inputs to MSNs, the amplitude of electrically evoked EPSPs were compared before and after bath application of galanin. Initial studies using 100 nM galanin did not result in consistent responses (not shown), so subsequent experiments were performed using 1 μM galanin. In MSNs of the DS, a significant decrease in EPSP amplitude was seen after galanin application. Repeated measures ANOVA did not reveal a significant effect of time or a significant interaction of time and treatment (F(4,48) = 1.951, p = 0.1171 and F(4,48) = 0.6389, p = 0.6373), but there was a significant effect of galanin treatment (F(1,12) = 92.48, p < 0.0001), which reduced the slope to to 71% of baseline response (n = 7 cells from 6 male mice; t6 = 8.625, p = 0.0001; Fig. 5). Recordings from MSNs in the NAc also revealed a significant decrease in EPSP amplitude after galanin application. In NAc MSNs, repeated measures ANOVA again did not reveal a significant effect of time or a significant interaction of time and treatment (F(4,64) = 0.7856, p = 0.5388 and F(4,64) = 0.9662, p = 0.4322), but did show a significant effect of galanin treatment (F(1,16) = 39.35, p < 0.0001), which reduced the slope to 72% of baseline response (n = 9 cells from 6 males, 3 females; t8 = 5.1410, p = 0.0009; Fig. 6). No significant changes in input resistance or membrane potential were observed in the DS or NAc. In the DS, membrane potential was −89.7mV ± 1.9 and input resistance was 91MΩ± 8.3, and in the NAc, membrane potential was −84.1 mV ± 2.4 and input resistance was 130MΩ± 16.0. Paired-pulse ratio was also assessed in both brain regions, and no significant difference was observed after application of galanin.
Fig. 5.
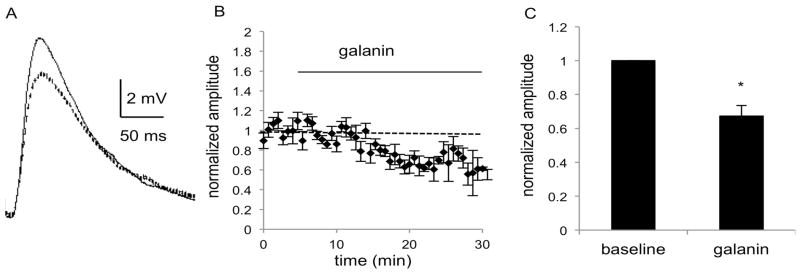
MSNs in the DS of C57BL/6J mice show a decrease in EPSP amplitude after application of galanin (1 μM), as seen in A) the average of 5 consecutive traces during the baseline (solid line) and galanin (dashed line) epochs of a representative experiment. B) The combined normalized amplitudes (n = 7 cells, 6 mice (m)) before and after galanin (1 μM) application at t = 0 and C) the mean of the averaged last five responses of baseline and galanin epochs. Data are expressed as mean ± SEM. *, p<0.05
Fig. 6.
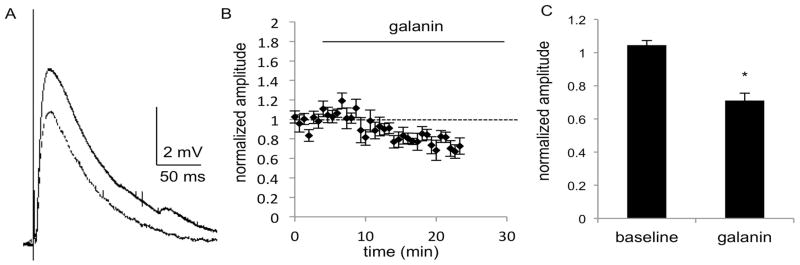
MSNs in the NAc of C57BL/6J mice show a decrease in EPSP amplitude after application of galanin (1 μM), as seen in A) the average of 5 consecutive traces during the baseline (solid line) and galanin (dashed line) epochs of a representative experiment. B) The combined normalized amplitudes (n = 9 cells, 7m, 2f) before and after galanin (1μM) application at t = 0 and C) the mean of the averaged last five responses of baseline and galanin epochs. Data are expressed as mean ± SEM. *, p<0.05
In order to identify the GalR subtypes involved in the ability of galanin to decrease the amplitude of the EPSP in NAc, galanin was applied to slices from GalR1and GalR2 KO mice. Galanin had no effect on EPSP amplitude in NAc in the absence of GalR1 or GalR2. ANOVA showed no effect of time or treatment in slices from GalR1KO mice (F(4,32) = 0.9825, p = 0.4309, F(1,8) = 0.2345, p = 0.6412; n = 5 cells; 3 females, 2 males; Fig. 7) or GalR2KO mice (F(4,40) = 1.497, p = 0.2214, F(1,10) = 0.3637, p = 0.5599; n = 6 cells from 2 females, 4 males; Fig. 8). There was an interaction of time and treatment in GalR1KO recordings (F(4,32) = 4.775, p = 0.0039). No sex differences were observed, so data were pooled for subsequent analysis. While the effects of galanin on EPSP amplitude do not wash out, as is often the case with large peptides in brain slices, the lack of effect of galanin in slices from GalR1KO and GalR2KO slices suggests that the effect of galanin on EPSP amplitude in WT slices is not due to rundown in these cells. These data suggest that signaling through both GalR1 and GalR2 is required for galanin-mediated suppression of glutamateric excitation of MSNs.
Fig. 7.
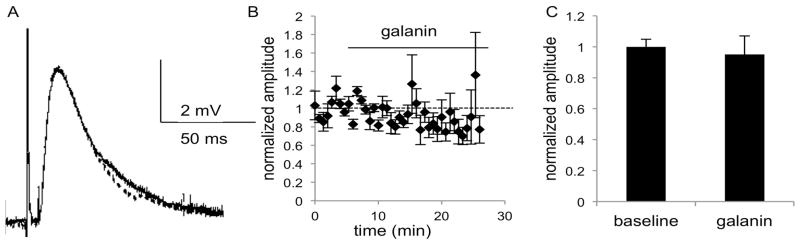
MSNs in the NAc of GalR1 KO mice show no change in EPSP amplitude after application of galanin (1 μM), as seen in A) the average of 5 consecutive traces during the baseline (solid line) and galanin (dashed line) epochs of a representative experiment. B) The combined normalized amplitudes (n = 5 cells, 2m, 3f) before and after galanin (1 μM) application at t = 0 and C) the mean of the averaged last five responses of baseline and galanin epochs. Data are expressed as mean ± SEM.
Fig. 8.
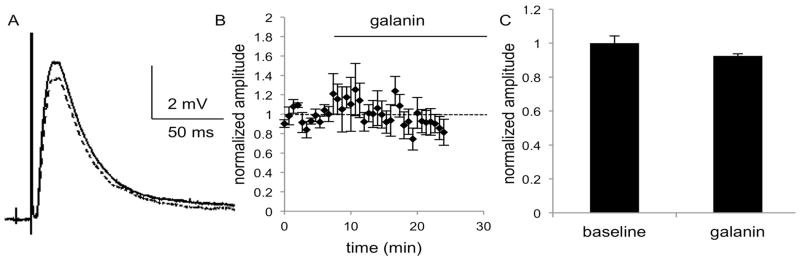
MSNs in the NAc of GalR2 knockout mice show no change in EPSP amplitude after application of galanin (1 μM), as seen in A) the average of 5 consecutive traces during the baseline (solid line) and galanin (dashed line) epochs of a representative experiment. B) The combined normalized amplitudes (n = 6 cells, 4m,2f) before and after galanin (1μM) application at t=0 and C) the mean of the averaged last five responses of baseline and galanin epochs. Data are expressed as mean ± SEM.
Conditioned Place Preference
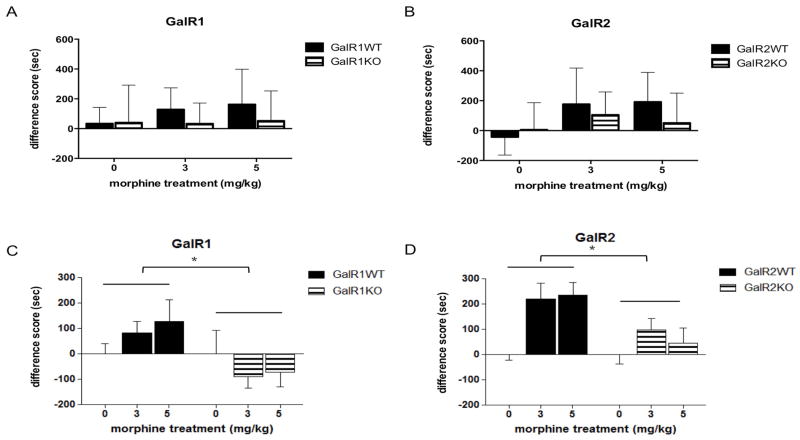
To examine whether the GalR subtypes required for the physiological effects of galanin are also important in a behavior dependent upon the activity of the NAc, GalR1 and GalR2 KO mice and their WT littermate controls were tested for morphine CPP (3 and 5 mg/kg). The groups were composed as follows: GalR1WT saline n = 7 (4 males, 3 females), GalR1WT 3mg/kg n = 9 (4m, 5f), GalR1WT 5mg/kg n = 8 (4m, 4f), GalR1KO saline n = 11 (3m, 8f), GalR1KO 3mg/kg n =11 (4m, 7f), GalR1KO 5 mg/kg n = 12 (5m, 7f), GalR2WT saline n = 14 (7m, 7f), GalR2WT 3mg/kg n = 14 (6m, 8f), GalR2WT 5mg/kg n = 15 (8m, 7f), GalR2KO saline n = 12 (5m, 7f), GalR2KO 3 mg/kg n = 14 (5m, 9f), GalR2KO 5mg/kg n = 12 (4m, 8f). ANOVA revealed a main effect of genotype (F1,51 = 4.952, p = 0.0305; Fig. 9) in the GalR1 KO experiment and main effects of treatment (F2,70 = 5.728, p = 0.0050; Fig. 9) and genotype (F1,70 = 6.278, p = 0.0146) in the GalR2 KO experiment. Posthoc analyses indicated that GalR2WT animals develop a significant preference for the morphine-paired chamber, while the GalR2KO mice did not. The GalR1WT mice showed a significantly increased preference for the morphine-paired chamber as compared to the GalR1KO mice at the 3 mg/kg dose (p = 0.016), and an almost significant increase at the 5 mg/kg dose (p = 0.056). Analysis of post-test drug-paired chamber time including sex as a factor revealed a three-way interaction of treatment by genotype by sex (F2,112 = 3.8, p = 0.0253). This effect was driven by a somewhat larger difference between genotypes at 3 mg/kg morphine in female mice and at 5 mg/kg morphine in male mice; since the directionality of the KO effect was the same in males and females, data were pooled for subsequent analyses. These data demonstrate that neither GalR1 nor GalR2 KO mice respond to morphine as their WT littermates do.
Fig. 9.
Raw GalR1 (A) and GalR2 (B) difference scores and difference scores normalized to saline (C, D) demonstrate that GalR1 and GalR2 knockout KO mice do not show significant morphine CPP at 3 or 5 mg/kg. Data are expressed as mean difference score ± SEM. *, p<0.05
Discussion
These NAc and DS recordings provide the first evidence that galanin acts directly on neurons whose activity is critical for the integration of reward signals (Carlezon and Thomas, 2009; Stuber et al., 2012). Application of the galanin peptide reduces both field potentials and individual MSN synaptic responses to electrical stimulation. The inclusion of GABA receptor antagonists in the recording solution and the abolition of the signal in the presence of the AMPA receptor blocker CNQX (Supplemental Figure) indicate that the reduction in fEPSPs could be representative of reduced glutamate signaling, although changes in cellular input resistance or cellular depolarization cannot be ruled out. The ability of galanin to reduce EPSPs is absent in MSNs in the NAc of GalR1 and GalR2 KO mice. This indicates that both of these GalR subtypes are necessary for galanin modulation of glutamatergic input to the NAc. It is worth noting that GalR3 manipulations were not included in our study; however, while GalR3 is expressed in brain, it is more abundant in the periphery (Smith et al., 1998), suggesting that it may contribute less to these brain mechanisms than the GalR1 and GalR2 subtypes. Interestingly, galanin acting through the GalR1 and GalR2 subtypes to reduce striatal EPSPs has the potential to lead to either enhancement or dampening of drug reward as measured by CPP. The reduction of EPSP amplitude decreases the likelihood of NAc cell firing, potentially supporting reward processes (such as CPP), consistent with the theory that reduced NAc activity disinhibits downstream reward signaling via the ventral pallidum (Carlezon and Thomas, 2009). However, reduction of EPSPs could also result in reduced afferent connection to the prefrontal cortex, amygdala, and hippocampus, potentially reducing cue-related processes (such as CPP) via the uncoupling of affective, cognitive, and spatial information from DA signaling (O’Donnell, Greene, et al., 1999). It should be noted that a direct comparison between the behavioral and electrophysiological results presented here is complicated by the difference in mouse ages at the time of testing, although the ages of animals used in the current studies are standard for the respective experimental paradigms (Narasimhaiah et al., 2009; Higley et al., 2011; Neugebauer et al., 2011).
Contrary to what has been seen in mice lacking the galanin peptide, mice lacking either the GalR1 or the GalR2 subtype showed greatly decreased morphine CPP. While all of these studies confirm a role for the galanin system in morphine reward processing, the discrepancies in directionality are likely accounted for by differences in experimental approach. Evidence for a galanin-induced reduction in morphine conditioning comes from a study in which the galanin peptide was infused intracerebroventricularly (Zachariou et al., 1999). Extensive studies have suggested that peptides with limited lipid solubility, such as galanin, will diffuse from a ventricular injection site in a manner strongly limited to the ventricular system (Yan et al., 1994; Pardridge, 1997; Francis et al., 2006). This indicates that an intracerebroventricular galanin infusion would act on galanin targets associated with the ventricular system, such as the locus coeruleus (Parker et al., 1995; Kolakowski et al., 1998; O’Donnell, Ahmad, et al., 1999). Indeed, a reduction in morphine reward as a result of galanin activity in the locus coeruleus would be consistent with the idea that galanin reduces tonic locus coeruleus activity that encodes the presence of rewarding stimuli (Grenhoff et al., 1993; Pieribone et al., 1995; Ma et al., 2001; Aston-Jones and Cohen, 2005).
A study of the effects of galanin peptide knockout in mice on a 129 Ola/Hsd background showed that these animals showed normal morphine CPP at 3 and 5 mg/kg and were somewhat more sensitive to a very low dose of morphine (0.25 mg/kg) (Hawes et al., 2008). While strain differences could explain an altered dose-response relationship for morphine, the fact that galanin peptide knockout has differential effects compared to knockout of individual receptor subtypes suggests that the balance of signaling between GalR subtypes is likely to be important for the overall effect of galanin signaling in particular brain areas. One possibility that could explain the unexpected observation that GalR1 and GalR2 KO mice show reduced morphine CPP is recent studies showing that GalR1 can heterodimerize with D1 and D5 receptors (Moreno et al., 2011). This interaction alters the signaling downstream of GalR1, reversing the effect of galanin from inhibitory to excitatory (Moreno et al., 2011). Removal of one galanin receptor subtype could therefore cause a reversal in signaling that would mimic the removal of the galanin peptide. Although the dimerization with D1 and D5 receptors was only shown for GalR1, it is possible that this type of dimerization is critical for GalR signaling in general, and that GalR2 may also dimerize with particular receptors, potentially altering its signaling.
Another consideration is that galanin peptide knockout mice lack all products of the galanin precursor protein including the galanin-like peptide (GALP) (Wynick et al., 1998), removing any signaling through galanin receptors. Such thorough disruption of the galanin system has been shown to result in compensatory signaling through neuropeptide Y (Hohmann et al., 2003, 2004), which limits the interpretation of these results in the context of galanin alone. The GalR1 and GalR2 KO mouse strains contain deletions of only one receptor subtype, leaving the other receptors and the peptide itself intact (Jacoby et al., 2002; Hobson et al., 2006). This more targeted disruption of galanin signaling clearly demonstrates that expression of both GalR1 and GalR2 are required for a normal conditioning response to morphine.
The importance of galanin signaling to morphine reward suggests that galanin acts on reward circuitry, and previous studies provide additional evidence to support this idea. Galanin modulates responses to many drugs of abuse, including cocaine (Narasimhaiah et al., 2009), amphetamine (Kuteeva et al., 2005), alcohol (Karatayev et al., 2009, 2010), and nicotine (Neugebauer et al., 2011). Mice lacking the gene for galanin show increased sensitivity to cocaine reward as measured by CPP (Narasimhaiah et al., 2009), though not as measured by self-administration (Brabant et al., 2010), and decreased sensitivity to nicotine reward (Neugebauer et al., 2011). Mice with elevated levels of galanin display an attenuated locomotor response to amphetamine (Kuteeva et al., 2005) and higher levels of alcohol consumption (Karatayev et al., 2009; McNamara and Robinson, 2010), while the peptide knockout mice show reduced levels of alcohol consumption (Karatayev et al., 2010). These studies demonstrate that galanin can both attenuate (morphine, cocaine, amphetamine) and potentiate (nicotine, ethanol) drug reward. While central galanin infusion has been shown to increase dopamine synthesis in the forebrain and striatum as a response to decreased dopaminergic tone (Ericson and Ahlenius, 1999), and galanin reduces dopamine release in striatal slices (Tsuda et al., 1998), the varied directionality of galanin’s effects on reward behaviors suggests a more complicated role for the peptide in reward circuitry than the straightforward reduction of dopamine release.
Our electrophysiological data suggest a nuanced role for galanin in reward circuitry, as has been suggested previously by the varied directionality of galanin’s effects on behaviors associated with drug reward. We have provided the first evidence that galanin acts directly on neurons of the mesocorticolimbic system, and have shown that its effects require the presence of both GalR1 and GalR2. In addition, we have shown that both of these receptor subtypes are required for morphine CPP. Increased understanding of the modulatory effects of peptides such as galanin on the cellular mechanisms of reward is vital for future development of interventions to protect against the addictive properties of opioids.
Supplementary Material
Acknowledgments
The authors would like to thank Nadia Gavrilova, Samantha Sheppard for technical assistance with this project and Dr. Yann Mineur for helpful conversations. This work was supported by grant DA15425 from the National Institutes of Health and the State of Connecticut, Department of Mental Health and Addiction Services (MRP), and by the Smith Family Foundation (MJH).
Abbreviations
- ACSF
Artificial cerebrospinal fluid
- CPP
Conditioned place preference
- DS
Dorsal striatum
- EPSP
Excitatory post-synaptic potential
- fEPSP
Excitatory post-synaptic field potential
- GalR
Galanin receptor
- KO
Knockout
- MSN
Medium spiny neuron
- NAc
Nucleus accumbens
- WT
Wildtype
Footnotes
The authors affirm that they have no conflicts of interest related to this work.
References
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Brabant C, Kuschpel AS, Picciotto MR. Locomotion and self-administration induced by cocaine in 129/OlaHsd mice lacking galanin. Behav Neurosci. 2010;124:828–838. doi: 10.1037/a0021221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branchek TA, Smith KE, Gerald C, Walker MW. Galanin receptor subtypes. Trends in Pharmacological Sciences. 2000;21:109–117. doi: 10.1016/s0165-6147(00)01446-2. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Thomas MJ. Biological substrates of reward and aversion: a nucleus accumbens activity hypothesis. Neuropharmacology. 2009;56(Suppl 1):122–132. doi: 10.1016/j.neuropharm.2008.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Wise RA. Rewarding actions of phencyclidine and related drugs in nucleus accumbens shell and frontal cortex. J Neurosci. 1996;16:3112–3122. doi: 10.1523/JNEUROSCI.16-09-03112.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao J, Nestler EJ. Molecular neurobiology of drug addiction. Annu Rev Med. 2004;55:113–132. doi: 10.1146/annurev.med.55.091902.103730. [DOI] [PubMed] [Google Scholar]
- Compton WM, Volkow ND. Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug Alcohol Depend. 2006;81:103–107. doi: 10.1016/j.drugalcdep.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Ericson E, Ahlenius S. Suggestive evidence for inhibitory effects of galanin on mesolimbic dopaminergic neurotransmission. Brain Res. 1999;822:200–209. doi: 10.1016/s0006-8993(99)01144-0. [DOI] [PubMed] [Google Scholar]
- Francis AB, Pace TWW, Ginsberg AB, Rubin BA, Spencer RL. Limited brain diffusion of the glucocorticoid receptor agonist RU28362 following i. c.v administration: Implications for i.c.v drug delivery and glucocorticoid negative feedback in the hypothalamic–pituitary–adrenal axis. Neuroscience. 2006;141:1503– 1515. doi: 10.1016/j.neuroscience.2006.04.067. [DOI] [PubMed] [Google Scholar]
- Grenhoff J, Nisell M, Ferré S, Aston-Jones G, Svensson TH. Noradrenergic modulation of midbrain dopamine cell firing elicited by stimulation of the locus coeruleus in the rat. J Neural Transm Gen Sect. 1993;93:11–25. doi: 10.1007/BF01244934. [DOI] [PubMed] [Google Scholar]
- Hawes JJ, Brunzell DH, Narasimhaiah R, Langel U, Wynick D, Picciotto MR. Galanin protects against behavioral and neurochemical correlates of opiate reward. Neuropsychopharmacology. 2008;33:1864–1873. doi: 10.1038/sj.npp.1301579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley MJ, Gittis AH, Oldenburg IA, Balthasar N, Seal RP, Edwards RH, Lowell BB, Kreitzer AC, Sabatini BL. Cholinergic Interneurons Mediate Fast VGluT3-Dependent Glutamatergic Transmission in the Striatum. PLoS ONE. 2011;6:e19155. doi: 10.1371/journal.pone.0019155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson SA, Holmes FE, Kerr NCH, Pope RJP, Wynick D. Mice deficient for galanin receptor 2 have decreased neurite outgrowth from adult sensory neurons and impaired pain-like behaviour. J Neurochem. 2006;99:1000–1010. doi: 10.1111/j.1471-4159.2006.04143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann JG, Krasnow SM, Teklemichael DN, Clifton DK, Wynick D, Steiner RA. Neuroendocrine profiles in galanin-overexpressing and knockout mice. Neuroendocrinology. 2003;77:354–366. doi: 10.1159/000071308. [DOI] [PubMed] [Google Scholar]
- Hohmann JG, Teklemichael DN, Weinshenker D, Wynick D, Clifton DK, Steiner RA. Obesity and endocrine dysfunction in mice with deletions of both neuropeptide Y and galanin. Mol Cell Biol. 2004;24:2978–2985. doi: 10.1128/MCB.24.7.2978-2985.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua XY, Hayes CS, Hofer A, Fitzsimmons B, Kilk K, Langel U, Bartfai T, Yaksh TL. Galanin acts at GalR1 receptors in spinal antinociception: synergy with morphine and AP-5. J Pharmacol Exp Ther. 2004;308:574–582. doi: 10.1124/jpet.103.058289. [DOI] [PubMed] [Google Scholar]
- Hulse RP, Wynick D, Donaldson LF. Activation of the galanin receptor 2 in the periphery reverses nerve injury-induced allodynia. Mol Pain. 2011;7:26. doi: 10.1186/1744-8069-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby AS, Hort YJ, Constantinescu G, Shine J, Iismaa TP. Critical role for GALR1 galanin receptor in galanin regulation of neuroendocrine function and seizure activity. Brain Res Mol Brain Res. 2002;107:195–200. doi: 10.1016/s0169-328x(02)00451-5. [DOI] [PubMed] [Google Scholar]
- Karatayev O, Baylan J, Leibowitz SF. Increased intake of ethanol and dietary fat in galanin overexpressing mice. Alcohol. 2009;43:571–580. doi: 10.1016/j.alcohol.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatayev O, Baylan J, Weed V, Chang S, Wynick D, Leibowitz SF. Galanin knockout mice show disturbances in ethanol consumption and expression of hypothalamic peptides that stimulate ethanol intake. Alcohol Clin Exp Res. 2010;34:72–80. doi: 10.1111/j.1530-0277.2009.01068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolakowski LF, Jr, O’Neill GP, Howard AD, Broussard SR, Sullivan KA, Feighner SD, Sawzdargo M, Nguyen T, Kargman S, Shiao LL, Hreniuk DL, Tan CP, Evans J, Abramovitz M, Chateauneuf A, Coulombe N, Ng G, Johnson MP, Tharian A, Khoshbouei H, George SR, Smith RG, O’Dowd BF. Molecular characterization and expression of cloned human galanin receptors GALR2 and GALR3. J Neurochem. 1998;71:2239–2251. doi: 10.1046/j.1471-4159.1998.71062239.x. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neural mechanisms of drug reinforcement. Ann N Y Acad Sci. 1992;654:171–191. doi: 10.1111/j.1749-6632.1992.tb25966.x. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn BM. Opioid prescriptions soar: increase in legitimate use as well as abuse. JAMA. 2007;297:249–251. doi: 10.1001/jama.297.3.249. [DOI] [PubMed] [Google Scholar]
- Kuteeva E, Hökfelt T, Ogren SO. Behavioural characterisation of transgenic mice overexpressing galanin under the PDGF-B promoter. Neuropeptides. 2005;39:299–304. doi: 10.1016/j.npep.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Lang R, Gundlach AL, Kofler B. The galanin peptide family: receptor pharmacology, pleiotropic biological actions, and implications in health and disease. Pharmacol Ther. 2007;115:177–207. doi: 10.1016/j.pharmthera.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, Tyler E, Fidler S, Merritt A. Properties of a presynaptic metabotropic glutamate receptor in rat neostriatal slices. J Neurophysiol. 1993;69:1236–1244. doi: 10.1152/jn.1993.69.4.1236. [DOI] [PubMed] [Google Scholar]
- Ma X, Tong YG, Schmidt R, Brown W, Payza K, Hodzic L, Pou C, Godbout C, Hökfelt T, Xu ZQ. Effects of galanin receptor agonists on locus coeruleus neurons. Brain Res. 2001;919:169–174. doi: 10.1016/s0006-8993(01)03033-5. [DOI] [PubMed] [Google Scholar]
- Matthews RT, German DC. Electrophysiological evidence for excitation of rat ventral tegmental area dopamine neurons by morphine. Neuroscience. 1984;11:617–625. doi: 10.1016/0306-4522(84)90048-4. [DOI] [PubMed] [Google Scholar]
- McNamara IM, Robinson JK. Conditional stimulation by galanin of saccharin and ethanol consumption under free and response contingent access. Neuropeptides. 2010;44:445–451. doi: 10.1016/j.npep.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Moreno E, Vaz SH, Cai NS, Ferrada C, Quiroz C, Barodia SK, Kabbani N, Canela EI, McCormick PJ, Lluis C, Franco R, Ribeiro JA, Sebastiao AM, Ferre S. Dopamine-Galanin Receptor Heteromers Modulate Cholinergic Neurotransmission in the Rat Ventral Hippocampus. Journal of Neuroscience. 2011;31:7412–7423. doi: 10.1523/JNEUROSCI.0191-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhaiah R, Kamens HM, Picciotto MR. Effects of galanin on cocaine-mediated conditioned place preference and ERK signaling in mice. Psychopharmacology (Berl) 2009;204:95–102. doi: 10.1007/s00213-008-1438-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer NM, Henehan RM, Hales CA, Picciotto MR. Mice lacking the galanin gene show decreased sensitivity to nicotine conditioned place preference. Pharmacol Biochem Behav. 2011;98:87–93. doi: 10.1016/j.pbb.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell D, Ahmad S, Wahlestedt C, Walker P. Expression of the novel galanin receptor subtype GALR2 in the adult rat CNS: distinct distribution from GALR1. J Comp Neurol. 1999;409:469–481. [PubMed] [Google Scholar]
- O’Donnell P, Greene J, Pabello N, Lewis BL, Grace AA. Modulation of Cell Firing in the Nucleus Accumbens. Annals of the New York Academy of Sciences. 1999;877:157–175. doi: 10.1111/j.1749-6632.1999.tb09267.x. [DOI] [PubMed] [Google Scholar]
- Pardridge WM. Drug Delivery to the Brain. Journal of Cerebral Blood Flow & Metabolism. 1997:713–731. doi: 10.1097/00004647-199707000-00001. [DOI] [PubMed] [Google Scholar]
- Parker EM, Izzarelli DG, Nowak HP, Mahle CD, Iben LG, Wang J, Goldstein ME. Cloning and characterization of the rat GALR1 galanin receptor from Rin14B insulinoma cells. Brain Res Mol Brain Res. 1995;34:179–189. doi: 10.1016/0169-328x(95)00159-p. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Brabant C, Einstein EB, Kamens HM, Neugebauer NM. Effects of galanin on monoaminergic systems and HPA axis: Potential mechanisms underlying the effects of galanin on addiction- and stress-related behaviors. Brain Res. 2010;1314:206–218. doi: 10.1016/j.brainres.2009.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieribone VA, Xu ZQ, Zhang X, Grillner S, Bartfai T, Hökfelt T. Galanin induces a hyperpolarization of norepinephrine-containing locus coeruleus neurons in the brainstem slice. Neuroscience. 1995;64:861–874. doi: 10.1016/0306-4522(94)00450-j. [DOI] [PubMed] [Google Scholar]
- Przewłocka B, Machelska H, Rekowski P, Kupryszewski G, Przewłocki R. Intracerebroventricular galanin and N-terminal galanin fragment enhance the morphine-induced analgesia in the rat. J Neural Transm Gen Sect. 1995;102:229–235. doi: 10.1007/BF01281157. [DOI] [PubMed] [Google Scholar]
- Saar K, Mazarati AM, Mahlapuu R, Hallnemo G, Soomets U, Kilk K, Hellberg S, Pooga M, Tolf BR, Shi TS, Hökfelt T, Wasterlain C, Bartfai T, Langel U. Anticonvulsant activity of a nonpeptide galanin receptor agonist. Proc Natl Acad Sci USA. 2002;99:7136–7141. doi: 10.1073/pnas.102163499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeeva OA, Chepkova AN, Doreulee N, Eriksson KS, Poelchen W, Monnighoff I, Heller-Stilb B, Warskulat U, Haussinger D, Haas HL. Taurine-Induced Long-Lasting Enhancement of Synaptic Transmission in Mice: Role of Transporters. The Journal of Physiology. 2003;550:911–919. doi: 10.1113/jphysiol.2003.045864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shippenberg TS, Elmer GI. The neurobiology of opiate reinforcement. Crit Rev Neurobiol. 1998;12:267–303. doi: 10.1615/critrevneurobiol.v12.i4.10. [DOI] [PubMed] [Google Scholar]
- Smith KE, Forray C, Walker MW, Jones KA, Tamm JA, Bard J, Branchek TA, Linemeyer DL, Gerald C. Expression cloning of a rat hypothalamic galanin receptor coupled to phosphoinositide turnover. J Biol Chem. 1997;272:24612–24616. doi: 10.1074/jbc.272.39.24612. [DOI] [PubMed] [Google Scholar]
- Smith KE, Walker MW, Artymyshyn R, Bard J, Borowsky B, Tamm JA, Yao WJ, Vaysse PJ, Branchek TA, Gerald C, Jones KA. Cloned human and rat galanin GALR3 receptors. Pharmacology and activation of G-protein inwardly rectifying K+ channels. J Biol Chem. 1998;273:23321–23326. doi: 10.1074/jbc.273.36.23321. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Britt JP, Bonci A. Optogenetic Modulation of Neural Circuits that Underlie Reward Seeking. Biological Psychiatry. 2012;71:1061–1067. doi: 10.1016/j.biopsych.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YG, Yu LC. Interactions of galanin and opioids in nociceptive modulation in the arcuate nucleus of hypothalamus in rats. Regul Pept. 2005;124:37–43. doi: 10.1016/j.regpep.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Tsuda K, Tsuda S, Nishio I, Masuyama Y, Goldstein M. Effects of galanin on dopamine release in the central nervous system of normotensive and spontaneously hypertensive rats☆. American Journal of Hypertension. 1998;11:1475– 1479. doi: 10.1016/s0895-7061(98)00168-x. [DOI] [PubMed] [Google Scholar]
- Wiesenfeld-Hallin Z, Xu XJ, Villar MJ, Hökfelt T. Intrathecal galanin potentiates the spinal analgesic effect of morphine: electrophysiological and behavioural studies. Neurosci Lett. 1990;109:217–221. doi: 10.1016/0304-3940(90)90566-r. [DOI] [PubMed] [Google Scholar]
- Wynick D, Small CJ, Bacon A, Holmes FE, Norman M, Ormandy CJ, Kilic E, Kerr NC, Ghatei M, Talamantes F, Bloom SR, Pachnis V. Galanin regulates prolactin release and lactotroph proliferation. Proc Natl Acad Sci USA. 1998;95:12671–12676. doi: 10.1073/pnas.95.21.12671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu ZQD, Zheng K, Hökfelt T. Electrophysiological studies on galanin effects in brain--progress during the last six years. Neuropeptides. 2005;39:269–275. doi: 10.1016/j.npep.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Yan Q, Matheson C, Sun J, Radeke MJ, Feinstein SC, Miller JA. Distribution of intracerebral ventricularly administered neurotrophins in rat brain and its correlation with trk receptor expression. Exp Neurol. 1994;127:23–36. doi: 10.1006/exnr.1994.1076. [DOI] [PubMed] [Google Scholar]
- Zachariou V, Parikh K, Picciotto MR. Centrally administered galanin blocks morphine place preference in the mouse. Brain Res. 1999;831:33–42. doi: 10.1016/s0006-8993(99)01476-6. [DOI] [PubMed] [Google Scholar]
- Zhang YP, Lundeberg T, Yu LC. Interactions of galanin and morphine in the spinal antinociception in rats with mononeuropathy. Brain Res. 2000;852:485–487. doi: 10.1016/s0006-8993(99)02236-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.