Abstract
Pathological neovascularization relies on an imbalance between potent pro-angiogenic agents and equally effective anti-angiogenic cues. Collectively, these factors contribute to an angiogenic niche within the tumor microenvironment. Oncogenic events and hypoxia contribute to augmented levels of angiokines and thereby activate the so-called angiogenic switch to promote aggressive tumorigenic and metastatic growth. Soluble decorin functions as a paracrine pan-inhibitor of receptor tyrosine kinases (RTK), such as Met and EGFR, and thus is capable of suppressing angiogenesis under normoxia. This leads to a non-canonical repression of HIF1-α and VEGFA and a concurrent induction of thrombospondin-1. The substantial induction of endogenous tumor cell-derived thrombospondin-1, a potent anti-angiogenic effector, led us to the discovery of an unexpected secretory phenotype occurring very rapidly (within 5 min) after decorin treatment of the triple-negative basal breast carcinoma cell line, MDA-MB-231. Surprisingly, the effect was not mediated by Met receptor antagonism, as initially hypothesized, but required EGFR signaling to achieve swift and robust thrombospondin-1 release. Furthermore, this effect was ultimately dependent on the prompt degradation of RhoA, via the 26S proteasome, leading to direct inactivation of ROCK1. The latter led to a derepression of thrombospondin-1 secretion. Collectively, these data provide a novel mechanistic role for the ROCK1 kinase in addition of providing the first conclusive evidence of decorin exclusively targeting a RTK to achieve a specific effect. The overall effects of soluble decorin on the tumor microenvironment would cause an immediately-early as well as sustained anti-angiogenic response in vivo.
Keywords: Proteoglycan, angiogenesis, epidermal growth factor receptor, Met, receptor tyrosine kinase
Introduction
An intricate balance exists between pro- and anti-angiogenic factors that either promote or impede, respectively, pathological angiogenesis within the tumor microenvironment [1]. When this balance is altered as a consequence of oncogenesis to be in favor of pro-angiokines, an angiocompetent milieu is formed via the so-called angiogenic switch [2–6]. This pathobiological imbalance within the tumor microenvironment allows for neovascularization of the tumorigenic tissue, leading to enhanced aggressive behavior, increased metastasis, and poorer prognosis [1]. This balance of angiocrine factors is intimately orchestrated by the plethora of functions conveyed by the surrounding extracellular matrix constituents that are able to differentially, and often are the critical determinants, that regulate angiogenesis [7].
Proteoglycan-mediated control of tumor angiogenesis exemplifies this principle [8–10]. Of particular interest is the inherently powerful anti-angiogenic properties exerted by the small leucine rich proteoglycan (SLRP) decorin [11,12], a paradigmatic member of the SLRP superfamily. Decorin, originally identified as a regulator of type I collagen fibrillogenesis and [13–20] and as highly-induced gene in the stroma of human colon carcinomas [21,22], is encoded by a large and highly-conserved gene with a complex promoter structure [23–25]. Soluble, monomeric decorin [26] certainly functions in a paracrine manner to bind, with high affinity, multiple receptor tyrosine kinases (RTKs) including members of the ErbB family, such as the EGFR and ErbB4 [27–29], and has been identified as the only antagonistic ligand of Met, the hepatocyte growth factor receptor [30,31]. In addition, decorin binds to the insulin-like growth factor receptor I [32–34] and attenuates its activity in bladder cancer cells [35]. These diverse bioactivities convey highly effective anti-tumorigenic [31,36–39] and anti-metastatic [40,41] properties onto decorin. Decorin is also involved in innate immunity and hypersensitivity reactions [42–44], and in bone pathophysiology [45]. Genetic ablation of the decorin gene favor lymphomagenesis in a p53-null background [46], induces intestinal tumor formation in a C57Bl/6 background [47,48] and plays a role in wound healing [49]. Concurrent with these activities is the induction of cyclin-dependent kinase inhibitors such as p21WAF1 [50,51] coincident with the degradation, in a non-canonical fashion, of potent oncoproteins such as β-catenin and Myc [52]. Therefore, decorin acts as a paracrine tumor repressor by acting as a pan-RTK inhibitor at the cell surface of tumor cells [10,53].
Decorin represses pro-angiogenic factors (HIF-1α and VEGFA) concurrent with simultaneous transcriptional induction of anti-angiogenic molecules such tissue inhibitor of metalloprotease 3 (TIMP3) and thrompspondin-1(TSP-1) under normoxia via suppression of pro-angiogenic HGF/Met signaling [11,54–56]. Previous work [56] has demonstrated an acute transcriptional response for THBS1 expression that correlated with increased TSP-1 protein in the triple-negative basal breast carcinoma cell lines, MDA-MB-231, as well as in tumor xenograft models composed of the same cell type. TSP-1 is an archetypical matricellular component of a gene family that encodes five large, modular, calcium-binding, secreted glycoproteins [57]. TSP-1 is a long, filamentous protein capable of binding several cell surface receptors enabling diverse regulation of cellular function among many different cell types [57]. Although originally identified as a secreted monomeric glycoprotein of ~140 kDa, TSP-1 functions primarily as a trimer and is derived from thrombin-stimulated platelets and plateletα-granules, accounting for ~3% and ~25% of total protein content, respectively [58]. It is now well established that TSP-1 is expressed by a wide variety of cell types, including predominant expression from vascular smooth muscle cells and endothelial cells [59]. Functionally, TSP-1 inhibits wound healing, inactivates MMP-9 and VEGFA liberation, triggers endothelial cell apoptosis via engagement of CD36 and signaling via Jun N-terminal kinase and p38 stress activated protein kinases and modulates adhesion [58–60]. Additional functions of TSP-1 include regulation of NO/cGMP signaling via engagement and ligation of CD47 with VEGFR2 within the cardiovascular system [61], regulation of synaptogenesis in the central nervous system [62], and modulation of TGF-β activation and fibrosis [63] and wound healing [64]. Moreover, TSP-1 inhibits angiogenesis via a direct effect on endothelial cell migration and survival, and by affecting VEGFA availability and VEGFR2 activity [65,66]. Notably, TSP-1 deficient mice display a lordotic curvature of the spine, increases in the number of circulating monocytes and eosinophils, and pulmonary inflammation [67]. Interestingly, the TSP-1 null mouse was not embryonic lethal, perhaps due to redundancy among the other TSP gene members [58].
In the context of cancer, oncogenic Ras signaling [68] and altered Myc activity, downstream of Ras [69] combinatorially repress TSP-1 expression. The transcriptional inhibitor Id1 was recently shown to repress THBS1 expression as Id1 deficiency is associated with increased TSP-1 levels [70]. As decorin is capable of unconventionally downregulating Myc [52], in conjunction with the concept that Myc drives Id1 induction [71], we sought to further characterize the mechanism for decorin-mediated induction of TSP-1 in the MDA-MB-231 cell line, presumably downstream of Met [56]. Unexpectedly, we found a prompt and robust secretory phenotype mediated by decorin that requires EGFR signaling, independently of Met, and orchestrated through the concerted degradation of RhoA and subsequent inactivation of ROCK1 to allow for rapid secretion of TSP-1 from MDA-MB-231 cells. Collectively, our results provide a novel secretory-inducing role for decorin and offer new perspective and understanding on the ability of this SLRP to attenuate the pro-angiogenic niche of the surrounding tumor microenvironment.
Results
Decorin evokes a rapid and biphasic release of TSP-1 in MDA-MB-231 breast carcinoma cells
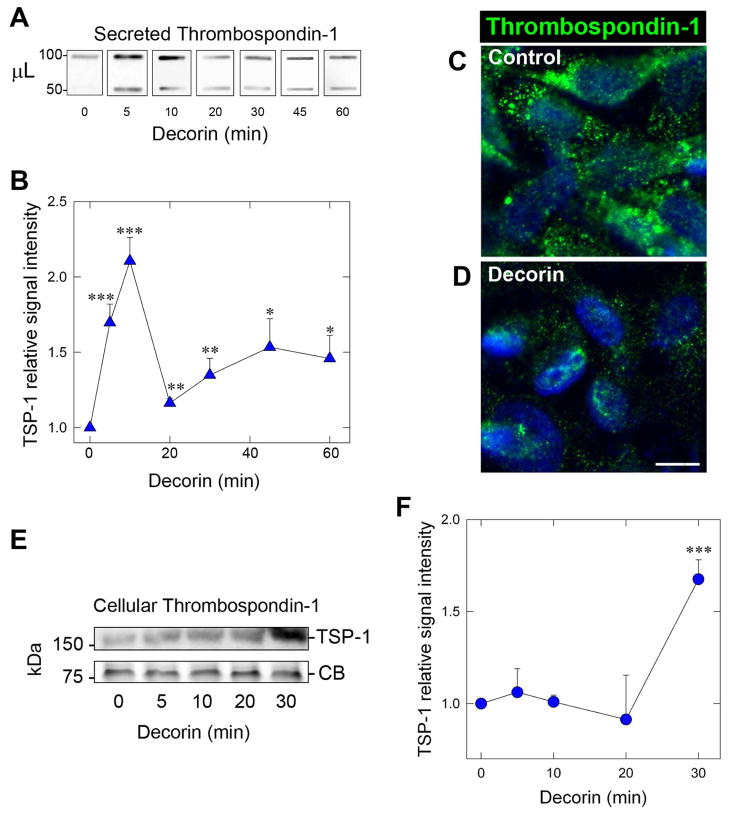
We have previously described a function for exogenous soluble decorin to induce expression of THBS1 mRNA and protein in MDA-MB-231 cells following engagement of Met as part of a potent angiostatic program [56]. Therefore, in the current study, we aimed to further characterize the mechanism of TSP-1 regulation and subsequent secretion while under the influence of decorin. Analysis of tumor cell-conditioned medium revealed a rapid mobilization of TSP-1 in as little as 5 min (~1.7-fold) following decorin exposure (Fig. 1A,B). It should be noted that decorin protein core was utilized throughout the study (hereafter referred to as decorin) unless otherwise indicated. Interestingly, the levels of TSP-1 spiked at 10min (~2.2-fold) then returned to near baseline levels at 20 min post treatment (Fig. 1B). However, TSP-1 began to increase a second time at 30 min and plateaued after 1 h (Fig. 1A,B). We believe that this biphasic release of TSP-1 reflects an initial burst of TSP-1 release most likely from pre-formed TSP-1 cytoplasmic storage followed by enhanced expression of THBS1 mRNA for the second phase in a manner analogous to platelet derived growth factor (PDGF)-mediated induction of THBS1 [72].
Fig. 1.
Decorin evokes a rapid and biphasic release of Thrombospondin-1 (TSP-1) in MDA-MB-231 breast carcinoma cells. (A) Slot blot analysis of secreted TSP-1 in triple negative breast carcinoma MDA-MB-231 tumor conditioned media following exposure to 200 nM decorin at the indicated time points. Indicated on the left are the volumes of conditioned media in μL. Each sample was diluted with DMEM to reach a constant volume of 400 μL. (B) Quantification of secreted TSP-1 following normalization to total cell number over time. Values represent the mean ±SEM of three independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001. (C,D) Representative confocal images of MDA-MB-231 cells of control (C) or decorin (200 nM, 40 min) treated (D) following immunostaining for TSP-1 (green) or stained with DAPI (blue) to visualize nuclei. Images were captured with the same exposure, gain, and intensity. Bar ~ 10 μm. (E) Immunoblot analysis via SDS-PAGE of cellular TSP-1 following treatment with decorin (200 nM) at the indicated time points. Equal loading of the cell lysates was determined by Coomassie Blue (CB) staining. (F) Immunoblot quantification of cellular TSP-1 at the time intervals reported. Normalization of signal intensities was achieved based on Coomassie blue staining. Slot blots and immunoblots were visualized via HRP-conjugated secondary antibodies. The data are representative of at least 3 independent experiments and reported as fold change ±SEM. *P < 0.05; **P < 0.01; ***P < 0.001 as determined by the Student’s t-test.
To further corroborate these finding, we performed confocal microscopy wherein MDA-MB-231 cells were exposed to decorin for 40 min and probed for TSP-1. The distribution of TSP-1 in control cells (Fig. 1C) was primarily arranged in a uniformly granular pattern. The appearance of TSP-1 staining insofar as both the number of granules and signal intensity of TSP-1 deposits in the decorin treated cells after 40 min were significantly diminished (Fig. 1D), in accordance with the above data detailing rapid release of TSP-1 from the cell.
In contrast to the rapid release evoked by decorin of secreted TSP-1, cellular TSP-1 at early time points (5–20 min) revealed no substantial changes in the intracellular stores of TSP-1 (Fig. 1E,F). However, at 30 min, there was a significant accumulation (~1.8-fold) of cellular TSP-1. These intracellular levels of TSP-1 remained high at 1 and 2 h post treatment (not shown).
Collectively, our results show an unexpected and novel role for decorin in evoking a rapid, biphasic, and time-dependent release of TSP-1 from quiescent (serum starved) MDA-MB-231 cells.
Decorin requires EGFR signaling, but not Met, to evoke rapid TSP-1
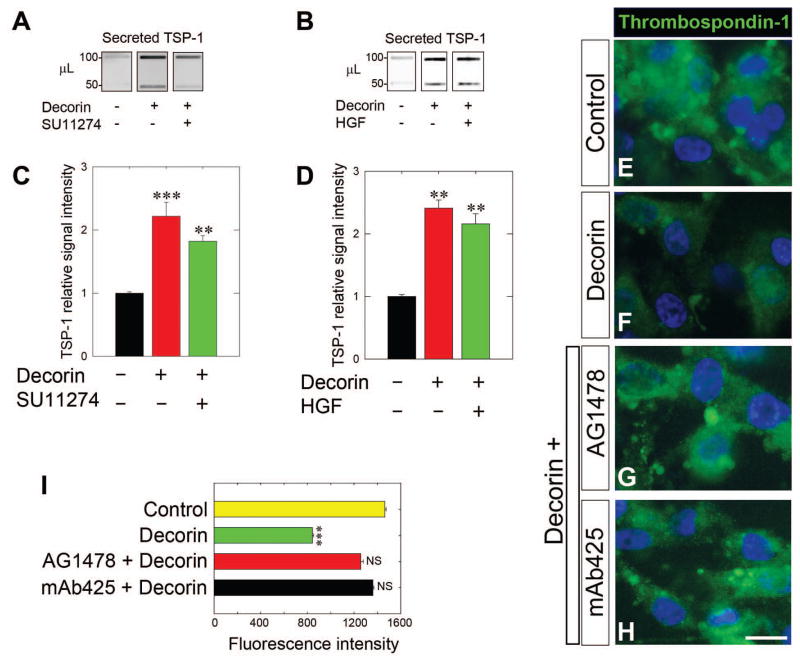
Next, we sought to identify the mechanism of this biological activity, i.e. we tested which of the known decorin RTK was responsible for coordinating this swift release of TSP-1 in response to soluble decorin. Our main hypothesis was that Met would be the primary receptor responsible for this activity, as Met has been previously implicated in coordinating the other aspects of decorin mediated angiostasis [56]. Therefore, we performed similar experiments in the presence or absence of the Met tyrosine kinase inhibitor SU11274, a highly selective inhibitor of the ATP binding domain for various Met mutants [73]. To this end, we pre-treated MDA-MB-231 cells for 30 min with SU11274 followed by an incubation with decorin for 10 min. Consistently, decorin facilitated a rapid burst of TSP-1 into the media; surprisingly, this effect was not blocked by SU11274 (Fig. 2A) as decorin still evoked TSP-1 secretion (~2-fold). Quantification of TSP-1 secretion in SU11274 pre-treated cells as well decorin alone revealed a marked induction of TSP-1 levels in both cases (P<0.001, Fig. 2C), but no significant differences between the two experimental conditions (P = 0.21). Next, in an attempt to link these effects to Met, we pre-treated MDA-MB-231 cells with hepatocyte growth factor (HGF) for 30 min [74]. Similarly, decorin was able to induce secretion while HGF pre-treatment failed to block decorin mediated secretion of TSP-1 (Fig. 2B). Quantification of secreted TSP-1 revealed a significant induction of TSP-1 secretion in the presence or absence of HGF (P<0.001, Fig, 2D) and no significant differences between the two experimental conditions (P = 0.35). Collectively, these results indicate that decorin engagement of Met is not involved in the secretion of TSP-1 by MDA-MB-231 cells.
Fig. 2.
Decorin requires EGFR signaling, but not Met, to evoke rapid TSP-1 release in MDA-MB-231 cells. (A,B) Slot blot analysis of secreted TSP-1 following treatment with either decorin alone (200 nM, 10 min) or in combination with a 30 min pre-treatment with the Met kinase inhibitor SU11274 (1 μM) as in (A) or HGF (50 ng·mL−1) as in (B) followed by a 10 min incubation with decorin (200 nM). (C,D) Quantification of secreted TSP-1 signal intensity following normalization to total cell number for either decorin alone or in combination with SU11274 (C) or HGF (D) pre-treatments. (E–H) Representative immunofluorescence images of TSP-1 (green) in control cells (E), cells treated with 200 nM decorin for 10 min (F), or in conjunction with a 30-min pretreatment with the EGFR kinase inhibitor AG1478 (1 μM, G) or with the EGFR blocking antibody mAb425 (10 μg·mL−1, H) followed by a 10-min decorin treatment (200 nM). All images shown were taken with the same exposure, gain, and intensity. Nuclei appear blue after DAPI staining. Bar ~ 10 μm (I) Average TSP-1 fluorescence intensity was quantified (n = 10/treatment). Data are representative of at least 2–3 independent experiments and reported as normalized fold changes ±SEM (C,D) or as average fluorescence intensity ±SEM (I). **P < 0.01; ***P < 0.001; NS, not significant.
The original identification of decorin mediated antagonism towards RTKs began with EGFR and upon decorin binding there is receptor dimerization, and rapid trans-autophosphorylation reminiscent of the effect on Met within 10 min [27]. We therefore interrogated the role of EGFR signaling on TSP-1 secretion, as MDA-MB-231 do express EGFR, but not HER2/Neu. Utilizing immunofluorescence to probe TSP-1 in response to decorin, pre-treatment with the EGFR small tyrosine kinase inhibitor AG1478 [75], abrogated the ability of decorin to deplete TSP-1 (compare Fig. 2F with Fig. 2G), making control (Fig. 2E) and AG1478 pretreated cells look virtually indistinguishable. Further, application of the EGFR-specific monoclonal blocking antibody mAb425 [76] recapitulated the blockade of decorin-mediated secretion of TSP-1 and mimicked control conditions as determined by immunofluorescence. Application of either AG1478 or mAb425 prior to decorin treatment almost completely restored the punctate pattern of TSP-1 to control signatures and significantly obviated the ability of decorin to induce TSP-1 release as determined by the average TSP-1 fluorescence signal (Fig. 2I).
Taken together, these data advocate for the ability of decorin to differentially signal and integrate specific aspects of the angiostatic program across different RTKs expressed by the tumor cell. As such, rapid secretion of TSP-1 is almost exclusively dependent on EGFR, but not Met, in these basal breast carcinoma cells.
Rapid secretion of TSP-1 depends on ROCK1 inhibition
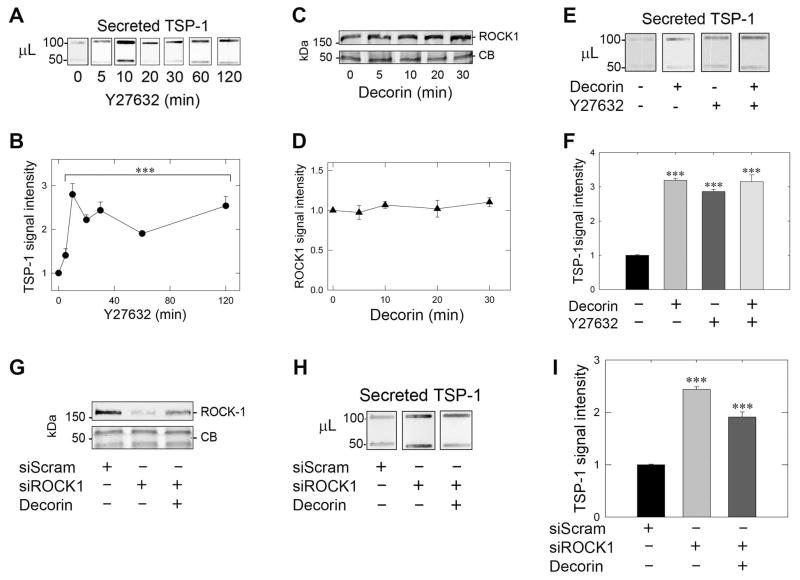
The role of p160-ROCK1 (Rho-associated coiled-coil containing protein kinases) in the control of TSP-1 has been evaluated and assigned an inhibitory role as a downstream effector of the Ras/PI3K/RhoA signaling pathway to achieve THBS1 transcriptional repression [69]. Indeed, treatment with the ROCK1 inhibitor Y27632 derepresses TSP-1 repression following an 8-h treatment [69]. Suppression of ROCK1 by a variety of mechanisms reduces cancer metastasis and invasion in lung adenocarcinoma [60] and gastric cancers via miRNA-148a [77]. However, the role of ROCK1 in regulating secretion has been limited and surprisingly has focused on the effects of ROCK1 inhibition functionally linked to hyperinsulinemia [78]. Therefore, we hypothesized that Y27632, a specific small molecule inhibitor of ROCK1, would be able to recapitulate the rapid pattern of decorin-evoked TSP-1 release from MDA-MB-231 cells. Incubation of MDA-MB-231 cells with Y27632 alone evoked a rapid and robust secretion of TSP-1 starting as early as 5 min, peaking at 10 min (~2.7-fold), with a plateau (~2.5-fold) that was sustainable for up to 2 h post-treatment with Y27632 (Fig. 3 A,B). These results recapitulate the decorin-evoked secretion of TSP-1 with comparable kinetics (cfr. Fig. 1A, B). Interestingly, no significant modulation of ROCK1 protein levels was seen with decorin up to 30 min (Fig. 3C,D) as well as up to 2 h (data not shown). Therefore, the activity of ROCK1, potentially via RhoA, must be curtailed to allow for rapid TSP-1 secretion and thus independent of ROCK1 protein level.
Fig. 3.
Rapid secretion of TSP-1 depends on ROCK1 inhibition. (A) Analysis of secreted TSP-1 in MDA-MB-231 tumor conditioned media via slot blot analysis in the presence of the ROCK1 inhibitor Y27632 (10 μM) at the indicated time points. (B) Quantification of secreted TSP-1 probed as in (A) at the reported time points following averaging of both bands and normalization to total cell number. (C) Immunblotting of ROCK1 via SDS-PAGE in MDA-MB-231 at the time intervals noted with 200 nM decorin. Coomassie blue (CB) stained portions of the gel served as equal loading controls for each lysate. (D) ROCK1 quantification following decorin (200 nM) over time as determined in (C) and normalized to the signal intensities given by Coomassie blue. (E) Slot blot determination of TSP-1 in MDA-MB-231 following individual treatment for 10 min with decorin (200 nM), Y27632 (10 μM) or pre-treatment (30 min) with Y27632 (10 μM) followed by decorin (200 nM) for 10 min. (F) Quantification of secreted TSP-1 in response to the applied treatments as described in (E). Secreted TSP-1 intensity was normalized to total cell number. (G) Immunoblot verification of ROCK1 depletion following transfection with siRNA (60 pM) specific for ROCK1 (siROCK1) relative to scramble siRNA (2 pM) notated as siScram in the absence or presence of 200 nM decorin for 10 min. Coomassie blue served as positive loading control for the siRNA-mediated knockdown of ROCK1 in MDA-MB-231 cells. (H) Effect of ROCK1 depletion on secreted TSP-1 assayed via slot blot analysis of the tumor-conditioned media harvested from the same samples as reported in (G) with corresponding quantification of secreted TSP-1 following normalization to total cell number in (I). Three independent experiments were performed for the above studies and reported as normalized fold changes ±SEM. *** P < 0.001; NS, not significant.
Next, we established that ROCK1 inhibition was the primary effector for decorin mediated TSP-1 release insofar as that treatment with either decorin or Y27632 alone achieved maximal TSP-1 secretion after 10 min (Fig. 3E,F). Further, pre-treatment with Y27632 followed by decorin application did not induce further liberation of TSP-1, indicating that ROCK1 inhibition is the primary target for decorin to evoke rapid TSP-1 mobilization (Fig. 3E,F).
The role of ROCK1 was functionally tied to the control of TSP-1 secretion through siRNA-mediated knockdown of ROCK1 (denoted as siROCK1). We achieved significant depletion of ROCK1 as detected by immunoblots (>85% of control levels, Fig. 3G) when compared to siScramble (siScram) transfected controls. Secreted TSP-1 in the presence of siROCK1 alone was significantly increased relative to siScram controls, indicating a role for ROCK1 to suppress basal TSP-1 secretion (Fig. 3H, I), and consistent with the above results that inhibition with Y27632 increased secreted TSP-1. Finally, decorin was unable to induce a further increase of secreted TSP-1 when in the presence of siROCK1 (Fig. 3H,I). These results indicate and further confirm that ROCK1 is epistatic to the bioactivity of decorin mediated secretion of TSP-1 and that upon inhibition or depletion of ROCK1, secreted TSP-1 is rapidly released.
Collectively, these data ascertain a novel role for ROCK1 in controlling basal levels of a highly anti-angiogenic molecule. Furthermore, ROCK1 serves as the main downstream effector in the mechanism of decorin-evoked TSP-1 release that emanates from positive EGFR signaling.
Decorin depends on EGFR to rapidly degrade RhoA and to evoke TSP-1 secretion
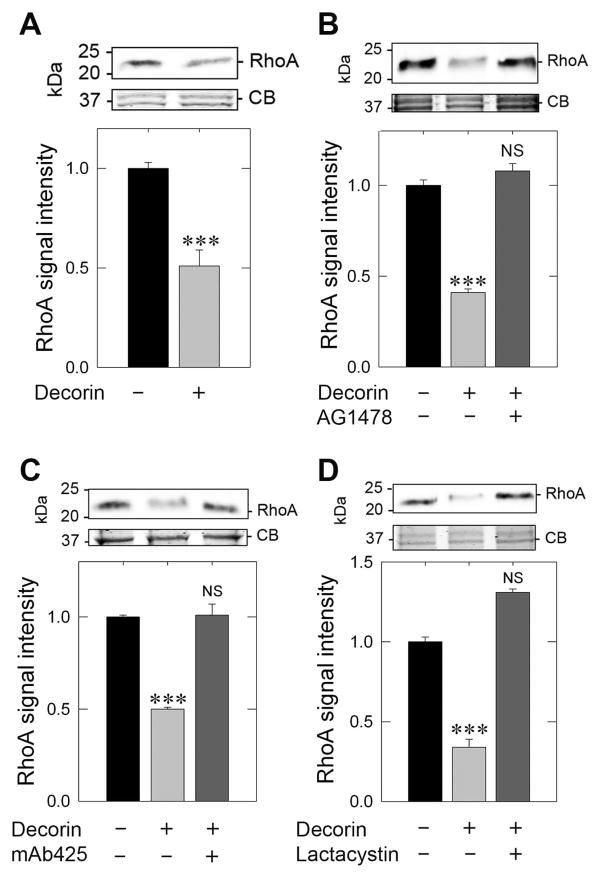
The cellular role of ROCK1 is activated and thus orchestrated by a direct and putative binding of RhoA [79], a small GTPase implicated in motility, invasion, and proliferation [80] as well as promoting oncogenic transformation and a more aggressive tumor phenotype [81]. Further, it has been found that increased RhoA and RhoC expression ultimately correlates with poorer prognoses in breast cancers [82]. Thus, as inhibition of ROCK1 with Y27632 even in the presence of decorin did not synergistically or additively increase total secreted TSP-1 together with the lack of ROCK1 modulation at the protein levels as assayed for up to 2 h, we hypothesized that ROCK1 would be unable to be activated via antagonism of RhoA, stipulated by decorin engaging EGFR. Immunoblotting revealed a stark reduction (< 50%) of RhoA in MDA-MB-231 within just 10 min of decorin treatment (Fig. 4 A). This observation supports the hypothesis that ROCK1 fails to become activated due to a pronounced reduction in RhoA levels. This effect was sensitive to EGFR kinase inhibition insofar as pre-treatment with AG1478 followed by decorin (10 min) failed to reduce total RhoA levels as compared to decorin treated samples (Fig. 4 B). This effect was further verified following quantification revealing a decorin dependent decrease (Fig. 4B) in RhoA (by over 60%) that was wholly abrogated by pretreating with AG1478 (Fig. 4B). Moreover, pretreatment with the EGFR monoclonal blocking antibody, mAb425 elicited identical effects by abolishing suppression of RhoA when compared to decorin alone (Fig. 4C). This rapid decrease (within 10 min of decorin application) of RhoA is the result of active proteolysis via the 26S proteasome since lactacystin, a powerful proteasome inhibitor, was able to completely attenuate the decorin-evoked degradation of RhoA (Fig. 4D).
Fig. 4.
Decorin depends on EGFR to rapidly degrade RhoA and evoke rapid TSP-1 secretion. (A) Immunoblot analysis of RhoA in response to 200 nM decorin for 10 min in MDA-MB-231 cells (top panel) and quantification of RhoA levels (bottom panel). (B) Immunoblot analysis of RhoA after exposure to decorin alone (200 nM, 10 min) or following pretreatment (30 min) with the EGFR inhibitor, AG1478 (1 μM) (top panel), with accompanying quantification of RhoA levels (bottom panel). (C) RhoA analysis following decorin treatment (200 nM, 10 min) or after pretreatment (30 min) with the EGFR blocking antibody, mAb425 (10 μg·mL−1) (top panel) and corresponding quantification (bottom panel). (D) RhoA exposed to decorin alone (200 nM, 10 min) or in conjunction with a 30 min pretreatment of the proteasome inhibitor, lactacystin (10 μM) (top panel) and quantification (bottom panel). In all instances, the upper portion of the gel was Coomassie blue stained for equal loading and normalization of RhoA signal intensity. The data reported are representative of at least 3 independent experiments and expressed as the average fold change ±SEM. *** P < 0.001.
Taken together, these data advocate for a prompt and vigorous decorin-mediated response to degrade RhoA via the 26S proteasome dependent on both EGFR signaling and the caveat that decorin binds EGFR, and not Met.
Decorin depends on classical secretory pathways for rapid TSP-1 secretion
Exosomes constitute an emerging class of secreted, membrane bound vesicles that allow for intercellular communication by delivering a host of bioactive molecules ranging from proteins, lipids, RNA, and DNA that seem to play an immunosuppressive role [83]. These nanovesicular bodies have been shown to play a functionally important role in both the initiation and progression of pathobiological states, including cancer. Interestingly enough, tumorigenic cells secrete increased amount of exosomes relative to non-tumorigenic counterparts and display a distinct proteomic signature representative of the host cell [84]. We, therefore, hypothesized that exosomes might be responsible for the rapid en masse secretion of TSP-1 from MDA-MB-231 cells. To this end, we subjected tumor cell conditioned media following incubation with decorin for 6 h to differential ultracentrifugation to isolate exosomal fractions followed by immunoblotting for the detection of exosomal TSP-1. It is important to note that the decorin treatment time was significantly increased to allow for the maximal collection of exosomes from the media. Thus, using caveolin-1 (Cav-1) as an exosomal marker [85], we found no significant change between control and decorin exosomal isolates (Fig. 5A). Quantification of three independent experiments normalized to the fetal bovine serum detected in the media showed no significant changes in TSP-1 levels (Fig. 5B). Paradoxically, exosomal Cav-1 was increased relative to control (Fig. 5A) suggesting decorin triggers an increase in exosomal secretion, but this does not underlie the secretory mechanism for TSP-1.
Fig. 5.
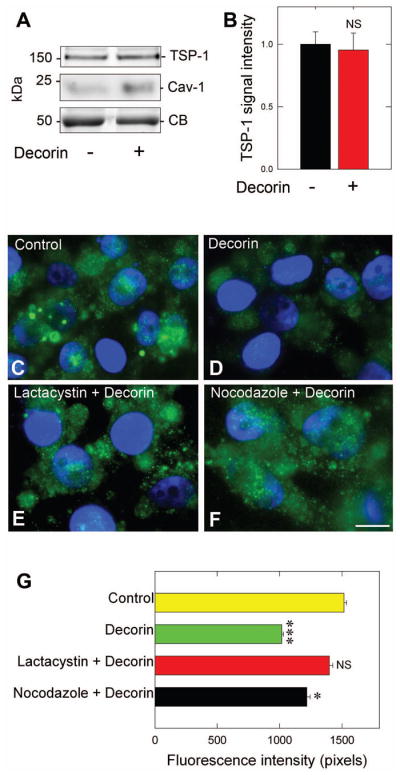
Decorin depends on classical secretory pathway for rapid TSP-1 secretion. (A) Immunoblot analysis of TSP-1 positive exosomes from MDA-MB-231 in response to 200 nM decorin (6 h) followed by ultracentrifugation (100,000 · g, 90 min) to isolate the exosome-containing fraction. (B) Quantification of exosomal TSP-1 after normalization to Coomassie blue. (C–F) Representative immunofluorescence images of MDA-MB-231 cells probe for TSP-1 (green) for control (C), decorin-only treated (200 nM, 10 min) or after pre-treatment (30 min) with the proteasome inhibitor, lactacystin (10 μM) as in (E) or with the microtubule inhibitor nocodazole (100 ng·mL−1) as in (F). The nuclei appear blue due to DAPI. All images were collected at the same exposure, gain, and intensity. (G) Quantification of average TSP-1 fluorescence intensity (n=10/treatment). Data are representative of at least three independent experiments and reported as normalized fold changes ±SEM (B), or as the average fluorescence intensity ±SEM for the reported immunofluorescence as in (G). * P < 0.05; ***P < 0.001; NS, not significant.
Classical secretion from the cell depends on the movement of vesicles via the microtubular network followed by fusion with the plasma membrane and secretion of the contents to the extracellular space. Thus, pre-incubating MDA-MB-231 cells with nocodazole, which interferes with microtubule polymerization, was able to block decorin mediated release of TSP-1 (Fig. 5F) when compared to decorin alone (Fig. 5D) and was similar to control (Fig. 5C). There was a small, but significant reduction in TSP-1 levels following pretreatment with nocodazole and ensuing decorin incubation (P = 0.041, Fig. 5G), when compared to controls suggesting alternative release mechanisms. However, when compared to decorin alone, a significant block (P = 0.009, Fig. 5G) occurred indicating a requirement for microtubules as mediating the release of TSP-1 under the influence of decorin. Finally, RhoA is potently degraded by decorin via the 26S proteasome (cfr. Fig. 4D), and this has functional consequences on the ability of decorin to trigger release of TSP-1. As shown (Fig. 5 E), pre-treatment with lactacystin (which attenuates RhoA degradation) was able to hinder the decorin-mediated release of TSP-1, suggesting a requirement for RhoA degradation as part of the mechanism to allow for accelerated TSP-1 release. Indeed, quantification of TSP-1 fluorescence revealed an almost complete block in TSP-1 release when compared to decorin (P = 0.001, Fig. 5G) and virtually identical to control (P = 0.29, Fig. 5G).
Overall, we demonstrate that decorin utilizes the classical secretory pathway dependent on microtubules to elicit TSP-1 discharge. Further, this pathway is profoundly sensitive to the levels of RhoA, as preventing degradation of this cytoskeletal mediator completely blocked decorin triggered release of TSP-1.
Discussion
A fragile balance among the angiokine signaling factors as well as the tumor parenchyma and endothelial cells residing within the tumor microenvironment mediates the tumor angiogenic switch. Angiogenesis critically relies on matrix derived cues for coordination, particularly small leucine-rich proteoglycans [30]. From the perspective of the tumor proper, decorin is a potent inhibitor of tumor angiogenesis [55]. Recently, we found a potent transcriptional repression of numerous genes encoding soluble pro-angiogenic mediators responsible for initial sculpting and continued maintenance of the angiogenic response such as VEGFA, FGF2, and ENG with concurrent induction of soluble anti-angiogenic effectors including THBS1 and TIMP3 [56]. These data demonstrated, for the first time, a potent induction of soluble angiostatic gene products from MDA-MB-231 in the presence of decorin in both in vitro and in vivo models of triple negative breast carcinoma [56].
In the present report, we have extended our studies concerning the underlying mechanism of decorin-mediated angiostasis by further characterizing thrombospondin-1 regulation, as this matricellular component has a profound influence on the state of the angiogenic niche by compromising the viability of endothelial cells [86] We discovered a novel mechanism for control of TSP-1 secretion (Fig. 6). In contrast to the protracted transcriptional response of THBS1 and TSP-1 protein that occurs following an 8-h incubation with decorin [56], we found a very rapid secretion (within 5 min) of presumably preformed TSP-1 from MDA-MB-231 cells that appear to be biphasic in nature. The second phase of TSP-1 release might be mediated via the induction of THBS1 mRNA expression or the consequent accumulation of the TSP-1 oligomeric form within the endoplasmic reticulum of cellular TSP-1 begins to increase, consistent with the ~30 min maturation time for TSP-1 accumulation within this compartment. These data outline an immediate-early transition from the tumor parenchyma to start reprogramming the otherwise pro-angiogenic niche.
Fig. 6.
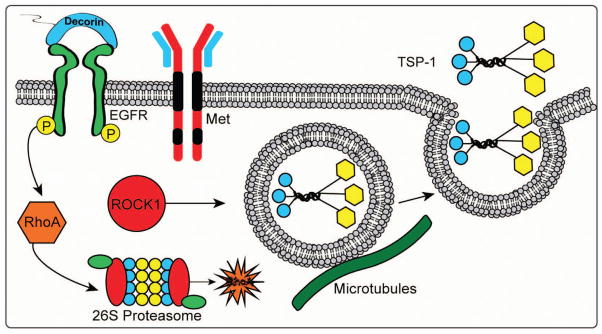
Diagram depicting the role of decorin in inducing rapid release of thrombospondin-1 (TSP-1) from breast cancer cells via the EGFR. Soluble decorin functions as a partial agonist of EGFR to elicit RhoA degradation via the 26S proteasome. Loss of RhoA leads to deactivation of ROCK1 signaling and mobilization of TSP-1-enriched vesicles. This novel decorin-evoked bioactivity is mediated exclusively through the EGFR but not via the Met receptor. Please refer to text for additional details.
We initially hypothesized that this effect would be coordinated by Met as decorin, which behaves as a partial agonist of Met, triggers rapid phosphorylation of the Met catalytic domain (at Tyr-1234/5) within five to ten min before returning to baseline [31]. Rapid TSP-1 release might be related to this spike in phosphorylation of the intracellular domains of Met immediately prior to receptor internalization. However, this was found to not be the case. Instead, decorin requires signaling via EGFR, as AG1478 and function blocking antibody mAb425 abrogated the immediate early release of TSP-1 (Fig. 6). This is consistent with the role of decorin in also triggering EGFR phosphorylation upon binding. This phosphorylation signature allows for the swift release of TSP-1 from MDA-MB-231 cells. This effect is intriguing insofar as this particular cell type expresses both Met and EGFR; however, decorin coordinates specific cellular events depending on the receptor it engages and thus integrates the signal over the repertoire of receptors known to bind decorin. This is thought to be mediated by differential binding affinities decorin has for EGFR and Met (Kd of ~80 nM and 2 nM, respectively). Despite the fact that decorin has a higher binding affinity for Met when compared to EGFR, we have clearly shown that Met does not play a role in TSP-1 release. Intrinsic differences that exist between EGFR and Met may influence decorin binding to EGFR, such as the topological requirements conferred by EGFR and/or the manifestation of fundamentally different phosphorylation patterns from that of Met to allow for TSP-1 release. Domain swapping experiments to precisely identify the bioactive determinants responsible for these differences between EGFR and Met will be of great importance for this phenomenon. Further, these data establish for the first time a role of decorin to not only bind, but also as a requirement to signal via an RTK to elicit downstream cellular events to achieve angiostasis.
Our results indicate that decorin-evoked TSP-1 release is not due to enhanced early transcriptional activity as TSP-1 levels peaked within 10 min of stimulation. Thus, we began to analyze downstream effectors of EGFR that could coordinate TSP-1 release. It has been established that Ras/RhoA/ROCK1 signaling is important in the control of Myc to repress THBS1, [69]. Increases in general protein secretion has been achieved through the inhibition of ROCK1 [87] and has been found to also be the case for decorin evoked TSP-1 secretion as no further secretion of TSP-1 was found to occur either upon small molecule inhibition or siRNA-mediated silencing of ROCK1. Furthermore, as no apparent changes in total ROCK1 levels occurred, we found a prominent degradation via the 26S proteasome of RhoA, the key small GTPase that activates ROCK1 to be required for this bioactivity (Fig. 6). Moreover, blocking degradation of RhoA prevented decorin-dependent release of TSP-1. Consistent with the finding of EGFR-dependent signaling, blocking with AG1478 also prevented RhoA degradation. These data identify not only a further anti-metastatic role for decorin through powerful suppression and degradation of RhoA, but also through derepression of potent angiostatic agent secretion in a non-canonical RhoA/ROCK1 dependent manner. Therefore, an immediate early loss of RhoA would presumably preclude activation of ROCK1 leading to the swift derepression of TSP-1 release and thus provide a mechanistic basis for this phenomenon downstream of EGFR signaling.
Finally we demonstrate the TSP-1 release does not involve secretion of TSP-1 from nanovesicles such as exosomes, but does depend on a stable microtubule network that is highly sensitive to the levels of RhoA and dependent on EGFR. This has several ramifications for the overall ability of decorin to impede tumorigenesis and metastatic capacity. Since decorin requires competent microtubule polymerization to drive secretion of TSP-1 (Fig. 6), stable microtubule growth is also a major detriment to cell motility and migration, as a decrease in microtubule stabilization leads to increased tumor cell invasion [88]. Interestingly, this is dependent on the activity of p27Kip1 [89], a cyclin-dependent kinase inhibitor inducible by decorin [90]. Therefore, rapid secretion of TSP-1 via RhoA/ROCK1 inhibition might be dependent on the activity of p27Kip1 thus leading to microtubule stabilization.
These data indicate an exceptionally early ability of decorin to begin to transform the surrounding tumor microenvironment by inducing secretion of soluble angiokine factors that inhibit tumor angiogenesis from the perspective of the tumor proper. Indeed, the profound effect decorin has on the tumor microenvironment is further exemplified in a recent study via systemic administration of decorin protein core to a triple negative orthotopic breast carcinoma xenograft model [91]. Decorin protein core was able to exclusively modulate stroma-specific (Mus musculus origin) genes without significant modulation of tumor xenograft (Homo sapiens) transcripts and thus resulting in a defined tumor microenvironment gene signature [91]. The mechanism of action remains quite unclear as to how soluble decorin, canonically viewed as a pan-RTK inhibitor acting on the tumor proper, is able to reprogram the surrounding tumor microenvironment through transcriptional induction of potent tumor suppressor genes. It is conceivable that decorin, acting on the tumor proper via RTK engagement, is able to prime the tumor microenvironment through the release of anti-angiogenic mediators to initiate this signature obtained from transcriptomic profiling. This might be of utmost importance for the contributions of endothelial cell-mediated angiogenesis following TSP-1 binding to CD36 and CD47.
In conclusion, our data further establish decorin as a key inhibitor of tumorigenesis by contributing to the so-called “tumor secretome” and further ameliorating the competency of the tumor proper to initiate and sustain competent angiogenic processes from the stroma.
Materials and methods
Cells, inhibitors and antibodies
MDA-MB-231 triple negative breast carcinoma cells were obtained from the American Type Culture Collection (Manassas, VA). The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) that was supplemented with 5% fetal bovine serum (FBS)(SAFC Biosciences, Lenexa, KS) and 100μg·mL−1 penicillin/streptomycin (MediaTech, Manassas, VA). Primary antibodies against ROCK1 (H-85), used at a 1:1000 dilution, RhoA (26C4), used at a 1:500 dilution, and Caveolin-1 (1-C), also used at a 1:500 dilution, were purchased from Santa Cruz Biotechnology (Santa Cruz, CA); mouse monoclonal anti-thrombospondin-1 primary antibody (used at 1:500) came from Abcam (Boston, MA). Secondary antibodies, Goat-α-Rabbit IgG HRP (AP307P) and Donkey-α-mouse IgG HRP (AP192P) were from Millipore (Billerica, MA) and used at 1:2000 and 1:6000 dilutions, respectively. Hepatocyte growth factor (H9661), lactacystin (L6785), and nocodazole (M1404) were purchased from Sigma-Aldrich (St. Louis, MO). The Met kinase inhibitor SU11274 (448101), the ROCK1 inhibitor Y27632 (68800) and EGFR kinase inhibitor AG1478 (658552) were from Calbiochem (Darmstadt, Germany). The mouse monoclonal EGFR blocking antibody was a generous gift from Dr. Ulrich Rodeck (Kimmel Cancer Center, Thomas Jefferson University). The purification of decorin protein core has been described extensively elsewhere [41,91]. Concisely recombinant human decorin, existing as a poly-His6 fusion protein, was expressed in 293-EBNA cells. The 293-EBNA cells were serum starved for maximal secretion of both decorin species: the glycanated and unglycanated forms of human decorin. This material was then passed on an Ni-NTA chelating column followed by exposure to increasing concentrations of imidazole (up to 250 mM) in 20 mM Tris-HCl, 500 mM NaCl, 0.2% CHAPS, pH 8.0 for elution. Finally, decorin was separated via anion-exchange chromatography on Q-Sepharose.
Immunoblotting
Following the end points of the specified treatments, MDA-MB-231 cells were briefly washed in ice-cold phosphate-buffered-saline and lysed in radioimmune precipitation assay (RIPA) buffer (1% Trition X-100, 20 mM Tris-HCl, pH 8.0, 137 mM NaCl, 10% glycerol, 2 mM EDTA, 1 mM Na3VO4, 10 μg/ml aprotinin, and 10 μg/ml leupeptin) for 20 min on ice. Samples were resolved by SDS-PAGE on 8%, 10%, or 12% gels subsequent to transfer on nitrocellulose for immunoblotting against the desired protein target. Equivalent loading and normalization of the samples were confirmed following Coomassie Blue staining carried out on a non-transferred portion of the SDS-PAGE gel.
Slot blot assays for detection of secreted TSP-1
Slot blot analysis of secreted TSP-1 in MDA-MB-231 cells entailed treating with 200 nM decorin protein core at the indicated time points as required by the experimental condition, following a 2 h serum starvation. MDA-MD-231 cells were seeded at a density of approximately 3×105 cells within 6-well dishes and allowed to grow to around 85% confluency (reflects about 1×106 cells). Next, tumor conditioned media (TCM) was then collected, centrifuged, and passed through disposable 0.22 μm syringe-driven filter unit (Millipore). Serial dilutions (maintaining a total volume of 400 μL) of the conditioned media were applied to the sample acceptor of the slot blot apparatus with suction (60 mBar) for a minimum of 30 min to ensure sample adsorption to the nitrocellulose membrane. The resulting membrane was blocked overnight in 1% BSA and incubated with a 1:500 dilution of the mouse monoclonal anti-thrombospondin-1 primary antibody, followed by incubation with a 1:6000 dilution of the Donkey-α-mouse IgG HRP-conjugated secondary, with visualization via enhanced chemiluminescence on an ImageQuant LAS-4000 (GE Healthcare). We quantified, via densitometry on the ImageJ program (NIH, Bethesda, MD), the top band (as reported above) which represents a 1:2 dilution (200 μL of TCM) while the bottom band is a 1:4 dilution (100 μL of TCM) within a constant volume of 400 μL (DMEM was used as diluent) loaded into each slot and reacted with the anti-TSP-1 antibody. Quantification of secreted TSP-1 bands was normalized to total cell number over time. The 1:2 and 1:4 dilutions were averaged and reported. This method was applied for all subsequent slot blot analyses.
Confocal microscopy, immunofluorescence imaging and quantification
Confocal microscopy and immunofluorescence studies were performed as described previously [52,92,93]. Briefly, approximately 5×104 MDA-MB-231 cells were seeded on 4-well glass chamber slides (BD Biosciences), which had been previously coated with 0.2% gelatin. MDA-MB-231 cells were serum starved (2 h) prior to incubation with decorin (200 nM). Cells were then washed with PBS and fixed using 4% paraformaldehyde on ice for 20 min, blocked in PBS 1% BSA overnight at 4°C, incubated with appropriate primary antibodies (1 h) at room temperature, washed in PBS, and then incubated in secondary antibody (goat anti-rabbit IgG Alexa-Fluor®488, Invitrogen) [94]. The nuclei were stained with DAPI (Vector Laboratories, Inc., Burlingame, CA). Images were obtained with a 63x, 1.3 oil-immersion objectives on a Zeiss LSM-780 confocal laser scanning microscope. The images were acquired with the ZEN 2010 software with the filters set at 488 nm for imaging and further analyzed with ImageJ and Adobe Photoshop CS5.1 (Adobe Systems, San Jose, CA). For the Immunofluorescence imaging studies, the slides were visualized using a Leica DM5500B microscope equipped with a Leica D-LUX3 camera in conjunction with the Advanced Fluorescence 1.8 software (Leica Microsystems, Inc.). Quantification of the relative fluorescence intensity of the TSP-1 signal was done via ImageJ [95]. Representative images were converted to black and white images, using the split channel option. The background was then subtracted and the threshold adjusted so that only fluorescent particles remained. The total amount of fluorescent particles was then quantified using the analyze particle option in ImageJ.
siRNA-mediated silencing of ROCK1
Transient transfection utilizing siRNA has been described elsewhere [56]. Briefly, ROCK1 was silenced though utilization of a cocktail comprised of three validated siRNAs specific for ROCK1 mRNA (ROCK1 siRNA sc-29473 Santa Cruz Biotechnology). Six-well plates of MDA-MB-231 cells (containing ~ 2×105 cells) were transfected with either the siScramble control (sc-37007, Santa Cruz Biotechnology) at 20 pM or siROCK1 at 60 pM using diluted Lipofectamine 2000 (Invitrogen) in transfection medium (1% BCS-DMEM) at ~70% confluency. The transfection was carried out for a total of 48 h at 37°C followed by treatment with decorin (200 nM) at the indicated time point. Validation of ROCK1 depletion was verified by immunoblotting using ROCK1 specific primary antibodies prior to slot blot analysis of the tumor-conditioned media for TSP-1.
Exosomal Isolation and Purification
Exosome isolation and purification was carried as described elsewhere [96]. Briefly, conditioned media from four 10-cm dishes with ~4 × 107 MDA-MB-231 cells was collected. Cells were treated for 6 h with 200 nM decorin in 1% BCS-DMEM whereupon media was pooled and centrifuged initially at 2000 · g for 15 min at 4°C, then 4000 · g for 15 min at 4°C in order to remove debris. Finally, exosomes were collected from the supernatant by ultracentrifugation at 105,000 · g for 90 min at 4°C. The resulting pellet was resuspended in PBS and subjected to immunoblotting analysis.
Statistical Analysis
Each experiment presented herein was repeated three or more times with a comparable pattern of responses. All data were expressed as means ±SEM. Results were statistically analyzed with the Student’s t-test or paired t-test using Sigma-Stat Software 11.0 (SPSS Inc). A probability value of P<0.05 was considered statistically significant.
Acknowledgments
We thank Ulrich Rodeck (Kimmel Cancer Center, Thomas Jefferson University) for the generous gift of the EGFR monoclonal blocking antibody, mAb425. This work was supported in part by the National Institutes of Health grants RO1 CA39481, RO1 CA47282, and RO1 CA120975 (to R.V.I.). T. Neill was supported by NIH training grant T32 AA07463 and this work is a partial fulfillment of T. Neill’s doctoral thesis in Cell and Developmental Biology, Thomas Jefferson University.
Abbreviations
- TSP-1
thrombospondin-1 protein
- VEGFA
vascular endothelial growth factor A
- RTK
receptor tyrosine kinase
- EGFR
Epidermal Growth Factor Receptor
- SLRP
small leucine rich proteoglycan
- ROCK1
Rho-associated coiled-coil containing protein kinase 1
- RhoA
Ras homolog gene family, member A
- mAb
monoclonal antibody
- CB
Coomassie Blue
- HGF
hepatocyte growth factor
- HIF-1α
hypoxia inducible factor 1-alpha
References
- 1.Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29:15–18. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- 2.Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8:464–478. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D, Winberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Butler JM, Kobayashi H, Rafii S. Instructive role of the vascular niche in promoting tumour growth and tissue repair by angiocrine factors. Nat Rev Cancer. 2010;10:138–146. doi: 10.1038/nrc2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Theocharis AD, Tzanakakis G, Karamanos NK. Proteoglycans in health and disease: Novel proteoglycan roles in malignancy and their pharmacological targeting. FEBS J. 2010;277:3904–3923. doi: 10.1111/j.1742-4658.2010.07800.x. [DOI] [PubMed] [Google Scholar]
- 6.Karamanos NK, Tzanakakis GN. Glycosaminoglycans: from ‘cellular glue” to novel therapeutical agents. Curr Opin Pharmacol. 2012;12:220–222. doi: 10.1016/j.coph.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Sottile J. Regulation of angiogenesis by extracellular matrix. Biochim Biophys Acta. 2004;1654:13–22. doi: 10.1016/j.bbcan.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Iozzo RV. Basement membrane proteoglycans: from cellar to ceiling. Nat Rev Mol Cell Biol. 2005;6:646–656. doi: 10.1038/nrm1702. [DOI] [PubMed] [Google Scholar]
- 9.Iozzo RV, Zoeller JJ, Nyström A. Basement membrane proteoglycans: Modulators par excellence of cancer growth and angiogenesis. Mol Cells. 2009;27:503–513. doi: 10.1007/s10059-009-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iozzo RV, Sanderson RD. Proteoglycans in cancer biology, tumour microenvironment and angiogenesis. J Cell Mol Med. 2011;15:1013–1031. doi: 10.1111/j.1582-4934.2010.01236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neill T, Schaefer L, Iozzo RV. Decorin, a guardian from the matrix. Am J Pathol. 2012;181:380–387. doi: 10.1016/j.ajpath.2012.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schaefer L, Iozzo RV. Biological functions of the small leucine-rich proteoglycans: from genetics to signal transduction. J Biol Chem. 2008;283:21305–21309. doi: 10.1074/jbc.R800020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vogel KG, Paulsson M, Heinegård D. Specific inhibition of type I and type II collagen fibrillogenesis by the small proteoglycan of tendon. Biochem J. 1984;223:587–597. doi: 10.1042/bj2230587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol. 1997;136:729–743. doi: 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Häkkinen L, Strassburger S, Kahari VM, Scott PG, Eichstetter I, Iozzo RV, Larjava H. A role for decorin in the structural organization of periodontal ligament. Lab Invest. 2000;80:1869–1880. doi: 10.1038/labinvest.3780197. [DOI] [PubMed] [Google Scholar]
- 16.Kalamajski S, Oldberd Å. The role of small leucine-rich proteoglycans in collagen fibrillogenesis. Matrix Biol. 2010;29:248–253. doi: 10.1016/j.matbio.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Robinson PS, Lin TW, Jawad AF, Iozzo RV, Soslowsky LJ. Investigating tendon fascicle structure-function relationship in a transgenic age mouse model using multiple regression models. Ann Biomed Eng. 2004;32:924–931. doi: 10.1023/b:abme.0000032455.78459.56. [DOI] [PubMed] [Google Scholar]
- 18.Robinson PS, Huang TF, Kazam E, Iozzo RV, Birk DE, Soslowsky LJ. Influence of decorin and biglycan on mechanical properties of multiple tendons in knockout mice. J Biomechanical Eng. 2005;127:181–185. doi: 10.1115/1.1835363. [DOI] [PubMed] [Google Scholar]
- 19.Rühland C, Schönherr E, Robenek H, Hansen U, Iozzo RV, Bruckner P, Seidler DG. The glycosaminoglycan chain of decorin plays an important role in collagen fibril formation at the early stages of fibrillogenesis. FEBS J. 2007;274:4246–4255. doi: 10.1111/j.1742-4658.2007.05951.x. [DOI] [PubMed] [Google Scholar]
- 20.Reed CC, Iozzo RV. The role of decorin in collagen fibrillogenesis and skin homeostasis. Glycoconj J. 2002;19:249–255. doi: 10.1023/A:1025383913444. [DOI] [PubMed] [Google Scholar]
- 21.Adany R, Heimer R, Caterson B, Sorrell JM, Iozzo RV. Altered expression of chondroitin sulfate proteoglycan in the stroma of human colon carcinoma. Hypomethylation of PG-40 gene correlates with increased PG-40 content and mRNA levels. J Biol Chem. 1990;265:11389–11396. [PubMed] [Google Scholar]
- 22.Iozzo RV. Proteoglycans and neoplasia. Cancer Metastasis Rev. 1988;7:39–50. doi: 10.1007/BF00048277. [DOI] [PubMed] [Google Scholar]
- 23.Danielson KG, Fazzio A, Cohen I, Cannizzaro LA, Eichstetter I, Iozzo RV. The human decorin gene: intron-exon organization, discovery of two alternatively spliced exons in the 5′ untranslated region, and mapping of the gene to chromosome 12q23. Genomics. 1993;15:146–160. doi: 10.1006/geno.1993.1022. [DOI] [PubMed] [Google Scholar]
- 24.Santra M, Danielson KG, Iozzo RV. Structural and functional characterization of the human decorin gene promoter. A homopurine-homopyrimidine S1 nuclease-sensitive region is involved in transcriptional control. J Biol Chem. 1994;269:579–587. [PubMed] [Google Scholar]
- 25.Mauviel A, Korang K, Santra M, Tewari D, Uitto J, Iozzo RV. Identification of a bimodal regulatory element encompassing a canonical AP-1 binding site in the proximal promoter region of the human decorin gene. J Biol Chem. 1996;271:24824–24829. doi: 10.1074/jbc.271.40.24824. [DOI] [PubMed] [Google Scholar]
- 26.Goldoni S, Owens RT, McQuillan DJ, Shriver Z, Sasisekharan R, Birk DE, Campbell S, Iozzo RV. Biologically active decorin is a monomer in solution. J Biol Chem. 2004;279:6606–6612. doi: 10.1074/jbc.M310342200. [DOI] [PubMed] [Google Scholar]
- 27.Iozzo RV, Moscatello D, McQuillan DJ, Eichstetter I. Decorin is a biological ligand for the epidermal growth factor receptor. J Biol Chem. 1999;274:4489–4492. doi: 10.1074/jbc.274.8.4489. [DOI] [PubMed] [Google Scholar]
- 28.Santra M, Eichstetter I, Iozzo RV. An anti-oncogenic role for decorin: downregulation of ErbB2 leads to growth suppression and cytodifferentiation of mammary carcinoma cells. J Biol Chem. 2000;275:35153–35161. doi: 10.1074/jbc.M006821200. [DOI] [PubMed] [Google Scholar]
- 29.Santra M, Reed CC, Iozzo RV. Decorin binds to a narrow region of the epidermal growth factor (EGF) receptor, partially overlapping with but distinct from the EGF-binding epitope. J Biol Chem. 2002;277:35671–35681. doi: 10.1074/jbc.M205317200. [DOI] [PubMed] [Google Scholar]
- 30.Goldoni S, Iozzo RV. Tumor microenvironment: Modulation by decorin and related molecules harboring leucine-rich tandem motifs. Int J Cancer. 2008;123:2473–2479. doi: 10.1002/ijc.23930. [DOI] [PubMed] [Google Scholar]
- 31.Goldoni S, Humphries A, Nyström A, Sattar S, Owens RT, McQuillan DJ, Ireton K, Iozzo RV. Decorin is a novel antagonistic ligand of the Met receptor. J Cell Biol. 2009;185:743–754. doi: 10.1083/jcb.200901129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schönherr E, Sunderkötter C, Iozzo RV, Schaefer L. Decorin, a novel player in the insulin-like growth factor system. J Biol Chem. 2005;280:15767–15772. doi: 10.1074/jbc.M500451200. [DOI] [PubMed] [Google Scholar]
- 33.Schaefer L, Tsalastra W, Babelova A, Baliova M, Minnerup J, Sorokin L, Gröne H-J, Reinhardt DP, Pfeilschifter J, Iozzo RV, Schaefer RM. Decorin-mediated regulation of fibrillin-1 in the kidney involves the insulin-like growth factor-1 receptor and mammalian target of rapamycin. Am J Pathol. 2007;170:301–315. doi: 10.2353/ajpath.2007.060497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Merline R, Lazaroski S, Babelova A, Tsalastra-Greul W, Pfeilschifter J, Schluter KD, Gunther A, Iozzo RV, Schaefer RM, Schaefer L. Decorin deficiency in diabetic mice: aggravation of nephropathy due to overexpression of profibrotic factors, enhanced apoptosis and mononuclear cell infiltration. J Physiol Pharmacol. 2009;60 (suppl 4):5–13. [PMC free article] [PubMed] [Google Scholar]
- 35.Iozzo RV, Buraschi S, Genua M, Xu S-Q, Solomides CC, Peiper SC, Gomella LG, Owens RT, Morrione A. Decorin antagonizes IGF receptor I (IGF-IR) function by interfering with IGF-IR activity and attenuating downstream signaling. J Biol Chem. 2011;286:34712–34721. doi: 10.1074/jbc.M111.262766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Csordás G, Santra M, Reed CC, Eichstetter I, McQuillan DJ, Gross D, Nugent MA, Hajnóczky G, Iozzo RV. Sustained down-regulation of the epidermal growth factor receptor by decorin. A mechanism for controlling tumor growth in vivo. J Biol Chem. 2000;275:32879–32887. doi: 10.1074/jbc.M005609200. [DOI] [PubMed] [Google Scholar]
- 37.Seidler DG, Goldoni S, Agnew C, Cardi C, Thakur ML, Owens RA, McQuillan DJ, Iozzo RV. Decorin protein core inhibits in vivo cancer growth and metabolism by hindering epidermal growth factor receptor function and triggering apoptosis via caspase-3 activation. J Biol Chem. 2006;281:26408–26418. doi: 10.1074/jbc.M602853200. [DOI] [PubMed] [Google Scholar]
- 38.Schaefer L, Iozzo RV. Small leucine-rich proteoglycans, at the crossroad of cancer growth and inflammation. Curr Opin Genet Dev. 2012;22:56–57. doi: 10.1016/j.gde.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 39.Reed CC, Gauldie J, Iozzo RV. Suppression of tumorigenicity by adenovirus-mediated gene transfer of decorin. Oncogene. 2002;21:3688–3695. doi: 10.1038/sj.onc.1205470. [DOI] [PubMed] [Google Scholar]
- 40.Goldoni S, Seidler DG, Heath J, Fassan M, Baffa R, Thakur ML, Owens RA, McQuillan DJ, Iozzo RV. An anti-metastatic role for decorin in breast cancer. Am J Pathol. 2008;173:844–855. doi: 10.2353/ajpath.2008.080275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reed CC, Waterhouse A, Kirby S, Kay P, Owens RA, McQuillan DJ, Iozzo RV. Decorin prevents metastatic spreading of breast cancer. Oncogene. 2005;24:1104–1110. doi: 10.1038/sj.onc.1208329. [DOI] [PubMed] [Google Scholar]
- 42.Merline R, Moreth K, Beckmann J, Nastase MV, Zeng-Brouwers J, Tralhão JG, Lemarchand P, Pfeilschifter J, Schaefer RM, Iozzo RV, Schaefer L. Signaling by the matrix proteoglycan decorin controls inflammation and cancer through PDCD4 and microRNA-21. Sci Signal. 2011;4:ra75. doi: 10.1126/scisignal.2001868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seidler DG, Mohamed NA, Bocian C, Stadtmann A, Hermann S, Schäfers K, Schäfers M, Iozzo RV, Zarbock A, Götte M. The role for decorin in delayed-type hypersensitivity. J Immunol. 2011;187:6108–6199. doi: 10.4049/jimmunol.1100373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moreth K, Iozzo RV, Schaefer L. Small leucine-rich proteoglycans orchestrate receptor crosstalk during inflammation. Cell Cycle. 2012;11:2084–2091. doi: 10.4161/cc.20316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nikitovic D, Aggelidakis J, Young MF, Iozzo RV, Karamanos NK, Tzanakakis GN. The biology of small leucine-rich proteoglycans in bone pathophysiology. J Biol Chem. 2012;287:33926–33933. doi: 10.1074/jbc.R112.379602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iozzo RV, Chakrani F, Perrotti D, McQuillan DJ, Skorski T, Calabretta B, Eichstetter I. Cooperative action of germline mutations in decorin and p53 accelerates lymphoma tumorigenesis. Proc Natl Acad Sci USA. 1999;96:3092–3097. doi: 10.1073/pnas.96.6.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bi X, Tong C, Dokendorff A, Banroft L, Gallagher L, Guzman-Hartman G, Iozzo RV, Augenlicht LH, Yang W. Genetic deficiency of decorin causes intestinal tumor formation through disruption of intestinal cell maturation. Carcinogenesis. 2008;29:1435–1440. doi: 10.1093/carcin/bgn141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bi X, Pohl NM, Yang GR, Gou Y, Guzman G, Kajdacsy-Balla A, Iozzo RV, Yang W. Decorin-mediated inhibition of colorectal cancer growth and migration is associted with E-cadherin in vitro and in mice. Carcinogenesis. 2012;33:326–330. doi: 10.1093/carcin/bgr293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Järveläinen H, Puolakkainen P, Pakkanen S, Brown EL, Höök M, Iozzo RV, Sage H, Wight TN. A role for decorin in cutaneous wound healing and angiogenesis. Wound Rep Reg. 2006;14:443–452. doi: 10.1111/j.1743-6109.2006.00150.x. [DOI] [PubMed] [Google Scholar]
- 50.De Luca A, Santra M, Baldi A, Giordano A, Iozzo RV. Decorin-induced growth suppression is associated with upregulation of p21, an inhibitor of cyclin-dependent kinases. J Biol Chem. 1996;271:18961–18965. doi: 10.1074/jbc.271.31.18961. [DOI] [PubMed] [Google Scholar]
- 51.Santra M, Mann DM, Mercer EW, Skorski T, Calabretta B, Iozzo RV. Ectopic expression of decorin protein core causes a generalized growth suppression in neoplastic cells of various histogenetic origin and requires endogenous p21, an inhibitor of cyclin-dependent kinases. J Clin Invest. 1997;100:149–157. doi: 10.1172/JCI119507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buraschi S, Pal N, Tyler-Rubinstein N, Owens RT, Neill T, Iozzo RV. Decorin antagonizes Met receptor activity and downregulates β-catenin and Myc levels. J Biol Chem. 2010;285:42075–42085. doi: 10.1074/jbc.M110.172841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iozzo RV, Schaefer L. Proteoglycans in health and disease: Novel regulatory signaling mechanisms evoked by the small leucine-rich proteoglycans. FEBS J. 2010;277:3864–3875. doi: 10.1111/j.1742-4658.2010.07797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grant DS, Kleinman HK, Goldberg ID, Bhargava MM, Nickoloff BJ, Kinsella JL, Polverini P, Rosen EM. Scatter factor induces blood vessel formation in vivo. Proc Natl Acad Sci USA. 1993;90:1937–1941. doi: 10.1073/pnas.90.5.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grant DS, Yenisey C, Rose RW, Tootell M, Santra M, Iozzo RV. Decorin suppresses tumor cell-mediated angiogenesis. Oncogene. 2002;21:4765–4777. doi: 10.1038/sj.onc.1205595. [DOI] [PubMed] [Google Scholar]
- 56.Neill T, Painter H, Buraschi S, Owens RT, Lisanti MP, Schaefer L, Iozzo RV. Decorin antagonizes the angiogenic network. Concurrent inhibition of Met, hipoxia inducible factor-1α and vascular endothelial growth factor A and induction of thrombospondin-1 and TIMP3. J Biol Chem. 2012;287:5492–5506. doi: 10.1074/jbc.M111.283499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Murphy-Ullrich JE, Iozzo RV. Thrombospondins in physiology and disease: New tricks for old dogs. Matrix Biol. 2012;31:152–154. doi: 10.1016/j.matbio.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mosher DF, Adams JC. Adhesion-modulating/matricellular ECM protein families: A structural, functional and evolutionary appraisal. Matrix Biol. 2012;31:155–161. doi: 10.1016/j.matbio.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 59.Armstrong LC, Bornstein P. Thrombospondins 1 and 2 function as inhibitors of angiogenesis. Matrix Biol. 2003;22:63–71. doi: 10.1016/s0945-053x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 60.Hosono Y, Yamaguchi T, Mizutani E, Yanagisawa K, Arima C, Tomida S, Shimada Y, Hiraoka M, Kato S, Yokoi K, Suzuki M, Takahashi T. MYBPH, a transcriptional target of TTF-1, inhibits ROCK1, and reduces cell motility and metastasis. EMBO J. 2012;31:481–493. doi: 10.1038/emboj.2011.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roberts DD, Miller TW, Rogers NM, Yao M, Isenberg JS. The matricellular protein thrombospondin-1 globally regulates cardiovascular function and responses to stress via CD47. Matrix Biol. 2012;31:162–169. doi: 10.1016/j.matbio.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Risher WC, Eroglu C. Thrombospondins as key regulators of synaptogenesis in the central nervous system. Matrix Biol. 2012;31:170–177. doi: 10.1016/j.matbio.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sweetwyne MT, Murphy-Ullrich JE. Thrombospondin 1 in tissue repair and fibrosis: TGF-β-dependent and independent mechanisms. Matrix Biol. 2012;31:178–186. doi: 10.1016/j.matbio.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kyriakides TR, MacLauchlan S. The role of thrombospondins in wound healing, ischemia, and the foreign body reaction. J Cell Commun Signal. 2009;3:215–225. doi: 10.1007/s12079-009-0077-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kazerounian S, Yee KO, Lawler J. Thrombospondins in cancer. Cell Mol Life Sci. 2008;65:700–712. doi: 10.1007/s00018-007-7486-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang X, Kazerounian S, Duquette M, Peruzzi C, Nagy JA, Dvorak HF, Parangi S, Lawler J. Thrombospondin-1 modulates vascular endothelial growth factor activity at the receptor level. FASEB J. 2009;23:3368–3376. doi: 10.1096/fj.09-131649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Adams JC, Lawler J. The thrombospondins. Cold Spring Harb Perspect Biol. 2011;3:1–29. doi: 10.1101/cshperspect.a009712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kalas W, Yu JL, Milson C, Rosenfield J, Benezra R, Bornstein P, Rak J. Oncogenes and angiogenesis: down-regulation of thrombospondin-1 in normal fibroblasts exposed to factors from cancer cells harboring mutant Ras. Can Res. 2005;65:8878–8886. doi: 10.1158/0008-5472.CAN-05-1479. [DOI] [PubMed] [Google Scholar]
- 69.Watnick RS, Cheng Y-N, Rangarajan A, Ince TA, Weinberg RA. Ras modulates myc activity to repress thrombospondin-1 expression and increase tumor angiogenesis. Cancer Cell. 2003;3:219–231. doi: 10.1016/s1535-6108(03)00030-8. [DOI] [PubMed] [Google Scholar]
- 70.Volpert O, Pili R, Sikder HA, Nelius T, Zaichuk T, Morris C, Shiflett CB, Devlin MK, Conant K, Alani RM. Id1 regulates angiogenesis through transcriptional repression of thrombospondin-1. Cancer Cell. 2002;2:473–483. doi: 10.1016/s1535-6108(02)00209-x. [DOI] [PubMed] [Google Scholar]
- 71.Swarbrick A, Åkerfeldt MC, Lee CSL, Sergio CM, Caldon CE, Hunter L-JK, Sutherland RL. Regulation of cyclin expression and cell cycle progression in breast epithelial cells by helix-loop-helix protein Id1. Oncogene. 2005;24:381–389. doi: 10.1038/sj.onc.1208188. [DOI] [PubMed] [Google Scholar]
- 72.Majack RA, Mildbrandt J, Dixit VM. Induction of thrombospondin messenger RNA levels occurs as an immediate primary response to platelet-derived growth factor. J Biol Chem. 1987;262:8821–8825. [PubMed] [Google Scholar]
- 73.Berthou S, Aebersold DM, Schimdt LS, Stroka D, Heigl C, Streit B, Stalder D, Gruber G, Liang S, Howlett AR, Candinas D, Greiner RH, Lipson KE, Zimmer Y. The Met kinase inhibitor SU11274 exhibits a selective inhibition pattern toward different receptor mutated variants. Oncogene. 2004;23:5387–5393. doi: 10.1038/sj.onc.1207691. [DOI] [PubMed] [Google Scholar]
- 74.Zhang Y-W, Su Y, Volpert OV, Vande Woude GF. Hepatocyte growth factor/scatter factor mediates angiogenesis through positive VEGF and negative thrombospondin 1 regulation. Proc Natl Acad Sci USA. 2003;100:12718–12723. doi: 10.1073/pnas.2135113100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Levitzki A, Gazit A. Tyrosine kinase inhibition:an approach to drug development. Science. 1995;267:1782–1788. doi: 10.1126/science.7892601. [DOI] [PubMed] [Google Scholar]
- 76.Rodeck U, Herlyn M, Herlyn D, Molthoff C, Atkinson B, Varello M, Steplewski Z, Koprowski H. Tumor growth modulation by a monoclonal antibody to the epidermal growth factor receptor: immunologically mediated and effector cell-independent effects. Cancer Res. 1987;47:3692–3696. [PubMed] [Google Scholar]
- 77.Zheng B, Liang L, Wang C, Huang S, Cao X, Zha R, Liu L, Jia D, Wu J, Ye Y, Wang Q, Long Z, Zhou Y, Du C, He X, Shi Y. MicroRNA-148a suppresses tumor cell invasion and metastasis by downregulating ROCK1 in gastric cancer. Clin Cancer Res. 2011;17:7574–7583. doi: 10.1158/1078-0432.CCR-11-1714. [DOI] [PubMed] [Google Scholar]
- 78.Lee DH, Shi J, Jeoung NH, Kim MS, Zabolotny JM, Lee SW, White MF, Wei L, Kim B-K. Targeted disruption of ROCK1 causes insulin resistance in vivo. J Biol Chem. 2009;284:11776–11780. doi: 10.1074/jbc.C900014200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ishizaki T, Maekawa M, Fujisawa K, Okawa K, Iwamatsu A, Fujita A, Watanabe N, Saito Y, Kakizuka A, Morii N, Narumiya S. The small GTP-binding protein Rho binds to and activates a 160 kDa Ser/Thr protein kinase homologous to myotonic dystrophy kinase. EMBO J. 1996;15:1885–1893. [PMC free article] [PubMed] [Google Scholar]
- 80.Aznar S, Lacal JC. Rho signals to cell growth and apoptosis. Cancer Lett. 2001;165:1–10. doi: 10.1016/s0304-3835(01)00412-8. [DOI] [PubMed] [Google Scholar]
- 81.Sahai E, Marxhall CJ. RHO-GTPases and cancer. Nature Rev Cancer. 2002;21:133–142. doi: 10.1038/nrc725. [DOI] [PubMed] [Google Scholar]
- 82.Fritz G, Brachetti C, Bahlmann F, Schmidt M, Kaina B. Rho GTPases in human breast tumours: expression and mutation analyses and correlation with clinical parameters. Brit J Cancer. 2002;87:635–644. doi: 10.1038/sj.bjc.6600510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Filipazzi P, Bürdek M, Villa A, Rivoltini L, Huber V. Recent advances on the role of tumor exosomes in immunosuppression and disease progression. Semin Cancer Biol. 2012;22:342–349. doi: 10.1016/j.semcancer.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 84.Henderson MC, Azorsa DO. The genomic and proteomic content of cancer cell-derived exosomes. Front Oncol. 2012;2 (Article 38):1–19. doi: 10.3389/fonc.2012.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Logozzi M, De Milito A, Lugini L, Borghi M, Calabrò L, Spada M, Perdicchio M, Marino ML, Federici C, Lessi E, Brambilla D, Venturi G, Lozupone F, Santinami M, Huber V, Maio M, Rivoltini L, Fais S. High levels of exosomes expressing CD36 and caveolin-1 in plasma of melanoma patients. PLoS ONE. 2009;4:e5219. doi: 10.1371/journal.pone.0005219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hamano Y, Sugimoto H, Soubasakos MA, Kieran M, Olsen BR, Lawler J, Sudhakar A, Kalluri R. Thrombospondin-1 associated with tumor microenvironment contributes to low-dose cyclophosphamide-mediated endothelial cell apoptosis and tumor growth suppression. Cancer Res. 2004;64:1570–1574. doi: 10.1158/0008-5472.can-03-3126. [DOI] [PubMed] [Google Scholar]
- 87.Hodges RR, Guilbert E, Shatos MA, Natarajan V, Dartt DA. Phospholipase D1, but not D2, regulates protein secretion via Rho/ROCK in a Ras/Raf-independent, MEK-dependent manner in rat lacrimal gland. Inv Ophtalm Vis Sci. 2011;52:2199–2210. doi: 10.1167/iovs.10-6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wong CCL, Gilkes DM, Zhang H, Chen J, Wei H, Chaturvedi P, Fraley SI, Wong C-M, Khoo U-S, Ng IOL, Wirtz D, Semenza GL. Hypoxia-inducible factor 1 is a master regulator of breast cancer metastatic niche formation. Proc Natl Acad Sci USA. 2011;108:16369–16374. doi: 10.1073/pnas.1113483108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Belletti B, Pellizzari I, Berton S, Fabris L, Wolf K, Lovat F, Schiappacassi M, D’Andrea S, Nicoloso MS, Lovisa S, Sonego M, Defilippi P, Vecchione A, Colombatti A, Friedl P, Baldassarre G. p27Kip1 controls cell morphology and motility by regulating microtubule-dependent lipid raft recycling. Mol Cell Biol. 2010;30:2229–2240. doi: 10.1128/MCB.00723-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xaus J, Comalada M, Cardó M, Valledor AF, Celada A. Decorin inhibits macrophage colony-stimulating factor proliferation of macrophages and enhances cell survival through induction of p27Kip1 and p21Waf1. Blood. 2001;98:2124–2133. doi: 10.1182/blood.v98.7.2124. [DOI] [PubMed] [Google Scholar]
- 91.Buraschi S, Neill T, Owens RT, Iniguez LA, Purkins G, Vadigepalli R, Evans B, Schaefer L, Peiper SC, Wang Z, Iozzo RV. Decorin protein core affects the global gene expression profile of the tumor microenvironment in a triple-negative orthotopic breast carcinoma xenograft model. PLoS ONE. 2012;7:e45559. doi: 10.1371/journal.pone.0045559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gonzalez EM, Reed CC, Bix G, Fu J, Zhang Y, Gopalakrishnan B, Greenspan DS, Iozzo RV. BMP-1/Tolloid-like metalloproteases process endorepellin, the angiostatic C-terminal fragment of perlecan. J Biol Chem. 2005;280:7080–7087. doi: 10.1074/jbc.M409841200. [DOI] [PubMed] [Google Scholar]
- 93.Goyal A, Pal N, Concannon M, Paulk M, Doran M, Poluzzi C, Sekiguchi K, Whitelock JM, Neill T, Iozzo RV. Endorepellin, the angiostatic module of perlecan, interacts with both the α2β1 integrin and vascular endothelial growth factor receptor 2 (VEGFR2) J Biol Chem. 2011;286:25947–25962. doi: 10.1074/jbc.M111.243626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ryynänen M, Ryynänen J, Solberg S, Iozzo RV, Knowlton RG, Uitto J. Genetic linkage of Type VII collagen (COL7A1) to dominant dystrophic epidermolysis bullosa in families with abnormal anchoring fibrils. J Clin Invest. 1992;89:974–980. doi: 10.1172/JCI115680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Goyal A, Poluzzi C, Willis AC, Smythies J, Shellard A, Neill T, Iozzo RV. Endorepellin affects angiogenesis by antagonizing diverse VEGFR2- evoked signaling pathways: transcriptional repression of HIF-1α and VEGFA and concurrent inhibition of NFAT1 activation. J Biol Chem. 2012;287:43543–43556. doi: 10.1074/jbc.M112.401786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lo Cicero A, Majkowska I, Nagase H, Di Liegro I, Troeberg L. Microvescicles shed by oligodendroglioma cells and rheumatoid synovial fibroblasts contain aggrecanase activity. Matrix Biol. 2012;31:229–233. doi: 10.1016/j.matbio.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]