Abstract
Antidepressant treatments enhance plasticity and increase neurogenesis in the adult brain, but it has been unclear how these effects influence mood. We propose that like environmental enrichment and exercise, antidepressant treatments enhance adaptability by increasing structural variability within the nervous system at many levels, from proliferating precursors to immature synaptic contacts. Conversely, sensory deprivation and chronic stress reduce this structural variability. Activity-dependent competition within the mood-related circuits, guided by rehabilitation, then selects for the survival and stabilization of those structures that best represent the internal or external milieu. Increased variability together with competition-mediated selection facilitates normal function, such as pattern separation within the dentate gyrus and other mood-related circuits, thereby enhancing adaptability towards novel experiences.
Keywords: Antidepressants, enrichment, stress, neurogenesis, synaptogenesis, plasticity
Neuronal plasticity: growth and change
The extrinsic and intrinsic milieu becomes represented in the structure and function of neuronal networks during development through neuronal plasticity. In adulthood, this representation is continuously optimized and modulated through plasticity and learning [1]. Plasticity occurs at several levels, from neurogenesis to the adjustment of synaptic weights, and modulates both the structure and function of neuronal networks (Figure 1). Changes in function of the nervous system are essentially always based on a structural change at some level (neuronal, synaptic, protein, or genomic structure), and it is therefore difficult to make clear distinctions between functional and structural plasticity.
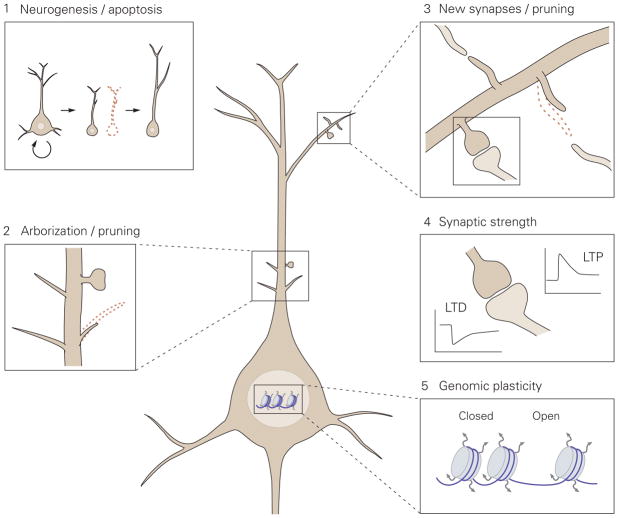
Figure 1. Proposed model for the levels of neuronal plasticity in antidepressant activity.
Neuronal plasticity acts at different structural levels and bidirectionally to influence variability and selection within neuronal networks. Increased formation of structures at any of these levels by, for example, environmental enrichment (EE) or chronic antidepressant treatment, generates variability and promotes competition between similar structures for stabilization, thereby enhancing adaptability, even if the total number of structures is not increased due to the simultaneous increase in structural elimination. 1. Neurogenesis and selective apoptosis. Increased precursor proliferation in the dentate gyrus, induced by antidepressants and EE, leads to the increased survival of newborn neurons that successfully mediate activity within the hippocampal circuitry. Neurons that fail to functionally integrate into the hippocampal circuitry are eliminated through apoptosis. 2. Arborization and pruning of axonal and dendritic branches. The increased dynamics of nascent branches promotes the stabilization of branches containing synapses that successfully represent environmental conditions, whereas arbors without active synapses remain short-lived and are pruned. 3. Synaptogenesis and synaptic elimination. Immature “trial synapses” between two neurons are initiated by filopodial extension from pre- or postsynaptic sites. Synapses that are successfully activated during the trial period are preferentially selected for stabilization, whereas contacts that fail to mediate activity collapse and are eliminated. 4. Plastic regulation of synaptic strength. Information transfer through active synapses is potentiated through the process of long-term potentiation (LTP), whereas inactive or inappropriately active synapses are suppressed through long-term depression (LTD). 5. Environmental activity regulates the transcription and translation of effector genes involved in neuronal plasticity through transcriptional control and epigenetic mechanisms, such as remodeling of chromatin structure from a closed to an open state.
Plasticity can be conceptualized as two distinct processes. First, structural variability is generated through the overproduction of immature neuronal structures, which occurs at many levels from new-born neurons to the outgrowth of filopodia (Figure 1). Second, selective stabilization among the overproduced structures retains those that best represent the internal or external milieu [2]. Structural variability may be stochastic, although it may also be genetically tuned, but selection is an active process driven by neuronal activity that reflects both extrinsic and intrinsic stimuli. Activity-dependent selection guides the stabilization of functionally relevant neurons and connections by utilizing genes involved in survival and synaptogenesis, or it eliminates weakly or incoherently active structures through the use of genes that underlie apoptosis and pruning [1,3]. The elimination of weakly active neurons and connections is critical for optimizing the signal-to-noise ratio in neuronal networks. Neuronal plasticity can be compared to auditions for a Broadway show: if many candidates audition, the production team can select an optimal performer for each role (and send those not suitable back home), but if only a few people show up, almost all have to be utilized regardless of their talent.
During the past few years, neuronal plasticity, and in particular adult neurogenesis, has been implicated in the beneficial effects of antidepressant drugs and electroconvulsive shock treatment (ECS) [4–6][7]. However, it has remained unclear how plasticity and neurogenesis impact mood and anxiety-related behaviors.
Here, we provide a framework for how chronic antidepressant drug treatment and ECS might utilize neurogenesis and other forms of neuronal plasticity to influence mood. We argue that antidepressants, environmental enrichment, and exercise increase structural variability at various levels in the nervous system, thereby offering more substrates for the selection process (Figure 1) [7]. Conversely, sensory deprivation and chronic stress would reduce variability and impair adaptability. Whereas acute stress may promote variability and adaptation, chronic stress is considered maladaptive and is associated with a loss of neurons and synapses [8,9]. Furthermore, stress may increase activity in certain brain regions, such as the amygdala and the mesolimbic dopaminergic system, leading to hypertrophy of these structures.
We argue that chronic antidepressant treatments and ECS act - at least in part - by utilizing a similar mechanism to increase adaptability and facilitate structural and functional reorganization in neuronal networks that have evolved to boost the effects of environmental enrichment, although the cellular and molecular mechanisms may be different. We further suggest that the effects of enrichment and antidepressant treatments that increase plasticity take place at many levels within neuronal networks (Figure 1). Because experience-guided selection constantly eliminates inactive structures, an increase or decrease in variability does not need to influence the mean number of these structures in brain (like counting the number of actors of the final cast does not tell you how many have auditioned), which makes detecting such a change in variability difficult. Therefore, it is currently unclear whether the effects of antidepressants are confined to particular brain areas or whether they occur more ubiquitously throughout the brain. Nevertheless, tougher competition between variable structures increases adaptability towards changes in the external or internal milieu, analogous to the way increased genetic variability contributes to the survival of a species facing a changing environment in the context of evolution.
Neurogenesis in the dentate gyrus
Adult neurogenesis is an exceptional form of plasticity in which entire neurons are generated and selected for survival in only a very few regions of adult mammalian brain [10]. The two main areas where neurogenesis occurs are the subventricular zone that lines the ventricles and gives rise to neuronal precursors that migrate toward the olfactory bulb, and the subgranular zone that lines the dentate gyrus (DG) of the hippocampus and gives rise to DG granule cells (Figure 2) [10].
Figure 2.
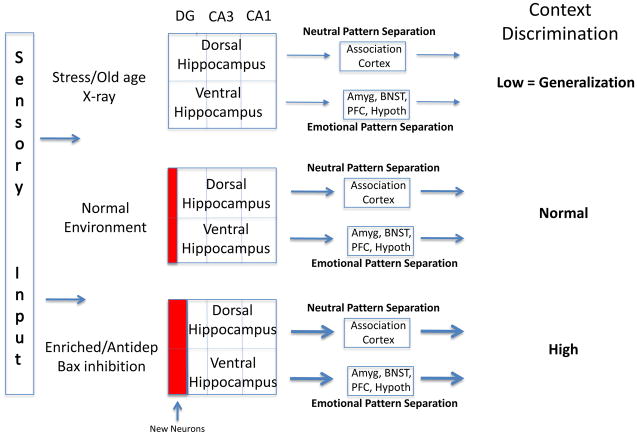
Effects of mood and environment on pattern separation and generalization. Information processing within the trisynaptic loop of the hippocampus [dentate gyrus (DG) => CA3 => CA1] is required for the discrimination between similar contexts. This process is termed pattern separation and is modulated by adult neurogenesis within the dentate gyrus (red boxes). Through different connectivity of the dorsal and ventral hippocampus (association cortices versus amygdala, bed nucleus of stria terminalis (BNST), prefrontal cortex, and hypothalamus), both neutral and emotionally charged contextual information is processed and discriminated. When adult neurogenesis is reduced or blocked by stress, aging, or experimental manipulations (such as X-ray treatment), discrimination is impaired, leading to generalization [17,19]. In contrast, increased neurogenesis, promoted by environmental enrichment, antidepressant treatment, or genetic manipulations (eg. Bax inhibition), improves pattern separation and discrimination [19,36]. Amyg, amygdala; BNST, bed nucleus of stria terminalis; PFC, Prefrontal cortex; Hypoth, hypothalamus; Antidep, antidepressant treatment.
Neurogenesis is a plastic process that is regulated by environmental factors. In the hippocampus, various stages of neurogenesis are stimulated by enriched environments, exercise, learning, and antidepressant drugs, and they are inhibited by chronic stress and aging [5,6,11]. Enrichment, antidepressants, and ECS stimulate the proliferation of neural stem cells, their differentiation into neurons, and the survival of the resulting young neurons (Figure 2) [4,12]. Conversely, both acute and chronic stress decrease proliferation and the survival of the newborn neurons in several species, including nonhuman primates [13,14], although increased neurogenesis has not been confirmed in all stress studies [13,14]. Newborn neurons have also been implicated in the adaptation to stress by buffering against it [16]. Furthermore, sensory deprivation decreases proliferation, the choice of a neuronal fate, and the survival of the young neurons [15]. These observations raise the possibility that changes in neurogenesis allow for a better adaptation to a changing environment either instructively by encouraging adaptive behavior [17] or permissively by increasing variability that is then utilized in experience-dependent selection. However, it is also possible that changes in neurogenesis are merely a consequence of these environmental changes. By reviewing the proposed functions of adult-born neurons, we can attempt to resolve these options.
Neurogenesis, pattern separation, and generalization
Recent functional studies of adult-born dentate granule neurons have focused on their potential role in pattern separation because increasing evidence from electrophysiological studies indicates that the DG is involved in pattern separation [18]. It has been proposed that pattern separation enables the processing of similar experiences as distinct memories and is critical for memory formation, although direct evidence for this mechanism is still missing. For example, pattern separation may enable us to remember two distinct beach vacations or where we last parked our car even though the parking garage and general context may be the same each morning.
In a number of rodent models, both loss and gain of function studies show that increases in neurogenesis improve, and decreases in neurogenesis impair, pattern separation [19,20]. Furthermore, some evidence exists indicating that young neurons are involved in discriminating between complex odor mixtures in the olfactory bulb, which may involve a process similar to pattern separation [21].
Pattern separation appears to be impaired both during normal aging and in individuals with Mild Cognitive Impairment [22]. In addition, functional imaging studies have identified abnormal activity in the dentate gyrus and CA3 of individuals with age related memory impairments when they perform a pattern separation task [23].
In the psychiatric literature, the term pattern separation is rarely used because it refers to a cognitive process that is usually not tested in psychiatric patients. However, generalization is a phenotype that is often associated with anxiety and mood disorders [24]. Generalization can be defined as our tendency to lump together similar experiences particularly when they have a strong emotional content. For example the sight of a plane flying over a skyscraper may remind us of 9/11. Generalization may therefore be considered the opposite of pattern separation. Generalization is a double-edged sword: in small doses it is clearly protective to avoid similar dangerous situations, for example, if you were hit by a car when you crossed a dangerous intersection a little generalization will enable you to be careful each time you cross this intersection; however, too much generalization may result in the fear to cross any street, which would clearly be maladaptive. Like pattern separation, generalization may be critical for memory formation because it may allow us to link similar memories rather than store them in unrelated categories. In the cognitive domain, the process that allows generalization is pattern completion, which has been proposed to take place in CA3 [25]. Thus, when new memories are encoded two processes are simultaneously at work: pattern separation to disambiguate similar situations and pattern completion (or generalization) to link similar or related events, particularly when they have a strong valence. There is indeed evidence that during memory encoding a balance between separation and completion allows for similar experiences to be stored either together or separately. For example, from an evolutionary point of view it is clearly important to store aversive memories together because any experience that is similar to a traumatic memory should be avoided.
Therefore, the DG appears to function in different regimes in different environmental situations. In a safe and rich environment, high pattern separation is advantageous because it increases the ability to discriminate between similar experiences, which may be optimal for a situation of high exploratory activity aimed at locating food and mates. In contrast, in a dangerous environment low pattern separation or generalization may be preferable because it will increase fear and promote avoidance rather than exploration [17,19].
The idea that variable levels of pattern separation have adaptive value raises the possibility that variable levels of neurogenesis may promote adaptation to a changing environment. The mechanisms through which increased neurogenesis contributes to pattern separation are still unclear (Box 1), but the fact that a major period of cell death occurs around the time that the neurons have established both pre and postsynaptic contacts suggests that the ability to contribute to the activity of the dentate gyrus is critical. This further suggests that adult neurogenesis may select the surviving neurons using principles similar to those that have been thoroughly investigated in the context of peripheral nervous system development. During early development, sensory and sympathetic neurons are produced in excess, and when their axons reach their target tissues, newborn neurons compete for access to a neurotrophic factor produced by the target cells. Those neurons that establish an optimal connection with the target cells receive a sufficient amount of the trophic factor and survive, whereas those that fail to optimally innervate the target are eliminated by programmed cell death [26,27].
Box 1. Outstanding questions.
The hypothesis that increase in structural variability induced by enrichment and antidepressant treatments promotes adaptability after activity-dependent selection is largely based on information derived from experiments performed in the peripheral nervous system and in primary sensory systems. Several important questions remain to be addressed before these principles can be extended to the higher cortical regions, such as those involved in the regulation of mood. These questions include:
Do antidepressants and enrichment promote synapse turnover in higher brain regions, such as the prefrontal cortex or the hippocampal CA1 area?
If enrichment and antidepressant treatments enhance variability, does this promote adaptability in mood-related behavior?
Are the effects of antidepressants confined to particular brain areas or do they occur more ubiquitously throughout the brain?
What mechanisms select newborn neurons for survival?
Enrichment and antidepressants reactivate critical period-like plasticity in the adult visual cortex in rodents, but do they produce similar reactivation in the human brain?
It is conceivable that a similar principle of redundancy and competition governs the selection of surviving neurons in the mammalian dentate gyrus, where neurogenesis continues into adulthood and where neuronal production, selection and elimination are continuously taking place. Although the hypothetical target-derived neurotrophic factor for the newborn DG neurons has not been identified, this factor should be regulated and released in an activity-dependent manner [28]. Indeed, brain-derived neurotrophic factor (BDNF), one of the prime candidates for this function, is regulated by neuronal activity [29]. We propose that when environmental conditions are changing, a newborn DG neuron with a slightly different activity pattern, reflecting different patterns of sensory input, has a higher probability to be selected for survival than newborn cells displaying activities very similar to the neurons that already exist in the mature DG. This idea is consistent with the increased survival of newborn neurons in an enriched environment. At least in the case of antidepressant treatment, there is evidence that increased survival of newborn neurons is balanced by an increase in the total number of apoptotic neurons within the DG, suggesting that although the newborn neurons are surviving, older neurons are eliminated [30]. Analogously, a Broadway producer planning for a new show looks forward to a large number of auditionees with diverse talent to replace at least some members of the current cast, even if the current cast had been excellent in the previous show. Therefore, when the input to the DG is variable, enhanced neurogenesis may be beneficial because it favors pattern separation.
Neurogenesis and antidepressant action
Plasticity that is based on neurogenesis operates on a different time scale than traditional forms of plasticity, such as spine or dendritic rearrangements that can occur much faster than the generation of new neurons. If variable levels of neurogenesis have an adaptive value it is likely to be in response to long lasting environmental changes such as those resulting from changing seasons. For example, neurogenesis varies in the hippocampus of birds that store food in hidden caches for retrieval in the winter. In these birds, neurogenesis is highest in the fall and winter when they hide and retrieve their food [31]. Similarly, treatments that increase neurogenesis are unlikely to produce a rapid behavioral response. This may be one reason why antidepressants have a delayed onset of therapeutic effect.
Evidence from animal models of anxiety and depression indicate that neurogenesis is necessary for some but not all effects of antidepressants [32,33]. Given the role of neurogenesis in pattern separation, we hypothesize that an improvement in pattern separation, particularly for situations and contexts that are emotionally charged, will impact mood and anxiety-related behaviors. Such an effect may be achieved by connections between the ventral hippocampus and the limbic system [34]. Unlike the dorsal part of the hippocampus that sends projections primarily to association cortices, the ventral hippocampus also sends projections to the amygdala, bed nucleus of stria terminalis, hypothalamus, and prefrontal cortex (PFC). In the PFC, projections from the ventral hippocampus have recently been shown to activate neurons that fire in response to anxiety-related modalities [35]. It is therefore possible that a particular context acquires a valence by virtue of the connections between the ventral hippocampus and the limbic system.
The process of neurogenesis may be harnessed to improve cognition and mood. A recent study demonstrated that inhibition of apoptotic cell death from the progeny of hippocampal neural stem cells significantly increases neurogenesis and improves pattern separation [36]. Interestingly this genetic manipulation had no impact on anxiety-related behaviors unless it was combined with exercise, which is consistent with the idea that enhanced survival benefits from the increased variability provided by exercise-induced precursor proliferation. Future studies in animal models are needed to investigate whether the combination of strategies aimed at stimulating neurogenesis (by inhibiting cell death or other means), together with behavioral enrichment or exercise, will result in antidepressant or anxiolytic-like effects.
Plasticity outside the DG
Increasing evidence suggests that enhanced neuronal plasticity induced by enrichment and chronic antidepressant treatment may not be restricted to neurogenesis (Figure 1). Furthermore, the mechanisms that mediate the effects of these treatments at smaller structural scales may be conceptually similar to those reviewed above for neurogenesis. However, technical limitations have hampered the recognition of these effects; it is difficult to detect changes in turnover of dendritic branches and spines when there are simultaneous changes in the rates these structures are produced and retracted without any change in the net number of structures. Therefore, it is currently unclear whether the effects of antidepressants are confined to mood-related circuits or are more widespread (Box 1). Nevertheless, at least in primary sensory areas, a change in turnover has significant functional consequences [37,38]. Developments in intravital microscopy of behaving animals is now circumventing these technical difficulties, and an increasing number of reports have focused on the dynamic effects of environmental manipulation on the structure of dendrites and axons [39,40].
Axonal and dendritic branches
It has been proposed that the construction of neural circuits proceeds through concurrent and nearly balanced growth and retraction of axonal and dendritic branches [3,41–43]. Nascent dendritic branches have been proposed to produce “trial synapses”, and only those trial synapses that receive appropriate synaptic input are preserved and the corresponding dendritic branch is stabilized [3]. Therefore, activity-dependent synapse stabilization appears to direct axonal and dendritic arbor selection and elimination (Figure 1).
There is evidence that antidepressant treatment and ECS increase the variability and turnover in the braches of dendrites and axons (Figure 1). Chronic fluoxetine administration simultaneously increases the elongation and retraction of branch tips in the mouse visual cortex [40] and ECS promotes axonal sprouting in the hippocampus [44], consistent with the idea that antidepressants increase variability in branch dynamics. Conversely, chronic mild stress has been shown to reduce volume as well as length and branching of apical dendrites within the DG, CA3 area, and the PFC in rats [45]. All of these effects were reversed by antidepressant treatment [45].
Synaptic connections
During early postnatal development, the density of synapses in the human cortex exceeds that found in adult brain by about twofold [46,47]. Although brain growth contributes to reduced spine density, it is thought that a net loss of synapses brings the synaptic density to adult level at adolescence. At least in the human PFC, synaptic pruning continues well into the 3rd decade [47]. It has been proposed that synaptic activity selects from the overproduced synapses those that are stabilized [2]. This activity-dependent process ensures that only those synapses that optimally represent external or internal input are retained and that those mediating random noise are eliminated [3,42].
Even in adulthood, synaptogenesis continues at a lower level, but if synaptic elimination occurs at a matching rate, the net number of synapses remains stable. Evidence from sensory cortices suggest that a simultaneous increase in spine formation and retraction increases adaptability by making a higher number of trial contacts available for selection (Figure 1) [40,48,49], as when a larger number of auditionees helps in selecting the optimal cast for a show. In higher cortical areas, evidence for a correlation between increased synapse turnover and improved function is lacking, due at least in part to technical difficulties (Box 1). It is possible that currently available methods underestimate the dynamic changes in the turnover of synaptic contacts that may take place after environmental changes or antidepressant drug administration (Figure 1C).
ECS increases the number of synapses in the hippocampus, but antidepressant treatment has only a minor effect on the net number of dendritic spines in the hippocampal CA1 area [50,51]. However, when the number of spines and synapses is abnormally downregulated by stress [52] or ovariectomy [53], the increasing effect of antidepressants on spine number becomes unmasked and fluoxetine treatment increases spine number back to the baseline level. This suggests that in normal hippocampus fluoxetine might simultaneously increase spine formation and elimination, thereby having only a minor effect on the net number of spines, but fluoxetine may produce this effect also indirectly through other mechanisms. A recent study that observed dendritic contacts repeatedly using 2-photon microscopy in the mouse visual cortex reported that chronic fluoxetine treatment simultaneously increased the elongation and retraction of dendritic branch tips [40].
Chronic stress and long-term glucocorticoid treatment leads to the loss of dendritic spines and synaptic contacts in the hippocampus and the PFC [8,54–56]. Chronic mild stress also increases the number of immature spines at the expense of mushroom-like mature spines in the apical dendrites of pyramidal neurons in the hippocampus and PFC, and these changes are largely reversible by antidepressant drug treatment [45]. A recent study reported that short-term glucocorticoid treatment increased spine dynamics in the mouse somatosensory cortex by simultaneously increasing spine formation and retraction and that inhibition of endogenous glucocorticoids reduces spine dynamics [9]. Consistent with earlier findings, long-term glucocorticoid treatment increased net spine elimination.
Plasticity of synaptic strength
In addition to synapse number, synaptic strength is also dynamically regulated by environmental experiences, including enriched environment, exercise, and antidepressant drugs. Chronic fluoxetine administration increases long-term potentiation (LTP) in the DG elicited in the absence of GABAA receptor (GABAAR) inhibitors, and this effect depends on the newborn neurons [12]. In the presence of GABAAR inhibitors, DG LTP is reduced, perhaps due to occlusion, and long-term depression (LTD) is enhanced [12,57,58]. Enrichment and fluoxetine enable LTP in the adult rat visual cortex [48,59], and a similar effect of fluoxetine treatment on LTP was observed in the murine amygdala [60]. These findings may be related to the “dematuration” process observed after chronic fluoxetine administration in the dentate granule neurons [58], indicating that antidepressant treatment reactivated a juvenile-like plasticity in brain [48,60]. Enriched environment and perhaps also fluoxetine treatment during early life accelerate cortical maturation [61–63]. Conversely, chronic mild stress facilitates LTD in the CA1 area and chronic antidepressant treatment blocked this LTD facilitation and enhanced LTP [64]. Thus, chronic antidepressant treatment and enriched environment may increase synaptic plasticity in several brain areas (Figure 1), which may be consistent with the increased dendritic spine dynamics and turnover induced by antidepressant treatment [40].
Genomic plasticity
The regulated expression and translation of specific genes by experience-dependent neuronal activity is a critical mechanism of neuronal plasticity [65–67]. Neuronal activity can influence gene expression by activating transcription factors or by inducing epigenetic changes in chromatin structure or DNA methylation [68–73] (Figure 1). Mutations in several genes regulated by activity are associated with neurodevelopmental disorders, which underlines the critical importance of this process for proper network connectivity [65,66].
BDNF is a critical mediator of neuronal activity and synaptic structure [74,75], and the production and release of BDNF is regulated by neuronal activity [74]. BDNF signaling through TrkB receptors promotes the survival of newborn neurons in the DG [30,76,77], enhances the outgrowth of axons and dendrites [43,78], stabilizes synapses, and promotes synaptic transmission [79,80]. The effects of enrichment and antidepressant drugs are at least partially mediated by BDNF signaling [30,48,77,81,82], although in brain areas that are activated by aversive stimuli, BDNF has pro-depressive effects [83]. Thus, the activity-dependent regulation of BDNF is a critical molecular mediator through which experience-dependent plasticity is translated into structural and functional changes in neuronal networks. If this were a Broadway show, BDNF would be a producer that selects actors and actresses for the cast from among the auditioning candidates.
Recent experimental and theoretical work suggests that environmental conditions may have relatively small or variable effects on the expression of individual genes, but they reliably increase the large-scale variability in gene expression, or the “genomic tone” [84,85]. High local variability in the DNA methylation rate without any change in the mean methylation level was recently discovered within variably methylated regions of genomes from several species [86] and may provide a mechanism for changes in the genomic tone [85]. This altered variation in methylation and gene expression without any change in the mean resembles the increase in structural variation and turnover in neurons and synapses discussed above, but it is not clear whether and to what extent the genome-level variation is causally related to variation in network dynamics. However, variability in gene expression levels does correlate with behavior [84]. Intriguingly, genes adjacent to the variably methylated regions have often been found to be functionally related to brain development and plasticity, even if the tissue analyzed was liver [86]. This suggests that variably methylated regions are associated with many genes involved in neuronal plasticity, which may induce variability in their expression levels throughout the organism.
The visual cortex as an example of experience-dependent plasticity
Critical period plasticity in the mammalian visual cortex is a well-characterized model for cortical development and plasticity [37,38,87,88] (Figure 3). It is widely thought that similar processes govern the development and tuning of neuronal connectivity in other cortical areas as well [1,88]. Recent studies have revealed that critical period-like plasticity can be reactivated in the adult visual cortex by a number of treatments, including enrichment and chronic fluoxetine treatment (Figure 3D) [40,48,59,62,89,90].
Figure 3.
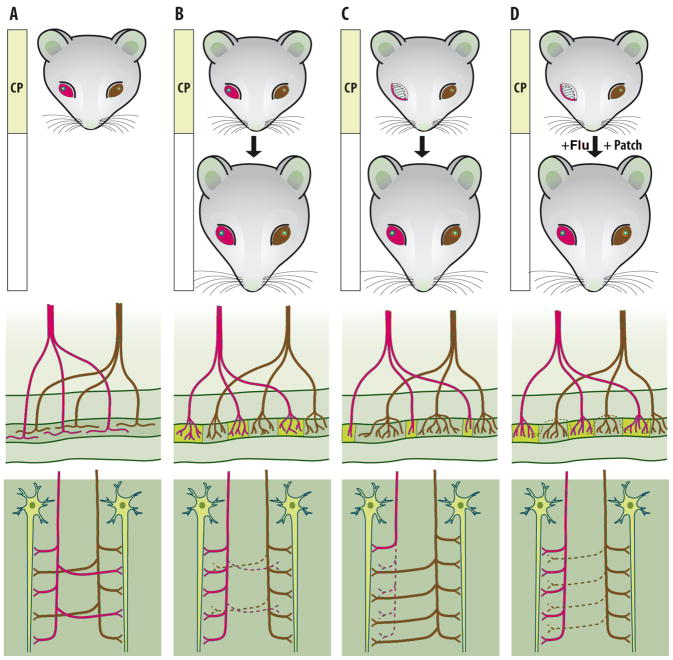
Plasticity of neuronal networks in mammalian primary visual cortex. A) Soon after eye-opening, inputs that mediate visual information from the left (blue) and right eye (red) diffusely innervate the entire layer IV of the primary visual cortex. B) During the postnatal critical period (CP), activity-dependent competition leads to the segregation of inputs from either eye into eye-specific regions, the ocular dominance (OD) columns. As a consequence, at the end of critical period, cortical neurons within a single column in the layer IV receive innervation predominantly from a single eye (middle panel). This segregation requires vision-induced neuronal activity such that axonal branches from one eye withdraw from the regions initially dominated by the other eye and elaborate connections in the own territory (lower panel). It should be noted that the OD columns cannot be detected in the primary visual cortex as any visible structures, even though they are differentially colored in the figure for the sake of clarity. C) Development of a normal network requires balanced use of both eyes. If one eye is deprived of vision during the critical period, the inputs mediating information from it lose in activity-dependent competition and withdraw [38]. The inputs mediating visual information from the open eye are active and overcome most of the visual cortex. If the vision of the deprived eye is not corrected and encouraged during the critical period, the network guided by only one open eye remains permanent even if the deprived eye was opened in adulthood. D) Fluoxetine treatment (Flu)[48], or exposure to an enriched environment in adulthood [59], reopens the critical period-like plasticity. This enables a reorganization of the network, if the deprived eye is opened in adulthood and encouraged by a temporary patching of the previously open eye. Under these conditions, the now more active deprived eye can regain territories within the layer IV, which gradually leads to a normal network guided by two open eyes. Note that under all these conditions, the net number of synapses remains constant (lower panel), only the source of visual information varies.
Reactivation of developmental plasticity in adult brain is apparently not restricted to the visual cortex. Chronic fluoxetine treatment induces a dematuration of neurons in the mouse DG that extends to the already matured granule neurons [58]. A recent study used the fear-conditioning paradigm to show that chronic fluoxetine treatment increases neuronal plasticity in the amygdala and leads to the long-term removal of conditioned fear response when fluoxetine treatment is combined with extinction training; neither fluoxetine treatment nor extinction training alone produced a long-term fear removal [60]. These findings demonstrate that enriched environment or fluoxetine treatment in adult animals reactivates a critical period-like plasticity, which facilitates the reorganization and functional recovery of a network miswired during development.
Conclusions
Taken together, we suggest that plasticity-inducing treatments, such as exercise, enrichment, or chronic antidepressant treatments increase variability at several structural levels of the nervous system. At all levels, new neurons or new synapses are produced in excess and compete for survival or stabilization. Neurons and synapses that contribute to the activity within neuronal networks are selected for survival and this experience-dependent competition guides the network structure to better represent the external and internal milieu. These data suggest that the combination of enrichment or fluoxetine treatment together with rehabilitation could be useful in a number of conditions where the activation of adult plasticity would be desired. Indeed, recent studies have shown the usefulness of antidepressant treatment for recovery from stroke in humans [91], as well as in animal models of Alzheimer’s disease [92,93] and traumatic brain injury [94]. For the treatment of depression, the data reviewed above suggest that the antidepressant treatment is effective only when combined with rehabilitation, such as psychotherapy. Clinical trials testing the necessity of rehabilitation in the antidepressant effect should be designed, and the combination of antidepressants and psychotherapy, which is recommended by treatment guidelines but too often not followed in clinical practice, should be promoted.
Acknowledgments
We would like to thank Sarah Mack for drawing the Figure 1. This manuscript was written during the sabbatical of EC at the lab of RH, supported by the Senior investigator grant of the Academy of Finland and the Schaefer Scholarship of Columbia University. Original work was supported by Sigrid Jusélius Foundation, Academy of Finland Center of Excellence program (for EC), National Institute for Mental Health, New York Stem Cell Initiative, Hope for Depression Research Foundation (for RH).
Footnotes
Disclosure statement:
EC is co-founder and advisor of Hermo Pharma that is running a clinical trial on the use of fluoxetine on amblyopia in adult humans. RH is a member of the scientific advisory board of Lundbeck and Roche.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- 2.Changeux JP, Danchin A. Selective stabilisation of developing synapses as a mechanism for the specification of neuronal networks. Nature. 1976;264:705–712. doi: 10.1038/264705a0. [DOI] [PubMed] [Google Scholar]
- 3.Hua JY, Smith SJ. Neural activity and the dynamics of central nervous system development. Nat Neurosci. 2004;7:327–332. doi: 10.1038/nn1218. [DOI] [PubMed] [Google Scholar]
- 4.Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci. 2007;10:1110–1115. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- 5.Warner-Schmidt JL, Duman RS. Hippocampal neurogenesis: opposing effects of stress and antidepressant treatment. Hippocampus. 2006;16:239–249. doi: 10.1002/hipo.20156. [DOI] [PubMed] [Google Scholar]
- 6.David DJ, et al. Implications of the functional integration of adult-born hippocampal neurons in anxiety-depression disorders. Neuroscientist. 2010;16:578–591. doi: 10.1177/1073858409360281. [DOI] [PubMed] [Google Scholar]
- 7.Castrén E. Is mood chemistry? Nat. Rev Neurosci. 2005;6:241–246. doi: 10.1038/nrn1629. [DOI] [PubMed] [Google Scholar]
- 8.McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- 9.Liston C, Gan WB. Glucocorticoids are critical regulators of dendritic spine development and plasticity in vivo. Proc Natl Acad Sci U S A. 2011;108:16074–16079. doi: 10.1073/pnas.1110444108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ming GL, Song H. Adult neurogenesis in the Mammalian brain: significant answers and significant questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Praag H, et al. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- 12.Wang JW, et al. Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. J Neurosci. 2008;28:1374–1384. doi: 10.1523/JNEUROSCI.3632-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schoenfeld TJ, Gould E. Stress, stress hormones, and adult neurogenesis. Exp Neurol. 2012;233:12–21. doi: 10.1016/j.expneurol.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vollmayr B, et al. Neurogenesis and depression: what animal models tell us about the link. Eur Arch Psychiatry Clin Neurosci. 2007;257:300–303. doi: 10.1007/s00406-007-0734-2. [DOI] [PubMed] [Google Scholar]
- 15.Dranovsky A, et al. Experience dictates stem cell fate in the adult hippocampus. Neuron. 2011;70:908–923. doi: 10.1016/j.neuron.2011.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dranovsky A, Leonardo ED. Is there a role for young hippocampal neurons in adaptation to stress? Behav Brain Res. 2012;227:371–375. doi: 10.1016/j.bbr.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glasper ER, et al. Adult neurogenesis: Optimizing hippocampal function to suit the environment. Behav Brain Res. 2012;227:380–383. doi: 10.1016/j.bbr.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 18.Leutgeb JK, et al. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315:961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- 19.Sahay A, et al. Pattern separation: a common function for new neurons in hippocampus and olfactory bulb. Neuron. 2011;70:582–588. doi: 10.1016/j.neuron.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakashiba T, et al. Young Dentate Granule Cells Mediate Pattern Separation, whereas Old Granule Cells Facilitate Pattern Completion. Cell. 2012;149:188–201. doi: 10.1016/j.cell.2012.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson DA. Pattern separation and completion in olfaction. Ann N Y Acad Sci. 2009;1170:306–312. doi: 10.1111/j.1749-6632.2009.04017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yassa MA, et al. Age-related memory deficits linked to circuit-specific disruptions in the hippocampus. Proc Natl Acad Sci U S A. 2011;108:8873–8878. doi: 10.1073/pnas.1101567108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yassa MA, et al. High-resolution structural and functional MRI of hippocampal CA3 and dentate gyrus in patients with amnestic Mild Cognitive Impairment. Neuroimage. 2010;51:1242–1252. doi: 10.1016/j.neuroimage.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lissek S, et al. Overgeneralization of conditioned fear as a pathogenic marker of panic disorder. Am J Psychiatry. 2010;167:47–55. doi: 10.1176/appi.ajp.2009.09030410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leutgeb S, Leutgeb JK. Pattern separation, pattern completion, and new neuronal codes within a continuous CA3 map. Learn Mem. 2007;14:745–757. doi: 10.1101/lm.703907. [DOI] [PubMed] [Google Scholar]
- 26.Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987;237:1154–1162. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- 27.Deppmann CD, et al. A model for neuronal competition during development. Science. 2008;320:369–373. doi: 10.1126/science.1152677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Castrén E. Neurotrophic effects of antidepressant drugs. Curr Opin Pharmacol. 2004;4:58–64. doi: 10.1016/j.coph.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 29.Thoenen H. Neurotrophins and neuronal plasticity. Science. 1995;270:593–598. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- 30.Sairanen M, et al. Brain-Derived Neurotrophic Factor and Antidepressant Drugs Have Different But Coordinated Effects on Neuronal Turnover, Proliferation, and Survival in the Adult Dentate Gyrus. Journal of Neuroscience. 2005;25:1089–1094. doi: 10.1523/JNEUROSCI.3741-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barnea A, Nottebohm F. Seasonal recruitment of hippocampal neurons in adult free-ranging black-capped chickadees. Proc Natl Acad Sci U S A. 1994;91:11217–11221. doi: 10.1073/pnas.91.23.11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santarelli L, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 33.David DJ, et al. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62:479–493. doi: 10.1016/j.neuron.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adhikari A, et al. Synchronized activity between the ventral hippocampus and the medial prefrontal cortex during anxiety. Neuron. 2010;65:257–269. doi: 10.1016/j.neuron.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sahay A, et al. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472:466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- 38.Hubel DH, et al. Plasticity of ocular dominance columns in monkey striate cortex. Philos Trans R Soc Lond B Biol Sci. 1977;278:377–409. doi: 10.1098/rstb.1977.0050. [DOI] [PubMed] [Google Scholar]
- 39.Holtmaat A, et al. Long-term, high-resolution imaging in the mouse neocortex through a chronic cranial window. Nat Protoc. 2009;4:1128–1144. doi: 10.1038/nprot.2009.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen JL, et al. Structural basis for the role of inhibition in facilitating adult brain plasticity. Nat Neurosci. 2011;14:587–594. doi: 10.1038/nn.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vaughn JE. Fine structure of synaptogenesis in the vertebrate central nervous system. Synapse. 1989;3:255–285. doi: 10.1002/syn.890030312. [DOI] [PubMed] [Google Scholar]
- 42.Lichtman JW, Colman H. Synapse elimination and indelible memory. Neuron. 2000;25:269–278. doi: 10.1016/s0896-6273(00)80893-4. [DOI] [PubMed] [Google Scholar]
- 43.Alsina B, et al. Visualizing synapse formation in arborizing optic axons in vivo: dynamics and modulation by BDNF. Nat Neurosci. 2001;4:1093–1101. doi: 10.1038/nn735. [DOI] [PubMed] [Google Scholar]
- 44.Chen AC, et al. ECS-Induced mossy fiber sprouting and BDNF expression are attenuated by ketamine pretreatment. J Ect. 2001;17:27–32. doi: 10.1097/00124509-200103000-00006. [DOI] [PubMed] [Google Scholar]
- 45.Bessa JM, et al. The mood-improving actions of antidepressants do not depend on neurogenesis but are associated with neuronal remodeling. Mol Psychiatry. 2009;14:764–73. 739. doi: 10.1038/mp.2008.119. [DOI] [PubMed] [Google Scholar]
- 46.Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 47.Petanjek Z, et al. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci U S A. 2011;108:13281–13286. doi: 10.1073/pnas.1105108108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maya Vetencourt JF, et al. The Antidepressant Fluoxetine Restores Plasticity in the Adult Visual Cortex. Science. 2008;320:385–388. doi: 10.1126/science.1150516. [DOI] [PubMed] [Google Scholar]
- 49.Trachtenberg JT, et al. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature. 2002;420:788–794. doi: 10.1038/nature01273. [DOI] [PubMed] [Google Scholar]
- 50.Chen F, et al. Repeated electroconvulsive seizures increase the total number of synapses in adult male rat hippocampus. Eur Neuropsychopharmacol. 2009;19:329–338. doi: 10.1016/j.euroneuro.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 51.Chen F, et al. Changes in rat hippocampal CA1 synapses following imipramine treatment. Hippocampus. 2008;18:631–639. doi: 10.1002/hipo.20423. [DOI] [PubMed] [Google Scholar]
- 52.Hajszan T, et al. Remodeling of hippocampal spine synapses in the rat learned helplessness model of depression. Biol Psychiatry. 2009;65:392–400. doi: 10.1016/j.biopsych.2008.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hajszan T, et al. Short-term treatment with the antidepressant fluoxetine triggers pyramidal dendritic spine synapse formation in rat hippocampus. European Journal of Neuroscience. 2005;21:1299–1303. doi: 10.1111/j.1460-9568.2005.03968.x. [DOI] [PubMed] [Google Scholar]
- 54.Cerqueira JJ, et al. The prefrontal cortex as a key target of the maladaptive response to stress. J Neurosci. 2007;27:2781–2787. doi: 10.1523/JNEUROSCI.4372-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hajszan T, et al. Effects of estradiol on learned helplessness and associated remodeling of hippocampal spine synapses in female rats. Biol Psychiatry. 2010;67:168–174. doi: 10.1016/j.biopsych.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Radley JJ, et al. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex. 2006;16:313–320. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- 57.Stewart CA, Reid IC. Repeated ECS and fluoxetine administration have equivalent effects on hippocampal synaptic plasticity. Psychopharmacology (Berl) 2000;148:217–223. doi: 10.1007/s002130050045. [DOI] [PubMed] [Google Scholar]
- 58.Kobayashi K, et al. Reversal of hippocampal neuronal maturation by serotonergic antidepressants. Proc Natl Acad Sci U S A. 2010;107:8434–8439. doi: 10.1073/pnas.0912690107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sale A, et al. Environmental enrichment in adulthood promotes amblyopia recovery through a reduction of intracortical inhibition. Nat Neurosci. 2007;10:679–681. doi: 10.1038/nn1899. [DOI] [PubMed] [Google Scholar]
- 60.Karpova NN, et al. Fear erasure in mice requires synergy between antidepressant drugs and extinction training. Science. 2011;334:1731–1734. doi: 10.1126/science.1214592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kirkwood A, et al. Co-regulation of long-term potentiation and experience-dependent synaptic plasticity in visual cortex by age and experience. Nature. 1995;375:328–331. doi: 10.1038/375328a0. [DOI] [PubMed] [Google Scholar]
- 62.Sale A, et al. Enrich the environment to empower the brain. Trends Neurosci. 2009;32:233–239. doi: 10.1016/j.tins.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 63.Weikum WM, et al. Prenatal exposure to antidepressants and depressed maternal mood alter trajectory of infant speech perception. Proc Natl Acad Sci U S A. 2012 doi: 10.1073/pnas.1121263109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Holderbach R, et al. Enhanced long-term synaptic depression in an animal model of depression. Biol Psychiatry. 2007;62:92–100. doi: 10.1016/j.biopsych.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 65.West AE, Greenberg ME. Neuronal activity-regulated gene transcription in synapse development and cognitive function. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a005744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Flavell SW, Greenberg ME. Signaling mechanisms linking neuronal activity to gene expression and plasticity of the nervous system. Annu Rev Neurosci. 2008;31:563–590. doi: 10.1146/annurev.neuro.31.060407.125631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Castrén E, et al. Treatment of neurodevelopmental disorders in adulthood. J Neurosci. 2012;32:14074–14079. doi: 10.1523/JNEUROSCI.3287-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Borrelli E, et al. Decoding the epigenetic language of neuronal plasticity. Neuron. 2008;60:961–974. doi: 10.1016/j.neuron.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jiang Y, et al. Epigenetics in the nervous system. J Neurosci. 2008;28:11753–11759. doi: 10.1523/JNEUROSCI.3797-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tsankova N, et al. Epigenetic regulation in psychiatric disorders. Nat Rev Neurosci. 2007;8:355–367. doi: 10.1038/nrn2132. [DOI] [PubMed] [Google Scholar]
- 72.Duman RS, Newton SS. Epigenetic marking and neuronal plasticity. Biol Psychiatry. 2007;62:1–3. doi: 10.1016/j.biopsych.2007.04.037. [DOI] [PubMed] [Google Scholar]
- 73.Guo JU, et al. Neuronal activity modifies the DNA methylation landscape in the adult brain. Nat Neurosci. 2011;14:1345–1351. doi: 10.1038/nn.2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thoenen H. Neurotrophins and Neuronal Plasticity. Science. 1995;270:593–598. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- 75.Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- 76.Bergami M, et al. Deletion of TrkB in adult progenitors alters newborn neuron integration into hippocampal circuits and increases anxiety-like behavior. Proceedings of the National Academy of Sciences. 2008;105:15570–15575. doi: 10.1073/pnas.0803702105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li Y, et al. TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron. 2008;59:399–412. doi: 10.1016/j.neuron.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McAllister AK, et al. Neurotrophins and synaptic plasticity. Annu Rev Neurosci. 1999;22:295–318. doi: 10.1146/annurev.neuro.22.1.295. [DOI] [PubMed] [Google Scholar]
- 79.Kang H, Schuman EM. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science. 1995;267:1658–1662. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- 80.Lu B. BDNF and activity-dependent synaptic modulation. Learn Mem. 2003;10:86–98. doi: 10.1101/lm.54603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Saarelainen T, et al. Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J Neurosci. 2003;23:349–357. doi: 10.1523/JNEUROSCI.23-01-00349.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rossi C, et al. Brain-derived neurotrophic factor (BDNF) is required for the enhancement of hippocampal neurogenesis following environmental enrichment. Eur J Neurosci. 2006;24:1850–1856. doi: 10.1111/j.1460-9568.2006.05059.x. [DOI] [PubMed] [Google Scholar]
- 83.Berton O, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- 84.Alter MD, et al. Variation in the large-scale organization of gene expression levels in the hippocampus relates to stable epigenetic variability in behavior. PLoS One. 2008;3:e3344. doi: 10.1371/journal.pone.0003344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Alter MD, Hen R. Is there a genomic tone? Implications for understanding development, adaptation and treatment. Dev Neurosci. 2009;31:351–357. doi: 10.1159/000216546. [DOI] [PubMed] [Google Scholar]
- 86.Feinberg AP, Irizarry RA. Stochastic epigenetic variation as a driving force of development, evolutionary adaptation, and disease. Proc Natl Acad Sci U S A. 2010;107(Suppl 1):1757–1764. doi: 10.1073/pnas.0906183107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Berardi N, et al. Molecular basis of plasticity in the visual cortex. Trends in Neurosciences. 2003;26:369–378. doi: 10.1016/S0166-2236(03)00168-1. [DOI] [PubMed] [Google Scholar]
- 88.Mountcastle VB. The columnar organization of the neocortex. Brain. 1997;120:701–722. doi: 10.1093/brain/120.4.701. [DOI] [PubMed] [Google Scholar]
- 89.Maya Vetencourt JF, et al. Serotonin triggers a transient epigenetic mechanism that reinstates adult visual cortex plasticity in rats. Eur J Neurosci. 2011;33:49–57. doi: 10.1111/j.1460-9568.2010.07488.x. [DOI] [PubMed] [Google Scholar]
- 90.Morishita H, Hensch TK. Critical period revisited: impact on vision. Curr Opin Neurobiol. 2008;18:101–107. doi: 10.1016/j.conb.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 91.Chollet F, et al. Fluoxetine for motor recovery after acute ischaemic stroke (FLAME): a randomised placebo-controlled trial. Lancet Neurol. 2011;10:123–130. doi: 10.1016/S1474-4422(10)70314-8. [DOI] [PubMed] [Google Scholar]
- 92.Aboukhatwa M, et al. Antidepressants are a rational complementary therapy for the treatment of Alzheimer’s disease. Mol Neurodegener. 2010;5:10. doi: 10.1186/1750-1326-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chadwick W, et al. Amitriptyline-mediated cognitive enhancement in aged 3xtg Alzheimer’s disease mice is associated with neurogenesis and neurotrophic activity. PLoS One. 2011;6:e21660. doi: 10.1371/journal.pone.0021660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Han X, et al. Imipramine treatment improves cognitive outcome associated with enhanced hippocampal neurogenesis after traumatic brain injury in mice. J Neurotrauma. 2011;28:995–1007. doi: 10.1089/neu.2010.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]