Abstract
Oxidative stress has been implicated in the pathogenesis of heart failure and atrial fibrillation and can result in increased peroxynitrite production in the myocardium.. Atrial and ventricular canine cardiac myocytes were superfused with SIN-1 (3-morpholinosydnonimine N-ethylcarbamide), a peroxynitrite donor, to evaluate the acute electrophysiologic effects of peroxynitrite. Perforated whole cell patch clamp techniques were used to record action potentials. SIN-1 (200 μM) increased the action potential duration (APD) in atrial and ventricular myocytes; however, in the atria, APD prolongation was rate-independent, while in the ventricle APD prolongation was rate dependent. In addition to prolongation of the action potential, beat to beat variability of repolarization was significantly increased in ventricular, but not atrial myocytes. We examined the contribution of intracellular calcium cycling to the effects of SIN-1 by treating myocytes with the SERCA blocker, thapsigargin (5-10 μM). Inhibition of calcium cycling prevented APD prolongation in the atrial and ventricular myocytes, and prevented the SIN-1 induced increase in ventricular beat to beat APD variability. Collectively, these data demonstrate that peroxynitrite affects atrial and ventricular electrophysiology differentially. A detailed understanding of oxidative modulation of electrophysiology in specific chambers is critical to optimize therapeutic approaches for cardiac diseases.
Keywords: electrophysiology, cardiac myocyte, peroxynitrite
Introduction
Reactive nitrogen species (RNS) and reactive oxygen species (ROS) are normally produced by cellular metabolism where they serve as cell signaling moieties. Nitrosative and/or oxidative stress occur when there is an imbalance between ROS/RNS production and their reduction by antioxidant agents or enzymes. Oxidative and nitrosative stress have been suggested to participate in the pathophysiology of numerous cardiovascular diseases including: progression of heart failure (HF), myocardial infarction (MI) and atrial fibrillation (AF) 1-4.
ROS and RNS are produced by multiple sources, among them uncoupling of mitochondrial electron transport, cytokine activity, NADH/NADPH oxidase and uncoupled nitric oxide synthases. Endogenous antioxidant defenses include superoxide dismutase, catalase, and glutathione, among others 5. When nitric oxide (NO) and superoxide anion are produced in close proximity they interact to form a very powerful oxidant called peroxynitrite (OONO−) 6.
Peroxynitrite rapidly causes nitration of proteins, including tryptophan and tyrosine residues.7, As a result of tyrosine nitration, 3-nitrotyrosine (3-NT) is formed, a modification which is either irreversible or slowly reversible 8. Increased 3-NT is therefore a biomarker for the presence of OONO−. Increased peroxynitrite has been associated with the pathology of a number of cardiac diseases, as recently reviewed 9. Specifically, increased 3-NT has been found in the atria of patients with persistent atrial fibrillation, and is thought to contribute to the impaired contractility associated with atrial fibrillation 10. In human ventricle, increased SERCA 3-NT content has been reported during heart failure 11. Thus, 3-NT is increased in both atrial fibrillation and heart failure. In addition, peroxynitrite has been implicated in the modulation of excitation-contraction coupling 11-14. Notably, there may be differential chamber-specific effects on action potential duration, the integrated cellular response to sarcolemmal ion currents, induced by cardiac diseases. Specifically, in chronic heart failure, the ventricular action potential is prolonged while the atrial action potential is shortened 15-17,18. The potential for peroxynitrite to elicit chamber-specific changes in action potential are undefined. In the present study we tested the hypothesis that there are differential effects of peroxynitrite on atrial and ventricular myocyte electrophysiology. SIN-1, a molsidomine metabolite, releases both superoxide and NO which react in a diffusion limited manner to produce ONOO-; thus, SIN-1 was used to test the acute electrophysiologic effects of OONO-.
Methods
Myocyte Isolation
All animal procedures were approved by the Institutional Animal Care and Use Committee of the Ohio State University. Twenty adult mixed breed dogs (male/female, age 9 months to 5 years) weighing between 8 and 20 kg with normal cardiac function were used for the experiments. On the day of the experiment, dogs where anesthetized with pentobarbital sodium (50 mg/kg IV; Nembutal, Abbott Laboratories). The heart was rapidly removed and perfused with cold cardioplegia solution (containing: 5% glucose, 0.1% mannitol, 22.4 mM NaHCO3 and 30 mM of KCl) injected into the coronary ostia.
The left circumflex artery was cannulated for myocyte isolation as previously described 19. Collagenase (Worthington, type 2; 0.65 mg/ml) was added to the perfusate for enzymatic dispersion of the myocytes. After 30-45 minutes of enzyme perfusion, the left atrial appendage was separated from the left ventricle. The left atrial appendage was minced and placed into 5 mL of perfusate in a shaking water bath at 37°C for 5-15 minutes as previously described 18. Simultaneously, the digested left ventricular mid-myocardial section of the left ventricular free wall was separated from the epicardial and endocardial section; digested tissue was shaken in a water bath at 37° C for an additional 5 minutes. After secondary digestion the myocytes were washed and stored at room temperature, as previously described 20. Storage solution contained (in mM): 118 NaCl, 4.8 MgCl2, 1.2 KH2PO4, 0.68 glutamine, 10 glucose, 5 pyruvate, 1 CaCl2; 1 μM insulin and 1% BSA. This isolation procedure typically yielded 40-60% and 70-90% of rod shaped atrial and ventricular myocytes, respectively, with sharp margins and clear striations.
Electrophysiological Studies
Data acquisition was performed with Clampex version 9.0 software (Axon Instruments Union City, CA, USA) and Axopatch 200A patch clamp amplifiers (Axon Instruments Inc.) Perforated whole cell patch clamp (using amphotericin B) was used to minimize alterations in intracellular milieu during action potential (AP) and K+ current recordings. Myocytes were placed in a laminin coated cell chamber (Cell Microcontrols, Norfolk, VA, USA) and superfused (1 mL/min) with bath solution containing the following (in mM): 135 NaCl, 5 KCl, 5 MgCl2, 10 D-glucose, 1.8 CaCl2, 5 HEPES, pH adjusted at 7.40 with NaOH, was used. After obtaining baseline data, the cells were superfused with bath solution supplemented with SIN-1 (200 μM), a concentration previously reported to inhibit phospholamban function in isolated cardiac myocytes 21as well as NOS activity22 which is relevant as we previously demonstrated that NOS activity is an important mediator of atrial electrophysiology 20 Specificity of SIN-1 effects through peroxynitrite formation was evaluated by co-superfusion with Uric Acid (500 μM), a peroxynitrite scavenger.
To assess the contribution of calcium cycling to the observed SIN-1 effects, the SERCA inhibitor, thapsigargin was used. Thapsigargin (5-10 μM) was added to the bath solution and recordings were taken at baseline, after thapsigargin, and after subsequent SIN-1 superfusion. Recordings were obtained a minimum of five minutes of drug.
Action potentials were recorded at 0.5 Hz, 1 Hz and 2 Hz applied in a random order. The average of the last 10 (steady state) action potentials, recorded during a train of 25 action potentials at each stimulation rate were averaged and used to calculate the action potential duration at 50% repolarization (APD50) and 90% of repolarization (APD90). To analyze beat to beat variability in the action potential recordings we measured the standard deviation (SD) of the action potential duration at 90% of repolarization (APD90) of the last ten traces (i.e. from trace 15-25) as previously described 23.
For K+ current recordings, the bath solution CaCl2 was reduced to 1 mM, and 2 μM of nifedepine was added to the bath solution to block the L-type calcium current. K+ currents were recorded at baseline and after SIN-1 perfusion as previously described 18. Action potentials and current recordings were made at 35 ± 0.5 °C.
Statistical Analysis
Acquired data were analyzed using Clampfit 8.0 software (Axon Instruments) and Origin 8.6 software (OriginLab, Northampton, MA, USA). Currents were normalized to cell capacitance in picofarads (pF) and are expressed as pA/pF. Comparisons between baseline and drug exposed cells were made by the appropriate t-test (Origin Pro 8.6, OriginLab). Comparison between baseline, thapsigargin and SIN-1 post thapsigargin were made using one way ANOVA with post hoc least significant difference testing. (Origin 8.6, Origin Lab). Rate- dependence of drug effects was analyzed by two way ANOVA with post hoc least significant difference testing (Originpro 8.6, OriginLab). All data are presented as mean ± SE and p<0.05 was the criterion for statistical significance for all comparisons.
Chemicals
All chemicals used were purchased from Sigma-Aldrich (St. Louis, MO, USA) and Fisher Scientific (Pittsburg, PA, USA). All buffers and solutions were prepared daily.
Results
Specificity of effects of SIN-1 on action potential duration
Pilot experiments were conducted to assure that SIN-1 effects observed in the cardiac myocytes result from the production of ONOO-. For this myocytes were co-superfused with the peroxynitrite scavenger, uric acid (500 μM) and SIN-1 200μm. Atrial action potentials were recorded at baseline, with uric acid and with uric acid + SIN-1 (Figure 1A). No statistical change in APD90 was observed between the baseline, uric acid and uric acid + SIN-1 treatments (p>0.05). Thus, any effects observed with SIN-1 exposure are consistent with peroxynitrite-dependent effects. SIN-1 treatment caused a significant (p<0.05 vs baseline) prolongation in the action potential (APD90) in both atrial and ventricular myocytes (Figure 1 Panels B and C, Figure 2 Panels A and B). The action potential prolongation observed in the atria was rate independent (p = 0.98 at rates of 0.5 Hz, 1 Hz and 2 Hz) while the observed prolongation in the ventricle was rate dependent (p<0.05, Figure 2 panels A and B, respectively). A significant increase in APD50 was only observed at 2 Hz in the atria (p<0.05 vs baseline Figure 2 panel A). Ventricular APD50 was unaffected by SIN-1 perfusion (p=0.73 Figure 2 panel B). The resting membrane potential was not affected by SIN-1 in either atrial (Baseline −78.82 ± 2.20 vs. SIN-1 −75.5 ± 3.30 p= 0.43) or ventricular myocytes (Baseline −80.40 ± 0.96 vs. SIN-1 −79.78 ± 0.76 p=0.41).
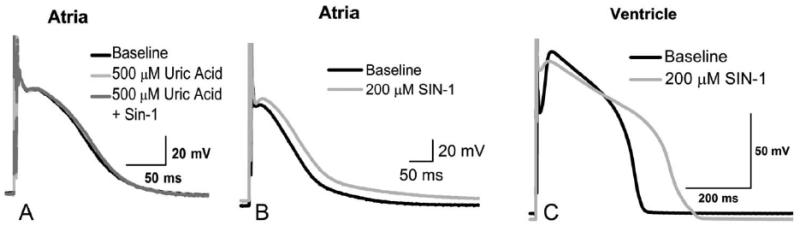
Figure 1.
SIN-1 prolongs the atrial and ventricular action potential. (A) The peroxynitrite scavenger uric acid was used to evaluate specificity of SIN-1. Atrial action potentials were recorded at baseline, with uric acid and with uric acid + SIN-1. No statistical change in atrial APD90 was observed after co-superfusion. (B) SIN-1 prolongs the atrial action potential and (C) SIN-1 prolongs the action potential and decreases the phase 1 notch in ventricular myocytes.
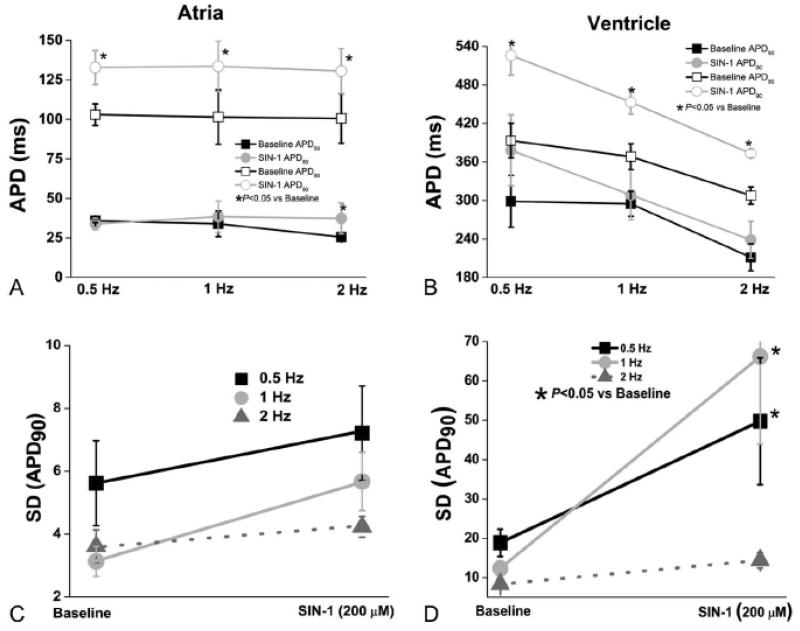
Figure 2.
SIN-1 affects atrial and ventricular repolarization. (A) SIN-1 causes a significant (p<0.05 vs baseline) prolongation in the atrial APD50 only at 2 Hz while the APD90 is significantly increased (p<0.05 vs baseline) after SIN-1 in a rate independent manner. (B) Contrary to the atria, no change was observed in the ventricular APD50 after SIN-1. At baseline ventricular APD90 was significantly shorter at 2 Hz than 0.5 Hz. A significant increase (p<0.05 vs baseline) was observed in the ventricular APD90 at all rates during SIN-1 superfusion. While SIN-1 prolonged the ventricular APD90, in contrast to the atria, APD90 retains rate-dependence, with APD90 being significantly shorter at 2 Hz, compared to 0.5 or 1 Hz (P<0.05). (C) SIN-1 does not affect atrial beat to beat variability of repolarization (SD APD90). (D) A significant (p<0.05 vs baseline) increase in beat to beat variability of repolarization (SD APD90) occurs during SIN-1 superperfusion in ventricular myocytes at 0.5 and 1 Hz, but not at 2 Hz. (n=5-8 cells in each group, N= 3-4 animals per group).
Beat to beat variability
Instability of repolarization is associated with an increased propensity to arrhythmias. We assessed beat to beat variability of APD90, and found it was increased only in ventricular myocytes after SIN-1 treatment at 1 and 2 Hz (p <0.05 vs baseline Figure 2 panels C and D).
Potassium currents
Potassium currents measured in the atria were: inward rectifier (IK1) at −80 mV, transient outward (Ito) and IKsus (sustained potassium current which contains IKur, IKs and IKr).24 In the ventricle: IK1, Ito and the fast component of the delayed rectifier potassium current (IKr) were measured. No significant differences (p>0.05) were found in any of the currents measured in the atria (Table 1). In the ventricle the only current was affected by SIN-1 was Ito which was significantly decreased after SIN-1 treatment (p<0.05 vs baseline, Table 1).
Table 1.
Electrophysiologic effects of SIN-1 in cardiac myocytes
| Ventricle (n=6, N=3) | Control | SIN-1 | P value |
|---|---|---|---|
| Ik1 at -100mV(pA/pF) | -6.552 ±1.911 | -6.584 ± 0.548 | 0.185 |
| IK1 Peak Outward (pA/pF) | 1.774 ± 0.127 | 1.688 ± 0.214 | 0.393 |
| Ito at +50 (pA/pF) | 7.143 ±1.664 | 6.803 ±1.585 | 0.003 |
| Ikr Peak (pA/pF) | 1.533 ± 0.147 | 1.502 ± 0.115 | 0.365 |
| Atria (n=8, N=3) | Control | SIN-1 | P value |
|---|---|---|---|
| Ik1 at -80mV (pA/pF) | -0.328 ± 0.264 | -0.239 ± 0.306 | 0.348 |
| Ito at +50mV (pA/pF) | 5.842±0.654 | 5.206± 0.775 | 0.597 |
| Iksus (pA/pF) | 6.271 ± 0.926 | 5.834 ± 1.088 | 0.326 |
IK1 (inward rectifier potassium current), Ito (transient outward potassium current), IKr (fast component of the delayed rectifier potassium current), IKsus (sustained outward potassium current which contains IKur, IKs and IKr), n (number of cells per group), N (number of animal per group)
Thapsigargin experiments
To assess the specificity of the effects observed after SIN-1 exposure, a comparison between baseline and thapsigargin were made. No significant difference between the groups in either cell type was found (p>0.05 data not shown). Thus, thapsigargin itself did not have direct electrophysiologic effects on the APD.
After thapsigargin pre-treatment, the cells were superfused with SIN-1. Thapsigargin pre-treatment prevented the SIN-1 dependent prolongation of atrial APD50 previously observed at 2 Hz (p=0.09 with thapsigargin + SIN-1 vs baseline, data not shown). Thapsigargin pretreatment abolished the previously observed APD90 prolongation in both atrial and ventricular myocytes (P=0.85 and P=0.68, respectively). In summary, no statistical differences between baseline and SIN-1 + thapsigargin were found in atrial or ventricular cells. (Figure 3 Panels A and B, respectively).
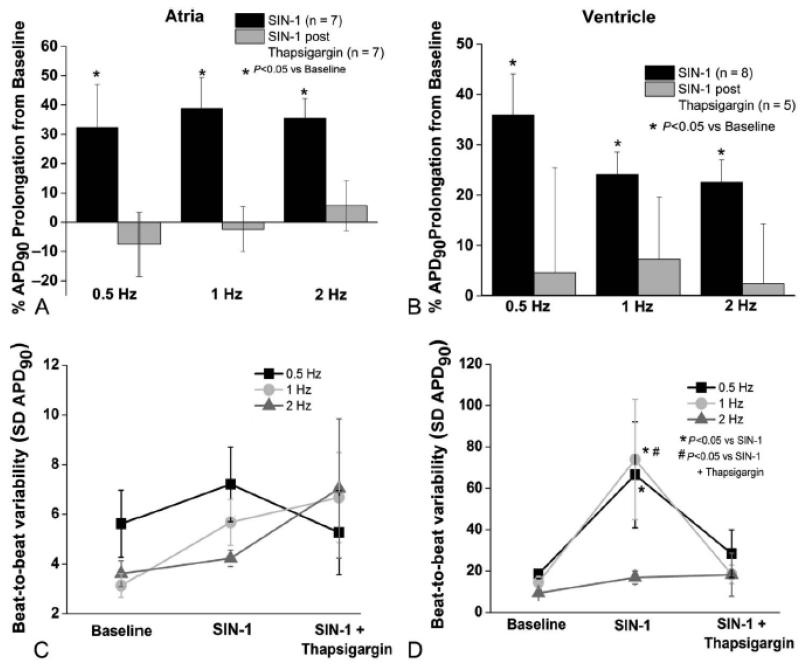
Figure 3.
Peroxynitrite-induced changes in atrial and ventricular repolarization and variability of repolarization are prevented with thapsigargin pre-treatment. (A) and (B) Thapsigargin pre-treatment (to deplete SR calcium stores and prevent calcium cycling) prevented both atrial and ventricular SIN-1 dependent APD90 prolongation (p>0.05 from baseline). (C) Atrial beat to beat variability in repolarization is unchanged by peroxynitrite (p=NS vs baseline). (D) Peroxynitrite-induced increase in ventricular beat to beat variability in repolarization is prevented by thapsigargin (p=NS vs baseline). (n=5-8 cells in each group and N= 3-5 animals per group)
The SIN-1 dependent increase in beat to beat variability (SD APD90) previously observed in ventricular myocytes was prevented by thapsigargin pretreatment. Therefore SERCA inhibition prevented ONOO- dependent ventricular repolarization instability (p>0.05 vs baseline Figure 3 Panels D).
Discussion
The main findings of this study are that peroxynitrite causes a significant prolongation of atrial and ventricular myocyte repolarization, increases repolarization instability in ventricular myocytes, and decreases Ito current density only in ventricular myocytes. Surprisingly, we found no inhibition of delayed rectifier repolarizing potassium currents in either atrial or ventricular myocytes with SIN-1. However, SERCA blockade and the resulting depletion of calcium in the sarcoplasmic reticulum with thapsigargin prevented the peroxynitrite-dependent effects on repolarization.
In the present study SIN-1 was used as a peroxynitrite donor as it releases both superoxide anion and NO, which at physiological pH combine to form peroxynitrite6. We ascertained that the effects observed after SIN-1 were due to peroxynitrite action and not due a non-specific effect, by conducting co-superperfusion experiments with uric acid (a natural peroxynitrite scavenger). Thus, the effects we observed are attributable to peroxynitrite.
The role of increased production of reactive species in the pathogenesis of several cardiovascular diseases is a well-established phenomenon. Increased peroxynitrite production has been shown to occur in diseases such as chronic heart failure, myocardial infarction and atrial fibrillation. Indeed 3-NT (commonly used as a biomarker for the production of ONOO−) has been found to be increased in patients with persistent atrial fibrillation and patients with inflammatory heart disease 10;14;25. It is formed when ONOO− modifies the tyrosine residues of proteins via nitration. When proteins are nitrated and 3-NT is formed it can cause a loss or gain of function or increased degradation of nitrated protein 8. Interestingly, another common finding in heart disease, uncoupling of inducible nitric oxide synthase (iNOS) has also been shown to result in an increase in peroxynitrite production in cardiovascular diseases 14;20. In addition to tyrosine it is notable that methionine, cysteine and tryptophan are also susceptible to nitration, therefore, it is possible that part of the differential effects of ONOO- between chambers observed in the present study are due to differential nitration of key protein residues, 7;26 as recently reviewed.27
Differential responses to SIN-1 between atria and ventricle were observed, and atrial specific changes in APD90 manifested as rate independent prolongation. The prolongation of APD in the atrial cells suggests that acute or transient increases in peroxynitrite are unlikely to participate in the shortening of the APD observed in chronic heart failure 18 or in atrial fibrillation15,28,29. In contrast to the atria, ventricular effects of SIN-1 on APD90 prolongation were rate dependent, accompanied by a significant increase in the beat to beat variability and a significant decrease in Ito current density.
The differential response between atrial and ventricular myocytes is not unexpected as the chambers differ in many ways. The action potential morphology and duration, gene and protein expression and calcium handling mechanisms are some of the differences between atrial and ventricular myocytes 30. In addition it has been reported that electrical remodeling during HF (tachypacing induced congestive HF in canine model) also differs between the chambers 31;32. Chronic HF is well known to prolong ventricular repolarization, 17;33 while in the atria, chronic HF shortens repolarization 15;18;20. Because of the differences between disease-induced electrical remodeling between chambers, it is not surprising to observe variable effects with SIN-1. The prolongation in the action potential and the increased beat to beat variability observed in this study are very common findings observed in the pathogenesis of heart failure and ventricular fibrillation canine models and have been linked to increased arrhythmogenesis 18;34.
SERCA inhibition and subsequent calcium depletion of the sarcoplasmic reticulum with thapsigargin before SIN-1 perfusion in ventricular myocytes prevented the SIN-1 dependent APD90 prolongation and increase in beat to beat variability, suggesting that calcium cycling might be responsible for the observed SIN-1 dependent effects in the ventricle. The effects of SIN-1 (ONOO−) on calcium handling have been previously described as affecting phospholamban (PLB) phosphorylation in murine ventricular myocytes 13;35 SERCA pump is regulated by PLB phosphorylation to increase the SR Ca2+ load and decrease cytosolic Ca2+ concentration under normal conditions (for a review see 36). In our experiments with thapsigargin we are not only inhibiting SERCA, but by depletion of calcium stores also inhibiting calcium handling in the myocyte. Thus, we suggest that SIN-1 is exerting electrophysiologic effects via a component of the Ca2+ handling system: SERCA, ryanodine receptor, PLB, etc.
Cardiac myocyte calcium handling has been reported to be redox sensitive in canine ventricular myocytes; reactive species cause an increase in spontaneous calcium release and calcium alternans, and are associated with heart failure and ventricular tachyarrhythmias 37;38. Specifically, ROS are known to activate serine/ threonine kinases such as CAMKII, which in turn which can phosphorylates RyR, PLB, sodium Channels and L-type calcium channels, as recently reviewed39 Under normal conditions CAMKII activation and subsequent phosphorylation of proteins are calcium-dependent, but in the presence of ROS, CAMKII may be oxidized resulting in calcium-independent activation40, possibly leading to abnormal phosphorylation of key components of the calcium handling system. Another mechanism for calcium-handling dependent effects of peroxynitrite may be through oxidation of specific components of the calcium-handling apparatus, as L-type calcium channels, sodium channels, RyR, Sodium calcium exchanger (NCX) and SERCA39. Thus, inhibition of calcium-cycling by thapsigargin may inhibit the acute effects of peroxynitrite through multiple mechanisms.
The basis of the atrio-ventricular differential effects of thapsigargin in mediating SIN-1 effects are not clear. Possible explanations might be that peroxynitrite targets proteins that are differentially expressed in the ventricle and the atria: e.g. PLB is the primary regulator of SERCA pump function in the ventricle, while sarcolipin is an atrial-specific regulator of SERCA pump function 41. Another possible explanation for the chamber specific responses to SIN-1 exposure is the potential for differential expression of the intracellular antioxidant defenses such as superoxide dismutase, catalase and glutathione in cardiac myocytes, or differential expression of proteins that detoxify peroxynitrite such as hemoglobin or peroxiredoxins,26;42 any of which could give rise to differential reactions during exposure to reactive species.
Reactive oxygen species and peroxynitrite reportedly decrease Ito density and prolong the action potential in rat ventricular myocytes 43. Whether the observed decrease in Ito current density would cause prolongation in the canine action potential is a matter of debate. Some reports suggest that this is only true in rodents, suggesting that in larger mammals (canines, humans) the effect of decreased Ito current density alters the notch observed in phase 1 of the action potential 44-46. Another possible explanation for the chamber specific differences we observed after thapsigargin may be that Ito is reduced in the ventricular, but not the atrial myocytes by peroxynitrite. Ito is known to modulate calcium current in very complex ways 47-49 and the effects of SIN-1 on ventricular Ito may be contributing to the differential modulation of beat to beat variability. Further investigation will be needed to determine the specific target(s) and mechanism(s) by which SIN-1 modulates calcium handling with secondary effects on repolarization.
Limitation
Prolonged (2-24h) SIN-1 exposure in cardiac myocytes has been reported to induce caspases and subsequent apoptosis.50,51 The time course from caspase activation to apoptotic signaling in adult cardiac myocytes appears to be at least 2 hours,52 while our maximum duration of exposure to SIN-1 was 15 minutes. Thus, apoptotic signaling is unlikely to contribute to the altered electrophysiology during SIN-1 exposure. The contribution of the late sodium, L-type calcium, and NCX currents to the effects of peroxynitrite were not taken into consideration in the present study. It is well known that late sodium current and L-type calcium current contribute to rate adaptation of the action potential 53. However, our goals were to focus primarily on the action potential duration, the integrated response to all ion currents, and action potential duration instability, a measure of arrhythmic potential, in the present study.
Myocytes were isolated from specific regions of the two chambers, and do not fully reflect the spatial differences occurring within the heart. All studies were conducted in myocytes from normal controls, and thus, the effects of peroxynitrite we observed may vary in heart diseases such as heart failure and atrial fibrillation.
Conclusions
Cardiac remodeling is comprised of multiple complex processes, and an increase in reactive species is only one component of the remodeling. Examining the changes that occur due to the increase in peroxynitrite provides potential insights into the pathophysiology of heart disease. Our data suggests a potential antiarrhythmic role of acute increases in peroxynitrite in the atria; however, it has proarrhythmic potential in the ventricle due to enhanced action potential instability54. A complete understanding of alterations due to peroxynitrite requires further study in the context of overall redox modulation of electrophysiology.
Acknowledgments
The authors thank Ms. Jeanne Green for excellent technical support.
Funding: This project was supported by NIH/NHLBI grant HL089836.
Footnotes
Conflict of interest: None of the authors in the present manuscript have any conflict of interest to declare.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Sugamura K, Keaney JF., Jr. Reactive oxygen species in cardiovascular disease. Free Radic Biol Med. 2011 Sep;51(5):978–992. doi: 10.1016/j.freeradbiomed.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Han W, Fu S, Wei N, Xie B, Li W, Yang S, Li Y, Liang Z, Huo H. Nitric oxide overproduction derived from inducible nitric oxide synthase increases cardiomyocyte apoptosis in human atrial fibrillation. Int J Cardiol. 2008 Nov;130(2):165–173. doi: 10.1016/j.ijcard.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 3.Sun Y. Oxidative stress and cardiac repair/remodeling following infarction. Am J Med Sci. 2007 Sep;334(3):197–205. doi: 10.1097/MAJ.0b013e318157388f. [DOI] [PubMed] [Google Scholar]
- 4.Kameda K, Matsunaga T, Abe N, Hanada H, Ishizaka H, Ono H, Saitoh M, Fukui K, Fukuda I, Osanai T, Okumura K. Correlation of oxidative stress with activity of matrix metalloproteinase in patients with coronary artery disease. Possible role for left ventricular remodelling. Eur Heart J. 2003 Dec;24(24):2180–2185. doi: 10.1016/j.ehj.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 5.Wattanapitayakul SK, Bauer JA. Oxidative pathways in cardiovascular disease: roles, mechanisms, and therapeutic implications. Pharmacol Ther. 2001 Feb;89(2):187–206. doi: 10.1016/s0163-7258(00)00114-5. [DOI] [PubMed] [Google Scholar]
- 6.Reiter CD, Teng RJ, Beckman JS. Superoxide reacts with nitric oxide to nitrate tyrosine at physiological pH via peroxynitrite. J Biol Chem. 2000 Oct;275(42):32460–32466. doi: 10.1074/jbc.M910433199. [DOI] [PubMed] [Google Scholar]
- 7.Nuriel T, Hansler A, Gross SS. Protein nitrotryptophan: formation, significance and identification. J Proteomics. 2011 Oct;74(11):2300–2312. doi: 10.1016/j.jprot.2011.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Souza JM, Peluffo G, Radi R. Protein tyrosine nitration--functional alteration or just a biomarker? Free Radic Biol Med. 2008 Aug;45(4):357–366. doi: 10.1016/j.freeradbiomed.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 9.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007 Jan;87(1):315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mihm MJ, Yu F, Carnes CA, Reiser PJ, McCarthy PM, Van Wagoner DR, Bauer JA. Impaired myofibrillar energetics and oxidative injury during human atrial fibrillation. Circulation. 2001 Jul;104(2):174–180. doi: 10.1161/01.cir.104.2.174. [DOI] [PubMed] [Google Scholar]
- 11.Lokuta AJ, Maertz NA, Meethal SV, Potter KT, Kamp TJ, Valdivia HH, Haworth RA. Increased nitration of sarcoplasmic reticulum Ca2+-ATPase in human heart failure. Circulation. 2005 Mar;111(8):988–995. doi: 10.1161/01.CIR.0000156461.81529.D7. [DOI] [PubMed] [Google Scholar]
- 12.Chesnais JM, Fischmeister R, Mery PF. Positive and negative inotropic effects of NO donors in atrial and ventricular fibres of the frog heart. J Physiol. 1999 Jul;518(Pt 2):449–461. doi: 10.1111/j.1469-7793.1999.0449p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kohr MJ, Davis JP, Ziolo MT. Peroxynitrite Increases Protein Phosphatase Activity and Promotes the Interaction of Phospholamban with Protein Phosphatase 2a in the Myocardium. Nitric Oxide. 2009 Apr;20(3):217–221. doi: 10.1016/j.niox.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonilla IM, Sridhar A, Gyorke S, Cardounel AJ, Carnes CA. Nitric oxide synthases and atrial fibrillation. Front Physiol. 2012;3:105. doi: 10.3389/fphys.2012.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Workman AJ, Pau D, Redpath CJ, Marshall GE, Russell JA, Norrie J, Kane KA, Rankin AC. Atrial cellular electrophysiological changes in patients with ventricular dysfunction may predispose to AF. Heart Rhythm. 2009 Apr;6(4):445–451. doi: 10.1016/j.hrthm.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Wagoner DR, Pond AL, Lamorgese M, Rossie SS, McCarthy PM, Nerbonne JM. Atrial L-type Ca2+ currents and human atrial fibrillation. Circ Res. 1999 Sep;85(5):428–436. doi: 10.1161/01.res.85.5.428. [DOI] [PubMed] [Google Scholar]
- 17.Nishijima Y, Sridhar A, Viatchenko-Karpinski S, Shaw C, Bonagura JD, Abraham WT, Joshi MS, Bauer JA, Hamlin RL, Gyorke S, Feldman DS, Carnes CA. Chronic cardiac resynchronization therapy and reverse ventricular remodeling in a model of nonischemic cardiomyopathy. Life Sci. 2007 Sep;81(14):1152–1159. doi: 10.1016/j.lfs.2007.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sridhar A, Nishijima Y, Terentyev D, Khan M, Terentyeva R, Hamlin RL, Nakayama T, Gyorke S, Cardounel AJ, Carnes CA. Chronic heart failure and the substrate for atrial fibrillation. Cardiovasc Res. 2009 Nov;84(2):227–236. doi: 10.1093/cvr/cvp216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kubalova Z, Terentyev D, Viatchenko-Karpinski S, Nishijima Y, Gyorke I, Terentyeva R, da Cunha DN, Sridhar A, Feldman DS, Hamlin RL, Carnes CA, Gyorke S. Abnormal intrastore calcium signaling in chronic heart failure. Proc Natl Acad Sci U S A. 2005 Sep;102(39):14104–14109. doi: 10.1073/pnas.0504298102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishijima Y, Sridhar A, Bonilla I, Velayutham M, Khan M, Terentyeva R, Li C, Kuppusamy P, Elton TS, Terentyev D, Gyorke S, Zweier JL, Cardounel AJ, Carnes CA. Tetrahydrobiopterin depletion and NOS2 uncoupling contribute to heart failure-induced alterations in atrial electrophysiology. Cardiovasc Res. 2011 Jul;91(1):71–79. doi: 10.1093/cvr/cvr087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kohr MJ, Wang H, Wheeler DG, Velayutham M, Zweier JL, Ziolo MT. Targeting of phospholamban by peroxynitrite decreases beta-adrenergic stimulation in cardiomyocytes. Cardiovasc Res. 2008 Jan;77(2):353–361. doi: 10.1093/cvr/cvm018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen W, Druhan LJ, Chen CA, Hemann C, Chen YR, Berka V, Tsai AL, Zweier JL. Peroxynitrite induces destruction of the tetrahydrobiopterin and heme in endothelial nitric oxide synthase: transition from reversible to irreversible enzyme inhibition. Biochemistry. 2010 Apr;49(14):3129–3137. doi: 10.1021/bi9016632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sridhar A, da Cunha DN, Lacombe VA, Zhou Q, Fox JJ, Hamlin RL, Carnes CA. The plateau outward current in canine ventricle, sensitive to 4-aminopyridine, is a constitutive contributor to ventricular repolarization. Br J Pharmacol. 2007 Nov;152(6):870–879. doi: 10.1038/sj.bjp.0707403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Wagoner DR, Pond AL, McCarthy PM, Trimmer JS, Nerbonne JM. Outward K+ current densities and Kv1.5 expression are reduced in chronic human atrial fibrillation. Circ Res. 1997 Jun;80(6):772–781. doi: 10.1161/01.res.80.6.772. [DOI] [PubMed] [Google Scholar]
- 25.Kooy NW, Lewis SJ, Royall JA, Ye YZ, Kelly DR, Beckman JS. Extensive tyrosine nitration in human myocardial inflammation: evidence for the presence of peroxynitrite. Crit Care Med. 1997 May;25(5):812–819. doi: 10.1097/00003246-199705000-00017. [DOI] [PubMed] [Google Scholar]
- 26.Alvarez B, Radi R. Peroxynitrite reactivity with amino acids and proteins. Amino Acids. 2003 Dec;25(3-4):295–311. doi: 10.1007/s00726-003-0018-8. [DOI] [PubMed] [Google Scholar]
- 27.Jones LH. Chemistry and biology of biomolecule nitration. Chem Biol. 2012 Sep;19(9):1086–1092. doi: 10.1016/j.chembiol.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 28.Franz MR, Karasik PL, Li C, Moubarak J, Chavez M. Electrical remodeling of the human atrium: similar effects in patients with chronic atrial fibrillation and atrial flutter. J Am Coll Cardiol. 1997 Dec;30(7):1785–1792. doi: 10.1016/s0735-1097(97)00385-9. [DOI] [PubMed] [Google Scholar]
- 29.Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995 Oct;92(7):1954–1968. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- 30.Kaab S, Barth AS, Margerie D, Dugas M, Gebauer M, Zwermann L, Merk S, Pfeufer A, Steinmeyer K, Bleich M, Kreuzer E, Steinbeck G, Nabauer M. Global gene expression in human myocardium-oligonucleotide microarray analysis of regional diversity and transcriptional regulation in heart failure. J Mol Med (Berl) 2004 May;82(5):308–316. doi: 10.1007/s00109-004-0527-2. [DOI] [PubMed] [Google Scholar]
- 31.Hanna N, Cardin S, Leung TK, Nattel S. Differences in atrial versus ventricular remodeling in dogs with ventricular tachypacing-induced congestive heart failure. Cardiovasc Res. 2004 Aug;63(2):236–244. doi: 10.1016/j.cardiores.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 32.Cardin S, Pelletier P, Libby E, Le BS, Xiao L, Kaab S, Demolombe S, Glass L, Nattel S. Marked differences between atrial and ventricular gene-expression remodeling in dogs with experimental heart failure. J Mol Cell Cardiol. 2008 Dec;45(6):821–831. doi: 10.1016/j.yjmcc.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 33.Beuckelmann DJ, Nabauer M, Erdmann E. Alterations of K+ currents in isolated human ventricular myocytes from patients with terminal heart failure. Circ Res. 1993 Aug;73(2):379–385. doi: 10.1161/01.res.73.2.379. [DOI] [PubMed] [Google Scholar]
- 34.Sridhar A, Nishijima Y, Terentyev D, Terentyeva R, Uelmen R, Kukielka M, Bonilla IM, Robertson GA, Gyorke S, Billman GE, Carnes CA. Repolarization abnormalities and afterdepolarizations in a canine model of sudden cardiac death. Am J Physiol Regul Integr Comp Physiol. 2008 Nov;295(5):R1463–R1472. doi: 10.1152/ajpregu.90583.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Snook JH, Li J, Helmke BP, Guilford WH. Peroxynitrite inhibits myofibrillar protein function in an in vitro assay of motility. Free Radic Biol Med. 2008 Jan;44(1):14–23. doi: 10.1016/j.freeradbiomed.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frank KF, Bolck B, Erdmann E, Schwinger RH. Sarcoplasmic reticulum Ca2+-ATPase modulates cardiac contraction and relaxation. Cardiovasc Res. 2003 Jan;57(1):20–27. doi: 10.1016/s0008-6363(02)00694-6. [DOI] [PubMed] [Google Scholar]
- 37.Terentyev D, Gyorke I, Belevych AE, Terentyeva R, Sridhar A, Nishijima Y, de Blanco EC, Khanna S, Sen CK, Cardounel AJ, Carnes CA, Gyorke S. Redox modification of ryanodine receptors contributes to sarcoplasmic reticulum Ca2+ leak in chronic heart failure. Circ Res. 2008 Dec;103(12):1466–1472. doi: 10.1161/CIRCRESAHA.108.184457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Belevych AE, Terentyev D, Viatchenko-Karpinski S, Terentyeva R, Sridhar A, Nishijima Y, Wilson LD, Cardounel AJ, Laurita KR, Carnes CA, Billman GE, Gyorke S. Redox modification of ryanodine receptors underlies calcium alternans in a canine model of sudden cardiac death. Cardiovasc Res. 2009 Dec;84(3):387–395. doi: 10.1093/cvr/cvp246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wagner S, Rokita AG, Anderson ME, Maier LS. Redox Regulation of Sodium and Calcium Handling. Antioxid Redox Signal. 2012 Oct; doi: 10.1089/ars.2012.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, Oddis CV, Bartlett RK, Lowe JS, O’Donnell SE, Aykin-Burns N, Zimmerman MC, Zimmerman K, Ham AJ, Weiss RM, Spitz DR, Shea MA, Colbran RJ, Mohler PJ, Anderson ME. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008 May;133(3):462–474. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Babu GJ, Bhupathy P, Carnes CA, Billman GE, Periasamy M. Differential expression of sarcolipin protein during muscle development and cardiac pathophysiology. J Mol Cell Cardiol. 2007 Aug;43(2):215–222. doi: 10.1016/j.yjmcc.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dhalla NS, Temsah RM, Netticadan T. Role of oxidative stress in cardiovascular diseases. J Hypertens. 2000 Jun;18(6):655–673. doi: 10.1097/00004872-200018060-00002. [DOI] [PubMed] [Google Scholar]
- 43.Fernandez-Velasco M, Ruiz-Hurtado G, Hurtado O, Moro MA, Delgado C. TNF-alpha downregulates transient outward potassium current in rat ventricular myocytes through iNOS overexpression and oxidant species generation. Am J Physiol Heart Circ Physiol. 2007 Jul;293(1):H238–H245. doi: 10.1152/ajpheart.01122.2006. [DOI] [PubMed] [Google Scholar]
- 44.Priebe L, Beuckelmann DJ. Simulation study of cellular electric properties in heart failure. Circ Res. 1998 Jun;82(11):1206–1223. doi: 10.1161/01.res.82.11.1206. [DOI] [PubMed] [Google Scholar]
- 45.Winslow RL, Rice J, Jafri S, Marban E, O’Rourke B. Mechanisms of altered excitation-contraction coupling in canine tachycardia-induced heart failure, II: model studies. Circ Res. 1999 Mar;84(5):571–586. doi: 10.1161/01.res.84.5.571. [DOI] [PubMed] [Google Scholar]
- 46.Sun X, Wang HS. Role of the transient outward current (Ito) in shaping canine ventricular action potential--a dynamic clamp study. J Physiol. 2005 Apr;564(Pt 2):411–419. doi: 10.1113/jphysiol.2004.077263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fulop L, Banyasz T, Magyar J, Szentandrassy N, Varro A, Nanasi PP. Reopening of L-type calcium channels in human ventricular myocytes during applied epicardial action potentials. Acta Physiol Scand. 2004 Jan;180(1):39–47. doi: 10.1046/j.0001-6772.2003.01223.x. [DOI] [PubMed] [Google Scholar]
- 48.Banyasz T, Fulop L, Magyar J, Szentandrassy N, Varro A, Nanasi PP. Endocardial versus epicardial differences in L-type calcium current in canine ventricular myocytes studied by action potential voltage clamp. Cardiovasc Res. 2003 Apr;58(1):66–75. doi: 10.1016/s0008-6363(02)00853-2. [DOI] [PubMed] [Google Scholar]
- 49.Sah R, Ramirez RJ, Oudit GY, Gidrewicz D, Trivieri MG, Zobel C, Backx PH. Regulation of cardiac excitation-contraction coupling by action potential repolarization: role of the transient outward potassium current (I(to)) J Physiol. 2003 Jan;546(Pt 1):5–18. doi: 10.1113/jphysiol.2002.026468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rabkin SW, Klassen SS. Nitric oxide differentially regulates the gene expression of caspase genes but not some autophagic genes. Nitric Oxide. 2007 May;16(3):339–347. doi: 10.1016/j.niox.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 51.Tao L, Gao E, Hu A, Coletti C, Wang Y, Christopher TA, Lopez BL, Koch W, Ma XL. Thioredoxin reduces post-ischemic myocardial apoptosis by reducing oxidative/nitrative stress. Br J Pharmacol. 2006 Oct;149(3):311–318. doi: 10.1038/sj.bjp.0706853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suzuki K, Kostin S, Person V, Elsasser A, Schaper J. Time course of the apoptotic cascade and effects of caspase inhibitors in adult rat ventricular cardiomyocytes. J Mol Cell Cardiol. 2001 May;33(5) doi: 10.1006/jmcc.2001.1364. [DOI] [PubMed] [Google Scholar]
- 53.Guo D, Lian J, Liu T, Cox R, Margulies KB, Kowey PR, Yan GX. Contribution of late sodium current (I(Na-L)) to rate adaptation of ventricular repolarization and reverse use-dependence of QT-prolonging agents. Heart Rhythm. 2011 May;8(5):762–769. doi: 10.1016/j.hrthm.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 54.Varkevisser R, Wijers SC, van der Heyden MA, Beekman JD, Meine M, Vos MA. Beat-to-beat variability of repolarization as a new biomarker for proarrhythmia in vivo. Heart Rhythm. 2012 Oct;9(10):1718–1726. doi: 10.1016/j.hrthm.2012.05.016. [DOI] [PubMed] [Google Scholar]