Abstract
Binge eating disorders are characterized by discrete episodes of rapid and excessive food consumption. In rats, giving intermittent access to sweet fat food mimics this aspect of binge eating. These models typically employ solid food; however, the total amount consumed depends on motivation, palatability and satiety, which are difficult to dissociate with solid food. In contrast, lick microstructure analysis can be used to dissociate these parameters when the ingestant is a liquid. Therefore, we developed a binge model using a liquid emulsion composed of corn oil, heavy cream and sugar. We show that rats given intermittent access to this high-fat emulsion develop binge-like behavior comparable to that previously observed with solid high-fat food. One feature of this behavior was a gradual escalation in consumption across 2.5 weeks of intermittent access, which was not apparent in rats given lower-fat liquid on the same access schedule. Lick microstructure analysis suggests that this escalation was due at least in part to increases in both motivation to consume and palatability-driven consumption.
1. Introduction
Binge eating disorders are widespread and complex psychiatric illnesses [1, 2]. The search for effective treatments depends on a greater understanding of the underlying neural mechanisms. To this end, several animal models of binge eating have been developed. Although they differ substantially in their details, common to many of them is the intermittent presentation of highly palatable fatty and/or sweet food [2–4]. Over several weeks of access, rats’ consumption of palatable food during the access periods gradually escalates [5–12], such that calorie inake on access days approaches that of rats given continuous access to the same calorie-dense food [7, 8, 10, 13–15]. Furthermore, if access to high-fat food is provided every other day, rats reduce their consumption of less caloric food available at other times [10, 11, 16]. This pattern is similar to the binge-abstinence cycle characteristic of binge eating disorders, and meets an operational definition of binge eating: consumption of a greater quantity of food than would otherwise be consumed in a similar period in the absence of an intermittent access schedule [3, 4].
Although rodent models of binge eating do not capture the complex social and psychological issues that contribute to human eating disorders, they do provide a means of studying the effects of intermittent bingeing on the brain processes that regulate consumption. A number of neurochemical changes have been associated with intermittent access binge consumption for prolonged periods (i.e., several weeks) [17, 18]. For instance, in the nucleus accumbens (NAc), bingeing on sweet or high fat food results in elevated dopamine release [14, 19, 20], upregulation of the dopamine transporter [21], decreased D2 dopamine receptor binding [22], and increased expression of D1 dopamine receptors [8]. Because NAc dopamine promotes food-seeking [23], these results suggest that motivation to seek food may be altered in bingeing subjects. In addition, expression of μ opioid receptors in the NAc is increased in bingeing animals. As the behavioral role of opioidergic neurotransmission in the NAc may be to regulate the palatability of consumed foods [24, 25] or to limit the effects of satiety [26], changes in the neural mechanisms responsible for palatability-driven consumption and satiety may also contribute to regulation of consumption during bingeing. Therefore, to gain a further understanding of the behavioral and neural processes underlying bingeing, it is necessary to dissociate the contributions of motivation, palatability and satiety to binge consumption.
Several means are available to accomplish this dissociation. For instance, sham feeding [27] and intragastric nutrient loading [28, 29] can be used to isolate effects of satiety; taste reactivity tests measure palatability [30]; and operant tasks such as progressive ratio schedules provide direct measures of motivation [31–33]. Alternatively, the temporal pattern of consumption during access periods can be measured in detail, providing insight into all three processes in a single simple experiment. Specifically, the rate of decline in consumption from the start of a meal, the latency to initiate consumption, and the initial rate of consumption serve as relatively independent measures of satiety, motivation and palatability, respectively [34–37]. These parameters are most easily measured for liquid ingestants, as commercially available lickometers provide an inexpensive and efficient means to obtain exact timestamps of consummatory behavior. In addition, this method allows the analysis of lick burst number and length, which are related to motivation and palatability, respectively [36–38]. Thus, providing binge-eating rats with a liquid ingestant in lickometers would allow the experimenter to rapidly obtain evidence for the differential influence of neural circuit manipulations (e.g., brain microinjections) on specific aspects of binge consumption.
Therefore, the goal of this study is to develop an intermittent access binge model using a calorie-dense liquid. Although previous studies have used sucrose solutions to establish bingeing [7, 39, 40], a long-term goal of our experiments is to examine the contribution of μ opioid receptors to binge consumption, and these receptors may preferentially regulate the palatability and intake of fat [25]. High-fat food, including shortening, mixtures of shortening and sugar, and fat-enriched chow, have been used to establish binge eating [5, 10, 11, 13, 16, 41, 42]. However, although previous studies have provided intermittent access to high-fat liquid [35], to our knowledge a binge model using sweet high-fat liquid has not been characterized in detail. Here, we demonstrate such a model based on the protocol developed by Corwin and colleagues, in which rats are given access on three days per week to a mixtures of solid fat and sugar [16]. We modified this procedure by using a liquid emulsion of heavy cream, corn oil and sugar (COS), delivered in lickometer-equipped sipper tubes, instead of a solid fat/sugar mixture. Chow and COS consumption, as well as body weights, were compared to a control group given continuous access to COS or no access to COS.
We predicted that consumption in the intermittent access group would rapidly increase from the first to subsequent access periods as rats learn to consume the liquid and overcome neophobia. However, prolonged intermittent access to high-fat ingestant results in gradually increasing consumption that clearly occurs after initial learning is complete [5, 6, 10–12]. It is not known whether this gradual escalation is due to changes in motivation, palatability or satiety. To test the hypothesis that the increased consumption is at least partially due to elevated motivation and palatability, we compared lick patterns in the rats given COS to a control group given a less caloric solution of cream and sugar (CS) under the same access schedule. Although both groups showed early rapid increases in consumption, the COS group showed a further gradual increase across 7 intermittent access periods, and this gradual increase was at least partially the result of increasing motivation and palatability. The results demonstrate that binge intake of high-calorie ingestant is due in part to elevated motivation- and palatability-driven consumption, and that these increases are the result of a long-term process distinct from initial learning and reduction of neophobia.
2. Materials and methods
2.1 Animals
Male Long–Evans rats (n=144) weighing 275–300 g were obtained from Harlan and housed in a room with a 12 h on, 12 h off light cycle. Experiments were conducted during the light phase. Animals were habituated to handling daily for at least one week before beginning experiments. During this time, chow intake and body weight were measured daily. Prior to the start of the experiment, three groups of rats were matched by average amount of chow consumed and average body weight. All animal procedures were consistent with the U.S. National Institutes of Health Guide for the Care and Use of Laboratory Animals, and were approved by the Institutional Animal Care and Use Committee of the Albert Einstein College of Medicine.
2.2 Behavior
2.2.1. Operant chambers
All behavioral experiments were run in standard Med Associates operant chambers (30 x 25 cm). The chambers were illuminated with one 28 V white house light, and at all times during the experiment, white noise (65 dB) was played through a dedicated speaker. This, and the melamine cabinet enclosing each box, ensured that minimal outside noise distracted the animals. Operant chambers were equipped with two lickometers, one empty and one filled with a cream-oil-sucrose liquid emulsion (COS). In all experiments, photobeams across the lickometers were used to detect the times of licks with 1 msec temporal resolution.
2.2.2. Ingestants
The heavy cream used for COS and CS contained 5 g fat, 1 g carbohydrate and 1 g protein per 15 mL. The COS emulsion was prepared daily by mixing 500 mL each of heavy cream and corn oil with 80 g sucrose and 1 g sodium stearoyl lactylate (Niacet Corporation), an emulsifier. The emulsion, prepared using a wire whisk, was stable for > 24 hr; after 24 hr, visible separation of oil and water phases could be observed. A less fatty and less caloric cream-sucrose (CS) solution was used in a separate group of animals; CS was prepared by mixing 1 L heavy cream with 80 g sucrose. The calorie content of COS was 5.99 kCal/mL; the value for CS was 3.65 Kcal/mL; and for chow (PMI LabDiet 5001), it was 3.02 kCal/g.
2.2.3. Intermittent access procedure
Rats were divided into three groups: the intermittent access (binge) group (ICOS; n=46), the continuous access group (CCOS; n=36) and a control group given no access to COS (NCOS; n=38). (The number of rats used is large because rats were subsequently used for pharmacological experiments to compare the effects of drugs on the different groups; these results are not reported here.) For 5 weeks, the ICOS and CCOS groups had access to a lickometer containing COS in the operant chambers three times per week (Monday, Wednesday and Friday; M-W-F) for 90 min. The CCOS group also had ad libitum access to COS at all times in their home cages. Lick timestamps and COS intake were recorded during the access sessions. Body weight, food intake and (for the CCOS group) COS intake in the home cage were recorded daily from Monday to Friday; therefore, 25 measurements are reported for the 5 week experiment (Figs. 2A,D,B,E; 3A,C). On Mondays, these measures were divided by three to normalize for the weekend. Comparisons of chow consumption on the day before vs the day after the access session (Figs. 2C,F; 3B,D) were made twice per week (for a total of 10 comparisons in 5 weeks). The NCOS rats were purchased at the same time as the other groups and were maintained in the same colony, but were not given access to COS. All rats had ad libitum access to chow and water at all times in the home cage.
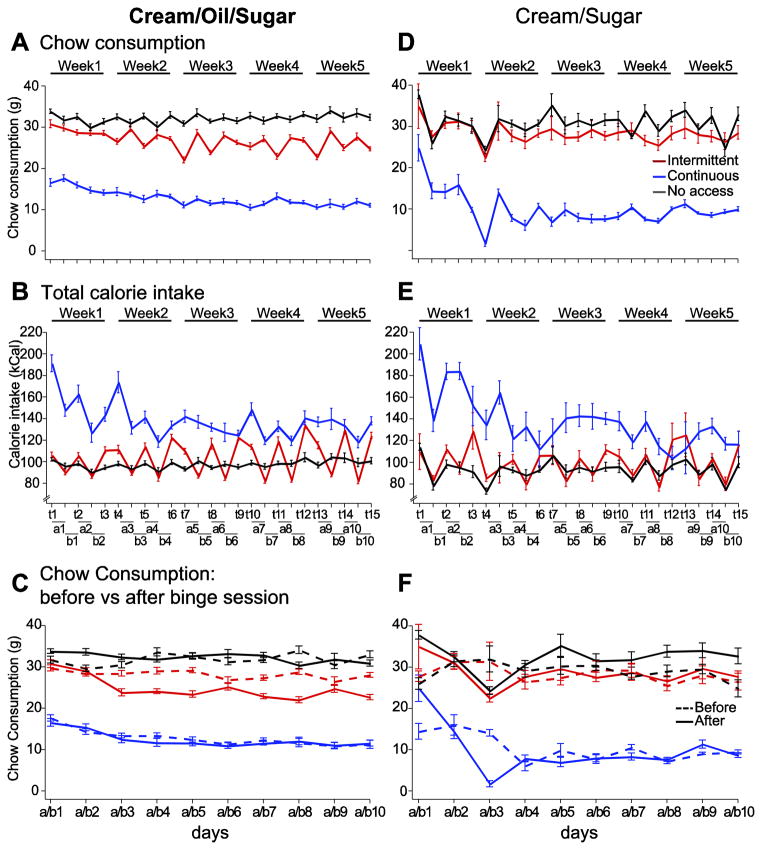
Figure 2. Calorie intake and chow consumption were influenced by access schedule and ingestant.
Chow consumption in the home cage (A, D) was more variable across days in the ICOS group compared to all other groups. ICOS rats always consumed less chow than the NCOS group (A), and consumed even less on the days after COS access sessions (days a1 to a10) (C); this effect was not observed for the ICS group (D, F). Total calorie intake from all sources was also highly variable in the ICOS group: it approached levels achieved by the CCOS group on COS access days, but was lower than the NCOS group consumed on intervening days (B). These effects were not as prominent in the comparison between ICS, CCS and NCS rats (E). In the X axis legends, “t1” through “t15” refer to test days on which ICOS and ICS rats were given access to COS and CS, respectively (and on which CCOS and CCS rats were placed in the same chambers). In addition, “a” and “b” refer to the days after and before access days. For instance, “a1” refers to the 24 hours after access to COS or CS was given on t1, and b1 refers to the 24 hours prior to access on day t2.
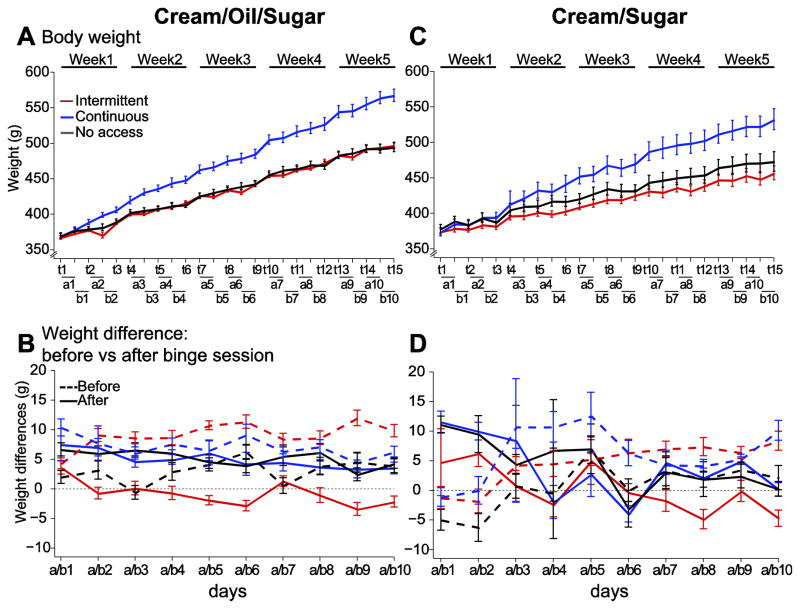
Figure 3. Intermittent access to COS or CS does not affect weight gain.
The body weight increase in the CCOS group was faster than in both the NCOS and ICOS groups, whereas the ICOS rats did not gain weight faster than the NCOS group (A). In the ICOS group, the body weight increase was lower on the day after the COS access session and higher on the day before (B). Although the rate of weight gain in the CCS rats exceeded that of ICS and NCS rats (C), it was not as rapid as that of CCOS rats. Unlike the ICOS rats, weight gain in the ICS rats did not vary across days before and after the CS access period (D). X axis conventions are as described in the legend for Fig. 2.
A separate experiment utilized the less caloric CS solution instead of COS. Three groups of rats (n=8 each) were used in this experiment, ICS, CCS and NCS, and these were treated exactly as the ICOS, CCOS and NCOS groups described above, respectively, except that CS was substituted for COS. NCOS and NCS groups were treated identically (no access to COS or CS); the difference was that the NCOS group was maintained in the colony simultaneously with the ICOS and CCOS groups, whereas the NCS group was maintained simultaneously with the ICS and CCS groups.
2.3. Data analysis
2.3.1. Statistics
Repeated-measures ANOVA with one factor (days) or two factors (days and group) were used to compare the effects of bingeing and control procedures. ANOVAs were followed by Holm–Sidak post hoc tests; an adjusted p < 0.05 was considered a significant difference. The ANOVA results are reported in Table 1. To determine whether consumption increased over successive access sessions, data from day 2 to day 8 were fitted to a linear model using least squares regression. The slope across these days was assessed for significant difference from 0, and slopes were compared between the ICOS and ICS groups using ANCOVA. All analyses were performed using the R software environment [43].
Table 1.
ANOVA results.
| Figure | Dependent variable | factor(s) | COS | Cream/sugar |
|---|---|---|---|---|
| 1A/B | consumption | days | F(14,1165) = 14.7;p<0.001 | F(14,195) = 0.9;p>0.1 |
| groups | F(1,1165) = 759.6; p<0.001 | F(1,195) = 126.1; p<0.001 | ||
|
| ||||
| 2A/D | chow consumption | days | F(24,2661) = 1.4; p<0.1 | F(24,525) = 3.1; p<0.001 |
| groups | F(2,2661) = 395.7; p<0.001 | F(2,525) = 12.9; p<0.001 | ||
| days x groups | F(48,2661) =5.1; p<0.001 | F(48,525) = 3.8; p<0.001 | ||
| 2B/E | chow intake before vs after: before | days | F(9,1074) = 0.2; p>0.1 | F(9,210) = 3.1; p<0.01 |
| groups | F(2,1074) = 168.7; p<0.001 | F(2,210) =4.8; p<0.05 | ||
| days x groups | F(18,1074) = 2.7; p<0.001 | F(18,210) = 6.4; p<0.001 | ||
| chow intake before vs after: after | days | F(9,1026) = 0.6; p>0.1 | F(9,210) = 5.2; p<0.001 | |
| groups | F(2,1026) = 101.4; p<0.001 | F(2,210) = 7.7; p<0.001 | ||
| days x groups | F(18,1026) = 4.0; p<0.001 | F(18,210) = 3.7; p<0.001 | ||
| chow intake before vs after | days x groups | F(2,2154) = 30.6; p<0.001 | F(2,474) = 1.3; p>0.1 | |
| 2C/F | calorie intake | days | F(24,2634) = 3.8; p<0.001 | F(24,525) = 1.9; p<0.01 |
| groups | F(2,2634) = 24.9; p<0.001 | F(2,525) = 25.5; p<0.001 | ||
|
| ||||
| 3A/C | weight vs after: before | days | F(24,2636) = 269.9; p<0.001 | F(24,500) = 43.6; p<0.001 |
| groups | F(2,2636) = 536.6; p<0.001 | F(2,500) = 124.2; p<0.001 | ||
| 3B/D | weight gain before | days | F(9,1048) = 2.3; p<0.05 | F(9,200) = 4.2; p<0.001 |
| groups | F(2,1048) = 17.7; p<0.001 | F(2,200) = 7.6; p<0.001 | ||
| weight gain before vs after: after | days | F(9,929) = 1.1; p>0.1 | F(9,200) = 3.3; p<0.001 | |
| groups | F(2,929) = 29.4; p<0.001 | F(2,200) = 2.2; p>0.1 | ||
| days x groups | F(18,929) = 1.6; p<0.05 | F(18,200) = 1.7; p<0.05 | ||
| weight gain before vs after | days x groups | F(2,2031) = 34.5; p<0.001 | F(2,454) =7.1; p<0.001 | |
|
| ||||
| 5A/B | latency | days | F(14,1006) = 5.0; p<0.001 | F(14,149) = 2.8; p<0.01 |
| groups | F(1,1006) = 193.0; p<0.001 | F(1,149) = 72.9; p<0.001 | ||
| 5D/E | initial licking rate | days | F(14,1011) = 6.9; p<0.001 | F(14,153) = 1.5; p>0.1 |
| groups | F(1,1011) = 7.1; p<0.01 | F(1,153) = 0.7; p>0.1 | ||
|
| ||||
| 6A/B | number of burst first 1/4 | days | F(14,1195) = 5.6; p<0.001 | F(14,204) = 0.8; p>0.1 |
| groups | F(1,1195) = 425.0; p<0.001 | F(1,204) = 179.7; p<0.001 | ||
| 6D/E | number of burst last 3/4 | days | F(14,1195) = 5.1; p<0.001 | F(14,204) =1.9; p<0.05 |
| groups | F(1,1195) = 44.6; p<0.001 | F(1,204) = 15.4; p<0.001 | ||
| 6G/H | burst duration | days | F(14,1005) = 5.1; p<0.001 | F(14,148) = 1.3; p>0.1 |
| groups | F(1,1005) = 17.1; p<0.001 | F(1,148) = 0.3; p>0.1 | ||
2.3.2. Licking microstructure
Lick rate was defined as the number of licks per second. The initial lick rate was defined as the lick rate during the minute beginning with the first lick of the first burst of the session [35–37, 44], and the latency to the first lick was defined as the time from start of the session to this lick. Bursts were defined as groups of licks separated by ILI > 1 s (i.e., termination of a burst was defined by the onset of an ILI > 1 s). Burst duration and burst size refer to the time spanned by a burst and the number of licks in that burst, respectively. Only bursts of three or more licks were considered. As most consumption occurred towards the beginning of the session, licking microstructure analyses were performed not only for the session as a whole, but also separately for two phases: the beginning of the session (first quarter, or 22.5 min, of the 90 min session) and the end of the session (last 3 quarters).
3. Results
3.1. Overall consumption and body weight
Rats in the ICOS (Intermittent access to COS) group had 5 weeks of access to a cream (50% v/v), oil (50% v/v) and sugar (8% w/v) liquid emulsion (COS) on test days: Mondays, Wednesdays and Fridays (and no access to COS at any other time). COS was delivered through a lickometer in 90 min access periods in operant chambers. Over the 5 weeks of intermittent access, the volume of COS consumed increased steadily: on access day 15 (at the end of the 5th week), the amount consumed was significantly higher than on days 1 to 10, and the amounts consumed on days 6 to 15 was greater than on days 1, 2 or 3 (Fig. 1A). In contrast to the ICOS group, rats in the CCOS group (Continuous access to COS) had access to COS not only in the operant chamber during the 90 min access period, but also in the home cage at all other times. As expected, the CCOS group consumed far less COS than the ICOS group in the operant chambers in all sessions after the first, and showed no consumption differences in the 15 days of access (Fig. 1A).
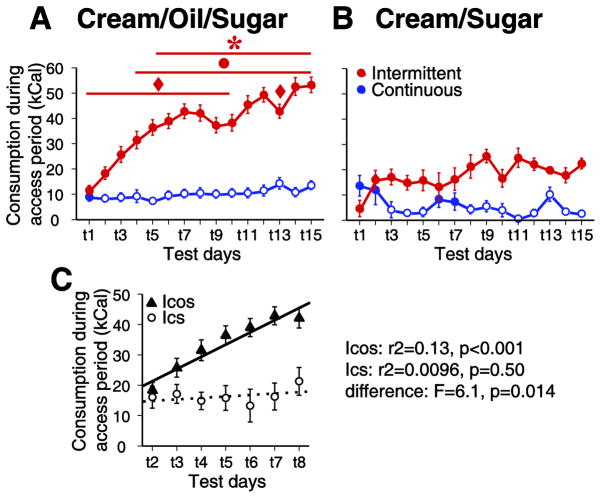
Figure 1. COS, but not CS consumption increased across successive intermittent access sessions.
Consumption increased over test days (t1 through t15) for the ICOS group, which had intermittent access to COS (A, C), but not the ICS group, which had intermittent access to lower fat CS (B,C). COS consumption during the session remained stable in the CCOS group, which had continuous access to COS in the home cage (A). Neither ICS nor CCS groups showed significant increases in consumption across days, although the CCS group typically consumed less than the ICS group (B). In this and subsequent figures, data shown in the left column shows results for the ICOS and CCOS groups, and the right column shows results for the ICS and CCS groups. The slope of consumption across test days 2 to 8 was significantly greater than 0 for the ICOS rats, but not the ICS rats; moreover, the slopes of the two were significantly different by ANCOVA (C).
For all figures, ANOVA results are reported in Table 1, and significance symbols (based on post-hoc tests) have the following meanings:
◆: significant difference from day 15
●: significant difference from day 2
*: significant difference from day 3
open symbols in the CCOS and CCS plots: significant difference from the corresponding I group (ICOS or ICS).
In contrast to the effects observed in the ICOS group, the amount consumed did not significantly increase across weeks for the ICS group, which had access to CS, a lower fat cream/sugar solution. These rats exhibited a low level of consumption in the first access session, and although consumption tended to be greater on subsequent sessions (and usually greater than in-chamber consumption by the CCS group), there were no further increases across days (Fig. 1B). The absence of a statistically significant effect of days in the ICS group contrasts with the strong effect of days in the ICOS group (Table 1); however, this difference could, in theory, arise from the smaller number of subjects (and hence statistical power) in the ICS group. Therefore, we broke the ICOS group into 6 sub-groups of comparable size to the ICS group (N = 8 for 5 ICOS subgroups, N = 6 for one sub-group; subjects in each sub-group were trained together). There was a significant increase in COS consumption in 5 of the 6 ICOS sub-groups (effect of days: group 1: F(14,211) = 5.87, P < 0.001; group 2: F(14,210) = 4.06, p < 0.001; group 3: F(14,200) = 4.83, p < 0.001; group 4: F(14,196) = 7.98, p< 0.001; group 5: F(14,74) = 1.87, p < 0.05); in the remaining sub-group, the effect approached significance (F(14,141) = 1.72, p= 0.058). In the ICS group, however, there was no evidence of even a trend towards a significant effect (p = 0.56; Table 1). Therefore, the absence of a significant effect of days in the ICS rats is unlikely to be due to lack of statistical power.
These results suggest that ICS rats learned to consume CS and to overcome neophobia within one session, with the result that their consumption reached a plateau by the second session. Because initial learning and neophobia effects should be similar for the ICOS and ICS groups, the large increase in consumption in the ICOS group from day 1 to day 2 (Fig. 1A) is likely to be due to initial learning and reduction of neophobia. However, the more gradual increase on subsequent days is inconsistent with these processes. Therefore, to isolate the gradual increase in intake (Fig. 1A) and other consummatory measures (Figs. 5 and 6) from initial learning and neophobia effects, we compare consumption on all individual days to baseline consumption on days 2 and 3.
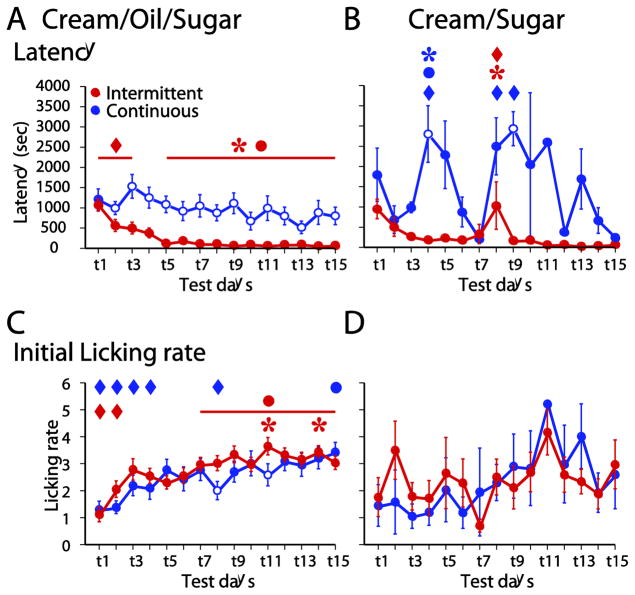
Figure 5. The latency to lick and initial licking rate changed over days of intermittent access to COS.
The latency to the first lick became reduced over days in the ICOS group, but not the CCOS group (A). There was no consistent change in latency in the ICS or CCS groups (B). The initial lick rate (in the 1 min after the first lick) increased for ICOS and CCOS groups (C), but not the ICS and CCS groups (D).
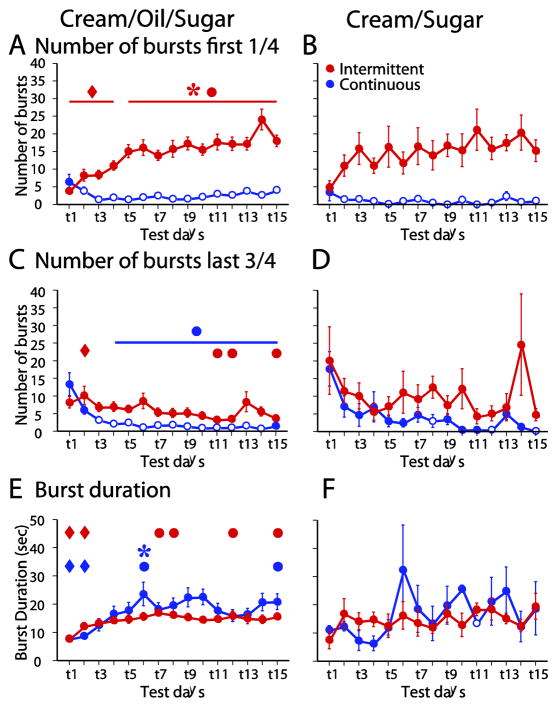
Figure 6. Burst microstructure changed across days of access to COS, but not CS.
During the first quarter of the session, the number of licks bursts increased across days for the ICOS group (A). During the last three quarters of the session, the burst number decreased for the ICOS and CCOS groups (C). The burst duration increased across days for the ICOS and CCOS groups (E). No changes in burst number (B, D) or burst duration (F) across days were observed in the ICS and CCS groups.
The early plateau effect in the ICS group stands in striking contrast to the gradual escalation observed in the ICOS group, which reached a plateau by day 8. To directly compare the rate of increase in consumption after the first day in the ICOS and ICS groups, we calculated the slope of the increase from day 2 to day 8 (Fig. 1C). Whereas the slope was positive and significantly different from 0 for the ICOS group, it did not differ significantly from 0 for the ICS group. Moreover, a significantly positive slope was observed in 5 of the 6 ICOS sub-groups described above (not shown). In addition, comparison of slopes in the ICOS and ICS groups using ANCOVA showed a significant difference between the two groups (Fig. 1C). Therefore, the ICOS group demonstrated a gradual escalation in consumption across intermittent access periods that cannot easily be explained as a consequence of initial learning or diminishment of neophobia. This gradual escalation is similar to that observed previously for rats given intermittent access to solid high-fat food [5, 6, 10–12].
A potential alternative explanation for the gradual escalation of consumption in the ICOS group is that the rats gradually learned to obtain more COS per lick. To test this hypothesis, we compared the number of licks per ml of liquid consumed across ICOS and ICS groups and across access days, using a two factor ANOVA. The ICOS group licked more efficiently than the ICS group (overall average across 15 sessions: COS: 315.0 ± 52.3 licks/mL; CS: 418.3 ± 106.4 licks/mL; F(1,719) = 13.6; p < 0.001) and there was an effect of access days (F(14,719) = 1.8; p = 0.04), but no interaction (F(14,719) = 0.5; p = 0.9). Post-hoc tests showed that the effect of days was due to a significant increase in efficiency from the first day to the second, but no further increases in later sessions. Therefore, the gradual escalation in consumption in the ICOS group cannot be attributed to changes in lick efficiency.
Another potentially confounding factor is the presence of the emulsifier (sodium stearoyl lactylate) in COS, but not CS: the ICOS rats may have initially avoided COS because of the presence of the emulsifier. Because it is impossible to make COS without an emulsifier, we tested the hypothesis that rats avoid the emulsifier used to prepare COS by comparing consumption of CS and CS + 0.1% sodium stearoyl lactylate (weight/volume). Six naïve male rats were presented simultaneously with bottles containing both liquids for 1 hr in the home cage. This experience was repeated twice after one day of habituation; the relative bottle position was changed between days to avoid a side bias. In the final two access sessions, rats drank similar amounts of CS (4.2 ± 0.8 ml) and CS + emulsifier (5.4 ± 0.4 ml; t-test p > 0.05). Therefore, rats do not avoid the emulsifier.
Over the 5 weeks, the ICOS and CCOS groups consumed progressively less freely available chow in the home cage. The CCOS group consumed relatively little chow (Fig. 2A) but, due to consumption of large amounts of COS, still consumed more calories per day than the NCOS control group, which did not have access to COS (Fig. 2B). The ICOS group showed large and regular swings in chow consumption (Fig. 2A), with rats in this group consuming more chow the day before access to COS and less in the 24 hr following COS access (Fig. 2C). In contrast, neither the NCOS nor the CCOS groups differed in their chow consumption immediately before and after the access period (Fig. 2C). For ICOS rats, the net result was that on COS access days, this group consumed total calories similar to the amount consumed by the CCOS group, and on days without COS, the ICOS group consumed slightly less than the NCOS group. The large number of calories consumed by the ICOS group on access days was due to intake of COS: by the end of the 5 weeks, the ICOS group consumed in 90 min slightly more than half the amount of COS that the CCOS group consumed in an entire day (on access day 15: ICOS: 54.2 ± 4.0 kCal of COS consumed; CCOS: 97.7 ± 5.7 kCal of COS consumed). These effects resulted in a “saw tooth” pattern of total caloric intake across days in the ICOS group (Fig. 2B). The latter effect can also be seen in Fig. 2C (the ICOS group’s chow consumption prior to binge days was lower than that of the NCOS group). These results are consistent with the findings of others using intermittent access to solid high-fat food as a rodent model of binge eating behavior [5, 10, 11, 16, 41, 42].
Importantly, this feeding pattern was not observed in the ICS rats, which had access to a lower fat cream/sugar solution instead of the COS emulsion: their daily chow intake was not affected by whether the rats had a CS access session (Fig. 2D,F). In addition, the “saw tooth” pattern of caloric intake was much less pronounced in ICS rats than in ICOS rats (compare Fig. 2E with Fig. 2B). Thus, a binge-like consumption pattern was observed with intermittent access to COS, but not with CS.
Although the body weight of rats in all groups increased over the 5 weeks, the CCOS group’s weight increased faster than that of the other groups (Fig. 3A). Strikingly, despite access to COS 3 days/week, the ICOS group did not gain more weight than the NCOS group (Fig. 3A). The ICOS rats’ seemingly normal regulation of body weight may be related to their reduced consumption of chow after intake of COS (Fig. 2C): their weight gain was substantially greater in the day prior to COS access than in the day following access (Fig. 3B). In contrast, the CCOS and NCOS groups gained weight at a constant rate (Fig. 3B). Similarly, with the cream/sugar solution, the CCS group’s weight increased faster than that of the ICS and NCS groups (Fig. 3C,D). These results are consistent with prior observations with solid high-fat food [10, 11, 41]. They indicate that, as is often the case in human binge eating disorders [45, 46], bingeing rats are able to restrict their consumption after large intake of highly caloric food, even after repeated intermittent access to such food across 5 weeks.
3.2. Within-session time course of consumption
When given access to a highly palatable liquid, rats’ initially avid consumption tapers off as the ingested nutrient (and the conditioned taste stimulus associated with it) activates satiety mechanisms [34]. Therefore, we asked whether COS consumption followed a similar pattern. As shown in plots of the time course of licking over the 90 min session (in 5 min bins), the ICOS group exhibited a greatly increased overall rate of licking in the first ~20 min of the session in week 5 compared with week 1 (Fig. 4A,B). The ICOS rats’ lick rates returned to low, sporadic levels by approximately 30 min after the start of the session (Fig. 4A,B), consistent with previous observations [34]. Because of the dramatic difference in lick rates in initial and later parts of the access session, in subsequent lick pattern analyses we examine consumption in the first quarter of the session (22.5 min) and last three quarters (68.5 min) separately.
Figure 4. The increase in COS consumption over weeks of intermittent access was concentrated within the first quarter of the session.
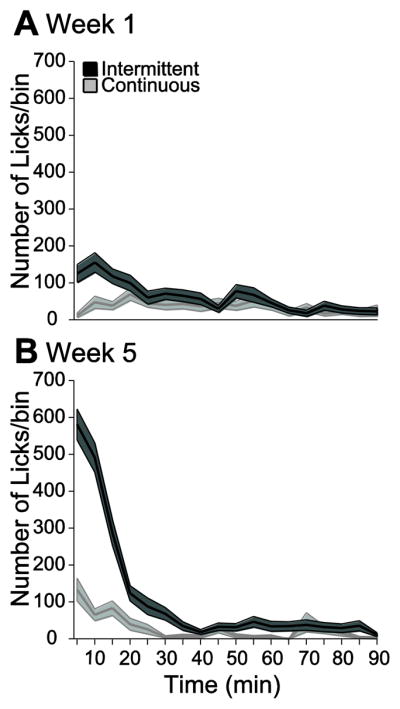
The number of licks is plotted against time in 5 min bins for week 1 (A) and week 5 (B) for ICOS and CCOS groups. The shaded region around the lines represents the SEM.
As expected, the increased rate of consumptions in the first quarter of the session was of much smaller magnitude in the CCOS group (Fig. 4B). Indeed, it is somewhat surprising that the CCOS rats drank much COS at all in the lickometer chambers, as COS was freely available in their home cages. One possibility is that the fresh COS was more avidly consumed than the COS in the home cage, which was up to 24 hr old. However, a naïve group of 6 rats given fresh COS and day-old COS in 1 hr two bottle choice tests in the home cage showed no preference for fresh COS (fresh COS: 2.9 ± 0.9 mL; day-old COS: 6.0 ± 1.8; t-test p > 0.05). Therefore, the increased initial consumption of COS in the CCOS group may be a function of behavioral activation following placement into the relatively novel (compared with the home cage) lickometer chamber.
3.3. Latency to first lick and initial lick rate
The gradual escalation in consumption across intermittent access to COS (Fig. 1) could be due in part to increased motivation to consume and/or to increased palatability-driven consumption. To test these hypotheses, we examined the latency to the first lick of the session (an index of motivation) and the initial lick rate (thought to reflect palatability-driven consumption) [35–37]. Over successive access sessions, the ICOS group’s latency to initiate COS consumption became shorter, whereas the CCOS group’s latency was unchanged (Fig. 5A). Moreover, the latency to first lick was consistently significantly longer in the CCOS group than the ICOS group (Fig. 5A), consistent with the differences in motivation that would be expected between animals given continuous and restricted access to a favored food. Notably, however, the initial lick rate increased across sessions in both ICOS and CCOS rats, and was typically not significantly different across groups on a given day (Fig. 5C). Thus, unlike the measure of motivation, the measure of palatability-driven consumption increased in parallel in the two groups.
As expected given the lack of an escalation in consumption in the ICS group, neither the ICS nor the CCS groups exhibited a significant decrease in first lick latency (Fig. 5B) or an increase in initial lick rate (Fig. 5D). In summary, intermittent access to COS (but not CS) resulted in apparent increases in motivation and palatability that accompanied the increase in COS consumption.
3.4. Burst number and length
Rats lick at a stereotyped rate of ~7 Hz. During a meal, bursts of licks at this rate are interrupted by pauses, which are usually >1 sec [36, 37]. From the second day of access to the fifteenth, the within-burst lick rate for the ICOS group remained stable (6.7 ± 0.2 vs 6.9 ± 0.1 Hz, p > 0.05), indicating that the gradual increase in consumption evident in this group (Fig. 1A) was not due to a change in the central pattern generator that controls within-burst lick rate.
The number of bursts during access to a palatable liquid is influenced by motivation to consume, whereas the length of bursts is more strongly influenced by the palatability of the liquid [36, 37]. Consistent with this idea, CCOS rats generally exhibited lower burst numbers than ICOS rats (Fig. 6A,C). In the ICOS group, the number of bursts was strongly dependent on the time within the access session: in the first quarter, burst number increased substantially across the 5 weeks of access, whereas it tended to decrease in the last three quarters (Fig. 6A,C). In contrast, ICS rats, which did not escalate their consumption (Fig. 1B), did not show changes in burst number in either the early or the late phase (Fig. 6B,D).
Unlike burst number, burst duration did not differ significantly between ICOS and CCOS groups on any day (Fig. 6E). Indeed, for both the ICOS and CCOS groups, burst duration increased across the 5 weeks of intermittent access (Fig. 6E). These escalation effects were absent in ICS and CCS rats (Fig. 6F).
In sum, the results show that weeks of intermittent access to COS resulted in a gradual increase in consumption in the early phase of each access session. This increase was accompanied by shorter latency to begin consumption and greater number of lick bursts (consistent with an increased motivation to consume), as well as greater initial lick rate and longer burst duration (consistent with increased palatability-driven consumption). Neither gradually escalating consumption nor increases in motivation and palatability indices were observed when rats were given CS instead of COS. Taken together, the results suggest that binge-like consumption is at least in part due to a gradual escalation in motivation and palatability beyond that expected from initial learning and diminishment of neophobia.
4. Discussion
4.1. Summary
Binge eating is characterized by discrete episodes (2 hours or less) of rapid and excessive food consumption that is not driven by hunger or metabolic need [2]. To study the effects of binge-like consumption on the neural processes that regulate food intake, previous animal studies have used intermittent access to foods high in caloric content, especially fat [5, 10, 11, 16, 41], because humans suffering from binge eating disorder or bulimia typically binge on sweet high-fat food [47–49]. Intake of nutrient-rich food and drink is governed by three inter-related processes: motivation, palatability and satiety. To study the neural mechanisms underlying binge consumption using invasive techniques (such as intracranial microinjection of drugs), it would be useful to examine these processes in isolation. One means of doing so is to examine the temporal pattern of consumption, a procedure that is greatly facilitated by delivering liquid ingestant through a lickometer. Therefore, we developed an intermittent access model of binge consumption of high-fat liquid, based on the binge models originally developed by Corwin and colleagues using solid fat [11, 12] and later extended to solid fat/sugar mixtures [10, 13, 16]. Because the ingestant is composed of inexpensive, widely available ingredients (supermarket cream, corn oil and sugar) and requires no special equipment to prepare, our model can be used in subsequent experiments to obtain evidence for the effects of neural manipulations on satiety, motivation and palatability during binge consumption.
4.2. Pattern of consumption and relationship to previous intermittent access binge models
Our results are similar to those established earlier for rats given intermittent access to solid sweet-fat food. In particular, when rats were given intermittent access to COS (cream/oil/sugar, ICOS group), consumption during the access period increased gradually across days of access, consistent with prior observations [5–12]. Furthermore, ICOS rats showed a “saw tooth” pattern of calorie intake, with decreased chow consumption following access to COS. These effects were observed despite procedural differences from the earlier models, including intermittent home cage access to the high-calorie ingestant and concurrent chow availability in previous studies but not ours (in which COS access was provided in separate chambers). Intriguingly, caloric intake on intermittent COS access days reached levels similar to those observed in 24 hr in rats given continuous access to COS, and chow intake after COS access was reduced to levels below those seen in rats given no access to COS at all. These results are consistent with previous findings using solid high-fat food [5, 10, 13, 50]. The large fluctuations in calorie intake induced parallel fluctuations in weight, with the net result that the ICOS rats gained weight no faster than rats that had access to chow only. Taken together, these results mirror previous observations using intermittent access to solid high-fat food [5, 10–13, 50] and suggest that intermittent access to liquid COS induces binge behavior that is remarkably similar to that induced using solid high-fat food.
Strikingly, rats given intermittent access to a less-caloric solution of cream and sugar (CS; ICS group) showed neither the gradual escalation in consumption nor the pronounced saw-tooth pattern observed in rats given COS. These results suggest that high fat and/or caloric content is necessary to develop binge-like behavior on this schedule. However, the results do not rule out the possibility that the development of bingeing is a function of lick efficiency (amount of fluid obtained per lick), which was greater for COS than for CS. The reason for this difference is unknown, but likely has to do with the different physical properties of COS and CS. The higher rate of calorie intake promoted by greater lick efficiency may contribute to the escalation of consumption in the ICOS group. In addition, the specific types of fat found in corn oil may contribute to rats’ susceptibility to develop bingeing, as may the form of the fat (an emulsion). Indeed, rats consume corn oil emulsions even more avidly than 100% corn oil, corn oil emulsions evoke a similar degree of consumption as solid fat, and sweetened corn oil emulsions evoke even greater consumption [51–53]. Thus, because many previous studies of bingeing used a mixture of solid fat and sugar [5, 16], the presence of emulsified corn oil may be the critical factor that bestows upon COS, but not CS, the ability to evoke binge consumption. This hypothesis could be tested by determining whether sweetened corn oil/water emulsions (without cream) induce binge-like behavior similar to that induced by COS. However, even if that were the case, COS may be preferable for use in future experiments because preparation of corn oil/water emulsions with long-term stability requires expensive equipment (a microfluidizer), whereas COS preparation requires no more sophisticated apparatus than a wire whisk.
Because our COS binge model provides rats access to high-fat ingestant three days per week, we were able to observe a reduction in chow consumption during the next 24 hr, which then recovered over the subsequent 24 hr. Chow consumption in the 24 hr after intermittent access to COS (but not CS) was significantly lower than 24 hr consumption by the control group with no access to COS at all (NCOS group), consistent with earlier reports with fat and sweet-fat food [10, 12, 13]. Together with our findings, these results suggest that binge intake activates a long-term satiety process that limits consumption for hours after the very short episode of consumption during the access period. These results are intriguing in light of findings that rats overconsume high-fat food compared with high-carbohydrate food of equal calorie density [28, 29, 54–58]. Intragastric preload with fat results in less inhibition of subsequent consumption than preload with an isocaloric dose of carbohydrate [29], and these effects are long lasting (up to one day) [28]. Thus, rats overconsume fat in part because fat is less efficacious (per calorie) in activating postingestive satiety mechanisms. These studies suggest that the sugar content of COS and other fat/sugar mixtures used in intermittent access binge procedures may contribute significantly to the post-binge inhibition of chow consumption. Further studies that directly compare the consequences of sugar- and fat-containing ingestants are required to clarify the differential roles of fat and sugar in the long-term satiety evoked by binge intake.
Consumption of COS gradually increased across the first 8 days (2.5 weeks) of intermittent access. Notably, rats given intermittent access to CS did not show a similar gradual increase, although both COS and CS intermittent access groups increased their consumption substantially from the first to second day of access. Because rats in both groups must learn how to access the lickometers and overcome initial neophobia, the initial (day 1 to day 2) increase in consumption can be ascribed to these processes. However, the further gradual increase in the ICOS group is inconsistent with these processes because it was not observed in the ICS group. Similar gradual increases have been reported in rats given sweet and/or high fat food or liquid on either 3 days/week or daily intermittent access schedules [9–12, 14, 59]. These results contrast remarkably with those from rats given continuous access to high-calorie food: daily consumption either declines gradually from an initial peak [5, 6, 10], as in our study (Fig. 3), or remains constant [12, 14]. Thus, overconsumption during intermittent access binges is due in part to an escalation in consumption that is absent in unrestricted subjects; because this effect depends on the fat and/or calorie content of the ingestant, our results suggest that the increase is not a simple consequence of learning to consume or overcoming neophobia. Rather, the gradually increasing consumption is likely due to other processes that are a consequence of the physical and/or nutrient properties of COS that differentiate it from CS.
Previous rat binge models have used daily intermittent access to high fat food as a control for 3 days/week “binge” access [6, 11, 12, 15, 16, 40, 42, 60–63]. However, other studies have used daily intermittent access to model bingeing [9, 10, 13, 14, 35, 59]; notably, consumption of high calorie ingestant often escalates across daily access periods, although this escalation may be less pronounced than for 3 days/week access [6]. Thus, there is some confusion as to what constitutes ideal binge and control access protocols. Our results provide a potential resolution: they indicate that a control group given intermittent access to CS on the same schedule as the COS binge group does not show escalated consumption. Experienced ICOS rats consume more calories during access periods than ICS rats, and therefore the comparison between these groups allows the conclusion that ICOS rats meet the operational definition of binge eating (consuming greater calories in a restricted period than non-bingers consume in a similar period). Thus, use of COS and CS (or, more generally, high- and low-calorie or high- and low-fat liquids) may prove useful for further experiments in which a control for binge consumption is required. This model has the additional advantage that it mirrors human binge eating in that humans binge on high-fat, high-calorie food, while non-binge eating typically involves consumption of less calorie-dense food. On the other hand, human binge eaters consume solid and semi-solid food, whereas COS and CS are liquids.
4.3. Motivation, palatability and satiety in binge consumption
Broadly considered, changes in three distinct processes could underlie the gradual increase in consumption across intermittent access days: (1) palatability-driven consumption could increase; (2) motivation to begin consumption could increase; and/or (3) satiety mechanisms could become reduced in efficacy, thereby prolonging the “meal” taken during the access period. Our results provide suggestive evidence for increases in both palatability-driven consumption and motivation to consume. In particular, the gradual escalation in COS consumption across days of intermittent access was accompanied by decreased latency to the first lick, greater number of lick bursts, increased rate of licking in the first minute of access, and longer lick bursts. The former two are considered indices of motivation, and the latter two are indices of palatability [36, 37, 64]. Each of these increased significantly across days as consumption increased dramatically. As each of these changes in lick pattern would tend to increase consumption, we conclude that escalated consumption across access sessions is at least partially a consequence of these structural changes, and therefore of increased motivation and palatability. Furthermore, these increases in measures of motivation and palatability were not observed in the ICS group, which did not show increased consumption. Therefore, the increased motivation and palatability are likely to be a consequence of the high calorie and/or fat content of COS compared with CS.
What could underlie the observed gradual increases in motivation and palatability? Motivation is not a singular brain process, but a psychological construct that reflects the activity of multiple neural mechanisms. For instance, hunger induces motivation to approach food, but the same motivated behavior can be induced by a stimulus associated with food even in subjects that are not hungry, as in conditioned eating [65]. The specific neural mechanism underlying increased motivation in bingeing rats is unknown. It is certainly possible that a conditioning process is involved, such that the stimuli in the access chamber become associated with COS and consequently, in later sessions, induce motivation to seek, approach and initiate consumption of COS as soon as the animal is placed in the chamber. Thus, the gradually increasing motivation displayed by the ICOS rats could be a function of the reinforcing strength of the unconditioned stimulus (COS); the CS solution may be of insufficient reinforcing strength to promote an increase in consumption beyond initial learning.
Stimulus-induced approach behavior is often dependent on the dopamine projection to the nucleus accumbens [66–69], and binge consumption of sucrose is accompanied by elevations in extracellular dopamine in the accumbens [19]. Furthermore, binge-like consumption induces several long-term changes in the accumbens dopamine system, including elevated D1 receptor and dopamine transporter expression, and decreased D2 receptor expression [8], suggesting that long-term changes in dopamine activity are involved in binge eating. Indeed, rats given prolonged intermittent access to fat exhibited higher breakpoints on a progressive ratio schedule of fat reinforcement [70]. This observation not only strongly supports our conclusion that motivation for palatable food is enhanced in binge eating, but provides further evidence for the involvement of mesolimbic dopamine, as performance of progressive ratio tasks depends on accumbens dopamine [71]. However, further studies are required to determine whether there is a causal link between dopamine activity in the accumbens and increased motivation to consume as bingeing develops.
In addition to behavioral changes consistent with increased motivation, we also observed effects that are most readily explained by an increase in palatability-driven consumption (increased initial lick rate and increased lick burst length). A number of brain structures are involved in palatability-driven consumption, including the nucleus accumbens, the nucleus of the solitary tract, the amygdala, the ventral pallidum, the ventral tegmental area, and the lateral hypothalamus [72–74]. In particular, μ opioid receptors, especially in the accumbens [44, 75–78] may contribute to palatability-driven consumption. Interestingly, binge-like consumption is associated with upregulated μ receptors in the accumbens [8], suggesting that increased opioidergic neurotransmission may be responsible for the increased palatability-driven consumption observed during binge access. These hypotheses await further testing with invasive manipulations of opioid receptor function in bingeing animals.
Intriguingly, measures of palatability increased not only in the ICOS rats, but in the CCOS group as well. These results therefore suggest that it is the prolonged access to COS, not the intermittency of access, that causes increased palatability-driven consumption. One difference between the CCOS and ICOS groups is that the CCOS rats gained weight, as would be expected given that ad libitum access to a high-fat diet induces obesity [41, 79, 80]. Thus, our results suggest that palatability-driven consumption of sweet high-fat foods may be increased in obese subjects. Previous studies have shown complex effects of obesity on palatability. In one case, lower concentrations of fat and sucrose were less palatable in obese than lean rats, whereas higher concentrations were slightly more palatable [79]. In another study, sucrose palatability measures (burst size) were not affected at all by obesity [81], and in yet another, palatability measures were decreased in a strain of obese rats [82]. An important difference between our study and previous studies is that we tested the palatability of the same high-fat ingestant to which animals had 24 hr continuous access (i.e., we tested the palatability of the diet that induced the obesity), whereas other studies gave animals high fat food in the home cage, but tested palatability of oil or sucrose solutions. These designs may introduce negative contrast effects, which could prevent detection of a heightened palatability mechanism. Experimental designs that minimize contrast effects should be employed to more extensively test the hypothesis that palatability-driven consumption is potentiated in obese subjects.
The observation that rats given continuous access to COS exhibited increased palatability-driven consumption, yet showed a decline in overall caloric intake (and COS consumption) from the first day (Fig. 2) suggests that some other process, such as satiety, counteracts the palatability increase to limit overall intake. This raises the question of whether satiety mechanisms are more efficacious in experienced bingers than in rats naïve to COS. By the 5th week of intermittent access, ICOS rats exhibited a sharp decline in consumption in the first 20–30 min after the access period began. This decline is likely due to conditioned and unconditioned satiety [34], and demonstrates that satiety mechanisms are robust in bingeing rats, consist with findings in human bulimia patients [83]. Although the rate of decline is faster in week 5 than in previous weeks (especially week 1), this measure could be influenced by the different rates of initial intake in early vs late sessions (which are functions of motivation and palatability). Therefore, additional work, perhaps using intragastric preloading, is necessary to assess whether differences in satiety mechanisms could contribute to the gradual increase in consumption across intermittent access days. Nevertheless, the robust within-session decline in consumption can be used in future studies as a measure of satiety when examining effects of neural manipulations on established binge consumption.
4.4. Conclusions
We demonstrate that a sweet high-fat liquid can be used in rats to induce binge-like consumption similar to that observed previously in intermittent access models using solid high-fat food. The use of liquid ingestant is advantageous in several ways. First, a less caloric liquid can be given on the same access schedule as a control for binge consumption. Second, the temporal precision afforded by measuring consumption with lickometers will facilitate further investigations of binge behavior, particularly using electrophysiology in behaving animals. Finally, use of a liquid high-fat ingestant enables lick microstructure analysis, which can be used to gather evidence for or against differences in satiety, motivation and palatability in bingeing rats following invasive neural manipulations (e.g., local microinjection of neuroactive compounds). Here, we use microstructural analysis to demonstrate that the gradual escalation in consumption across successive access periods is due at least in part to increasing motivation to initiate consumption and increasing palatability-driven consumption. Further investigation of these processes in COS bingeing rats will lead to enhanced understanding of the neural mechanisms underlying binge consumption.
Highlights.
We establish an intermittent access model of binge eating using sweet high-fat liquid
Rats escalate their consumption across 2.5 weeks of intermittent access sessions
Lick microstructure analysis dissociates motivation and palatability
Escalated consumption is due to increases in both motivation and palatability
Rats given continuous fatty liquid access become obese and show increased palatability
Acknowledgments
We thank Drs. Gary Schwartz and Nicole Avena for helpful discussions, Dr. Schwartz and the Animal Energy Balance Phenotyping Core of the New York Obesity Nutrition Research Center for the use of operant chambers, and Niacet Corporation for their generous gift of sodium stearoyl lactylate. This research was supported by grants from the Klarman Family Foundation, NARSAD and NIH (MH092757) to SMN, and by a Hilda and Preston Davis Foundation fellowship to SL.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bulik CM, Reichborn-Kjennerud T. Medical morbidity in binge eating disorder. Int J Eat Disord. 2003;34 (Suppl):S39–46. doi: 10.1002/eat.10204. [DOI] [PubMed] [Google Scholar]
- 2.Mathes WF, Brownley KA, Mo X, Bulik CM. The biology of binge eating. Appetite. 2009;52:545–53. doi: 10.1016/j.appet.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corwin RL, Avena NM, Boggiano MM. Feeding and reward: perspectives from three rat models of binge eating. Physiol Behav. 2011;104:87–97. doi: 10.1016/j.physbeh.2011.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corwin RL, Buda-Levin A. Behavioral models of binge-type eating. Physiol Behav. 2004;82:123–30. doi: 10.1016/j.physbeh.2004.04.036. [DOI] [PubMed] [Google Scholar]
- 5.Bello NT, Guarda AS, Terrillion CE, Redgrave GW, Coughlin JW, Moran TH. Repeated binge access to a palatable food alters feeding behavior, hormone profile, and hindbrain c-Fos responses to a test meal in adult male rats. Am J Physiol Reg Integr Comp Physiol. 2009;297:R622–31. doi: 10.1152/ajpregu.00087.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Babbs RK, Wojnicki FH, Corwin RL. Assessing binge eating. An analysis of data previously collected in bingeing rats. Appetite. 2012;59:478–82. doi: 10.1016/j.appet.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colantuoni C, Rada P, McCarthy J, Patten C, Avena NM, Chadeayne A, et al. Evidence that intermittent, excessive sugar intake causes endogenous opioid dependence. Obes Res. 2002;10:478–88. doi: 10.1038/oby.2002.66. [DOI] [PubMed] [Google Scholar]
- 8.Colantuoni C, Schwenker J, McCarthy J, Rada P, Ladenheim B, Cadet JL, et al. Excessive sugar intake alters binding to dopamine and mu-opioid receptors in the brain. Neuroreport. 2001;12:3549–52. doi: 10.1097/00001756-200111160-00035. [DOI] [PubMed] [Google Scholar]
- 9.Avena NM, Rada P, Hoebel BG. Underweight rats have enhanced dopamine release and blunted acetylcholine response in the nucleus accumbens while bingeing on sucrose. Neuroscience. 2008;156:865–71. doi: 10.1016/j.neuroscience.2008.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berner LA, Avena NM, Hoebel BG. Bingeing, self-restriction, and increased body weight in rats with limited access to a sweet-fat diet. Obesity (Silver Spring) 2008;16:1998–2002. doi: 10.1038/oby.2008.328. [DOI] [PubMed] [Google Scholar]
- 11.Corwin RL, Wojnicki FH, Fisher JO, Dimitriou SG, Rice HB, Young MA. Limited access to a dietary fat option affects ingestive behavior but not body composition in male rats. Physiol Behav. 1998;65:545–53. doi: 10.1016/s0031-9384(98)00201-7. [DOI] [PubMed] [Google Scholar]
- 12.Dimitriou SG, Rice HB, Corwin RL. Effects of limited access to a fat option on food intake and body composition in female rats. Int J Eat Disord. 2000;28:436–45. doi: 10.1002/1098-108x(200012)28:4<436::aid-eat12>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 13.Kinzig KP, Hargrave SL, Honors MA. Binge-type eating attenuates corticosterone and hypophagic responses to restraint stress. Physiol Behav. 2008;95:108–13. doi: 10.1016/j.physbeh.2008.04.026. [DOI] [PubMed] [Google Scholar]
- 14.Rada P, Avena NM, Hoebel BG. Daily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience. 2005;134:737–44. doi: 10.1016/j.neuroscience.2005.04.043. [DOI] [PubMed] [Google Scholar]
- 15.Wojnicki FH, Johnson DS, Corwin RL. Access conditions affect binge-type shortening consumption in rats. Physiol Behav. 2008;95:649–57. doi: 10.1016/j.physbeh.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong KJ, Wojnicki FH, Corwin RL. Baclofen, raclopride, and naltrexone differentially affect intake of fat/sucrose mixtures under limited access conditions. Pharmacol Biochem Behav. 2009;92:528–36. doi: 10.1016/j.pbb.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis C, Carter JC. Compulsive overeating as an addiction disorder. A review of theory and evidence. Appetite. 2009;53:1–8. doi: 10.1016/j.appet.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 18.Avena NM. Binge eating: neurochemical insights from animal models. Eat Disord. 2009;17:89–92. doi: 10.1080/10640260802371604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Avena NM, Rada P, Moise N, Hoebel BG. Sucrose sham feeding on a binge schedule releases accumbens dopamine repeatedly and eliminates the acetylcholine satiety response. Neuroscience. 2006;139:813–20. doi: 10.1016/j.neuroscience.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 20.Liang NC, Hajnal A, Norgren R. Sham feeding corn oil increases accumbens dopamine in the rat. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1236–9. doi: 10.1152/ajpregu.00226.2006. [DOI] [PubMed] [Google Scholar]
- 21.Bello NT, Sweigart KL, Lakoski JM, Norgren R, Hajnal A. Restricted feeding with scheduled sucrose access results in an upregulation of the rat dopamine transporter. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1260–8. doi: 10.1152/ajpregu.00716.2002. [DOI] [PubMed] [Google Scholar]
- 22.Bello NT, Lucas LR, Hajnal A. Repeated sucrose access influences dopamine D2 receptor density in the striatum. Neuroreport. 2002;13:1575–8. doi: 10.1097/00001756-200208270-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berridge KC. ‘Liking’ and ‘wanting’ food rewards: brain substrates and roles in eating disorders. Physiol Behav. 2009;97:537–50. doi: 10.1016/j.physbeh.2009.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelley AE, Bakshi VP, Haber SN, Steininger TL, Will MJ, Zhang M. Opioid modulation of taste hedonics within the ventral striatum. Physiol Behav. 2002;76:365–77. doi: 10.1016/s0031-9384(02)00751-5. [DOI] [PubMed] [Google Scholar]
- 25.Taha SA. Preference or fat? Revisiting opioid effects on food intake. Physiol Behav. 2010;100:429–37. doi: 10.1016/j.physbeh.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katsuura Y, Heckmann JA, Taha SA. mu-Opioid receptor stimulation in the nucleus accumbens elevates fatty tastant intake by increasing palatability and suppressing satiety signals. Am J Physiol. 2011;301:R244–54. doi: 10.1152/ajpregu.00406.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith GP. John Davis and the meanings of licking. Appetite. 2001;36:84–92. doi: 10.1006/appe.2000.0371. [DOI] [PubMed] [Google Scholar]
- 28.Warwick ZS. Dietary fat dose dependently increases spontaneous caloric intake in rat. Obes Res. 2003;11:859–64. doi: 10.1038/oby.2003.118. [DOI] [PubMed] [Google Scholar]
- 29.Warwick ZS, McGuire CM, Bowen KJ, Synowski SJ. Behavioral components of high-fat diet hyperphagia: meal size and postprandial satiety. American J Physiol Reg Integ Comp Physiol. 2000;278:R196–200. doi: 10.1152/ajpregu.2000.278.1.R196. [DOI] [PubMed] [Google Scholar]
- 30.Grill HJ, Norgren R. The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res. 1978;143:263–79. doi: 10.1016/0006-8993(78)90568-1. [DOI] [PubMed] [Google Scholar]
- 31.Hodos W. Progressive ratio as a measure of reward strength. Science. 1961;134:943–4. doi: 10.1126/science.134.3483.943. [DOI] [PubMed] [Google Scholar]
- 32.Hodos W, Kalman G. Effects of increment size and reinforcer volume on progressive ratio performance. J Exp Anal Behav. 1963;6:387–92. doi: 10.1901/jeab.1963.6-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stewart WJ. Progressive reinforcement schedules: a review and evaluation. Aust J Psychol. 1975;27:9–22. [Google Scholar]
- 34.Davis JD, Smith GP. Learning to sham feed: behavioral adjustments to loss of physiological postingestional stimuli. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 1990;259:R1228–R35. doi: 10.1152/ajpregu.1990.259.6.R1228. [DOI] [PubMed] [Google Scholar]
- 35.Bocarsly ME, Berner LA, Hoebel BG, Avena NM. Rats that binge eat fat-rich food do not show somatic signs or anxiety associated with opiate-like withdrawal: implications for nutrient-specific food addiction behaviors. Physiol Behav. 2011;104:865–72. doi: 10.1016/j.physbeh.2011.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davis JD, Perez MC. Food deprivation- and palatability-induced microstructural changes in ingestive behavior. Am J Physiol. 1993;264:R97–103. doi: 10.1152/ajpregu.1993.264.1.R97. [DOI] [PubMed] [Google Scholar]
- 37.Spector AC, Klumpp PA, Kaplan JM. Analytical issues in the evaluation of food deprivation and sucrose concentration effects on the microstructure of licking behavior in the rat. Behav Neurosci. 1998;112:678–94. doi: 10.1037//0735-7044.112.3.678. [DOI] [PubMed] [Google Scholar]
- 38.Baird JP, St John SJ, Nguyen EA. Temporal and qualitative dynamics of conditioned taste aversion processing: combined generalization testing and licking microstructure analysis. Behav Neurosci. 2005;119:983–1003. doi: 10.1037/0735-7044.119.4.983. [DOI] [PubMed] [Google Scholar]
- 39.Cottone P, Sabino V, Steardo L, Zorrilla EP. Opioid-dependent anticipatory negative contrast and binge-like eating in rats with limited access to highly preferred food. Neuropsychopharmacology. 2008;33:524–35. doi: 10.1038/sj.npp.1301430. [DOI] [PubMed] [Google Scholar]
- 40.Wojnicki FH, Stine JG, Corwin RL. Liquid sucrose bingeing in rats depends on the access schedule, concentration and delivery system. Physiol Behav. 2007;92:566–74. doi: 10.1016/j.physbeh.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 41.Boggiano MM, Artiga AI, Pritchett CE, Chandler-Laney PC, Smith ML, Eldridge AJ. High intake of palatable food predicts binge-eating independent of susceptibility to obesity: an animal model of lean vs obese binge-eating and obesity with and without binge-eating. Int J Obes (Lond) 2007;31:1357–67. doi: 10.1038/sj.ijo.0803614. [DOI] [PubMed] [Google Scholar]
- 42.Rao RE, Wojnicki FH, Coupland J, Ghosh S, Corwin RL. Baclofen, raclopride, and naltrexone differentially reduce solid fat emulsion intake under limited access conditions. Pharmacol Biochem Behav. 2008;89:581–90. doi: 10.1016/j.pbb.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R foundation for Statistical Computing; 2012. [Google Scholar]
- 44.Taha SA, Katsuura Y, Noorvash D, Seroussi A, Fields HL. Convergent, not serial, striatal and pallidal circuits regulate opioid-induced food intake. Neuroscience. 2009;161:718–33. doi: 10.1016/j.neuroscience.2009.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Engelberg MJ, Gauvin L, Steiger H. A naturalistic evaluation of the relation between dietary restraint, the urge to binge, and actual binge eating: A clarification. International Journal of Eating Disorders. 2005;38:355–60. doi: 10.1002/eat.20186. [DOI] [PubMed] [Google Scholar]
- 46.Fedoroff IDC, Polivy J, Herman CP. The Effect of Pre-exposure to Food Cues on the Eating Behavior of Restrained and Unrestrained Eaters. Appetite. 1997;28:33–47. doi: 10.1006/appe.1996.0057. [DOI] [PubMed] [Google Scholar]
- 47.Bartholome LT, Raymond NC, Lee SS, Peterson CB, Warren CS. Detailed analysis of binges in obese women with binge eating disorder: Comparisons using multiple methods of data collection. Int J Eat Disord. 2006;39:685–93. doi: 10.1002/eat.20289. [DOI] [PubMed] [Google Scholar]
- 48.Guss JL, Kissileff HR, Devlin MJ, Zimmerli E, Walsh BT. Binge size increases with body mass index in women with binge-eating disorder. Obes Res. 2002;10:1021–9. doi: 10.1038/oby.2002.139. [DOI] [PubMed] [Google Scholar]
- 49.Kales EF. Macronutrient analysis of binge eating in bulimia. Physiology & Behavior. 1990;48:837–40. doi: 10.1016/0031-9384(90)90236-w. [DOI] [PubMed] [Google Scholar]
- 50.Bello NT, Patinkin ZW, Moran TH. Opioidergic consequences of dietary-induced binge eating. Physiology & Behavior. 2011;104:98–104. doi: 10.1016/j.physbeh.2011.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Castonguay TW, Burdick SL, Guzman MA, Collier GH, Stern JS. Self-selection and the obese Zucker rat: the effect of dietary fat dilution. Physiology & behavior. 1984;33:119–26. doi: 10.1016/0031-9384(84)90022-2. [DOI] [PubMed] [Google Scholar]
- 52.Lucas F, Ackroff K, Sclafani A. Dietary fat-induced hyperphagia in rats as a function of fat type and physical form. Physiology & behavior. 1989;45:937–46. doi: 10.1016/0031-9384(89)90218-7. [DOI] [PubMed] [Google Scholar]
- 53.Lucas F, Sclafani A. Hyperphagia in rats produced by a mixture of fat and sugar. Physiol Behav. 1990;47:51–5. doi: 10.1016/0031-9384(90)90041-2. [DOI] [PubMed] [Google Scholar]
- 54.Lucas F, Ackroff K, Sclafani A. High-fat diet preference and overeating mediated by postingestive factors in rats. Am J Physiol. 1998;275:R1511–22. doi: 10.1152/ajpregu.1998.275.5.R1511. [DOI] [PubMed] [Google Scholar]
- 55.Synowski SJ, Smart AB, Warwick ZS. Meal size of high-fat food is reliably greater than high-carbohydrate food across externally-evoked single-meal tests and long-term spontaneous feeding in rat. Appetite. 2005;45:191–4. doi: 10.1016/j.appet.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 56.Warwick ZS, Synowski SJ, Bell KR. Dietary fat content affects energy intake and weight gain independent of diet caloric density in rats. Physiol Behav. 2002;77:85–90. doi: 10.1016/s0031-9384(02)00816-8. [DOI] [PubMed] [Google Scholar]
- 57.Warwick ZS, Synowski SJ, Rice KD, Smart AB. Independent effects of diet palatability and fat content on bout size and daily intake in rats. Physiol Behav. 2003;80:253–8. doi: 10.1016/j.physbeh.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 58.Warwick ZS, Weingarten HP. Determinants of high-fat diet hyperphagia: experimental dissection of orosensory and postingestive effects. Am J Physiol. 1995;269:R30–7. doi: 10.1152/ajpregu.1995.269.1.R30. [DOI] [PubMed] [Google Scholar]
- 59.Avena NM, Rada P, Hoebel BG. Sugar and fat bingeing have notable differences in addictive-like behavior. J Nutr. 2009;139:623–8. doi: 10.3945/jn.108.097584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Buda-Levin A, Wojnicki FH, Corwin RL. Baclofen reduces fat intake under binge-type conditions. Physiol Behav. 2005;86:176–84. doi: 10.1016/j.physbeh.2005.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Corwin RL, Wojnicki FH. Binge eating in rats with limited access to vegetable shortening. Curr Protoc Neurosci. 2006;Chapter 9(Unit9 23B) doi: 10.1002/0471142301.ns0923bs36. [DOI] [PubMed] [Google Scholar]
- 62.Wojnicki FH, Charny G, Corwin RL. Binge-type behavior in rats consuming trans-fat-free shortening. Physiol Behav. 2008;94:627–9. doi: 10.1016/j.physbeh.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Corwin RL, Wojnicki FH. Baclofen, raclopride, and naltrexone differentially affect intake of fat and sucrose under limited access conditions. Behav Pharmacol. 2009;20:537–48. doi: 10.1097/FBP.0b013e3283313168. [DOI] [PubMed] [Google Scholar]
- 64.Davis JD, Smith GP. Analysis of the microstructure of the rhythmic tongue movements of rats ingesting maltose and sucrose solutions. Behav Neurosci. 1992;106:217–28. [PubMed] [Google Scholar]
- 65.Weingarten HP. Conditioned cues elicit feeding in sated rats: a role for learning in meal initiation. Science. 1983;220:431–3. doi: 10.1126/science.6836286. [DOI] [PubMed] [Google Scholar]
- 66.Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res Brain Res Rev. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- 67.Nicola SM. The flexible approach hypothesis: unification of effort and cue-responding hypotheses for the role of nucleus accumbens dopamine in the activation of reward-seeking behavior. J Neurosci. 2010;30:16585–600. doi: 10.1523/JNEUROSCI.3958-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Parkinson JA, Olmstead MC, Burns LH, Robbins TW, Everitt BJ. Dissociation in effects of lesions of the nucleus accumbens core and shell on appetitive pavlovian approach behavior and the potentiation of conditioned reinforcement and locomotor activity by D-amphetamine. J Neurosci. 1999;19:2401–11. doi: 10.1523/JNEUROSCI.19-06-02401.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yun IA, Wakabayashi KT, Fields HL, Nicola SM. The ventral tegmental area is required for the behavioral and nucleus accumbens neuronal firing responses to incentive cues. J Neurosci. 2004;24:2923–33. doi: 10.1523/JNEUROSCI.5282-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wojnicki FH, Babbs RK, Corwin RL. Reinforcing efficacy of fat, as assessed by progressive ratio responding, depends upon availability not amount consumed. Physiol Behav. 2010;100:316–21. doi: 10.1016/j.physbeh.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bari AA, Pierce RC. D1-like and D2 dopamine receptor antagonists administered into the shell subregion of the rat nucleus accumbens decrease cocaine, but not food, reinforcement. Neuroscience. 2005;135:959–68. doi: 10.1016/j.neuroscience.2005.06.048. [DOI] [PubMed] [Google Scholar]
- 72.Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: Integration of energy, action and reward. Physiology & Behavior. 2005;86:773–95. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 73.Smith KS, Tindell AJ, Aldridge JW, Berridge KC. Ventral pallidum roles in reward and motivation. Behavioural Brain Research. 2009;196:155–67. doi: 10.1016/j.bbr.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yamamoto T. Central mechanisms of roles of taste in reward and eating. Acta Physiologica Hungarica. 2008;95:165–86. doi: 10.1556/APhysiol.95.2008.2.2. [DOI] [PubMed] [Google Scholar]
- 75.Bakshi VP, Kelley AE. Feeding induced by opioid stimulation of the ventral striatum: role of opiate receptor subtypes. J Pharmacol Exp Ther. 1993;265:1253–60. [PubMed] [Google Scholar]
- 76.Taha SA, Norsted E, Lee LS, Lang PD, Lee BS, Woolley JD, et al. Endogenous opioids encode relative taste preference. Eur J Neurosci. 2006;24:1220–6. doi: 10.1111/j.1460-9568.2006.04987.x. [DOI] [PubMed] [Google Scholar]
- 77.Zhang M, Gosnell BA, Kelley AE. Intake of high-fat food is selectively enhanced by mu opioid receptor stimulation within the nucleus accumbens. J Pharmacol Exp Ther. 1998;285:908–14. [PubMed] [Google Scholar]
- 78.Zhang M, Kelley AE. Opiate agonists microinjected into the nucleus accumbens enhance sucrose drinking in rats. Psychopharmacology (Berl) 1997;132:350–60. doi: 10.1007/s002130050355. [DOI] [PubMed] [Google Scholar]
- 79.Shin AC, Townsend RL, Patterson LM, Berthoud HR. “Liking” and “wanting” of sweet and oily food stimuli as affected by high-fat diet-induced obesity, weight loss, leptin, and genetic predisposition. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1267–80. doi: 10.1152/ajpregu.00314.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Furnes M, Zhao CM, Chen D. Development of Obesity is Associated with Increased Calories per Meal Rather than per Day. A Study of High-Fat Diet-Induced Obesity in Young Rats. Obesity Surgery. 2009;19:1430–8. doi: 10.1007/s11695-009-9863-1. [DOI] [PubMed] [Google Scholar]
- 81.Johnson AW. Dietary manipulations influence sucrose acceptance in diet induced obese mice. Appetite. 2012;58:215–21. doi: 10.1016/j.appet.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 82.Marco A, Schroeder M, Weller A. Microstructural pattern of palatable food intake from weaning to adulthood in male and female OLETF rats. Behav Neurosci. 2009;123:1251–60. doi: 10.1037/a0017740. [DOI] [PubMed] [Google Scholar]
- 83.Zimmerli EJ, Devlin MJ, Kissileff HR, Walsh BT. The development of satiation in bulimia nervosa. Physiology & behavior. 2010;100:346–9. doi: 10.1016/j.physbeh.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]