Abstract
PCBs are carcinogens, but for many decades it was assumed that PCBs may not possess initiating activity. Initiation is a process that involves changes in the DNA sequence, often, but not exclusively produced through DNA adduction by a reactive compound or reactive oxygen species (ROS). DNA adducts can be detected by 32P-postlabeling, a method that Dr. Ramesh Gupta co-developed and refined. Today these types of assays together with other mechanistic studies provide convincing evidence that specific PCB congeners can be biotransformed to genotoxic and therefore potentially initiating metabolites. This review will provide an overview of our current knowledge of PCBs genotoxic potential and mechanism of action, emphasizing the contributions of Dr. Ramesh Gupta during his tenures at the Universities of Kentucky and Louisville.
Keywords: PCB, 32P-postlabeling, metabolic activation, gene mutation, genotoxicity
1. Introduction to PCBs and the process of carcinogenesis
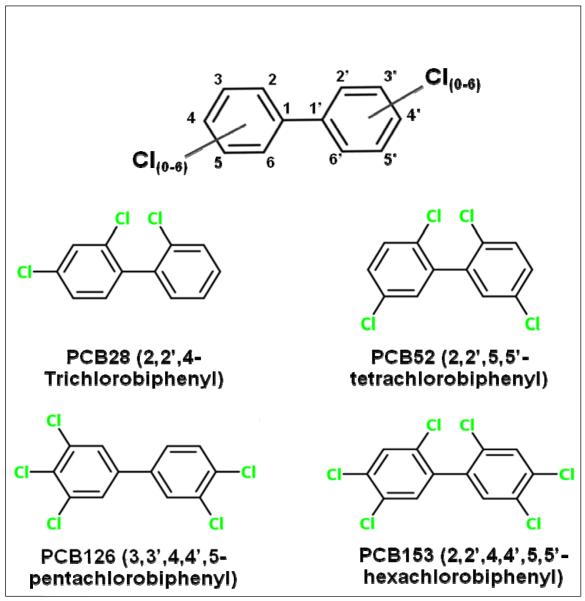
Polychlorinated biphenyls (PCBs), widely used as plasticizers, flame retardants, lubricants, and for many other applications, are no longer commercially produced in the USA, but still in use in closed applications. PCBs are mixtures of the total 209 possible congeners which differ in the number and position of chlorines on the biphenyl rings (Figure 1). Due to their chemical stability, PCBs have become ubiquitous as environmental and human contaminants. In rodents, PCB mixtures are complete carcinogens, producing lesions primarily in the liver [28, 52, 92], but other organs are also affected. Examination of the mortality rate of the Japanese Yusho PCB poisoning victims 40 years after the event found increased risk for all types of cancer, liver cancer, and lung cancer in men, but only for liver cancer in women [68]. A similar 24-year follow-up study of Taiwanese Yucheng PCB poisoning victims found increased mortality from liver disease, but no increase in liver cancer [104]. However, since in both cases a coexposure to PCDFs, PCB heat degradation products, occurred, the relative contributions of PCBs or PCDFs to the observed long term effects remain elusive. PCBs are classified as probable human carcinogens, but one of the congeners, 3,3′,4,4′,5-pentachlorobiphenyl (PCB126), is now classified by the International Agency of Research on Cancer (IARC) as a human carcinogen [38] and a new, complete reevaluation of PCBs is currently under way.
Figure 1.
Chemical Structures of PCBs
Chemcial carcinogensis is a multistep process. The first step, initiation, is a heritable, irreversible change in the DNA sequence or quantity. A single exposure to an initiator may be sufficient if the mutagenic changes occur in oncogenes and/or tumor suppressor genes. The second step, promotion, provides a growth advantage to such initiated cells, and requires repeated, long term exposure to the promoter. Promotion is a non-genotoxic process and, up to a certain point, reversible. The final step, progression, is characterized by increasing changes in the karyotype and a phenotypic change of the cells from preneoplastic to benign and further to malignant. It is assumed that compounds that are clastogens (chromosome breaking) or aneugens (causing changes in chromosome number) may be involved in progression. Experiments with multistage carcinogenesis models have shown that some compounds may act as an initiator or promoter, but not both [29, 35, 113]. In that case consecutive exposure to both types of carcinogens is required. In the case of PCBs it appears now that some congeners, particularly higher chlorinated ones, are efficacious promoters while others, the lower chlorinated congeners, more prone to be biotransformed and thereby bioactivated, are potential initiators [49]. In this publication we will describe the evidence for initiating activity of PCBs and discuss some, but not all, possible mechanisms.
2. Compounds may require metabolic activation to reactive intermediates and/or byproducts to act as initiators: example lower chlorinated biphenyls
Often a compound itself has no initiating activity, but a metabolite or degradation intermediate or byproduct may. PCBs, especially those PCBs with more chlorine atoms, are fairly resistant to chemical or metabolic attack, which along with their lipophilicity, poor water solubility, and low vapor pressure, contributes to their propensity for accumulation and biomagnification. This also means that they are not very reactive themselves and therefore were generally seen as not being genotoxic. Recent results with lower chlorinated biphenyls have changed this perception.
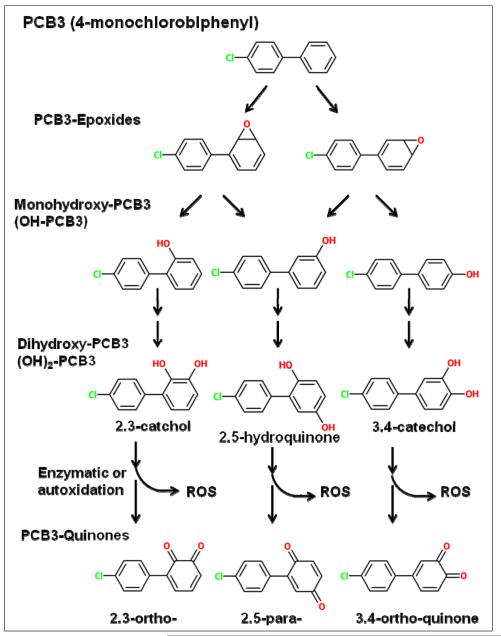
The 209 PCB congeners vary greatly in their propensity for metabolic attack, the first line of which is monooxygenation by members of the cytochrome P-450 (CYP) superfamily. This can theoretically produce no less than 837 possible monohydroxylated products [77]. The enzymatic hydroxylation may occur via a “direct insertion” mechanism or via the intermediacy of an arene oxide [4, 5, 26, 43, 100, 111]. Epoxides are usually fairly reactive, electrophilic species. The number of chlorines present determines stability, i.e. the greater the number of chlorines, the more stable the arene oxide intermediates.
Monohydroxylated PCBs may undergo an additional hydroxylation catalyzed primarily by cytochromes P-450 producing di-hydroxylated PCB derivatives [53]. Depending on the relative position of the hydroxyl groups, catechols (ortho position) or hydroquinones (para position) may be formed (Figure 2). The further oxidation of PCB catechols and hydroquinones may be catalyzed by peroxidases [1, 66], prostaglandin synthase [106] or other enzymes and non-enzymatic processes, yielding highly reactive electrophilic PCB quinones [1, 106]. Alternatively, hydroxylated PCBs may be substrates for glucuronidation [101] and sulfonation [19, 47, 48], resulting in less reactive, more easily excretable metabolites.
Figure 2.
Biotransformation pathways of PCB3
Arene oxides and quinones are chemically reactive metabolites and therefore prime suspects for having initiating activity since they may be direct acting genotoxic intermediates. But sometimes it is not the compound itself but a byproduct that is producing the carcinogenic damage. The autoxidation/enzymatic oxidation of a PCB hydroquinone may produce reactive oxygen species (ROS; oxygen ions and peroxides) [1, 66]. ROS are considered to be active in initiation, promotion, and progression [44] and evidence for their involvement in PCB-related genotoxicity will be discussed later.
Many higher chlorinated PCB congeners are potent and/or efficacious inducers of xenobiotic metabolizing enzymes [69], a change that may alter the metabolism of endogenous or other exogenous compounds. For example, PCBs induce CYPs in the liver which may change the metabolism of endogenous estrogen to more harmful estrogen catechols [37] or ROS producing estrogen quinones [10]. However, these possible indirect mechanisms will not be addressed further in this review.
3. Pathways to an initiated cell
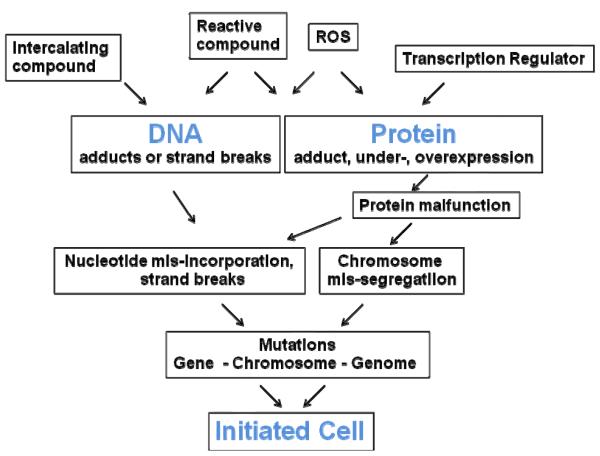
Initiation involves a change in the DNA sequence or quantity, i.e. either a gene mutation, chromosome mutation (breaks, rearrangements), or genome mutation (change in the number of chromosomes). Different pathways may result in these types of changes (Figure 3). A reactive compound or metabolite may bind covalently to the DNA forming a DNA-adduct or ROS may react with and damage the DNA. If not repaired, these adducts may cause misreading by polymerases resulting in a gene mutations like basepair changes. Non-covalent interactions of a compound, for example by intercalation, may also cause gene mutations, particularly insertions or deletions of basepairs [2, 24, 93]. ROS and other DNA-reactive chemicals may also cause DNA strand breaks. Reactive compounds or intermediates or ROS may also damage DNA-related proteins like topoisomerases, tubulin, etc. The result may be DNA strand breaks and errors in chromosome distribution during mitosis. Changes in gene regulation of such proteins, for example of telomerase, may also result in DNA alterations. These gene, chromosome, and genome mutations may result in the formation of an initiated cell, the first step in carcinogenesis.
Figure 3.
Pathways to Initiation
A multitude of assays have been developed to detect compounds which cause mutations by interacting with DNA or protein. Several of these assays were used to examine the genotoxic potential of PCBs and possible mechanisms. A brief description of the results and their interpretation is provided below.
4. Adduct formation of PCBs with DNA and protein – experimental results
4.1. 32P postlabeling
A method to identify covalent binding of a compound specifically with DNA is the 32P-postlabeling technique. This highly sensitive method was developed by Randerath and Gupta [31] and further improved by Gupta, particularly for the detection of ROS damage to the DNA [32]. A major advantage of this technique is that it does not require prior knowledge of the structure of the adducts or labeled test compounds. A disadvantage is that the chemical nature of the adducts cannot be identified easily unless standards are available.
The first publication with 32P-postlabeling and PCBs appeared in 1989. In monolayer cultures of human Chang liver cells, low levels of photo-induced 2,2′,4,4′,5,5′-hexachlorobiphenyl (PCB153) DNA adducts could be detected within 30 minutes of incubation using the 32P-postlabeling method [11]. The results showed the fast transfer of PCB153 into the cell nucleus. Most of the compound was bound to nuclear protein, only a very small amount of the photo-induced adducts was associated with purine nucleotides.
In 1995 it was reported that after exposure of primary fetal rat and quail egg hepatocytes and human HepG2 hepatoma cells to 3,3′,4,4′-tetrachlorobiphenyl (PCB77), a significant level of DNA adducts was seen, i.e. 37 with quail and 20 adducts per 109 nucleotides with the rat and human cells [18]. Interestingly, the level of EROD (CYP1A1) activity did not correlate with the level of adduct formation, suggesting that other CYPs may have been involved in the bioactivation of PCB77. Also, although the major adduct was the same for all 3 species, there were species-specific adducts with each cell type, another indication for different activation pathways. Northern blot analysis showed hybridization with CYPlA1, 2B1 and 3A1 mRNA in fetal rat hepatocytes, CYP3A and 1A mRNA in HepG2, and a CYP2 type mRNA closely related to homolog rat CYP2E or CYP2C in fetal quail hepatocytes [17]. Adduct analysis using different CYPs from supersomes for bioactivation should now be able to identify the most potent activation pathway, but this mehod was not available at that time. Aroclor 1254 (Aroclors are commercial PCB mixtures produced from about 1930-1979) did not produce significant levels of adducts [18]. However, in human primary hepatocytes from 5 different donors, 4-monochlorobiphenyl (PCB3) and, to a lesser degree Aroclors 1016 and 1254 produced a significant increase in DNA adducts [9]. Noteworthy is that the level of adduct formation differed strongly from donor to donor, from single digits to 36 to over 200 adducts per 109 nucleotides, indicating huge inter-individual differences in susceptibility to DNA damage by PCBs. Differing CYP activity levels may be responsible for this variation, although differences in other enzymes may also play a role.
Starting in the mid 1990s, a cooperation between Drs. Robertson and Gupta produced a series of reports with PCBs and the 32P-postlabeling technique. To gain more insight into the metabolic activation pathway, Mitch McLean, a graduate student in their laboratories, incubated PCB3 with hepatic microsomes from rats treated with 3-methylcholanthrene (3-MC), a CYP1A and 1B inducer, or phenobarbital (PB), which induces CYP2B. The formation of 5 different mono- and 3 dihydroxy-metabolites was reported [53]. Incubation of 15 different mono- and dichlorinated biphenyls with deoxyguanosine (dG) in vitro resulted in 1-8 different dG adducts with each PCB, except 2,3′- and 2,4′-dichlorobiphenyl, but a microsomal activation system plus an oxidation system with horseradish peroxidase and hydrogen peroxide (HRP/H2O2) was required [54]. This gave an indication that it is not a primary CYP metabolite, but rather secondary oxidation products, possibly semiquinones or quinones, that are the most DNA-reactive species. Only 4-mono-, 3,4-di- and 3,5-dichlorobiphenyl also produced some (fewer) adducts in the absence of HRP/H2O2, suggesting that these congeners build DNA-reactive epoxide intermediates. Additional experiments conducted by another jointly mentored graduate student, Greg Oakley, showed that these PCB congeners do not form adducts with dC or dT, and only 3,4-dichlorobiphenyl formed adducts with dA [67]. With calf thymus DNA, the adduction rate was about 60-80% lower than with dG. Tri-chlorinated congeners produced l-higher adduct levels than di- or monochlorinated biphenyls, i.e. 3,4,5-tri > 3,4-di > 2-mono or 3-monochlorobiphenyl. Since it was suspected that a semiquinone or quinone is the ultimate reactive intermediate, experiments with synthesized hydroquinones and para-quinones of the 3 monochlorinated biphenyls as well as 3,4-di and 3,4,5-trichlorobiphenyl were performed. In these assays a strong pH dependency of adduct formation was observed, with an optimum at pH 9.5 with the hydroquinones and pH 5.0 with the para-quinones, and the least reactivity at pH 7.2 [3]. The mechanisms of activation of PCB-derived (hydro)quinones to semiquinone free radicals and the effect of pH on these reactions have been subsequently examined in details [94-96].
Daria Pereg, a graduate student in the Robertson and Gupta laboratories, examined the extent and limits of bioactivation of PCBs with a series of synthetic mono- to hexachlorinated biphenyls. Those PCBs were exposed to liver microsomes from rat, mouse and humans in the presence of calf thymus DNA. Adduct formation was seen with congeners containing 1-3 chlorines, but not above [70]. 2-monochlorobiphenyl produced similar adduct patters with all 3 microsomal systems, while 4-mono-, 3,4-dichloro and 3,4,5-trichlorobiphenyl showed similar patterns with two of the three microsomal systems. With 4,4′-dichlorobiphenyl a different adduct pattern was seen for each microsomal system. The rat microsomal system had a higher CYP activity than the human system and produced higher levels of DNA adducts. Co-chromatography of adducts derived from synthetic PCB3-para-quinone with DNA with those from metabolically activated PCB3 DNA adducts showed that one of the adducts was in common. These results indicate that most lower chlorinated biphenyls can be bioactivated by mammalian livers, including humans, to DNA-binding metabolites and at least some of these metabolites are probably quinones. The reactivity of PCB-quinones with isolated DNA was further demonstrated in a quantitative study with synthetic PCB-quinones using HPLC/ESI-MS/MS and 32P-postlabeling. These experiments obtained 3 – 1200 adducts per 108 nucleotides, a stunningly high level of adduct formation [116]. Most adducts were found with guanosine, and even though 32P-postlabeling was the much more sensitive method, the authors reported that it nevertheless “severely underestimated” the number of adducts compared to the MS technique.
Not all adducts in the experiments described above may be derived from covalent binding of a PCB metabolite to DNA. The dihydroxy/quinone metabolites are redox pairs and during the oxidation of dihydroxy-biphenyls to the quinone, superoxide is formed [55]. To analyze whether reactive oxygen species (ROS) are involved, Dr. Gupta and his coworkers developed a new newly, highly sensitive, 32P-postlabeling assay for 8-oxo-deoxyguanosine (8-oxodG) [15]. The incubation of 3,4-dichloro-2′,5′-dihydroxybiphenyl with calf thymus DNA in the presence of the breast tissue-associated enzyme, lactoperoxidase, and H2O2 resulted in a significant increase in ROS-induced DNA damage to 253 8-oxodG per 106 nucleotides compared to 118 per 106 in the solvent controls [66]. Addition of CuCl2 instead of lactoperoxidase/H2O2 resulted in a 10× higher level (2669 per 106 nucleotides). FeCl3 was ineffective. Catalase and sodium azide reduced, while superoxide dismutase and particularly glutathione substantially increased 8-oxodG formation. These results demonstrate that biotransformation of lower chlorinated biphenyls can result in the production of free radicals and oxidative DNA damage. Dr. Gupta further improved the 32P-postlabeling method so that it is now possible to detect a large number of different types of ROS-derived nucleotide modulations [76]. Using this technique and Cu2+-mediated activation of different PCB metabolites incubated with DNA, over a dozen novel polar oxidative DNA adducts were observed [98]. These adducts were chromatographically similar for all active agents, but different metabolites had different potency. The highest adduct levels were observed with the hydroquinones (55 to 142 polar oxidation adducts/106 nucleotides), while up to 40% lower levels were seen with PCB catechols and no significant increase with monohydroxylated and quinone metabolites. The PCB29-HQ (2,4,5-trichloro-) was unusually potent, but otherwise the oxidative DNA damage was inversely related to the chlorination level. Experiments with various scavengers suggest that H2O2, singlet oxygen, hydroxyl radical, and superoxide may be involved in this PCB-mediated oxidative DNA damage and that copper plays a major role in the ROS generation [98].
One of these unusual adducts formed by oxidative DNA damage is M1dG [3-(2′-deoxy-beta-D-erythro-pentofuranosyl)-pyrimido[1,2-a]-purin-10(3H)-one]. A single exposure to PCB153 or PCB126 or a mixture of the two did not increase the level of M1dG adducts in rats [41]. However, exposure for 1 year to 1000 ng/kg/day of PCB126 resulted in M1dG adduct accumulation in the liver. PCB153 alone had no effect, but coexposure to equal amounts of PCB153 and PCB126 resulted in dose-dependent increases in M1dG adduct accumulation even with the lower dose of 300 ng/kg/day. Varying the dose of PCB153 with a fixed dose of PCB126 showed a dose-dependent effect of PCB153. These results are consistent with the results from cancer bioassays that demonstrated a synergistic effect between PCB126 and PCB153 [65]. This positive correlation between M1dG adducts and liver carcinogenesis needs further study to test a cause and effect relationship, which would suggest that these PCB congeners are carcinogenic through ROS production and ROS-mediated DNA damage.
Overall these 32P-postlabeling experiments show that lower chlorinated PCBs are metabolized by microsomal systems and liver cells to highly reactive species, probably epoxides, quinones, and various reactive oxygen species, that can efficiently modify nucleotides and DNA which may lead to mutations, while higher chlorinated PCBs may produce hepatocarcinogensis through ROS-mediated DNA damage.
4.2. Radioactive labeled PCBs for the detection of DNA and protein adducts
A second, older method to detect covalent binding of a test compound to cellular macromolecules uses 14C or 3H-labeled compounds. This technique is frequently used in in vivo assays with rodents to gain information about the ADME (absorption-distribution-metabolism-excretion) of a compound, but can also provide valuable information about mechanistic aspects when used in vitro or with cells.
Steve Safe and coworkers published a series of papers in the 1970s about PCB-binding to macromolecules. Using 3H-PCB3 and rat liver microsomes for metabolic activation they found strong covalent binding to protein and RNA [110]. They suspected an arene oxide as the binding intermediate since they observed an NIH shift of the isotope [111]. PCB3 was easier to oxidize by microsomes than 4′-monohydroxy-PCB3 (4′OH-PCB3).Also, protein binding by PCB3, but not by 4′OH-PCB3 metabolites, was greatly reduced if CYP1A was inhibited. Microsomes from 3-MC-treated animals which are rich in CYP 1A produced higher protein binding levels than microsomes from untreated animals [13]. These results suggest the existence of at least 2 reactive intermediates, one of them an arene oxide, a second one that does not depend on CYP1A for its formation. If DNA or polynucleotides were added to the incubation mixture, strong binding to poly(G) was seen and some binding to poly(A), accompanied by a reduction of protein binding [111]. Both nucleotides are more nucleophilic than the other nucleotides since they possess a primary amine moiety, which may be involved in the PCB binding. To test if metabolic activation and binding to proteins and DNA could also occur in cells, the group exposed Chinese hamster ovary cells to 3H-PCB3. They observed the formation of hydroxylated metabolites and covalent binding to macromolecules [109]. Even though 85% of binding was to proteins, the specific binding to DNA was 3.5 times higher than to protein and 1.4 times higher than to RNA, suggesting that PCB3 may very well be activated to DNA damaging metabolites.
To gain more insight into the chemical nature of the adducted PCB, Hesse and coworkers incubated 14C-PCB4 (2,2′-dichloro-) with PB-induced liver microsomes in a carefully controlled time study. They observed linearly increasing binding over a time span of 120 minutes [36, 88]. Since monohydroxy-metabolites were formed within 10 minutes and disappeared quickly during the next 10 minutes and because glutathione strongly reduced protein adduct formation, they concluded that “there is strong evidence that the majority of bound PCB4 metabolites do not originate from arene oxides, but from other reactive species, possibly semiquinones or quinones”. They even quantified the contribution of arene oxides, if any, to no greater than 25% [36].
Shimada and coworkers also employed 14C-PCBs to analyze their metabolic activation and covalent binding to proteins [85, 87]. They observed that some lower chlorinated congeners (3 chlorines or less) are better activated to covalent binding species by 3-MC inducible CYPs, while those with 4 chlorines were better substrates for PB inducible CYPs [86, 88]. In the in vitro system with microsomal proteins PCBs bound to cytochrome P-450 reductase and cytochrome P-450, but not cytochrome b5. If cytosolic proteins were present as well, strong binding to many cytosolic proteins was observed and it was hypothesized that cysteine moieties were the major binding sites [86, 89].
The question whether PCBs with 6 or more chlorines can be metabolically bioactivated to covalent binding species remains controversial. Several groups did not see protein binding with PCB153 (2,2′,4,4′,5,5′-hexachlorobiphenyl) and a microsomal activation system [36, 88], others observed efficient binding to protein and DNA in vitro and even into the nuclear fraction of liver cells in vivo [14, 64]. In an in vivo experiment with rats, about 70% of the bound PCB153 was bound to protein, 30% to DNA, and nothing to RNA. Overall PCB153 is more likely to bioaccumulate, but congeners with 2 free adjacent carbon atoms, like PCB136 (2,2′,3,3′,6,6′-hexachlorobiphenyl) are more prone to be biotransformed and to bind to macromolecules, in this case RNA > protein > DNA [57]. Similarly experiments with PCB77 (3,3′,4,4′-tetrachlorobiphenyl) and PCB47 (2,2′,4,4′-tetrachlorobiphenyl) showed higher accumulation of PCB47 in adipose tissue, but higher levels and covalent binding of PCB77 in the liver and blood, including erythrocytes [90, 91]. A study with 14C-radiolabeled PCB3 and PCB77 in mice found significant amounts of both PCBs in various tissues, with liver > kidneys > lung [71]. Bound PCBs were mostly localized in the cytosol or organelles, less in the microsomes, and significant amounts of PCB3 and PCB77 were covalently bound to proteins in the nucleus of the liver of these animals, with PCB3 >> PCB77. There was also a substantial covalent binding of these PCBs to liver DNA, but it was below the significance level. In addition, significant binding of PCB3 and PCB77 to hemoglobin of peripheral red blood cells was observed [102]. Binding levels peaked 3-6h after IV injection with PCB3 and after 18h with PCB77. Calculation of the molecular weights indicated that part of the hemoglobin adducts were likely due to binding of an arene oxide metabolite while others were most likely due to binding of a PCB quinone.
The observation that PCB52 formed quinonoid protein adducts in the liver and brain of exposed rats indicates that a major bioactivation pathway for PCBs leads indeed to reactive quinones and that these metabolites can reach and bind to proteins and probably DNA in such distant and protected tissues as our brain [46].
Protein binding of PCB quinones occurs preferably to cysteines, but also to arginine and histidine [1]. Binding of PCB metabolites to proteins, including nuclear proteins, was observed in vitro and in vivo. If binding inhibits the function of an essential DNA-related protein, this may lead to DNA strand breaks or chromosome mis-segregation. It is therefore noteworthy that binding of PCB3 quinone to topoisomerase I, a nuclear protein that is essential for proper DNA maintenance and replication, was observed [7, 8, 99]. Thus, besides binding to DNA, PCB quinones bind to cellular proteins, a process that may also lead to genotoxicity and cancer initiation.
5. Mutation Induction by PCBs - experimental results
Adduct formation is only the first step in cancer initiation. It has to be followed by a change in the DNA sequence or quantity. Unfortunately, very few reports directly correlate a type of DNA adduct with a specific type of mutation, and none exist for PCBs. However, correlation between the propensity of a metabolite to produce adducts and to result in some form of mutation may allow careful conclusions about the causal relationship between those two events.
5.1. DNA strand breaks and chromosome maldistribution
DNA strand breaks and anomalous segregation of chromosomes represent major forms of genotoxicity and can be induced by DNA and protein adduction.
The COMET assay measures migration of genomic DNA in an electrical field, which may be increased by compounds that produce DNA strand breaks and alkali-labile sites. ., This assay showed a positive response with PCB52 and PCB77 in human lymphocytes [78], and PCB101 and PCB118 in fish cells in vitro [51]. A modified version of the COMET assay indicated ROS as the DNA breaking species in the fish cells. In human HL60 promyelocytic leukemia cells, the redox cycling pair PCB3-hydroquinone and PCB3-p-quinone both induced COMETs [112]. The hydroquinone required cellular myeloperoxidase activity for COMET induction, while the quinone’s ability to induce COMETs was independent of the peroxidase activity. For both compounds about half of the breaks were due to ROS, while the other half was most likely produced by a direct interaction of the quinone with the DNA or DNA-related proteins.
The micronucleus assay detects chromosome breaks and chromosome loss. PCB 153, 138, 101, and 118 induced micronuclei in fish cells in vitro [51] and PCB-contaminated soil from industrial or irrigation sites induced micronuclei in plant species [12, 97]. However, no increase was seen after exposure of human lymphocytes and human keratinocytes to commercial PCB mixtures in vitro [6, 105]. This unresponsiveness could be the result of incomplete metabolic activation. When V79 Chinese hamster lung fibroblasts were exposed to the 3 mono-, 2 dihydroxy-, and 2 quinone metabolites of PCB3, PCB3 itself was negative in the assay, but the mono- and, more efficiently the dihydroxylated and quinoid metabolites induced micronuclei formation [115]. The para-quinone produced micronuclei mostly by chromosome breaks, the monohydroxylated and to a lesser extent the other metabolites mostly by chromosome loss, pointing towards different mechanisms of genotoxicity. The highly reactive quinone may interact with the DNA directly, or with DNA maintenance proteins like topoisomerase, leading to chromosome breaks, while the other compounds may react with the cytoskeleton or other proteins involved in chromosome distribution.
Recent results indicate that telomeres, the very ends of chromosomes, are particularly sensitive to DNA damage since telomeres are not efficiently repaired. Severely shortened telomeres cause a cellular crisis and after this the emergence of immortal cells with aberrant karyotypes, believed to be created by the stickiness of chromosome ends with short telomeres, fusion of chromosomes and missegregation during following cell divisions [30]. Several PCB congeners and PCB3 p-quinone induce telomere shortening, the congeners most likely by reducing telomerase activity, the PCB3-quinone most likely via ROS generation [39, 83, 84]. This area of research is only in its infancy.
Most studies with rats and commercial PCB mixtures did not find chromosome aberrations in bone marrow or spermatogonia, while studies with fish were positive [reviewed in [49]]. Sargent, however, did report chromosome breaks and rearrangements in human lymphocytes exposed to Arochlor 1254 or PCB77 or PCB153 [79, 80]. PCB52 was negative, but a combination of PCB52 with PCB77 acted synergistically, producing more aberrations than the sum. Similar results were obtained in bone marrow and liver cells of rats treated with PCB52 and PCB77 in vivo [56, 79, 81]. Besides chromosome aberrations, an increase in polyploid cells was observed in V79 hamster cells in vitro, where PCB52 and PCB105 were strong inducers of aberrant mitosis, while PCB77 and PCB153 were inactive [40]. In addition, a strong synergistic action was seen when PCB105 (2,3,3′,4,4′-pentachlorobiphenyl) was combined with the spindle poison triphenyltin, indicating an interaction with the cytoskeleton as mechanism of this effect [40]. Interestingly, hydroquinones of PCB2 and PCB3, but not the PCB3 3,4-catechol or the PCB1 hydroquinone, induced an up to 90% polyploidization of V79 cells [25]. Further studies indicate an interaction of these metabolites with the cytoskeleton. These results point to a strict structure-dependency in the genotoxicity of PCB-metabolites.
Sister chromatid exchanges (SCE) are formed only during the S-phase of the cell cycle at/near the replication fork by a double strand break and reciprocal rejoining of the strands. A positive linear relationship was observed between cell transformation in vitro and SCE induction indicating the potential value of this short term test for genotoxicity testing. Of three individual and a concordance of 0.59 was seen for SCE induction and rodent carcinogenicity [73, 114], PCB congeners that were tested in vitro for SCE induction (4-monochloro, 2,2′,5,5′- and 3,3′,4,4′-tetrachlorobiphenyl, i.e. PCB3, 52,and 77, respectively), none was positive [25, 80]. However, in human lymphocytes, 2,3,7,8-Tetrachlorodibenzodioxin (TCDD), 2,3,4,7,8-pentachlorodibenzofuran (PenCDF) and PCB126 (3, 3′,4,4′,5-pentachlorobiphenyl), three potent AhR agonists, significantly increased the frequency of SCEs with almost the same dose-relationship with respect to their TEQ and they acted in an additive way with other SCE-inducers [59]. Also, two of five methylsulfonate metabolites of PCBs (MSF-PCBs), i.e. 3-MSF-2,2′,4′,5,5′- and 4-MSF-2,2′,3′,4′,5-PenCB, enhanced SCE levels in lymphocytes in vitro, although very high doses (>5 ppm) were needed to produce significant effects [60]. Of several monochlorinated biphenyl metabolites, only the PCB3-3,4-catechol induced SCE in V79 cells, while the structurally closely related PCB1, PCB2, or PCB3-hydroquinones were negative, supporting a very strict structure-activity relationship for SCE induction [25]. These results suggest that most PCB congeners and their metabolites may not be efficient inducers of SCE, but that their enzyme-inducing activity may make cells more susceptible to the effect of other endogenous or exogenous compounds.
5.2. Gene mutations
Bacterial mutation assays like the Ames test rely on extracellular metabolic activation and are therefore often not appropriate for compounds with multistep activation pathways or short lived active intermediates. Not surprisingly, most bacterial mutagenicity tests with commercial PCB mixtures or the few congeners that were tested were negative [49].
In vitro gene mutation assays with mammalian cells used PCB mixtures or a few congeners and were always negative [33]. However, the cell lines used in such mutation studies like the Chinese hamster lung fibroblast (V79), Chinese hamster ovary fibroblast (CHO), and mouse lymphoma (L5178Y) cell lines, have only very limited or no biotransformation capability. To overcome this problem, a series of synthetic PCB3 metabolites was tested in the V79 gene mutation assay [115]. Both the ortho (3,4-) and para- (2,5-) quinone efficiently induced mutations at the HPRT locus, while none of the tested mono- or dihydroxylated metabolites or PCB3 itself were mutagenic. This is in agreement with the hypothesis that the PCB quinones are genotoxic and potentially initiating carcinogens.
To analyze the mutagenic activity of PCB3, a transgenic in vivo assay was employed. The BigBlue rat (Stratagene) is a transgenic rat that Male rats were treated weekly for 4 weeks with i.p. injections of 3-carries multiple copies of an indicator gene. methylcholantrene (3-MC), PCB3, 4-OH-PCB3 or vehicle alone (corn oil) and killed after a period of additional 10 days. The mutation frequency per 100,000 pfu was 1.7 in negative control, and 8.8 (3-MC), 4.8 (PCB3), and 4.0 (4-HO-PCB3) [45]. This mutation statistically significant. Moreover, in these from predominantly transitions to predominant treatment groups the mutation spectrum was altered similar, but smaller effect, causing a doubling of the mutation rate that was below the level of statistical significance [45]. This demonstrates that this PCB congener is mutagenic in vivo in the target organ, the liver. Unfortunately, these results cannot definitely point out the ultimate mutagenic species, but narrows the possibilities to an ortho- or para-quinone, an epoxide or ROS. All indications from other assays point towards adduct formation by a quinone, most likely the ortho (3,4-) quinone as a highly likely culprit.
5.3. In vivo initiation test
Very few assays exist that examine the initiating activity of a compound in vivo. In the mouse two-stage skin carcinogenesis model, the commercial PCB mixture Arochlor 1254, with predominantly tetra- and pentachlorainted biphenyls, acted as a weak tumor initiator [16]. In the rat liver initiation assay, the Solt Farber protocol with male F344 rats, Aroclor 1254 and the congeners PCB153, PCB52, and PCB47 showed only negative results [34]. A possible explanation for this is that PCB congeners with four or more chlorine atoms are poor substrates for metabolic activation. Therefore lower chlorinated biphenyl congeners, more easily biotranformed to potential reactive and genotoxic metabolites were tested for their ability to induce preneoplastic foci. The tested PCB congeners included PCB3 (4-Cl-,), PCB12 (3,4-diCl-) and PCB38 (3,4,5-triCl-BP) [21]. No nodules were apparent in animals in the vehicle control group, or groups receiving PCB12 or PCB38 as initiators. However, the PCB3 induced grossly visible nodules in 50% of the rats treated. In a second experiment PCB15 (4,4′-diCl-), PCB77 (3,3′,4,4′-tetraCl-), PCB52 (2,2′,5,5′-tetraCl-BP) and a combination of PCB77& PCB52 all increased the number of foci per cm3 in the liver, with PCB15 >> PCB52 > PCB77 > PCB52/77 [21]. The conclusion from these experiments is that lower chlorinated PCBs are able to initiate hepatocarcinogenesis in the rat, but from these few congeners tested, a strict structure-activity relationship could not be assigned.
A series of experiments with synthetic PCB3 metabolites were tested in an effort to determine the metabolic activation pathway and the ultimate initiating agent [23]. Test compounds included the 2-OH-, 3-OH-, 4-OH-, 2,3-diOH-, 3,4-diOH-, 2,5-diOH-, 2,3-quinone, 3,4-quinone, and 2,5-quinone metabolites of PCB3. The 4-OH- and 3,4-quinone metabolites of PCB3 significantly increased the number of foci/cm3, the number of foci per liver and the focal volume (% of liver), while none of the other PCB3 metabolites had a significant effect [22]. These results point towards the 3,4-ortho-quinone of PCB3 as the ultimate initiating metabolite in rat liver.
6. Relevance – evidence for DNA damage by PCBs in nature and humans
Human epidemiological studies and observations in nature may alert us to the possible carcinogenic activity of a compound. Laboratory experiments can provide indications for initiating activity of a compound, the type of genotoxicity, and are essential to elucidate the activation pathway and mechanism(s) of action. The missing link between these two types of studies, epidemiology and laboratory research, are biomonitoring studies in animals and humans. Most of these focus on the detection and quantification of a compound in biological samples. With PCBs some, few attempts were made to detect genotoxic damage in humans and wildlife and to relate it to exposure.
6.1. DNA strand breaks and chromosome number changes in biospecimens
Very few studies exist that address genotoxicity in PCB-exposed wildlife species. Among these, increased levels of micronuclei were observed in several fish species after exposure to commercial PCB mixtures or to PCBs and dichlorodiphenyltrichloroethane (DDT) in contaminated areas [reviewed in [49]].
Some epidemiological studies observe abnormal karyotypes in workers exposed to PCBs [42, 103], while others reported negative effects [20]. The challenge with human studies is that workers and other contaminated population groups are usually exposed to multiple compounds, while their exposure to the study compound may vary greatly.
SCE is an assay that may be useful for human bi omonitoring purposes since it is easy to perform using blood peripheral lymphocytes, but it may have limited sensitivity [108]. Such biomonitoring studies from the 1980s and 1990s reported higher SCE levels in peripheral lymphocytes of PCB exposed workers and of victims of the 1978 (Yu-Cheng) rice oil PCB poisoning in Taiwan [42, 50]. Usually the effects were only visible after a long occupational exposure or co-exposure to cigarette smoke. Recent short term exposure to PCBs in a fire did not elevate SCE levels [20]. Sometimes the basic SCE level was not elevated, but cells were “sensitized”, i.e. demonstrated SCE if a second compound like α-naphthoflavone (ANF) was added [63]. Interestingly, several concentrations of a reconstructed organochlorine mixtures of PCBs, polychlorinated dibenzodioxins (PCDDs), polychlorinated dibenzofurans (PCDFs), DDT and others similar to the ones found in humans around 1990 induced SCE in vitro and enhanced SCE induction by ANF in cultured lymphocytes [61]. The EC50 for SCE induction by this mixture was only 3 times higher than the average concentration in healthy humans at that time. Recent studies did not find elevated SCE levels in the lymphocytes of teachers in a PCB-contaminated school [107]. Also, a retesting of Yusho patients who had been contaminated with PCB in rice oil in 1968 did not show elevated SCE levels even though their blood still had seven times higher toxic equivalent (TEQ) levels than in healthy Japanese controls, indicating he continuous presence of PCDDs, PCDFs, and coplanar PCBs [62]. This suggests that the legacy of “weathererd” PCBs does not produce SCE in the current human population.
6.2. Detection and monitoring of adduct formation
Measuring DNA and protein adduction by an environmental compound in tissues form wildlife or humans is a challenging task, but would be extremely helpful for pollution biomonitoring and to provide to connection between exposure and toxic effects. Only the very sensitive 32P-postlabeling technique could provide such data.
A pollution biomonitoring study for the river Rhone, France, found different numbers and patterns of DNA adducts in the livers of carp upstream and downstream of a PCB incineration plant, suggesting different kinds of exposure [72]. However, since no chemical analysis of the tissue, water, or soil from the collection sites was performed, no statement about the chemical nature of he adducts can be made. A study with English sole collected from different sites in the Puget Sound, Washington, USA, found a highly significant correlation between the concentration of total PCBs in the liver and the hepatic DNA adduct level [58]. Similarly, a study with crayfish from the river Meuse, Netherlands, found a strong positive correlation between DNA adducts in the hepatopancreatic tissues and body burden of PCB28, PCB52, PCB101, but not with the higher chlorinated congeners PCB118, PCB138, PCB153, PCB180 [82].
The detection of DNA adducts in human samples is even more challenging due to the limitation of available tissue, the usually extremely low exposure levels to the test compound, and the existence of co-exposures that are related to lifestyle choices and environment. Thus Gallagher and coworkers did not see elevated DNA adduct levels in the placentas of non-smoking Taiwanese women who had been exposed to PCBs and polychlorinated dibenzofurans compared to non (less)-exposed non-smoking women from Taiwan or the USA [27]. They also measured arylhydrocarbon hydroxylase (AHH) activity in these tissues and found similar levels in placentas from American smokers and Taiwanese PCB-exposed non-smokers, but higher DNA adduct levels only in the American smoker samples, indicating that AHH induction alone is not sufficient to increase DNA adduct levels. Most recently Dr. Gupta and coworkers analyzed 108 samples from Inuit in Canada for PCB levels in blood and polar and lipophilic DNA adducts in white blood cells. This population is exposed to PCBs through their diet of whale blubber and other seafood. Although they observed an apparent accumulation of specific adducts with increasing PCB levels, no definite association could be made [74]. Inuit diet is also rich in selenium. A follow-up study with 83 Inuit samples found a negative correlation in the high ratio of Se/PCB group with 8-oxo-dG and total adducts, suggesting that high blood selenium levels may have a protective effect against oxidative DNA damage [75]. These studies demonstrate the complexity of human biomonitoring studies, while also showing that they can provide us with valuable information about individual susceptibility and/or protective factors.
7. Summary and Conclusions
The above results clearly demonstrate that lower chlorinated biphenyls may be readily bioactivated to reactive intermediates, arene oxides and particularly quinones, with the generation of ROS. These reactive species can interact with DNA and with protein forming covalently bound adducts which are most likely the cause for the observed gene, chromosome, and genome mutations. There are indications for adduct formation and mutagenesis in human and environmental biospecimens, highlighting the relevance of laboratory research to provide advanced warning and an understanding of possible mechanisms of bioactivation. Such research may also inform and aid in the design of efficient tools for biomonitoring, treatment, and chemoprevention. Dr. Ramesh Gupta had a significant influence in advancing these goals by developing highly sensitive methods for DNA adduct detection which he and his coworkers then successfully applied in PCB research [3, 41, 54, 66, 67, 70, 74, 75, 98]. This research has significantly advanced our understanding of the mechanisms of cancer initiation by PCBs.
Acknowledgements
The authors and their research were supported by funding from the NIH (ES 07380 and ES 013661). The opinions expressed are solely those of the authors, and do not reflect an official policy of the NIH.
Footnotes
Conflict of Interest Statement The authors have no personal or financial conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature
- [1].Amaro AR, Oakley GG, Bauer U, Spielmann HP, Robertson LW. Metabolic activation of PCBs to quinones: reactivity toward nitrogen and sulfur nucleophiles and influence of superoxide dismutase. Chem Res Toxicol. 1996;9:623–629. doi: 10.1021/tx950117e. [DOI] [PubMed] [Google Scholar]
- [2].Anderson RD, Berger NA. International Commission for Protection Against Environmental Mutagens and Carcinogens. Mutagenicity and carcinogenicity of topoisomerase-interactive agents. Mutat Res. 1994;309:109–142. doi: 10.1016/0027-5107(94)90048-5. [DOI] [PubMed] [Google Scholar]
- [3].Arif JM, Lehmler HJ, Robertson LW, Gupta RC. Interaction of benzoquinone- and hydroquinone-derivatives of lower chlorinated biphenyls with DNA and nucleotides in vitro. Chem Biol Interact. 2003;142:307–316. doi: 10.1016/s0009-2797(02)00141-2. [DOI] [PubMed] [Google Scholar]
- [4].Ariyoshi N, Koga N, Yoshimura H, Oguri K. Metabolism of 2,4,5,2′,4′,5′-hexachlorobiphenyl (PCB153) in guinea pig. Xenobiotica. 1997;27:973–983. doi: 10.1080/004982597240136. [DOI] [PubMed] [Google Scholar]
- [5].Ariyoshi N, Yoshimura H, Oguri K. Identification of in vitro metabolites of 2,4,6,2′,4′,6′-hexachlorobiphenyl from phenobarbital-treated dog liver microsomes. Biological & pharmaceutical bulletin. 1993;16:852–857. doi: 10.1248/bpb.16.852. [DOI] [PubMed] [Google Scholar]
- [6].Belpaeme K, Delbeke K, Zhu L, Kirsch-Volders M. PCBs do not induce DNA breakage in vitro in human lymphocytes. Mutagenesis. 1996;11:383–389. doi: 10.1093/mutage/11.4.383. [DOI] [PubMed] [Google Scholar]
- [7].Bender RP, Lehmler HJ, Robertson LW, Ludewig G, Osheroff N. Polychlorinated biphenyl quinone metabolites poison human topoisomerase IIalpha: altering enzyme function by blocking the N-terminal protein gate. Biochemistry. 2006;45:10140–10152. doi: 10.1021/bi0524666. [DOI] [PubMed] [Google Scholar]
- [8].Bender RP, Osheroff N. Mutation of cysteine residue 455 to alanine in human topoisomerase IIalpha confers hypersensitivity to quinones: enhancing DNA scission by closing the N-terminal protein gate. Chem Res Toxicol. 2007;20:975–981. doi: 10.1021/tx700062t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Borlak J, Hock A, Hansen T, Richter E. DNA adducts in cultures of polychlorinated biphenyl-treated human hepatocytes. Toxicol Appl Pharmacol. 2003;188:81–91. doi: 10.1016/s0041-008x(02)00075-3. [DOI] [PubMed] [Google Scholar]
- [10].Brown JF, Jr., Mayes BA, Silkworth JB, Hamilton SB. Polychlorinated biphenyls modulated tumorigenesis in Sprague Dawley rats: correlation with mixed function oxidase activities and superoxide (O2* ) formation potentials and implied mode of action. Toxicol Sci. 2007;98:375–394. doi: 10.1093/toxsci/kfm122. [DOI] [PubMed] [Google Scholar]
- [11].Buff K, Wegenke M, Brundl A. Photo-induced formation of DNA adducts of 2,2′,4,4′,5,5′-hexachlorobiphenyl in cultured human cells. Biochem Pharmacol. 1989;38:2773–2779. doi: 10.1016/0006-2952(89)90430-9. [DOI] [PubMed] [Google Scholar]
- [12].Cotelle S, Masfaraud JF, Ferard JF. Assessment of the genotoxicity of contaminated soil with the Allium/Vicia-micronucleus and the Tradescantia-micronucleus assays. Mutat Res. 1999;426:167–171. doi: 10.1016/s0027-5107(99)00063-9. [DOI] [PubMed] [Google Scholar]
- [13].Crawford A, Safe S. 4-chlorobiphenyl metabolism: the effects of chemical inducers. Gen Pharmacol. 1979;10:227–231. doi: 10.1016/0306-3623(79)90094-6. [DOI] [PubMed] [Google Scholar]
- [14].Daubeze M, Narbonne JF. Incorporation of labeled 2,4,5,2′,4′,5′-hexachlorobiphenyl into the nuclear fraction of rat hepatocytes in vivo. Toxicology. 1984;31:315–318. doi: 10.1016/0300-483x(84)90112-4. [DOI] [PubMed] [Google Scholar]
- [15].Devanaboyina U, Gupta RC. Sensitive detection of 8-hydroxy-2′deoxyguanosine in DNA by 32P-postlabeling assay and the basal levels in rat tissues. Carcinogenesis. 1996;17:917–924. doi: 10.1093/carcin/17.5.917. [DOI] [PubMed] [Google Scholar]
- [16].DiGiovanni J, Viaje A, Berry DL, Slaga TJ, Juchau MR. Tumor-initiating ability of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and Arochlor 1254 in the two-stage system of mouse skin carcinogenesis. Bull Environ Contam Toxicol. 1977;18:552–557. doi: 10.1007/BF01684000. [DOI] [PubMed] [Google Scholar]
- [17].Dubois M, De Waziers I, Thome JP, Kremers P. P450 induction by Aroclor 1254 and 3,3′,4,4′-tetrachlorobiphenyl in cultured hepatocytes from rat, quail and man: interspecies comparison, Comparative biochemistry and physiology. Part C. Pharmacology, toxicology & endocrinology. 1996;113:51–59. doi: 10.1016/0742-8413(95)02037-3. [DOI] [PubMed] [Google Scholar]
- [18].Dubois M, Pfohl-Leszkowicz A, Grosse Y, Kremers P. DNA adducts and P450 induction in human, rat and avian liver cells after exposure to polychlorobiphenyls. Mutat Res. 1995;345:181–190. doi: 10.1016/0165-1218(95)90053-5. [DOI] [PubMed] [Google Scholar]
- [19].Ekuase EJ, Liu Y, Lehmler HJ, Robertson LW, Duffel MW. Structure-activity relationships for hydroxylated polychlorinated biphenyls as inhibitors of the sulfation of dehydroepiandrosterone catalyzed by human hydroxysteroid sulfotransferase SULT2A1. Chem Res Toxicol. 2011;24:1720–1728. doi: 10.1021/tx200260h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Elo O, Vuojolahti P, Janhunen H, Rantanen J. Recent PCB accidents in Finland. Environ Health Perspect. 1985;60:315–319. doi: 10.1289/ehp.8560315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Espandiari P, Glauert HP, Lehmler HJ, Lee EY, Srinivasan C, Robertson LW. Polychlorinated biphenyls as initiators in liver carcinogenesis: resistant hepatocyte model. Toxicol Appl Pharmacol. 2003;186:55–62. doi: 10.1016/s0041-008x(02)00018-2. [DOI] [PubMed] [Google Scholar]
- [22].Espandiari P, Glauert HP, Lehmler HJ, Lee EY, Srinivasan C, Robertson LW. Initiating activity of 4-chlorobiphenyl metabolites in the resistant hepatocyte model. Toxicol Sci. 2004;79:41–46. doi: 10.1093/toxsci/kfh097. [DOI] [PubMed] [Google Scholar]
- [23].Espandiari P, Robertson LW, Srinivasan C, Glauert HP. Comparison of different initiation protocols in the resistant hepatocyte model. Toxicology. 2005;206:373–381. doi: 10.1016/j.tox.2004.07.014. [DOI] [PubMed] [Google Scholar]
- [24].Ferguson LR, Denny WA. Genotoxicity of non-covalent interactions: DNA intercalators. Mutat Res. 2007;623:14–23. doi: 10.1016/j.mrfmmm.2007.03.014. [DOI] [PubMed] [Google Scholar]
- [25].Flor S, Ludewig G. Polyploidy-induction by dihydroxylated monochlorobiphenyls: structure-activity-relationships. Environ Int. 2010;36:962–969. doi: 10.1016/j.envint.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Forgue ST, Allen JR. Identification of an arene oxide metabolite of 2,2′,5-5′-tetrachlorobiphenyl by gas chromatography-mass spectroscopy. Chem Biol Interact. 1982;40:233–245. doi: 10.1016/0009-2797(82)90103-x. [DOI] [PubMed] [Google Scholar]
- [27].Gallagher JE, Everson RB, Lewtas J, George M, Lucier GW. Comparison of DNA adduct levels in human placenta from polychlorinated biphenyl exposed women and smokers in which CYP 1A1 levels are similarly elevated. Teratog Carcinog Mutagen. 1994;14:183–192. doi: 10.1002/tcm.1770140405. [DOI] [PubMed] [Google Scholar]
- [28].Glauert HP, Robertson LW, Silberhorn EM. PCBs and tumor promotion. In: Robertson LW, Hansen LG, editors. PCBs: Recent Advances in Environmental Toxicology and Health Effects. University Press of Kentucky; Lexington, KY: 2001. pp. 355–371. [Google Scholar]
- [29].Goldsworthy TL, Hanigan MH, Pitot HC. Models of hepatocarcinogenesis in the rat--contrasts and comparisons. Crit Rev Toxicol. 1986;17:61–89. doi: 10.3109/10408448609037071. [DOI] [PubMed] [Google Scholar]
- [30].Greenberg RA. Telomeres, crisis and cancer. Curr Mol Med. 2005;5:213–218. doi: 10.2174/1566524053586590. [DOI] [PubMed] [Google Scholar]
- [31].Gupta RC. 32P-postlabeling assay to measure carcinogen-DNA adducts. Prog Exp Tumor Res. 1987;31:21–32. doi: 10.1159/000413900. [DOI] [PubMed] [Google Scholar]
- [32].Gupta RC, Arif JM. An improved (32)P-postlabeling assay for the sensitive detection of 8-oxodeoxyguanosine in tissue DNA. Chem Res Toxicol. 2001;14:951–957. doi: 10.1021/tx000131d. [DOI] [PubMed] [Google Scholar]
- [33].Hattula ML. Mutagenicity of PCBs and their pyrosynthetic derivatives in cell-mediated assay. Environ Health Perspect. 1985;60:255–257. doi: 10.1289/ehp.8560255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hayes MA, Safe SH, Armstrong D, Cameron RG. Influence of cell proliferation on initiating activity of pure polychlorinated biphenyls and complex mixtures in resistant hepatocyte in vivo assays for carcinogenicity. J Natl Cancer Inst. 1985;74:1037–1041. [PubMed] [Google Scholar]
- [35].Hecker E. Three stage carcinogenesis in mouse skin--recent results and present status of an advanced model system of chemical carcinogenesis. Toxicol Pathol. 1987;15:245–258. doi: 10.1177/019262338701500221. [DOI] [PubMed] [Google Scholar]
- [36].Hesse S, Mezger M, Wolff T. Activation of [14C] chlorobiphenyls to protein-binding metabolites by rat liver microsomes. Chem Biol Interact. 1978;20:355–365. doi: 10.1016/0009-2797(78)90113-8. [DOI] [PubMed] [Google Scholar]
- [37].Ho PW, Garner CE, Ho JW, Leung KC, Chu AC, Kwok KH, Kung MH, Burka LT, Ramsden DB, Ho SL. Estrogenic phenol and catechol metabolites of PCBs modulate catechol-O-methyltransferase expression via the estrogen receptor: potential contribution to cancer risk. Curr Drug Metab. 2008;9:304–309. doi: 10.2174/138920008784220600. [DOI] [PubMed] [Google Scholar]
- [38].IARC A Review of Human Carcinogens: Chemical Agents and Related Occupations. 2012. [DOI] [PubMed]
- [39].Jacobus JA, Flor S, Klingelhutz A, Robertson LW, Ludewig G. Chlorophenyl)-1,4-Benzoquinone Increases the Frequency of Micronuclei and Shortens Telomeres. Environ Toxicol Pharmacol. 2008;25:267–272. doi: 10.1016/j.etap.2007.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Jensen KG, Wiberg K, Klasson-Wehler E, Onfelt A. Induction of aberrant mitosis with PCBs: particular efficiency of 2, 3,3′,4,4′-pentachlorobiphenyl and synergism with triphenyltin. Mutagenesis. 2000;15:9–15. doi: 10.1093/mutage/15.1.9. [DOI] [PubMed] [Google Scholar]
- [41].Jeong YC, Walker NJ, Burgin DE, Kissling G, Gupta M, Kupper L, Birnbaum LS, Swenberg JA. Accumulation of M1dG DNA adducts after chronic exposure to PCBs, but not from acute exposure to polychlorinated aromatic hydrocarbons. Free Radic Biol Med. 2008;45:585–591. doi: 10.1016/j.freeradbiomed.2008.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kalina I, Sram RJ, Konecna H, Ondrussekova A. Cytogenetic analysis of peripheral blood lymphocytes in workers occupationally exposed to polychlorinated biphenyls. Teratog Carcinog Mutagen. 1991;11:77–82. doi: 10.1002/tcm.1770110203. [DOI] [PubMed] [Google Scholar]
- [43].Kaminsky LS, Kennedy MW, Adams SM, Guengerich FP. Metabolism of dichlorobiphenyls by highly purified isozymes of rat liver cytochrome P-450. Biochemistry. 1981;20:7379–7384. doi: 10.1021/bi00529a009. [DOI] [PubMed] [Google Scholar]
- [44].Klaunig JE, Wang Z, Pu X, Zhou S. Oxidative stress and oxidative damage in chemical carcinogenesis. Toxicol Appl Pharmacol. 2011;254:86–99. doi: 10.1016/j.taap.2009.11.028. [DOI] [PubMed] [Google Scholar]
- [45].Lehmann L, Esch H, Kirby P, Robertson LW, Ludewig G. 4-monochlorobiphenyl (PCB3) induces mutations in the livers of transgenic Fisher 344 rats. Carcinogenesis. 2007;28:471–478. doi: 10.1093/carcin/bgl157. [DOI] [PubMed] [Google Scholar]
- [46].Lin PH, Sangaiah R, Ranasinghe A, Upton PB, La DK, Gold A, Swenberg JA. Formation of quinonoid-derived protein adducts in the liver and brain of Sprague-Dawley rats treated with 2,2′,5, 5′-tetrachlorobiphenyl. Chem Res Toxicol. 2000;13:710–718. doi: 10.1021/tx000030f. [DOI] [PubMed] [Google Scholar]
- [47].Liu Y, Apak TI, Lehmler HJ, Robertson LW, Duffel MW. Hydroxylated polychlorinated biphenyls are substrates and inhibitors of human hydroxysteroid sulfotransferase SULT2A1. Chem Res Toxicol. 2006;19:1420–1425. doi: 10.1021/tx060160+. [DOI] [PubMed] [Google Scholar]
- [48].Liu Y, Smart JT, Song Y, Lehmler HJ, Robertson LW, Duffel MW. Structure-activity relationships for hydroxylated polychlorinated biphenyls as substrates and inhibitors of rat sulfotransferases and modification of these relationships by changes in thiol status. Drug metabolism and disposition: the biological fate of chemicals. 2009;37:1065–1072. doi: 10.1124/dmd.108.026021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ludewig G. In: Cancer Initiation by PCBs. Robertson LW, Hansen JC, editors. The University Press of Kentucky; Lexington, KY: 2001. [Google Scholar]
- [50].Lundgren K, Collman GW, Wang-Wuu S, Tiernan T, Taylor M, Thompson CL, Lucier GW. Cytogenetic and chemical detection of human exposure to polyhalogenated aromatic hydrocarbons. Environ Mol Mutagen. 1988;11:1–11. doi: 10.1002/em.2850110103. [DOI] [PubMed] [Google Scholar]
- [51].Marabini L, Calo R, Fucile S. Genotoxic effects of polychlorinated biphenyls (PCB 153, 138, 101, 118) in a fish cell line (RTG-2) Toxicol In Vitro. 2011;25:1045–1052. doi: 10.1016/j.tiv.2011.04.004. [DOI] [PubMed] [Google Scholar]
- [52].Mayes BA, McConnell EE, Neal BH, Brunner MJ, Hamilton SB, Sullivan TM, Peters AC, Ryan MJ, Toft JD, Singer AW, Brown JF, Jr., Menton RG, Moore JA. Comparative carcinogenicity in Sprague-Dawley rats of the polychlorinated biphenyl mixtures Aroclors 1016, 1242, 1254, and 1260. Toxicol Sci. 1998;41:62–76. doi: 10.1093/toxsci/41.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].McLean MR, Bauer U, Amaro AR, Robertson LW. Identification of catechol and hydroquinone metabolites of 4-monochlorobiphenyl. Chem Res Toxicol. 1996;9:158–164. doi: 10.1021/tx950083a. [DOI] [PubMed] [Google Scholar]
- [54].McLean MR, Robertson LW, Gupta RC. Detection of PCB adducts by the 32P-postlabeling technique. Chem Res Toxicol. 1996;9:165–171. doi: 10.1021/tx9500843. [DOI] [PubMed] [Google Scholar]
- [55].McLean MR, Twaroski TP, Robertson LW. Redox cycling of 2-(x’-mono, -di, -trichlorophenyl)-1, 4-benzoquinones, oxidation products of polychlorinated biphenyls. Archives of biochemistry and biophysics. 2000;376:449–455. doi: 10.1006/abbi.2000.1754. [DOI] [PubMed] [Google Scholar]
- [56].Meisner LF, Roloff B, Sargent L, Pitot H. Interactive cytogenetic effects on rat bone-marrow due to chronic ingestion of 2,5,2′,5′ and 3,4, 3′,4′ PCBs. Mutat Res. 1992;283:179–183. doi: 10.1016/0165-7992(92)90105-q. [DOI] [PubMed] [Google Scholar]
- [57].Morales NM, Matthews HB. In vivo binding of 2,3,6,2′,3′,6′-hexachlorobiphenyl and 2,4,5,2′,4′,5′-hexachlorobiphenyl to mouse liver macromolecules. Chem Biol Interact. 1979;27:99–110. doi: 10.1016/0009-2797(79)90153-4. [DOI] [PubMed] [Google Scholar]
- [58].Myers MS, Johnson LL, Hom T, Collier TK, Stein JE, Varanasi U. Toxicopathic hepatic lesions in subadult English sole (Pleuronectes vetulus) from Puget Sound, Washington, USA: Relationship with other biomarkers of contaminant exposure. Marine Environmental Research. 1998;45:47–67. [Google Scholar]
- [59].Nagayama J, Nagayama M, Haraguchi K, Kuroki H, Masuda Y. Effect of 2, 3, 4, 7, 8-pentachlorodibenzofuran and its analogues on induction of sister chromatid exchanges in cultured human lymphocytes. Fukuoka Igaku Zasshi. 1995;86:184–189. [PubMed] [Google Scholar]
- [60].Nagayama J, Nagayama M, Haraguchi K, Kuroki H, Masuda Y. Induction of sister chromatid exchanges in cultured human lymphocytes with methylsulphonyl PCB congeners. Fukuoka Igaku Zasshi. 1999;90:238–245. [PubMed] [Google Scholar]
- [61].Nagayama J, Nagayama M, Iida T, Hirakawa H, Matsueda T, Masuda Y. Effects of highly toxic organochlorine compounds retained in human body on induction of sister chromatid exchanges in cultured human lymphocytes. Chemosphere. 1994;29:2349–2354. doi: 10.1016/0045-6535(94)90403-0. [DOI] [PubMed] [Google Scholar]
- [62].Nagayama J, Nagayama M, Iida T, Hirakawa H, Matsueda T, Ohki M, Tsuji H. Comparison between “Yusho” patients and healthy Japanese in contamination level of dioxins and related chemicals and frequency of sister chromatid exchanges. Chemosphere. 2001;43:931–936. doi: 10.1016/s0045-6535(00)00453-7. [DOI] [PubMed] [Google Scholar]
- [63].Nagayama J, Nagayama M, Wada K, Iida T, Hirakawa H, Matsueda T, Masuda Y. [The effect of organochlorine compounds on the induction of sister chromatid exchanges in cultured human lymphocytes] Fukuoka Igaku Zasshi. 1991;82:221–227. [PubMed] [Google Scholar]
- [64].Narbonne JF, Daubeze M. In vitro binding of hexachlorobiphenyl to DNA and proteins. Toxicology. 1980;16:173–175. doi: 10.1016/0300-483x(80)90047-5. [DOI] [PubMed] [Google Scholar]
- [65].NTP, Toxicology and carcinogenesis studies of a binary mixture of 3,3′,4,4′,5-pentachlorobiphenyl (PCB 126) (Cas No. 57465-28-8) and 2,2′,4,4′,5,5′-hexachlorobiphenyl (PCB 153) (CAS No. 35065-27-1) in female Harlan Sprague-Dawley rats (gavage studies) Natl Toxicol Program.Tech Rep Ser. 2006:1–258. [PubMed] [Google Scholar]
- [66].Oakley GG, Devanaboyina U, Robertson LW, Gupta RC. Oxidative DNA damage induced by activation of polychlorinated biphenyls (PCBs): implications for PCB-induced oxidative stress in breast cancer. Chem Res Toxicol. 1996;9:1285–1292. doi: 10.1021/tx960103o. [DOI] [PubMed] [Google Scholar]
- [67].Oakley GG, Robertson LW, Gupta RC. Analysis of polychlorinated biphenyl-DNA adducts by 32P-postlabeling. Carcinogenesis. 1996;17:109–114. doi: 10.1093/carcin/17.1.109. [DOI] [PubMed] [Google Scholar]
- [68].Onozuka D, Yoshimura T, Kaneko S, Furue M. Mortality after exposure to polychlorinated biphenyls and polychlorinated dibenzofurans: a 40-year follow-up study of Yusho patients. Am J Epidemiol. 2009;169:86–95. doi: 10.1093/aje/kwn295. [DOI] [PubMed] [Google Scholar]
- [69].Parkinson A, Safe SH, Robertson LW, Thomas PE, Ryan DE, Reik LM, Levin W. Immunochemical quantitation of cytochrome P-450 isozymes and epoxide hydrolase in liver microsomes from polychlorinated or polybrominated biphenyl-treated rats. A study of structure-activity relationships. J Biol Chem. 1983;258:5967–5976. [PubMed] [Google Scholar]
- [70].Pereg D, Robertson LW, Gupta RC. DNA adduction by polychlorinated biphenyls: adducts derived from hepatic microsomal activation and from synthetic metabolites. Chem Biol Interact. 2002;139:129–144. doi: 10.1016/s0009-2797(01)00292-7. [DOI] [PubMed] [Google Scholar]
- [71].Pereg D, Tampal N, Espandiari P, Robertson LW. Distribution and macromolecular binding of benzo[a]pyrene and two polychlorinated biphenyl congeners in female mice. Chem Biol Interact. 2001;137:243–258. doi: 10.1016/s0009-2797(01)00256-3. [DOI] [PubMed] [Google Scholar]
- [72].Pfohl-Leszkowicz A, Weber-Lotfi F, Masfaraud JF, Devaux A, Laouedj A, Guillemaut P, Malaveille C, Rether B, Monod G, Dirheimer G. DNA adduct detection: some applications in monitoring exposure to environmental genotoxic chemicals. IARC Sci Publ. 1993:373–378. [PubMed] [Google Scholar]
- [73].Popescu NC, Amsbaugh SC, DiPaolo JA. Relationship of carcinogen-induced sister chromatid exchange and neoplastic cell transformation, International journal of cancer. Journal international du cancer. 1981;28:71–77. doi: 10.1002/ijc.2910280113. [DOI] [PubMed] [Google Scholar]
- [74].Ravoori S, Ayotte P, Srinivasan C, Pereg D, Robertson LW, Russell GK, Jeyabalan J, Gupta RC. DNA damage associated with PCBs in the whole blood cells of Inuit. Environ Toxicol Pharmacol. 2008;25:273–276. doi: 10.1016/j.etap.2007.10.027. [DOI] [PubMed] [Google Scholar]
- [75].Ravoori S, Srinivasan C, Pereg D, Robertson LW, Ayotte P, Gupta RC. Protective effects of selenium against DNA adduct formation in Inuit environmentally exposed to PCBs. Environ Int. 2010;36:980–986. doi: 10.1016/j.envint.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Ravoori S, Vadhanam MV, Davey DD, Srinivasan C, Nagarajan B, Gupta RC. Modulation of novel DNA adducts during human uterine cervix cancer progression. Int J Oncol. 2006;29:1437–1443. [PubMed] [Google Scholar]
- [77].Rayne S, Forest K. pK(a) values of the monohydroxylated polychlorinated biphenyls (OH-PCBs), polybrominated biphenyls (OH-PBBs), polychlorinated diphenyl ethers (OH-PCDEs), and polybrominated diphenyl ethers (OH-PBDEs) Journal of environmental science and health. 2010;45:1322–1346. doi: 10.1080/10934529.2010.500885. [DOI] [PubMed] [Google Scholar]
- [78].Sandal S, Yilmaz B, Carpenter DO. Genotoxic effects of PCB 52 and PCB 77 on cultured human peripheral lymphocytes. Mutat Res. 2008;654:88–92. doi: 10.1016/j.mrgentox.2008.05.005. [DOI] [PubMed] [Google Scholar]
- [79].Sargent L, Dragan YP, Erickson C, Laufer CJ, Pitot HC. Study of the separate and combined effects of the non-planar 2,5,2′,5′- and the planar 3,4,3′,4′-tetrachlorobiphenyl in liver and lymphocytes in vivo. Carcinogenesis. 1991;12:793–800. doi: 10.1093/carcin/12.5.793. [DOI] [PubMed] [Google Scholar]
- [80].Sargent L, Roloff B, Meisner L. In vitro chromosome damage due to PCB interactions. Mutat Res. 1989;224:79–88. doi: 10.1016/0165-1218(89)90006-2. [DOI] [PubMed] [Google Scholar]
- [81].Sargent LM, Sattler GL, Roloff B, Xu YH, Sattler CA, Meisner L, Pitot HC. Ploidy and specific karyotypic changes during promotion with phenobarbital, 2,5,2′,5′-tetrachlorobiphenyl, and/or 3,4,3′4′-tetrachlorobiphenyl in rat liver. Cancer Res. 1992;52:955–962. [PubMed] [Google Scholar]
- [82].Schilderman PA, Moonen EJ, Maas LM, Welle I, Kleinjans JC. Use of crayfish in biomonitoring studies of environmental pollution of the river Meuse. Ecotoxicol Environ Saf. 1999;44:241–252. doi: 10.1006/eesa.1999.1827. [DOI] [PubMed] [Google Scholar]
- [83].Senthilkumar PK, Klingelhutz AJ, Jacobus JA, Lehmler H, Robertson LW, Ludewig G. Airborne polychlorinated biphenyls (PCBs) reduce telomerase activity and shorten telomere length in immortal human skin keratinocytes (HaCat) Toxicol Lett. 2011;204:64–70. doi: 10.1016/j.toxlet.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Senthilkumar PK, Robertson LW, Ludewig G. PCB153 reduces telomerase activity and telomere length in immortalized human skin keratinocytes (HaCaT) but not in human foreskin keratinocytes (NFK) Toxicol Appl Pharmacol. 2012;259:115–123. doi: 10.1016/j.taap.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Shimada T. Metabolic activation of [14C] polychlorinated biphenyl mixtures by rate liver microsomes. Bull Environ Contam Toxicol. 1976;16:26–32. doi: 10.1007/BF01753101. [DOI] [PubMed] [Google Scholar]
- [86].Shimada T, Imai Y, Sato R. Covalent binding of polychlorinated biphenyls to proteins by reconstituted monooxygenase system containing cytochrome P-450. Chem Biol Interact. 1981;38:29–44. doi: 10.1016/0009-2797(81)90151-4. [DOI] [PubMed] [Google Scholar]
- [87].Shimada T, Sato R. Covalent binding in vitro of polychlorinated biphenyls to microsomal macromolecules. Involvement of metabolic activation by a cytochrome P-450-linked mono-oxygenase system. Biochem Pharmacol. 1978;27:585–593. doi: 10.1016/0006-2952(78)90399-4. [DOI] [PubMed] [Google Scholar]
- [88].Shimada T, Sato R. Covalent binding of polychlorinated biphenyls to rat liver microsomes in vitro: nature of reactive metabolites and target macromolecules. Toxicol Appl Pharmacol. 1980;55:490–500. doi: 10.1016/0041-008x(80)90051-4. [DOI] [PubMed] [Google Scholar]
- [89].Shimada T, Sawabe Y. Activation of 3,4,3′,4′-tetrachlorobiphenyl to protein-bound metabolites by rat liver microsomal cytochrome P-448-containing monooxygenase system. Toxicol Appl Pharmacol. 1983;70:486–493. doi: 10.1016/0041-008x(83)90166-7. [DOI] [PubMed] [Google Scholar]
- [90].Shimada T, Sawabe Y. Comparative studies on distribution and covalent tissue binding of 2,4,2′,4′- and 3,4,3′,4′-tetrachlorobiphenyl isomers in the rat. Arch Toxicol. 1984;55:182–185. doi: 10.1007/BF00316125. [DOI] [PubMed] [Google Scholar]
- [91].Shimada T, Sawabe Y, Nakano Y. Interaction of 3,4,3′,4′-tetrachlorobiphenyl metabolites formed by cytochrome P-450 in vitro with rat erythrocytes. Arch Toxicol. 1985;58:20–26. doi: 10.1007/BF00292611. [DOI] [PubMed] [Google Scholar]
- [92].Silberhorn EM, Glauert HP, Robertson LW. Carcinogenicity of polyhalogenated biphenyls: PCBs and PBBs. Crit. Rev. Toxicol. 1990;20:439–496. doi: 10.3109/10408449009029331. [DOI] [PubMed] [Google Scholar]
- [93].Snyder RD, McNulty J, Zairov G, Ewing DE, Hendry LB. The influence of N-dialkyl and other cationic substituents on DNA intercalation and genotoxicity. Mutat Res. 2005;578:88–99. doi: 10.1016/j.mrfmmm.2005.03.022. [DOI] [PubMed] [Google Scholar]
- [94].Song Y, Buettner GR. Thermodynamic and kinetic considerations for the reaction of semiquinone radicals to form superoxide and hydrogen peroxide. Free Radic Biol Med. 2010;49:919–962. doi: 10.1016/j.freeradbiomed.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Song Y, Buettner GR, Parkin S, Wagner BA, Robertson LW, Lehmler HJ. Chlorination increases the persistence of semiquinone free radicals derived from polychlorinated biphenyl hydroquinones and quinones. J Org Chem. 2008;73:8296–8304. doi: 10.1021/jo801397g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Song Y, Wagner BA, Witmer JR, Lehmler HJ, Buettner GR. Nonenzymatic displacement of chlorine and formation of free radicals upon the reaction of glutathione with PCB quinones. Proc Natl Acad Sci U S A. 2009;106:9725–9730. doi: 10.1073/pnas.0810352106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Song YF, Wilke BM, Song XY, Gong P, Zhou QX, Yang GF. Polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs) and heavy metals (HMs) as well as their genotoxicity in soil after long-term wastewater irrigation. Chemosphere. 2006;65:1859–1868. doi: 10.1016/j.chemosphere.2006.03.076. [DOI] [PubMed] [Google Scholar]
- [98].Spencer WA, Lehmler HJ, Robertson LW, Gupta RC. Oxidative DNA adducts after Cu(2+)-mediated activation of dihydroxy PCBs: role of reactive oxygen species. Free Radic Biol Med. 2009;46:1346–1352. doi: 10.1016/j.freeradbiomed.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Srinivasan A, Robertson LW, Ludewig G. Sulfhydryl binding and topoisomerase inhibition by PCB metabolites. Chem Res Toxicol. 2002;15:497–505. doi: 10.1021/tx010128+. [DOI] [PubMed] [Google Scholar]
- [100].Stadnicki SS, Lin FS, Allen JR. DNA single strand breaks caused by 2,2′,5,5′-tetrachlorobiphenyl and its metabolites. Res Commun Chem Pathol Pharmacol. 1979;24:313–327. [PubMed] [Google Scholar]
- [101].Tampal N, Lehmler HJ, Espandiari P, Malmberg T, Robertson LW. Glucuronidation of hydroxylated polychlorinated biphenyls (PCBs) Chem Res Toxicol. 2002;15:1259–1266. doi: 10.1021/tx0200212. [DOI] [PubMed] [Google Scholar]
- [102].Tampal N, Myers S, Robertson LW. Binding of polychlorinated biphenyls/metabolites to hemoglobin. Toxicol Lett. 2003;142:53–60. doi: 10.1016/s0378-4274(02)00484-8. [DOI] [PubMed] [Google Scholar]
- [103].Tretjak Z, Volavsek C, Beckmann SL. Structural chromosome aberrations and industrial waste. Lancet. 1990;335:1288. doi: 10.1016/0140-6736(90)91362-e. [DOI] [PubMed] [Google Scholar]
- [104].Tsai PC, Ko YC, Huang W, Liu HS, Guo YL. Increased liver and lupus mortalities in 24-year follow-up of the Taiwanese people highly exposed to polychlorinated biphenyls and dibenzofurans. Sci Total Environ. 2007;374:216–222. doi: 10.1016/j.scitotenv.2006.12.024. [DOI] [PubMed] [Google Scholar]
- [105].van Pelt FN, Haring RM, Weterings PJ. Micronucleus formation in cultured human keratinocytes: Involvement of intercellular bioactivation. Toxicol In Vitro. 1991;5:515–518. doi: 10.1016/0887-2333(91)90084-q. [DOI] [PubMed] [Google Scholar]
- [106].Wangpradit O, Mariappan SV, Teesch LM, Duffel MW, Norstrom K, Robertson LW, Luthe G. Oxidation of 4-chlorobiphenyl metabolites to electrophilic species by prostaglandin H synthase. Chem Res Toxicol. 2009;22:64–71. doi: 10.1021/tx800300t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Wiesner G, Wild KJ, Gruber M, Lindner R, Taeger K. A cytogenetic study on the teaching staff of a polluted school with a questionable increased incidence of malignancies. Int J Hyg Environ Health. 2000;203:141–146. doi: 10.1078/S1438-4639(04)70019-X. [DOI] [PubMed] [Google Scholar]
- [108].Willems MI, Roggeband R, Baan RA, Wilmer JW, de Raat WK, Lohman PH. Monitoring the exposure of rats to benzo[a]pyrene by the determination of mutagenic activity in excreta, chromosome aberrations and sister chromatid exchanges in peripheral blood cells, and DNA adducts in peripheral blood lymphocytes and liver. Mutagenesis. 1991;6:151–158. doi: 10.1093/mutage/6.2.151. [DOI] [PubMed] [Google Scholar]
- [109].Wong A, Basrur P, Safe S. The metabolically mediated DNA damage and subsequent DNA repair by 4-chlorobiphenyl in Chinese hamster ovary cells. Res Commun Chem Pathol Pharmacol. 1979;24:543–550. [PubMed] [Google Scholar]
- [110].Wyndham C, Devenish J, Safe S. The in vitro metabolism, macromolecular binding and bacterial mutagenicity of 4-chloribiphenyl, a model PCB substrate. Res Commun Chem Pathol Pharmacol. 1976;15:563–570. [PubMed] [Google Scholar]
- [111].Wyndham C, Safe S. In vitro metabolism of 4-chlorobiphenyl by control and induced rat liver microsomes. Biochemistry. 1978;17:208–215. doi: 10.1021/bi00595a002. [DOI] [PubMed] [Google Scholar]
- [112].Xie W, Wang K, Robertson LW, Ludewig G. Investigation of mechanism(s) of DNA damage induced by 4-monochlorobiphenyl (PCB3) metabolites. Environ Int. 2010;36:950–961. doi: 10.1016/j.envint.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Yamasaki H, Mesnil M, Nakazawa H. Interaction and distinction of genotoxic and non-genotoxic events in carcinogenesis. Toxicol Lett. 1992;64-65(Spec No):597–604. doi: 10.1016/0378-4274(92)90237-e. [DOI] [PubMed] [Google Scholar]
- [114].Zeiger E, Haseman JK, Shelby MD, Margolin BH, Tennant RW. Evaluation of four in vitro genetic toxicity tests for predicting rodent carcinogenicity: confirmation of earlier results with 41 additional chemicals. Environ Mol Mutagen. 1990;16(Suppl 18):1–14. doi: 10.1002/em.2850160502. [DOI] [PubMed] [Google Scholar]
- [115].Zettner MA, Flor S, Ludewig G, Wagner J, Robertson LW, Lehmann L. Quinoid metabolites of 4-monochlorobiphenyl induce gene mutations in cultured Chinese hamster v79 cells. Toxicol Sci. 2007;100:88–98. doi: 10.1093/toxsci/kfm204. [DOI] [PubMed] [Google Scholar]
- [116].Zhao S, Narang A, Ding X, Eadon G. Characterization and quantitative analysis of DNA adducts formed from lower chlorinated PCB-derived quinones. Chem Res Toxicol. 2004;17:502–511. doi: 10.1021/tx034245b. [DOI] [PubMed] [Google Scholar]