Abstract
Bacteria cooperate to form multicellular communities and compete against one another for environmental resources. Here, we review recent advances in our understanding of bacterial competition mediated by contact-dependent growth inhibition (CDI) systems. Different CDI+ bacteria deploy a variety of toxins to inhibit neighboring cells and protect themselves from autoinhibition by producing specific immunity proteins. The genes encoding CDI toxin–immunity pairs appear to be exchanged between cdi loci and are often associated with other toxin-delivery systems in diverse bacteria. CDI also appears to facilitate cooperative behavior between kin, suggesting that these systems may have other roles beyond competition.
Keywords: Bacterial competition, biofilm, cellular communication, contact-dependent growth inhibition, toxic nuclease, two-partner secretion proteins
Bacterial competition
Bacterial cells are often regarded as isolated and autonomous entities, yet they exhibit a number of cooperative and competitive behaviors. Bacteria collaborate to assemble multicellular biofilm communities and secrete small diffusible signaling molecules to coordinate activities [1, 2]. Soluble factors are also used for intercellular competition, with some bacteria releasing microcins and bacteriocins to inhibit the growth of competitors [3, 4]. Other inhibitory systems require direct cell-to-cell contact between competing bacteria. Contact-dependent growth inhibition (CDI) was first observed in Escherichia coli isolate EC93, which deploys a two-partner (type V) secretion system to inhibit other E. coli strains [5]. Subsequently, type VI secretion systems were also found to mediate interbacterial competition in a contact-dependent manner [6–8]. Thus, Gram-negative bacteria possess at least two general mechanisms to inhibit neighboring cells. Both systems confer a substantial competitive growth advantage, suggesting that contact-dependent inhibition plays a significant role in shaping bacterial communities. In this review, we outline recent advances in our understanding of CDI mediated by the CdiAB family of two-partner secretion proteins. Readers are referred to a recent comprehensive review of type VI secretion for its role in interbacterial competition [9].
Contact-dependent growth inhibition (CDI) in E. coli EC93
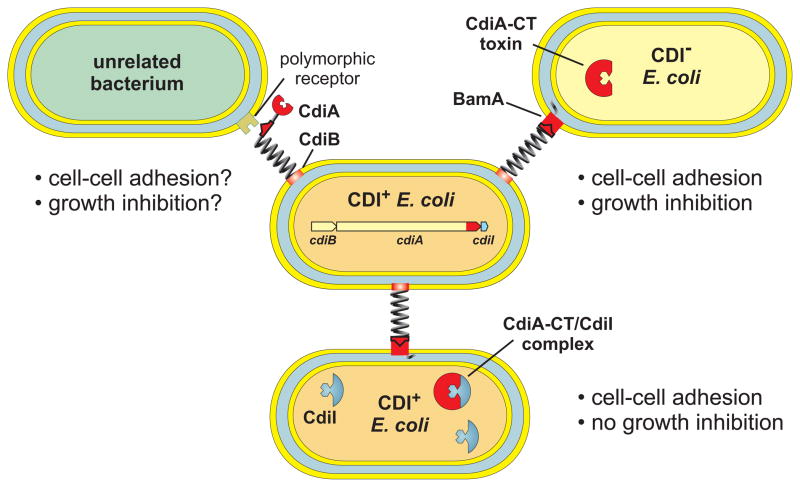
CDI was discovered in E. coli EC93, an isolate from rat intestine that inhibits the growth of laboratory E. coli K-12 strains [5]. Enteric bacteria commonly produce soluble antibacterial toxins, but E. coli EC93 requires direct contact with target cells to inhibit growth. CDI is mediated by the cdiBAI gene cluster, which is sufficient to confer the CDI+ inhibitor phenotype to E. coli K-12 cells. The cdiB and cdiA genes encode a two-partner secretion system [10, 11]. CdiB is a β-barrel protein that exports CdiA across the outer membrane. CdiA is a very large (~319 kDa) hemagglutinin-repeat protein that carries the CDI growth inhibition activity. Based on its similarity to filamentous hemagglutinin (FHA) from Bordetella species [12], CdiA is predicted to extend several hundred Å from the surface of CDI+ cells to bind receptors on target bacteria (Figure 1). Upon contact with target cells CdiA appears to be cleaved to release a C-terminal toxin domain (CdiA-CT) for translocation into target cells. Expression of CdiA-CT inside E. coli K-12 leads to dissipation of the proton motive force, decreased ATP pools and growth inhibition [13], suggesting that the toxin forms a pore in the inner membrane. The CdiA receptor, BamA, was identified in genetic selections for E. coli K-12 mutants that are resistant to CDI [14]. BamA is a highly conserved, outer membrane β-barrel protein that is required for the assembly of other β-barrel proteins [15–17]. BamA is present in all Gram-negative bacteria, raising the possibility that E. coli EC93 uses CDI to inhibit other species. However, the predicted extracellular loops of BamA are highly variable between species [18], suggesting that unrelated bacteria are resistant to E. coli EC93 (Figure 1). The cdiI gene is tightly linked to cdiA and encodes an immunity protein that protects E. coli EC93 from autoinhibition [5]. CdiI expression is also sufficient to protect E. coli K-12 from CDI. CdiI is small (8.9 kDa) and contains two predicted transmembrane regions suggesting that it is localized to the inner membrane, where it could potentially block the assembly or opening of the CdiA-CT pore. Thus, the E. coli EC93 CDI system encodes a toxin–immunity pair that confers a competitive growth advantage over other E. coli strains.
Figure 1. Contact-dependent growth inhibition (CDI) in Escherichia coli.
CDI+ E. coli express cdiBAI gene clusters and present CdiB/CdiA on the cell surface. CdiA binds receptors on neighboring E. coli cells and delivers a toxin derived from its C-terminus (CdiA-CT) into the target cell. CdiA-CT toxins inhibit the growth of CDI− cells, but isogenic CDI+ inhibitors produce CdiI immunity proteins that protect them from toxin activity. The extracellular residues of most outer membrane proteins are highly variable between species, suggesting that a variety of specific receptors may be targeted by CDI.
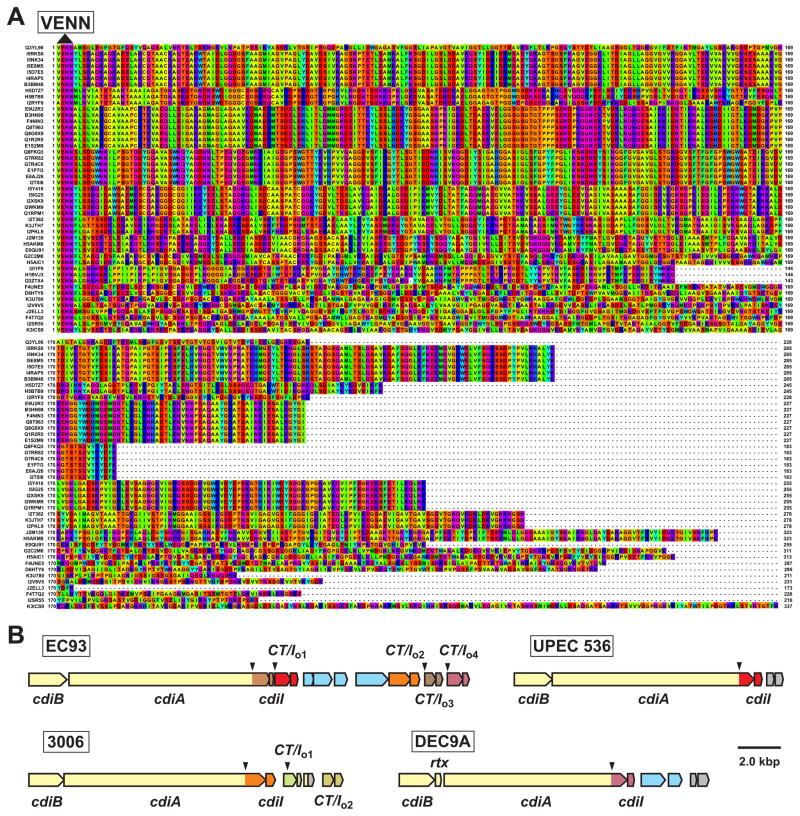
CDI diversity in other bacteria
Genes encoding CDI systems are found in many different α-, β- and γ-proteobacteria [19]. Most cdi loci are organized in the same cdiBAI gene order as E. coli EC93, but the systems from Burkholderia, Cupravidus and Variovorax species are arranged as cdiAIB clusters [19–21]. CDI systems are usually encoded within genomic or pathogenicity islands. Therefore, not all strains of a given species necessarily contain cdi genes and some strains carry multiple loci [19, 22]. For example, cdi loci are found in ~90 of the 576 E. coli genomes that have been sequenced to date. E. coli CdiA proteins share large regions of sequence identity, but their C-terminal regions diverge abruptly after a common VENN peptide motif [19, 23], suggesting that CDI+ strains deploy many different toxins. There are at least 17 distinct E. coli CdiA-CT sequence types based on pair-wise alignments (Figure 2A); however it is unclear whether each toxin type has a unique activity. CdiA-CT polymorphism is a hallmark of CDI in other bacteria as well [19, 22]. In Burkholderia systems, the variable CdiA-CT region is demarcated by a (Q/E)LYN motif, which appears to be analogous to the VENN sequence [20, 21]. These findings imply that CDI+ bacteria exploit a common secretion mechanism to deploy a variety of toxins. In accord with toxin diversity, CdiI immunity proteins are also variable and specific for cognate CdiA-CT. CdiIEC93 provides immunity to CdiA-CTEC93 activity but not to the toxic CdiA-CTUPEC536 tRNase from uropathogenic E. coli 536 (UPEC 536) [19]. Similarly, CdiIUPEC536 protects cells from UPEC 536, but is ineffective against the CDI system from E. coli EC93 [19]. Thus, CDI constitutes a network of cognate toxin–immunity pairs, each with the potential to mediate interstrain competition.
Figure 2. E. coli CdiA-CT toxin diversity.
A) CdiA-CT sequences are presented beginning with the conserved VENN peptide motif. UniProt accession numbers are provided to the left of each sequence and residues are colored according to the Taylor scheme. B) The cdi loci from E. coli EC93, UPEC 536, E. coli 3006 and E. coli O157:H7 DEC9A are depicted with cdiA-CT–cdiI pairs color-coded to indicate family types. The downward pointing arrows indicate VENN-encoding regions. The EC93 orphan-1 (CT/Io1), orphan-2 (CT/Io2) and orphan-4 (CT/Io4) pairs share sequence identity with the main toxin–immunity sequences from UPEC 536, E. coli 3006 and E. coli DEC9A, respectively. Open reading frames in light blue correspond to insertion sequence elements and transposase genes, rtx encodes a predicted toxin acyltransferase family member and genes depicted in gray are unrelated to CDI.
Although CDI-associated toxins are highly variable, related CdiA-CT–CdiI pairs are often found in diverse bacterial clades. For example, the CdiA-CT from E. coli TA271 (UniProt: F4UNE5) shares identity with corresponding sequences from Neisseria lactamica str. 020-06 (E4ZCK5), Gallibacterium anatis UMN179 (F4HC25) and Acinetobacter sp. RUH2624 (C0B226). These observations suggest that cdiA-CT–cdiI pairs are horizontally exchanged between bacteria. If so, then CdiA should be modular and capable of delivering many different CdiA-CT toxins. This hypothesis is supported by work with experimentally generated CdiA chimeras. The CdiA-CTEC93 toxin region can be fused to CdiAUPEC536 at the common VENN sequence to generate a functional CdiA protein [19]. Moreover, CdiA-CTs from Yersinia pestis and Dickeya dadantii can be delivered into E. coli target cells when grafted onto CdiAUPEC536. Remarkably, CDI-associated toxins are found in several other protein families as well. Members of the Rhs/YD-repeat (Pfam: PF05593), WXG (PF04740), Neisserial MafB and Mycobacterial Ala-Pro-rich protein families all share C-terminal sequences with CdiA, suggesting that these proteins also mediate intercellular competition [22, 24, 25]. Analysis of the C-terminal regions of selected Rhs and WXG proteins has confirmed that these domains are indeed toxins [22, 25]. Like CdiA-CT toxins, the Rhs-CT and WXG-CT activities are specifically blocked by immunity proteins encoded immediately downstream of each toxin gene. Thus, bacteria collectively carry a large repertoire of toxin–immunity genes that are shared between different delivery systems.
Orphan cdiA-CT–cdiI pairs
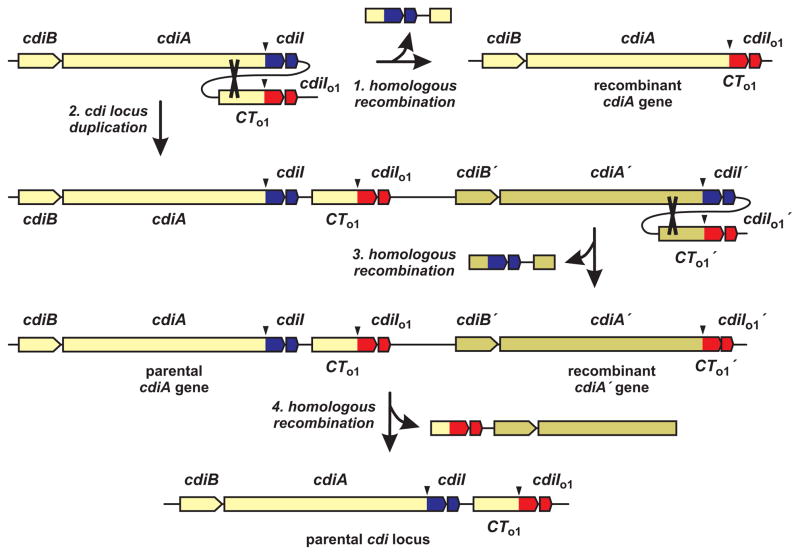
cdi loci often contain additional cdiA-CT–cdiI gene pairs in tandem arrays downstream of the main cdiBAI cluster (Figure 2B). These toxin–immunity pairs have been termed ‘orphan’ modules because they appear to be DNA fragments displaced from full-length cdiA genes [22]. Orphan regions typically contain insertion sequence (IS) elements and transposon-related genes (Figure 2B), suggesting that the modules are horizontally transferred and integrated sequentially into the cdi locus. The function of orphan toxin–immunity modules and the selective pressure to retain them are unknown. Orphan cdiA-CT fragments often contain conserved cdiA coding sequences upstream of the VENN-encoding region, but do not encode secretion signal sequences or N-terminal TPS transport domains. Therefore, orphan toxins are not likely to be exported. Moreover, most orphan cdiA-CT sequences lack translation initiation signals, so it is unclear whether these toxins are synthesized. In contrast, orphan cdiI genes appear fully functional and provide immunity in at least one instance [22]. This latter observation suggests that orphan modules could persist to confer immunity to multiple CDI toxins. However, a number of orphan cdiA-CT sequences produce active toxins when expressed in E. coli cells [22], indicating that toxin activity is often retained. Orphan modules could also represent an arsenal of diverse toxins held in reserve. It may be advantageous for CDI+ cells to deploy alternative toxins under certain circumstances – perhaps to outcompete bacteria that have acquired a cdiI immunity gene. Because orphan cdiA-CT fragments often share conserved sequences with the upstream full-length cdiA gene [22], homologous recombination could fuse orphan modules to cdiA and allow expression of new toxins (Figure 3, step 1). However, simple recombination would delete the parental cdiI gene and leave the recombinant susceptible to inhibition by neighboring wild-type siblings. Alternatively, recombination could occur subsequent to duplication of the cdi locus (Figure 3, steps 2 & 3). Large duplications are common in bacterial chromosomes [26, 27] and this phenomenon could provide an opportunity for CDI+ cells to deploy new toxins yet remain immune to the parental system. Importantly, the duplicated cdi locus could readily revert to its original structure through homologous recombination (Figure 3, step 4). Thus, iterative cycles of duplication and recombination could promote orphan toxin expression and perhaps account for the selective pressure to retain these gene pairs.
Figure 3. Models of orphan toxin–immunity gene rearrangement.
Orphan cdiA-CT genes that contain conserved sequences upstream of the VENN-encoding region (downward arrow) can undergo homologous recombination with the full-length cdiA gene (indicated by cross-over in step 1). Recombination would delete the parental toxin–immunity coding sequences and fuse the orphan module (red) to cdiA. Alternatively, the cdi locus could undergo spontaneous duplication (step 2) followed by homologous recombination (step 3) to generate a recombinant cdiA gene. Further recombination between the orphan cdiA-CT and the recombined cdiA could regenerate the original parental genotype (step 4).
Structure and function of CDI toxin–immunity pairs
The first CDI toxin activity was identified before the systems were known to mediate bacterial competition. Kleanthous and colleagues discovered that the HecA (CdiAEC16) adhesin from Erwinia chrysanthemi EC16 carries a C-terminal domain that resembles the toxic rRNase domain of colicin E3 [28]. A few other CdiA-CT toxins share obvious homology with known bacteriocins. CdiA-CT3937-2 from D. dadantii 3937 is related to pyocin S3, and CdiA-CTK96243 from Burkholderia pseudomallei K96243 is related to colicin E5 [19]. In each instance, CdiA-CT activity is very similar to its colicin homologue [19, 21]. Aravind and colleagues have recently published a series of comprehensive analyses that predict many CDI toxins are nucleases, adenosine deaminases, ADP-ribosyl cyclases and metallopeptidases [24, 29, 30]. This broad range of biochemical activities is consistent with toxin sequence diversity, and the predictions are supported by biochemical studies showing that many CdiA-CTs are RNases with unique substrate specificities. For example, the CDI toxin from B. pseudomallei isolate E479 cleaves tRNA between the highly conserved T54 and Ψ55 residues, and another toxin from B. pseudomallei 1026b cleaves within the aminoacyl acceptor stem of tRNAAla [21]. Given the diversity of CdiA-CT sequences, it seems likely that many other novel activities will be characterized, some of which may have practical applications in nucleic acids research and biotechnology.
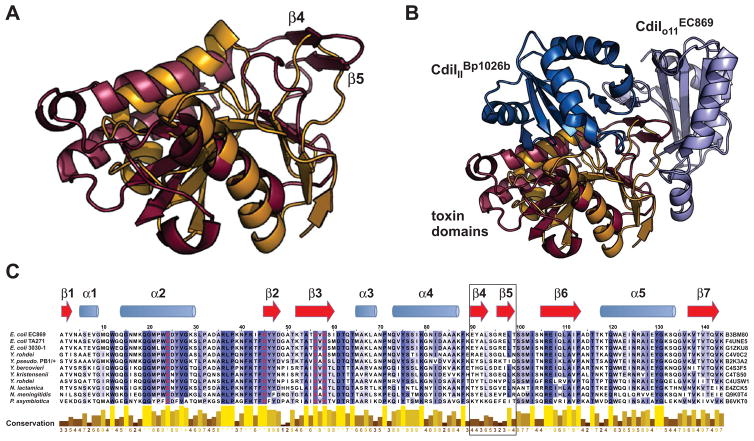
High-resolution structural analysis has recently provided the first detailed glimpse into the CDI toxin–immunity network. Morse et al. solved crystal structures of the CdiA-CTIIBp1026b–CdiIIIBp1026b complex encoded by chromosome II of B. pseudomallei 1026b and the CdiA-CTo11EC869–CdiIo11EC869 complex encoded by the orphan-11 module of E. coli O157:H7 strain EC869 [31]. Both CdiA-CTs comprise at least two domains. The N-terminal domains are flexible and not completely resolved in the final models, whereas the compact C-terminal domains are responsible for toxin activity and mediate all interactions with immunity proteins. A two-domain structure is also suggested by sequence alignments, which indicate that many CdiA-CTs are assembled from independently assorting N-terminal and C-terminal regions [21, 32]. The CdiA-CTo11EC869 and CdiA-CTIIBp1026b toxin domains share only ~15% sequence identity but both fold into similar structures that resemble type IIS restriction endonucleases (Figure 4A). Although the two toxins have very similar structures and active sites, each exhibits a distinct nuclease activity. CdiA-CTo11EC869 is a Zn2+-dependent DNA endonuclease, whereas CdiA-CTIIBp1026b is a specific tRNase as described above [21, 31]. In contrast, the CdiIIIBp1026b and CdiIo11EC869 immunity proteins are not structurally related to one another and bind to non-overlapping sites on the nuclease domain (Figure 4B). CdiIIIBp1026b binds directly over the toxin active site, forming an intricate network of direct and water-mediated hydrogen bonds with CdiA-CTIIBp1026b [31]. In contrast, CdiIo11EC869 binds its toxin through an unusual βcomplementation interaction. CdiA-CTo11EC869 extends a β-hairpin structure (β4-β5) that inserts like a lock-in-key into CdiIo11EC869 to complete a six-stranded β-sheet (Figure 4B). This interaction leaves the CdiA-CTo11EC869 active site exposed, but DNase activity is effectively neutralized in the complex [31]. Notably, CdiA-CTIIBp1026b lacks the extended β-hairpin (Figure 4A), and there is no detectable interaction between the non-cognate toxin and immunity proteins [31]. These initial studies show that toxins with diverse sequences can adopt similar structures, yet still exhibit unique growth inhibition activities and retain highly specific interactions with immunity proteins.
Figure 4. CdiA-CT–CdiI complex structures.
A) Structural alignment of the CdiA-CTo11EC869 (maroon) and CdiA-CTIIBp1026b (orange) nuclease domains. The β4-β5 hairpin in CdiA-CTo11EC869 interacts with the CdiIo11EC869 immunity protein. B) CdiIo11EC869 and CdiIIIBp1026b immunity proteins bind to distinct sites on the toxin nuclease domains. Structures correspond to 4G6V and 4G6U in the Protein Data Bank and were rendered using PyMol. C) Alignment of CdiA-CTo11EC869 toxin homologues. The CdiA-CTo11EC869 nuclease domain sequence is aligned with related toxin sequences from the indicated bacterial species. UniProt accession numbers are given to the right of each sequence. Secondary structure elements (blue α-helices and red β-strands) from CdiA-CTo11EC869 are indicated above the alignment. The alignment was rendered with Jalview 2.8 at 30% sequence identity with progressively darker shades of purple indicating greater residue conservation. The conservation index is based on [38] and values are provided below each residue. Predicted toxin active site residues are rendered in red, and the β4-β5 hairpin (boxed) mediates interactions with the CdiIo11EC869 immunity protein.
Evolution of CDI toxin–immunity pairs
Toxins within a given CdiA-CT family typically share significant sequence identity but also show regions of divergence. This is particularly apparent in the CdiA-CTo11EC869–CdiIo11EC869 toxin–immunity family, which is present in several E. coli strains and a variety of Yersinia, Neisseria and Photorhabdus species (Figure 4C). Sequence alignments show that residues within the β4-β5 hairpin are the least conserved amongst CdiA-CTo11EC869 family members (Figure 4C). Because the β4-β5 hairpin interacts directly with CdiIo11EC869 [31], these observations suggest that the toxin–immunity protein interface is diversifying rapidly. This hypothesis is supported by sequence alignments of CdiIo11EC869 homologues, which show that toxin-interacting residues are also poorly conserved. The same phenomenon is observed with homologues of the CdiA-CTIIBp1026b–CdiIIIBp1026b complex, though fewer sequences are available for comparison. It appears that CdiA-CT–CdiI pairs diversify by mutation to form families of near-cognate toxin–immunity proteins. Further genetic drift likely prevents the cross-binding of diverging CdiA-CT–CdiI pairs, generating distinct immunity groups.
Within each CdiA-CT–CdiI family, the immunity proteins usually exhibit much greater sequence diversity than the toxins. This suggests that CdiI evolution is rapid and may represent the mechanism for overall CdiA-CT–CdiI diversification. CdiI proteins are presumably free to diverge as long as they maintain sufficient affinity for CdiA-CT to provide immunity. Because CdiA-CT toxins are typically enzymes, their evolution is constrained by the need to retain catalytic activity. Of course, if CdiI evolution is too rapid (or radical), the cell would be exposed to unopposed CdiA-CT toxin activity. Therefore, diversification probably proceeds through iterative cycles of cdiI drift followed by reciprocal changes in cdiA-CT, such that the encoded proteins retain binding interactions through evolution. This model assumes that some missense mutations will weaken but not completely disrupt the CdiA-CT–CdiI interaction. This assumption seems reasonable for the CdiA-CTIIBp1026b–CdiIIIBp1026b complex, which is held together by an extensive network of interacting residues. Additionally, there is evidence that a diverging CdiI protein can provide cross-immunity to a near-cognate toxin. The orphan-1 module from E. coli EC93 is related to the CdiA-CT–CdiI pair from UPEC 536 (see Figure 3B), with 77% identity between CdiA-CT sequences and 35% identity between CdiI proteins. Both immunity proteins have significantly lower affinity for near-cognate CdiA-CT compared to their cognate toxins. Despite this lower binding affinity, CdiIUPEC536 blocks the tRNase activity of near-cognate CdiA-CTo1EC93 in vitro [22]. Conversely, the CdiIo1EC93 immunity protein is unable to neutralize the CdiA-CTUPEC536 toxin. These results support the feasibility of the reciprocal mutation model. Moreover, it is tempting to speculate that cdiA-CT–cdiI modules may diversify more rapidly when present in orphan clusters. Orphan cdiA-CT sequences usually lack translation signals, indicating that the toxins are probably produced at lower levels than orphan immunity proteins. Excess orphan CdiI could provide a buffer against mutations that reduce affinity for toxin and thereby allow compensatory mutations to be acquired by the orphan toxin gene. The selective pressure to fix these mutations could arise through periodic expression of the orphan module following genetic rearrangement of the CDI locus (as outlined in Figure 3). Thus, competitive pressures within a CDI+ population may drive both the assimilation of new toxins as well as the gradual restructuring of existing toxin–immunity protein interactions.
Regulation and restriction of growth inhibition
E. coli EC93 is unusual amongst CDI+ bacteria because its system is expressed constitutively under laboratory growth conditions. Other bacteria tightly regulate CDI expression. For example, plant pathogens appear to express their cdi genes only when colonizing specific hosts. Before CDI had been characterized, Collmer and colleagues found that disruption of the virA gene adversely affected the growth of E. chrysanthemi EC16 on a variety of plants [33]. Based on these results, virA was postulated to encode a virulence factor required for host colonization. However, virA lies immediately downstream of a cdiA homologue (hecA) and is predicted to encode a CdiI immunity protein. Therefore, an alternative explanation is that E. chrysanthemi EC16 only expresses HecA/CdiAEC16 when invading plants, and the apparent virulence phenotype reflects autoinhibition in the absence of VirA/CdiIEC16 immunity protein. This latter model is supported by work with another plant pathogen, D. dadantii 3937, which selectively activates one of its two cdi loci when cultured on chicory [19]. Similarly, CDI is largely repressed in Burkholderia thailandensis cells grown in liquid batch cultures, but strongly induced during growth in biofilms on solid media [20]. Thus, CDI expression tends to be activated a high cell densities when cell-cell contact is common. The molecular cues that induce CDI and the downstream regulatory pathways are unknown and remain outstanding problems in the field.
In at least one instance, CdiA-CT toxin delivery into target cells is not sufficient to inhibit growth. The UPEC 536 system deploys a tRNA anticodon nuclease, but this toxin is only active when bound to the metabolic enzyme CysK [32]. CysK has O-acetyl-L-serine sulfhydrylase activity and functions with CysE (L-serine-O-acetyltransferase) to synthesize L-cysteine from L-serine. CysE and CysK bind one another to form the highly conserved cysteine synthase complex [34, 35]. CdiA-CTUPEC536 and CysE share a common C-terminal peptide motif and thus the toxin appears to mimic the CysE binding interaction with CysK. CysK–CdiA-CTUPEC536 binding is required for growth inhibition and E. coli ΔcysK mutants are completely resistant to CDIUPEC536 [32]. These findings suggest that the UPEC 536 CDI system is only conditionally effective, allowing target cells to avoid inhibition through decreased expression or mutation of cysK. Alternatively, the CysK–CdiA-CTUPEC536 interaction may serve another unknown function. CysK and the CdiIUPEC536 immunity protein can bind CdiA-CTUPEC536 simultaneously. Therefore, toxins exchanged between UPEC 536 cells exist as ternary complexes with CysK and CdiIUPEC536. These complexes lack toxic tRNase activity, but have the potential to influence metabolism by modulating CysK activity. Therefore, CdiA-CT exchange between isogenic, immune bacteria could serve an intercellular signaling function.
Beyond growth inhibition
CDI has a well-established role in bacterial competition, but recent findings suggest these systems may also mediate cooperative behavior. Disruption of the cdi locus in B. thailandensis E264 abrogates biofilm formation, suggesting the system helps to establish and maintain communities in mixed microbial populations [20]. The mechanism underlying the biofilm phenotype in not known, but CDI expression in E. coli causes cells to auto-aggregate in a BamA-dependent manner [14]. Thus, biofilm formation may be promoted through CDI mediated cell-cell adhesion. Moreover, CDI could facilitate kin discrimination by preventing related, but non-isogenic, strains from participating in the group behavior. CDI may play a similar role in host invasion and colonization by plant pathogens. E. chrysanthemi EC16 hecA/cdiA mutants are defective in adhesion to host cells [36], suggesting that HecA/CdiAEC16 plays a direct role in pathogenesis. However, hecA mutants also fail to auto-aggregate; and this phenotype contributes to the virulence defect [36]. Bacterial auto-aggregation is associated with the killing of plant epidermal cells and may be important for host colonization. Perhaps the pathogen evades antibacterial defenses more effectively as an aggregated mass or must attain a critical cell density prior to invasion. Additionally, the coupling of cdi and virulence gene expression may provide a mechanism to suppress ‘cheaters’ that forgo production of virulence factors, yet seek to exploit the niche created by their virulent siblings. This problem may be particularly acute for soft-rot pathogens, which liquefy plant tissues thereby releasing nutrients for cheaters and other competitors [37]. CDI has only been characterized in one pathogenesis model, but these systems are commonly present in a variety of bacterial pathogens [23]. We suspect that CDI coordinates multicellular activities in other pathogens as well and speculate that this may represent the primary function of these systems.
Concluding remarks
CDI represents a nexus between the competitive and cooperative forces that shape bacterial populations. CDI systems deploy a diverse array of toxin domains that mediate interstrain competition. CDI toxin–immunity evolution appears to be rapid and is likely driven by competition, horizontal gene transfer and orphan toxin–immunity modules. CDI also appears to play roles in cooperative behavior such as biofilm formation. The coincident expression of CDI systems with bacterial group behaviors suggests that growth inhibition is used to enforce cooperation and punish cheaters. Important areas for further research include determination of the regulatory networks that govern CDI expression and the mechanisms by which CDI modulates interactions in microbiomes. Additionally, metagenomic studies indicate that bacteria contain several other analogous competition systems. Most of these systems have yet to be characterized experimentally and it is not known whether they require direct cell-cell contact. However, the existence of so many systems implies that intercellular toxin delivery is a fundamental and ubiquitous process in bacterial biology.
Acknowledgments
We thank Kiel Nikolakakis and Steve Poole for assistance with figures. Research in the Low and Hayes laboratories is supported by grant 0642052 (D.A.L.) from the National Science Foundation, and grants U54 AI065359 (D.A.L), R21 AI099687 (C.S.H.), U01 GM102318 (D.A.L. & C.S.H.) and R01 GM078634 (C.S.H.) from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lopez D, et al. Biofilms. Cold Spring Harb Perspect Biol. 2010;2:a000398. doi: 10.1101/cshperspect.a000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ng WL, Bassler BL. Bacterial quorum-sensing network architectures. Annu Rev Genet. 2009;43:197–222. doi: 10.1146/annurev-genet-102108-134304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Destoumieux-Garzon D, et al. Focus on modified microcins: structural features and mechanisms of action. Biochimie. 2002;84:511–519. doi: 10.1016/s0300-9084(02)01411-6. [DOI] [PubMed] [Google Scholar]
- 4.Cascales E, et al. Colicin biology. Microbiol Mol Biol Rev. 2007;71:158–229. doi: 10.1128/MMBR.00036-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aoki SK, et al. Contact-dependent inhibition of growth in Escherichia coli. Science. 2005;309:1245–1248. doi: 10.1126/science.1115109. [DOI] [PubMed] [Google Scholar]
- 6.Hood RD, et al. A type VI secretion system of Pseudomonas aeruginosa targets a toxin to bacteria. Cell Host Microbe. 2010;7:25–37. doi: 10.1016/j.chom.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacIntyre DL, et al. The Vibrio cholerae type VI secretion system displays antimicrobial properties. Proc Natl Acad Sci U S A. 2010;107:19520–19524. doi: 10.1073/pnas.1012931107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng J, et al. Genetic analysis of anti-amoebae and anti-bacterial activities of the type VI secretion system in Vibrio cholerae. PLoS One. 2011;6:e23876. doi: 10.1371/journal.pone.0023876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silverman JM, et al. Structure and regulation of the type VI secretion system. Annu Rev Microbiol. 2012;66:453–472. doi: 10.1146/annurev-micro-121809-151619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayes CS, et al. Bacterial contact-dependent delivery systems. Annu Rev Genet. 2010;44:71–90. doi: 10.1146/annurev.genet.42.110807.091449. [DOI] [PubMed] [Google Scholar]
- 11.Leo JC, et al. Type V secretion: mechanism(s) of autotransport through the bacterial outer membrane. Philos Trans R Soc Lond B Biol Sci. 2012;367:1088–1101. doi: 10.1098/rstb.2011.0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kajava AV, et al. Beta-helix model for the filamentous haemagglutinin adhesin of Bordetella pertussis and related bacterial secretory proteins. Mol Microbiol. 2001;42:279–292. doi: 10.1046/j.1365-2958.2001.02598.x. [DOI] [PubMed] [Google Scholar]
- 13.Aoki SK, et al. Contact-dependent growth inhibition causes reversible metabolic downregulation in Escherichia coli. J Bacteriol. 2009;191:1777–1786. doi: 10.1128/JB.01437-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aoki SK, et al. Contact-dependent growth inhibition requires the essential outer membrane protein BamA (YaeT) as the receptor and the inner membrane transport protein AcrB. Mol Microbiol. 2008;70:323–340. doi: 10.1111/j.1365-2958.2008.06404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paschen SA, et al. Evolutionary conservation of biogenesis of beta-barrel membrane proteins. Nature. 2003;426:862–866. doi: 10.1038/nature02208. [DOI] [PubMed] [Google Scholar]
- 16.Voulhoux R, et al. Role of a highly conserved bacterial protein in outer membrane protein assembly. Science. 2003;299:262–265. doi: 10.1126/science.1078973. [DOI] [PubMed] [Google Scholar]
- 17.Wu T, et al. Identification of a multicomponent complex required for outer membrane biogenesis in Escherichia coli. Cell. 2005;121:235–245. doi: 10.1016/j.cell.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 18.Smith DL, et al. Short-tailed stx phages exploit the conserved YaeT protein to disseminate Shiga toxin genes among enterobacteria. J Bacteriol. 2007;189:7223–7233. doi: 10.1128/JB.00824-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aoki SK, et al. A widespread family of polymorphic contact-dependent toxin delivery systems in bacteria. Nature. 2010;468:439–442. doi: 10.1038/nature09490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson MS, et al. The Burkholderia bcpAIOB genes define unique classes of two-partner secretion and contact dependent growth inhibition systems. PLoS Genet. 2012;8:e1002877. doi: 10.1371/journal.pgen.1002877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nikolakakis K, et al. The toxin/immunity network of Burkholderia pseudomallei contact-dependent growth inhibition (CDI) systems. Mol Microbiol. 2012;84:516–529. doi: 10.1111/j.1365-2958.2012.08039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poole SJ, et al. Identification of functional toxin/immunity genes linked to contact-dependent growth inhibition (CDI) and rearrangement hotspot (Rhs) systems. PLoS Genet. 2011;7:e1002217. doi: 10.1371/journal.pgen.1002217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aoki SK, et al. Toxin on a stick: modular CDI toxin delivery systems play roles in bacterial competition. Virulence. 2011;2:356–359. doi: 10.4161/viru.2.4.16463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang D, et al. A novel immunity system for bacterial nucleic acid degrading toxins and its recruitment in various eukaryotic and DNA viral systems. Nucleic Acids Res. 2011;39:4532–4552. doi: 10.1093/nar/gkr036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holberger LE, et al. A novel family of toxin/antitoxin proteins in Bacillus species. FEBS Lett. 2012;586:132–136. doi: 10.1016/j.febslet.2011.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nasvall J, et al. Real-time evolution of new genes by innovation, amplification, and divergence. Science. 2012;338:384–387. doi: 10.1126/science.1226521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reams AB, et al. Duplication frequency in a population of Salmonella enterica rapidly approaches steady state with or without recombination. Genetics. 2010;184:1077–1094. doi: 10.1534/genetics.109.111963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walker D, et al. Identification of the catalytic motif of the microbial ribosome inactivating cytotoxin colicin E3. Protein Sci. 2004;13:1603–1611. doi: 10.1110/ps.04658504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iyer LM, et al. Evolution of the deaminase fold and multiple origins of eukaryotic editing and mutagenic nucleic acid deaminases from bacterial toxin systems. Nucleic Acids Res. 2011;39:9473–9497. doi: 10.1093/nar/gkr691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang D, et al. Polymorphic toxin systems: comprehensive characterization of trafficking modes, processing, mechanisms of action, immunity and ecology using comparative genomics. Biol Direct. 2012;7:18. doi: 10.1186/1745-6150-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morse RP, et al. Structural basis of toxicity and immunity in contact-dependent growth inhibition (CDI) systems. Proc Natl Acad Sci U S A. 2012;109:21480–21485. doi: 10.1073/pnas.1216238110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diner EJ, et al. Identification of a target cell permissive factor required for contact-dependent growth inhibition (CDI) Genes Dev. 2012;26:515–525. doi: 10.1101/gad.182345.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rojas CM, et al. The Erwinia chrysanthemi EC16 hrp/hrc gene cluster encodes an active Hrp type III secretion system that is flanked by virulence genes functionally unrelated to the Hrp system. Mol Plant Microbe Interact. 2004;17:644–653. doi: 10.1094/MPMI.2004.17.6.644. [DOI] [PubMed] [Google Scholar]
- 34.Wirtz M, et al. Structure and function of the hetero-oligomeric cysteine synthase complex in plants. J Biol Chem. 2010;285:32810–32817. doi: 10.1074/jbc.M110.157446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao C, et al. On the interaction site of serine acetyltransferase in the cysteine synthase complex from Escherichia coli. Biochem Biophys Res Commun. 2006;341:911–916. doi: 10.1016/j.bbrc.2006.01.054. [DOI] [PubMed] [Google Scholar]
- 36.Rojas CM, et al. HecA, a member of a class of adhesins produced by diverse pathogenic bacteria, contributes to the attachment, aggregation, epidermal cell killing, and virulence phenotypes of Erwinia chrysanthemi EC16 on Nicotiana clevelandii seedlings. Proc Natl Acad Sci U S A. 2002;99:13142–13147. doi: 10.1073/pnas.202358699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Charkowski A, et al. The role of secretion systems and small molecules in soft-rot enterobacteriaceae pathogenicity. Annu Rev Phytopathol. 2012;50:425–449. doi: 10.1146/annurev-phyto-081211-173013. [DOI] [PubMed] [Google Scholar]
- 38.Livingstone CD, Barton GJ. Protein sequence alignments: a strategy for the hierarchical analysis of residue conservation. Comput Appl Biosci. 1993;9:745–756. doi: 10.1093/bioinformatics/9.6.745. [DOI] [PubMed] [Google Scholar]