Abstract
Previously, we have demonstrated that inhibition of Hedgehog pathway induces predominantly apoptosis in diffuse large B-cell lymphoma (DLBCL) cell lines of activated B-cell (ABC) type but predominantly cell cycle arrest in those of germinal center (GC). Here, we explored the possibility of overcoming the resistance to apoptosis to SMO inhibitors in 5 DLBCL cells of GC type using the combination of the SMO inhibitor HhAntag (Genentech Inc) with the BH3 mimetic ABT-737 (Abbott Laboratories). As controls we have used 2 DLBCL of ABC type (OCI-LY10 and OCI-LY3). Combinatorial treatments were performed with increasing concentrations of the HhAntag with low-doses (equal or less than the IC20) of ABT-737. MTT assays were used to detect changes in cell viability and Annexin-V and PARP1 cleavage assays were used to detect apoptosis. Combining low-doses of ABT-737 with increasing concentrations of HhAntag in GC DLBCL cell lines resulted in significantly increase of apoptosis in comparison to treatments with the SMO inhibitor alone. We concluded that in GC DLBCL cell lines, in contrast to those of ABC type, functional inhibition of BCL2 family members is usually needed to overcome the resistance to apoptosis to SMO inhibitors. These findings provide a rationale to explore the use of SMO and BCL2 inhibitors as adjuvant therapy for treatment of DLBCL of GC type.
Keywords: Smoothened (SMO) inhibitors, ABT-737, BH3 mimetics, HhAntag, Hedgehog signaling, diffuse large B-cell lymphoma
INTRODUCTION
Diffuse large B-cell lymphoma (DLBCL) is the most common lymphoid malignancy in adults, representing almost 40% of cases of non-Hodgkin’s lymphoma [1]. There are 2 main molecular subtypes of DLBCL, germinal center (GC) and activated B-cell (ABC) that have distinctive cytogenetic and molecular features supporting the rationale that these 2 subtypes are distinct entities [2–5]. It has been proven that DLBCL is one of the neoplasms most sensitive to chemotherapy. With standard treatments, consisting of combination of chemoimmunotherapy, certain percentage of patients can be cured. However many (~50%) of newly diagnosed patients will not be cured with the available protocols and treatment options are limited [1, 6]. Therefore, novel treatment strategies based on better understanding of disease biology are needed.
Antiapoptotic proteins are key mediators of programmed cell death that are frequently expressed in malignant lymphomas [7]. For example, BCL2 is constitutively overexpressed in almost all follicular lymphomas and in almost 40% of DLBCL as a consequence of BCL2 gene amplification or the t(14;18) [8, 9]. Proteins of the BCL2 family are key regulators of apoptosis and the role of these proteins in inhibition of apoptosis and resistance to chemotherapy treatments has been widely demonstrated [10, 11]. The antiapoptotic proteins BCL2, MCL1, BCL-XL, and BFL-1/A1 share homology in the BH1, BH2, BH3, and BH4 domains. The propapototic BH3-only family members (BAD, BIK, NOXA, HRK, BMF, and PUMA) share homology only in the BH3 domain, which is essential for pro-death function. These proteins cause mitochondrial permeabilization and commitment the cell to death (apoptosis).
ABT-737 (Abbott Laboratories) is a BH3 mimetic that inhibits pro-survival members of the BCL2 family, including BCL-XL, BCL-2 and BCL-W, and induces BAX/BAK-dependent apoptosis [12–14]. Targeting antiapoptotic BCL2 family members with small molecule inhibitors such as ABT-737 to lower the threshold to induce apoptosis represents a new opportunity to overcome chemotherapy resistance making these compounds potentially complimentary to conventional or targeted cytotoxic drugs.
Previously, we have demonstrated that Hedgehog (Hh) signaling is functional in DLBCL and that its inhibition, using the smoothened (SMO) inhibitor cyclopamine-KAAD, induced apoptosis in DLBCL cell lines of ABC type but not or little apoptosis, in those of GC subtype [15, 16]. The main effect of inhibition of Hh signaling in DLBCL of GC type was cell cycle arrest [15]. We have also found that inhibition of Hh pathway signaling in DLBCL of ABC type was associated with decrease of mRNA and protein levels of BCL2, a direct downstream target of GLI1 [17, 18]; however in GC DLBCL cells inhibition of Hh pathway resulted in little or no decrease of BCL2 expression [15]. These findings suggest that the lack of decrease in the expression of BCL2 seen in GC DLBCL cells after inhibiting Hh signaling may be one factor contributing to apoptosis resistance to SMO inhibitors in DLBCL of GC type.
Here, we sought to investigate if adding low-doses of a functional BCL2 inhibitor along with a Hh inhibitor may increase the susceptibility to apoptosis in GC DLBCL cell lines. To inhibit SMO we used HhAntag, drug developed by Genentech (Genentech Inc, San Diego, CA), and for the functional inhibition of BCL2 family members we used the BH3 mimetic ABT-737 developed by Abbott Laboratories (Abbott Lab, IL). Our findings indicate that functional inhibition of BCL2 family members is needed to increase the susceptibility to apoptosis to SMO inhibitors in DLBCL of GC type.
MATERIALS AND METHODS
Cell lines and drugs
Five GC DLBCL cell lines, DOHH2, SuDHL4, OCI-LY19, HT (from DSMZ; Germany) and Toledo (ATCC; Manassas, VA) were used. Two ABC DLBCL (OCI-LY3 and OCI-LY10) cell lines were also used. OCI-LY3 and OCI-LY10 were kindly provided by Michael G Rosenblum (Department of Experimental Therapeutics, MD Anderson Cancer Center). These cell lines have been validated using conventional karyotyping at the core facility in our institution. All the GC DLBCL cell lines with the exception of HT had the t(14;18)(q32;q21). All cell lines were maintained at 37°C in RPMI 1640 (ATCC) with 10% heat inactivated fetal bovine serum (FBS; Sigma, St Louis, MO) in a humidified atmosphere containing 5% CO2.
We used a novel Hh inhibitor named HhAntag developed by Genentech (Genentech Inc) and the BH3 mimetic ABT-737 (Abbott lab). HhAntag is a small molecule that belongs to the benzimidazole chemical family (C24H23CLN4O3-2HCL) [18]. ABT-737 is a BH3-only mimetic with high affinity to BCL-XL, BCL-2 and BCL-W [13, 14].
Cell viability and apoptosis assays
ABT-737 and HhAntag were diluted in DMSO. Typically, for each condition 20 × 103 cells were plated in triplicates in RPMI 1640 media with 2% FBS and desired concentrations of ABT-737 and HhAntag. After 48 hours, cell viability was assessed using a MTT kit (Cell Titer 96 Aqueous One Solution from Promega, Madison, WI). For Toledo and OCI-LY19 cell lines, higher number of cells (70 × 103) was needed in order to get measurable optical density readings for the MTT assay. For each cell line MTT assays were done in triplicate and repeated at least twice. For Toledo cells the cell count assay Vi-Cell viability analyzer (Beckman coulter) was also used.
Apoptosis was detected by using the Annexin V and PI staining kit (BD Biosciences PharMingen, San Jose, CA) according to the manufacturer’s instructions. Briefly, cells were plated at a density of 0.75 × 106 cells/mL in RPMI 1640 media with 2% FBS with desired concentrations of ABT-737 and HhAntag. At 48 hours post-treatment the cells were harvested and tested for apoptosis by Annexin V and PI staining. For each cell line Annexin V assays were performed in triplicate and repeated at least two times. Apoptosis was also determined by the cleavage of the polyADP-ribose polymerase 1 (PARP1) nuclear enzyme (Cell Signaling Technology, Beverly, MA). When the cells undergo apoptosis, PARP1 (116 kDa) is cleaved by caspase-3 and -7 to generate 2 fragments of 85 and 24 kDa [19].
Western blot analysis
Western blotting was performed as described previously [15]. Briefly, 0.75 × 106 cells/mL were cultured in RPMI 1640 media with 2% FBS with the desired concentrations of each drug alone or in combination (ABT-737 & HhAntag) and harvested 24 or 48 hours post-treatment. Proteins were extracted from total cell lysates and resolved on 10% to 15% SDS-polyacrylamide gel electrophoresis. The following monoclonal and polyclonal antibodies were used: BCL2, BCL-XL, BCL-W, BIM, BAX (Cell Signaling Technology) and β-actin (Sigma, St Louis, MO).
Statistical Analysis
A two-tailed paired Student’s t-test was used to evaluate the statistical significance of the changes noted in cell death after drug treatments. A p-value < 0.05 was considered to be statistically significant.
RESULTS
Combined treatments using IC20 doses of ABT-737 with increasing concentrations of HhAntag resulted in decreased cell viability in a subset of GC DLBCL cell lines
Using MTT assays, we calculated the IC20 of ABT-737 for each cell line at 48 hours. These data are shown in table 1. The IC20 of ABT-737 for the different cell lines range from 1 nM to 2.5 µM. For the co-treatment experiments with the HhAntag we chose an ABT-737 dose not higher than the IC20. Co-treatments with the fixed dose of ABT-737 (≤IC20) along with increasing concentrations of HhAntag ranging between 1.0 to 7.5 µM were performed as previously published [15, 20]. All the DLBCL cell lines used in this study express SMO and GLI1 [15]. The effect of HhAntag on the activation status of Hh signaling was determined by qRT-PCR measuring levels of expression of GLI1, the most reliable indicator of the activation status of the Hh signaling pathway. Treatments with SMO inhibitors, including HhAntag, reduced GLI1 mRNA in a concentration dependent manner [15, 20].
Table 1.
ABT-737 concentrations used for each DLBCL cell line to approximate the inhibitory concentration of 20% and 50% of cells (IC20 and IC50). The IC20 doses for each cell line were used for the combined treatments with the HhAntag.
| Cell lines | IC20 | IC50 |
|---|---|---|
| DOHH2 | 1 nM | 5 nM |
| SuDHL4 | 10 nM | 50 nM |
| OCI-LY19 | 2.5 nM | 10 nM |
| TOLEDO | 10 nM | 50 nM |
| HT | 2.5 µM | 7.5 µM |
| OCI-LY3 | 20 nM | 50 nM |
| OCI-LY10 | 5 nM | 25 nM |
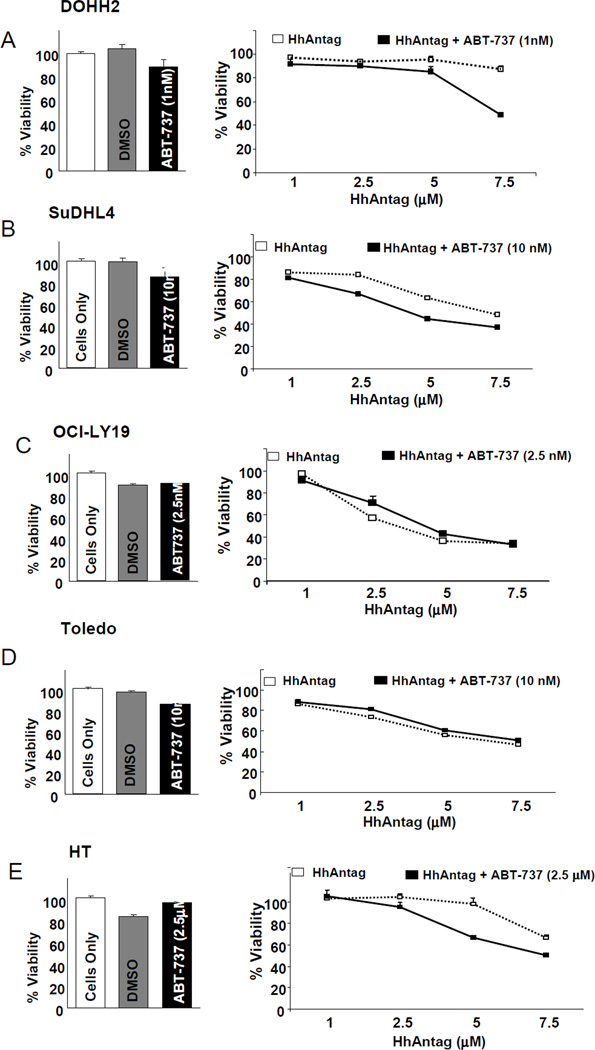
Co-treatments with ABT-737 and increasing concentrations of HhAntag for 48 hours resulted in a modest decrease in cell viability in 3 cell lines DOHH2, SuDHL4 and HT, in comparison with HhAntag alone (Figures 1A–C). In DOHH2, a decrease on cell viability was seen by adding 1nM of ABT-737 to 7.5 µM of the HhAntag. In SuDHL4 cells, a modest decrease on cell viability was seen by adding 10 nM of ABT-737 along with 2.5, 5 and 7.5 µM of the HhAntag, and, in HT cells, a decrease on cell viability was mainly seen by adding 10 nM of ABT-737 to 5 and 7.5 µM of the HhAntag. No effects on cell viability were seen combining ABT-737 with HhAntag in OCI-LY19 and Toledo (Figures 1D and E. However, these 2 cell lines have a low-metabolic activity that may decrease the sensitivity to detect differences on cell viability using MTT assays. In Toledo cells, an additional viability assay based on tryphan blue incorporation, Vi-Cell viability analyzer (Beckman coulter), was used. Using this assay, a decrease on cell viability was seen by adding 10 nM of ABT-737 to increasing concentrations of HhAntag (Supplementary figure 1).
Figure 1. Combining low-doses of ABT-737 and increasing concentrations of HhAntag resulted in decreased cell viability in 3 out of 5 GC-DLBCL cells.
MTT assays showed that the combined treatment of IC20-doses of ABT-737 with increasing concentrations of HhAntag for 48 hours resulted in modest decreased cell viability in comparison with treatments of HhAntag alone in DOHH2 (A), SuDHL4 (B), and HT cells (C). No effect on cell viability combining ABT-737 with HhAntag was seen in OCI-LY19 (D) and Toledo (E). DOHH2 and HT were the cell lines showing the lowest sensitivity to the treatments with the HhAntag alone. The minimally lethal effect of the concentrations of ABT-737 used for the combinatorial treatments is evident by the little decrease in the cell viability after treatment with ABT-737 alone for 48 hours (left panels).
Combined treatments of IC20-doses of ABT-737 with increasing concentrations of HhAntag resulted in significant increased apoptosis in GC DLBCL cell lines
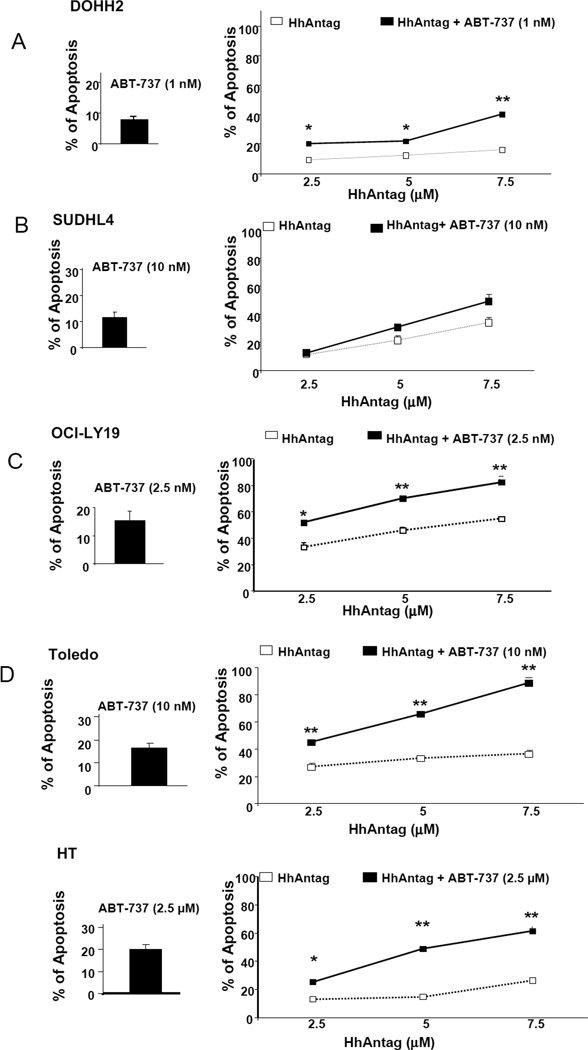
The IC20 for ABT-737 at 48 hours for each cell line was calculated using Annexin V and PI assays and these doses were used for the combined treatments with the HhAntag. As shown in figure 2, co-treatments with IC20 doses of ABT-737 along with increasing concentrations of the HhAntag for 48 hours resulted in a statistically significant increase of apoptosis in 4 out of 5 cell lines analyzed (Table 2 and Figures 2A to E).
Figure 2. Combining low-doses of ABT-737 and increasing concentrations of HhAntag resulted in increased apoptosis in GC-DLBCL cells.
Annexin V and PI labeling assay showed that the combined treatments of fixed IC20 doses of ABT-737 and increasing concentrations of the HhAntag resulted in a statistically significant increase of apoptosis in comparison to treatments with HhAntag alone in 4 of 5 cell lines, DOHH2 (A), HT (C), OCI-Ly19 (D) and Toledo (E). A significant increased in apoptosis combining ABT-737 with HhAntag was not detected SuDHL4 (B). Similarly that with the MTT assays, DOHH2 and HT were the cell lines showing the lowest sensitivity to the treatments with the HhAntag alone. The percentage of apoptosis induced by the concentrations of ABT-737 used for the combinatorial treatments is shown in the left panels (all of them were below 20%). * p<0.05; **p<0.01.
Table 2.
Summary of the apoptotic rates detected for each DLBCL cell line as result of the treatment with HhAntag alone and with the combination of IC20 doses of ABT-737 with increasing concentrations of HhAntag.
| Cell Type | Doses of “Hh- Antagonist” (µM) |
% of Apoptosis “Hh-Antagonist” Alone |
% of Apoptosis “Hh-Antagonist” + ABT-737 |
p-value |
|---|---|---|---|---|
| DOHH2 | 2.5 | 9.3 | 20.4 | 0.011 |
| 5.0 | 12.3 | 22 | 0.017 | |
| 7.5 | 16.2 | 40.4 | 0.009 | |
| SuDHL4 | 2.5 | 11.5 | 12.8 | 0.7 |
| 5.0 | 21.8 | 31.1 | 0.1 | |
| 7.5 | 34.5 | 49.5 | 0.119 | |
| HT | 2.5 | 12.8 | 25.4 | 0.008 |
| 5.0 | 14.7 | 48.9 | 0.002 | |
| 7.5 | 26.4 | 61.7 | 0.011 | |
| OCI-LY19 | 2.5 | 33.2 | 46 | 0.011 |
| 5.0 | 46 | 70.5 | 0.001 | |
| 7.5 | 55 | 82.3 | 0.002 | |
| TOLEDO | 2.5 | 27 | 45.4 | 0.001 |
| 5.0 | 33.4 | 66 | 0.002 | |
| 7.5 | 36.8 | 89.4 | 0.001 | |
| OCI-LY3 | 2.5 | 35.5 | 51.2 | 0.053 |
| 5.0 | 58.8 | 65.8 | 0.125 | |
| 7.5 | 73.5 | 77.1 | 0.280 | |
| OCI-LY10 | 1.0 | 12.2 | 15.0 | 0.246 |
| 2.5 | 34.1 | 55.7 | 0.015 | |
| 5.0 | 79.5 | 88.6 | 0.074 |
When DOHH2 cells were treated with 1 nM of ABT-737 only 7% of the cells were apoptotic. However, adding 1 nM of ABT-737 to 2.5 and 7.5 µM of HhAntag resulted in at least 2 fold increased in the apoptotic rate compared with the same concentrations of the SMO inhibitor alone. In HT cells adding 2.5 µM of ABT-737 to 5µM HhAntag resulted in a 3-fold increase of the apoptotic rate. In OC-LY19 cells, combining 2.5 nM of ABT-737 with 5 µM of HhAntag resulted in 1.5 fold increased in the apoptotic rate with an apoptosis rate of 70%. Toledo was the cell line more sensitive to the addition of ABT-737 to the HhAntag as adding 10 nM ABT-737 to 5 µM or 7.5 µM of HhAntag resulted in at least 2 fold increased in the apoptotic rate and an apoptotic rate of 66% and 89.4%, respectively. In SUDHL4 cells adding 10nM of ABT-737 to increasing concentrations of HhAntag resulted in slight increase of the apoptosis rate but these differences were not statistically significant.
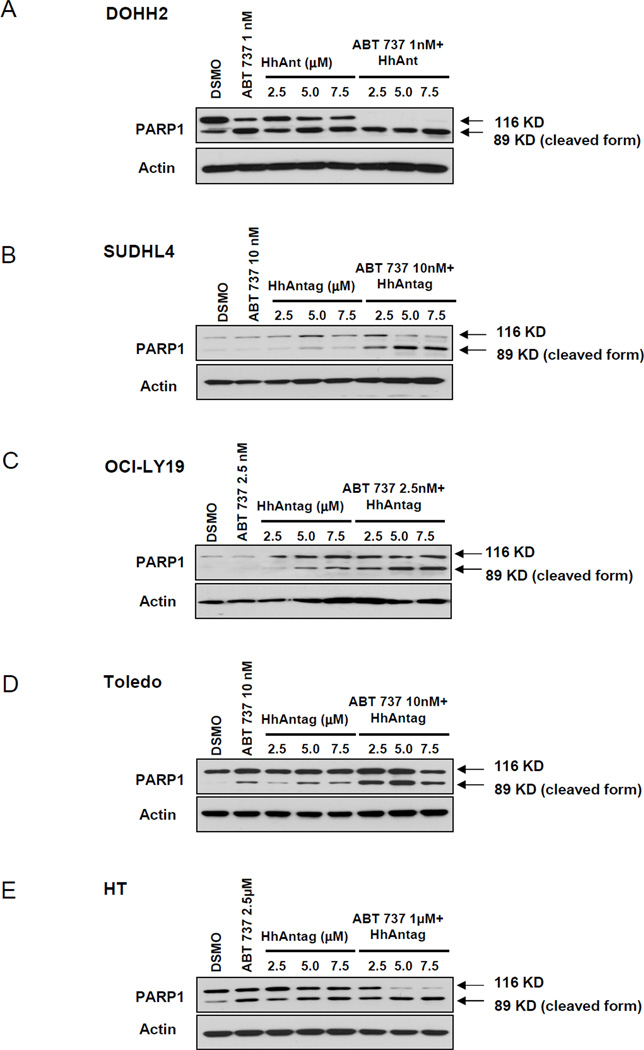
The increased apoptotic rate consequence of combining low-doses of ABT-737 with HhAntag was further supported by the increased cleavage of PARP1 seen with the drug combination in comparison to the cleavage of PARP1 obtained with each drug alone (Figures 3A–E). Altogether, these data show that combining a SMO inhibitor with low-doses of a functional BCL2 inhibitor, significantly increase the apoptotic ratio in most of GC DLBCL cells.
Figure 3. Combining low-doses of ABT-737 and increasing concentrations of HhAntag resulted in increased cleavage of PARP1, indication of increase of susceptibility of apoptosis in all DLBCL cell lines.
Combined treatment with ABT-737 and HhAntag resulted in increased susceptibility to apoptosis in comparison to the treatments with either inhibitor alone as determined by increased cleavage of PARP1 resulting in increase of the cleaved form (89 kDa). In DOHH2 cells (A) complete cleavage of PARP1 was observed on combined treatments, whereas in SUDHL4 (B), HT (C), OCI-LY19 (D) and Toledo (E), there is an increase of cleaved PARP1 in the three different combined treatment conditions tested in comparison with the HhAntag alone.
Effect of the HhAntag treatment on the expression levels of BCL2 family members on GC DLBCL cell lines
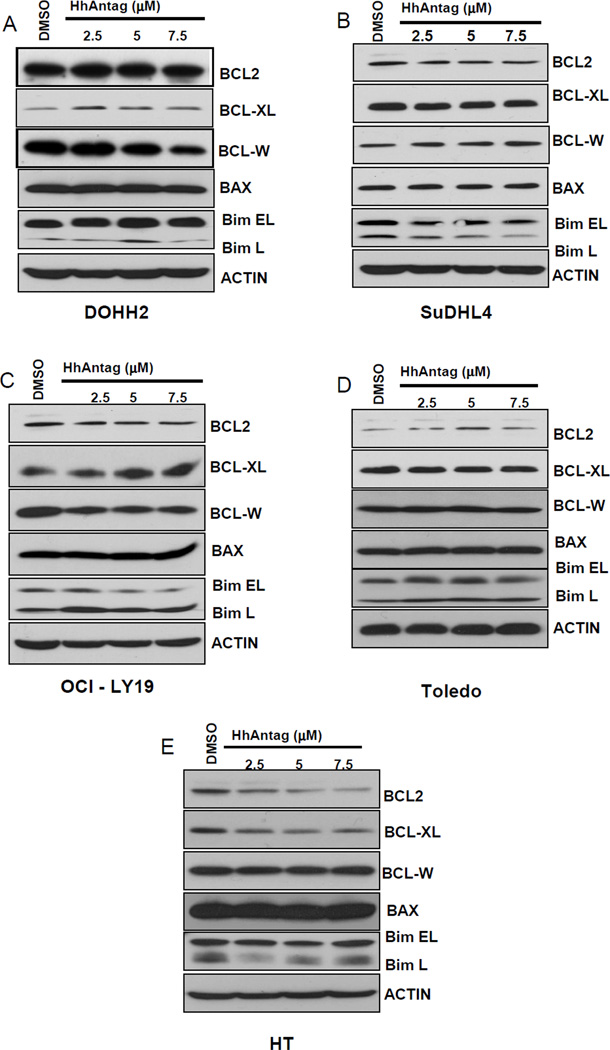
We investigated if differences in the expression of the pro-survival BCL2-like proteins BCL2, BCL-XL, and BCL-W and pro-apoptotic relatives BIM and BAX may explain the baseline susceptibility and differences in the apoptotic rate observed among the different GC DLBCL cell lines after treatment with HhAntag. As shown in figure 4A, variable baseline expression levels of the pro-survival proteins BCL2, BCL-XL and BCL-W and pro-apoptotic proteins BIM and BAX were seen in all GC DLBCL cell lines. DOHH2 was the cell line with the highest expression levels of BCL2 and BCL-W and the cell line showing the lowest sensitivity to HhAntag (only ~16% of the cells were apoptotic when treated with the highest concentration of HhAntag). Toledo was the cell line with the lowest baseline expression of BCL2 and one of the cell lines more sensitive to apoptosis induced the combination of ABT-737 with HhAntag.
Figure 4. Treatment with increasing concentrations of HhAntag resulted in no changes in the protein levels of BCL2 in those DLBCL cell lines with the t(14;18) but induced a concentration-dependent decrease of BCL2 protein in HT cells, GC cells without the t(14;18).
The basal expression levels of the anti-apoptotic (BCL2, BCL-XL and BCL-W) and pro-apoptotic (BAX, BIM) in the 5 GC-DLBCL cell lines used in the study are shown (A). DOHH2 was the cell line was the highest levels of BCL2 expression and the cell line with the less sensitivity for the SMO inhibitor. Treatment with increasing concentrations of HhAntag resulted in no significant changes in the protein levels of BCL2 in cell lines with the t(14;18) (DOHH2, SuDHL4, OCI-LY19, and Toledo) (B–E). However, treatments with increasing concentrations of HhAntag resulted in decrease in the protein levels of BCL2 in HT cells (F), cells without the t(14;18). No significant changes in the protein expression levels of the anti-apoptotic (BCL-XL and BCL-W) or the pro-apoptotic (BAX, BIM) BCL2 family members were seen after treatments with all concentrations of HhAntag.
We found that increasing concentrations of HhAntag did not significantly decreased the expression levels of BCL2 in GC DLBCL cell lines carrying the t(14;18) (DOHH2, SuDHL-4, OCI-LY19, and Toledo) (Figures 4B–F). However, treatments with HhAntag in HT-cells, GC DLBCL cells without the t(14;18), resulted in decreased of BCL2 protein levels in a concentration dependent manner. These findings support the concept that the presence of the t(14;18) may be one factor that explain the lack of modulation of BCL2 after Hh signaling inhibition [15].
Combined treatments of IC20-doses of ABT-737 with increasing concentrations of HhAntag and effect of the HhAntag treatment on the expression levels of BCL2 family members on ABC DLBCL cell lines
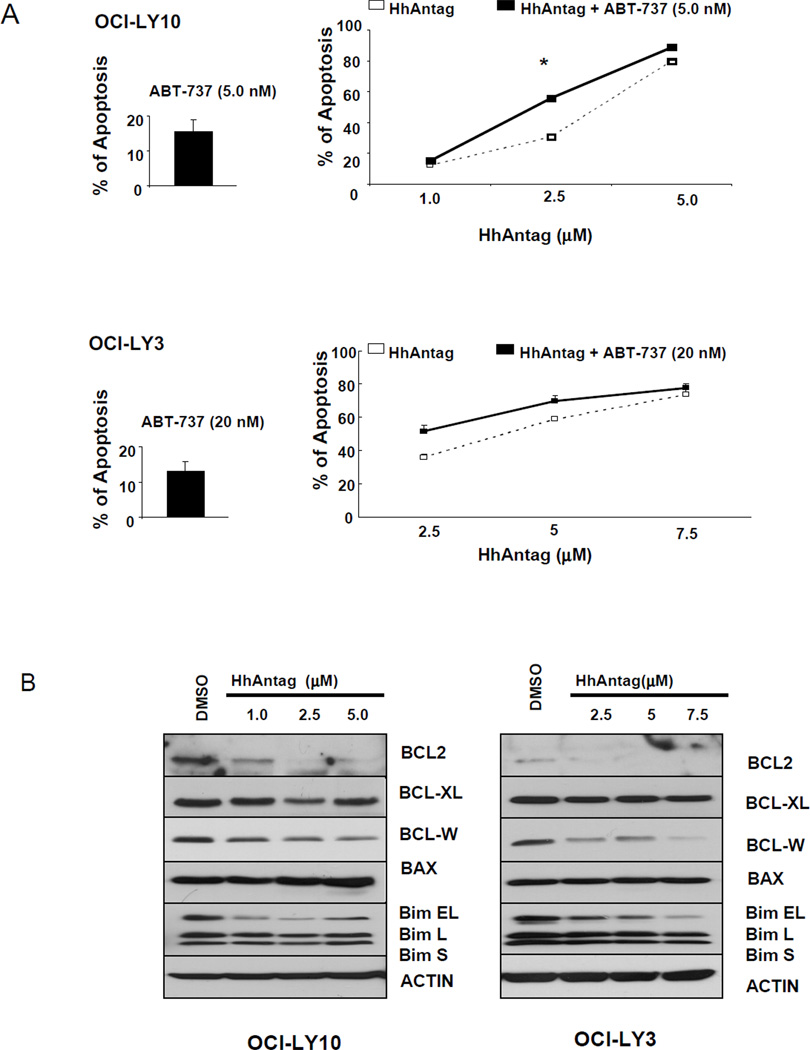
The IC20 for ABT-737 at 48 hours for OCI-LY10 and OCI-LY3 (ABC DLBCL cell lines) was calculated using Annexin V and PI assays and these doses were used for the combined treatments with HhAntag. As previously reported for cyclopamine-KAAD [15], we noticed that ABC DLBCL cells were more sensitive to HhAntag than GC DLBCL cells as 5 µM of HhAntag resulted in almost 80% of apoptosis in OCI-LY10 and in almost 60% in OCI-LY3 (Figure 5A) In these 2 ABC DLBCL cell lines, adding small doses (<IC20) of ABT-737 to increasing concentrations of HhAntag resulted in slight increase of the apoptosis rate but these differences (although statistically significant at the dose of 2.5 µM for OCI-LY10; p=0.015) were less pronounced than those seen in GC DLBCL cells (Figure 5A).
Figure 5. ABC DLBCL cells lines were more sensitive to HhAntag than GC DLBCL cells. HhAntag induced a concentration-dependent decrease of the anti-apoptotic proteins BCL2 and BCL-W in ABC DLBCL cells.
Annexin V and PI labeling assays showed that the beneficial effect of adding fixed IC20 doses of ABT-737 to increasing concentration of HhAntag on the percentage of apoptosis was less pronounced in OCI-LY10 and OCI-LY3 cells than that seen in GC DLBCL cells (A). In contrast to those GC DLBCL cell lines with the t(14;18), treatment with increasing concentrations of HhAntag resulted in decrease of the expression levels of BCL2 and BCL-W in ABC DLBCL cell lines. In contrast to the GC DLBCL cell lines, OCI-LY3 and OCI-LY10 show the shortest form of BIM (BIM S). Three major isoforms of BIM are generated by alternative splicing: BIM EL, BIM L and BIM S [28].
We previously have reported that the SMO inhibitor, cyclopamine-KAAD, decrease the mRNA and protein expression levels of BCL2 in ABC DLBCL cell lines but not in those of GC type with the t(14;18) [15]. Here, we also found that increasing concentrations of HhAntag decrease the expression levels of the prosurvival proteins BCL2 and BCL-W in OCI-LY10 and OCI-LY3 cell lines (Figure 5B). The decrease in the expression of BCL2 in DLBCL of ABC type is not an unexpected finding as Hh signaling is a direct transcriptional regulator of BCL2 expression [17, 18]. BCL-W (BCL2-like 2; BCL2L2) is a prosurvival protein located at chromosome 14. The reason of the modulation in the expression of this gene after treatment with HhAntag is unclear. If BC2L2 gene is a downstream target of Hh signaling is unknown at this time.
Altogether, these data support the concept that the resistance to apoptosis observed in most of the GC DLBCL cell lines to SMO inhibitors is determined, at least in part, by the expression of functional BCL2 family members and that the susceptibility of ABC DLBCL cells to apoptosis after SMO inhibitors is related to the decreased expression of prosurvival proteins.
DISCUSSION
A large number of cancers have been shown to have Hh pathway activation. Recently, we have found that Hh signaling is active and functional in DLBCL and that pharmacologic inhibition of Hh pathway induced cell cycle arrest in GC DLBCL cell lines and mainly apoptosis in those of ABC type [15]. However, the reasons for these differences are not clear.
Based on these previous findings, we sought to investigate if functional inhibition of BCL2 may increase apoptosis-induced by a Hh pathway inhibitor in GC DLBCL cell lines. For this, we used 5 GC DLBCL cell lines and SMO and BCL2 inhibitors developed by Genentech and Abbott, respectively. The 5 DLBCL cell lines used in this study express SMO and treatments with increasing concentrations of HhAntg resulted in a concentration dependent decrease of GLI1 mRNA levels indicating inhibition of Hh signaling. We have also shown expression of GLI1 and SMO in DLBCL tumors and provided evidence that the effects of SMO inhibitors in DLBCL are due to inhibition of Hh signaling pathway and not exclusively due to off-target effects [15, 16, 20].
We found that combining low-doses of ABT-737 with HhAntag resulted in a significant increase of apoptosis in comparison with treatments with HhAntag alone. Our data support that the functionality of BCL2 family proteins is an important factor in determining the susceptibility to apoptosis induced by a Hh pathway inhibitor in DLBCL cell lines.
There were some discrepancies in the results obtained by the MTT assay and by the apoptotic assays, Annexin V and PARP1. MTT assays are based on the conversion of the tretrazolium salt, MTT, into fromazan crystals by living cells, which are analyzed colorimetrically to determine mitochondrial activity [21]. Toledo and OCI-LY19 cell lines demonstrated consistently a very low baseline metabolic activity as we had to use high number of cells to get measurable optical density readings. We think that the baseline low-metabolic activity of these 2 cell lines contributes to explain the low sensitivity of the MTT assays to detect differences in cell viability between treatments. In Toledo cells, using a viability assay based on tryphan blue incorporation, Vi-Cell viability analyzer (Beckman coulter), we confirmed the significant effect on cell death of combining HhAntag with ABT-737 observed by Annexin and PI assays (Supplementary figure 1).
Several studies have highlighted the importance of BCL2 in the tolerance to apoptosis against Hh inhibitors and described that inhibition of Hh signaling decreased the expression of BCL2 in chronic myeloid leukemia [22], low-grade B-cell lymphomas/plasma cell myeloma [23], and pancreatic cancer [24] among others. Dierks et al [23] showed that Hh pathway inhibition downregulates BCL2 expression in stroma-dependant lymphoma cells whereas overexpression of BCL2 rescues lymphoma cells from cyclopamine-induced apoptosis. The modulation of BCL2 expression by the activation status of Hh signaling is an expected finding as BCL2 is a direct downstream target of Hh pathway. Bigelow et al and Regl et al, were the first in establishing a direct link between Hh and BCL2 expression, showing that the Hhrelated transcription factors GLI1 and GLI2, but not GLI3, were able to activate the BCL2 promoter and that one important consequence of the deregulation of Hh signaling in basal cell carcinomas of the skin was the up-regulation of BCL2 [17, 18].
In a previous study, we found that Hh pathway inhibition with cyclopamine-KAAD induced decrease of BCL2 expression in ABC DLBCL cell lines but not in those of GC type [15]. These findings were also confirmed in the current study using HhAntag in 2 ABC DLBCL cell lines (OCI-LY10 and OCI-LY3). We hypothesize that one factor that may explain the differences seen in the modulation of BCL2 expression after Hh pathway inhibition between GC and ABC DLBCL cells could be the presence of the t(14;18). The rationale behind this hypothesis is that in the absence of the t(14;18), BCL2 expression is controlled by the BCL2 promoter and its transcriptional regulatory elements, including GLI1. However, in the presence of the t(14;18), one of the BCL2 gene moves from chromosomal 18 to chromosomal 14 to be transcriptionally overexpressed and controlled by the regulatory elements of the IgH promoter independently of GLI1 [25]. Supporting this interpretation, we found that in HT-cells [GC DLBCL cells without the t(14;18)] and in ABC DLBCL cell lines [cells without t(14;18)], increasing concentrations of HhAntag decreased, in a concentration-dependent manner, the expression levels of BCL2. Modulation levels of BCL2 expression by HhAntag was not seen in cell lines with the t(14;18). However, additional factors may be also involved. The presence of somatic mutations of the BCL2 promoter, that frequently occur in follicular lymphoma or GC DLBCL [26], may also prevent the control of the regulation of BCL2 expression by its regulatory elements and to contribute to explain the lack of the effect of inhibitors of Hh pathway on BCL2 expression [27].
In summary, combining an Hh signaling inhibitor with low-doses of ABT-737 resulted in increased apoptosis in most of the DLBCL cells of GC type. Our data support that the functionality of BCL2 family members is a factor in determining susceptibility to apoptosis induced by SMO inhibitors. These findings provide a rationale to explore the use of combining both inhibitors as adjuvant therapeutic agents to increase chemotherapy susceptibility to current or future protocols for GC DLBCL.
Supplementary Material
Acknowledgements
This work was supported by funds from The Translational Grant of The Leukemia & Lymphoma Society (to RS and FV), and K08 Physician-Scientist Award 1 K08 CA143151-01 (NIH) (to FV), and SPORE Lymphoma Grant 1P50CA136411-01A1 (to FV).
Footnotes
Competing Interests: The authors have declared that no competing interest exist.
REFERENCES
- 1.Armitage JO, Weisenburger DD. New approach to classifying non-Hodgkin's lymphomas: clinical features of the major histologic subtypes. Non-Hodgkin's Lymphoma Classification Project. J Clin Oncol. 1998;16:2780–2795. doi: 10.1200/JCO.1998.16.8.2780. [DOI] [PubMed] [Google Scholar]
- 2.Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–511. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 3.Rosenwald A, Wright G, Chan WC, Connors JM, Campo E, Fisher RI, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:1937–1947. doi: 10.1056/NEJMoa012914. [DOI] [PubMed] [Google Scholar]
- 4.Savage KJ, Monti S, Kutok JL, Cattoretti G, Neuberg D, De Leval L, et al. The molecular signature of mediastinal large B-cell lymphoma differs from that of other diffuse large B-cell lymphomas and shares features with classical Hodgkin lymphoma. Blood. 2003;102:3871–3879. doi: 10.1182/blood-2003-06-1841. [DOI] [PubMed] [Google Scholar]
- 5.Fu K, Weisenburger DD, Choi WW, Perry KD, Smith LM, Shi X, et al. Addition of rituximab to standard chemotherapy improves the survival of both the germinal center B-cell-like and non-germinal center B-cell-like subtypes of diffuse large B-cell lymphoma. J Clin Oncol. 2008;26:4587–4594. doi: 10.1200/JCO.2007.15.9277. [DOI] [PubMed] [Google Scholar]
- 6.Coiffier B. Treatment of diffuse large B-cell lymphoma. Curr Hematol Rep. 2005;4:7–14. [PubMed] [Google Scholar]
- 7.Zhang H, Nimmer PM, Tahir SK, Chen J, Fryer RM, Hahn KR, et al. Bcl-2 family proteins are essential for platelet survival. Cell Death Differ. 2007;14:943–951. doi: 10.1038/sj.cdd.4402081. [DOI] [PubMed] [Google Scholar]
- 8.Yunis JJ, Frizzera G, Oken MM, McKenna J, Theologides A, Arnesen M. Multiple recurrent genomic defects in follicular lymphoma. A possible model for cancer. N Engl J Med. 1987;316:79–84. doi: 10.1056/NEJM198701083160204. [DOI] [PubMed] [Google Scholar]
- 9.Yang E, Korsmeyer SJ. Molecular thanatopsis: a discourse on the BCL2 family and cell death. Blood. 1996;88:386–401. [PubMed] [Google Scholar]
- 10.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2005;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 11.Fesik SW. Promoting apoptosis as a strategy for cancer drug discovery. Nat Rev Cancer. 2005;5:876–885. doi: 10.1038/nrc1736. [DOI] [PubMed] [Google Scholar]
- 12.Mason KD, Khaw SL, Rayeroux KC, Chew E, Lee EF, Fairlie WD, et al. The BH3 mimetic compound, ABT-737, synergizes with a range of cytotoxic chemotherapy agents in chronic lymphocytic leukemia. Leukemia. 2009;23:2034–2041. doi: 10.1038/leu.2009.151. [DOI] [PubMed] [Google Scholar]
- 13.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 14.van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10:389–399. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh RR, Kim JE, Davuluri Y, Drakos E, Cho-Vega JH, Amin HM, et al. Hedgehog signaling pathway is activated in diffuse large B-cell lymphoma and contributes to tumor cell survival and proliferation. Leukemia. 2010;24:1025–1036. doi: 10.1038/leu.2010.35. [DOI] [PubMed] [Google Scholar]
- 16.Kim JE, Singh RR, Cho-Vega JH, Drakos E, Davuluri Y, Khokhar FA, et al. Sonic hedgehog signaling proteins and ATP-binding cassette G2 are aberrantly expressed in diffuse large B-cell lymphoma. Mod Pathol. 2009;22:1312–1320. doi: 10.1038/modpathol.2009.98. [DOI] [PubMed] [Google Scholar]
- 17.Bigelow RL, Chari NS, Unden AB, Spurgers KB, Lee S, Roop DR, et al. Transcriptional regulation of bcl-2 mediated by the sonic hedgehog signaling pathway through gli-1. J Biol Chem. 2004;279:1197–1205. doi: 10.1074/jbc.M310589200. [DOI] [PubMed] [Google Scholar]
- 18.Regl G, Kasper M, Schnidar H, Eichberger T, Neill GW, Philpott MP, et al. Activation of the BCL2 promoter in response to Hedgehog/GLI signal transduction is predominantly mediated by GLI2. Cancer Res. 2004;64:7724–7731. doi: 10.1158/0008-5472.CAN-04-1085. [DOI] [PubMed] [Google Scholar]
- 19.Lazebnik YA, Kaufmann SH, Desnoyers S, Poirier GG, Earnshaw WC. Cleavage of poly(ADP-ribose) polymerase by a proteinase with properties like ICE. Nature. 1994;371:346–347. doi: 10.1038/371346a0. [DOI] [PubMed] [Google Scholar]
- 20.Singh RR, Kunkalla K, Qu C, Schlette E, Neelapu SS, Samaniego F, et al. ABCG2 is a direct transcriptional target of hedgehog signaling and involved in stroma-induced drug tolerance in diffuse large B-cell lymphoma. Oncogene. 2011;30:4874–4886. doi: 10.1038/onc.2011.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Meerloo J, Kaspers GJ, Cloos J. Cell sensitivity assays: the MTT assay. Methods Mol Biol. 2011;731:237–245. doi: 10.1007/978-1-61779-080-5_20. [DOI] [PubMed] [Google Scholar]
- 22.Su W, Meng F, Huang L, Zheng M, Liu W, Sun H. Sonic hedgehog maintains survival and growth of chronic myeloid leukemia progenitor cells through beta-catenin signaling. Exp Hematol. 2012;40:418–427. doi: 10.1016/j.exphem.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Dierks C, Grbic J, Zirlik K, Beigi R, Englund NP, Guo GR, et al. Essential role of stromally induced hedgehog signaling in B-cell malignancies. Nat Med. 2007;13:944–951. doi: 10.1038/nm1614. [DOI] [PubMed] [Google Scholar]
- 24.Singh BN, Fu J, Srivastava RK, Shankar S. Hedgehog signaling antagonist GDC-0449 (Vismodegib) inhibits pancreatic cancer stem cell characteristics: molecular mechanisms. PLoS One. 2011;6:e27306. doi: 10.1371/journal.pone.0027306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Graninger WB, Seto M, Boutain B, Goldman P, Korsmeyer SJ. Expression of Bcl-2 and Bcl-2-Ig fusion transcripts in normal and neoplastic cells. J Clin Invest. 1987;80:1512–1515. doi: 10.1172/JCI113235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schuetz JM, Johnson NA, Morin RD, Scott DW, Tan K, Ben-Nierah S, et al. BCL2 mutations in diffuse large B-cell lymphoma. Leukemia. 2012;26:1383–1390. doi: 10.1038/leu.2011.378. [DOI] [PubMed] [Google Scholar]
- 27.Saito M, Novak U, Piovan E, Basso K, Sumazin P, Schneider C, et al. BCL6 suppression of BCL2 via Miz1 and its disruption in diffuse large B cell lymphoma. P Natl Acad Sci USA. 2009;106:11294–11299. doi: 10.1073/pnas.0903854106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Connor L, Strasser A, O'Reilly LA, Hausmann G, Adams JM, Cory S, et al. Bim: a novel member of the BCL-2 family that promotes apoptosis. EMBO J. 1998;17:384–395. doi: 10.1093/emboj/17.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.