Abstract
Metabolites of tobacco smoke constituents can be quantified in urine and other body fluids providing a realistic measure of carcinogen and toxicant dose in a smoker. Many previous studies have demonstrated that these metabolites – referred to as biomarkers in this paper – are related to tobacco smoke exposure. The studies reviewed here were designed to answer another question: are these substances also biomarkers of cancer risk? Using a prospective study design comparing biomarker levels in cancer cases and controls, all of whom were smokers, the results demonstrate that several of these biomarkers – total cotinine, total 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL), r-1-,t-2,3,c-4-tetrahydroxy-1,2,3,4-tetrahydrophenanthrene (PheT), and total N’-nitrosonornicotine (NNN) - are biomarkers of cancer risk. Therefore, these biomarkers have the potential to become part of a cancer risk prediction algorithm for smokers.
Keywords: tobacco smoke, biomarkers, cotinine, NNAL, PheT, NNN
1. Introduction
Tobacco products are responsible for 22% of all cancer death worldwide and 30% of cancer mortality in the United States http://www.who.int/mediacentre/factsheets/fs297/en/index.html [1]. At least eighteen different types of cancer are caused to varying extents by tobacco products [2]. This paper focuses on lung and esophageal cancer. Approximately 90% of lung cancer mortality in populations with prolonged use is attributed to cigarette smoking [3]. More than 3000 people in the world succumb to this deadly and relatively incurable disease daily [4]. Cigarette smoking is also an important cause of esophageal cancer, both squamous cell carcinoma and adenocarcinoma [3].
In addition to the addictive but non-carcinogenic compound nicotine, cigarette smoke contains over 70 established carcinogens, including tobacco-specific nitrosamines, polycyclic aromatic hydrocarbons (PAH), and volatile carcinogens such as 1,3-butadiene and benzene [5]. There is no doubt that daily exposure to these compounds is a major cause of cancer in smokers. Professor Ramesh Gupta has made important contributions to our understanding of the relevant DNA damage mechanisms [6-9]. However, we have little ability to predict which of the 1.3 billion smokers in the world will actually get cancer. It is axiomatic in toxicology that “the dose makes the poison” and therefore it is logical that susceptibility to cancer in smokers is a function of carcinogen dose. Tobacco carcinogen and toxicant biomarkers – quantitative measures of tobacco constituents or their metabolites in body fluids – can inform us about dose much more accurately than counting numbers of cigarettes smoked, even if that information could be reliably obtained [10]. This is because there are wide differences in carcinogen and toxicant delivery among different tobacco products depending on their characteristics and the ways in which they are smoked. In the studies reviewed here, we focused on a panel of tobacco carcinogen and toxicant biomarkers representing some of the most important compounds in cigarette smoke.
The structures of the tobacco carcinogen and toxicant biomarkers considered here are shown in Figure 1 [10]. Cotinine and cotinine-N-glucuronide are metabolites of nicotine; their sum is “total cotinine.” Total cotinine is a reliable measure of uptake of the addictive compound nicotine. 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) and its glucuronides are metabolites of the potent tobacco-specific lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK); their sum is “total NNAL.” Total NNAL is a well established biomarker of NNK uptake. NNN-N-glucuronide is a metabolite of the tobacco-specific esophageal and oral cavity carcinogen N’-nitrosonornicotine (NNN); values for each of these are reported. r-1,t-2,3,c-4-Tetrahydroxy-1,2,3,4-tetrahydrophenanthrene (PheT) is a metabolite of phenanthrene, structurally related to carcinogenic PAH [11]. This metabolite is formed by the diol epoxide metabolism pathway, believed to be important in the metabolic activation to DNA binding intermediates of carcinogenic PAH. The mercapturic acids shown in Figure 1 are metabolites of the tobacco smoke volatiles 1,3-butadiene, ethylene oxide, benzene, acrolein, and crotonaldehyde, respectively [12]. 1,3-Butadiene, ethylene oxide, and benzene are considered carcinogenic to humans by the International Agency for Research on Cancer, based mainly on occupational studies and mechanistic data related to hematopoietic malignancies [13;14]. Acrolein is a highly toxic but marginally carcinogenic compound while crotonaldehyde is a relatively weak hepatocarcinogen [15]. Many studies have shown that concentrations of all of the biomarkers discussed here are higher in the urine and other body fluids of smokers than non-smokers and that in most cases their levels significantly decrease upon cessation of smoking [10]. Therefore, these substances are clearly biomarkers of tobacco smoke exposure and dose. But are they also biomarkers of cancer risk? We addressed this question in the studies reviewed here by quantifying tobacco carcinogen and toxicant biomarkers in pre-diagnostic urine samples from the Shanghai Cohort Study [16-20].
Fig. 1.
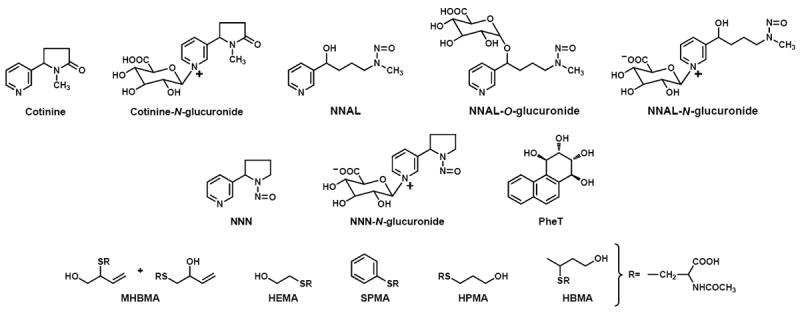
Chemical structures of the tobacco carcinogen and toxicant biomarkers discussed in the text.
2. Approach
The Shanghai Cohort Study enrolled 18,244 men between January 1, 1986 and September 30, 1989 [20;21]. They were between 45 and 64 years old and lived in one of four geographically defined communities in Shanghai, China. In-person interviews were conducted and a urine sample was obtained from each subject upon enrollment. Cases of lung cancer or esophageal cancer were identified annually by in-person re-interviews of all surviving cohort members and review of reports from the Shanghai Cancer Registry and the Shanghai Municipal Vital Statistics Office. For the lung cancer studies, we identified cases who smoked cigarettes at baseline and then randomly selected one control subject for each case from all cohort members who were current smokers at enrollment, free of cancer, and alive at the time of cancer diagnosis of the index case. Controls were matched to the index case by age at enrollment, date of urine collection, and neighborhood of residence at recruitment. The selection of controls was the same for the esophageal cancer study, except that 3 control subjects were randomly selected for each case in order to increase statistical power due to the smaller number of esophageal cancer than lung cancer cases.
Urine samples were retrieved from the biospecimen bank and analyzed for the following tobacco smoke carcinogen and toxicant biomarkers (Figure 1): total cotinine (the sum of cotinine and cotinine-N-glucuronide); total NNAL (the sum of NNAL, NNAL-O-glucuronide and NNAL-N-glucuronide); PheT; free NNN and total NNN (the sum of free NNN and NNN-N-glucuronide); 1-hydroxy-2-(N-acetylcysteinyl)-3-butene and 1-(N-acetylcysteinyl)-2-hydroxy-3-butene (collectively called MHBMA for monohydroxybutyl mercapturic acid, metabolites of 1,3-butadiene); 2-hydroxyethyl mercapturic acid (HEMA), a metabolite of ethylene oxide; S-phenyl mercapturic acid (SPMA), a metabolite of benzene; 3-hydroxypropyl mercapturic acid (HPMA), a metabolite of acrolein; and 4-hydroxybut-2-yl mercapturic acid (HBMA), a metabolite of crotonaldehyde. The analyses were carried out as previously described [10;16-19].
For total cotinine, total NNAL, and PheT there were urine samples from 476 lung cancer cases and 476 controls. Due to sample depletion, there were 392 urine samples from lung cancer cases and 343 from controls for measurement of mercapturic acids. For total NNN, there were urine samples from 74 esophageal cancer cases and 218 from controls.
3. Results
Urinary levels of all biomarkers increased significantly with numbers of cigarettes smoked per day. Furthermore, levels of all urinary biomarkers were significantly higher in cancer cases than in controls.
The results of the lung cancer study are summarized in Table 1. Total cotinine, total NNAL, and PheT were all independently and significantly associated with lung cancer risk, even after adjustment for number of cigarettes smoked per day, number of years of smoking, and the other two biomarkers (e.g., results for total cotinine adjusted for total NNAL and PheT). The odds ratios in the highest versus the lowest tertile in the adjusted models were 2.97 for total cotinine, 1.67 for total NNAL, and 1.69 for PheT. The trend for increasing odds ratios with increasing tertile of biomarker were statistically significant in all cases, P<0.0001 for total cotinine, P = 0.0436 for total NNAL, and P = 0.0157 for PheT.
Table 1.
Urinary levels of tobacco carcinogen and toxicant biomarkers in relation to risk of lung cancer in current smokers, the Shanghai Cohort Study
| Levels of urinary biomarkers in tertiles
|
P for trend | |||
|---|---|---|---|---|
| 1st tertile | 2nd tertile | 3rd tertile | ||
| Total cotinine (nmol/mg creatinine) | <5.58 | 5.58 – 13.35 | >13.35 | |
| No. Controls/No. Cases | 158/47 | 161/146 | 157/283 | |
| Smoking-Adjusted OR (95% CI) 1 | 1.00 | 2.33 (1.52 – 3.57) | 3.70 (2.42 – 5.65) | <0.0001 |
| Biomarkers-Adjusted OR (95% CI) 2 | 1.00 | 2.05 (1.33 – 3.18) | 2.97 (1.89 – 4.65) | <0.0001 |
| Total NNAL (pmol/mg creatinine) | <0.14 | 0.14 – 0.28 | >0.28 | |
| No. Controls/No. Cases | 158/76 | 161/166 | 157/234 | |
| Smoking-Adjusted OR (95% CI) 1 | 1.00 | 1.89 (1.24 – 2.87) | 2.60 (1.68 – 4.04) | <0.0001 |
| Biomarkers-Adjusted OR (95% CI) 2 | 1.00 | 1.46 (0.94 – 2.27) | 1.67 (1.04 – 2.69) | 0.0436 |
| Total PheT (pmol/mg creatinine) | <21.7 | 21.7 – 35.3 | >35.3 | |
| No. Controls/No. Cases | 158/121 | 161/151 | 157/204 | |
| Smoking-Adjusted OR (95% CI) 1 | 1.00 | 1.37 (0.94 – 1.98) | 2.01 (1.34 – 3.02) | 0.0008 |
| Biomarkers-Adjusted OR (95% CI) 2 | 1.00 | 1.23 (0.83 – 1.82) | 1.69 (1.10 – 2.60) | 0.0157 |
| MHBMA (pmol/mg creatinine) | <5.53 | 5.53 – 15.39 | >15.39 | |
| No. Controls/No. Cases | 130/86 | 133/111 | 129/146 | |
| Smoking-Adjusted OR (95% CI) 3 | 1.00 | 1.14 (0.77 – 1.68) | 1.44 (0.98 – 2.11) | 0.0582 |
| Cotinine-Adjusted OR (95% CI) 4 | 1.00 | 0.87 (0.58 – 1.32) | 0.97 (0.64 – 1.46) | 0.9331 |
| HEMA (pmol/mg creatinine) | <11.1 | 11.1 – 22.6 | >22.6 | |
| No. Controls/No. Cases | 130/73 | 133/114 | 129/156 | |
| Smoking-Adjusted OR (95% CI) 3 | 1.00 | 1.32 (0.88 – 1.97) | 1.81 (1.22 – 2.68) | 0.0029 |
| Cotinine-Adjusted OR (95% CI) 4 | 1.00 | 1.01 (0.66 – 1.56) | 1.05 (0.67 – 1.64) | 0.8248 |
| SPMA (pmol/mg creatinine) | <1.65 | 1.65 – 3.56 | >3.56 | |
| No. Controls/No. Cases | 130/78 | 133/115 | 129/150 | |
| Smoking-Adjusted OR (95% CI) 3 | 1.00 | 1.23 (0.84 – 1.85) | 1.59 (1.08 – 2.34) | 0.0184 |
| Cotinine-Adjusted OR (95% CI) 4 | 1.00 | 1.01 (0.67 – 1.53) | 1.11 (0.73 – 1.68) | 0.6088 |
| HPMA (pmol/mg creatinine) | <5946 | 5946 – 11852 | >11852 | |
| No. Controls/No. Cases | 130/74 | 133/108 | 129/161 | |
| Smoking-Adjusted OR (95% CI) 3 | 1.00 | 1.18 (0.79 – 1.77) | 1.67 (1.12 – 2.49) | 0.0099 |
| Cotinine-Adjusted OR (95% CI) 4 | 1.00 | 0.83 (0.53 – 1.29) | 0.96 (0.61 – 1.52) | 0.9849 |
| HBMA (pmol/mg creatinine) | <4340 | 4340 – 10380 | >10380 | |
| No. Controls/No. Cases | 130/63 | 133/124 | 129/156 | |
| Smoking-Adjusted OR (95% CI) 3 | 1.00 | 1.60 (1.06 – 2.41) | 1.87 (1.24 – 2.83) | 0.0041 |
| Cotinine-Adjusted OR (95% CI) 4 | 1.00 | 1.22 (0.79 – 1.89) | 1.12 (0.70 – 1.77) | 0.7491 |
Odds ratios (ORs) were derived from conditional logistic regression models that retained the case-control matched pairs, of which controls were matched to the index cases on current smoking status, age, neighborhood of residence, and year and month of urine collection, and adjusted for number of cigarettes/day and number of years of smoking; CI, confidence interval.
Further adjusted for total NNAL and PheT in the case of total cotinine, PheT and total cotinine in the case of total NNAL, and total NNAL and total cotinine in the case of PheT.
Odds ratios were derived from unconditional logistic regression models that included matching factors (age, neighborhood of residence, and duration of biospecimen storage before assays for urinary biomarkers), number of cigarettes smoked per day and number of years of smoking at baseline.
In addition to matching factors, number of cigarettes smoked per day, number of years of smoking, odds ratios were adjusted for urinary total cotinine.
All of the mercapturic acids were significantly associated with lung cancer risk after correction for number of cigarettes smoked per day and number of years of smoking. The odds ratios in the highest versus the lowest tertile were 1.44 for MHBMA, 1.81 for HEMA, 1.59 for SPMA, 1.67 for HPMA, and 1.87 for HBMA. However, adjustment for cotinine resulted in a null association for all of the mercapturic acids with lung cancer risk.
The results of the esophageal cancer study are summarized in Table 2. Total NNN and free NNN were significantly associated with esophageal cancer risk, even after adjustment for number of cigarettes per day, number of years of smoking, number of alcoholic drinks per day, urinary total cotinine, and urinary total NNAL. The odds ratios in the upper tertile compared to the lowest tertile were 17.0 for total NNN and 15.8 for free NNN (P<0.0001). A higher percentage of NNN-N-glucuronide was also significantly associated with a lower risk of esophageal cancer, after adjustment for the same factors listed above.
Table 2.
Urinary levels of total NNN, free NNN, and the percentage of NNN-N-Gluc in relation to risk of esophageal cancer among current smokers, the Shanghai Cohort Study
| Levels of urinary biomarkers in tertiles
|
P for trend | |||
|---|---|---|---|---|
| 1st tertile | 2nd tertile | 3rd tertile | ||
| Total NNN (fmol/mg creatinine) | <29.2 | 29.2 – 66.5 | >66.5 | |
| No. Controls/No. Cases | 74/6 | 76/23 | 73/48 | |
| Matched OR (95% CI) 1 | 1.00 (referent) | 4.51 (1.64 – 12.36) | 13.4 (4.64 – 38.76) | <0.0001 |
| Adjusted OR (95% CI) 2 | 1.00 (referent) | 4.17 (1.32 – 13.17) | 18.3 (4.40 – 76.32) | <0.0001 |
| NNAL-adjusted OR (95% CI) 3 | 1.00 (referent) | 3.99 (1.25 – 12.72) | 17.0 (3.99 – 72.80) | <0.0001 |
| Free NNN (fmol/mg creatinine) | <10.4 | 10.4 – 28.5 | >28.5 | |
| No. Controls/No. Cases | 74/7 | 76/15 | 73/55 | |
| Matched OR (95% CI) 1 | 1.00 (referent) | 2.97 (1.02 – 8.65) | 15.5 (5.18 – 46.62) | <0.0001 |
| Adjusted OR (95% CI) 2 | 1.00 (referent) | 3.30 (0.95 – 11.52) | 16.6 (4.29 – 64.43) | <0.0001 |
| NNAL-adjusted OR (95% CI) 3 | 1.00 (referent) | 3.24 (0.92 – 11.36) | 15.8 (4.02 – 62.28) | <0.0001 |
| Percentage of NNN-N-Gluc (%) | <50.5 | 50.5 – 62.2 | >62.2 | |
| No. Controls/No. Cases | 74/45 | 76/18 | 73/14 | |
| Matched OR (95% CI) 1 | 1.00 (referent) | 0.35 (0.18 – 0.69) | 0.24 (0.11 – 0.52) | <0.0001 |
| Adjusted OR (95% CI) 2 | 1.00 (referent) | 0.36 (0.17 – 0.77) | 0.25 (0.11 – 0.59) | 0.0007 |
| NNAL-adjusted OR (95% CI) 3 | 1.00 (referent) | 0.37 (0.17 – 0.80) | 0.27 (0.11 – 0.62) | 0.0013 |
Odds ratios (ORs) were derived from conditional logistic regression models that retained the case-control matched sets, of which controls were matched with index cases on current smoking status, age, neighborhood, and year and month of urine collection; CI, confidence interval.
In addition to matching factors, ORs were adjusted for number of cigarettes per day, number of years of smoking, number of alcoholic drinks per day, and urinary total cotinine.
Further adjusted for urinary total NNAL.
4. Discussion
The studies summarized here indicate that the tobacco smoke carcinogen and toxicant biomarkers total cotinine, total NNAL, PheT, NNN, and total NNN are risk biomarkers for cancer in addition to their well established roles as exposure biomarkers. These findings have considerable implications for future research directions, in which these biomarkers may ultimately contribute to prediction of individual cancer risk in smokers, an area of great importance in prevention and possibly early detection of cancer.
The results for total cotinine and total NNAL are consistent with previous work. Several studies including ours have noted a relationship between cotinine levels in serum or urine and lung cancer risk in smokers [16;22-24], although one previous report was negative in this respect [25]. Cotinine and cotinine glucuronide are metabolites of the non-carcinogen nicotine, so the relationship of their levels to lung cancer is an indirect one. It results from the fact that smokers regulate their use of cigarettes to achieve a certain level of nicotine, thus satisfying their addiction. When they smoke, they are simultaneously exposed to nicotine and multiple carcinogens.
Two previous smaller studies, nested in the Singapore Chinese Health Study and the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial, demonstrated a significant relationship between total NNAL and lung cancer, in agreement with the results summarized here [16;25]. The relationship between total NNAL levels and lung cancer is more direct than that of total cotinine because NNAL and its glucuronides are metabolites of the tobacco-specific lung carcinogen NNK which readily induces lung tumors in rats, mice, hamsters, and ferrets after systemic treatment with even low doses [26].
This was the first large study to demonstrate a relationship between a specific biomarker that integrates PAH exposure and metabolism – PheT - and lung cancer in smokers. PAH are classic carcinogens widely considered to be important causative factors for lung cancer in smokers based on their occurrence in cigarette smoke, strong carcinogenic activities in rodent models including lung cancer models, and metabolic, DNA binding, and genetic consequences in humans [27-30]. Thus, our results are consistent with a large body of experimental data on PAH. One previous smaller study did not detect a significant relationship between serum PheT and lung cancer [25].
While the relationships of total NNAL and PheT to lung cancer in the Shanghai Cohort Study were significant, they were not as strong as one might have expected based on various lines of evidence connecting NNK and PAH to lung cancer in smokers. With respect to total NNAL, it is important to note that NNK levels in Chinese cigarettes are relatively low compared to a typical American blend [31]. The mean levels of total NNAL in the urine of our subjects were 0.20 pmol/mg creatinnine in controls and 0.28 pmol/mg creatinine in cases, 4-5 times less than the amounts of total NNAL typically observed in the urine of U.S. cigarette smokers [10;17]. We have also observed that levels of PheT are considerably higher in non-smokers from Shanghai than in non-smokers from the U.S. [32]. Exposure to higher levels of PAH among non-smokers in Shanghai could be due to air pollution, diet, or other factors. These exposures, particularly from diet, would tend to blunt the effects of PAH exposure specifically from cigarette smoke and consequently diminish the utility of PheT as a specific biomarker of lung cancer risk in smokers from Shanghai.
Perhaps the most remarkable finding in this study was the strong relationship of total NNN to esophageal cancer risk – 17-fold higher for the upper tertile of exposure after adjustment for cotinine and total NNAL, the latter showing no relationship to esophageal cancer. These results demonstrate coherence between epidemiologic data in smokers and rat carcinogenicity studies, in which NNN but not NNK, is a potent esophageal carcinogen [26]. The results therefore add considerable support to the role of NNN as a major cause of esophageal cancer in smokers, proposed previously based on its levels in cigarette smoke and on metabolism, DNA binding, and carcinogenicity studies mainly in rats [28;33]. These results underline the urgent need for reduction of NNN and NNK levels in tobacco products. The technology to do this exists and is well validated but has not been applied to most tobacco products [33]. The results also point to a possible previously unrecognized role of nornicotine in tobacco carcinogenesis. Nornicotine is easily nitrosated to produce NNN [34], and some studies indicate that this occurrs in humans [35;36]. The strong relationship of total NNN to esophageal cancer in the Shanghai cohort study could result partially from endogenous formation of NNN from nornicotine and dietary nitrite, particularly since levels of tobacco-specific nitrosamines in the smoke of cigarettes used in Shanghai are relatively low, as noted above.
We did not observe a significant relationship between the volatile toxicants and carcinogens and lung cancer in smokers, after adjustment for cotinine. Our interpretation of these data is that the lung carcinogenicity of these compounds, if it exists in humans, is not strong enough to overcome the power of cotinine as a representative of all other smoke constituents [19]. Among these volatiles, only 1,3-butadiene, ethylene oxide, and benzene have been shown to induce lung tumors in laboratory animals, and then only in mice [19]. Overall, their potency as lung carcinogens appears to be considerably weaker than those of PAH or NNK [19]. Although 1,3-butadiene, ethylene oxide, and benzene are recognized as human carcinogens, the data from occupational studies indicate that their effects are not mainly on lung cancer [13;14].
Levels of the biomarkers quantified in this study will depend on carcinogen or toxicant dose plus metabolism (with the exception of free NNN, which represents the parent compound in smoke). Thus, there are two variables which affect the final value: dose and metabolism. Since levels of all of these biomarkers are higher in smokers than non-smokers, and in most cases their levels decrease upon smoking cessation, dose is a more important factor than metabolism. But differences in metabolic pathways for each of these compounds could differentially affect cancer risk in individuals exposed to the same dose. A variety of enzymes are involved in the metabolism of the carcinogens and toxicants considered here, and further studies are required to determine their effects on cancer risk.
The studies reviewed here have certain limitations. The cohort consists only of males from Shanghai and the results may not be representative of female smokers, or smokers from other parts of the world where different types of cigarettes are smoked. There was only a single urine sample collected at baseline from all subjects. That urine sample may not be representative of the subjects’ continuing exposure, although longitudinal studies of some of these biomarkers have shown only relatively minor variation over time [37].
In summary, the results reviewed here demonstrate that several tobacco smoke toxicant and carcinogen biomarkers – total cotinine, total NNAL, PheT, and total NNN – are not only biomarkers of exposure but also are biomarkers of cancer risk. Based on comparison of cases and controls who were all smokers, total cotinine, total NNAL, and PheT were risk biomarkers for lung cancer while total NNN was a risk biomarker for esophageal cancer. These results provide new directions for possible early identification of smokers at high risk for cancer.
Acknowledgments
These studies were supported by U.S. National Institutes of Health grants CA-129534, CA-144034, CA-92025 and CA-81301. We thank Bob Carlson for editorial assistance.
Footnotes
Conflict of Interest Statement.
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.American Cancer Society. Cancer Facts & Figures 2010. American Cancer Society; Atlanta: 2010. pp. 1–72. [Google Scholar]
- 2.Secretan B, Straif K, Baan R, Grosse Y, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, Cogliano V. A review of human carci nogens--Part E: tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol. 2009;10:1033–1034. doi: 10.1016/s1470-2045(09)70326-2. [DOI] [PubMed] [Google Scholar]
- 3.International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 83. IARC; Lyon, FR: 2004. Tobacco Smoke and Involuntary Smoking; pp. 53–1187. [PMC free article] [PubMed] [Google Scholar]
- 4.Boyle P, Levin P. World Cancer Report, 2008. IARC; Lyon, FR: 2008. pp. 110–117. [Google Scholar]
- 5.Hecht SS. Research opportunities related to establishing standards for tobacco products under the family smoking prevention and tobacco control act. Nicotine Tob Res. 2012;14:18–28. doi: 10.1093/ntr/ntq216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arif JM, Dresler C, Clapper ML, Gairola CG, Srinivasan C, Lubet RA, Gupta RC. Lung DNA adducts detected in human smokers are unrelated to typical polyaromatic carcinogens. Chem Res Toxicol. 2006;19:295–299. doi: 10.1021/tx0502443. [DOI] [PubMed] [Google Scholar]
- 7.Gupta RC, Arif JM, Gairola CG. Enhancement of pre-existing DNA adducts in rodents exposed to cigarette smoke. Mutat Res. 1999;424:195–205. [PubMed] [Google Scholar]
- 8.Gupta RC, Sopori ML, Gairola CG. Formation of cigarette smoke-induced DNA adducts in the rat lung and nasal mucosa. Cancer Res. 1989;49:1916–1920. [PubMed] [Google Scholar]
- 9.Randerath K, Reddy MV, Gupta RC. 32P-labeling test for DNA damage. Proc Natl Acad Sci U S A. 1981;78:6126–6129. doi: 10.1073/pnas.78.10.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hecht SS, Yuan J-M, Hatsukami DK. Applying tobacco carcinogen and toxicant biomarkers in product regulation and cancer prevention. Chem Res Toxicol. 2010;23:1001–1008. doi: 10.1021/tx100056m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hecht SS, Carmella SG, Villalta PW, Hochalter JB. Analysis of phenanthrene and benzo[a]pyrene tetraol enantiomers in human urine: relevance to the bay region diol epoxide hypothesis of benzo[a]pyrene carcinogenesis and to biomarker studies. Chem Res Toxicol. 2010;23:900–908. doi: 10.1021/tx9004538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carmella SG, Chen M, Han S, Briggs A, Jensen J, Hatsukami DK, Hecht SS. Effects of smoking cessation on eight urinary tobacco carcinogen and toxicant biomarkers. Chem Res Toxicol. 2009;22:734–741. doi: 10.1021/tx800479s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 97. IARC; Lyon, FR: 2008. 1,3-Butadiene, ethylene oxide and vinyl halides (vinyl fluoride, vinyl chloride and vinyl bromide) pp. 45–309. [PMC free article] [PubMed] [Google Scholar]
- 14.International Agency for Research on Cancer. Overall Evaluations of Carcinogenicity: An Updating of IARC Monographs. Supplement 7. 1-42. IARC; Lyon, FR: 1987. pp. 120–122. [Google Scholar]
- 15.Zhang S, Balbo S, Wang M, Hecht SS. Analysis of acrolein-derived 1,N2-propanodeoxyguanosine adducts in human leukocyte DNA from smokers and nonsmokers. Chem Res Toxicol. 2011;24:119–124. doi: 10.1021/tx100321y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuan JM, Koh WP, Murphy SE, Fan Y, Wang R, Carmella SG, Han S, Wickham K, Gao YT, Yu MC, Hecht SS. Urinary levels of tobacco-specific nitrosamine metabolites in relation to lung cancer development in two prospective cohorts of cigarette smokers. Cancer Res. 2009;69:2990–2995. doi: 10.1158/0008-5472.CAN-08-4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuan J-M, Gao Y-T, Murphy SE, Carmella SG, Wang R, Zhong Y, Moy KA, Davis AB, Tao L, Chen M, Han S, Nelson HH, Yu MC, Hecht SS. Urinary levels of cigarette smoke constituent metabolites are prospectively associated with lung cancer development in smokers. Cancer Res. 2011;71:6749–6757. doi: 10.1158/0008-5472.CAN-11-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuan JM, Knezevich AD, Wang R, Gao YT, Hecht SS, Stepanov I. Urinary levels of the tobacco-specific carcinogen N’-nitrosonornicotine and its glucuronide are strongly associated with esophageal cancer risk in smokers. Carcinogenesis. 2011;32:1366–1371. doi: 10.1093/carcin/bgr125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuan JM, Gao YT, Wang R, Chen M, Carmella SG, Hecht SS. Urinary levels of volatile organic carcinogen and toxicant biomarkers in relation to lung cancer development in smokers. Carcinogenesis. 2012 Feb 21; doi: 10.1093/carcin/bgs026. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ross RK, Yuan JM, Yu MC, Wogan GN, Qian GS, Tu JT, Groopman JD, Gao YT, Henderson BE. Urinary aflatoxin biomarkers and risk of hepatocellular carcinoma. The Lancet. 1992;339:943–946. doi: 10.1016/0140-6736(92)91528-g. [DOI] [PubMed] [Google Scholar]
- 21.Yuan JM, Ross RK, Wang XL, Gao YT, Henderson BE, Yu MC. Morbidity and mortality in relation to cigarette smoking in Shanghai, China A prospective male cohort study. JAMA. 1996;275:1646–1650. [PubMed] [Google Scholar]
- 22.de Waard F, Kemmeren JM, van Ginkel LA, Stolker AAM. Urinary cotinine and lung cancer risk in a female cohort. Br J Cancer. 1995;72:784–787. doi: 10.1038/bjc.1995.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boffetta P, Clark S, Shen M, Gislefoss R, Peto R, Andersen A. Serum cotinine level as predictor of lung cancer risk. Cancer Epidemiol Biomarkers Prev. 2006;15:1184–1188. doi: 10.1158/1055-9965.EPI-06-0032. [DOI] [PubMed] [Google Scholar]
- 24.Timofeeva MN, McKay JD, Smith GD, Johansson M, Byrnes GB, Chabrier A, Relton C, Ueland PM, Vollset SE, Midttun O, Nygard O, Slimani N, Romieu I, Clavel-Chapelon F, Boutron-Ruault MC, Fagherazzi G, Kaaks R, Teucher B, Boeing H, Weikert C, Bueno-de-Mesquita HB, van Gils C, Peeters PH, Agudo A, Barricarte A, Huerta JM, Rodriguez L, Sanchez MJ, Larranaga N, Khaw KT, Wareham N, Allen NE, Travis RC, Gallo V, Norat T, Krogh V, Masala G, Panico S, Sacerdote C, Tumino R, Trichopoulou A, Lagiou P, Trichopoulos D, Rasmuson T, Hallmans G, Riboli E, Vineis P, Brennan P. Genetic polymorphisms in 15q25 and 19q13 loci, cotinine levels, and risk of lung cancer in EPIC. Cancer Epidemiol Biomarkers Prev. 2011;20:2250–2261. doi: 10.1158/1055-9965.EPI-11-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Church TR, Anderson KE, Caporaso NE, Geisser MS, Le C, Zhang Y, Benoit AR, Carmella SG, Hecht SS. A prospectively measured serum biomarker for a tobacco-specific carcinogen and lung cancer in smokers. Cancer Epidemiol Biomarkers Prev. 2009;18:260–266. doi: 10.1158/1055-9965.EPI-08-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hecht SS. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem Res Toxicol. 1998;11:559–603. doi: 10.1021/tx980005y. [DOI] [PubMed] [Google Scholar]
- 27.Hecht SS. Tobacco smoke carcinogens and lung cancer. J Natl Cancer Inst. 1999;91:1194–1210. doi: 10.1093/jnci/91.14.1194. [DOI] [PubMed] [Google Scholar]
- 28.Hecht SS. Tobacco carcinogens, their biomarkers, and tobacco-induced cancer. Nature Rev Cancer. 2003;3:733–744. doi: 10.1038/nrc1190. [DOI] [PubMed] [Google Scholar]
- 29.International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. IARC; Lyon, FR: 2004. Tobacco Smoke and Involuntary Smoking; pp. 33–1187. [PMC free article] [PubMed] [Google Scholar]
- 30.International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 92. IARC; Lyon, FR: 2010. Some Non-Heterocyclic Polycyclic Aromatic Hydrocarbons and Some Related Exposures; pp. 35–818. [PMC free article] [PubMed] [Google Scholar]
- 31.Wu W, Zhang L, Jain RB, Ashley DL, Watson CH. Determination of carcinogenic tobacco-specific nitrosamines in mainstream smoke from U.S.-brand and non-U.S.-brand cigarettes from 14 countries. Nicotine Tob Res. 2005;7:443–451. doi: 10.1080/14622200500125898. [DOI] [PubMed] [Google Scholar]
- 32.Kensler TW, Ng D, Carmella SG, Chen M, Jacobson LP, Munoz A, Egner PA, Chen JG, Qian GS, Chen TY, Fahey JW, Talalay P, Groopman JD, Yuan JM, Hecht SS. Modulation of the metabolism of airborne pollutants by glucoraphanin-rich and sulforaphane-rich broccoli sprout beverages in Qidong. China, Carcinogenesis. 2012;33:101–107. doi: 10.1093/carcin/bgr229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 89. IARC; Lyon, FR: 2007. Smokeless tobacco and some tobacco-specific N-nitrosamines. [PMC free article] [PubMed] [Google Scholar]
- 34.Mirvish SS, Sams J, Hecht SS. Kinetics of nornicotine and anabasine nitrosation in relation to N’-nitrosonornicotine occurrence in tobacco and to tobacco-induced cancer. J Natl Cancer Inst. 1977;59:1211–1213. doi: 10.1093/jnci/59.4.1211. [DOI] [PubMed] [Google Scholar]
- 35.Stepanov I, Carmella SG, Han S, Pinto A, Strasser AA, Lerman C, Hecht SS. Evidence for endogenous formation of N’-nitrosonornicotine in some long term nicotine patch users. Nicotine Tob Res. 2009;11:99–105. doi: 10.1093/ntr/ntn004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stepanov I, Carmella SG, Briggs A, Hertsgaard L, Lindgren B, Hatsukami DK, Hecht SS. Presence of the carcinogen N’-nitrosonornicotine in the urine of some users of oral nicotine replacement therapy products. Cancer Res. 2009;69:8236–8240. doi: 10.1158/0008-5472.CAN-09-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Church TR, Anderson KE, Le C, Zhang Y, Kampa DM, Benoit AR, Yoder AR, Carmella SG, Hecht SS. Temporal stability of urinary and plasma biomarkers of tobacco smoke exposure among cigarette smokers. Biomarkers. 2010;15:345–352. doi: 10.3109/13547501003753881. [DOI] [PMC free article] [PubMed] [Google Scholar]


