Abstract
We examined whether infants’ preference for speech at 12 months is associated with autistic-like behaviors at 18 months in infants who are at increased risk for autism spectrum disorder (ASD) because they have an older sibling diagnosed with ASD and in low-risk infants. Only low-risk infants listened significantly longer to speech than to nonspeech at 12 months. In both groups, relative preference for speech correlated positively with general cognitive ability at 12 months. However, in high-risk infants only, preference for speech was associated with autistic-like behavior at 18 months, while in low-risk infants, preference for speech correlated with language abilities. This suggests that in children at risk for ASD an atypical species-specific bias for speech may underlie atypical social development.
Keywords: speech preference, language development, high-risk infant siblings, autism spectrum disorders
Early diagnosis of autism is key to treatment and outcomes for people living with Autism Spectrum Disorders (ASD; Dawson, 2008; Motiwala, Gupta, Lilly, Ungar, & Coyte, 2006; National Research Council, 2001). But indices of autism-like behaviors in the first year of life remain elusive. Currently, concerns about atypical language and social function, two of the main characteristics of ASD, rely on children’s explicit productive abilities, which only emerge reliably after the first year of life (DeGiacomo & Fombonne, 1998). As a result, ASD is typically diagnosed around the age of 3-5 years (Mandell, Novake, & Zubritsky, 2005; Ozonoff et al., 2009). Recently an observational checklist (the CSBS-DP Infant Toddler Checklist; Pierce et al., 2011; Wetherby & Prizant, 2002) shows promise for identifying ASD relevant behaviors at 1 year of age, suggesting that certain behaviors may be present early in development. In this study, we examine whether atypical behaviors that emerge in infants at risk for ASD at 1 year of age can predict autistic-like behaviors.
To successfully acquire language, infants must orient to the relevant signals in the environment early in development. Typically developing (TD) infants have fundamental species-typical perceptual biases that direct their attention to socially relevant stimuli such as voices and faces. The biases that human infants show for biological stimuli at birth such as speech and faces over non-speech and non-faces (Butterfield & Siperstein, 1970; Johnson et al., 1991; Valenza, Simion, Cassia, & Umilta, 1996; Vouloumanos & Werker, 2007) are sharpened further over 3 months, with TD infants attending preferentially to the vocalizations and faces of conspecifics over those of a closely-related primate, the rhesus macaque (Heron, Wirth, & Pascalis, 2011; Vouloumanos, Hauser, Werker, & Martin, 2010). By 5 months, infants connect conspecific voices and faces–they expect speech to be produced by humans, and not other animals (Vouloumanos, Druhen, Hauser, & Huizink, 2009), and by 12 months, infants understand that speech has a specific communicative function that other vocalizations lack (Martin, Onishi, & Vouloumanos, 2012). Thus, the trajectory of typical human development is characterized by early perceptual biases for orienting to speech and faces, which may have functional consequences for learning about and interacting with others.
Children diagnosed with ASD show apparent deficits in basic processing of the voices and faces of their own species (Behrmann, Thomas & Humphreys, 2006; Chawarska, Volkmar, & Klin, 2010; Kuhl, Coffey, Padden, & Dawson, 2005; Paul, Chawarska, Fowler, Cicchetti, & Volkmar, 2007; Whitehouse & Bishop, 2008). Children at risk for ASD might also show atypical speech preferences early in development. This suggests a key theoretical proposal: that an early basic impairment in preferential processing of speech could form the basis of deficits in social communication skills that are characteristic of ASD (Kuhl, et al., 2005; Surian & Siegal, 2008). As such, examining early emerging differences between at-risk and TD infants’ processing of relevant social stimuli such as speech may provide fundamental insights into divergent developmental behaviors.
Despite the relatively high prevalence of ASD, studying a sufficient number of individuals within the general population to characterize early individual differences that can lead to ASD behaviors is not feasible. However, familial transmission rates are notably higher: the recurrence risk in a later-born sibling of a child diagnosed with ASD was recently estimated at 19% (Ozonoff et al., 2011), with an additional 15% probability of cognitive or language difficulties (Elsabbagh & Johnson, 2010; Ritvo et al., 1989; Zwaigenbaum et al., 2005). The higher prevalence of ASD behaviors in high-risk later-born siblings allows us to identify a reasonable number of cases from a feasible sample size (Zwaigenbaum et al., 2007). Here we examine whether an atypical preference for speech at 12 months is associated with language ability and autistic-like behaviors at 18 months of age.
Specifically, we tested infants who are at elevated risk for ASD because they have an older sibling diagnosed with ASD (SIBS-A) and infant siblings of TD children (SIBS-TD) on their preference for speech over non-speech sounds. We hypothesize that deficits in a bias for speech may be associated with autistic-like behaviors at 18 months of age. Discovering that autistic-like behaviors correlate with earlier atypical preferences for speech could help inform early detection strategies and better target early interventions.
Method
Participants
Participants were 62 healthy, full term infants (32 females) with at least one older sibling (Mean age: 12.45 months, SD = .27). High-risk infants with an older sibling diagnosed with ASD (SIBS-A; N = 31; 15 females) were recruited from several local ASD treatment agencies and pediatric clinics including: Health Services’ Early Child Development Team, Society for Treatment of Autism, Renfrew Educational Services, Parent-Link, and other local service organizations. Low-risk infant siblings (SIBS-TD; N = 31; 17 females) of typically developing children were recruited from local parent fairs, flyers, and advertisements. Parents of the low-risk infants completed a questionnaire to ensure that ASD was not present in any family members. Data were collected from infants at 12 and 18 months of age. Exclusionary criteria included the presence of a neurological disorder of known etiology, significant sensory or motor impairment, major physical abnormalities, and history of serious head injury and/or neurological disease. Hearing status of the infants was confirmed with an otoacoustic emissions screening procedure at each session prior to the observational tasks. There were no infants who were unable to be tested due to middle ear fluid.
Infants’ general development at 12 months was assessed using the Mullen Scales of Early Learning (Mullen, 1995), a laboratory-based observational measure. The Mullen is a comprehensive assessment of language, motor, and perceptual abilities for children of all ability levels. The Mullen consists of five scales: visual reception, receptive language, expressive language, fine motor and gross motor skills. The Mullen Early Learning Composite (ELC) score is based on the first 4 scales, which are designed to measure cognitive ability and has a mean of 100 and a standard deviation of 15. This is standardized for children aged 0-69 months. The gross motor scale covers children from 0-29 months of age. We included only those infants who had a standard score of at least 70 (two SD below the mean) on the Mullen ELC (n= 31 per group), which excluded 1 infant in the high-risk group. As a result, our risk groups did not differ on the Mullen ELC score at 12 months (see Table 1 for details).
Table 1.
Participant Information and Mullen Scores
| SIBS-TD | SIBS-A | |
|---|---|---|
| Number of Participants | 31 | 31 |
| Number of Females | 17 | 15 |
|
Chronological Age in
months |
12.47 (.26) | 12.43 (.28) |
| Gross Motor T Score | 49.73 (13.54) | 51.53 (11.50) |
| Fine Motor T Score | 55.10 (9.96) | 57.10 (11.71) |
| Visual Reception T Score | 51.33 (7.82) | 50.10 (8.12) |
|
Receptive Language T
Score |
45.97 (7.11) | 42.16 (7.14) * |
|
Expressive Language T
Score |
53.93 (10.47) | 49.87 (11.04) |
|
Early Learning Composite
Standard Score |
103.47 (9.52) | 99.90 (11.75) |
Note. For age and Mullen scores, we report the mean and standard deviation (in parentheses). Measures for which means differ between groups are noted with symbols
p < .05.
Data collection varied for each task depending on whether the infant was able to complete all tasks at each visit. As such, sample size varied for each task. Of the 62 infants, 53 completed the Speech/NonSpeech task (25 SIBS-A). See Table 2 for participant numbers for measures of language (MacArthur Bates-Communicative Development Inventories; Feldman et al., 2000; Fenson et al., 1994) and autistic behaviors (Autism Observation Scales Infancy; Bryson, Zwaigenbaum, McDermott, Rombough, & Brian, 2008; Zwaigenbaum et al., 2005).
Table 2.
Descriptive statistics for observational and experimental tasks.
| SIBS-TD | SIBS-A | |||||
|---|---|---|---|---|---|---|
| 12 months | Language (MB- CDI) |
Receptive Language | 68.1 (48.3) | 27 | 62.8 (72.5) | 28 |
| Expressive Language | 7.6 (7.2) | 27 | 4.9 (5.3) | 28 | ||
| Gestures | 25.2 (8.0) | 27 | 21.6 (9.9) | 27 | ||
|
| ||||||
| Autism (AOSI) |
AOSI - Score | 4.8 (3.8) | 27 | 6.0 (4.3) | 25 | |
| AOSI - Markers | 2.9 (2.0) | 27 | 3.7 (2.4) | 25 | ||
|
| ||||||
| Listening Time (s) |
Speech | 11.7 (5.3) | 28 | 11.4 (6.5) | 25 | |
| Non-Speech | 9.5 (3.8) | 28 | 10.3 (7.2) | 25 | ||
|
| ||||||
| 18 months | Language (MB- CDI) |
Receptive Language | 214.2 (88.5) | 25 | 136.1 (102.1) | 17 * |
| Expressive Language | 75.2 (72.4) | 26 | 37.5 (55.7) | 25 * | ||
| Gestures | 44.3 (7.8) | 24 | 52.2 (49.3) | 17 | ||
|
| ||||||
| Autism (AOSI) |
AOSI - Score | 4.7 (4.1) | 26 | 6.4 (3.3) | 26 † | |
| AOSI - Markers | 3.0 (2.1) | 26 | 4.1 (2.1) | 26 † | ||
Note. Means, standard deviations (in parentheses), and sample size (offset) for MB-CDI, AOSI and speech and non-speech listening task. Parents did not always complete the full MB-CDI forms for receptive language and gestures, especially at 18 months.Measures for which means differ between groups are noted with symbols
p < .05
p ≤ .10.
Stimuli
Infants heard two types of auditory stimuli: a speech set composed of nonsense words, and a non-speech set composed of complex non-speech analogues (used in Vouloumanos & Curtin, under review; Vouloumanos & Werker, 2004; 2007), all delivered at 65 dB +/− 5 dB. Speech stimuli included 12 tokens of monosyllabic nonsense words spoken by a female native English speaker. Tokens varied in intonational contour (average minimum and maximum pitch: 197 Hz and 350 Hz, respectively) and duration (525–1155 ms). Complex non-speech stimuli included 12 time-varying sinusoidal waves tracking regions of significant energy in natural speech (i.e., the fundamental frequency and the first three formants). Non-speech analogues retained the amplitude envelope, relative formant amplitude, relative intensity and pitch contour of their speech counterparts.
Procedure
Infants were tested using a modified version of the sequential looking preference (SLP) procedure (Cooper & Aslin, 1990, 1994, Vouloumanos & Curtin, under review; Vouloumanos & Werker, 2004). Infants sat on a parent’s lap in front of a monitor. A movie of a flashing light attracted the infant’s attention to the screen. Once the infant fixated on the screen, the experimenter initiated the trial. A black and white checkerboard was displayed concurrently with each of the two sound types. Five speech trials and five non-speech trials were presented, with order counterbalanced across infants. A full trial consisted of different tokens of either speech or nonspeech separated by 300 to 450 ms of silence, for a full trial length of 40 s. Stimulus presentation was of a fixed length. Auditory and visual stimuli were presented using Habit X (Cohen, Atkinson, & Chaput 2000). Looking time was scored online, and then coded offline from infant videos collected during the study. Since the visual stimulus was always a checkerboard, but the sounds varied in each trial, the main dependent measure was looking time to the checkerboard for each sound type: speech and non-speech.
Observational Measures of linguistic development and autistic-like behavior
We assessed language development using the MacArthur-Bates Communicative Development Inventory (MB-CDI; Feldman et al., 2000; Fenson et al., 1994). This is a parental report that measures children’s word comprehension and production, and gesture production (e.g., giving, showing, pointing). The MB-CDI is a widely used parental report measure that has been validated in both typically developing and in high-risk populations.
To assess autistic-like behavior we used the Autism Observation Scales Infancy (AOSI: Bryson et al., 2008; Zwaigenbaum et al., 2005). The AOSI is an 18-item direct observational measure of autistic symptomatology, developed to detect and monitor early signs of autism as they emerge in high-risk infants (having an older sibling diagnosed with ASD) in infants aged 6 to 18 months. The administration of the standard set of semi-structured activities allows for an interactive context in which the infant is engaged in play, while the examiner conducts a set of systematic presses to elicit particular target behaviors. Behaviors are rated on a scale from 0 to 3. A score of 0 suggests typical function with higher scores indicating increased deviation from typical behavior (Bryson et al., 2008). Furthermore the number of markers is calculated by adding up the number of items with a non-zero score. Inter-rater reliability is excellent (0.94 for total score at 18 months), and the AOSI has fair to good test–retest reliability at 12 months (0.61 for total score; Bryson et al., 2008). Using a cut-off point of 7 markers, the AOSI has shown sensitivity of 84% and specificity of 98% for autism in siblings (Zwaigenbaum et al., 2005). Six of our participants, 2 low-risk infants and 4 high-risk infants, presented 7 or more risk markers on the AOSI.
Results
We examine whether an atypical preference for speech at 12 months is associated with language ability and autistic-like behaviors at 18 months of age. We first test for group differences for each of the factors of interest: preference for speech assessed using the experimental task, linguistic development assessed with the MB-CDI, and autistic behaviors assessed with the AOSI while reporting each factor’s relationship with general development using the Mullen ELC scores. We then examine, within separate sections, how speech preference relates to linguistic development and autistic behaviors, adjusting for general development if necessary.
Experimental speech/non-speech task
Overall, infants listened to speech significantly longer than to non-speech. A 2 (Sound Type: speech, nonspeech) × 2 (Group: SIBS-A, SIBS-TD) univariate analysis of variance (ANOVA) revealed a main effect of sound type at 12 months, F (1,51) = 6.23, p = .016, ηp2= .109, but no group differences or interactions. However, there was a significant difference in the amount of variance observed across groups (p = .004), suggesting that despite no overall group differences, groups differed in their intragroup variability. To examine whether one group was driving the preference for speech, we looked at each group’s performance individually (see Table 2). Infants in the SIBS-TD group listened to speech significantly longer overall than to non-speech (t(27) = 2.16, p = .040, Cohen’s d = .48), but infants in the SIBS-A group did not listen reliably longer to speech than to non-speech (t(24) = 1.37, p = .19).
We next created a speech preference index for each infant by subtracting the overall looking time during nonspeech trials from overall looking time during speech trials (speech minus nonspeech). A positive score reflects a preference for listening to speech while a negative score reflects a preference for listening to nonspeech. This speech preference index was used in subsequent analyses.
Speech preference index correlated positively with Mullen ELC scores, r(51) = .432, p = .001, across the entire sample, and within each group (SIBS-A: r(25) = .405, p = .040; SIBS-TD: r(27) = .480, p = .011).
Observational tasks
Linguistic development
MB-CDI scores varied considerably between individuals and groups (see Table 2). Groups did not differ at 12 months, however, at 18 months, groups differed on receptive language, F(1,40) = 6.96, p = .012, η2 = .148, and expressive language, F(1,49) = 4.32, p = .043, η2 = .08 (see Table 2).
Across the entire sample, the Mullen ELC score at 12 months correlated with parental reports of expressive vocabulary on the MB-CDI at both ages (12 months: r(54) = .486, p < .001; 18 months: r(49) = .368, p = .009), and with infants’ total gestures at 12 months, r(54) = .453, p = .001. This suggests that infants with higher Mullen standard scores had greater expressive vocabularies at 12 and 18 months and produced more gestures at 12 months.
Autism-like behaviors
AOSI scores differed marginally between groups at 18 months for the number of markers, F(1,50) = 3.70, p = .058, η2 = .07, and for the total score F(1,50) = 2.78, p = .102, η2 = .053 (see Table 2).
Across the entire sample, the Mullen ELC Score at 12 months negatively correlated with the number of AOSI markers at both ages (12 months: r(51) = −.305, p = .029; 18 months: r(51) = −.488, p < .001), and with the total AOSI score marginally at 12 months r(50) = −.265, p = .060, and reliably at 18 months, r(51) = −.446, p < .001, suggesting that infants with higher Mullen standard scores produced fewer autistic-like behaviors.
Relationships between speech preference index and language
Speech preference index correlated positively with the MB-CDI expressive language score at 18 months, r(46) = .386, p = .008, across the entire sample, although this relationship did not reach significance within each group.
After adjusting for the Mullen ELC score, speech preference index was only marginally correlated with the MB-CDI expressive language measures at 18 months, r(43) = .290, p = .056, across the entire sample. While this appears surprising, the Mullen ELC includes 2 scales measuring receptive and expressive language. Thus some of the variance that is partialled out when we adjust for the Mullen ELC score necessarily reflects language skill (as measures by the Mullen subscales).
Specific relationship between speech preference index and autistic-like behavior
Our primary question is whether a relationship exists between a preference for speech and atypical behaviors associated with ASD. We explored relationships within the groups separately because the sibling groups differ in their risk profile: the SIBS-A group is at elevated risk for ASD. Since all of our measures significantly correlated with the Mullen ELC score obtained at 12 months, we conducted partial correlations adjusting for this factor. We included all infants for whom we had AOSI data at 12 and at 18 months (SIBS-A: n= 22; SIBS-TD: n = 21).
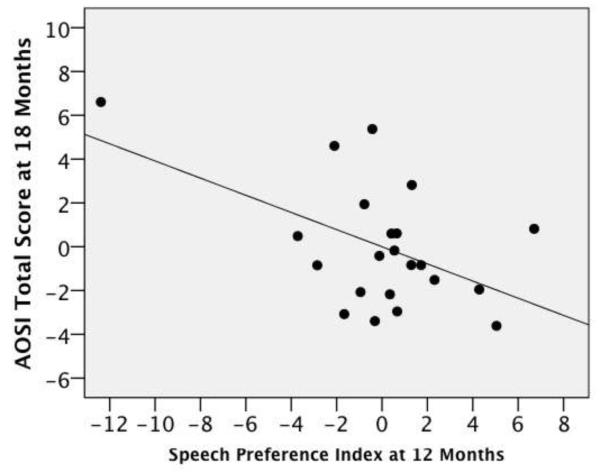
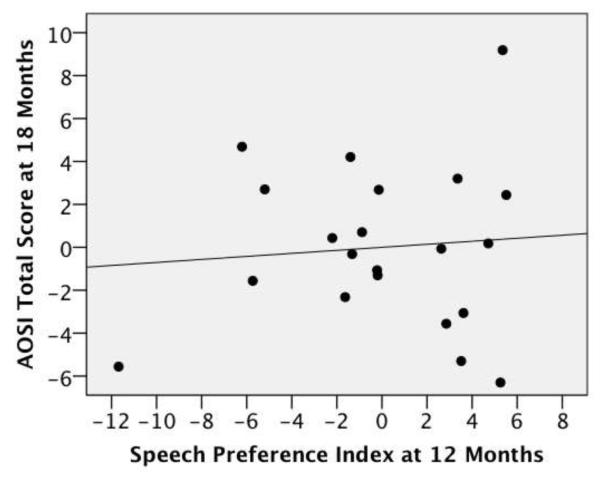
In the SIBS-A group, preference for speech correlated negatively with AOSI scores at 18 months of age (Figure 1). That is, infants who preferred listening to speech at 12 months had lower AOSI total scores (r(19) = −.512, p = .018), and fewer total markers (r(19) = −.424, p = .056) at 18 months. There was no relationship between preference for speech and AOSI scores at 18 months in the SIBS-TD group (Figure 2; AOSI total score: r(18) = .084, p = .725; AOSI total markers: r(18) = .163, p = .494). No other significant correlations were obtained. This suggests that even when adjusting for general cognitive ability (i.e., Mullen ELC scores) infants who are at greater risk for ASD who show a greater preference for speech at 12 months are less likely to exhibit autistic behaviors at 18 months of age.
Figure 1.
Partial regression plot of speech preference index on AOSI total score (centered) adjusting for Mullen ELC score for the SIBS-A group, (r(19) =−.512, p = .018).
Figure 2.
Partial regression plot of speech preference index on AOSI total score (centered) adjusting for Mullen ELC score for the SIBS-TD group, r(18) = .084, p = .725.
Of the 6 infants who scored above the 7-marker tentative cut-off for the AOSI (Zwaigenbaum et al., 2005), the 2 low risk infants both preferred speech, while 3 of 4 infants in the high-risk group preferred nonspeech, with the remaining infant showing a looking time difference of less than 1 s.
Discussion
Infants’ relative preference for speech at 12 months significantly correlated with performance on the AOSI at 18 months for infants at elevated risk for ASD. Although the groups did not differ on their overall preference for speech over nonspeech, the correlation between speech preference and AOSI suggests that, in some siblings at elevated risk for ASD, a basic bias for listening to speech might be atypical and related to autistic-type behaviors. This relationship, however, did not surface for siblings of TD infants, suggesting that speech preference is not associated with autistic-type behaviors in this group.
As a group, SIBS-TD showed an overall listening preference for speech over nonspeech (as in other studies, e.g., Vouloumanos & Curtin, under review; Vouloumanos & Werker, 2004, 2007). In contrast, despite being in the same direction of preference, the SIBS-A as a group did not listen reliably longer to speech. This lack of preference at the group level likely reflects greater heterogeneity in the SIBS-A group: only a subset of SIBS-A, but not all, will develop ASD (Ozonoff et al., 2011; Young, Merin, Rogers, & Ozonoff, 2009), and within the subset of SIBS-A who will not develop ASD, only some will have language impairments (Toth, Dawson, Melztoff, Greenson, & Fein, 2007). Moreover, the difference between listening patterns between groups is striking: rather than listening less to speech, SIBS-A tended to listen longer to non-speech (although this difference was not significant) with greater variance in listening times to nonspeech. This parallels recent findings on looking times for faces and checkerboards in which we find that SIBS-A and SIBS-TD look at faces equally, but SIBS-A look at non-social checkerboards more than SIBS-TD do (Droucker, Curtin, & Vouloumanos, in press). Some studies of children with ASD show impaired attention to social stimuli (e.g., Chawarska, et al., 2010; Dawson, Meltzoff, Osterling, Rinaldi, & Brown, 1998; Osterling, Dawson, & Munson, 2002; Whitehouse & Bishop, 2008), while others show unimpaired processing of social stimuli (e.g., van der Geest, Kemner, Verbaten, & van Engeland, 2002). Indeed, studies investigating selective attention for social (faces) and non-social (houses) visual stimuli have found that unlike controls, individuals with ASD only show attentional modulation for non-social stimuli (Bird, Catmur, Silani, Frith, & Frith, 2006). We tentatively suggest that SIBS-A, as a group, might be more interested in non-social stimuli (nonspeech and checkerboards), rather than being less interested in social stimuli (speech and faces).
Two different and separable aspects of listening to speech at 12 months may predict different aspects of development at 18 months. Overall attention to speech predicts language ability at 18 months in TD infants (Vouloumanos & Curtin, under review). In contrast, relative preference for speech over non-speech more specifically predicts autistic-like behaviors at 18 months in high-risk infants in the current study. This latter pattern of findings is consistent with studies showing that infants’ ability to inhibit attention to irrelevant stimuli is correlated with general indices of development (e.g., Lalonde & Werker, 1995; Conboy, Sommerville, & Kuhl, 2008). Overall attention and relative preference for speech may be separable aspects that might reflect two different underlying processes: a mechanism for fixating attention on particular stimuli and a mechanism for selecting between competing stimuli (see also Vouloumanos & Curtin, under review). Attention to speech may direct attention to linguistically relevant information and facilitates communicative development, while relative preference for speech in infants at risk for ASD may facilitate social development.
Although infants in our study have not yet been assessed for ASD, and atypical speech preference cannot yet be linked to ASD diagnosis, our findings provide a compelling association between atypical speech preference and autistic-like behaviors that can be assessed in 18-month-olds at risk.
Our findings provide support for the role of early perceptual biases in directing attention to linguistic and socially relevant information. Atypical attention to speech in children with ASD is correlated with delays or impairments in language development (e.g., Kuhl et al., 2005; Paul, et al., 2007), supporting a link between speech biases and language development. Our results are consistent with the possibility that an atypical bias for speech in a high-risk population may have cascading effects on development, specifically on autistic-like behaviors, and these effects may be observable prior to 2 years of age.
Acknowledgments
This research was supported by an Alberta Centre for Child, Family, and Community Research grant awarded to SC and by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number R01HD072018 awarded to AV and SC. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Further support was provided by NYU research funds to AV. We would like to thank the members of the University of Calgary Speech Development Lab and the members of the NYU Infant Cognition and Communication Lab for their help with this research as well as all the families who participated in this study.
Footnotes
Author Note Suzanne Curtin, Department of Psychology, University of Calgary, 2500 University Drive NW, Calgary AB, T2N 1N4, Canada. Athena Vouloumanos, Department of Psychology, New York University, 6 Washington Place, New York NY, 10003, USA.
References
- Behrmann M, Thomas C, Humphreys K. Seeing it differently: Visual processing in autism. Trends in Cognitive Sciences. 2006;10:258–264. doi: 10.1016/j.tics.2006.05.001. doi:10.1016/j.tics.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Bird G, Catmur C, Silani G, Frith C, Frith U. Attention does not modulate neural responses to social stimuli in autism spectrum disorders. NeuroImage. 2006;31:1614–1624. doi: 10.1016/j.neuroimage.2006.02.037. doi:10.1016/j.neuroimage.2006.02.037. [DOI] [PubMed] [Google Scholar]
- Bryson SE, Zwaigenbaum L, McDermott C, Rombough V, Brian J. The autism observational scale for infants: Scale development and reliability data. Journal of Autism and Developmental Disabilities. 2008;38:731–738. doi: 10.1007/s10803-007-0440-y. doi:10.1007/s10803-007-0440-y. [DOI] [PubMed] [Google Scholar]
- Butterfield EC, Siperstein GN. Influence of contingent auditory stimulation upon non-nutritional suckle. In: Bosma JF, editor. Third symposium on oral sensation and perception: The mouth of the infant. Charles C. Thomas; Springfield, IL: 1970. pp. 313–334. [Google Scholar]
- Chawarska K, Volkmar F, Klin A. Limited attentional bias for faces in toddlers with autism spectrum disorders. Archives of General Psychiatry. 2010;67:178–185. doi: 10.1001/archgenpsychiatry.2009.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen LB, Atkinson DJ, Chaput HH. Habit X: A new program for obtaining and organizing data in infant perception and cognition studies. University of Texas; Austin: 2004. [Google Scholar]
- Conboy BT, Sommerville JA, Kuhl PK. Cognitive control factors in speech perception at 11 months. Developmental Psychology. 2008;44(5):1505–1512. doi: 10.1037/a0012975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper RP, Aslin RN. Preference for infant-directed speech in the first month after birth. Child Development. 1990;61:1584–1595. doi: 10.2307/1130766. [PubMed] [Google Scholar]
- Cooper RP, Aslin RN. Developmental differences in infant attention to the spectral properties of infant-directed speech. Child Development. 1994;65:1663–1677. doi: 10.1111/j.1467-8624.1994.tb00841.x. doi: 10.2307/1131286. [DOI] [PubMed] [Google Scholar]
- Dawson G. Early behavioural intervention, brain plasticity, and the prevention of autism spectrum disorder. Development and Psychopathology. 2008;20:775–803. doi: 10.1017/S0954579408000370. doi:10.1017/S0954579408000370. [DOI] [PubMed] [Google Scholar]
- Dawson G, Meltzoff AN, Osterling J, Rinaldi J, Brown E. Children with autism fail to orient to naturally occurring social stimuli. Journal of Autism and Developmental Disorders. 1998;28(6):479–485. doi: 10.1023/a:1026043926488. [DOI] [PubMed] [Google Scholar]
- DeGiacomo A, Fombonne E. Parental recognition of developmental abnormalities in autism. European Child & Adolescent Psychiatry. 1998;7:131–136. doi: 10.1007/s007870050058. doi:10.1007/s007870050058. [DOI] [PubMed] [Google Scholar]
- Droucker D, Curtin S, Vouloumanos A. Linking infant-directed-speech and face preferences to language outcomes in infants at risk for autism spectrum disorder. Journal of Speech, Language, and Hearing Research. doi: 10.1044/1092-4388(2012/11-0266). (in press) [DOI] [PubMed] [Google Scholar]
- Elsabbagh M, Johnson MH. Getting answers from babies about autism. Trends in Cognitive Sciences. 2010;14:81–87. doi: 10.1016/j.tics.2009.12.005. doi:10.1016/j.tics.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Feldman HM, Dollaghan CA, Campbell TF, Kurs-Lasky M, Janosky JE, Paradise JL. Measurement properties of the MacArthur communicative development inventories at ages one and two years. Child Development. 2000;71:310–22. doi: 10.1111/1467-8624.00146. doi:10.1111/1467-8624.00146. [DOI] [PubMed] [Google Scholar]
- Fenson L, Dale PS, Reznick JS, Thal D, Bates E, Hartung JP, Pethick S, Reilly JS. The MacArthur Communicative Development Inventories: User’s guide and technical manual. Singular Publishing Group; San Diego: 1993. [Google Scholar]
- Heron-Delaney M, Wirth S, Pascalis O. Infants’ knowledge of their own species. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2011;366(1571):1753–63. doi: 10.1098/rstb.2010.0371. doi:10.1098/rstb.2010.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MH, Dziurawiec S, Ellis H, Morton J. Newborns’ preferential tracking of face-like stimuli and its subsequent decline. Cognition. 1991;40:1–19. doi: 10.1016/0010-0277(91)90045-6. doi:10.1016/0010-0277(91)90045-6. [DOI] [PubMed] [Google Scholar]
- Kuhl PK, Coffey CS, Padden D, Dawson G. Links between social and linguistic processing of speech in preschool children with autism: Behavioral and electrophysiological measures. Developmental Science. 2005;8:F1–F12. doi: 10.1111/j.1467-7687.2004.00384.x. doi:10.1111/j.1467-7687.2004.00384.x. [DOI] [PubMed] [Google Scholar]
- Lalonde CE, Werker JF. Cognitive influences on cross-language speech perception in infancy. Infant Behavior & Development. 1995;18(4):459–475. [Google Scholar]
- Mandell DS, Novake MM, Zubritsky CD. Factors associated with age of diagnosis among children with autism spectrum disorders. Pediatrics. 2005;116:1480–1486. doi: 10.1542/peds.2005-0185. doi:10.1542/peds.2005-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marler P, Peters S. Selective vocal learning in a sparrow. Science. 1977;198:519–521. doi: 10.1126/science.198.4316.519. [DOI] [PubMed] [Google Scholar]
- Motiwala SS, Gupta S, Lilly MB, Ungar WJ, Coyte PC. The cost-effectiveness of expanding intensive behavioural intervention to all autistic children in Ontario: in the past year, several court cases have been brought against provincial governments to increase funding for Intensive Behavioural Intervention (IBI). This economic evaluation examines the costs and consequences of expanding an IBI program. Health Policy. 2006;1:135–51. [PMC free article] [PubMed] [Google Scholar]
- Martin A, Onishi KH, Vouloumanos A. Understanding the abstract role of speech in communication at 12 months. Cognition. 2012;123(1):50–60. doi: 10.1016/j.cognition.2011.12.003. doi:10.1016/j.cognition.2011.12.00. [DOI] [PubMed] [Google Scholar]
- Mullen E. Mullen Scales of Early Learning. American Guidance; Circle Pines, MN: 1995. [Google Scholar]
- National Research Council. Division of Behavioral and Social Sciences and Education - Committee on Educational Interventions for Children with Autism . In: Educating Children with Autism. Lord C, McGee JP, editors. National Academy Press; Washington DC: 2001. [Google Scholar]
- Osterling J, Dawson G, Munson J. Early recognition of one year old infants with autism spectrum disorder versus mental retardation: A study of first birthday party home videotapes. Development and Psychopathology. 2002;14:239–251. doi: 10.1017/s0954579402002031. DOI:10.1017/S0954579402002031. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Young G, Carter AS, Messinger D, Yirmiya N, Zwaigenbaum L, Bryson SE, Carver L, Constantino J, Dobkins K, Hutman T, Iverson J, Landa R, Rogers S, Sigman M, Stone W. Recurrence risk for autism spectrum disorders: A Baby Siblings Research Consortium study. Pediatrics. 2011;128(3):e488–e495. doi: 10.1542/peds.2010-2825. doi:10.1542/peds.2010-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R, Chawarska K, Fowler C, Cicchetti D, Volkmar F. Listen my children and you shall hear: Auditory preferences in toddlers with autism spectrum disorders. Journal of Speech Language Hearing Research. 2007;50:1350–1364. doi: 10.1044/1092-4388(2007/094). doi:10.1044/1092-4388(2007/094) [DOI] [PubMed] [Google Scholar]
- Pierce K, Carter C, Weinfeld M, Desmond J, Hazin R, Bjork R, Gallagher N. Detecting, studying, and treating autism early: the one-year well-baby check-up approach. Journal of Pediatrics. 2011 doi: 10.1016/j.jpeds.2011.02.036. Epub ahead of print. doi:10.1016/j.jpeds.2011.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritvo ER, Jorde LB, Mason-Brothers A, Freeman BJ, Pingree C, Jones MB, McMahon WM, Peterson PB, Jenson WR, Mo A. The UCLA - University of Utah epidemiologic survey of autism: Recurrence risk estimates and genetic counselling. American Journal of Psychiatry. 1989;146:1032–1036. doi: 10.1176/ajp.146.8.1032. PMid:2750975. [DOI] [PubMed] [Google Scholar]
- Surian L, Siegal M. Handbook of the neuroscience of language. Elsevier Ltd.; London, UK: 2008. Language and communication disorders in autism and asperger syndrome; pp. 377–385. [Google Scholar]
- Valenza E, Simion F, Cassia VM, Umilta C. Face preference at birth. Journal of Experimental Psychology: Human Perception and Performance. 1996;22:892–903. doi: 10.1037//0096-1523.22.4.892. doi: 10.1037/0096-1523.22.4.892. [DOI] [PubMed] [Google Scholar]
- van der Geest JN, Kemner C, Verbaten MN, van Engeland H. Gaze behavior of children with pervasive developmental disorder toward human faces: A fixation time study. Journal of Child Psychology and Psychiatry. 2002;43(5):669–678. doi: 10.1111/1469-7610.00055. [DOI] [PubMed] [Google Scholar]
- Vouloumanos A, Curtin S. Tuned to speech: How infants’ attention to speech predicts language development. Journal of Experimental Child Psychology. (Under Review) [Google Scholar]
- Vouloumanos A, Druhen MJ, Hauser MD, Huizink AT. Five-month-old infants’ identification of the sources of vocalizations. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:18867–72. doi: 10.1073/pnas.0906049106. doi: 10.1073/pnas.0906049106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vouloumanos A, Hauser M, Werker J, Martin A. The tuning of human neonates preference for speech. Child Development. 2010;81:517–527. doi: 10.1111/j.1467-8624.2009.01412.x. doi: 10.1111/j.1467-8624.2009.01412.x. [DOI] [PubMed] [Google Scholar]
- Vouloumanos A, Werker JF. Tuned to the signal: The privileged status of speech for young infants. Developmental Science. 2004;7:270–276. doi: 10.1111/j.1467-7687.2004.00345.x. doi:10.1111/j.1467-7687.2004.00345.x. [DOI] [PubMed] [Google Scholar]
- Vouloumanos A, Werker JF. Listening to language at birth: Evidence for a bias for speech in neonates. Developmental Science. 2007;10(2):159–164. doi: 10.1111/j.1467-7687.2007.00549.x. doi:doi:10.1111/j.1467-7687.2007.00549. [DOI] [PubMed] [Google Scholar]
- Wetherby AM, Prizant BM. CSBS DP Manual: Communication and Symbolic Behavior Scales Developmental Profile, first normed edition. Brookes; Baltimore: 2002. [Google Scholar]
- Whitehouse AJ, Bishop DV. Do children with autism ‘switch off’ to speech sounds? An investigation using event-related potentials. Developmental Science. 2008;11:516–524. doi: 10.1111/j.1467-7687.2008.00697.x. doi: 10.1111/j.1467-7687.2008.00697.x. [DOI] [PubMed] [Google Scholar]
- Young GS, Merin N, Rogers SJ, Ozonoff S. Gaze behavior and affect at 6 months: predicting clinical outcomes and language development in typically developing infants and infants at risk for autism. Developmental Science. 2009;12(5):798–814. doi: 10.1111/j.1467-7687.2009.00833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. International Journal of Developmental Neuroscience. 2005;23:143–152. doi: 10.1016/j.ijdevneu.2004.05.001. doi:10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Zwaigenbaum L, Thurm A, Stone W, Baranek G, Bryson S, Iverson J, Kau A, Klin A, Lord C, Landa R, Rogers S, Sigman M. Studying the emergence of autism spectrum disorders in high-risk infants: methodological and practical issues. Journal of Autism and Developmental Disorders. 2007;37(3):466–80. doi: 10.1007/s10803-006-0179-x. [DOI] [PubMed] [Google Scholar]