Abstract
Background
Childhood exposure to I-131 from the 1986 Chernobyl accident led to a sharp increase in papillary thyroid carcinoma (PTC) incidence in regions surrounding the reactor. Data concerning the association between genetic mutations in PTCs and individual radiation doses are limited.
Methods
We performed mutational analysis of 62 PTCs diagnosed in a Ukrainian cohort of patients who were <18 y.o. in 1986 and received 0.008-8.6 Gy of I-131 to the thyroid and explored associations between mutation types and I-131 dose and other characteristics.
Results
RET/PTC rearrangements were most common (35%), followed by BRAF (15%) and RAS (8%) point mutations. Two tumors carrying PAX8/PPARγ rearrangement were identified. We found a significant negative association with I-131 dose for BRAF and RAS point mutations and a significant concave association with I-131 dose, with an inflection point at 1.6 Gy and odds ratio 2.1, based on a linear-quadratic model for RET/PTC and PAX8/PPARγ rearrangements. The trends with dose were significantly different between tumors with point mutations and rearrangements. Compared to point mutations, rearrangements were associated with residence in the relatively iodine deficient Zhytomyr region, younger age at exposure or surgery, and male gender.
Conclusions
Our results provide the first demonstration of PAX8/PPARγ rearrangements in post-Chernobyl tumors and show different associations for point mutations and chromosomal rearrangements with I-131 dose and other factors. These data support the relationship between chromosomal rearrangements, but not point mutations, and I-131 exposure and point to a possible role of iodine deficiency in generation of RET/PTC rearrangements in these patients.
Keywords: Chernobyl, papillary thyroid carcinoma, iodine-131, RET/PTC, PAX8/PPARγ
INTRODUCTION
Exposure to ionizing radiation during childhood is known to cause thyroid cancer, with significant dose-dependently increased incidence, particularly in children and young adults.1 After the Chernobyl accident of 1986, residents of regions surrounding the Chernobyl nuclear power plant, including Ukraine, Belarus, and the Russian Federation, received variable doses of radioiodines through inhalation and ingestion of contaminated dairy products or vegetables. These regions experienced a dramatic rise in incidence of thyroid cancer,2 with at least 5,000 new cases observed in individuals exposed during childhood or adolescence.3 Papillary thyroid carcinoma (PTC) is known to be the principal type of thyroid carcinoma associated with radiation exposure and comprised the majority of pediatric thyroid tumors in residents of the regions surrounding Chernobyl.2,4,5
Case-control and cohort studies of post-Chernobyl thyroid cancers have demonstrated that the risk of thyroid carcinoma is strongly related to I-131 dose absorbed by the thyroid.6-9 The reported excess relative risk per unit of dose (Gy) are between 2 to 5. Additionally, age at exposure and iodine deficiency have been found to modify the I-131 related risk of thyroid cancer with higher risk per unit of dose observed in persons exposed as younger children, particularly infants,10 and in individuals living in areas with low soil iodine content.7
Somatic genetic alterations that activate the mitogen activated protein kinase (MAPK) signaling cascade are known to be “driver” mutations that play a crucial role in the development of PTC.11 These include point mutations in BRAF and RAS as well as chromosomal rearrangements such as RET/PTC. Whereas MAPK activation via point mutations is far more common in sporadic thyroid cancer, in tumors associated with radiation exposure, this pathway is most frequently activated via RET/PTC and other chromosomal rearrangement. Studies of post-Chernobyl and post-radiotherapy tumors have found RET/PTC rearrangements in up to of PTCs.12-14 The link between chromosomal rearrangements and exposure to ionizing radiation has also been supported by studies that have demonstrated induction of RET/PTC in human thyroid cell lines and tissue xenografts in SCID mice by X-ray or γ-radiation.15,16 Recent studies have led to better understanding of mechanisms by which radiation exposure induces chromosomal rearrangements. Studies of both RET/PTC and TRK rearrangements have shown that the gene loci involved in fusions lie in close spatial proximity to one another within the human thyroid cell nucleus at the time of exposure,17-19 likely predisposing to recombination of adjacent chromosomal regions radiation-induced DNA damage.
However, the association of chromosomal rearrangements or other mutational events with individual radiation doses in humans is not well established. Among PTCs that individuals exposed to predominantly γ-radiation from the atomic bombings in Hiroshima and Nagasaki, higher doses were associated with higher prevalence of RET/PTC rearrangements and lower prevalence of BRAF point mutations.20,21 By contrast, no significant association between RET/PTC activation and individual I-131doses was found in one post-Chernobyl study of cancer occurring in the Bryansk oblast of the Russian Federation.22 The prevalence of another rearrangement type, PAX8/PPARγ, known to occur in follicular thyroid cancer and only occasionally found in PTC,11 has not been studied in post-Chernobyl tumors.
Herein, we report the results of mutational analysis of a series of post-Chernobyl PTCs from Ukrainian individuals with measurement-based estimates of I-131 dose to the thyroid reconstructed as part of the Ukrainian-American cohort study.23 Based on the aforementioned experimental and human data, we hypothesized that RET/PTC rearrangements represent a common genetic events in these cancers and chromosomal rearrangements and point mutations have different association with I-131 dose. The obtained results demonstrate the predominance of chromosomal rearrangements in these tumors, show for the first time the occurrence of PAX8/PPARγ rearrangements in post-Chernobyl tumors, and establish associations of specific genetic alterations with I-131 doses and other patient characteristics.
MATERIALS AND METHODS
Patients and tissue samples
Cases included patients who were part of the Ukrainian-American cohort study and underwent surgery for suspected thyroid carcinoma.23 The cohort is composed of 13,243 Ukrainian residents, less than 18 years old at the time of the Chernobyl accident, with individual radioactivity measurements taken within two months after the accident. After four sequential screening examinations, 110 thyroid carcinomas, including 104 PTCs, were diagnosed between 1998 and 2008 at the Laboratory of Morphology of Endocrine System of the Institute of Endocrinology and Metabolism (IEM, Kyiv, Ukraine).24 The International Pathology Panel, within the Chernobyl Tissue Bank (CTB), reviewed all pathological diagnoses. Seventy five of 104 cases of PTC had at least one frozen tissue specimen from which DNA or RNA were extracted at IEM or Imperial College (London, UK). Nucleic acids from 74 PTCs were received through the CTB. Four cases from a separate cohort exposed in utero were excluded. Eight cases that lacked either DNA (n=3) or RNA (n=5) were also excluded.
Estimation of I-131 thyroid doses
Dosimetric methods have been described in detail.25,26 Briefly, individual I-131 thyroid doses and their uncertainties were estimated from thyroid radioactivity measurements, data on dietary and lifestyle habits, and environmental transfer models using a Monte-Carlo procedure with 1,000 realizations per individual.26 For the analysis, we used the arithmetic mean of each individual’s 1,000 realizations as the best estimate of I-131 dose corrected for thyroid masses typical of the Ukrainian population.6
Nucleic Acids
Tumor DNA and RNA were obtained through CTB. The samples were received with a CTB code that was later linked with a code for the individual in the Ukrainian-American study.23
Detection of point mutations
All tumors for which DNA was available were tested for point mutations in BRAF (V600E and K601E), NRAS (codon 61), HRAS (codon 61), and KRAS (codons 12/13) genes using fluorescence melting curve analysis as previously described.27 Briefly, the samples were amplified on the LightCycler (Roche Diagnostics, Indianapolis, IN) using the LightCycler FASTStart DNA Master Mix (Roche) and specific probes.27 Post-PCR melting curves were compared to those from control tumors known to have/lack specific point mutations. All mutations were confirmed by Sanger sequencing.
Detection of chromosomal rearrangements
All tumors for which RNA was available were screened for RET/PTC1, RET/PTC3, and PAX8/PPARγ using real-time RT-PCR. Tumor RNA was reverse transcribed and amplified on the 7500 Real Time PCR System (Applied Biosystems) using the Taqman Universal PCR Master Mix (Applied Biosystems) and specific probes.27 For PAX8/PPARγ, a ΔCt of <10 cycles, as compared to the amplification of GAPDH, was used as a cut-off. For PAX8/PPARγ and RET/PTC3, the presence of the rearrangement was confirmed by conventional RT-PCR and agarose gel electrophoresis. All chromosomal rearrangements were confirmed by Sanger sequencing.
Statistical analysis
Mutation prevalence data was analyzed using standard logistic regression modeling. The probability of a BRAF or RAS positive mutation for the relevant endpoint given effect modifying variables: age at surgery a, gender s, oblast O and Chernobyl-associated radiation dose D (in Gy), is given by:
The model is fitted by binomial maximum likelihood 28 using Epicure. Age (at surgery) was centered by subtracting 25 years to aid convergence of fitted models. For chromosomal rearrangements, the linear and quadratic terms for dose (dose+dose2) were used, while for point mutations a linear model in dose sufficed. Unless otherwise stated, all confidence intervals were profile-likelihood based.28 All tests were two-sided and based on the likelihood-ratio test.29 Adjustments were made for age at surgery, gender, oblast of residence at the time of screening and dose because of indications of significant or borderline significant effects on mutation rates. Oblast at the time of screening compared with that at the time of exposure differed for only three patients. Tests of heterogeneity were performed as described by Pierce and Preston.30 Analysis of time from exposure to surgery used a standard linear regression model, using log-transformed time since exposure. All linear regression analyses were performed using Stata. Mean age at exposure was compared by two sample t-test.
RESULTS
Case characteristics
The case series includes 26 males (42%) and 36 females (58%) living in areas surrounding Chernobyl, i.e. the Zhytomyr (n=17; 27.4%), Kyiv (n=12; 19.4%) or Chernihiv (n=33; 53.2%) oblasts, who were between 5 months and 17 years old (mean, 8.0 years) at the time of the Chernobyl accident. The estimated I-131 dose for patients in the study ranged from 0.008 Gy to 8.6 Gy, with a mean dose of 1.3 Gy. Surgical removal of PTCs occurred between October, 1998 and December, 2007, with time between exposure and surgery ranging from 12.5 to 21.6 years (mean, 16.5 years).
Mutation analysis
RET/PTC rearrangement was the most common genetic alteration and was found in 22 (35%) cases, including 14 RET/PTC1 and 8 RET/PTC3 rearrangements (Table 1). Point mutations in the BRAF and RAS genes were found in 9 (15%) and 5 (8%) of the tumors, respectively. All nine BRAF mutations were V600E. Four RAS mutations were detected in NRAS codon 61 and one in HRAS codon 61. No KRAS mutations were found in codons 12 and 13. Additionally, we studied these tumors for PAX8/PPARγ rearrangement, a prototypic genetic alteration found in thyroid follicular carcinoma that occurs with lower prevalence in the follicular variant of PTC. Two tumors were positive for PAX8/PPARγ; both were of the follicular variant of PTC. In both cases, the fusions were between exon 9 of PAX8 and exon 1 of PPARγ, with several expected splice variants of the chimeric PAX8/PPARγ transcripts detected. One tumor had more than one mutation, harboring an NRAS point mutation in codon 61 and PAX8/PPARγ rearrangement. Twenty-five (40%) tumors revealed none of the studied mutations.
Table 1.
Genetic alterations and exposure-related characteristics among cases of thyroid cancer developed after the Chernobyl accident in Ukraine
| Genetic Alteration | Mutation frequency |
I-131 dose, mean (Gy) |
Age at exposure, mean (yr) |
Age at surgery, mean (yr) |
Time since exposure, mean (yr) |
|---|---|---|---|---|---|
| RET/PTC1 | 14 (22%) | 1.04 | 6.4 | 23.6 | 16.9 |
| RET/PTC3 | 8 (13%) | 1.54 | 6.3 | 20.1 | 13.7 |
| BRAF | 9 (15%) | 0.27 | 10.2 | 27.1 | 16.8 |
| RAS * | 5 (8%) | 0.20 | 10.9 | 29.4 | 18.6 |
| PAX8/PPARγ * | 2 (3%) | 0.62 | 12.2 | 25.8 | 13.5 |
| No known mutation | 25 (40%) | 1.97 | 7.8 | 24.4 | 16.6 |
| Total/overall | 62 (100%) | 1.27 | 8.1 | 24.6 | 16.5 |
One case was found to be positive for both NRAS mutation and PAX8/PPARγ rearrangement.
Univariate analysis of mutation type and exposure-related characteristics
Patients with tumors positive for BRAF or RAS point mutations had the lowest average dose of I-131 (0.27 Gy and 0.20 Gy, respectively), significantly lower than that for all other patients (p<0.001). Patients with tumors harboring RET/PTC1 or RET/PTC3 rearrangements received average doses of 1.04 Gy and 1.54 Gy, respectively. Patients with tumors negative for any of these mutations had the highest average dose (1.97 Gy). Additionally, as compared to all other patients, patients with tumors harboring RET/PTC1 or RET/PTC3 were significantly younger at the time of exposure (6.4 and 6.3 years, respectively) whereas patients with BRAF or RAS mutations were significantly older at the time of exposure (10.2 and 10.9 years, respectively) (p=0.01 for both). In our case series, age at exposure correlated with age at surgery (or attained age) (Pearson correlation coefficient, r2=0.85). Thus, patients with tumors having RET/PTC1 or RET/PTC3 rearrangements were also younger at the time of surgery (mean age 23.6 years and 20.1 years, respectively) (p=0.007) and patients with tumors positive for BRAF or RAS mutations were older (mean age 27.1 years and 29.4 years, respectively) (p=0.002) than other cases. The mean time between exposure and surgery for RET/PTC3 positive cases was 13.7 years, significantly shorter than that for all other cases combined (p<0.001) (Table 1).
Multivariate analysis of tumors positive for BRAF or RAS point mutations
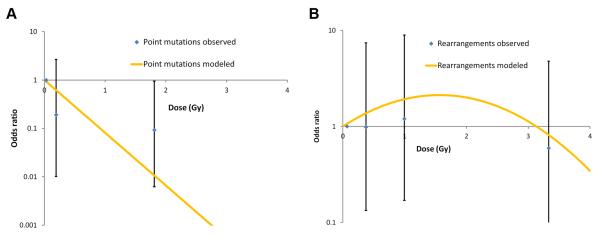
Factors independently associated with tumors harboring BRAF or RAS point mutations, as compared to all other tumors, are shown in Table 2. Adjusting for age at surgery, gender, and oblast of residence, there was a significant negative association between these point mutations and I-131 dose (p=0.001). The estimated regression coefficient for the dose term based on a log-linear model was −2.51 per Gy (95% CI: −5.42, −0.78) (Fig. 1A). In addition, the point mutations were associated with older age at surgery (p=0.014) and female gender (p=0.002).
Table 2.
Factors associated with BRAF or RAS point mutations in multivariate analysis
|
BRAF or RAS mutation positive |
BRAF or RAS mutation negative |
|||
|---|---|---|---|---|
| Characteristic | % or mean (SD) | % or mean (SD) | OR | 95% CI |
| I-131 dose, Gy | ||||
| 0.008-<0.05 | 35.7 | 3.9 | 1.00 | Referent |
| 0.05-<0.35 | 28.6 | 19.6 | 0.19 | (0.01, 2.67) |
| 0.35-8.60 | 35.7 | 76.5 | 0.09 | (0.01, 0.95) |
| P trend | 0.0011 | |||
| Oblast of residence | ||||
| Chernihiv | 71.4 | 47.1 | 1.00 | Referent |
| Kyiv | 21.4 | 21.6 | 0.24 | (0.02, 2.33) |
| Zhytomyr | 7.1 | 31.4 | 0.07 | (0.00, 0.82) |
| P heterogeneity | 0.086 | |||
| Age at surgery, yr | 28.1 (4.2) | 23.4 (4.9) | 1.26 | (1.05, 1.61) |
| P trend | 0.014 | |||
| Sex | ||||
| Male | 14.3 | 47.1 | 1.00 | Referent |
| Female | 85.7 | 52.9 | 21.59 | (2.69, 349.32) |
| P | 0.002 |
Based on linear dose-response model.
Figure 1. Dose-response relationship for point mutations and chromosomal rearrangements.
A. Dose-response relationship for patients with tumors harboring BRAF or RAS point mutations. Odds ratio data for the relationship between chromosomal rearrangement and estimated I-131 dose fitted to a linear quadratic model with adjustments for a sex, oblast, age of surgery, and dose. B. Dose-response relationship for patients with tumors harboring RET/PTC or PAX8/PPARγ chromosomal rearrangements. Odds ratio data for the relationship between chromosomal rearrangement and estimated I-131 dose fitted to a linear quadratic model with adjustments for a sex, oblast, age of surgery, and dose.
Multivariate analysis of tumors positive for RET/PTC or PAX8/PPARγ chromosomal rearrangements
There was a significant negative dose-response relationship using continuous I-131 dose (p=0.040, not shown), which was improved at marginal levels of statistical significance (p=0.053) by addition of a quadratic term in dose (dose2); overall the linear-quadratic dose response was statistically significant (2 df trend, p=0.019; Table 3) with estimated regression coefficients of 0.96 per Gy (95% CI: −0.54, 2.61) for the linear term and of −0.31 per Gy2 for the quadratic term (95% CI: −0.70, 0.00). While the parameters of the dose-response function are imprecise, as evidenced by wide confidence intervals, the data suggest a non-monotone relationship for RET/PTC or PAX8/PPARγ-positive tumors and dose with increased risk at low-to-moderate doses and decreased risk at high doses (Fig. 1B). The latter may reflect the fact that cases with no known mutation were associated with the highest average dose overall (p=0.012). Indeed, among the patients who received doses above 1.6 Gy, 10 out of 16 (62.5%), had tumors that were negative for all mutations studied.
Table 3.
Factors associated with RET/PTC or PAX8/PPARγ rearrangements in multivariate analysis
|
RET/PTC or PAX8/PPARγ positive |
RET/PTC or PAX8/PPARγ negative |
|||
|---|---|---|---|---|
| Characteristic | % or mean (SD) | % or mean (SD) | OR | 95% CI |
| I-131 dose, Gy | ||||
| 0.008-<0.4 | 25.0 | 39.5 | 1.00 | Referent |
| 0.4-<0.91 | 25.0 | 23.7 | 1.38 | (0.28, 7.00) |
| 0.91-<1.63 | 25.0 | 10.5 | 2.47 | (0.34, 19.05) |
| 1.63-8.60 | 25.0 | 26.3 | 0.77 | (0.13, 4.13) |
| P trend | 0.0191 | |||
| Oblast of residence | ||||
| Chernihiv | 45.8 | 57.9 | 1.00 | Referent |
| Kyiv | 12.5 | 23.7 | 0.88 | (0.13, 5.13) |
| Zhytomyr | 41.7 | 18.4 | 11.66 | (2.22, 82.52) |
| P heterogeneity | 0.007 | |||
| Age at surgery, yr | 22.6 (4.5) | 25.8 (5.3) | 0.79 | (0.66, 0.91) |
| P trend | 0.001 | |||
| Sex | ||||
| Male | 54.2 | 36.8 | 1.00 | Referent |
| Female | 45.8 | 63.2 | 0.27 | (0.06, 0.96) |
| P | 0.043 |
Based on linear quadratic model (dose+dose2).
In addition to dose, the presence of chromosomal rearrangement was positively associated with residence in the Zhytomyr oblast (p=0.007 for 2 df test of oblast differences) and negatively associated with female gender (p=0.043) and attained age (p=0.001) (Table 3). The presence of RET/PTC3 rearrangement compared to all other tumors was negatively associated with time between exposure and surgery, even when adjusted for I-131 dose and age at exposure (p=0.001) or attained age (p=0.012, not shown). No association with time from exposure to surgery was found for any other rearrangements or point mutations.
Comparison between tumors positive for point mutations and those harboring chromosomal rearrangements
Direct comparison of tumors with point mutations (BRAF or RAS) and chromosomal rearrangements (RET/PTC or PAX8/PPARγ) using multivariate logistic regression demonstrated a significant difference in trends with I-131 dose (p=0.020; Table 4). Additionally, age at surgery (p<0.001), gender (p<0.001), and oblast of residence (p=0.003) were significantly and independently associated with presence of point mutations relative to chromosomal rearrangements. Compared to BRAF or RAS positive tumors, RET/PTC or PAX8/PPARγ positive tumors were likely to occur in individuals with higher I-131 doses, younger age at surgery (and therefore younger at exposure), in males, and residents of Zhytomyr oblast (Table 4).
Table 4.
Comparison of RET/PTC or PAX8/PPARγ positive tumors with BRAF or RAS mutation positive tumors according to selected patient characteristics in thyroid cancers that developed after the Chernobyl accident
| Characteristic | Relative risk of rearrangements vs point mutations (95% CI) |
P heterogeneity1 | |
|---|---|---|---|
| I-131 dose, Gy | 8.00 (1.30, 150.96) | 0.020 | |
| Oblast of residence | Kyiv vs Chernihiv | 4.12 (0.22, 100.89) | 0.003 |
| Zhytomyr vs Chernihiv | 139.07 (7.13, 5843.00) | ||
| Age at surgery, yr | 0.62 (0.47, 0.79) | <0.001 | |
| Sex | Female vs Male | 0.01 (0.00, 0.16) | <0.001 |
Based on multivariate logistic regression models.
DISCUSSION
Our study of post-Chernobyl thyroid tumors confirm the high frequency of chromosomal rearrangements, particularly RET/PTC, and lower frequency of BRAF and RAS point mutations, compared to those typically observed in sporadic tumors,11 and report for the first time the occurrence of PAX8/PPARγ rearrangements in post-Chernobyl PTCs. Moreover, we identified significant independent differences between chromosomal rearrangements and point mutations with respect to I-131 thyroid doses, gender, oblast of residence, and age at exposure or surgery, suggesting that these tumors have distinct etiologies, i.e. tumors with chromosomal rearrangements, but not with point mutations, are likely to be radiation-related.
Unique features of this study include individual I-131 thyroid doses based on radioactivity measurements,6,25 well-characterized tumors detected through standardized medical examinations, and comprehensive molecular profiling. Consequently, we were able to draw associations between specific mutation types and I-131 dose and other contributing factors. The association between I-131 thyroid dose and presence of chromosomal rearrangements followed a linear quadratic function, indicating a positive relationship in the low-to-moderate dose range and a negative relationship at high doses. It is likely that tumors with no known mutations accounted for the observed downturn at high doses, as their proportion relative to other tumors reached 62% at doses of 1.6 Gy or higher. Interestingly, a positive linear dose-response for RET/PTC rearrangements has been reported in thyroid tumors that developed among the atomic bomb survivors of Hiroshima and Nagasaki,20,21 but very few cases in these studies received doses higher than 1.6 Gy.20 No significant association was found for RET/PTC activation with individual I-131 doses in the study of PTCs from Bryansk oblast of the Russian Federation.22 However, these patients were exposed to lower doses (mean 0.363 Gy for childhood cancers and 0.039 Gy for adult cancers) than those in the current study (mean 1.3 Gy). The inconsistencies in dose-response findings across these studies are likely to result from different dose and age range, geographic origin, uncertainties in dose estimates, or limited sample size.
By contrast, patients with tumors harboring BRAF and RAS point mutations received the lowest average I-131 doses, were oldest at the time of the Chernobyl accident or at surgery, and were predominantly female. The strong negative association for point mutation-positive tumors with dose found when comparing BRAF and RAS positive tumors against all others is consistent with that in atomic bomb survivors.20 The negative association with dose together with the fact that the BRAF mutation is most commonly found in sporadic thyroid cancer,11,31 incidence of which rapidly increases during the third decade of life and is three times as common in women,32,33 suggest that BRAF and RAS positive tumors found in this cohort were likely to develop via pathogenic mechanisms more typical of sporadic thyroid cancer.
More than one-third of the tumors in our study had no identifiable mutations, and exhibited their own unique characteristics. The age at exposure or surgery for individuals with such tumors was higher than that of patients with RET/PTC rearrangements, but lower than that of patients with point mutations. These patients also received the highest I-131 thyroid doses. Therefore, the development of these tumors is likely to be related to radiation exposure, but involved other, unknown mutations. A recent study of PTCs in atomic bomb survivors found ALK rearrangements, although they were detected at very low levels and using highly sensitive analyses, leaving the biological significance of this finding unclear.34 Other chromosomal rearrangements that occur very rarely in sporadic PTC but have been seen with higher frequency in PTCs following radiation exposure include BRAF/AKAP9 and TRK rearrangements.11 It is possible that these unmeasured, rare rearrangements may partially compose the set of tumors with as yet unknown mutations in our study.
Another unexpected finding in the current study was a strong association between RET/PTC and residence in Zhytomyr oblast. Although study participants from Zhytomyr received higher I-131 doses than those of the neighboring Ukrainian oblasts, the association with RET/PTC was independent of dose. The Zhytomyr oblast has no noticeable geographic or ethnic differences, but is associated with relative iodine deficiency. Indeed, residents of Zhytomyr have been shown to have lower levels of urinary iodine excretion than those of Kyiv or Chernihiv oblasts.35 Iodine deficiency has been shown to contribute to the risk of post-Chernobyl thyroid cancer in Belarus,3,7 and may possibly be responsible for the increased frequency of RET/PTC in tumors from Zhytomyr oblast residents. It is conceivable that more avid trapping and intracellular metabolism of I-131 by thyroid cells under conditions of high thyroid stimulating hormone (TSH) stimulation produce more extensive damage to the nuclear DNA, resulting in RET/PTC rearrangement. If confirmed, this would provide a biological basis for the higher risk of radiation-induced thyroid cancer in areas of relative iodine deficiency.
This study also identified PAX8/PPARγ rearrangements in post-Chernobyl tumors. This rearrangement is known to occur with high frequency in another type of thyroid cancer, follicular carcinoma, and with much lower frequency in the follicular variant of PTC.11 Both tumors positive for PAX8/PPARγ rearrangement in our study were follicular variant of PTC. In one large study, tumors with PAX8/PPARγ rearrangements were more common in patients with a history of preceding non-thyroid cancer,36 which may implicate therapeutic radiation. However, to our knowledge, PAX8/PPARγ has not been previously reported in post-Chernobyl or other radiation-associated cancers.
In summary, this study provides strong support for the association between chromosomal rearrangements and exposure to I-131 from the Chernobyl accident. Furthermore, our findings point to a possible role of iodine deficiency in generation of RET/PTC rearrangements. Finally, since a significant proportion of tumors in our study had no detectable mutations and were associated with high radiation doses, we hypothesize that undiscovered mutational events important in radiation-induced thyroid carcinogenesis must exist.
Acknowledgements
It is with great sadness that we report the death of our colleague Dr. Elaine Ron who was one of the original investigators involved in design of the study. We greatly appreciate her contribution and support. We thank Dr. Gerry Thomas and staff of the Chernobyl Tissue Bank for providing samples. We are grateful to dosimetry team including Drs. Ilya Likhtarev, Lina Kovgan (Radiation Protection Institute, Ukrainian Academy of Technological Sciences), Andre Bouville (formerly with the NCI, NIH), and Vladimir Drozdovich (NCI, NIH) for dose reconstruction efforts. We thank Dr. Alice Sigurdson (NCI, NIH) for critical review of the manuscript.
Financial support: Supported by the NIH grant R01 CA88041 and in part by the Intramural Research Program of the NIH, National Cancer Institute.
Footnotes
Conflict of interest, financial disclosures: The authors declare no conflict of interest or any financial disclosures.
REFERENCES
- 1.Ron E, Lubin JH, Shore RE, et al. Thyroid cancer after exposure to external radiation: a pooled analysis of seven studies. Radiat Res. 1995;141:259–277. [PubMed] [Google Scholar]
- 2.Stsjazhko VA, Tsyb AF, Tronko ND, Souchkevitch G, Baverstock KF. Childhood thyroid cancer since accident at Chernobyl. BMJ. 1995;310:801. doi: 10.1136/bmj.310.6982.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cardis E, Howe G, Ron E, et al. Cancer consequences of the Chernobyl accident: 20 years on. J Radiol Prot. 2006;26:127–140. doi: 10.1088/0952-4746/26/2/001. [DOI] [PubMed] [Google Scholar]
- 4.Pacini F, Vorontsova T, Demidchik EP, et al. Post-Chernobyl thyroid carcinoma in Belarus children and adolescents: comparison with naturally occurring thyroid carcinoma in Italy and France. J Clin Endocrinol Metab. 1997;82:3563–3569. doi: 10.1210/jcem.82.11.4367. [DOI] [PubMed] [Google Scholar]
- 5.LiVolsi VA, Abrosimov AA, Bogdanova T, et al. The Chernobyl thyroid cancer experience: pathology. Clin Oncol (R Coll Radiol) 2011;23:261–267. doi: 10.1016/j.clon.2011.01.160. [DOI] [PubMed] [Google Scholar]
- 6.Brenner AV, Tronko MD, Hatch M, et al. I-131 dose response for incident thyroid cancers in Ukraine related to the Chornobyl accident. Environ Health Perspect. 2011;119:933–939. doi: 10.1289/ehp.1002674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cardis E, Kesminiene A, Ivanov V, et al. Risk of thyroid cancer after exposure to 131I in childhood. J Natl Cancer Inst. 2005;97:724–732. doi: 10.1093/jnci/dji129. [DOI] [PubMed] [Google Scholar]
- 8.Davis S, Stepanenko V, Rivkind N, et al. Risk of thyroid cancer in the Bryansk Oblast of the Russian Federation after the Chernobyl Power Station accident. Radiat Res. 2004;162:241–248. doi: 10.1667/rr3233. [DOI] [PubMed] [Google Scholar]
- 9.Tronko MD, Howe GR, Bogdanova TI, et al. A cohort study of thyroid cancer and other thyroid diseases after the chornobyl accident: thyroid cancer in Ukraine detected during first screening. J Natl Cancer Inst. 2006;98:897–903. doi: 10.1093/jnci/djj244. [DOI] [PubMed] [Google Scholar]
- 10.Nikiforov YE. Radiation-induced thyroid cancer: what we have learned from chernobyl. Endocr Pathol. 2006;17:307–317. doi: 10.1007/s12022-006-0001-5. [DOI] [PubMed] [Google Scholar]
- 11.Nikiforov YE, Nikiforova MN. Molecular genetics and diagnosis of thyroid cancer. Nat Rev Endocrinol. 2011;7:569–580. doi: 10.1038/nrendo.2011.142. [DOI] [PubMed] [Google Scholar]
- 12.Bounacer A, Wicker R, Caillou B, et al. High prevalence of activating ret proto-oncogene rearrangements, in thyroid tumors from patients who had received external radiation. Oncogene. 1997;15:1263–1273. doi: 10.1038/sj.onc.1200206. [DOI] [PubMed] [Google Scholar]
- 13.Nikiforov YE, Rowland JM, Bove KE, Monforte-Munoz H, Fagin JA. Distinct pattern of ret oncogene rearrangements in morphological variants of radiation-induced and sporadic thyroid papillary carcinomas in children. Cancer Res. 1997;57:1690–1694. [PubMed] [Google Scholar]
- 14.Rabes HM, Demidchik EP, Sidorow JD, et al. Pattern of radiation-induced RET and NTRK1 rearrangements in 191 post-chernobyl papillary thyroid carcinomas: biological, phenotypic, and clinical implications. Clin Cancer Res. 2000;6:1093–1103. [PubMed] [Google Scholar]
- 15.Caudill CM, Zhu Z, Ciampi R, Stringer JR, Nikiforov YE. Dose-dependent generation of RET/PTC in human thyroid cells after in vitro exposure to gamma-radiation: a model of carcinogenic chromosomal rearrangement induced by ionizing radiation. J Clin Endocrinol Metab. 2005;90:2364–2369. doi: 10.1210/jc.2004-1811. [DOI] [PubMed] [Google Scholar]
- 16.Mizuno T, Iwamoto KS, Kyoizumi S, et al. Preferential induction of RET/PTC1 rearrangement by X-ray irradiation. Oncogene. 2000;19:438–443. doi: 10.1038/sj.onc.1203343. [DOI] [PubMed] [Google Scholar]
- 17.Zhu Z, Ciampi R, Nikiforova MN, Gandhi M, Nikiforov YE. Prevalence of RET/PTC rearrangements in thyroid papillary carcinomas: effects of the detection methods and genetic heterogeneity. J Clin Endocrinol Metab. 2006;91:3603–3610. doi: 10.1210/jc.2006-1006. [DOI] [PubMed] [Google Scholar]
- 18.Nikiforova MN, Stringer JR, Blough R, Medvedovic M, Fagin JA, Nikiforov YE. Proximity of chromosomal loci that participate in radiation-induced rearrangements in human cells. Science. 2000;290:138–141. doi: 10.1126/science.290.5489.138. [DOI] [PubMed] [Google Scholar]
- 19.Roccato E, Bressan P, Sabatella G, et al. Proximity of TPR and NTRK1 rearranging loci in human thyrocytes. Cancer Res. 2005;65:2572–2576. doi: 10.1158/0008-5472.CAN-04-4294. [DOI] [PubMed] [Google Scholar]
- 20.Hamatani K, Eguchi H, Ito R, et al. RET/PTC rearrangements preferentially occurred in papillary thyroid cancer among atomic bomb survivors exposed to high radiation dose. Cancer Res. 2008;68:7176–7182. doi: 10.1158/0008-5472.CAN-08-0293. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi K, Eguchi H, Arihiro K, et al. The presence of BRAF point mutation in adult papillary thyroid carcinomas from atomic bomb survivors correlates with radiation dose. Mol Carcinog. 2007;46:242–248. doi: 10.1002/mc.20277. [DOI] [PubMed] [Google Scholar]
- 22.Tuttle RM, Lukes Y, Onstad L, et al. ret/PTC activation is not associated with individual radiation dose estimates in a pilot study of neoplastic thyroid nodules arising in Russian children and adults exposed to Chernobyl fallout. Thyroid. 2008;18:839–846. doi: 10.1089/thy.2008.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stezhko VA, Buglova EE, Danilova LI, et al. A cohort study of thyroid cancer and other thyroid diseases after the Chornobyl accident: objectives, design and methods. Radiat Res. 2004;161:481–492. doi: 10.1667/3148. [DOI] [PubMed] [Google Scholar]
- 24.Bogdanova TI, Zurnadzhy LY, Greenebaum E, et al. A cohort study of thyroid cancer and other thyroid diseases after the Chornobyl accident: pathology analysis of thyroid cancer cases in Ukraine detected during the first screening (1998-2000) Cancer. 2006;107:2559–2566. doi: 10.1002/cncr.22321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Likhtarev I, Bouville A, Kovgan L, Luckyanov N, Voilleque P, Chepurny M. Questionnaire- and measurement-based individual thyroid doses in Ukraine resulting from the Chornobyl nuclear reactor accident. Radiat Res. 2006;166:271–286. doi: 10.1667/RR3545.1. [DOI] [PubMed] [Google Scholar]
- 26.Likhtarev I, Minenko V, Khrouch V, Bouville A. Uncertainties in thyroid dose reconstruction after Chernobyl. Radiat Prot Dosimetry. 2003;105:601–608. doi: 10.1093/oxfordjournals.rpd.a006310. [DOI] [PubMed] [Google Scholar]
- 27.Nikiforov YE, Steward DL, Robinson-Smith TM, et al. Molecular testing for mutations in improving the fine-needle aspiration diagnosis of thyroid nodules. J Clin Endocrinol Metab. 2009;94:2092–2098. doi: 10.1210/jc.2009-0247. [DOI] [PubMed] [Google Scholar]
- 28.McCullagh PNJ. Generalized linear models Monographs on statistics and applied probability. Vol. 37. Chapman and Hall/CRC; Boca Raton, FA: 1989. pp. 1–526. [Google Scholar]
- 29.Cox D, Hinkley DV. Theoretical statistics. Chapman and Hall; London: 1974. [Google Scholar]
- 30.Pierce DA, Preston DL. Joint analysis of site-specific cancer risks for the atomic bomb survivors. Radiat Res. 1993;134:134–142. [PubMed] [Google Scholar]
- 31.Kimura ET, Nikiforova MN, Zhu Z, Knauf JA, Nikiforov YE, Fagin JA. High prevalence of BRAF mutations in thyroid cancer: genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res. 2003;63:1454–1457. [PubMed] [Google Scholar]
- 32.Albores-Saavedra J, Henson DE, Glazer E, Schwartz AM. Changing patterns in the incidence and survival of thyroid cancer with follicular phenotype--papillary, follicular, and anaplastic: a morphological and epidemiological study. Endocr Pathol. 2007;18:1–7. doi: 10.1007/s12022-007-0002-z. [DOI] [PubMed] [Google Scholar]
- 33.Hayat MJ, Howlader N, Reichman ME, Edwards BK. Cancer statistics, trends, and multiple primary cancer analyses from the Surveillance, Epidemiology, and End Results (SEER) Program. Oncologist. 2007;12:20–37. doi: 10.1634/theoncologist.12-1-20. [DOI] [PubMed] [Google Scholar]
- 34.Hamatani K, Mukai M, Takahashi K, Hayashi Y, Nakachi K, Kusunoki Y. Rearranged anaplastic lymphoma kinase (ALK) gene in adult-onset papillary thyroid cancer among atomic-bomb survivors. Thyroid. 2012 doi: 10.1089/thy.2011.0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tronko M, Kravchenko V, Fink D, et al. Iodine excretion in regions of Ukraine affected by the Chornobyl Accident: experience of the Ukrainian-American cohort study of thyroid cancer and other thyroid diseases. Thyroid. 2005;15:1291–1297. doi: 10.1089/thy.2005.15.1291. [DOI] [PubMed] [Google Scholar]
- 36.Nikiforova MN, Biddinger PW, Caudill CM, Kroll TG, Nikiforov YE. PAX8-PPARgamma rearrangement in thyroid tumors: RT-PCR and immunohistochemical analyses. Am J Surg Pathol. 2002;26:1016–1023. doi: 10.1097/00000478-200208000-00006. [DOI] [PubMed] [Google Scholar]