Abstract
Background
Professional society guidelines recommend follow up colonoscopy in patients with resected colonic adenomas. However, adherence to guideline recommendations in routine clinical practice has not been well characterized.
Methods
Using a population-based sample of Medicare beneficiaries, we identified all patients aged 70 and older with a claim for colonoscopy with polypectomy or hot biopsy in 2001–2004. Medicare claims through 2009 identified colonoscopy within the following five years, as well as FOBT, sigmoidoscopy and barium enema.
Results
12,771 patients were included. At five years, 45.7% received another colonoscopy, with 32.3% of procedures including polypectomy. Rates of FOBT, flexible sigmoidoscopy and barium enema at five years were 54.0%, 3.8% and 2.9%, respectively. There was a marked decrease in repeat colonoscopy at 1, 3, and 5 years with more recent years of index procedures. Other predictors of undergoing repeat colonoscopy were younger age, African American race, and a colonoscopy prior to the index examination. There was no association with physician specialty. The decreasing use of colonoscopy with time was maintained in a multivariable analysis.
Conclusions
In this sample of elderly Medicare beneficiaries, there was underuse of follow up colonoscopy at 5 years after polypectomy, with fewer than half receiving a repeat examination. In particular, the use of this procedure has decreased over the four-year study period. Coupled with other data showing overuse of follow up colonoscopy in patients without polyps, there appears to be significant discordance between guidelines and actual practice.
Keywords: Colonic polyps, colonoscopy, Medicare, health services
Introduction
Colorectal cancer is currently the second leading cause of cancer mortality in the United States, accounting for approximately 52,000 deaths and 152,000 new cases yearly (1). The vast majority of colorectal cancers are thought to arise from colon adenomas (2), which are present is as many as 40% of patients in the targeted ages for colorectal cancer screening.
In addition to screening, patients with adenomas removed at an index colonoscopy are typically referred for follow up colonoscopies at a more frequent time interval than patients without adenomas. The goal of these examinations, termed surveillance, is to detect recurrent adenomas or polyps that were missed at the time of the initial colonoscopy. Colonoscopies for polyp surveillance as frequently as every two years have been reimbursed under Medicare since 1998, and have been recommended by clinical practice guidelines since 1997 (3). The specific intervals recommended by guidelines have generally been extended in more recent versions, but for most patients, a follow up interval of five years is prescribed (4). These recommendations are supported by the National Polyp Study, which provided evidence that longer follow up intervals were equally effective in detecting recurrent or missed polyps (5). More frequent intervals may be justified among patients with advanced adenomas, defined as polyps > 1 cm in size or containing focal high grade dysplasia or villous features (4), as these polyps are thought to have a higher rate of progression to cancer (6).
Despite recommendations for post-polypectomy follow up, there is evidence that clinicians may not adhere to practice guidelines. Surveys of practicing endoscopists (7) and primary care physicians who refer patients for colonoscopy (8) suggest that physicians may perform surveillance in excess of guidelines. In contrast, a recently published study that used Medicare claims data reported that 46% of average-risk patients with a colonoscopy that was negative for polyps underwent a repeat examination within 7 years (9). In a recent audit of post-polypectomy follow up practices in the Prostate, Lung, Colorectal and Ovarian Cancer screening trial, the cumulative probability of a surveillance colonoscopy within 5 years was 58% in patients with an advanced adenoma and 26% in patients with no adenomas, suggesting discordance from guidelines (10).
Given the evidence that receipt of follow up colonoscopy may be discordant from practice guidelines, we conducted the present study in a population-based cohort to ascertain the use of surveillance colonoscopy among patients with a history of colonic polyps. We hypothesized that a significant proportion of patients receive follow up colonoscopy in excess of recommended guidelines and the receipt would be associated with patient and endoscopist factors.
Methods
Data Sources
The data for the study were obtained from noncancer sample of the linked Surveillance Epidemiology and End Results (SEER)-Medicare database, developed in the 1990’s as a large population-based source of information for epidemiological and health services research (11,12). The files consist of a 5% random sample of Medicare beneficiaries without cancer who reside in one of the geographic areas contained in the SEER registries. The SEER Program captures approximately 26% of the US population and within each registry, approximately 93% of patients older than 64 years are included.
Medicare claims are contained in three different files, the Carrier file, which includes provider claims, the Outpatient file, which includes claims from institutional outpatient providers, and the Medicare Provider Analysis and Review (MEDPAR) files, which includes all hospitalizations. Each Medicare claim contains diagnoses coded by the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM), and procedures coded according to Common Procedural Terminology, 4th Edition (CPT-4) or ICD-9-CM. The Carrier and Outpatient claims also include physician specialty code and an encrypted version of the physician’s unique personal identifier (UPIN), which was also used to categorize practitioners according to specialty. In addition to Medicare claims, we used the Summarized Denominator (SUMDENOM) File, which contains demographic, enrollment and entitlement information, and the ZIP Code Census File, which includes selected sociodemographic information at the ZIP code level from the 2000 Census.
The cohort of patients undergoing colonoscopy with polypectomy was identified using the 2001 through 2004 Medicare Carrier, Outpatient, and MEDPAR files. These years were selected because they include a time period in which colonoscopy was reimbursed through Medicare and allowed for a minimum of five year follow up in all patients. Patients were eligible if they were 70 years of age and older (to allow ascertainment of procedure use in the previous five years) and were receiving Medicare benefits through Part A and Part B through the entire study period. Patients who were enrolled in Medicare sponsored managed care plans during the period from 1997–2009 were excluded because of the high likelihood of incomplete claims. Patients with a diagnosis code for carcinoma-in-situ (230.3, 230.4) within 6 months (before or after) of the index colonoscopy were also excluded from the analysis. Because of the different biological mechanisms of colon cancer and the goals of surveillance, patients with inflammatory bowel disease (ulcerative colitis and Crohn’s disease) were excluded. Finally, in order to obtain a cohort with complete examinations in whom follow up guidelines are more uniform, examinations with a designation as incomplete were excluded. Follow up among surviving patients occurred through the end of 2009.
All claims for colonoscopy with polypectomy were identified by procedure codes for one or more of the following: colonoscopy with snare polypectomy (CPT-4 44393, 44394, 45383, 45385; ICD-9-CM 45.42, 45.43, 48.36) which includes both snare cautery and “cold snare” polypectomy, colonoscopy with hot biopsy of polyp (CPT-4 44392, 45384), and colonoscopy with ablation of polyps (e.g., argon plasma coagulation) (CPT-4 45383). These codes were selected based on the results of preliminary studies and prior surveys of endoscopists (13) as having a high positive predictive value for the presence of a larger polyp which is much more likely to be adenomatous. Patients were followed from the index colonoscopy through the earliest of the following events: second colonoscopy, bowel resection, death, five-year interval after the initial examination, or end of calendar year 2009.
Demographic characteristics, including age, gender and race, were obtained from the SUMDENOM file. Medicare claims from the previous five years were searched for procedure codes for colonoscopy. Claims from the previous five years through the index colonoscopy date were searched for a diagnosis that constituted increased neoplasia risk or a prior history of polyp removal. These criteria included codes for a personal history of colon polyps (V12.72), family history of gastrointestinal neoplasm (V16.0), benign neoplasm (polyp) of colon (211.3) or rectum (211.4), and previous colonoscopy or sigmoidoscopy with polypectomy (CPT-4 44392, 44393, 44394, 45333, 45338, 45339, 45383, 45384, 45385; ICD-9-CM 45.42, 45.43, 48.36). Diagnosis codes according to according to ICD-9-CM during the 365 day to 30 day interval (total 11 months) prior to the index colonoscopy were searched to derive a previously validated, weighted comorbidity index (14).
In addition, MEDPAR, Carrier and Outpatient files from the date of the index colonoscopy through the end of the follow up period were searched for the presence of claims for other colorectal procedures including fecal occult blood testing (FOBT), flexible sigmoidoscopy, and barium enema.
As a proxy for individual level measures, which were unavailable in Medicare, the ZIP Code Census files contained ecological measures of socioeconomic status. Relevant measures at the time of the index colonoscopy included median income and proportion of persons 25 and older with a high school diploma. Measures for each patient in the cohort at the time of the index colonoscopy were rank ordered and divided into quartiles according to rank.
Geographic regions were divided into Northeast, South, Midwest and West. Using the encrypted UPIN and Medicare specialty codes, physician specialty at the index colonoscopy was coded as gastroenterology, colorectal surgery, general surgery, internal medicine, family medicine, and unknown.
Analysis
The major outcome of interest was repeat colonoscopy within five years of the index procedure. Given the variable length of follow up due to death or disenrollment, we used Kaplan Meier analysis. We also used Kaplan Meier analysis to measure colonoscopy use at one and three year follow up, as well as the use of FOBT, flexible sigmoidoscopy and barium enema. Variables examined for their association with repeat colonoscopy included demographic characteristics, geographic region, comorbidity, colonoscopy prior to the index examination, endoscopist specialty, and small area socioeconomic measures.
The log-rank test was used to examine the association of individual variables with receipt of colonoscopy at one, three and five years. We then used a multivariable Cox proportional hazards model to determine the independent association of individual covariates with time to repeat colonoscopy. As the major outcome of interest was receipt of colonoscopy at five years, we censored all patients beyond five years of follow up. In a secondary analysis, we also examined factors associated with FOBT use at five years.
The protocol was approved by the Institutional Review Board, University Hospitals Case Medical Center.
Results
From the Medicare data files, we identified 33,094 patients who underwent colonoscopy with polypectomy in 2001–2004. Patients were excluded for the following non-mutually exclusive reasons: age < 70 (n=15,511), lack of enrollment in Medicare Parts A and B (n=842), enrollment in Medicare managed care plans (n=3,984), a diagnosis of inflammatory bowel disease (n=227), and a diagnosis of carcinoma-in-situ (n=34). The remaining 12,771 patients comprised the study cohort (Table 1). Consistent with the demographics of the older Medicare population, the mean age of the sample was 78.1 ± 5.0 years, 56.3% were female and 86.4% were Caucasian. Most patients had comorbidity scores of 0, 24.6% were had additional cancer risk factors (prior polyp, family history) and 22.4% underwent at least one colonoscopy prior to the index examination. Gastroenterologists performed the index colonoscopy in 65%, and the procedure included one or more of snare polypectomy in 84.5%, hot biopsy in 14.7% and ablation of polyps in 3.5%.
Table 1.
Factors Associated with Repeat Colonoscopy at 1, 3 and 5 Years in 12,998 Medicare Beneficiaries
| Variable | N (%) | Colonoscopy Year 1 (%) |
P Value | Colonoscopy Year 3 (%) |
P Value | Colonoscopy Year 5 (%) |
P Value |
|---|---|---|---|---|---|---|---|
| Age Group | |||||||
| 70–74 | 4050 (31.7) | 6.9 | 0.17 | 25.3 | 0.37 | 50.5 | <0.0001 |
| 75–79 | 4666 (36.5) | 7.7 | 26.7 | 47.3 | |||
| 80–84 | 2697 (21.1) | 8.9 | 25.4 | 42.7 | |||
| ≥ 85 | 1358 (10.6) | 9.6 | 22.6 | 31.4 | |||
| Gender | |||||||
| Female | 7196 (56.3) | 8.0 | 0.02 | 24.7 | 0.03 | 43.6 | 0.0001 |
| Male | 5575 (46.7) | 7.8 | 27.2 | 49.5 | |||
| Race | |||||||
| White | 11036 (86.4) | 7.6 | 0.002 | 25.4 | 0.0009 | 46.2 | 0.02 |
| Black | 666 (5.2) | 11.3 | 34.3 | 53.8 | |||
| Other | 847 (6.6) | 9.1 | 25.7 | 42.6 | |||
| Unknown | 222 (1.7) | 7.7 | 25.6 | 48.3 | |||
| Procedure Year | |||||||
| 2001 | 3240 (25.4) | 9.3 | 0.0004 | 34.7 | <0.0001 | 57.4 | <0.0001 |
| 2002 | 3412 (26.7) | 7.9 | 26.3 | 48.6 | |||
| 2003 | 3270 (25.6) | 7.9 | 22.5 | 42.7 | |||
| 2004 | 2849 (22.3) | 6.4 | 19.1 | 36.2 | |||
| Comorbidity | |||||||
| 0 | 7063 (55.3) | 7.2 | 0.0004 | 24.6 | <0.0001 | 45.8 | 0.38 |
| 1 | 3389 (26.5) | 7.9 | 26.3 | 47.7 | |||
| ≥ 2 | 2319 (18.2) | 10.0 | 29.2 | 45.3 | |||
| Increased risk * | |||||||
| Yes | 3139 (24.6) | 7.9 | 0.89 | 32.8 | <0.0001 | 61.3 | <0.0001 |
| No | 9632 (75.4) | 7.9 | 23.6 | 41.2 | |||
| Prior Colonoscopy | |||||||
| Yes | 2863 (22.4) | 8.6 | 0.003 | 35.6 | <0.0001 | 65.3 | <0.0001 |
| No | 9908 (77.6) | 7.7 | 23.2 | 41.2 | |||
| Specialty | |||||||
| Gastroenterology | 8288 (64.9) | 7.7 | 0.12 | 25.5 | 0.02 | 46.1 | 0.17 |
| Family medicine | 1558 (12.2) | 8.9 | 30.5 | 53.5 | |||
| Internal medicine | 1226 (9.6) | 8.8 | 29.2 | 45.1 | |||
| General surgery | 1060 (8.3) | 6.8 | 26.0 | 42.1 | |||
| Colorectal surgery | 243 (1.9) | 8.1 | 24.9 | 50.3 | |||
| Unknown | 396 (3.1) | 7.8 | 18.7 | 48.9 | |||
| Geographic region | |||||||
| Northeast | 3697 (28.9) | 7.4 | <0.00001 | 25.7 | 0.002 | 46.1 | <0.0001 |
| South | 2060 (16.1) | 8.6 | 31.1 | 53.5 | |||
| Midwest | 1431 (11.2) | 7.5 | 24.6 | 45.2 | |||
| West | 4112 (32.2) | 7.6 | 23.2 | 42.1 | |||
| Other | 1471 (11.5) | 9.0 | 27.5 | 50.3 | |||
| Income Quintile | |||||||
| 1 | 3113 (24.4) | 8.9 | <0.0001 | 28.8 | 0.01 | 48.2 | 0.06 |
| 2 | 3130 (24.5) | 8.3 | 26.5 | 46.8 | |||
| 3 | 3120 (24.4) | 7.1 | 23.4 | 44.3 | |||
| 4 | 3125 (24.5) | 7.4 | 25.1 | 45.7 | |||
| Education Quintile | |||||||
| 1 | 3122 (24.4) | 8.9 | 0.02 | 29.2 | 0.007 | 48.3 | 0.15 |
| 2 | 3119 (24.4) | 7.4 | 26.1 | 44.5 | |||
| 3 | 3123 (24.5) | 8.5 | 25.2 | 46.8 | |||
| 4 | 3124 (24.5) | 6.9 | 23.6 | 45.3 |
Increased risk includes prior history of polyps, or family history of cancer.
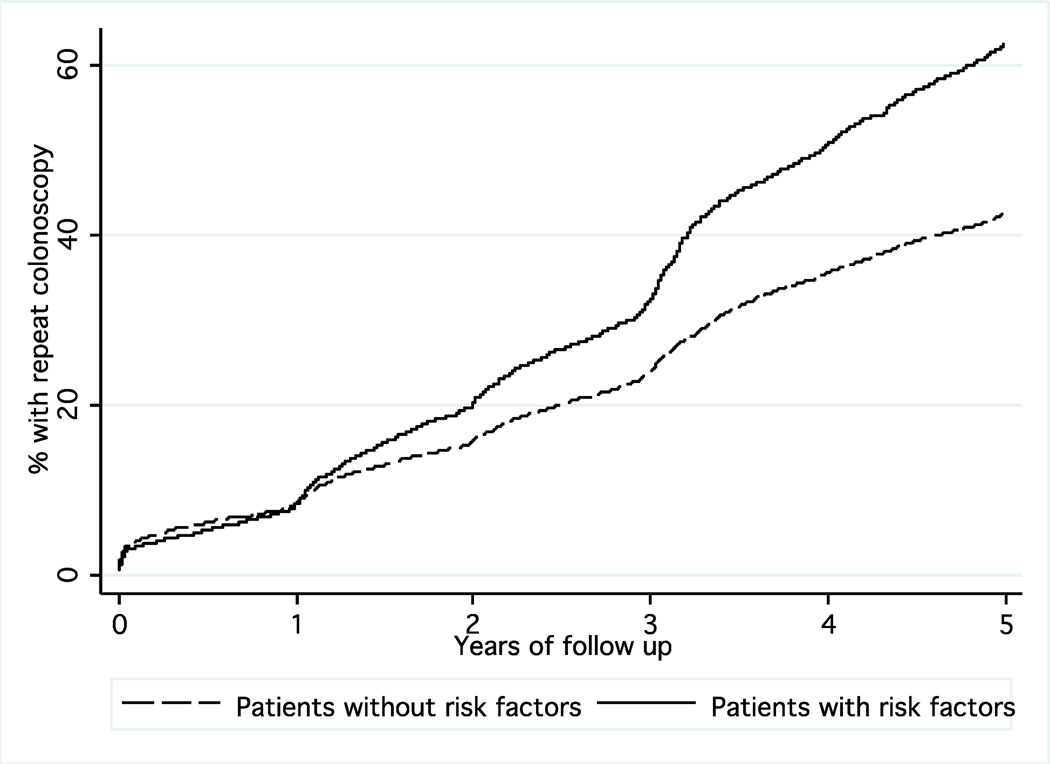
Among the eligible patients in the cohort, 7.9% underwent colonoscopy within 1 year of follow up, 25.5% received a colonoscopy within 3 years, and 45.7% underwent colonoscopy within 5 years (Figure 1). The follow up colonoscopy was diagnostic (no procedure or biopsy alone) in 67.7% and included polypectomy in 32.3%. The use of barium enema and flexible sigmoidoscopy at five years was low (2.9% and 3.8%, respectively), but FOBT was used commonly (16.4% at one year, 42.1% at three years, 54.0% at five years).
Figure 1.
Kaplan-Meier curve showing the proportion of patients with previous polypectomy undergoing repeat colonoscopy in follow up stratified by the presence or absence of previous risk factors for neoplasia (prior polyp, family history of colon cancer). There was a significantly greater use of colonoscopy in patients with risk factors (p<0.0001).
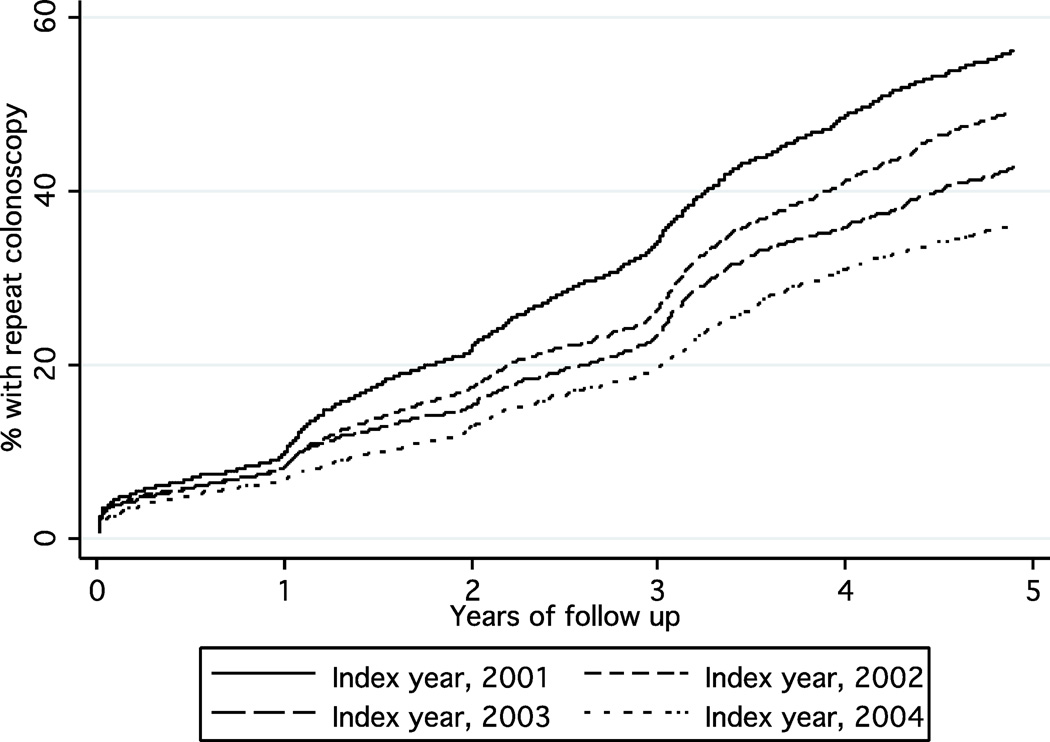
Predictors of colonoscopy receipt are shown in Table 1. In general, patients with more comorbidity were more likely to receive colonoscopy early in the follow up period, and advanced age was associated with a lower cumulative rate at five years. Male patients were more likely to undergo colonoscopy in years 3 and 5, and African Americans were more likely to undergo colonoscopy at all three time points. Repeat procedures were also more common in patients with a colonoscopy prior to the index examination and in patients with additional cancer risk factors (Figure 1). Among patients with a prior diagnosis of colon polyps and/or family history of cancer, the frequency of surveillance was 7.9%, 32.8% and 61.3% at 1, 3 and 5 years, respectively, compared to 7.9%, 23.6% and 41.2% in others. The frequency of repeat colonoscopy was highest in the Southern US but there were no consistent differences in colonoscopy receipt according to endoscopist specialty or socioeconomic factors. Importantly, the frequency of repeat colonoscopy significantly decreased with more recent time periods (Figure 2). For example, the rate of five-year colonoscopy decreased from 57.4% for index procedures performed in 2001 to 36.2% for procedures performed in 2004.
Figure 2.
Kaplan-Meier curves of the receipt of repeat colonoscopy following polypectomy stratified by year of index polypectomy. The frequency decreased with later years of the index colonoscopy (p<0.0001).
The results of the multivariable proportional hazards analysis are shown in Table 2. Independent predictors of repeat colonoscopy at five years included younger age, male gender, a diagnosis of colon polyps or other risk factors prior to the index examination, and a colonoscopy prior to the index examination. As in the univariate analysis, the likelihood of repeat colonoscopy within five years decreased with more recent time periods. Compared to 2001, the hazard ratio of undergoing repeat colonoscopy for 2004 index procedures was 0.61 (95% CI 0.55–0.68).
Table 2.
Multivariable Analysis for Predicting Repeat Colonoscopy at Five Years
| Variable | Hazard Ratio (95% CI) |
|---|---|
| Age Group | |
| 70–74 | 1.0 |
| 75–79 | 0.95 (0.89–1.02) |
| 80–84 | 0.89 (0.82–0.97) |
| ≥ 85 | 0.70 (0.62–0.80) |
| Gender | |
| Male | 1.0 |
| Female | 0.90 (0.84–0.97) |
| Race | |
| White | 1.0 |
| Black | 1.21 (1.02–1.44) |
| Other/Unknown | 1.19 (0.89–1.59) |
| Procedure Year | |
| 2001 | 1.0 |
| 2002 | 0.82 (0.74–0.91) |
| 2003 | 0.72 (0.65–0.80) |
| 2004 | 0.61 (0.55–0.68) |
| Comorbidity | |
| 0 | 1.0 |
| 1 | 1.01 (0.94–1.09) |
| ≥ 2 | 1.05 (0.96–1.15) |
| Increased Risk | |
| No | 1.0 |
| Yes | 1.47 (1.31–1.66) |
| Prior Colonoscopy | |
| No | 1.0 |
| Yes | 1.23 (1.09–1.38) |
| Specialty | |
| Gastroenterology | 1.0 |
| Family medicine | 1.15 (0.96–1.38) |
| Internal medicine | 1.09 (0.95–1.24) |
| General surgery | 1.02 (0.85–1.21) |
| Colorectal surgery | 1.04 (0.95–1.13) |
| Unknown | 1.23 (1.00–1.52) |
| Geographic region | |
| Northeast | 1.0 |
| South | 1.16 (1.03–1.32) |
| Midwest | 0.96 (0.83–1.11) |
| West | 0.93 (0.83–1.04) |
| Other | 1.13 (0.99–1.30) |
| Income Quintile | |
| 1 | 1.0 |
| 2 | 1.04 (0.93–1.16) |
| 3 | 0.98 (0.87–1.10) |
| 4 | 1.02 (0.90–1.14) |
| Education Quintile | |
| 1 | 1.0 |
| 2 | 0.91 (0.81–1.02) |
| 3 | 0.99 (0.88–1.11) |
| 4 | 1.00 (0.88–1.14) |
In a secondary analysis, we examined factors that were associated with FOBT at five years. In a univariate analysis, older age and increased comorbidity were inversely associated with FOBT use (odds ratio (OR) 75–79: 1.03 (95% CI 0.94–1.12), 80–84 OR 0.89 (95% CI 0.80–0.99), ≥ 85 OR 0.69 (95% CI 0.60–0.79), comorbidity score 1 OR 0.88 (95% CI 0.81–0.96, comorbidity score ≥ 2 OR 0.70 (95% CI 0.63–0.77)). As with colonoscopy, the use of FOBT declined with more recent years of the index colonoscopy (2002 OR (95% CI 0.85–1.03), 2003 OR 0.79 (95% CI 0.72–0.88), 2004 OR 0.66 (95% CI 0.59–0.73)). None of the other patient or provider factors were associated with FOBT.
Discussion
Colonoscopy is a commonly recommended procedure for screening in the asymptomatic at risk population as well as for the follow up or surveillance of patients with adenomatous polyps. Studies that have included medical record review (15), data from clinical trials (10,16), and surveys of endoscopists (7) and referring physicians (8) have all reported discordance between guideline recommendations and surveillance practices. In addition, the findings of a recently published study that used Medicare claims data (9) were suggestive of the overuse of follow up colonoscopy in polyp free individuals. The present study, which also included Medicare data, highlights another problem with colonoscopic practice, the underuse of surveillance in patients with polyps. We found that less than half of eligible patients underwent repeat colonoscopy within 5 years, a rate that was even lower than in previous studies. The frequency also appeared to diminish with more recent study years. This is a patient population thought to be at increased risk for development of advanced neoplasia and colorectal carcinoma, and thus, more frequent colonoscopic intervals than the average risk population is recommended. Indeed, we found that more than 30% of the follow up colonoscopies included polypectomy, suggesting a relatively high yield of surveillance in this patient population. We also noted that the frequency of a nonrecommended surveillance procedure, FOBT, exceeded that of colonoscopy. The latter practice is not recommended by guidelines and would serve little purpose in the detection of recurrent polyps.
Our study also found a somewhat higher frequency of polyp surveillance in African Americans, which differs from lower reported rates of screening colonoscopy in the general Medicare population (17) and in beneficiaries undergoing surveillance colonoscopy after cancer resection (18). The underlying reasons for this difference are not evident but could potentially be attributed to more advanced polyp characteristics in African Americans. These factors, which are not available in this database, could be associated with earlier recall for surveillance. The differences in surveillance use among SEER regions are also consistent with other studies in colon cancer survivors (18) as well as in the general Medicare population (19,20). The potential reasons for geographic variation are likely multifactorial, but given that surveillance colonoscopy is universally reimbursed, local practice patterns are likely important.
The underlying reasons for the underuse of surveillance are likely multifactorial and cannot be ascertained from this database. First, it is possible that because of changes in providers and/or imperfect communication systems, endoscopist recommendations from the initial colonoscopy were not disseminated to the patient’s primary physician. Second, for certain patient groups, such as the extreme elderly or patients with multiple comorbidities, the perceived benefit of repeat colonoscopy in prolonging life may have been limited (21). In contrast to the US Preventive Services Task Force screening guidelines, where an upper age limit of 75 is recommended for routine colorectal screening (22), similar guidelines do not exist for polyp surveillance. Our data used a previously validated comorbidity index (14), but Medicare claims do not include other measures of performance status such as frailty. There is also a higher risk of procedural complications in older individuals and patients with greater comorbidity (23) and for these reasons, endoscopists may have been reluctant to recommend surveillance. Third, the findings of a decreased frequency of five-year surveillance over time may have reflected changes in guidelines to some extent. Through 2003 (3), guidelines recommended a five-year surveillance interval for single small (<1 cm) tubular adenomas and a three-year follow up for all others. In 2003 (24), surveillance of two small adenomas was extended from three to five years. The guidelines from 2006 (4) suggested that individuals with 1–2 small adenomas could have surveillance after 5–10 years and a three year interval was recommended for all other scenarios. Our data did not include clinical details about polyp size or histology, and thus, in some instances the endoscopist may have adhered to a follow up that was closer to 10 years. Fourth, despite recommendations from health care providers, patients may have declined repeat colonoscopy.
Although Medicare claims data capture a large patient population and are thought to be accurate for measurement of procedure use, there are some potential limitations of the database. First, unlike cancer specific pathology data that are contained in SEER (11,12), we did not have access to pathology of the polyps that were removed, including number, size and presence of villous features. Based on preliminary studies in two local endoscopy units (25) and surveys of practicing endoscopists (13), we hypothesized that the use of snare polypectomy was relatively specific for adenomatous polyps. However, the validity of this hypothesis in the general Medicare population has not been assessed. It is possible that a large number of small, nonprecancerous hyperplastic polyps were removed using this technique and thus, lack of follow up deemed appropriate. Second, a subset of patients without polyps removed may have been erroneously coded as polypectomy and appropriately not followed up. However, an analysis which linked Medicare claims to procedure notes found a sensitivity and specificity of more than 97% for snare polypectomy (26). Third, the study was limited to Medicare beneficiaries aged 70 and older who were receiving benefits through fee-for-service arrangements. Thus, the generalizability of the findings to younger patients and managed care recipients is unknown. Conceivably, the frequency of polyp surveillance could have been much higher in younger patients with a greater perceived life expectancy, and members of managed care plans may have received different intensity of follow up compared to beneficiaries in fee-for-service plans. In addition, the study was limited to residents of geographic areas served by SEER registries, but these are thought to be representative of the general US population (12). Fourth, the study was limited to beneficiaries without a previous or future cancer diagnosis. Patients who receive ongoing follow up for cancer may have greater contact with the healthcare system and more opportunity for colonoscopy referral, or alternatively, a lower likelihood of referral if their malignant disease was deemed incurable. In addition, patients with high risk adenomas on index colonoscopy who subsequently develop colon cancer would also be excluded. Thus, the sample could be biased toward patients with lower risk polyps who may not require intensive follow up. Finally, given the large sample sizes, some of the differences which achieved statistical significance may not have been clinically relevant.
In summary, the findings of this population-based study suggest underuse of follow up colonoscopy in patients at increased risk for subsequent colonic neoplasia. The findings are particularly relevant given related work that is consistent with overuse of colonoscopy in patients without a history of neoplasia. Further patient and provider based research is needed to elucidate the underlying reasons for this discordance with clinical practice guidelines.
Acknowledgment
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
Supported by the National Cancer Institute at the National Institutes of Health, R01 CA132862
Footnotes
There are no financial disclosures.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Levine JS, Ahnen DJ. Adenomatous polyps of the colon. N Engl J Med. 2006;355:2551–2557. doi: 10.1056/NEJMcp063038. [DOI] [PubMed] [Google Scholar]
- 3.Winawer SJ, Fletcher RH, Miller L, et al. Colorectal cancer screening: clinical guidelines and rationale. Gastroenterology. 1997;112:594–642. doi: 10.1053/gast.1997.v112.agast970594. [DOI] [PubMed] [Google Scholar]
- 4.Winawer SJ, Zauber AG, Fletcher RH, et al. Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. Gastroenterology. 2006;130:1872–1875. doi: 10.1053/j.gastro.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 5.Winawer SJ, Zauber AG, O’Brien MJ, et al. Randomized comparison of surveillance intervals after colonoscopic removal of newly diagnosed adenomatous polyps. N Engl J Med. 1993;328:901–906. doi: 10.1056/NEJM199304013281301. [DOI] [PubMed] [Google Scholar]
- 6.Brenner H, Hoffmeister M, Stegmaier C, Brenner G, Altenhofen L, Houg U. Risk of progression of advanced adenomas to colorectal cancer by age and sex: estimates based on 840,149 screening colonoscopies. Gut. 2007;56:1585–1589. doi: 10.1136/gut.2007.122739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mysliwiec PA, Brown ML, Klabunde CN, Ransohoff DF. Are physicians doing too much colonoscopy? A national survey of colorectal surveillance after polypectomy. Ann Intern Med. 2004;141:264–271. doi: 10.7326/0003-4819-141-4-200408170-00006. [DOI] [PubMed] [Google Scholar]
- 8.Boolchand V, Singh J, Olds G, Singh P, Chak A, Cooper GS. Colorectal cancer screening after polypectomy: a national survey of primary care physicians. Ann Intern Med. 2006;145:654–659. doi: 10.7326/0003-4819-145-9-200611070-00007. [DOI] [PubMed] [Google Scholar]
- 9.Goodwin JS, Singh A, Reddy N, Riall TS, Kuo YF. Overuse of screening colonoscopy in the Medicare population. Arch Intern Med. 2011;171:1335–1343. doi: 10.1001/archinternmed.2011.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schoen RE, Pinsky PF, Weissfeld JL, et al. Utilization of surveillance colonoscopy in community practice. Gastroenterology. 2010;138:73–81. doi: 10.1053/j.gastro.2009.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Potosky AL, Riley GF, Lubitz JD, Mentnech RM, Kessler LG. Potential for cancer related health services research using a linked Medicare-tumor registry database. Med Care. 1993;31:732–748. [PubMed] [Google Scholar]
- 12.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data. Med Care. 2002;40(Suppl):3–18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 13.Singh N, Harrison M, Rex DK. A survey of colonoscopic polypectomy practices among clinical gastroenterologists. Gastrointest Endosc. 2004;60:414–418. doi: 10.1016/s0016-5107(04)01808-5. [DOI] [PubMed] [Google Scholar]
- 14.Klabunde CN, Warren JL, Legler JM. Assessing comorbidity using claims data. Med Care. 2002;40(Suppl):26–35. doi: 10.1097/00005650-200208001-00004. [DOI] [PubMed] [Google Scholar]
- 15.Krist AH, Jones RM, Woolf SH, et al. Timing of repeat colonoscopy: disparity between guidelines and endoscopists’ recommendation. Am J Prev Med. 2007;33:471–478. doi: 10.1016/j.amepre.2007.07.039. [DOI] [PubMed] [Google Scholar]
- 16.Laiyemo AO, Pinsky PF, Marcus PM, et al. Utilization and yield of surveillance colonoscopy in the continued follow-up study of the polyp prevention trial. Clin Gastroenterol Hepatol. 2009;7:62–67. doi: 10.1016/j.cgh.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooper GS, Koroukian SM. Racial disparities in the use of and indications for colorectal procedures in Medicare beneficiaries. Cancer. 2004;100:418–424. doi: 10.1002/cncr.20014. [DOI] [PubMed] [Google Scholar]
- 18.Cooper GS, Kou TD, Reynolds HL., Jr Receipt of guideline recommended follow up in older colorectal cancer survivors: a population based analysis. Cancer. 2008;113:2029–2037. doi: 10.1002/cncr.23823. [DOI] [PubMed] [Google Scholar]
- 19.Song Y, Skinner J, Bynum J, Sutherland J, Wennberg JE, Fisher ES. Regional variations in diagnostic practices. N Engl J Med. 2010;363:45–53. doi: 10.1056/NEJMsa0910881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Baicker K, Newhouse JP. Geographic variation in Medicare drug spending. N Engl J Med. 2010;363:405–409. doi: 10.1056/NEJMp1004872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ko CW, Sonnenberg A. Comparing risks and benefits of colorectal cancer screening in elderly patients. Gastroenterology. 2005;129:1163–1170. doi: 10.1053/j.gastro.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 22.U.S. Preventive Services Task Force. Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:627–637. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 23.Warren JL, Klabunde CN, Mariotto AB, et al. Adverse events after outpatient colonoscopy in the Medicare population. Ann Intern Med. 2009;150:849–857. doi: 10.7326/0003-4819-150-12-200906160-00008. [DOI] [PubMed] [Google Scholar]
- 24.Winawer S, Fletcher R, Rex D, et al. Colorectal cancer screening and surveillance: clinical guidelines and rationale – update based on new evidence. Gastroenterology. 2003;124:544–560. doi: 10.1053/gast.2003.50044. [DOI] [PubMed] [Google Scholar]
- 25.Okolie CN, Cooper GS. Administrative data for colonoscopy with polypectomy have a high level of accuracy (abstract) Am J Gastroenterol. 2007;102:S458. [Google Scholar]
- 26.Ko CW, Dominitz JA, Green P, Kreuter W, Baldwin LM. Accuracy of Medicare claims for identifying findings and procedures performed during colonoscopy. Gastrointest Endosc. 2011;73:447–453. doi: 10.1016/j.gie.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]