Abstract
Background
The neurosteroid allopregnanolone is a potent allosteric modulator of the GABA(A) receptor with anxiolytic properties. Exogenous administration of allopregnanolone reduces anxiety, and allopregnanolone blockade impairs social and affective functioning. However, the neural mechanism whereby allopregnanolone improves mood and reduces anxiety is unknown. In particular, brain imaging has not been used to link neurosteroid effects to emotion regulation neurocircuitry.
Methods
To investigate the brain basis of allopregnanolone’s impact on emotion regulation, participants were administered 400mg of pregnenolone (N=16) or placebo (N=15) and underwent 3T fMRI while performing the Shifted-Attention Emotion Appraisal Task (SEAT), which probes emotional processing and regulation.
Results
Compared to placebo, allopregnanolone was associated with reduced activity in the amygdala and insula across all conditions. During the appraisal condition, allopregnanolone increased activity in the dorsal medial prefrontal cortex and enhanced connectivity between the amygdala and dorsal medial prefrontal cortex, an effect that was associated with reduced self-reported anxiety.
Conclusions
These results demonstrate that in response to emotional stimuli, allopregnanolone reduces activity in regions associated with generation of negative emotion. Furthermore, allopregnanolone may enhance activity in regions linked to regulatory processes. Aberrant activity in these regions has been linked to anxiety psychopathology. These results thus provide initial neuroimaging evidence that allopregnanolone may be a target for pharmacological intervention in the treatment of anxiety disorders, and suggest potential future directions for research into neurosteroid effects on emotion regulation neurocircuitry.
Keywords: Allopregnanolone, neuroactive steroid, fMRI, pharmaco-fMRI, emotion regulation, anxiety, pregnenolone
Introduction
Allopregnanolone (ALLO) is a progesterone-derived neurosteroid with potent anxiolytic properties (1) that acts as a positive allosteric modulator at the GABA(A) receptor (2–4). Allopregnanolone is produced de novo in neurons and glia (2), in addition to synthesis in peripheral organs including the ovaries and adrenal glands (5). In tissue culture, it acts on GABA(A) receptors with 20-fold higher potency than benzodiazepines and barbiturates (3), and modulates a broader range of GABA(A) receptors than either of these compounds (6). Due to its pronounced anxiolytic-like actions in rodent models and GABA(A) receptor activity, allopregnanolone shows promise as a mechanism for anxiolysis in psychiatric disorders.
Convergent evidence from animal studies and human clinical research implicates allopregnanolone dysregulation in mood and anxiety symptomatology. In rats, blockade of metabolism from progesterone to allopregnanolone impairs social and affective (anxiety-related) behavior in rats (7, 8), which in turn is restored by allopregnanolone infusion (9). Consistent with these findings, allopregnanolone infusions have reliably been shown to reduce stress and anxiety-like behavior in rodents (2, 3, 10–15). Furthermore, exploratory and low-anxiety behavior in rats is correlated with circulating and hippocampal levels of allopregnanolone, but not with levels of estradiol, progesterone, or corticosterone (8), suggesting that allopregnanolone may show greater promise as an anxiolytic candidate than other pregnane neurosteroids. Allopregnanolone also reduces conditioned fear responding, facilitates fear extinction, and prevents the reinstatement of fear memory after extinction (16). Studies in humans are less abundant, but broadly consistent with animal findings. Cerebrospinal fluid levels of allopregnanolone are reduced in women with major depressive disorder and PTSD, and increase with successful pharmacological treatment (17–21). Multiple antidepressant agents (including fluoxetine, norfluoxetine, fluvoxamine and paroxetine) elevate allopregnanolone brain levels (19, 22–25), leading to the suggestion that allopregnanolone induction might be an important mechanism for the antidepressant effects of SSRIs (16, 18, 26, 27). Based on these observations, it has been suggested that allopregnanolone dysregulation may contribute to the development of neuropsychiatric disorders, and that restoration of allopregnanolone regulation may be a potential pathway for symptomatic improvement (1, 6, 23). However, the specific neural mechanisms whereby allopregnanolone improves mood and anxiety symptomatology are unknown.
One potential mechanism for allopregnanolone’s effects in the central nervous system is its ability to directly impact emotion neurocircuitry. Allopregnanolone acts directly on GABA(A) receptors, which are present throughout the cortex and limbic system (28). The anxiolytic actions of allopregnanolone likely involve the amygdala (29). In rats, microinfusions of allopregnanolone directly into the amygdala produce rapid anxiolytic (30), antidepressant (31), and anti-aggressive (32) effects. In humans, progesterone administration (which increases downstream allopregnanolone) modulates amygdala responses to emotional faces (33), and increases functional connectivity between amygdala and dorsal medial prefrontal cortex (dmPFC) (34), a regulatory region interconnected with limbic structures (35) and crucial to emotional regulation (36). However, no studies have yet examined the impact of allopregnanolone on emotion regulation, a function subserved by these neurocircuits and postulated to be disrupted in anxiety disorders (for reviews, see 37, 38). Since deficits in emotion regulation may contribute to or maintain anxiety (38), understanding allopregnanolone’s impact on emotion regulation neurocircuits may be central to understanding why allopregnanolone dysregulation is associated with distress and psychopathology.
In the current study, we used a probe of emotion processing, modulation, and regulation (39, 40) to examine the neural basis of allopregnanolone’s effects on emotion processing. Given previous behavioral and preclinical evidence of its anxiolytic and antidepressant effects, we expected allopregnanolone to diminish responses in emotion generation neurocircuits (e.g. amygdala and insula), and enhance activation in regions subserving regulatory control of emotions (e.g. dmPFC).
Methods and Materials
Participants
Study participants were 31 right-handed healthy male volunteers aged 18–32 years (mean ± SD=22 ± 3.38) recruited from the community via advertisement. Our investigation was restricted to males because allopregnanolone levels fluctuate over the course of the menstrual cycle and may have differential impact on mood depending on menstrual cycle phase (41), and it was not feasible within the limited scope of this project to recruit women in all phases of the menstrual cycle. Exclusion criteria were history of head injury, recent steroid use, and current or past psychiatric disorder, as assessed via the Mini-International Neuropsychiatric Interview (M.I.N.I.; 42). Participants were given full details of the study and provided written informed consent. The study was approved by the Institutional Review Board of the University of Michigan Medical School. All participants completed self-report measures of anxiety, sedation, and neurocognitive function. Details can be found in Supplemental Information.
Drug Administration
Study drug (pregnenolone) and matching placebo identical in appearance were obtained from Belmar Pharmacy (Lakewood, CO), which provided certificates of analysis. Participants were randomly assigned to receive a single oral dose of 400 mg pregnenolone (n=16), or placebo (n=15). Participants and investigators were blind to condition. Pregnenolone was administered as a precursor loading strategy to significantly increase downstream allopregnanolone levels. Pregnenolone is lipophilic and readily crosses the blood brain barrier. We have previously found that pregnenolone is preferentially metabolized to allopregnanolone, rather than other compounds such as cortisol or DHEA (43, 44); however these metabolites were also assayed. Allopregnanolone serum levels have been reported to triple two hours after oral administration of 400 mg pregnenolone (45). Thus, drug administration occurred two hours before neuroimaging to ensure elevated levels during the scan.
Steroid measurements
We used circulating serum levels of allopregnanolone and pregnenolone as indicators of central neurosteroid levels. In animal models, serum neurosteroid levels appear to be closely related to hippocampal levels (46). Serum samples for assay were collected once prior to drug administration and once after the scanning session. Pregnenolone and allopregnanolone levels in serum were determined by a highly sensitive and specific gas chromatography-mass spectrometry method as described previously (47, 48), with modifications (the electron impact ionization mode was utilized for this investigation, rather than negative ion chemical ionization). One ml of serum was extracted three times in ethyl acetate before high performance liquid chromatography (HPLC) purification using tetrahydrofuran, ethanol, and hexane in the mobile phase. All samples were injected in duplicate. Mean intra-assay coefficients of variation for pregnenolone and allopregnanolone were 0.9% and 2.9%, respectively. The limit of detection with this method was 1 pg for both pregnenolone and allopregnanolone. Serum DHEA levels were determined via enzyme immunoassay (ALPCO Diagnostics, Salem, NH), and serum levels of cortisol and DHEAS were determined by chemiluminescent enzyme immunoassay (IMMULITE) according to the manufacturer’s directions (Siemens Healthcare Diagnostics Inc., Tarrytown, NY). All neurosteroid values were natural log transformed prior to analyses.
Shifted-Attention Emotion Appraisal (SEAT) Paradigm
In order to investigate the brain basis of emotional response and regulation, our laboratory has developed an emotional appraisal task (39, 40) modifying the task of Anderson and colleagues (49). The SEAT taskpresents compound stimuli that include both emotional faces and neutral scenes (see Figure 1). Stimuli include composite pictures of superimposed faces (foreground) and buildings (background), as well as 20 pictures of faces or buildings only. The face pictures depict neutral, angry, or fearful expressions, and the building pictures depict indoor or outdoor scenes. In three different conditions, participants are asked to respond to three different questions: (1) ‘Gender’: Whether the face in the foreground is male or female; (2) ‘Inside/Outside’: Whether the scene in the background is indoors or outdoors; or (3) ‘Like/Dislike’: Whether the face in the foreground is liked or disliked. This probes multiple components of emotion regulation, including (1) implicit emotional processing, (2) attentional modulation of emotion, and (3) modulation of emotion by appraisal. Further details can be found in Supplemental Information.
Figure 1.

Task stimuli depict neutral, angry, or fearful expressions superimposed on building pictures of indoor or outdoor scenes. Faces reprinted with permission from the Paul Ekman Group, LLC.
Magnetic Resonance Imaging
Image Acquisition
MRI scanning occurred on a Philips 3.0 Tesla Achieva X-series MRI (Philips Medical Systems). After a T1 image (T1-overlay) was obtained, a T2*-weighted, echoplanar acquisition sequence [GRE; repetition time, 2000 ms; echo time, 25 ms; flip angle, 90°; field of view (FOV), 22 cm; 42 slice; thickness/skip, 3.0/0 mm matrix size equivalent to 64 × 64] was collected. After discarding three initial volumes to permit thermal equilibration of the MRI signal, 185 volumes were acquired per run. After acquiring the functional volumes, a high-resolution T1 scan was obtained for anatomic normalization [26 FOV; thickness/skip, 1.0/0 mm]. E-prime was used to present stimuli and record responses (Psychology Software Tools, Pittsburgh, PA). Participants viewed stimuli through MR-compatible liquid crystal display goggles (NordicNeuroLabs http://www.nordicneurolab.com) and responded to those stimuli using an MRI-compatible button box.
Preprocessing
A standard series of processing steps was performed using statistical parametric mapping (SPM8; www.fil.ion.ucl.ac.uk/spm). Scans were reconstructed, motion-corrected, slice-time corrected, realigned to the first scan in the experiment to correct for head motion, co-registered with the high-resolution sagittal images, anatomically normalized to the Montreal Neurological Institute (MNI) 152 template brain, resampled to 3×3×3 mm3 voxels, and smoothed with an 8×8×8 mm3 kernel. Motion parameters (mean displacement, mean angle) were compared across drug conditions via Independent-Samples Kruskal-Wallis tests, and runs with any movement greater than 3 mm were excluded.
Data Analysis
Maps of activation in each condition, as well as reaction time and on-line accuracy judgments were analyzed via a 2 (Drug Type: Pregnenolone or Placebo) × 3 (Face Type: Angry, Fearful, Neutral) × 3 (Condition: Male/Female, Inside/Outside, Like/Dislike) repeated measures ANOVA to assess for main effects and interaction effects. Follow-up simple effects analyses were performed with two-tailed t-tests, with significance threshold set to .05, corrected for multiple comparisons. Since 20 clusters were found, the significance threshold was set to .05/20 = .0025. Levels of allopregnanolone and pregnenolone (endpoint minus baseline) were entered as regressors in between-subject analyses.
Whole Brain and Region of Interest Analysis
Z-score images from the individual activation maps were entered into second-level random-effects analyses implemented in SPM8. Second-level maps were corrected for multiple comparisons using whole-brain family-wise error correction, p<.05. In addition, region of interest (ROI) analysis with small volume correction (SVC) was conducted with a priori brain areas identified in previous neuroimaging studies of allopregnanolone (33, 34) and studies using the SEAT task (40). Activation threshold and cluster size were determined using AlphaSim (50) to correspond to a false positive rate of p<0.05, corrected for multiple comparisons within ROIs. A priori ROIs of anatomical dmPFC (k=1473), insula (k=536), and amygdala (k=69) were used as masks. Images were thresholded using a voxelwise threshold of p<0.05 uncorrected with a minimum cluster size of 30 voxels for amygdala, 81 connected voxels for insula, and 170 voxels for dmPFC. Only the activations within the ROIs that survived the volume and voxel correction criteria were extracted and used for further analysis. Activation foci were labeled by comparison with the neuroanatomical atlas by Talairach and Tournoux (51). Reported voxel coordinates correspond to standardized Montreal Neurologic Institute (MNI) space.
The time series from significant clusters within regions of group difference were used in a psychophysiological interaction (PPI) analysis. Deconvolved time series in the anatomical dmPFC was extracted for each participant as the first regressor in the PPI analysis (physiological variable). The second regressor represented the experimental condition (appraisal; psychological variable). The regressor of interest was the interaction between the time series of the seed region and the experimental condition.
Results
Participants
Sixteen participants were administered pregnenolone and 15 were administered placebo. No participant met criteria for any psychiatric disorder, as assessed by the M.I.N.I. Groups did not differ by age or race. There were no significant differences between pregnenolone administration and placebo groups in self-reported anxiety, sedation, or neurocognitive function (in all cases p>0.2). Sample demographics and characteristics can be found in Table 1.
Table 1.
Sample Demographics and Characteristics
| Placebo (n=15) | Pregnenolone (n=16) | t/χ2 | p | |
|---|---|---|---|---|
| Age (Mean ± SD) | 23 ± 3.0 | 22 ± 3.7 | .94 | .36 |
| Race | 3A, 3AA, 9C | 2A, 14C | 4.26 | .12 |
| Digit Span | ||||
| Baseline | 19.6 ± 3.9 | 19.8 ± 3.5 | .16 | .87 |
| Change | 1.1 ± 2.4 | .31 ± 2.0 | 1.0 | .31 |
| Trail-Making Test | ||||
| Baseline | 51.4 ± 15.7 | 54.4 ± 9.9 | .65 | .52 |
| Change | −7.4 ± 11.5 | −10.6 ± 6.6 | .95 | .35 |
| PANAS-X Negative Affect | 1.4 ± .34 | 1.2 ± .25 | 1.8 | .08 |
| Drug Effects Questionnaire | 1.9 ± .66 | 1.7 ± 1.0 | .58 | .57 |
| VAS Anxiety | ||||
| Baseline | .83 ± .43 | 1.0 ± .87 | .80 | .43 |
| Change | .09 ± .66 | −.28 ± .78 | 1.4 | .18 |
| In-Scan Anxiety Survey | 7.4 ± 1.9 | 7.6 ± 1.8 | .25 | .81 |
A, Asian; AA, African-American; C, Caucasian; PANAS-X, Positive and Negative Affect Schedule – Expanded Form; VAS, Visual Analogue Scale. Change = Endpoint minus Baseline.
Intervention
At three hours post-administration, pregnenolone resulted in threefold elevations in serum levels of pregnenolone (paired t(15)=10.89, p<0.001), and increased allopregnanolone sevenfold (paired t(15)=13.59, p<0.001). Pregnenolone administration also increased levels of pregnanolone (allopregnanolone’s 5β-stereoisomer) [t(29)=3.17, p=.004] by approximately 60% and reduced DHEAS levels [t(29)=3.29, p=.003] by approximately 5%. Baseline and endpoint levels of pregnenolone, allopregnanolone, and pregnanolone can be found in Table 2. Compared to placebo, pregnenolone did not differentially alter serum cortisol or DHEA levels at three-hours post-administration (in all cases p>0.3). There were no significant differences in subjective drug effects (p>.5). Participants’ guesses of which drug they received did not deviate from chance (χ2(3)=1.34, p=.71). Pregnenolone administration was utilized as a precursor loading strategy to enhance allopregnanolone levels, however, for accuracy, we will use the term “PREG group” to refer to the participants who were administered pregnenolone. This issue is further detailed in the discussion.
Table 2.
Steroid and Metabolite Levels
| Steroid (pg/ml) | Placebo (n=15) | Pregnenolone Group (n=16) | t | p | ||
|---|---|---|---|---|---|---|
|
| ||||||
| Baseline (Mean ± SD) | Endpoint (Mean ± SD) | Baseline (Mean ± SD) | Endpoint (Mean ± SD) | |||
| Pregnenolone | 1378.8 ± 579.1 | 1190.8 ± 468.5 | 1238.0 ± 378.7 | 3943.6 ± 696.3 | 12.3* | <.001 |
| Allopregnanolone | 114.3 ± 49.6 | 118.1 ± 76.5 | 112.3 ± 40.9 | 802.2 ± 280.7 | 10.2 | <.001 |
| Pregnanolone | 470.6 ± 200.8 | 490.5 ± 135.6 | 352.0 ± 106.6 | 517.4 ± 96.2 | 3.2 | .004 |
T-tests were conducted on change scores (steroid levels at endpoint minus steroid levels at baseline).
SEAT Task
Behavioral Results
There were no significant differences between PREG and placebo groups in reaction time [F(1, 29)=1.72, p=.20) or accuracy [F(1, 29)=.031, p=.86). There was a significant main effect of task driven by increased accuracy (.81 ± .028 vs .70 ± .017) and decreased reaction time (1.34 ± .055 vs 1.44 ± .05) in the attention modulation task as compared to the implicit emotion processing task [reaction time F(2, 58)=6.57, p=.003); accuracy F(2, 58)=41.11, p<.001)].
fMRI Results
Examination of motion parameter summary statistics revealed there were no differences between groups in mean displacement or mean angle (in all cases p>0.07). Maximum displacement did not exceed 3 mm.
Main Effect of Drug
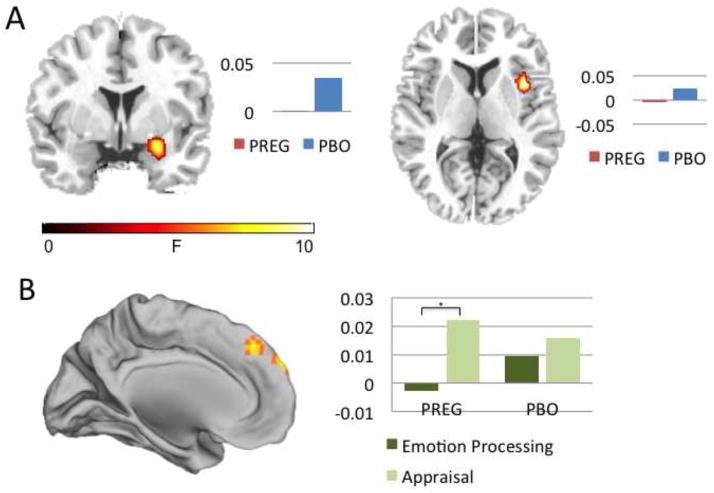
There was a main effect of drug in the amygdala ([27,−1,−17]; F(1,29)=9.97, p<.05, SVC) and the insula ([42,8,4]; F(1,29)=10.97, p<.05, SVC) such that pregnenolone administration decreased amygdala and insula activity across all conditions and face types (see Figure 2). Across all conditions and face types, change in serum allopregnanolone level was negatively associated with right amygdala activity (r=−.66, p<.001), and change in serum pregnenolone level was negatively associated with right insula activity (r=−.65, p<.001), indicating that peripheral increase in allopregnanolone and pregnenolone was associated with reduced activation in these emotion generation regions. Whole-brain correlations with steroid levels (pregnenolone, allopregnanolone, and pregnanolone) can be found in Table 3.
Figure 2.
(A) Compared to placebo, pregnenolone administration decreased activation in right amygdala (y=2) and right insula (z=-6) across conditions and face types. (B) Compared to placebo, pregnenolone administration increased dorsal medial prefrontal cortex activation during appraisal (x=0). Percent signal change is displayed next to each figure. PREG=pregnenolone administration group. PBO=placebo.
Table 3.
Whole-Brain Correlations with Steroid Levels
| Steroid (pg/ml) and Brain Region | Cluster Size | MNI coordinates (x,y,z) | Analysis (r) |
|---|---|---|---|
| Pregnenolone | |||
| Right Insula | 12 | 42, 11, 4 | −.65 |
| Right Inferior Frontal Gyrus | 12 | 48, 11, 37 | −.62 |
| Allopregnanolone | |||
| Right Amygdala | 10 | 27, −1, −17 | −.66 |
| Pregnanolone | |||
| Right Lingual Gyrus | 18 | 33, −70, 7 | .62 |
| Right Insula | 127 | 30, 8, 7 | −.68 |
| Medial Frontal Gyrus | 11 | 18, 38, 31 | −.56 |
Correlations were conducted with change scores (natural log transformed steroid levels at endpoint minus baseline), and are significant at p<.001, uncorrected, extent threshold k>10.
Main Effect of Condition: Effects of Appraisal
There was a main effect of condition in the dmPFC [(−12,32,58); F(2, 232)=6.71; k=255; p<.001], and left anterior insula [(−48,26,−2]; F=6.72; k=160; p<.001] such that activity in these regions was increased during the appraisal condition as compared to the implicit emotion processing condition (p<.001). This effect was present for both the PREG (p=0.0002) and placebo (p=0.003) groups.
Main Effect of Condition: Effects of Attention Modulation
There was a main effect of condition in the left parietal cortex ([−36,−82,28]; F=54.26; k=341; p<.001), left precuneus ([−15,−55,13]; F=45.02; k=173; p<.001), and bilateral parahippocampal gyrus (Right: [30,−40,−11]; F=56.94; k=169; p<.001; Left: [−27,−43,−11]; F=64.07; k=225; p<.001) such that activity in these regions was increased in the attention modulation condition as compared to the implicit emotion processing condition (p<.001). Parahippocampal activity showed significant overlap with Parahippocampal Place Area (Right: [30,−40,−14]; k=158; Left: [−27,−37,−14]; k=131), indicating heightened attention to location during this condition.
Drug by Condition Interaction
There was a significant drug by condition interaction in the dmPFC ([3,56,37]; F(2,232)=6.41, p<.05, SVC), such that pregnenolone administration increased dmPFC activity in the appraisal condition as compared to the implicit emotion processing condition (p=.002; see Figure 2). The placebo group showed no significant differences between conditions in this dmPFC region. DMPFC activation in the PREG group was positively correlated with self-reported anxiety (r=.50, p=.047), whereas there was no relationship between dmPFC activation and self-reported anxiety in the placebo group (r=.18, p=.52). Since this region showed differential activation due to drug (PREG>PBO) during appraisal, subsequent PPI analysis was performed with this dmPFC region as a seed during the appraisal condition to identify differential patterns of connectivity across the drug versus placebo groups.
Psychophysiological Interaction (PPI)
Compared to placebo, the PREG group showed significantly greater functional connectivity between the dmPFC and left amygdala ([−30,−1,−23]; t=4.8, p<.001; see Figure 3) during the appraisal condition as compared to implicit baseline. No other region showed differential connectivity with the dmPFC. Functional connectivity between dmPFC and amygdala in the PREG group was inversely correlated with self-reported anxiety (r=−.52, p=.046). There was no relationship between self-reported anxiety and dmPFC-amygdala connectivity in the placebo group (r=−.043, p=.88). We further hypothesized that ratios of neurosteroid levels could influence functional connectivity between the dmPFC and amygdala or anxiety ratings (e.g., 20). These exploratory analyses can be found in Table 4.
Figure 3.
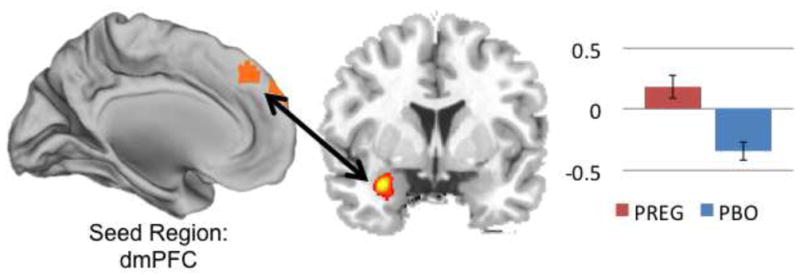
Pregnenolone administration increased functional connectivity between dorsal medial prefrontal cortex (dmPFC) and left amygdala (y=3) during appraisal.
PREG=pregnenolone administration group. PBO=placebo.
Table 4.
Correlations between steroid level, in-scan anxiety, and dmPFC-amygdala functional connectivity
| Steroid (pg/ml) | PREG/ALLO | DHEA/ALLO | DHEA/CORT |
|---|---|---|---|
| In-scan anxiety rating | .051 | −.089 | −.133 |
| dmPFC-amgydala connectivity | −.444* | −.337 | −.148 |
ALLO = allopregnanolone; CORT = cortisol; PREG = pregnenolone.
p<.05
Discussion
We used a novel probe of emotion processing, modulation and regulation to assess the neural basis of allopregnanolone’s impact on emotion processing. We demonstrate that elevation of allopregnanolone following pregnenolone administration is associated with reduced activity in regions linked to the generation of negative emotion, increased activity in regions linked to regulatory processes, and enhancement of functional connectivity; an effect that is associated with less self-reported anxiety. To our knowledge, this is the first neuroimaging study to demonstrate an effect of allopregnanolone on emotion regulation, a function likely dysregulated in anxiety disorders. These findings add to the current knowledge regarding the effects of allopregnanolone on emotion neurocircuits, and provide neuroimaging evidence that allopregnanolone may modulate neurocircuits in directions counter to those observed in anxiety psychopathology.
Pregnenolone administration reduced activity in neural circuits associated with the generation of negative emotions. Across all conditions and all face types, pregnenolone administration decreased right amygdala and right insula activity, and serum levels of pregnenolone and allopregnanolone were negatively correlated with amygdala and insula activation levels. The amygdala is a key region in threat detection (52), fear conditioning (53), and emotional salience (54). The insula is responsible for interoception (55), disgust (56), emotion processing (57), emotional recall (36), and anticipation of aversive stimuli (58). Both regions are associated with negative emotional response (57), and greater amygdala activation in response to the presentation of facial expressions is associated with greater magnitude of emotional response (59–63). Additionally, activation reductions in amygdala and insula are associated with down-regulation of negative emotions (64). Thus, allopregnanolone’s reduction of activity in amygdala and insula suggests that allopregnanolone may reduce emotional reactivity to aversive stimuli.
Pregnenolone administration also increased activity in the dmPFC, a region linked to regulatory control over emotion. This finding was specific to the appraisal condition. We have previously demonstrated that shifting attention to become aware of and evaluate the intensity of one’s emotional response to aversive stimuli leads to robust activation of dmPFC and rostral ACC (59, 65–67). Behavioral studies of emotional appraisal and labeling report that this strategy lowers distress (68) and facilitates habituation (69). Thus, allopregnanolone’s enhancement of dmPFC activity during appraisal suggests that allopregnanolone may facilitate the evaluation of one’s own emotional response and aid in successful down-regulation of negative emotions. Interestingly, greater dmPFC activity during appraisal was associated with greater self-reported anxiety. As the dmPFC is central to conscious threat appraisal (70), greater dmPFC activity in individuals with higher self-reported anxiety could reflect greater levels of threat processing. Alternatively, higher activity in this region could reflect greater task engagement in certain individuals.
Finally, pregnenolone administration increased connectivity between amygdala and dmPFC during appraisal, with greater connectivity associated with reduced self-reported anxiety. Psychophysiological interaction (PPI) reflects the connectivity between one region and another during a particular context, controlling for the baseline relationship between regions. Thus, the current results suggest that allopregnanolone is associated with greater functional coupling between amygdala and dmPFC during appraisal specifically. However, PPI does not assess the impact of third-party regions on the two regions of interest, and does not reflect causal relationships. While these correlational findings do not necessarily provide evidence of an inhibitory or excitatory relationship, they may suggest potential neural mechanisms of emotional regulation given known structural connections and reciprocal feedback loops between amygdala and mPFC (71–73). Previous research indicates that emotion regulation depends on interactions between dmPFC and amygdala (74). At least one previous study has demonstrated that enhanced connectivity between dmPFC and amygdala is associated with successful emotion regulation and less negative affect (75). Of note, some evidence suggests that successful regulation is associated with an inverse relationship (anti-correlation) between dmPFC and amygdala (64, 76). However, in our sample, greater connectivity between amygdala and dmPFC was associated with less self-reported anxiety, suggesting allopregnanolone’s modulatory effects on connectivity may aid dmPFC-mediated appraisal and/or reduce amygdala-mediated negative emotional responding. Our findings extend those of Van Wingen and colleagues (34) by demonstrating that allopregnanolone’s selective enhancement of dmPFC to amygdala connectivity is associated with reduced anxiety. Since several anxiety disorders are characterized by a lack of neural regulatory control (77) and impaired emotion regulation (38), future studies should examine allopregnanolone’s potential as a neurosteroid target for pharmacologic intervention for these individuals.
Allopregnanolone likely impacts emotion regulation neurocircuitry through GABAergic mechanisms, though it may also impact this circuitry through its enhancement of neurogenesis (78) myelination (79) or neuroprotection (80–83). Amygdala and mPFC are rich in GABA(A) receptors (28) and endogenous allopregnanolone (48), suggesting that allopregnanolone could feasibly have a direct impact on activity in these regions. Indeed, in our sample, allopregnanolone serum level was more strongly correlated to amygdala activity than activity in any other brain region. Preclinical research suggests that the amygdala may be a particular target of allopregnanolone’s anxiolytic effects (30). In rats, microinfusions of allopregnanolone directly into the amygdala produce anxiolytic (30) antidepressant (31) and anti-aggressive (32) effects. In previous neuroimaging studies, greater endogenous allopregnanolone has been reported to be associated with lower amygdala reactivity (33, 41) and greater coupling between amygdala and dmPFC (34). Though we did not directly test the GABAergic effect of our intervention, our findings illuminate potential neural pathways through which pregnenolone administration and resulting increases in allopregnanolone levels could feasibly impact GABAergic transmission in a manner that is relevant to pathological anxiety.
There are several limitations to this study. Limitations of our intervention include the fact that we measured serum levels of allopregnanolone, and not CSF or brain levels. However, in animals, neurosteroid levels appear to be highly correlated (46). Secondly, as steroid levels were only measured twice (once at baseline and once at the 3-hour endpoint), it is possible that steroids showing no change (including cortisol and DHEA) were in fact acutely changed but had returned to baseline by the endpoint of our experiment. Third, our event-related fMRI design is not well-suited to assess allopregnanolone’s potential impact on overall brain perfusion (82) or neurovascular coupling. Future studies should employ PET or arterial spin labeling to examine these issues. Sample limitations include the fact that our sample size was modest, thus our results should be considered preliminary. In particular, our power to detect differences between groups was limited by our small sample size; therefore, our study requires replication. Additionally, our sample consisted of healthy male individuals without mood or anxiety disorder diagnoses. Thus, extrapolations to women or to clinical populations should be made with caution. Potential behavioral data limitations include the fact that pregnenolone administration did not reduce overall self-reported anxiety, thus it is possible that the observed correlations between anxiety and amygdala-dmPFC coupling might be related to normal variations in anxiety levels rather than a drug induced effect per se. However, it may not be anticipated that participants without baseline anxiety symptoms would necessarily report decreases in self-reported anxiety. Future investigations in participants meeting criteria for anxiety disorders at study entry may help to clarify this issue. Finally, our drug manipulation involved the administration of pregnenolone, not allopregnanolone. Since allopregnanolone is not currently commercially available for clinical use, it was necessary to administer pregnenolone as a precursor loading strategy to increase downstream allopregnanolone levels. As our results demonstrate, oral administration of pregnenolone increases allopregnanolone levels sevenfold. We have framed our results in terms of an allopregnanolone manipulation, but our results may also be attributable to increases in pregnenolone. Pregnenolone levels are low in individuals with major depression (84) and anxiety disorders (85, 86), and are increased by fluoxetine administration in rats (22) and in humans (87). Therefore, pregnenolone may also be relevant to anxiety symptomatology, and may influence relevant neurocircuits. Thus, future studies should attempt to disentangle the emotion regulatory effects of pregnenolone versus its metabolite allopregnanolone.
In conclusion, we demonstrate that pregnenolone administration (leading to increased downstream allopregnanolone levels) reduces activity in regions associated with the generation of negative emotion and enhances activity in regions linked to regulatory control over emotion, as well as increasing connectivity between two of these regions (dmPFC and amygdala). Considering the wealth of evidence that neurocircuits involving these regions are altered in anxiety disorders, our results invite further investigation into the brain basis for allopregnanolone’s use as an anxiolytic pharmacological intervention.
Supplementary Material
Acknowledgments
The research reported in this article was supported by grants from the National Institute of Mental Health (R24 MH075999) to IL, from the Telemedicine and Advanced Technology Research Center (W81XWH-08-2-0208) to IL and AK, from a VA Career Development Transition Award to CM, and by the Veterans Affairs Mid-Atlantic Mental Illness, Research, Education and Clinical Center.
Footnotes
Financial Disclosures:
Dr. Marx discloses that she is an applicant or co-applicant on pending U.S. patent applications on the use of neurosteroids and derivatives for the treatment of central nervous system disorders and for lowering cholesterol (no patents issued, no licensing in place), and she is an unpaid scientific advisor to Sage Therapeutics.
The remaining authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Girdler SS, Klatzkin R. Neurosteroids in the context of stress: implications for depressive disorders. Pharmacol Ther. 2007;116:125–139. doi: 10.1016/j.pharmthera.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABA(A) receptor. Nat Rev Neurosci. 2005;6:565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- 3.Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- 4.Calogero AE, Palumbo MA, Bosboom AM, Burrello N, Ferrara E, Palumbo G, et al. The neuroactive steroid allopregnanolone suppresses hypothalamic gonadotropin-releasing hormone release through a mechanism mediated by the gamma-aminobutyric acidA receptor. J Endocrinol. 1998;158:121–125. doi: 10.1677/joe.0.1580121. [DOI] [PubMed] [Google Scholar]
- 5.Purdy RH, Morrow AL, Moore PH, Jr, Paul SM. Stress-induced elevations of gamma-aminobutyric acid type A receptor-active steroids in the rat brain. Proc Natl Acad Sci U S A. 1991;88:4553–4557. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pinna G, Rasmusson AM. Up-regulation of neurosteroid biosynthesis as a pharmacological strategy to improve behavioural deficits in a putative mouse model of post-traumatic stress disorder. J Neuroendocrinol. 2011;24:102–116. doi: 10.1111/j.1365-2826.2011.02234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frye CA, Paris JJ. Progesterone turnover to its 5alpha-reduced metabolites in the ventral tegmental area of the midbrain is essential for initiating social and affective behavior and progesterone metabolism in female rats. J Endocrinol Invest. 2011;34:e188–199. doi: 10.3275/7334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koonce CJ, Walf AA, Frye CA. Type 1 5alpha-reductase may be required for estrous cycle changes in affective behaviors of female mice. Behav Brain Res. 2012;226:376–380. doi: 10.1016/j.bbr.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frye CA, Paris JJ, Walf AA, Rusconi JC. Effects and Mechanisms of 3alpha,5alpha,-THP on Emotion, Motivation, and Reward Functions Involving Pregnane Xenobiotic Receptor. Front Neurosci. 2011;5:136. doi: 10.3389/fnins.2011.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harrison NL, Simmonds MA. Modulation of the GABA receptor complex by a steroid anaesthetic. Brain Res. 1984;323:287–292. doi: 10.1016/0006-8993(84)90299-3. [DOI] [PubMed] [Google Scholar]
- 11.Lambert JJ, Belelli D, Peden DR, Vardy AW, Peters JA. Neurosteroid modulation of GABAA receptors. Prog Neurobiol. 2003;71:67–80. doi: 10.1016/j.pneurobio.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Martin-Garcia E, Pallares M. Intrahippocampal nicotine and neurosteroids effects on the anxiety-like behaviour in voluntary and chronic alcohol-drinking rats. Behav Brain Res. 2005;164:117–127. doi: 10.1016/j.bbr.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 13.Frye CA, Rhodes ME. Infusions of 5alpha-pregnan-3alpha-ol-20-one (3alpha,5alpha-THP) to the ventral tegmental area, but not the substantia nigra, enhance exploratory, anti-anxiety, social and sexual behaviours and concomitantly increase 3alpha,5alpha-THP concentrations in the hippocampus, diencephalon and cortex of ovariectomised oestrogen-primed rats. J Neuroendocrinol. 2006;18:960–975. doi: 10.1111/j.1365-2826.2006.01494.x. [DOI] [PubMed] [Google Scholar]
- 14.Darbra S, Pallares M. Alterations in neonatal neurosteroids affect exploration during adolescence and prepulse inhibition in adulthood. Psychoneuroendocrinology. 2010;35:525–535. doi: 10.1016/j.psyneuen.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 15.Darbra S, Pallares M. Effects of early postnatal allopregnanolone administration on elevated plus maze anxiety scores in adult male Wistar rats. Neuropsychobiology. 2012;65:20–27. doi: 10.1159/000328161. [DOI] [PubMed] [Google Scholar]
- 16.Pibiri F, Nelson M, Guidotti A, Costa E, Pinna G. Decreased corticolimbic allopregnanolone expression during social isolation enhances contextual fear: A model relevant for posttraumatic stress disorder. Proc Natl Acad Sci U S A. 2008;105:5567–5572. doi: 10.1073/pnas.0801853105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amin Z, Mason GF, Cavus I, Krystal JH, Rothman DL, Epperson CN. The interaction of neuroactive steroids and GABA in the development of neuropsychiatric disorders in women. Pharmacol Biochem Behav. 2006;84:635–643. doi: 10.1016/j.pbb.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 18.van Broekhoven F, Verkes RJ. Neurosteroids in depression: a review. Psychopharmacology (Berl) 2003;165:97–110. doi: 10.1007/s00213-002-1257-1. [DOI] [PubMed] [Google Scholar]
- 19.Uzunova V, Sheline Y, Davis JM, Rasmusson A, Uzunov DP, Costa E, et al. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc Natl Acad Sci U S A. 1998;95:3239–3244. doi: 10.1073/pnas.95.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rasmusson AM, Pinna G, Paliwal P, Weisman D, Gottschalk C, Charney D, et al. Decreased cerebrospinal fluid allopregnanolone levels in women with posttraumatic stress disorder. Biol Psychiatry. 2006;60:704–713. doi: 10.1016/j.biopsych.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 21.Strohle A, Romeo E, Hermann B, Pasini A, Spalletta G, di Michele F, et al. Concentrations of 3 alpha-reduced neuroactive steroids and their precursors in plasma of patients with major depression and after clinical recovery. Biol Psychiatry. 1999;45:274–277. doi: 10.1016/s0006-3223(98)00328-x. [DOI] [PubMed] [Google Scholar]
- 22.Marx CE, Shampine LJ, Khisti RT, Trost WT, Bradford DW, Grobin AC, et al. Olanzapine and fluoxetine administration and coadministration increase rat hippocampal pregnenolone, allopregnanolone and peripheral deoxycorticosterone: implications for therapeutic actions. Pharmacol Biochem Behav. 2006;84:609–617. doi: 10.1016/j.pbb.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 23.Matsumoto K, Puia G, Dong E, Pinna G. GABA(A) receptor neurotransmission dysfunction in a mouse model of social isolation-induced stress: possible insights into a non-serotonergic mechanism of action of SSRIs in mood and anxiety disorders. Stress. 2007;10:3–12. doi: 10.1080/10253890701200997. [DOI] [PubMed] [Google Scholar]
- 24.Uzunov DP, Cooper TB, Costa E, Guidotti A. Fluoxetine-elicited changes in brain neurosteroid content measured by negative ion mass fragmentography. Proc Natl Acad Sci U S A. 1996;93:12599–12604. doi: 10.1073/pnas.93.22.12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romeo E, Strohle A, Spalletta G, di Michele F, Hermann B, Holsboer F, et al. Effects of antidepressant treatment on neuroactive steroids in major depression. Am J Psychiatry. 1998;155:910–913. doi: 10.1176/ajp.155.7.910. [DOI] [PubMed] [Google Scholar]
- 26.Pinna G, Costa E, Guidotti A. SSRIs act as selective brain steroidogenic stimulants (SBSSs) at low doses that are inactive on 5-HT reuptake. Curr Opin Pharmacol. 2009;9:24–30. doi: 10.1016/j.coph.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pinna G, Costa E, Guidotti A. Fluoxetine and norfluoxetine stereospecifically and selectively increase brain neurosteroid content at doses that are inactive on 5-HT reuptake. Psychopharmacology (Berl) 2006;186:362–372. doi: 10.1007/s00213-005-0213-2. [DOI] [PubMed] [Google Scholar]
- 28.Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- 29.Akwa Y, Purdy RH, Koob GF, Britton KT. The amygdala mediates the anxiolytic-like effect of the neurosteroid allopregnanolone in rat. Behav Brain Res. 1999;106:119–125. doi: 10.1016/s0166-4328(99)00101-1. [DOI] [PubMed] [Google Scholar]
- 30.Engin E, Treit D. The anxiolytic-like effects of allopregnanolone vary as a function of intracerebral microinfusion site: the amygdala, medial prefrontal cortex, or hippocampus. Behav Pharmacol. 2007;18:461–470. doi: 10.1097/FBP.0b013e3282d28f6f. [DOI] [PubMed] [Google Scholar]
- 31.Shirayama Y, Muneoka K, Fukumoto M, Tadokoro S, Fukami G, Hashimoto K, et al. Infusions of allopregnanolone into the hippocampus and amygdala, but not into the nucleus accumbens and medial prefrontal cortex, produce antidepressant effects on the learned helplessness rats. Hippocampus. 2011;21:1105–1113. doi: 10.1002/hipo.20824. [DOI] [PubMed] [Google Scholar]
- 32.Nelson M, Pinna G. S-norfluoxetine microinfused into the basolateral amygdala increases allopregnanolone levels and reduces aggression in socially isolated mice. Neuropharmacology. 2011;60:1154–1159. doi: 10.1016/j.neuropharm.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Wingen G, van Broekhoven F, Verkes RJ, Petersson KM, Backstrom T, Buitelaar J, et al. How progesterone impairs memory for biologically salient stimuli in healthy young women. J Neurosci. 2007;27:11416–11423. doi: 10.1523/JNEUROSCI.1715-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Wingen GA, van Broekhoven F, Verkes RJ, Petersson KM, Backstrom T, Buitelaar JK, et al. Progesterone selectively increases amygdala reactivity in women. Mol Psychiatry. 2008;13:325–333. doi: 10.1038/sj.mp.4002030. [DOI] [PubMed] [Google Scholar]
- 35.Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- 37.Dillon DG, Deveney CM, Pizzagalli DA. From Basic Processes to Real-World Problems: How Research on Emotion and Emotion Regulation Can Inform Understanding of Psychopathology, and Vice Versa. Emot Rev. 2011;3:74–82. doi: 10.1177/1754073910380973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amstadter A. Emotion regulation and anxiety disorders. J Anxiety Disord. 2008;22:211–221. doi: 10.1016/j.janxdis.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sripada RK, Marx CE, King AP, Rajaram N, Garfinkel SN, Abelson JL, et al. DHEA Enhances Emotion Regulation Neurocircuits and Modulates Memory for Emotional Stimuli. 2012. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klumpp H, Ho SS, Taylor SF, Phan KL, Abelson JL, Liberzon I. Trait anxiety modulates anterior cingulate activation to threat interference. Depress Anxiety. 2011;28:194–201. doi: 10.1002/da.20802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ossewaarde L, Hermans EJ, van Wingen GA, Kooijman SC, Johansson IM, Backstrom T, et al. Neural mechanisms underlying changes in stress-sensitivity across the menstrual cycle. Psychoneuroendocrinology. 2010;35:47–55. doi: 10.1016/j.psyneuen.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 42.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.) the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- 43.Marx CE, Keefe RS, Buchanan RW, Hamer RM, Kilts JD, Bradford DW, et al. Proof-of-Concept Trial with the Neurosteroid Pregnenolone Targeting Cognitive and Negative Symptoms in Schizophrenia. Neuropsychopharmacology. 2009 doi: 10.1038/npp.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marx CE, Bradford DW, Hamer RM, Naylor JC, Allen TB, Lieberman JA, et al. Pregnenolone as a novel therapeutic candidate in schizophrenia: emerging preclinical and clinical evidence. Neuroscience. 2011;191:78–90. doi: 10.1016/j.neuroscience.2011.06.076. [DOI] [PubMed] [Google Scholar]
- 45.Marx CE. 2007 unpublished data. [Google Scholar]
- 46.Marx CE, Shampine LJ, Duncan GE, VanDoren MJ, Grobin AC, Massing MW, et al. Clozapine markedly elevates pregnenolone in rat hippocampus, cerebral cortex, and serum: candidate mechanism for superior efficacy? Pharmacol Biochem Behav. 2006;84:598–608. doi: 10.1016/j.pbb.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 47.Marx CE, Trost WT, Shampine L, Behm FM, Giordano LA, Massing MW, et al. Neuroactive steroids, negative affect, and nicotine dependence severity in male smokers. Psychopharmacology (Berl) 2006;186:462–472. doi: 10.1007/s00213-005-0226-x. [DOI] [PubMed] [Google Scholar]
- 48.Marx CE, Trost WT, Shampine LJ, Stevens RD, Hulette CM, Steffens DC, et al. The neurosteroid allopregnanolone is reduced in prefrontal cortex in Alzheimer’s disease. Biol Psychiatry. 2006;60:1287–1294. doi: 10.1016/j.biopsych.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 49.Anderson AK, Christoff K, Panitz D, De Rosa E, Gabrieli JD. Neural correlates of the automatic processing of threat facial signals. J Neurosci. 2003;23:5627–5633. doi: 10.1523/JNEUROSCI.23-13-05627.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ward B. Simultaneous inference for fMRI data. 2000 http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf.
- 51.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. Thieme; 1988. [Google Scholar]
- 52.Adolphs R, Tranel D, Hamann S, Young AW, Calder AJ, Phelps EA, et al. Recognition of facial emotion in nine individuals with bilateral amygdala damage. Neuropsychologia. 1999;37:1111–1117. doi: 10.1016/s0028-3932(99)00039-1. [DOI] [PubMed] [Google Scholar]
- 53.Armony JL, LeDoux JE. How the brain processes emotional information. Ann N Y Acad Sci. 1997;821:259–270. doi: 10.1111/j.1749-6632.1997.tb48285.x. [DOI] [PubMed] [Google Scholar]
- 54.Whalen PJ, Shin LM, McInerney SC, Fischer H, Wright CI, Rauch SL. A functional MRI study of human amygdala responses to facial expressions of fear versus anger. Emotion. 2001;1:70–83. doi: 10.1037/1528-3542.1.1.70. [DOI] [PubMed] [Google Scholar]
- 55.Oppenheimer SM, Gelb A, Girvin JP, Hachinski VC. Cardiovascular effects of human insular cortex stimulation. Neurology. 1992;42:1727–1732. doi: 10.1212/wnl.42.9.1727. [DOI] [PubMed] [Google Scholar]
- 56.Britton JC, Phan KL, Taylor SF, Welsh RC, Berridge KC, Liberzon I. Neural correlates of social and nonsocial emotions: An fMRI study. Neuroimage. 2006;31:397–409. doi: 10.1016/j.neuroimage.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 57.Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am J Psychiatry. 2007;164:318–327. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- 58.Simmons AN, Paulus MP, Thorp SR, Matthews SC, Norman SB, Stein MB. Functional activation and neural networks in women with posttraumatic stress disorder related to intimate partner violence. Biol Psychiatry. 2008;64:681–690. doi: 10.1016/j.biopsych.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Phan KL, Taylor SF, Welsh RC, Ho SH, Britton JC, Liberzon I. Neural correlates of individual ratings of emotional salience: a trial-related fMRI study. Neuroimage. 2004;21:768–780. doi: 10.1016/j.neuroimage.2003.09.072. [DOI] [PubMed] [Google Scholar]
- 60.Schneider F, Grodd W, Weiss U, Klose U, Mayer KR, Nagele T, et al. Functional MRI reveals left amygdala activation during emotion. Psychiatry Res. 1997;76:75–82. doi: 10.1016/s0925-4927(97)00063-2. [DOI] [PubMed] [Google Scholar]
- 61.Carre JM, Fisher PM, Manuck SB, Hariri AR. Interaction between trait anxiety and trait anger predict amygdala reactivity to angry facial expressions in men but not women. SCAN. doi: 10.1093/scan/nsq101. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ewbank MP, Fox E, Calder AJ. The Interaction Between Gaze and Facial Expression in the Amygdala and Extended Amygdala is Modulated by Anxiety. Front Hum Neurosci. 2010;4:56. doi: 10.3389/fnhum.2010.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dyck M, Loughead J, Kellermann T, Boers F, Gur RC, Mathiak K. Cognitive versus automatic mechanisms of mood induction differentially activate left and right amygdala. Neuroimage. 2011;54:2503–2513. doi: 10.1016/j.neuroimage.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 64.Kanske P, Heissler J, Schonfelder S, Bongers A, Wessa M. How to regulate emotion? Neural networks for reappraisal and distraction. Cereb Cortex. 2011;21:1379–1388. doi: 10.1093/cercor/bhq216. [DOI] [PubMed] [Google Scholar]
- 65.Phan KL, Taylor SF, Welsh RC, Decker LR, Noll DC, Nichols TE, et al. Activation of the medial prefrontal cortex and extended amygdala by individual ratings of emotional arousal: a fMRI study. Biol Psychiatry. 2003;53:211–215. doi: 10.1016/s0006-3223(02)01485-3. [DOI] [PubMed] [Google Scholar]
- 66.Taylor SF, Phan KL, Decker LR, Liberzon I. Subjective rating of emotionally salient stimuli modulates neural activity. Neuroimage. 2003;18:650–659. doi: 10.1016/s1053-8119(02)00051-4. [DOI] [PubMed] [Google Scholar]
- 67.Liberzon I, Taylor SF, Fig LM, Decker LR, Koeppe RA, Minoshima S. Limbic activation and psychophysiologic responses to aversive visual stimuli. Interaction with cognitive task. Neuropsychopharmacology. 2000;23:508–516. doi: 10.1016/S0893-133X(00)00157-3. [DOI] [PubMed] [Google Scholar]
- 68.Lieberman MD, Inagaki TK, Tabibnia G, Crockett MJ. Subjective Responses to Emotional Stimuli During Labeling, Reappraisal, and Distraction. Emotion. 2011;11:468–480. doi: 10.1037/a0023503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tabibnia G, Lieberman MD, Craske MG. The lasting effect of words on feelings: Words may facilitate exposure effects to threatening images. Emotion. 2008;8:307–317. doi: 10.1037/1528-3542.8.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mechias ML, Etkin A, Kalisch R. A meta-analysis of instructed fear studies: implications for conscious appraisal of threat. Neuroimage. 2010;49:1760–1768. doi: 10.1016/j.neuroimage.2009.09.040. [DOI] [PubMed] [Google Scholar]
- 71.Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34:905–923. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bracht T, Tuscher O, Schnell S, Kreher B, Rusch N, Glauche V, et al. Extraction of prefronto-amygdalar pathways by combining probability maps. Psychiatry Res. 2009;174:217–222. doi: 10.1016/j.pscychresns.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 73.Robinson JL, Laird AR, Glahn DC, Lovallo WR, Fox PT. Metaanalytic connectivity modeling: delineating the functional connectivity of the human amygdala. Hum Brain Mapp. 2010;31:173–184. doi: 10.1002/hbm.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 75.Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL. Amygdala-frontal connectivity during emotion regulation. Soc Cogn Affect Neurosci. 2007;2:303–312. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee H, Heller AS, van Reekum CM, Nelson B, Davidson RJ. Amygdala-prefrontal coupling underlies individual differences in emotion regulation. Neuroimage. doi: 10.1016/j.neuroimage.2012.05.044. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang JM, Johnston PB, Ball BG, Brinton RD. The neurosteroid allopregnanolone promotes proliferation of rodent and human neural progenitor cells and regulates cell-cycle gene and protein expression. J Neurosci. 2005;25:4706–4718. doi: 10.1523/JNEUROSCI.4520-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ghoumari AM, Ibanez C, El-Etr M, Leclerc P, Eychenne B, O’Malley BW, et al. Progesterone and its metabolites increase myelin basic protein expression in organotypic slice cultures of rat cerebellum. J Neurochem. 2003;86:848–859. doi: 10.1046/j.1471-4159.2003.01881.x. [DOI] [PubMed] [Google Scholar]
- 80.Mellon SH, Gong W, Schonemann MD. Endogenous and synthetic neurosteroids in treatment of Niemann-Pick Type C disease. Brain Res Rev. 2008;57:410–420. doi: 10.1016/j.brainresrev.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Djebaili M, Guo Q, Pettus EH, Hoffman SW, Stein DG. The neurosteroids progesterone and allopregnanolone reduce cell death, gliosis, and functional deficits after traumatic brain injury in rats. J Neurotrauma. 2005;22:106–118. doi: 10.1089/neu.2005.22.106. [DOI] [PubMed] [Google Scholar]
- 82.Sayeed I, Guo Q, Hoffman SW, Stein DG. Allopregnanolone, a progesterone metabolite, is more effective than progesterone in reducing cortical infarct volume after transient middle cerebral artery occlusion. Ann Emerg Med. 2006;47:381–389. doi: 10.1016/j.annemergmed.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 83.Griffin LD, Gong W, Verot L, Mellon SH. Niemann-Pick type C disease involves disrupted neurosteroidogenesis and responds to allopregnanolone. Nat Med. 2004;10:704–711. doi: 10.1038/nm1073. [DOI] [PubMed] [Google Scholar]
- 84.George MS, Guidotti A, Rubinow D, Pan B, Mikalauskas K, Post RM. CSF neuroactive steroids in affective disorders: pregnenolone, progesterone, and DBI. Biol Psychiatry. 1994;35:775–780. doi: 10.1016/0006-3223(94)91139-8. [DOI] [PubMed] [Google Scholar]
- 85.Semeniuk T, Jhangri GS, Le Melledo JM. Neuroactive steroid levels in patients with generalized anxiety disorder. J Neuropsychiatry Clin Neurosci. 2001;13:396–398. doi: 10.1176/jnp.13.3.396. [DOI] [PubMed] [Google Scholar]
- 86.Heydari B, Le Melledo JM. Low pregnenolone sulphate plasma concentrations in patients with generalized social phobia. Psychol Med. 2002;32:929–933. doi: 10.1017/s0033291702005238. [DOI] [PubMed] [Google Scholar]
- 87.Bicikova M, Tallova J, Hill M, Krausova Z, Hampl R. Serum concentrations of some neuroactive steroids in women suffering from mixed anxiety-depressive disorder. Neurochem Res. 2000;25:1623–1627. doi: 10.1023/a:1026622704704. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.