Abstract
The cAMP signaling pathway mediates synaptic plasticity and is essential for memory formation in both vertebrate and invertebrates. In the fruit fly Drosophila melanogaster, mutations in the cAMP pathway lead to impaired olfactory learning. These mutant genes are preferentially expressed in the mushroom body (MB), an anatomical structure essential for learning. While cAMP-mediated synaptic plasticity is known to be involved in facilitation at the excitatory synapses, little is known about its function in GABAergic synaptic plasticity and learning. In this study, using whole-cell patch clamp technique on Drosophila primary neuronal cultures, we demonstrate that focal application of an adenylate cyclase activator forskolin (FSK) suppresses inhibitory GABAergic postsynaptic currents (IPSCs). We observed a dual regulatory role of FSK on GABAergic transmission, where it increases overall excitability at GABAergic synapses, while simultaneously acting on postsynaptic GABA receptors to suppress GABAergic IPSCs. Further we show that cAMP decreases GABAergic IPSCs in a PKA-dependent manner through a postsynaptic mechanism. PKA acts through the modulation of ionotropic GABA receptor sensitivity to the neurotransmitter GABA. This regulation of GABAergic IPSCs is altered in the cAMP pathway and short-term memory mutants dunce and rutabaga, with both showing altered GABA receptor sensitivity. Interestingly, this effect is also conserved in the MB neurons of both these mutants. Thus, our study suggests that alterations of cAMP-mediated GABAergic plasticity, particularly in the MB neurons of cAMP mutants, account for their defects in olfactory learning.
Keywords: cAMP-PKA signaling pathway, GABAergic IPSCs, fruit fly, forskolin, learning and memory
INTRODUCTION
Cyclic AMP signaling is the most well known second messenger system mediating synaptic plasticity, learning and memory in both vertebrates and invertebrates (Milner et al., 1998; Kandel, 2001). In Drosophila, a number of short-term memory genes are essential molecular components of the cAMP signaling cascade. For example, dunce encodes a phosphodiesterase which breaks down cytoplasmic cAMP (Dudai et al., 1976) and rutabaga codes for the cAMP synthesizing enzyme adenylate cyclase (Livingstone et al., 1984). These genes are expressed preferentially in the mushroom body (MB), a brain region involved in olfactory learning (Keene & Waddell, 2007). cAMP signaling has been implicated in regulating synaptic plasticity at glutamatergic neuromuscular junctions and at excitatory cholinergic synapses in Drosophila. Interestingly, the memory mutants show alterations in facilitation and/or post-tetanic potentiation (Zhong & Wu, 1991; Lee & O'Dowd, 2000; Rohrbough & Broadie, 2002). These findings demonstrate that cAMP signaling regulates plasticity at excitatory glutamate and cholinergic synapses and thus mediates learning and memory in Drosophila.
γ-aminobutyric acid (GABA), a major inhibitory neurotransmitter (Bormann, 1988), plays an important role in neuronal communication (Paulsen & Moser, 1998). In mammals, GABAergic neurons innervate the memory center hippocampus (Freund & Buzsaki, 1996) and GABA is known to be critical for higher brain functions such as learning/memory and coordinated behaviors (Floyer-Lea et al., 2006; Fernandez et al., 2007). Indeed, inhibitory synaptic plasticity was observed at GABAergic synapses in rodent hippocampus CA1 neurons (Lu et al., 2000; Chevaleyre & Castillo, 2003). In contrast to the excitatory synaptic plasticity (e.g. LTP), inhibitory GABAergic plasticity is relatively unexplored in the hippocampus. In particular, few studies explore inhibitory plasticity mediated by the cAMP-PKA pathway largely due to the complexity of hippocampal GABAergic circuits (Castillo et al., 2011) and GABAA receptor subunit composition (Nusser et al., 1999).
Similar to the hippocampus, the Drosophila mushroom body (MB) is extensively innervated by inhibitory GABAergic neurons (Yasuyama et al., 2002). The GABAergic neurons projecting on to the MB have also been implicated in olfactory learning (Liu & Davis, 2009). Further, it has been demonstrated that a Drosophila GABA receptor RDL (resistance to dieldrin) inhibits olfactory associative learning (Liu et al., 2007). RDL containing GABA receptors have been shown to regulate GABAergic synaptic currents at Drosophila central synapses (Lee et al., 2003; Su & O'Dowd, 2003). All these studies indicate an important role of GABAergic transmission in learning and memory. However, it remains unclear whether and how cAMP-dependent plasticity at inhibitory GABAergic synapses mediates learning and memory.
In the present study, we use whole-cell patch-clamp to examine physiological mechanisms underlying regulation of cAMP-mediated GABAergic plasticity in Drosophila primary neuronal cultures. Our results show that inhibitory GABAergic transmission is suppressed by the cAMP-PKA signaling pathway. We also examine alterations of GABAergic plasticity in the MB neurons of cAMP mutants dunce and rutabaga, and demonstrate that cAMP alters the sensitivity of ionotropic GABA receptors to modulate GABAergic transmission in the MB neurons of both learning mutants.
METHODS
Fly strains
The w1118 (a “Cantonized” white eye stock) and Canton-S flies were used as wild-type flies in all experiments. dunce (dnc1), rutabaga (rut1), UAS-GFP(T10) and Rdl1 null mutant (Rdl1/TM6B, ubi-GFP) strains were obtained from Bloomington stock center and maintained on standard fly food medium.
Drosophila embryonic neuronal cultures
Neuronal cultures were prepared from midgastrula stage embryos and grown in the culture medium (DDM1) as previously described (Lee & O'Dowd, 1999; Park & Lee, 2006). Cultures were maintained in an incubator supplied with 5% CO2 at 24–25°C for 3–6 days in vitro.
Drosophila pupal neuronal cultures
The pupal culture protocol was adapted from the one described earlier (Su & O'Dowd, 2003). The dissecting saline solution was composed of (in mM): 126 NaCl, 5.4 KCl, 0.17 NaH2PO4, 0.22 KH2PO4, 33.3 glucose, 43.8 sucrose, and 9.9 HEPES, pH 7.4. The enzyme solution in which the brains were incubated consisted of 5 U/ml papain, L-cysteine 5.5mM), EDTA (1.1mM) and 2-mercaptoethanol (0.067mM) in dissecting saline solution. Glass coverslips used to plate the neurons were coated with 0.01% of Poly-L-Lysine. 3–6 days in vitro pupal cultures were used for all electrophysiology experiments.
Pharmacology
Forskolin (Sigma), an activator of adenylate cyclase, the cAMP analog db-cAMP (Calbiochem) and GABA (Sigma) were focally applied to the patched neurons using Picospritzer III (Parker Hannifin Corp.). H-89 (Sigma) used to inhibit PKA was added to the external recording solution 10–15 min before recording. The membrane impermeable PKA blocker PKI (6–22) amide (Biomol) was added to the internal recording solution contained in the recording pipette. For perfusion experiments, forskolin was freshly made and added to the recording chamber by a perfusion system with vacuum/solution flow control valves (FR50, Warner Instruments Inc.) at a speed of 1 mL/min.
Electrophysiology
Each coverslip containing Drosophila neuronal cultures was transferred to a recording chamber with the external solution (mM): 140 NaCl, 1 CaCl2, 4 MgCl2, 3 KCl, and 5 HEPES, pH 7.2. Postsynaptic currents (PSCs) were recorded with whole-cell pipettes (tip resistance 4–6 MΩ for embryonic and 8–10 MΩ for pupal neuronal cultures) filled with internal solutions consisting of (mM): 120 CsOH, 120 D-gluconic acid, 0.1 CaCl2, 2 MgCl2, 20 NaCl, 1.1 EGTA, and 10 HEPES, pH 7.2. ATP (4 mM) was added to the internal solution to prevent rundown of the currents. For recording GABAergic IPSCs the voltage was held at 0 mV to prevent interference by cholinergic EPSCs (Lee et al., 2003). GABA-evoked currents were recorded in the whole-cell configuration, at a holding potential of 0 mV, in response to puffer application of GABA and forskolin. Axopatch 200B amplifier (Axon Instruments Inc.) was used to measure PSCs. Action potentials (APs) were recorded in the cell-attached mode, and were hence extracellular.
Data Analysis
Individual PSCs were analyzed using the Minianalysis detection software (Synaptosoft) with threshold criteria for individual events at 5pA and 5pF for GABAergic PSCs (two-fold greater than the 2.5 pA root mean square noise level). Events were accepted for kinetic analysis only if the shape was asymmetrical with a fast-rising and slowly decaying falling phase. Current traces were filtered at 2 kHz and digitized at 20 kHz using pClamp9 software. For each acquired data trace, all events were detected and the frequency was calculated every 5 sec. If the PSC frequency declined continuously over 20 sec before drug application, a rundown of PSCs was suspected and the data were not analyzed further. For the frequency analysis, only PSCs (before drug application) with a stable frequency longer than 20 sec were used.
All statistical analyses were carried out using Origin 7 (Origin Lab, MA). When comparing multiple genotypes we used One-way Anova analysis with appropriate post-hoc tests to compare groups. For all other experiments two tailed Student’s T-test was performed, unless otherwise noted in the text.
RESULTS
Drosophila inhibitory GABAergic synaptic transmission is suppressed by the adenylate cyclase activator forskolin
We examined modulatory effects of cAMP signaling on inhibitory GABAergic synaptic transmission in Drosophila primary cultured neurons. For this, GABAergic inhibitory postsynaptic currents (IPSCs) were recorded in cultured neurons using whole-cell patch technique as previously described (Lee et al., 2003). These spontaneous IPSCs are comprised of both action potential (AP)-dependent and -independent (miniature IPSC) synaptic currents that are almost completely Ca2+-dependent (Lee et al., 2003).
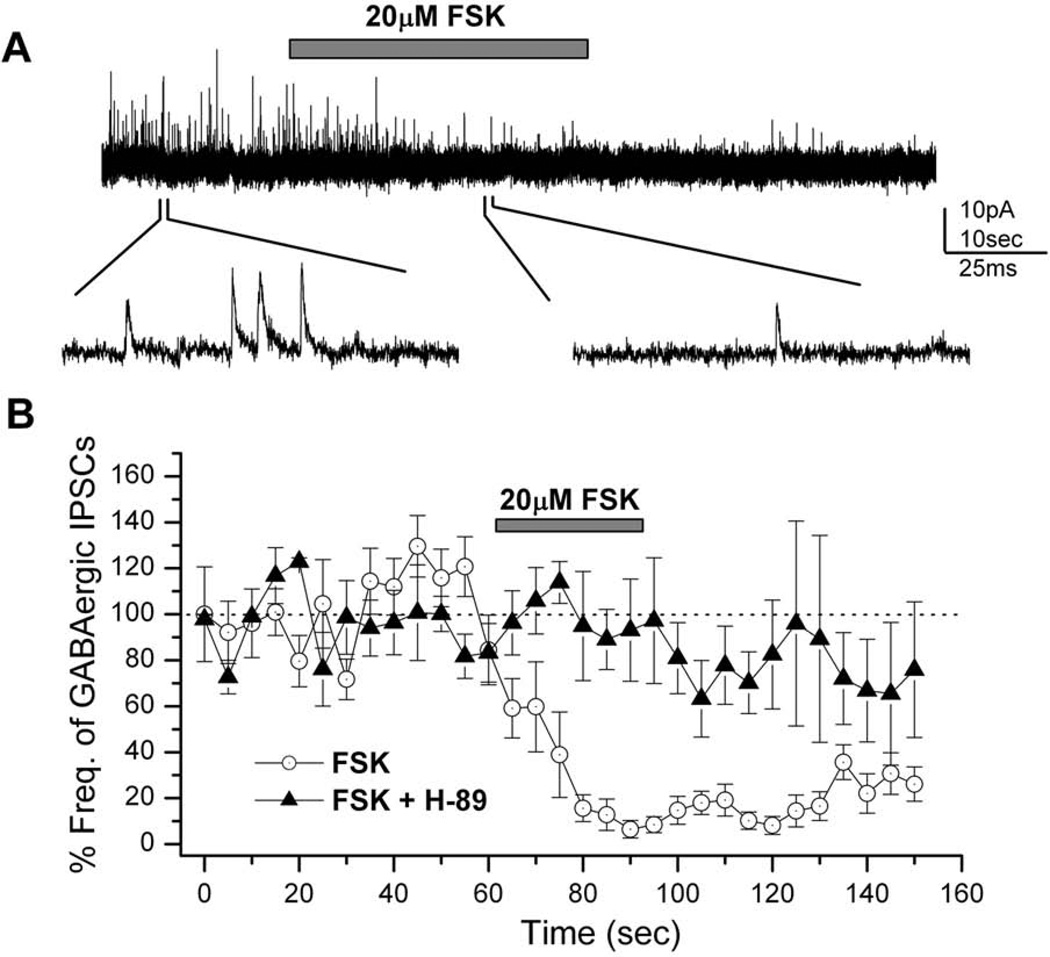
In order to eliminate interference by cholinergic synaptic currents neurons were held at 0 mV, the reversal potential of nicotinic acetylcholine receptors, as previously described (Lee & O'Dowd, 1999). The adenylyl cyclase (AC) activator forskolin (FSK; 20µM) was focally applied to a neuron showing GABAergic IPSCs. FSK strongly reduced the frequency of GABAergic IPSCs during its application and this suppression continued even after the end of FSK application (Figure 1A). Figure 1B shows the average reduction in the frequency of GABAergic IPSCs (n=9). To account for the variation in GABAergic IPSC frequency between different neurons, the frequency of IPSCs was calculated every 5 sec and normalized to the average frequency for 30–60 sec before FSK application (Yuan & Lee, 2007). The maximum reduction in the frequency of IPSCs was observed about 20 sec from the onset of FSK application as its effect was gradual for the first 20 sec. The time lag observed is probably due to the time taken by FSK to act on AC and to then increase the intracellular cAMP levels. We further observed a prolonged suppression of GABAergic IPSCs after the end of FSK application.
Figure 1. Suppression of inhibitory GABAergic postsynaptic currents (IPSCs) by forskolin (FSK).
A, FSK (20µM) was focally applied to a wild type neuron (3 days in culture) after obtaining a stable GABAergic IPSC trace. GABAergic IPSCs were almost completely suppressed by FSK. Example IPSC traces are shown on an expanded time scale below the complete recording trace. Holding potential (VH) = 0 mV. B, The graph shows the reduction in GABAergic IPSC frequency by the application of 20µM FSK in wild-type neurons in the absence (n=9; circles) and presence (n=4) of 50µM H-89 (a membrane-permeable PKA inhibitor). The reduction seen in IPSCs in the presence of H-89 is almost negligible as compared to the change in the absence of H-89. FSK was applied for 30 seconds (indicated by bar) at 62.5 seconds after the initiation of the IPSC recording. The IPSC frequency was calculated every 5 seconds and normalized to that of controls as described in materials and methods. Bars indicate SEM.
Since protein kinase A (PKA) is a known effector of cAMP, we examined its involvement in FSK-mediated suppression of GABAergic IPSCs using the PKA inhibitor H-89. Cultured neurons were incubated in the recording solution with H-89 for 10–15 minutes before whole-cell recordings of GABAergic IPSCs. In the presence of H-89, the focal application of 20µM FSK showed no decrease in the frequency of GABAergic IPSCs (n=5; Figure 1B). This attenuation of FSK-mediated GABAergic IPSCs by H-89 demonstrates that PKA is a key player in this process.
Dibutyric cAMP down-regulates Drosophila GABAergic IPSCs in a dose-dependent manner
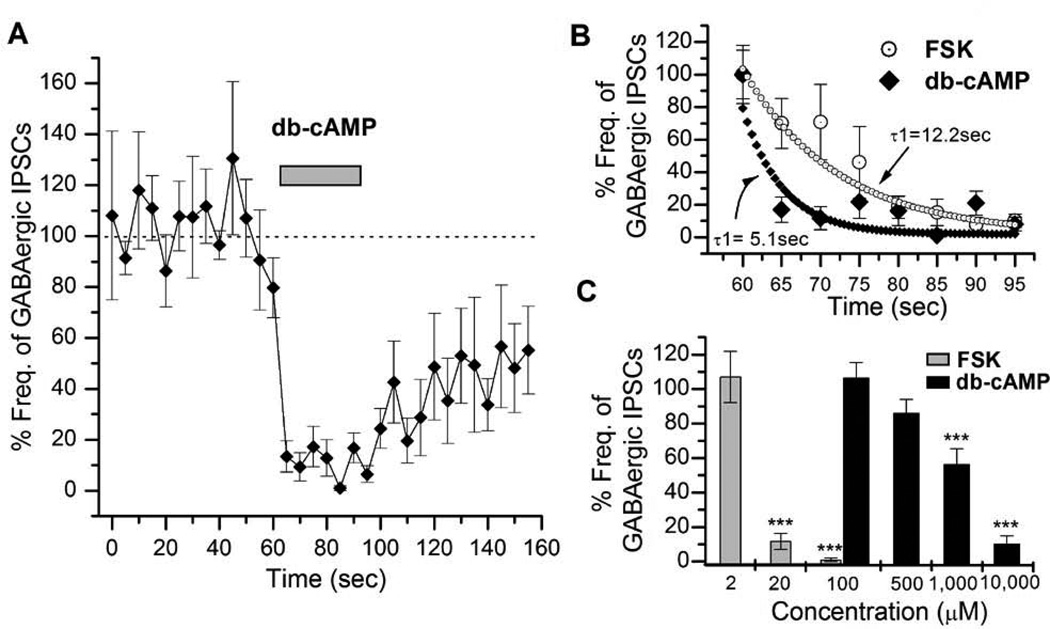
Our results with H-89 strongly indicate that GABAergic suppression by FSK is through the cAMP-PKA signaling pathway. However some studies have shown that FSK can also act through cAMP-independent mechanisms (Hoshi et al., 1988; Harris-Warrick, 1989; Gandia et al., 1997). We thus used a membrane-permeable cAMP analog (db-cAMP) to rule out such cAMP-independent effects of FSK. GABAergic IPSC frequency was markedly decreased by db-cAMP (n=6; Figure 2A) whereas it increased cholinergic EPSCs (data not shown, refer to Yuan & Lee, 2007). We observed no lag time for this suppression unlike that seen earlier with FSK application (Figure 1B).
Figure 2. Inhibitory actions of cAMP on GABAergic synaptic transmission.
A, The graph shows the reduction in GABAergic IPSCs (VH = 0mV) on the application of 10mM db-cAMP (n=6) in wild type neurons (n=9). Near complete suppression was seen during 30 seconds of db-cAMP application. In each experiment, db-cAMP was applied at 62.5 sec after initiation of IPSC recording. Bars indicate SEM. B, The plot shows a comparison of the suppression rate of GABAergic IPSCs by FSK (20µM) and db-cAMP (10mM). This rate was calculated using a double exponential fit. The rate of suppression for FSK action was two-fold slower than that of db-cAMP. C, The graph shows effects of different concentrations of db-cAMP (100µM - 10mM) and FSK (2 – 100µM) on GABAergic IPSCs. The suppression potency was determined by averaging percent frequencies for 20 sec from 70–90 sec in a graph similar to A. Error bars indicate SEM. ANOVA test, *** P< 0.001. Number of replicates: FSK – 2µM (n=2), 20µM (n=9), 100 µM (n=5); cAMP - 100µM (n=4), 500µM (n=5), 1,000µM (n=4), 10,000µM (n=6).
In order to examine the lag time seen during FSK application we compared the decrease in IPSC frequency by both FSK and db-cAMP. For this, we plotted the average frequency of IPSCs (n=9 for FSK; n=6 for db-cAMP) and fitted the curves of both the plots to determine their decay constants (Figure 2B). The FSK plot had a decay constant (τ1) of 12.2 sec, while the cAMP action was much faster (τ1=5.1 sec). This instantaneous action of db-cAMP is probably because it does not require the activation of intermediates such as AC. We also noted a faster rate of recovery of GABAergic IPSCs (Figure 2A) at the end of the db-cAMP puffing. This suggests that AC is active for a long duration even after the termination of FSK application while the effect of db-cAMP lasts only as long as the external signal is present. We then examined the dose-dependent effects of forskolin and db-cAMP on GABAergic IPSCs. The comparison of average frequencies between different db-cAMP concentration groups shows a significant effect on GABAergic IPSCs (One-way ANOVA F3,108=9.579, P< 0.001). The lower concentrations of db-cAMP, 100µM (106.26±9.20, n=4) and 500µM (85.78±8.38, n=5) showed a small reduction in the IPSC frequency. This was significantly reduced to 56.3 ± 8.99 (P< 0.01, n=4) by 1mM and to 10.2 ± 4.7% (P< 0.01, n=6) by 10mM db-cAMP (Figure 2C). Similarly, we noted that increasing the concentration of FSK had a significant effect on GABAergic IPSCs (One-way ANOVA F2,93=7.798, P< 0.001). While 2µM FSK did not show any reduction in GABAergic IPSCs, 20µM FSK strongly suppressed them (11.6±4.7%, P< 0.01, n=9). This effect was more pronounced with 100µM FSK (1.1±0.9%, P< 0.01, n=5). Thus 20µM FSK was used in all our experiments with embryonic neuronal cultures.
Suppression of GABAergic IPSCs by cAMP-PKA signaling is postsynaptic
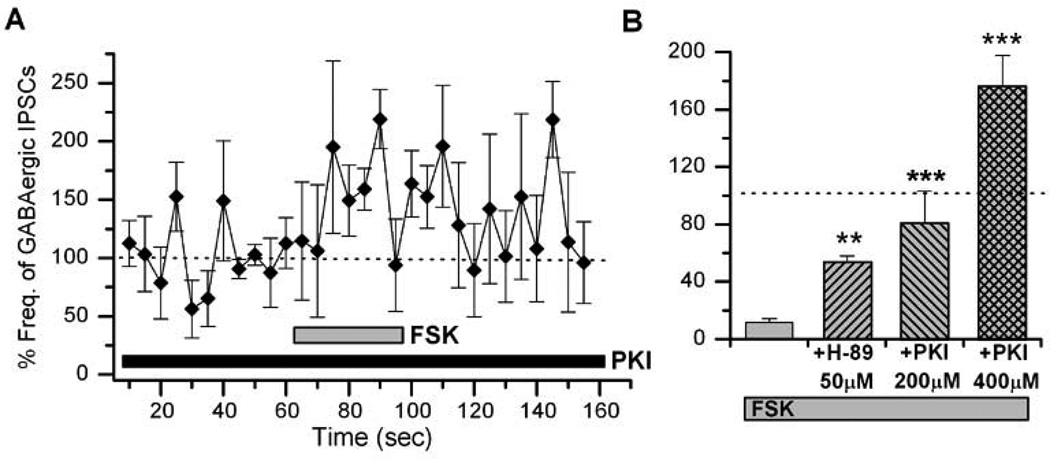
Previous reports have shown that the cAMP-dependent plasticity at excitatory cholinergic synapses in Drosophila is presynaptic (Lee & O'Dowd, 2000). In this study, we examined the locus of cAMP actions on GABAergic synaptic transmission. First, a PKA inhibitor H-89 was added to the whole-cell patch pipette in order to block possible postsynaptic actions of cAMP signaling. In the presence of 50µM H-89 we saw only 47% suppression of GABAergic IPSCs to 52.95±5.11% (P< 0.01, n=4) by FSK, compared to 89% by FSK alone, suggesting that the locus of action may be postsynaptic (Figure 3). However this experiment did not rule out the possibility of presynaptic origin because H-89 in the pipette can diffuse to the presynaptic terminal. We thus included a membrane-impermeable PKA blocker PKI (6–22) amide (200 and 400µM) in the internal recording pipette. This resulted in a significant attenuation of FSK-mediated GABAergic IPSCs by 200µM (79.97±23.12%, P< 0.001, n=4) and 400µM (175.76±21.82%, P< 0.001, n=4) PKI (6–22) amide as compared to FSK alone. This indicates that the locus of PKA action is postsynaptic (Figures 3A & B).
Figure 3. Postsynaptic addition of PKA blockers suppress the effect of forskolin (FSK) effects on GABAergic transmission.
A, FSK action on GABAergic transmission was suppressed by a membrane-impermeable PKA inhibitor PKI (6–22) amide added to the postsynaptic neurons through the whole-cell patch pipette. FSK (20µM) was focally applied as indicated. B, The graph shows suppression of the modulatory action of FSK by PKI amide and H-89. In all cases, 20µM FSK was applied for 30 sec. Student t-test, * P< 0.05, ** P< 0.01, *** P< 0.001. Number of replicates: 50µM H-89 (n=3), 200µM PKI (n=4), 400µM PKI (n=4).
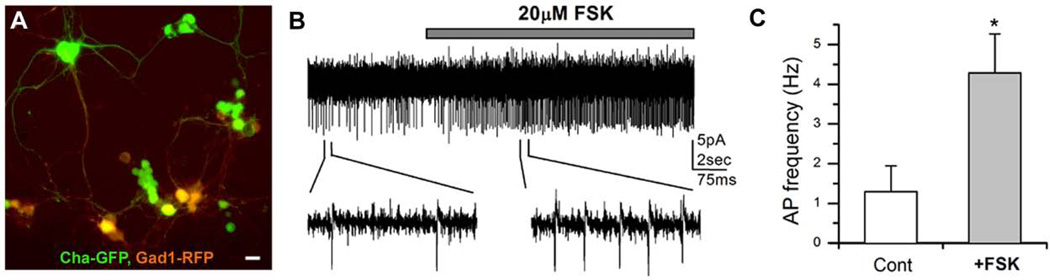
Interestingly, we observed that a high concentration of PKI (400µM) not only rescued the phenotype, but resulted in a significant increase in the frequency of sIPSCs as compared to our normalized control values (Figure 3B). This suggests that once all postsynaptic actions of PKA are blocked by PKI, the focally applied FSK can still act on presynaptic targets and lead to an increase in frequency of GABAergic IPSCs. To examine this possibility, we looked at the excitability of GABAergic neurons in the presence of FSK. GABAergic neurons were labeled with red fluorescent protein (RFP) driven by the GABA specific Gad1 promoter (i.e. Gad1-RFP), allowing us to visualize live cells (Figure 4A). Most RFP(+) neurons also stained positive with a GABA-specific antibody (data not shown). Extracellular action potentials (APs) were recorded from RFP(+) neurons (Figure 4B) in cell-attached mode since it is known to not interfere with the intracellular milieu and is a good indicator of cellular excitability (Hodges et al., 2002). Focal application of FSK significantly increased the frequency of APs in GABAergic neurons (4.28±0.98Hz, P< 0.001, n=5) compared to controls (1.29±0.65Hz, n=5), indicating an increase of GABAergic neuronal excitability by FSK. However, it may be possible that cAMP indirectly increases GABAergic excitability through activation of excitatory synaptic inputs to GABAergic neurons. Since the cholinergic currents are a major excitatory synaptic input in Drosophila, we repeated the same AP experiments using a specific cholinergic blocker, curare. We were able to record APs from RFP(+) GABAergic neurons in the presence of 1 µM curare although the number of GABA neurons showing APs were very few. In the presence of curare, FSK still increased AP frequency to about 2.3 fold (4.2±1.6Hz, P< 0.001, n=4) compared to controls (1.8±0.4Hz, n=4), demonstrating that cAMP signaling directly increases GABAergic excitability. Taken together, our results clearly show that cAMP-PKA signaling directly enhanced the excitability of not only inhibitory GABAergic (Figure 4) but also excitatory cholinergic neurons (Lee & O'Dowd, 2000).
Figure 4. Increased excitability of GABAergic neurons by forskolin (FSK).
A, A fluorescent image showing GFP-labeled cholinergic and RFP-labeled GABAergic neurons. Neuronal cultures were prepared from a fly line carrying Cha-Gal4, UAS-GFP and Gad1-RFP transgenes (Cha-GFP; Gad1-RFP). Scale bar = 10µm B, An extracellular action potential (AP) recording from a GABAergic neuron marked with Gad1-RFP. The AP frequency increased in the presence of FSK (20µM). C, Graph shows the average AP frequency before (control) or during focal application of FSK. Student t-test, * P< 0.05. Number of replicates: 20µM FSK (n=5).
Evoked IGABA is suppressed by forskolin
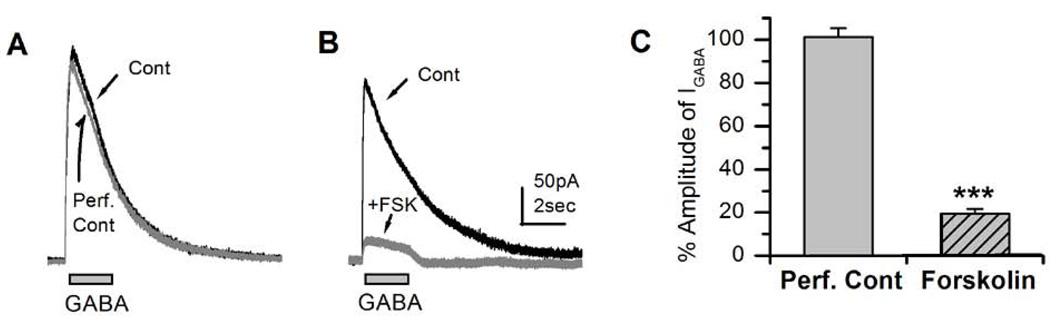
Our results in Figure 4 have shown that although FSK increases GABAergic excitability, it also decreases the frequency of IPSCs. GABAergic IPSCs result from the activation of ionotropic GABA receptors expressed in a postsynaptic density. Therefore, it was of interest to directly examine the effects of FSK on synaptic GABA receptors. However, it is still technically challenging to study evoked synaptic IPSCs in Drosophila neuronal cultures, which are mediated by activation of synaptic GABA receptors. Instead, we examined GABA-evoked currents (IGABA) in the presence of FSK. GABA-evoked currents in whole-cell mode were first recorded from a single neuron by focal application of 100µM GABA for 2 seconds (Figure 5A). Following this, 20µM FSK was bath-perfused and then 100µM GABA was puffed again on the same neuron (Figure 5B). We noted that GABA-evoked currents were strongly reduced after FSK perfusion (19.5 ± 2.1%, P< 0.001, n=5) compared to control (101.24±4.16%, n=5) IGABA (Figure 5B & C). These results support the notion that FSK-mediated suppression of GABAergic transmission is due to reduced response of the postsynaptic GABA receptors and not due to reduced presynaptic GABA release.
Figure 5. Suppresion of GABA-evoked currents (IGABA) by forskolin (FSK).
A, GABA-evoked currents (IGABA) before and after perfusion of the control recording solution. 100µM GABA was focally applied for 2 sec as indicated. B, Perfusion of FSK (20µM) markedly suppressed IGABA. C, Percent amplitude of IGABA after perfusion of control versus FSK-containing solution. Student t-test, ***P< 0.001. Number of replicates: 20µM FSK (n=5).
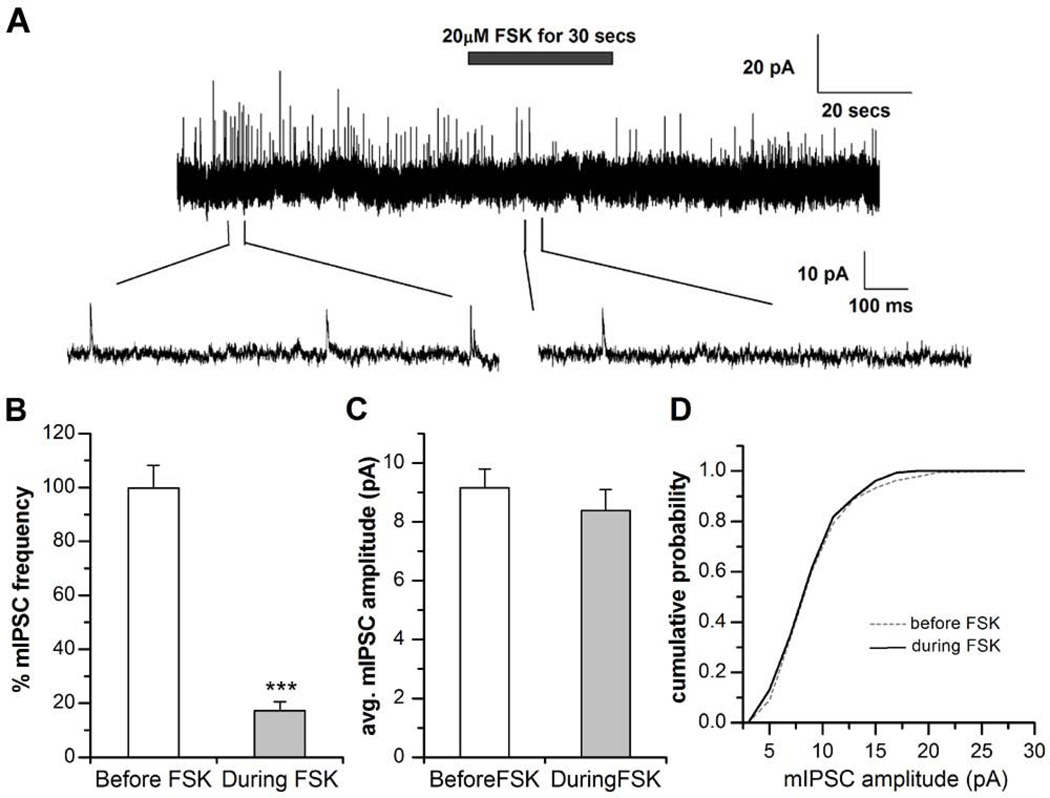
Frequency of mIPSCs is reduced by forskolin, but mIPSC amplitude remains unchanged
To further confirm the postsynaptic mode of suppression by cAMP signaling, we measured miniature IPSCs in the presence of 1 µM TTX. The frequency of mIPSCs was quantified as described earlier for sIPSCs (Figure 1). Then, 20µM FSK was focally applied for 30 seconds. Figure 6 shows that mIPSC frequency was significantly reduced to 17.3 ± 3.3% (P< 0.001, n=8) during FSK application compared to frequency before FSK application. However there was no change in the amplitude of mIPSCs before (9.15 ± 0.64pA, n=8) and during (8.38 ± 0.71pA; P=0.43, n=8) FSK application. A comparison of the cumulative distribution of mIPSC amplitude before (n=1170) and during (n=154) FSK application revealed no significant change (Kologorov-Smirnov test; D=0.33; P=0.30). The identical effect of FSK on mIPSCs and sIPSCs indicates that both AP-dependent and -independent mechanisms at the inhibitory GABAergic synapses are regulated similarly by FSK. No detectable change in mIPSC amplitude by FSK possibly rules out an exclusively post-synaptic mode of action at GABAergic synapses. However our results on the effect of FSK perfusion on GABA evoked currents (Figure 5) and the increase in frequency of sIPSCs on inclusion of a membrane-impermeable PKA blocker in the postsynaptic neuron (Figure 3) strongly support postsynaptic suppression of GABAergic transmission by FSK.
Figure 6. Suppression of miniature inhibitory GABAergic postsynaptic currents (mIPSCs) by forskolin (FSK).
A, FSK (20µM) was focally applied to a wild type neuron (3 days in culture) in the presence of 1µM tertrodotoxin (TTX) and 1µM curare after obtaining a stable GABAergic IPSC trace. Miniature IPSCs (mIPSCs) were recorded at holding potential of 0 mV. The frequency of GABAergic mIPSCs were significantly suppressed during the focal application of FSK. Example IPSC traces are shown on an expanded time scale below the complete recording trace. Holding potential (VH) = 0 mV. B, The graph shows the reduction in GABAergic mIPSC frequency by the application of 20µM FSK as compared to the % frequency of mIPSCs before the application of FSK (*** P< 0.001, n=8). C, The graph shows no reduction in the average GABAergic mIPSC amplitude during the application of 20µM FSK as compared to the amplitude of mIPSCs before the application of FSK (n=8). Paired T-test was used to distinguish between groups. Bars indicate SEM. D, Kologorov-Smirnov test was carried out to distinguish any difference in the cumulative probability of mIPSC amplitude between before and after FSK treatment. We observed no change in the cumulative probability distribution of mIPSCs before FSK (n=1170 events) and after FSK treatment (n=154 events) binned at 5pA for both the groups (P=0.308).
Majority of IPSCs is mediated by Rdl-containing GABA receptors
We next wanted to examine why there was no change observed in mIPSC amplitude during FSK application. It is possible that there may be two types of synaptic GABA receptors: PKA-sensitive versus –insensitive. If so, PKA-insensitive synaptic GABA receptors would still contribute to mIPSCs that show no change in the amplitude during FSK application. In Drosophila, there are three ionotropic GABA receptor subunits cloned so far (Rdl, LCCH2, and GRD; Hosie et al, 1997). Of these three known GABA receptor subunits, the Rdl subunit is best characterized and also widely expressed in several regions of the Drosophila brain (Harrison et al., 1996). Our previous study has shown that Rdl-containing GABA receptor plays a role in inhibitory synaptic transmission (Lee et al, 2003). However, the exact subunit composition of Drosophila synaptic GABA receptors is not known.
In this study, we examined whether there are different types of GABA receptors mediating synaptic currents using a Rdl null mutant line. Since the homozygous Rdl1 null mutant is lethal, this mutant fly line was maintained over a balancer chromosome (Goldstein & Fyrberg, 1994; Greenspan, 2004). For this we used a TM6B balancer carrying Ubi-GFP, in which GFP expression is controlled by the ubiquitin promoter. Three different genotypes (1:2:1 ratio) of embryos are expected from the heterozygous Rdl1/TM6B, Ubi-GFP fly: Rdl1/Rdl1, Rdl1/TM6B, Ubi-GFP, and TM6B, Ubi-GFP/ TM6B, Ubi-GFP. Therefore, each neuronal culture was prepared from a single embryo to avoid any intermingling of genotypes. Accordingly, homozygous Rdl1 null mutant neuronal cultures were identified as GFP-negative (refer to Darya et al., 2009 for further experimental details). Functional properties of Rdl1 null GABA synapses were examined using whole-cell recording technique (Table 1). Incidence rate (5.8%, n=3 out of 52) of GABAergic sIPSCs was markedly lower than that of wild type (29.4%, n= 10 out of 34). In contrast, the incidence rate (67%, n=42/63) of cholinergic EPSCs was increased in Rdl1 null mutant compared to that of wild type (~50%; Lee & O’Dowd, 2009). Interestingly, we also observed that the frequency of sIPSCs in the Rdl null mutant was at least six times lower than wild-type strains (Table 1). This suggests that there are at least two functionally different types of synaptic GABA receptors, one with and the other without Rdl subunits. Since the majority of functional synaptic GABA receptors contain Rdl subunit, it is plausible that Rdl is a molecular target of PKA-signaling. In contrast, synaptic GABA receptors without the Rdl subunit would be resistant to PKA-dependent suppression and thus contribute to GABAergic IPSC currents with no change in amplitude in the presence of PKA (Table 1).
Table 1.
Functional properties of GABAergic sIPSCs in wild type, Rdl1 null, dnc1 and rut1 neuronal cultures.
| strain | Incidence rate | Frequency (Mean±SEM) | Amplitude (Mean±SEM) |
|---|---|---|---|
| wild type | 29.4% (10/34) | 3.0±0.2 Hz (n=9) | 10.3±1.4 pA (n=9) |
| Rdl1 null | 5.7% (3/52) | 0.5±0.3 Hz (n=3) | 9.4±1.0pA (n=3) |
| dnc1 | 20.3% (11/54) | 1.7±0.2 Hz (n=11) | 10.2±1.9 pA (n=9) |
| rut1 | 27.9% (12/43) | 2.2±0.1Hz (n=11) | 9.4±1.0 pA (n=9) |
Memory mutants dunce and rutabaga show impaired cAMP-dependent GABAergic plasticity
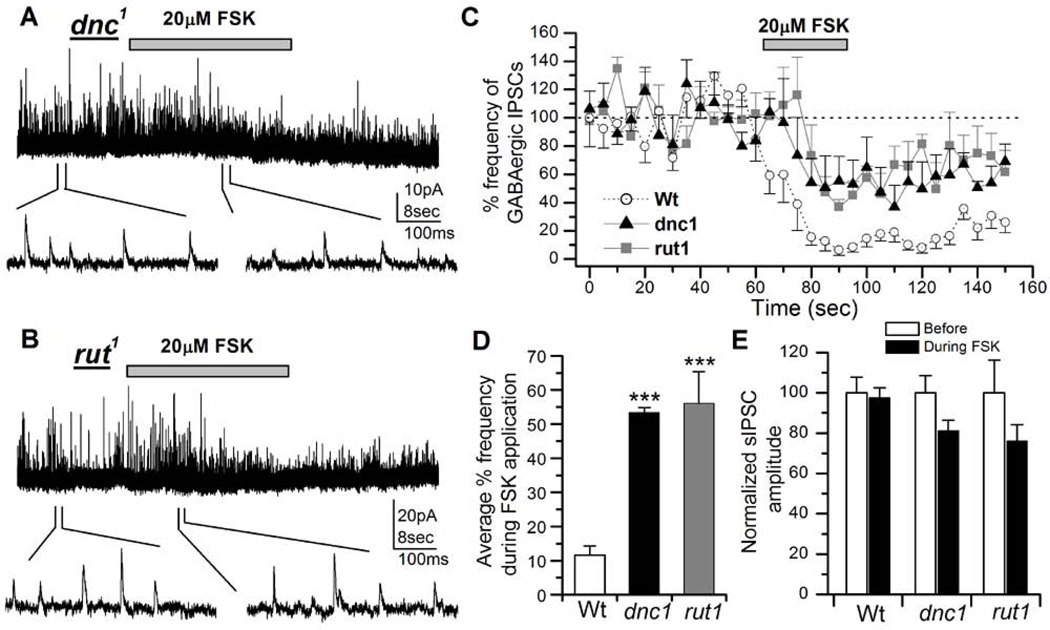
Many known olfactory learning and memory mutants in Drosophila have defective cAMP signaling (Keene & Waddell, 2007; Davis, 2011). We thus examined if the suppression of GABAergic IPSCs by cAMP signaling is altered in these learning and memory mutants. For this, two well established olfactory memory mutants, dunce1 (dnc1) (Dudai et al., 1976) and rutabaga1 (rut1) (Livingstone et al., 1984) were used. The dunce gene encodes the cAMP phosphodiesterase (PDE) enzyme (Byers et al., 1981) and this mutation results in an increase in the cytoplasmic cAMP levels. On the other hand, rut1 harbors a mutation in adenylyl cyclase (AC) enzyme (Levin et al., 1992), which results in a decrease in the cytoplasmic cAMP levels. Thus these two mutants provide an ideal background of intrinsically high and low levels of intracellular cAMP. 20µM FSK was applied on 3–6 days old cultured neurons of each of these two strains. FSK was focally applied for 30 seconds from 62.5–92.5 seconds and the frequency of events was plotted at 5 sec intervals as described earlier (Figure 1B). This resulted in only a modest decrease in GABAergic IPSC frequency in both dnc1 (n=6; Figure 7A) and rut1 neurons (n=7; Figure 7B) as compared to the wild type controls (Figure 7C). In addition, the rate of suppression of IPSCs by FSK had slowed down in the mutants as compared to the wild-type. To quantify this effect of FSK, we averaged the IPSC frequency from 80–95 sec. This showed that both mutants dnc1 (53.40±1.45%, P< 0.001, n=5) and rut1 (56.05±9.29%, P< 0.001, n=7) had similar levels of reduction in the IPSC frequencies when compared to the wild-type controls (Wt=11.63±2.70%, n=9). In contrast, the amplitude in dnc1 and rut1 was not significantly different before and after FSK application, although it was slightly reduced (Figure 7E).
Figure 7. Modulatory actions of forskolin (FSK) on inhibitory GABAergic postsynaptic currents (IPSCs) is attenuated in learning and memory mutants.
A, Effects of FSK on GABAergic IPSCs in a dnc1 neuron. After obtaining a stable IPSC trace from a 3 day old dnc1 neuron, 20µM FSK was focally applied for 30 seconds (indicated by bar). FSK suppression of GABAergic IPSC frequency was significantly attenuated compared to wild type (see Figure 1). GABAergic IPSCs are shown on an expanded time scale below the entire recording trace. VH = 0 mV. B, Effects of FSK on GABAergic IPSCs in a rut1 neuron (3 days old). C, The graph shows a comparison of the reduction of GABAergic IPSCs by 20µM FSK in wild type (n=9, circles), dnc1 (n=5, triangles) and rut1 (n=7, squares) neurons. D, The graph shows reduction in percent frequency of GABAergic IPSCs during the focal application of 20µM FSK in dnc1 and rut neurons compared to wild type. Bars indicate SEM. Students t-test, ***P< 0.001. E, The graph shows average sIPSC amplitude before and during FSK application from GABAergic IPSCs described in C. Although the amplitude in dnc1 and rut1 was slightly reduced the difference was not significant. Paired T-test was used to compare before and after treatment amplitudes in each genotype.
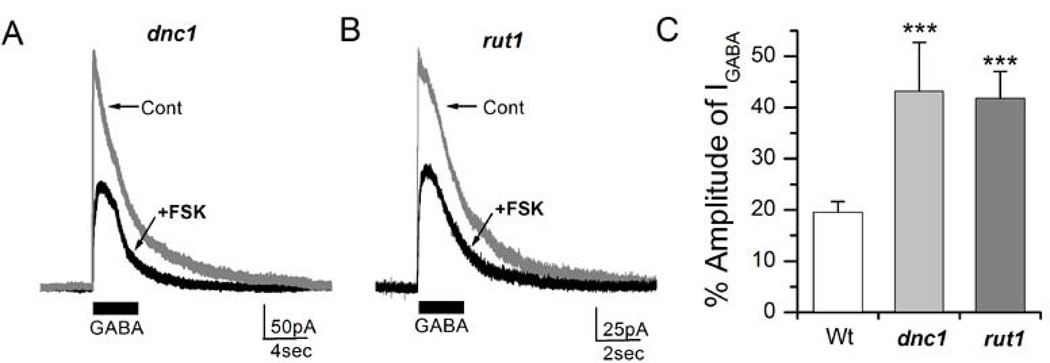
We further examined whether the alteration in GABAergic IPSCs seen in both dnc1 and rut1 mutants is due to reduced ionotropic GABA receptor sensitivity to FSK as noted earlier (Figure 5B). Cultured embryonic neurons from dnc1 and rut1 were whole-cell patched and GABA (100µM) was focally puffed for 2 sec to measure IGABA. Subsequently, FSK (20µM) was perfused in to the recording chamber and then GABA was puffed again. We noted that IGABA was reduced in neurons derived from dnc1 and rut1 after FSK perfusion (Figure 8A & B). However, the amount of suppression was significantly less in dnc1 (43.66±3.88%, P< 0.001, n=8) and rut1 (45.91±3.85%, P< 0.001, n=8) mutants (Figure 8C) when compared to wild-type neurons (19.54±2.10%, n=5).
Figure 8. Forskolin effects on GABA-evoked currents (IGABA) are attenuated in learning and memory mutants.
A, GABA-evoked currents (IGABA) were recorded from a dnc1 neuron before and after perfusion of FSK (20µM). 100µM GABA was focally applied for 2 sec as indicated. B, Perfusion of FSK (20µM) suppressed IGABA in a rut1 neuron. C, Percent amplitude of IGABA after perfusion of FSK. Data for wild type was from Figure 5C for comparison. Bars show SEM. Students t-test, ***P< 0.001. Number of replicates: Wt (n=5), dnc1 (n=8), rut1 (n=8).
Embryonic and late-stage pupal mushroom body (MB) neurons from memory mutants show altered GABA receptor response to forskolin
The cAMP signaling pathway is essential for memory formation in Drosophila. It is also known that the Drosophila mushroom body (MB) is the center for olfactory learning and memory (Davis, 2011). We thus examined whether GABA receptors in MB neurons are also modulated by cAMP signaling. In order to identify MB neurons for electrophysiology, the MB specific c309-Gal4 driver with strong expression in α, β and γ lobes (Joiner et al., 2006; Krashes et al., 2007) was used to drive GFP expression in MB neurons (i.e. c309-Gal4×UAS-GFP). GABA-evoked currents (IGABA) in embryonic MB neurons were reduced to 19.33±1.95% (n=8) after 20µM FSK perfusion (Figure 9). Interestingly, this reduction in MB neurons by FSK was very similar to that seen in the total population of neurons (Figure 5C).
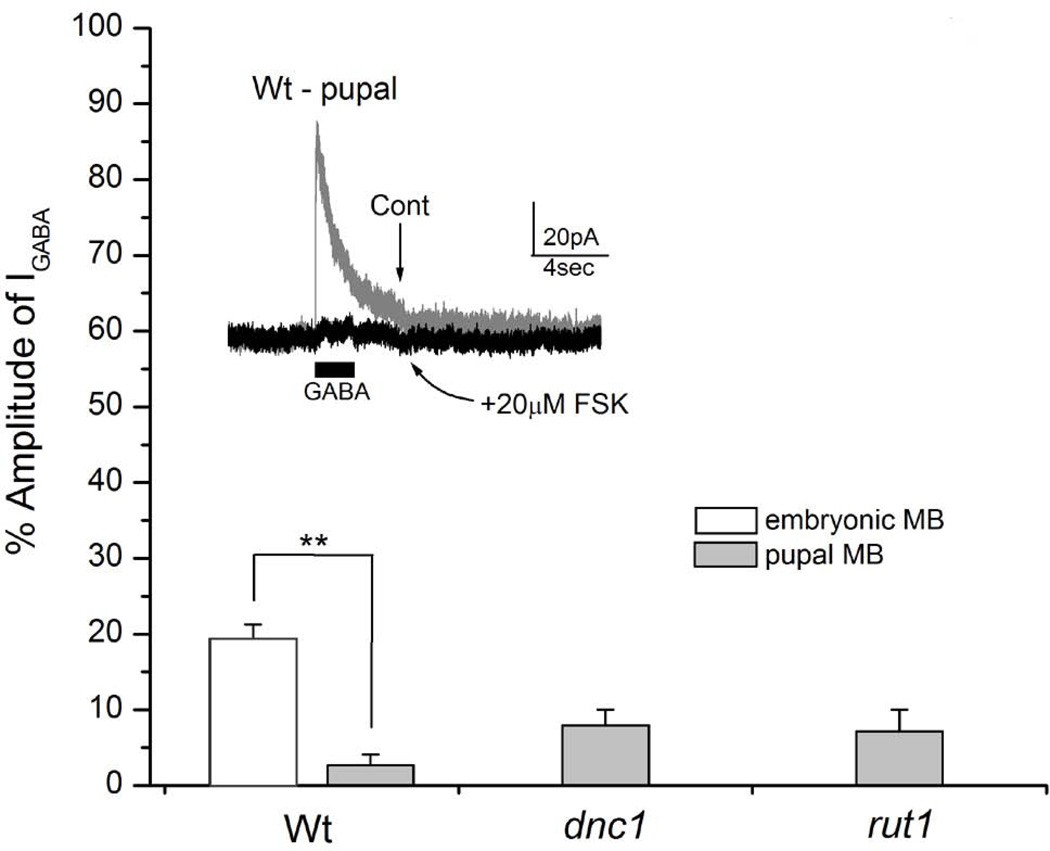
Figure 9. 20µM forskolin (FSK) markedly suppresses GABA-evoked currents (IGABA) recorded in wild type, dnc1 and rut1 pupal mushroom body (MB) neurons.
Cultured pupal MB neurons were prepared from wild type, dnc1 and rut1. MB neurons were identified by GFP expression under the control of a MB-specific driver c309-Gal4. GABA-evoked currents (IGABA) were suppressed in neurons derived from all three strains. Suppression for wild type, dnc1 and rut1 was 97.3, 92.0 and 92.9%, respectively. Although this suppression was slightly higher in wild type, there was no statistical difference between the three strains. In contrast, GABA-evoked currents (IGABA) from wild type embryonic MB neurons showed reduced sensitivity to FSK as it suppressed only 80.7%. Inset IGABA recorded from a wild type pupal MB neurons before and after 20µM FSK perfusion. Students t-test, **P< 0.01. Number of replicates: Wt embryonic (n=7) and pupal (n=4), dnc1 (n=4), rut1 (n=6).
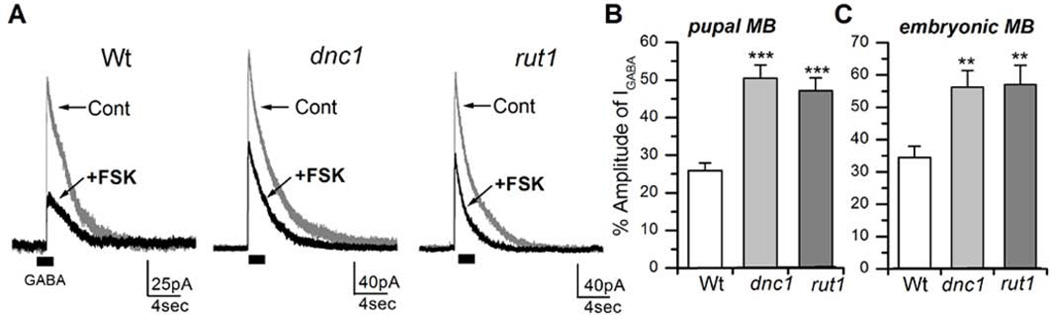
Since embryonic MB neurons are known to differentiate in culture, we examined already differentiated MB neurons to rule out the possibility that GABA receptors in the differentiated brain behaved differently from the embryonic cultures. Though there are studies showing electrophysiological recordings from intact fly brains, in vivo recording in Drosophila is very laborious and challenging. Thus for our experiments, we dissociated late stage c309-Gal4×UAS-GFP (T10) pupal brain neurons and cultured them as described earlier (Su & O'Dowd, 2003). GABA-evoked currents were successfully recorded from pupal MB neurons. When 20µM FSK was perfused, IGABA reduced to 2.65±1.44%, which was significant when compared to embryonic neurons (P< 0.001, n=4). Pupal brain neuronal cultures were also prepared from fly lines carrying c309-Gal4×UAS-GFP in both the dnc1 or rut1 background. 20µM FSK perfusion greatly reduced IGABA to 7.95±2.05 (n=4) in dnc1 and to 7.13±2.87 (n=7) in rut1 pupal MB neurons (Figure 9). This number was slightly higher than wild type neurons but not significantly different (P=0.08 for dnc1 and P=0.26 for rut1). These results showed that GABA receptors in pupal MB neurons were more sensitive than embryonic MB neurons. Since 20µM FSK appeared to be too strong for pupal MB neurons, lower concentrations of FSK were tested. We found that 2µM FSK reduced IGABA in wild type pupal MB neurons to 25.87±2.09% (n=6), which is less suppressive than 20µM FSK (Figure 9). Subsequently, 2µM FSK was tested on dnc1 and rut1 pupal MB neurons and a significantly lesser reduction to 50.52±3.5% (P< 0.001, n=6) and 47.19±3.39% (P<0.001, n=6), for dnc1 and rut1 respectively, was noted (Figure 10). These results confirm that GABA receptors in dnc1 and rut1 pupal MB neurons are significantly less sensitive to FSK. We also examined the effect of 2µM FSK on IGABA in embryonic MB neurons. Figure 10C shows that while FSK strongly suppressed wild type IGABA (34.45±1.97%, n=5). Its effect on dnc1 (53.19±3.55%, P< 0.01, n=5) and rut1 (56.78±5.00%, P< 0.01, n=5) embryonic MB neurons was significantly less pronounced. Thus, the effects of FSK on the embryonic MB neurons were very similar to those observed in pupal MB neurons, indicating that there is no stage-specific or developmental difference in FSK-mediated suppression of GABA receptors.
Figure 10. FSK (2µM) effects on GABA-evoked currents (IGABA) are attenuated in pupal and embryonic mushroom body (MB) neurons prepared from learning and memory mutants.
A, GABA-evoked currents (IGABA) were recorded from wild type, dnc1 and rut1 pupal MB neurons before and after perfusion of 2µM FSK. 100µM GABA was focally applied for 2 seconds as indicated. B, Percent amplitude of IGABA in pupal MB neurons after perfusion of FSK. C, Percent amplitude of IGABA in embryonic MB neurons after perfusion of FSK. Bar = SEM. Students t-test, **P< 0.01 and ***P< 0.001. Number of replicates: Wt (n=6), dnc1 (n=6), rut1 (n=6).
DISCUSSION
cAMP signaling inhibits frequency of GABAergic IPSCs
Ca2+/CaM dependent adenylate cyclase (AC) produces cAMP and is also known to function as a co-incidence detector during learning in both Drosophila (Tomchik & Davis, 2009; Gervasi et al., 2010) and Aplysia (Abrams et al., 1991). In addition, AC-dependent cAMP activation changes the strength of Drosophila excitatory synapses (Zhong & Wu, 1991; Lee & O'Dowd, 2000) which may be the cellular mechanism underlying learning and memory. Although inhibitory synaptic transmission is equally important for proper neuronal communication, the effects of cAMP at the inhibitory GABAergic synapses have remained unexplored. In this study we show that forskolin (FSK), an activator of cAMP, suppresses the frequency of inhibitory GABAergic IPSCs in Drosophila primary neuronal cultures (Figure 1). We observed a concentration dependent effect of FSK on GABAergic IPSCs (Figure 2) in the same physiological range as described in recent imaging studies in intact fly brains (Tomchik & Davis, 2009; Gervasi et al., 2010). Further we show that cAMP decreases GABAergic IPSCs in a PKA-dependent manner through a postsynaptic mechanism.
Sparsening of odor representation through GABAergic inhibition in the mushroom body (MB) neurons is thought to be a possible mechanism for information storage in locusts (Perez-Orive et al., 2002). GABAergic local neurons are known to be involved in olfactory information processing in Drosophila (Wilson & Laurent, 2005; Olsen & Wilson, 2008) indicating that GABAergic transmission plays a crucial role in shaping odor response. The MB shows extensive GABAergic innervation in both locusts (Perez-Orive et al., 2002) and Drosophila (Yasuyama et al., 2002). This, along with the observation that cAMP pathway genes like dunce and rutabaga are preferentially expressed in the MB (Davis, 2011), indicates that cAMP mediated GABAergic plasticity may be important for learning in Drosophila. Consistent with this hypothesis, we observe altered cAMP mediated GABAergic IPSCs in the cAMP mutants dnc1 and rut1 (Figure 6). The effect of cAMP on suppression of GABAergic currents was less pronounced in the mutants. This suggests that the altered inhibition contributes to their observed learning defects. In fact, recent studies have shown that GABAA RDL receptors expressed in the MB and GABAergic neurons projecting to the MB are essential for olfactory learning (Liu et al., 2007; Liu & Davis, 2009). It is thus possible that altered cAMP mediated GABAergic plasticity at the MB neurons may account for some forms of the learning defects in Drosophila.
Postsynaptic GABA receptors mediate the effect of cAMP on GABAergic IPSCs
GABAergic IPSCs are known to act through picrotoxin-sensitive postsynaptic GABA receptors in both Drosophila embryonic and pupal neuronal cultures (Lee et al., 2003; Su & O'Dowd, 2003). We observe that the suppression of GABAergic IPSCs by FSK is completely abolished in the presence of a membrane impermeable PKA inhibitor restricted to the postsynaptic neuron (Figure 3). This indicates that PKA may modulate GABAergic IPSCs by regulating GABA receptor sensitivity by phosphorylation, similar to what has been suggested in the mammalian hippocampus (Nusser et al., 1999).
There are three known ionotrophic GABA receptor gene homologs in Drosophila – RDL, LCCH3 and GRD (Harvey et al, 1994; Hosie et al., 1997). Amongst them, the GABA RDL subunit is widely expressed in several regions of the Drosophila brain (Harrison et al., 1996) and its expression in the MB is inversely correlated to olfactory learning (Liu et al., 2007). Therefore, RDL-containing GABA receptors may play an important role in cAMP-dependent synaptic plasticity and thus be involved in learning and memory. Our data suggests that the majority of synaptic GABA receptors contain the RDL subunit while a small fraction of synaptic GABA receptors lack RDL (Table 1), providing evidence of heterogeneous synaptic GABA receptors in Drosophila for the first time. However, it is still not known what particular subunit of GABA receptors is involved in regulation of cAMP-dependent GABAergic plasticity. Based on the observation that RDL containing GABA receptors mediate the majority of GABAergic IPSCs in Drosophila primary neuronal cultures, the action of FSK on GABAergic IPSCs is probably through the GABA RDL subunit. While the detailed molecular mechanism remains to be explored, we propose that PKA-mediated phosphorylation of RDL subunits and subsequent GABA receptor internalization may occur in the postsynaptic region (Mou et al., 2011; Vithlani et al., 2011). In this scenario, the only functional synaptic GABA receptors will be those lacking the RDL subunit at the postsynaptic regions. This will account for a decrease in mIPSC frequency with response to FSK, while leaving mIPSC amplitude almost unchanged (Figure 6).
Although GABA receptor subunits would be the target of cAMP-PKA signaling we still cannot rule out the possibility that other molecules can be phosphorylated and then indirectly regulate GABA receptor subunits. Future work using heterologous expression systems for GABA subunits will help to determine whether GABA receptors are directly phosphorylated.
Alterations in ionotropic GABA receptor sensitivity in learning mutants
Our recordings from embryonic and pupal MB neurons of both dunce and rutabaga mutants show a defect in ionotropic GABA receptor response in the presence of FSK (Figures 7, 9 & 10). Interestingly, this response to FSK is similar in both the mutants despite their contrasting levels of cellular cAMP. Recent imaging studies in the rutabaga mutant have shown that AC is required for co-incidence detection in the MB neurons (Tomchik & Davis, 2009; Gervasi et al., 2010). FSK application also fails to increase PKA to wild-type levels in the MB neurons of rutabaga (Gervasi et al., 2010). Thus in our experiments, the changes in receptor response in rutabaga can be explained by a lack of increase in cAMP/PKA levels due to defects in FSK-mediated AC activation.
The dunce mutants with high levels of cAMP also show defects in short-term memory due to alterations in the spatiotemporal restriction of dunce phosphodiesterase to the Drosophila MB (Gervasi et al., 2010). Further, the dunce MB neurons show an increase in PKA levels on FSK application similar to the wild-type strains. These findings suggest that FSK-mediated inhibition of GABA receptor should be greater in dunce neurons. However, in our results (Figure 7) the dunce and rutabaga mutants, despite having opposing effects on cellular cAMP levels, showed very similar FSK mediated effects on GABAergic IPSCs. Several other studies have also shown that dunce and rutabaga have similar defects in growth cone motility, excitatory synaptic plasticity and more importantly, short-term memory (Kim & Wu, 1996; Gasque et al., 2006). Even though the effect of FSK on GABAergic IPSCs in dunce and rutabaga mutants is similar, it is very likely that the molecular mechanisms underlying these responses differ in the two mutants. It has been shown that elevated cAMP signaling reduces phosphorylation in rat kidney cells through activation of protein phosphatase 2A (Feschenko et al., 2002). In addition, increased PKA activity in mouse hippocampus hyper-phosphorylates several downstream molecular targets including a tyrosine phosphatase (STEP), correlates with decreased phosphodiesterase protein (PDE4) levels and results in memory defects (Giralt et al., 2011). Therefore, it is tempting to speculate that high levels of cAMP due to the dunce mutation leads to the activation of phosphatase(s) and thus reduces the effects of FSK as seen in our study (Figure 8). Taken together, all these findings strongly suggest that the disruption of cellular cAMP homeostasis can alter inhibitory GABAergic synaptic plasticity and hence cause defects in olfactory learning, although the underlying mechanisms leading to this effect can be different (e.g. reduced PKA activity in rut1 versus increased phosphatase activity in dnc1).
Dual regulation of synaptic excitation and inhibition by cAMP
Strengthening in the efficacy of excitatory transmission underlies enhanced synaptic plasticity such as hippocampal long-term potentiation (LTP) and facilitation (LTF) in Aplysia (Milner et al., 1998). It is thus possible that the suppression of inhibitory transmission by a common second messenger like cAMP, which can enhance excitatory synaptic transmission, may lead to synaptic strengthening. Previous work has shown that the cAMP activator FSK increases excitability at the cholinergic synapses in Drosophila primary neuronal cultures (Yuan & Lee, 2007). However the effect of FSK on other synapses like the GABAergic synapses has not been explored. We show that FSK elevates overall cellular excitability at GABAergic synapses as demonstrated by the increase in spontaneous AP frequency (Figure 4). Moreover, when PKA in the postsynaptic neuron is completely blocked by an inhibitor, we see an increase in the frequency of GABAergic IPSCs (Figure 3A&B). Together with previous studies (Yuan & Lee, 2007), our results indicate that FSK/cAMP act as common molecules regulating globally presynaptic excitability at both the cholinergic as well as GABAergic synapses. We also note that FSK inhibits the response of postsynaptic GABA receptors in a specific manner leading to a decrease in GABAergic synaptic strength. Our studies demonstrate a novel dual regulatory role of cAMP by showing that it increases overall presynaptic function on one hand; and, acts specifically on postsynaptic GABA receptors to decrease GABAergic plasticity on the other. This action of cAMP could result in global increases in excitability and learning.
Acknowledgements
This work was partially supported by NIH grant NS050260. We thank Dr. R.A. Colvin for valuable inputs on previous versions of this manuscript.
REFERENCES
- Abrams TW, Karl KA, Kandel ER. Biochemical studies of stimulus convergence during classical conditioning in Aplysia: dual regulation of adenylate cyclase by Ca2+/calmodulin and transmitter. J Neurosci. 1991;11:2655–2665. doi: 10.1523/JNEUROSCI.11-09-02655.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronstein K, Auld V, Ffrench-Constant R. Distribution of two GABA receptor-like subunits in the Drosophila CNS. Invert Neurosci. 1996;2:115–120. doi: 10.1007/BF02214114. [DOI] [PubMed] [Google Scholar]
- Bormann J. Electrophysiology of GABAA and GABAB receptor subtypes. Trends Neurosci. 1988;11:112–116. doi: 10.1016/0166-2236(88)90156-7. [DOI] [PubMed] [Google Scholar]
- Byers D, Davis RL, Kiger JA., Jr Defect in cyclic AMP phosphodiesterase due to the dunce mutation of learning in Drosophila melanogaster. Nature. 1981;289:79–81. doi: 10.1038/289079a0. [DOI] [PubMed] [Google Scholar]
- Castillo PE, Chiu CQ, Carroll RC. Long-term plasticity at inhibitory synapses. Curr Opin Neurobiol. 2011;21:328–338. doi: 10.1016/j.conb.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevaleyre V, Castillo PE. Heterosynaptic LTD of hippocampal GABAergic synapses: a novel role of endocannabinoids in regulating excitability. Neuron. 2003;38:461–472. doi: 10.1016/s0896-6273(03)00235-6. [DOI] [PubMed] [Google Scholar]
- Davis RL. Traces of Drosophila memory. Neuron. 2011;70:8–19. doi: 10.1016/j.neuron.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darya K, Ganguly A, Lee D. Quantitative analysis of synaptic boutons in culturesDrosophilaneurons. Brain Research. 2009;1280:1–12. doi: 10.1016/j.brainres.2009.05.026. [DOI] [PubMed] [Google Scholar]
- Dudai Y, Jan YN, Byers D, Quinn WG, Benzer S. dunce, a mutant of Drosophila deficient in learning. Proc Natl Acad Sci U S A. 1976;73:1684–1688. doi: 10.1073/pnas.73.5.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigenspan A, Bormann J. Facilitation of GABAergic signaling in the retina by receptors stimulating adenylate cyclase. Proc Natl Acad Sci U S A. 1994;91:10893–10897. doi: 10.1073/pnas.91.23.10893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez F, Morishita W, Zuniga E, Nguyen J, Blank M, Malenka RC, Garner CC. Pharmacotherapy for cognitive impairment in a mouse model of Down syndrome. Nat Neurosci. 2007;10:411–413. doi: 10.1038/nn1860. [DOI] [PubMed] [Google Scholar]
- Feschenko MS, Stevenson E, Nairn AC, Sweadner KJ. A novel cAMP-stimulated pathway in protein phosphatase 2A activation. J Pharmacol Exp Ther. 2002;302:111–8. doi: 10.1124/jpet.302.1.111. [DOI] [PubMed] [Google Scholar]
- Floyer-Lea A, Wylezinska M, Kincses T, Matthews PM. Rapid modulation of GABA concentration in human sensorimotor cortex during motor learning. J Neurophysiol. 2006;95:1639–1644. doi: 10.1152/jn.00346.2005. [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Gandia L, Vitale ML, Villarroya M, Ramirez-Lavergne C, Garcia AG, Trifaro JM. Differential effects of forskolin and 1,9-dideoxy-forskolin on nicotinic receptor- and K+-induced responses in chromaffin cells. Eur J Pharmacol. 1997;329:189–199. [PubMed] [Google Scholar]
- Gasque G, Labarca P, Delgado R, Darszon A. Bridging behavior and physiology: ion-channel perspective on mushroom body-dependent olfactory learning and memory in Drosophila. J Cell Physiol. 2006;209:1046–1053. doi: 10.1002/jcp.20764. [DOI] [PubMed] [Google Scholar]
- Gervasi N, Tchenio P, Preat T. PKA dynamics in a Drosophila learning center: coincidence detection by rutabaga adenylyl cyclase and spatial regulation by dunce phosphodiesterase. Neuron. 2010;65:516–529. doi: 10.1016/j.neuron.2010.01.014. [DOI] [PubMed] [Google Scholar]
- Giralt A, Saavedra A, Carretón O, Xifró X, Alberch J, Pérez-Navarro E. Increased PKA signaling disrupts recognition memory and spatial memory: role in Huntington's disease. Hum Mol Genet. 2011;20:4232–47. doi: 10.1093/hmg/ddr351. [DOI] [PubMed] [Google Scholar]
- Goldstein LSB, Fyrberg EA. Methods Cell Biol. vol. 44. San Diego: Academic Press; 1994. Drosophila melanogaster: practical uses in cell and molecular biology. [Google Scholar]
- Greenspan RJ. Fly Pushing: The Theory and Practice of Drosophila Genetics. 2nd Ed. Cold Spring Harbor, New York: CSHL Press; 2004. [Google Scholar]
- Harris-Warrick RM. Forskolin reduces a transient potassium current in lobster neurons by a cAMP-independent mechanism. Brain Res. 1989;489:59–66. doi: 10.1016/0006-8993(89)90008-5. [DOI] [PubMed] [Google Scholar]
- Harrison JB, Chen HH, Sattelle E, Barker PJ, Huskisson NS, Rauh JJ, Bai D, Sattelle DB. Immunocytochemical mapping of a C-terminus anti-peptide antibody to the GABA receptor subunit, RDL in the nervous system in Drosophila melanogaster. Cell Tissue Res. 1996;284:269–278. doi: 10.1007/s004410050587. [DOI] [PubMed] [Google Scholar]
- Harvey RJ, Schmitt B, Hermans-Borgmeyer I, Gundelfinger ED, Betz H, Darlison MG. Sequence of a Drosophila ligand-gated ion-channel polypeptide with an unusual amino-terminal extracellular domain. J Neurochem. 1994;62:2480–2483. doi: 10.1046/j.1471-4159.1994.62062480.x. [DOI] [PubMed] [Google Scholar]
- Hodges DD, Lee D, Preston CF, Boswell K, Hall LM, O'Dowd DK. tipE regulates Na+-dependent repetitive firing in Drosophila neurons. Mol Cell Neurosci. 2002;19:402–416. doi: 10.1006/mcne.2001.1088. [DOI] [PubMed] [Google Scholar]
- Hoshi T, Garber SS, Aldrich RW. Effect of forskolin on voltage-gated K+ channels is independent of adenylate cyclase activation. Science. 1988;240:1652–1655. doi: 10.1126/science.2454506. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Aronstein K, Sattelle DB, ffrench-Constant RH. Molecular biology of insect neuronal GABA receptors. Trends Neurosci. 1997;20:578–583. doi: 10.1016/s0166-2236(97)01127-2. [DOI] [PubMed] [Google Scholar]
- Joiner WJ, Crocker A, White BH, Sehgal A. Sleep in Drosophila is regulated by adult mushroom bodies. Nature. 2006;441:757–760. doi: 10.1038/nature04811. [DOI] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- Keene AC, Waddell S. Drosophila olfactory memory: single genes to complex neural circuits. Nat Rev Neurosci. 2007;8:341–354. doi: 10.1038/nrn2098. [DOI] [PubMed] [Google Scholar]
- Kim YT, Wu CF. Reduced growth cone motility in cultured neurons from Drosophila memory mutants with a defective cAMP cascade. J Neurosci. 1996;16:5593–5602. doi: 10.1523/JNEUROSCI.16-18-05593.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ, Keene AC, Leung B, Armstrong JD, Waddell S. Sequential use of mushroom body neuron subsets during drosophila odor memory processing. Neuron. 2007;53:103–115. doi: 10.1016/j.neuron.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D, O'Dowd DK. Fast excitatory synaptic transmission mediated by nicotinic acetylcholine receptors in Drosophila neurons. J Neurosci. 1999;19:5311–5321. doi: 10.1523/JNEUROSCI.19-13-05311.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D, O'Dowd DK. cAMP-dependent plasticity at excitatory cholinergic synapses in Drosophila neurons: alterations in the memory mutant dunce. J Neurosci. 2000;20:2104–2111. doi: 10.1523/JNEUROSCI.20-06-02104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D, Su H, O'Dowd DK. GABA receptors containing Rdl subunits mediate fast inhibitory synaptic transmission in Drosophila neurons. J Neurosci. 2003;23:4625–4634. doi: 10.1523/JNEUROSCI.23-11-04625.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin LR, Han PL, Hwang PM, Feinstein PG, Davis RL, Reed RR. The Drosophila learning and memory gene rutabaga encodes a Ca2+/Calmodulin-responsive adenylyl cyclase. Cell. 1992;68:479–489. doi: 10.1016/0092-8674(92)90185-f. [DOI] [PubMed] [Google Scholar]
- Liu X, Davis RL. The GABAergic anterior paired lateral neuron suppresses and is suppressed by olfactory learning. Nat Neurosci. 2009;12:53–59. doi: 10.1038/nn.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Krause WC, Davis RL. GABAA receptor RDL inhibits Drosophila olfactory associative learning. Neuron. 2007;56:1090–1102. doi: 10.1016/j.neuron.2007.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone MS, Sziber PP, Quinn WG. Loss of calcium/calmodulin responsiveness in adenylate cyclase of rutabaga, a Drosophila learning mutant. Cell. 1984;37:205–215. doi: 10.1016/0092-8674(84)90316-7. [DOI] [PubMed] [Google Scholar]
- Lu YM, Mansuy IM, Kandel ER, Roder J. Calcineurin-mediated LTD of GABAergic inhibition underlies the increased excitability of CA1 neurons associated with LTP. Neuron. 2000;26:197–205. doi: 10.1016/s0896-6273(00)81150-2. [DOI] [PubMed] [Google Scholar]
- Milner B, Squire LR, Kandel ER. Cognitive neuroscience and the study of memory. Neuron. 1998;20:445–468. doi: 10.1016/s0896-6273(00)80987-3. [DOI] [PubMed] [Google Scholar]
- Mou L, Heldt SA, Ressler KJ. Rapid brain-derived neurotrophic factor-dependent sequestration of amygdala and hippocampal GABA(A) receptors via different tyrosine receptor kinase B-mediated phosphorylation pathways. Neuroscience. 2011;176:72–85. doi: 10.1016/j.neuroscience.2010.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Mody I. Differential regulation of synaptic GABAA receptors by cAMP-dependent protein kinase in mouse cerebellar and olfactory bulb neurones. J Physiol. 1999;521 Pt 2:421–435. doi: 10.1111/j.1469-7793.1999.00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen SR, Wilson RI. Lateral presynaptic inhibition mediates gain control in an olfactory circuit. Nature. 2008;452:956–960. doi: 10.1038/nature06864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SS, Lee D. Selective loss of dopaminergic neurons and formation of Lewy body-like aggregations in alpha-synuclein transgenic fly neuronal cultures. Eur J Neurosci. 2006;23:2908–2914. doi: 10.1111/j.1460-9568.2006.04844.x. [DOI] [PubMed] [Google Scholar]
- Paulsen O, Moser EI. A model of hippocampal memory encoding and retrieval: GABAergic control of synaptic plasticity. Trends Neurosci. 1998;21:273–278. doi: 10.1016/s0166-2236(97)01205-8. [DOI] [PubMed] [Google Scholar]
- Perez-Orive J, Mazor O, Turner GC, Cassenaer S, Wilson RI, Laurent G. Oscillations and sparsening of odor representations in the mushroom body. Science. 2002;297:359–365. doi: 10.1126/science.1070502. [DOI] [PubMed] [Google Scholar]
- Rohrbough J, Broadie K. Electrophysiological analysis of synaptic transmission in central neurons of Drosophila larvae. J Neurophysiol. 2002;88:847–860. doi: 10.1152/jn.2002.88.2.847. [DOI] [PubMed] [Google Scholar]
- Schwaerzel M, Monastirioti M, Scholz H, Friggi-Grelin F, Birman S, Heisenberg M. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J Neurosci. 2003;23:10495–10502. doi: 10.1523/JNEUROSCI.23-33-10495.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H, O'Dowd DK. Fast synaptic currents in Drosophila mushroom body Kenyon cells are mediated by alpha-bungarotoxin-sensitive nicotinic acetylcholine receptors and picrotoxin-sensitive GABA receptors. J Neurosci. 2003;23:9246–9253. doi: 10.1523/JNEUROSCI.23-27-09246.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomchik SM, Davis RL. Dynamics of learning-related cAMP signaling and stimulus integration in the Drosophila olfactory pathway. Neuron. 2009;64:510–521. doi: 10.1016/j.neuron.2009.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vithlani M, Terunuma M, Moss SJ. The dynamic modulation of GABA(A) receptor trafficking and its role in regulating the plasticity of inhibitory synapses. Physiol Rev. 2011;91:1009–1022. doi: 10.1152/physrev.00015.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI, Laurent G. Role of GABAergic inhibition in shaping odor-evoked spatiotemporal patterns in the Drosophila antennal lobe. J Neurosci. 2005;25:9069–9079. doi: 10.1523/JNEUROSCI.2070-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuyama K, Meinertzhagen IA, Schurmann FW. Synaptic organization of the mushroom body calyx in Drosophila melanogaster. J Comp Neurol. 2002;445:211–226. doi: 10.1002/cne.10155. [DOI] [PubMed] [Google Scholar]
- Yuan N, Lee D. Suppression of excitatory cholinergic synaptic transmission by Drosophila dopamine D1-like receptors. Eur J Neurosci. 2007;26:2417–2427. doi: 10.1111/j.1460-9568.2007.05870.x. [DOI] [PubMed] [Google Scholar]
- Zars T, Fischer M, Schulz R, Heisenberg M. Localization of a short-term memory in Drosophila. Science. 2000;288:672–675. doi: 10.1126/science.288.5466.672. [DOI] [PubMed] [Google Scholar]
- Zhang HG, Lee HJ, Rocheleau T, ffrench-Constant RH, Jackson MB. Subunit composition determines picrotoxin and bicuculline sensitivity of Drosophila gamma-aminobutyric acid receptors. Mol Pharmacol. 1995;48:835–840. [PubMed] [Google Scholar]
- Zhong Y, Wu CF. Altered synaptic plasticity in Drosophila memory mutants with a defective cyclic AMP cascade. Science. 1991;251:198–201. doi: 10.1126/science.1670967. [DOI] [PubMed] [Google Scholar]