Abstract
Objective
To examine how phenotype affects longitudinal decline on the Mini-Mental State Examination (MMSE) in patients with frontotemporal lobar degeneration (FTLD) and Alzheimer's disease (AD)
Background
The MMSE is the most commonly administered assessment for dementia severity; however, the effects of phenotype on longitudinal MMSE performance in FTLD and AD have not been extensively studied.
Methods
Data from 185 patients diagnosed with AD (n=106) and three FTLD (n=79) phenotypes (behavioral variant frontotemporal dementia [bvFTD], nonfluent agrammatic variant of primary progressive aphasia [nfaPPA], and semantic variant PPA [svPPA]) were collected for up to 52 months since initial evaluation.
Results
Differential rates of decline were noted in that MMSE scores declined more precipitously for AD and svPPA compared to bvFTD and nfaPPA patients (p=0.001). The absolute 4-year MMSE decline given median baseline MMSE for bvFTD (14.67, 95% confidence interval [CI]: 14.63-14.71) and nfaPPA (11.02, 95% CI: 10.98-11.06) were lower than svPPA (22.32, 95% CI: 22.29-22.34) or AD (22.24, 95% CI: 22.22-22.26).
Conclusions
These data suggest that within-group AD and FTLD phenotypes present distinct patterns of longitudinal decline on the MMSE. MMSE may not be adequately sensitive to track disease progression in some phenotypes of FTLD.
Keywords: MMSE, Alzheimer's disease, frontotemporal lobe dementia, longitudinal assessment
Introduction
Research over the past few decades has emphasized the heterogeneity of phenotypic expressions in patients with dementia. This is reflected, in part, by the differential rates of cognitive decline seen in Alzheimer's disease (AD) and frontotemporal lobar degeneration (FTLD).1,2 FTLD encompasses a range of progressive neurodegenerative disorders presenting with imaging and autopsy evidence of frontal and temporal atrophy.3-5 The major FTLD subtypes examined in this study include behavioral variant frontotemporal dementia (bvFTD), nonfluent and agrammatic variant of primary progressive aphasia (nfaPPA), and semantic variant of PPA (svPPA). Although FTLD phenotypes such as these share some clinical features with Alzheimer's disease (AD), research suggests that other behavioral and cognitive characteristics distinguish FTLD phenotypes from each other and from AD.6,7 Previous neuropsychological studies6,8 have focused on the cross-sectional distinctions between FTLD and AD and this research has augmented our understanding of the pathology and diagnoses of these dementia syndromes. By contrast, there has been much less research comparing and contrasting the longitudinal decline in AD and FTLD.1,2
Longitudinal designs are crucial to better understand the natural history of dementia syndromes. Greater knowledge of the natural history of dementia syndromes is critical for designing clinical trials for the pharmacological treatment of dementia and to better anticipate the caregiving needs of patients and their families. Since its emergence, the Mini-Mental State Examination (MMSE)9 has become the most widely administered neuropsychological test used to screen for cognitive impairment in both clinical and research settings because of its brevity and practicality.10 However, longitudinal change in AD and FTLD, as it is reflected by the MMSE, has not been thoroughly researched. Several studies11,12 have directly compared annual rates of cognitive decline within the context of the MMSE in AD and FTLD but results have been inconsistent. One study indicated that the rate of decline as measured by the MMSE is slower in patients with FTLD than AD.11 Conversely, a second study12 found significantly accelerated decline in patients with FTLD compared to AD. The cases in the second study were autopsy-confirmed, which may bias the result toward individuals who progress rapidly. The AD patients were matched to FTLD patients resulting in a sample of younger AD patients, which may impact generalization of the comparisons between FTLD and AD patients. A problem common to both of these prior studies was that patients with different FTLD phenotypes were combined into a single group and compared to AD patients, thereby obscuring potential differences within the FTLD spectrum of disorders. Prior research suggests different rates of decline within various dementia syndromes on the MMSE. Thus, the highly verbal nature of the MMSE may overestimate dementia severity in patients with PPA13 but may be less accurate in characterizing overall impairment in other FTLD phenotypes such as patients who present with bvFTD. This suggests that in addition to the comparative analysis of MMSE longitudinal between-group changes, MMSE longitudinal within-group changes may provide a useful and heuristically meaningful measure of disease progression. Thus, the current research tracked MMSE scores in three FTLD phenotypes (bvFTD, nfaPPA, and svPPA) and patients with AD for up to 52 months since initial visit. The goal of the current research was to obtain better estimates of both between-group and within-group longitudinal changes on the MMSE in three FTLD phenotypes and AD. This will allow us to examine if MMSE is sensitive to track disease progression in FTLD.
Methods
Participants
A total of 185 patients with clinical diagnoses of AD or FTLD were included in this study. Evaluation and recruitment were conducted at the Department of Neurology, University of Pennsylvania. A consensus committee consisting of at least two trained reviewers evaluated and confirmed the presence of diagnostic criteria based on a semi-structured history, a detailed neurologic exam, the Philadelphia Brief Assessment of Cognition (PBAC),7,14 and the MMSE. Cases with discrepancies between reviewers were presented to the entire committee to obtain a consensus diagnosis. Initial diagnostic decision-making did not include longitudinal change on the MMSE test performance. Detailed neuropsychological testing was obtained on different occasions, and reviewers were blinded to patients’ performance on the neuropsychological protocol. All study participants and their legal representatives agreed to the informed consent approved by the Institutional Review Board at the University of Pennsylvania.
Among the subjects in the cohort, 79 were clinically diagnosed with FTLD and further categorized into one of three subgroups based on a modification of published criteria.15,16 At the time when this analysis was performed and the manuscript was written, the recently published criteria17,18 were in the final stage of preparation for publication and had not yet been formally finalized. The modifications in diagnostic criteria used to select patients in this paper are consistent with these recently published criteria. The final sample consisted of 40 patients diagnosed with bvFTD and presented with significant social/behavioral difficulties and dysexecutive impairment; 23 patients were diagnosed with nfaPPA and presented with effortful speech that may have resulted from speech sound errors and impaired grammatical expression, but still retain relative single word comprehension; and 16 svPPA patients who exhibited naming deficits and difficulty understanding single words and objects. Other symptoms include fluent and circumlocutory spontaneous speech frequently empty of content. The clinical diagnosis of AD was rendered in 106 patients based on criteria from the National Institute of Neurologic and Communicative Disorders and Stroke – Alzheimer's Disease and Related Disorders Association.19 They were primarily characterized by impaired episodic memory along with weakened visuoconstruction and/ or executive control. We excluded AD patients with visual, aphasic or frontal lobe variants. This decision was made to limit skewed MMSE test performance because of possible outlying performance on selected MMSE test items related to language and motor production (e.g., writing a sentence, copying a figure). Moreover, these patients have unclear underlying pathology. Table 1 presents baseline demographic characteristics such as age, years of education, MMSE scores, and months between estimated onset of illness and initial evaluation.
Table 1.
Demographic characteristics of frontotemporal lobar degeneration (FTLD) subtypes and Alzheimer's disease (mean ± SD)
| bvFTD n = 40 | nfaPPA n = 23 | svPPA n = 16 | AD n = 106 | |
|---|---|---|---|---|
| Age at initial visit | 60.98 (9.66) | 66.83 (9.22) | 63.31 (9.60) | 71.78 (8.48) |
| Education | 15.18 (2.72) | 14.83 (2.71) | 15.56 (3.12) | 14.06 (3.35) |
| MMSE at baseline | 25.48 (3.64) | 26.17 (2.74) | 23.63 (6.46) | 21.10 (5.78) |
| Duration of illness at initial evaluation | 24.13 (12.79) | 23.04 (11.81) | 29.44 (12.61) | 25.47 (13.47) |
Abbreviation: bvFTD = behavioral variant frontotemporal dementia; nfaPPA = nonfluent agrammatic variant of primary progressive aphasia; svPPA = semantic variant PPA; AD = Alzheimer's disease
Subjects in this sample were followed up to 52 months since initial visit. The longitudinal follow-up time for each patient is defined as number of months since initial visit. Our sample included subjects who had at least two MMSE records. Overall, the average follow-up time between two visits is 6.98 months (SD= 5.10). Within each group, the average follow-up time between two visits is 7.20 months (SD = 5.45) for bvFTD, 8.07 months (SD = 5.25) for nfaPPA, 9.12 (SD = 5.21) for svPPA, and 6.41 (SD = 4.84) for AD. A one-way analysis of variance (ANOVA) was used to test differences in duration between visits among groups. Duration between two visits differed significantly among the four groups (F[3,413]=3.43, p= 0.017), but the Tukey post-hoc comparisons were not significant for any subsequent analyses. The average number of visits is 3.25 times (SD = 1.80) during the study. There were no significant differences across groups in terms of the number of visits. A total of 92 patients had only two visits, 34 patients had only three visits, and 59 patients had four or more visits. The average duration of follow-up is 15.7 months (SD=11.3, range from 1 to 52 months) since initial evaluation. Length of follow-up did not differ significantly among the groups (F[3,181]=1.73, p=0.162).
Measures
All subjects were tested with the MMSE as a component of their initial clinical assessment as well as any follow-up in this study. The total MMSE score ranges from 0 to a perfect score of 30, reflecting assessment on orientation, memory, naming, comprehension, repetition, figure copy, and sentence production. The total score was kept as a continuous measure in the analyses. Other clinical and demographic measures collected included age at initial visit of the patients and the number of years of school completed.
Statistical Analysis
ANOVA was used to compare continuous baseline demographic variables and MMSE scores at initial evaluation among the dementia groups. We constructed a mixed-effects model20 to examine patterns of cognition measured by the total MMSE score longitudinally from initial evaluation up to 52 months post initial visit. A mixed-effects model is an extension of a linear regression model that allows the calculation of mean trajectory of MMSE score for each group as well as the estimation of each patient's trajectory. One advantage of the mixed-effects model approach is its emphasis on individual patients’ trajectories over time rather than on mean values at each time point. The mixed-effects model includes three parts. The first part contains the random effect parameters. These are random variables and are assumed to follow a normal distribution with a correlation structure that can be estimated from the data. The second part includes fixed-effect parameters that are assumed to be non-random. The third component includes an error term that is assumed to follow a normal distribution.
The mixed-effects model takes into account within-subject correlations from repeated measurements of MMSE scores in the same subjects and for missing data points. It is also based on a time-in-study data structure that allows individually varying intervals between evaluation visits, so subjects can have missing data during follow-up, provided the missing data is missing-at-random. In this analysis, we only included patients with two or more MMSE records to allow for estimation of rate of decline and to not impose assumptions that those with only one evaluation are similar to those with two or more evaluations. The χ2- test was used to compare drop-out rates across diagnosis groups. T-test was used to compare baseline MMSE, duration of illness, and other demographic characteristics between drop-outs and non-drop-outs. All of these analyses comparing drop-outs and non-drop-outs were not significant.
Our model specified the intercept and the regression coefficient for the follow-up time as random effects such that subjects have a unique intercept and slope characterizing their individual trajectories. The independence covariance matrix was specified for the random effects, while an autoregressive AR(1) correlation structure was specified for the error terms. This correlation structure makes scientific sense as each subject has different intercepts and slopes, and observations within a subject that are closer in time are more similar than observations further apart in time.
Estimations in models used the restricted maximum likelihood procedure that ensures a less biased estimate since both fixed and random effects are specified as unknowns. We also applied the Kenward-Rogers correction21 for standard errors and F-statistics in the mixed-effects model. This is especially appropriate for unbalanced data where each group has different number of patients and each patient has varying number of observations.
Population mean coefficient for the follow-up time was estimated by averaging across the subject-specific regression coefficients for the follow-up time. This reflects the average monthly change in MMSE over time. Duration was treated as a continuous variable and measured as number of months since initial evaluation.
One of our main interests is the relationship between diagnosis and longitudinal measures of MMSE after adjusting for covariates. Fixed effects for adjustments included follow-up time since initial evaluation and diagnostic group assignments. We also adjusted for baseline MMSE measured at the initial visit for baseline cognitive impairment as well as age at baseline and education. However, the effects of baseline age and education were not significant in the presence of baseline MMSE and thus were removed from the final mixed-effects model. We also analyzed the interaction between dementia group and follow-up. If the interaction term were significant, the rate of decline for each disease group would be estimated separately.
Longitudinal decline may be linear or curvilinear, and we examined both longitudinal effects in our analyses. Previous work22 has shown that MMSE longitudinal decline displays a curvilinear trend, hence a quadratic duration term tested whether an accelerated nonlinear trend was present.
Extensive model diagnostics were performed through standardized residual plots. We found no evidence against the model assumptions: both the error term and random effects were normally distributed. The residual plots also indicated the appropriateness of the mean model and the covariance structure among repeated measurements. All analyses were conducted using the statistical software package SAS version 9.2 (SAS Institute Inc., Cary, North Carolina). All statistics tests were two-sided. Statistical significance was set at the p< .05 level unless otherwise noted.
Results
Demographic Data
Table 1 provides demographic information for the study cohort. At baseline (initial visit), there were significant differences between diagnosis groups for age (F[3,181]=16.18, p< 0.001) reflected by one-way ANOVA. Multiple comparisons using Tukey's adjustment suggest that AD patients were older than bvFTD and svPPA patients (p< 0.05). This is consistent with the key characteristics of younger age of onset among FTLD patients. There were also significant differences among the groups in baseline MMSE scores (F[3, 181]=10.81, p< 0.001, Table 1). AD patients were 4.3 points lower than bvFTD patients and 5.1 points lower than nfaPPA patients at the initial evaluation (p< 0.05). Tukey's pair-wise comparisons between all other pairs were not statistically significant at p< 0.05. There were no significant differences among groups with respect to education or duration of illness by initial evaluation.
Results from Mixed-Effects Model Analysis
Table 2 provides the results of the final mixed-effects model describing the longitudinal decline of MMSE for the four groups. We note that education and age at baseline were not significant and were not included in the final mixed-effects model. The model revealed a significant group by duration interaction (F[3, 231]=5.87, p<0.001) reflecting group differences in longitudinal decline after adjustment for baseline MMSE differences. Pair-wise comparisons found differences between bvFTD with AD (p= 0.039) and nfaPPA with AD (p<0.001), indicating MMSE scores declined significantly faster in patients with AD than in either of the two FTLD subtypes. svPPA patients declined faster on the MMSE than nfaPPA patients (p=0.006). Other dementia group comparisons were not statistically significant at p< 0.05.
Table 2.
Mixed-effects model: Effects of group and duration since initial visit on MMSE scores
| Predictors | F Value | DF [Num,Den] | Estimate |
|---|---|---|---|
| Intercept | 0.325 | ||
| Group | 1.50 | [3,231] | |
| bvFTD | 0.453 | ||
| nfaPPA | -0.080 | ||
| svPPA | -0.200 | ||
| AD | 0 [Reference] | ||
| Duration since initial visit | 9.83 | [1,181] | -0.224b |
| Duration × group | 5.87a | [3,231] | |
| Duration × bvFTD | 0.158a | ||
| Duration × nfaPPA | 0.234b | ||
| Duration × svPPA | -0.002 | ||
| Duration × AD | 0 [Reference] | ||
| Duration × duration | 11.80 | [1,231] | -0.005b |
| Baseline MMSE | 3298.54 | [1,231] | 0.982b |
Abbreviation: bvFTD = behavioral variant frontotemporal dementia; nfaPPA = nonfluent agrammatic variant of primary progressive aphasia; svPPA = semantic variant PPA; AD = Alzheimer's disease
p<.05
p<.01
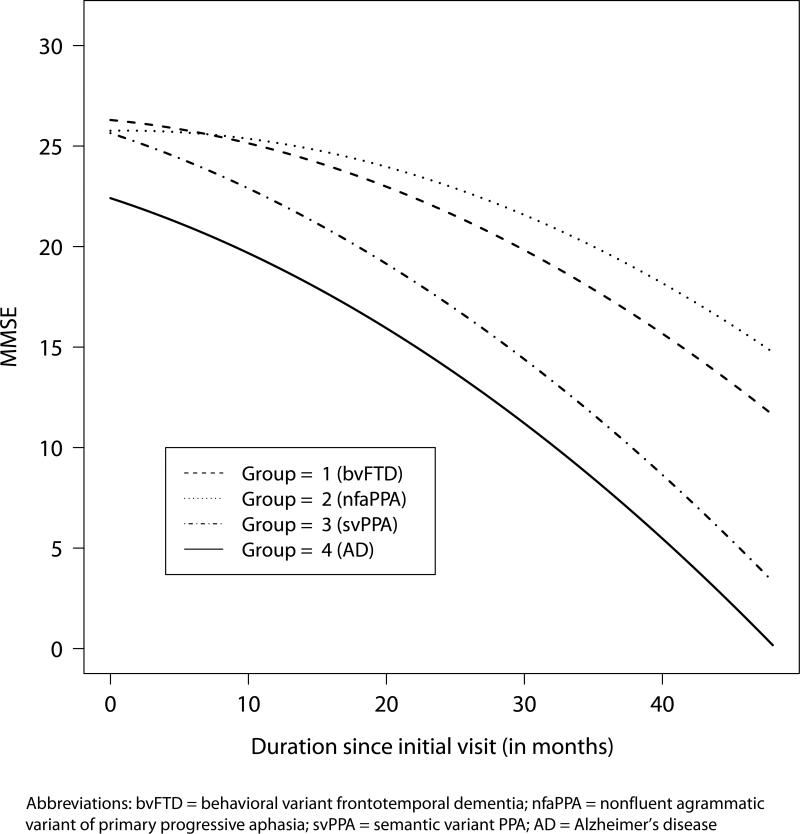
Figure 1 depicts the effect of group assignment on longitudinal decline for patients adjusting for baseline MMSE. Median baseline MMSE scores were identified for each of the four groups: bvFTD=26, nfaPPA=26, svPPA=26 and AD=22.5. The absolute decline of MMSE scores in the AD group was greater than those with bvFTD or nfaPPA, as presented in the estimated MMSE scores from the model. Patients with group-specific median baseline MMSE in the bvFTD and nfaPPA groups have predicted absolute decline of 14.67 (95% CI: 14.63-14.71) and 11.02 (95% CI: 10.98-11.06) points respectively, whereas the predicted absolute decline in the svPPA and AD groups are 22.32 (95% CI: 22.29-22.34) and 22.24 (95% CI: 22.22-22.26), respectively, over four years since initial visit. All groups show a curvilinear trend over four years post initial evaluation.
Figure 1.
Predicted MMSE scores over time from the mixed–effects model given group–specific median baseline MMSE
Table 3 presents within-group estimated annual rate of change in total MMSE given group-specific median baseline MMSE test scores. From year to year within-group analyses indicate little differences between AD and svPPA patients and little difference between nfaPPA and bvFTD groups. However, AD/svPPA patients decline faster on the MMSE than bvFTD/nfaPPA patient groups.
Table 3.
Estimated Annual Rate of Change in MMSE# (post-initial visit, between years)
| Groups | 0 & 1 | 1 & 2 | 2 & 3 | 3 & 4 |
|---|---|---|---|---|
| bvFTD | -1.51 | -2.95 | -4.39 | -5.82 |
| nfaPPA | -0.60 | -2.04 | -3.47 | -4.91 |
| svPPA | -3.42 | -4.86 | -6.30 | -7.73 |
| AD | -3.40 | -4.86 | -6.28 | -7.72 |
Abbreviation: bvFTD = behavioral variant frontotemporal dementia; nfaPPA = nonfluent agrammatic variant of primary progressive aphasia; svPPA = semantic variant PPA; AD = Alzheimer's disease
Based on group-specific median baseline MMSE (bvFTD = 26, nfaPPA = 26, svPPA = 26, AD = 22.5)
Discussion
The findings of this study highlight the impact of distinct dementia phenotypes regarding differential longitudinal decline as monitored with the MMSE.23-25 By analyzing three phenotypic presentations of FTLD against AD, we note that the cognitive decline in some patients progresses more rapidly (e.g., AD, svPPA) compared to other patient groups (e.g., bvFTD, nfaPPA) over four years. These findings suggest the need for a more sophisticated analysis when the MMSE is used to tact longitudinal decline both clinically and for clinical trials.
As suggested in previous research,25 MMSE is a brief assessment that weighs verbal and memory functions more heavily than nonverbal and behavioral functions. In addition, the MMSE differentially assesses the impact of dementia on orientation and visuospatial operations. These cognitive skills are known to be at risk in patients with AD but perhaps are not as prevalent in FTLD. A third of the items on the MMSE are orientation items that highly correlate with amnesiac disorders as seen in AD. Patients may have difficulty with items on the test for various reasons.26 For example, patients with a language disorder such as svPPA may have difficulty with orientation because of poor comprehension. AD may fail this item because of their episodic memory deficits.
Based on the predicted outcome of MMSE scores, bvFTD and nfaPPA displayed only an overall decline of 14.7 and 11.0 points respectively over four years post initial evaluation, which might suggest minimal cognitive decline over such a long disease duration. In fact, the dementia worsened clinically, as measured with the Philadelphia Brief Assessment of Cognition, (PBAC),7,14 another instrument used to screen for dementia, but this was not reflected by the total MMSE scores. This difference among groups is unlikely to be due to having examined patients at different points in their natural history: we adjusted for MMSE at the initial evaluation, and survival studies indicate more rapid decline in FTLD than AD.12 A recent study by Gordon et al27 showed evidence that commonly used cognitive tests, including the MMSE, lack sensitivity in detecting longitudinal cognitive decline in FTLD phenotypes as indexed by MRI measures and are highly dependent on the specific subtypes. Future studies should include direct comparisons of MMSE and PBAC in terms of their ability to track disease progression.
The MMSE is insensitive to the clinical characteristics now recognized as prominent features of some forms of dementia. In bvFTD, for example, the MMSE does not monitor the social changes that are so prominent in this subgroup. Pasquier et al.11,24 noted that MMSE scores remained high in the bvFTD group compared to an AD group, then abruptly dropped when patients showed signs of apathy and mutism, usually predicted by a MMSE score of 18. It has been shown that patients with bvFTD can have severe functional impairments despite high MMSE scores.12,28,29 Likewise, in nfaPPA, the MMSE does not monitor speech fluency or agrammatism, and may be insensitive to the progressive changes in this subgroup. Thus, if the MMSE is part of a clinical assessment of protocol for AD and FTLD, the relative change over time within a group may be as informative comparatively as absolute change over time measured by between-group analyses. The modest changes of MMSE in nfaPPA and bvFTD groups over time indicate that MMSE may not be sensitive to some FTLD subgroups and thus may not be a good tool for monitoring progression in FTLD.
Several expanded versions of the MMSE such as the Cognitive Abilities Screening Instrument (CASI)35 have been developed to address greater range of cognitive function or specific neurological deficits in diseases such as multiple sclerosis, but they have not been analyzed in the specific FTLD subtypes. The Addenbrooke's Cognitive Examination (ACE)31 is also a variant of the MMSE that can monitor disease progression in FTLD, but does not assess social functioning. The Mattis Dementia Rating Scale (MDRS)32 has also been shown to be a useful monitoring tool for disease progression in FTLD.33,34 The Montreal Cognitive Assessment (MoCA),35 a cognitive screening tool more sensitive to frontal lobe disturbances, incorporates tasks specific to frontal lobe function, such as Trail Making tests and tests of attention. The recently developed Philadelphia Brief Assessment of Cognition (PBAC)7,14 may serve as an effective alternative to the MMSE in screening for dementia severity and tracking longitudinal progression among these dementia subgroups as this test was designed to assess a broader range of neuropsychological and behavioral functions specific to AD and FTLD subtypes. Regardless of the test under consideration, future studies should incorporate longitudinal analysis both between and within patient groups.
Several limitations should be recognized when interpreting results from this study. First, our sample includes clinically-diagnosed patients instead of autopsy-confirmed cases. It should be noted, however, that the diagnostic and classification process adheres to strict guidelines and published criteria. Second, although patient groups were adjusted for MMSE at the time of initial assessment, the present results may have underestimated the progression of cognitive decline due to the possibility that we did not examine FTLD spectrum patients sufficiently late in the disease process, as prior research has shown more rapid disease progression in FTLD compared to AD.12 Thirdly, we recognize that the possible inclusion of phenocopy cases in our bvFTD cohort may reduce estimates of decline.33 We attempted to exclude phenocopy cases by inspecting MRI and having follow-up on cases to demonstrate decline, but not all patients had autopsy data to allow for complete pathologic evidence for FTLD. Fourth, MMSE subscale scores have been shown to differentiate dementia groups. Such data might have resulted in meaningful between-group differences. These data were not available in the current research and is a topic for future research. Lastly, patients may not be representative of the general population and appear to be better educated than population-based samples.24
Acknowledgments
Source of Funding Dr. Xie receives funding from National Institute of Health (AG-10124, AG-17586, and NS-53488). Dr. Xie serves as a consultant to Roche on a clinical trial unrelated to this study. Dr. Grossman receives funding from National Institute of Health (AG-17586, AG-15116, NS-44266, AG-32953, and NS-53488), has pending grant from Wyncote Foundation, and receives payment for lectures from University of Florida and Max-Planck Institute.
Footnotes
Conflicts of Interest Drs. Libon, Rascovsky, and Ms. Tan report no disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Libon DJ, Xie SX, Wang X, et al. Neuropsychological decline in frontotemporal lobe dementia: A longitudinal analysis. Neuropsychology. 2009;23:337–346. doi: 10.1037/a0014995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xie SX, Libon DJ, Wang X. Longitudinal patterns of semantic and episodic memory in frontotemporal lobar degeneration and Alzheimer's disease. J Int Neuropsychol Soc. 2010;16:278–286. doi: 10.1017/S1355617709991317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKhann G, Trojanowski JQ, Grossman M, et al. Clinical and pathological diagnosis of frontotemporal dementia: Report of a work group on frontotemporal dementia and Pick's disease. Arch Neurol. 2001;58:1803–1809. doi: 10.1001/archneur.58.11.1803. [DOI] [PubMed] [Google Scholar]
- 4.Knopeman DS, Jack CR, Jr, Kramer JH, et al. Brain and ventricular volumetric changes in frontotemporal lobar degeneration over 1 year. Neurology. 2009;72:1843–1849. doi: 10.1212/WNL.0b013e3181a71236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lund and Manchester Groups Clinical and neuropathological criteria for frontotemporal dementia. J Neurol Neurosurg Psychiatry. 1994;57:416–418. doi: 10.1136/jnnp.57.4.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kramer JH, Jurik J, Sha SJ. Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cogn Behav Neurol. 2003;16:211–218. doi: 10.1097/00146965-200312000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Libon DJ, Massimo L, Moore P, et al. Screening for frontotemporal dementias and Alzheimer's disease with the Philadelphia Brief Assessment of Cognition: a preliminary analysis. Dement Geriatr Cogn Disord. 2007;24:441–447. doi: 10.1159/000110577. [DOI] [PubMed] [Google Scholar]
- 8.Libon DJ, Xie S, Moore P, et al. Patterns of neuropsychological impairment associated with frontotemporal dementia: A factor analytic study. Neurology. 2007;68:368–375. doi: 10.1212/01.wnl.0000252820.81313.9b. [DOI] [PubMed] [Google Scholar]
- 9.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 10.Galasko D, Klauber MR, Hofstetter CR, et al. The Mini-Mental Examination in the early diagnosis of Alzheimer's disease. Arch Neurol. 1990;47:49–52. doi: 10.1001/archneur.1990.00530010061020. [DOI] [PubMed] [Google Scholar]
- 11.Pasquier F, Richard F, Lebert F. Natural history of frontotemporal dementia: comparison with Alzheimer's disease. Dementia Geriatr Cogn Disord. 2004;17:253–257. doi: 10.1159/000077148. [DOI] [PubMed] [Google Scholar]
- 12.Rascovsky K, Salmon DP, Lipton AM, et al. Rate of progression differs in frontotemporal dementia and Alzheimer disease. Neurology. 2005;65:397–403. doi: 10.1212/01.wnl.0000171343.43314.6e. [DOI] [PubMed] [Google Scholar]
- 13.Osher J, Wicklund A, Rademaker A, et al. The mini-mental state examination in behavioral variant frontotemporal dementia and primary progressive aphasia. Am J Alzheimers Dis Other Demen. 2007;22:468–473. doi: 10.1177/1533317507307173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Libon DJ, Rascovsky K, Gross RG, et al. The Philadelphia Brief Assessment of Cognition (PBAC): a validated screening measure for dementia. Clin Neuropsychol. 2011;25:1314–1330. doi: 10.1080/13854046.2011.631585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grossman M, Ash S. Primary progressive aphasia: A review. Neurocase. 2004;10:3–18. doi: 10.1080/13554790490960440. [DOI] [PubMed] [Google Scholar]
- 16.Neary D, Snowden JS, Gustafson L. Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 17.Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raskovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease: Report on the NINCDS-ADRDA work group under the auspices of the Department of Health and Human Services Task Force on Alzheimer's disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 20.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 21.Kenward MG, Roger JH. Small sample inference for Fixed Effects from Restricted Maximum Likelihood. Biometrics. 1997;53:983–997. [PubMed] [Google Scholar]
- 22.Grossman M, Xie SX, Libon DJ, et al. Longitudinal decline in autopsy-defined frontotemporal lobar degeneration. Neurology. 2008;70:2036–2045. doi: 10.1212/01.wnl.0000303816.25065.bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grossman M, Libon DJ, Forman MS, et al. Distinct antemortem profiles in patients with pathologically defined frontotemporal dementia. Arch Neurol. 2007;64:1601–1609. doi: 10.1001/archneur.64.11.1601. [DOI] [PubMed] [Google Scholar]
- 24.Pasquier F, Lebert F, Lavenu I, et al. The clinical picture of frontotemporal dementia: Diagnosis and follow-up. Dement Geriatr Cogn Disord. 1999;10(suppl 1):10–14. doi: 10.1159/000051206. [DOI] [PubMed] [Google Scholar]
- 25.Haxby JV, Raffaele K, Gillette J, Schapiro MB, Rapoport SI. Individual trajectories of cognitive decline in patients with dementia of the Alzheimer type. J Clin Exp Neuropsychol. 1992;14:575–592. doi: 10.1080/01688639208402846. [DOI] [PubMed] [Google Scholar]
- 26.Jefferson AL, Cosentino S, Ball SK, et al. Errors produced on the Mini-Mental State Examination and neuropsychological test performance among patients with Alzheimer's disease, ischaemic vascular dementia, and Parkinson's disease. J Neuropsychiatry Clin Neurosci. 2002;14:311–320. doi: 10.1176/jnp.14.3.311. [DOI] [PubMed] [Google Scholar]
- 27.Gordon E, Rohrer JD, Kim LG, et al. Measuring disease progression in frontotemporal lobar degeneration: A clinical and MRI study. Neurology. 2010;78:666–673. doi: 10.1212/WNL.0b013e3181d1a879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosen HJ, Narvaez JM, Hallam B, et al. Neuropsychological and functional measures of severity in Alzheimer disease, frontotemporal dementia, and semantic dementia. Alzheimer Dis Assoc Disord. 2004;18:202–207. [PubMed] [Google Scholar]
- 29.Mioshi E, Hsieh S, Savage S, et al. Clinical staging and disease progression in frontotemporal dementia. Neurology. 2010;74:1591–1597. doi: 10.1212/WNL.0b013e3181e04070. [DOI] [PubMed] [Google Scholar]
- 30.Teng EL, Hasegawa K, Homma A, et al. The Cognitive Abilities Screening Instrument (CASI): A practical test for cross-cultural epidemiologic studies of dementia. Int Psychogeriatr. 1994;6:45–56. doi: 10.1017/s1041610294001602. [DOI] [PubMed] [Google Scholar]
- 31.Larner AJ. Addenbrooke's Cognitive Examination (ACE) for the diagnosis and differential diagnosis of dementia. Clin Neurol Neurosurg. 2007;109:491–494. doi: 10.1016/j.clineuro.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 32.Mattis S. Dementia Rating Scale: Professional Manual. Psychological Assessment Resources; Odessa, FL: 1988. [Google Scholar]
- 33.Kipps CM, Nestor PJ, Dawson CE, et al. Measuring progression in frontotemporal dementia: implications for therapeutic interventions. Neurology. 2008;70:2046–2052. doi: 10.1212/01.wnl.0000313366.76973.8a. [DOI] [PubMed] [Google Scholar]
- 34.Rascovsky K, Salmon DP, Hansen L, et al. Distinct cognitive profiles and rates of decline on the Mattis Dementia Rating Scale in autopsy-confirmed frontotemporal dementia and Alzheimer's disease. J Int Neuropsychol Soc. 2008;14:373–383. doi: 10.1017/S135561770808051X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]