Abstract
Objective
We aimed to characterize severity and occurrence of knee osteoarthritis (OA), and effects of age, sex, body weight, and reproductive status on population-level normal variation in this condition in the baboon, a natural model of human knee OA.
Methods
We visually inspected articular cartilage of distal right femora of 464 baboons (309 females, 155 males) and assigned an OA severity score (comparable to a modified Outerbridge score) from 1 = unaffected to 4 = advanced OA (eburnation). Presence/absence of osteophytes was recorded. We tested for significant effects of age, sex, weight, and, in females, reproductive status (pre-, peri-, or post-menopausal) on OA. When appropriate, analyses were repeated on an age-matched subset (153 of each sex).
Results
Knee OA was more frequent and severe in older animals (p < 0.0001), but significant age variation was apparent in each severity grade. Sex differences within the younger and older age groups suggest that males develop knee OA earlier, but females progress more quickly to advanced disease. There is a strong relationship between reproductive status and OA severity grade in females (p = 0.0005) with more severe OA in peri- and post-menopausal female baboons, as in humans.
Conclusions
Idiopathic knee OA is common in adult baboons. Occurrence and severity are influenced strongly by reproductive status in females, and by sex with regard to patterns of disease progression – providing an animal model to investigate sex-specific variation in OA susceptibility in which the environmental heterogeneity inherent in human populations is vastly reduced.
Keywords: sex differences, spontaneous OA, animal model, retrospective study, crosssectional study
Introduction
Osteoarthritis (OA) affects populations worldwide1,2 but the causes of disease onset and interindividual variation in susceptibility and progression remain elusive. A suite of OA risk factors have been identified3,4, including genetic variation5,6, age, sex, obesity, reproductive status (e.g., post-menopausal) in females, mechanical stresses, leg muscle strength, mass, and related parameters7, and history of previous joint trauma. Significant further advancement in knowledge of OA pathogenesis is severely hindered, however, by a scarcity of established animal models that faithfully reflect human disease etiology and progression, and by logistical barriers to the study of early stage disease in humans.
Non-human primates share with humans many age-related degenerative conditions including naturally occurring idiopathic OA8, thereby serving as an appropriate model system for basic and translational OA research with high relevance to human disease. OA is particularly well documented in Old World Monkeys9-11 (i.e., Cercopithecidae), including macaques and baboons. This group is second only to non-human apes in phylogenetic proximity to humans, and, consequently, shares with us many aspects of skeletal anatomy, genetics, and disease12-14. Rhesus (Macaca mulatta) and cynomolgus macaques (Macaca fascicularis) have also been extensively studied with regard to OA9-11,15-24. We summarize general results in these species and in baboons in Table 1. Baboons commonly develop OA in multiple joints and are used in experimental studies of joint degeneration25-28, but until now have not been systematically examined with regard to prevalence of spontaneously occurring OA. Baboons (Papio hamadryas ssp.) from the Southwest National Primate Research Center (SNPRC)/Texas Biomedical Research Institute (TBRI) colony provide a unique opportunity to study normal population-level variation in all stages of OA progression (especially early stage disease) in a large, outbred pedigreed sample.
Table 1.
Comparison of knee OA in Old World Monkey species and humans.
| Humans | Baboons (based on this study) | Rhesus macaques | Cynomolgus macaques | |
|---|---|---|---|---|
|
Prevalence of knee OA with increasing age |
Prevalence increases with age – humans over the age of 65 have higher prevalence48 |
Disease severity differs significantly between younger and older (21.67 years +) baboons |
OA prevalence increases with age9,17,18 |
Knee OA prevalence increases with age11 |
|
Effects of weight on knee OA |
Heavy weight is risk factor for knee OA in multiple populations worldwide36 |
OA severity correlated with increased weight in females, but not in males |
Relationship between weight and OA severity is inconclusive17,18,49 |
Increased weight correlated with subchondral bone thickness, but not articular cartilage lesions in the tibial plateau11 |
|
Sex differences in prevalence of knee OA |
Significantly higher occurrence of knee OA in older women (> 55 years) than older men36,37 |
Males develop knee OA earlier, but females progress more rapidly to advanced disease |
Knee OA is more frequent in female than male rhesus macaques (10.9% vs. 23.5%, respectively)9 |
No significant sex difference in knee OA prevalence in cynomolgus monkeys11 |
|
Prevalence of knee OA in females relative to reproductive status |
Postmenopausal women show higher prevalence of OA than premenopausal women36,37 |
Post- and peri-menopausal females show higher prevalence of knee OA than pre- menopausal baboons |
Lumbar vertebrate OA is more common in postmenopausal monkeys than premenopausal ones49 |
Ovariectomized monkeys were examined with respect to knee OA, but not compared to intact females50 |
| Osteophytes | More prevalent in older individuals than younger ones44 |
Higher prevalence in older baboons than younger ones |
Associated with joints with limited passive excursion capabilities and with radiographic OA8,21 |
Osteophytes present in monkeys with knee OA11 |
|
Gross pathology/ Macroscopic assessment of OA |
Five OA stages in modified Outerbridge classification based on cartilage33 |
Four OA stages (unaffected, mild moderate, advanced) based on cartilage degradation |
Four OA stages (normal, mild, moderate, severe) applied to knees of rhesus macaques21 |
Gross anatomy examined on specimens but the grading system is unclear11 |
| Histology | OARSI grading system47 | OARSI grading system appears to work for baboons |
Modified Mankin grading scheme applied to histology from knee joint21 |
Semiquantitative histological grading scheme applied to cynomolgus monkeys11 |
|
Radiographic or MRI evidence of OA |
Assessment typically based on Kellgren and Lawrence34 |
Radiographic diagnosis based primarily on osteophytes |
OA diagnosed from MRI using osteophytes, articular cartilage thickness, joint space21 |
Radiographic evidence of knee OA in cynomolgus monkeys50 |
|
Symptomatic evidence of OA |
Inflammation, pain, stiffness, and loss of joint mobility2 |
Based on weight loss, reduced range of motion, & crepitation in knee joint; also limited observational data on baboons dragging their legs |
Obvious disability and gait abnormalities with advanced OA; also studies on passive joint excursions reveal OA in knee joint9,18 |
Some older animals were observed to have a stiff gait and/or a limp50 |
The aim of our study was to quantify occurrence and severity of knee OA in the SNPRC/TBRI baboons based on macroscopic assessment of distal femora and to characterize the relationship of OA to animal sex, body weight, and age. Specifically, we investigated the following questions: 1) Does knee OA prevalence and severity increase with advancing age and increasing weight as expected in baboons? 2) Are there differences between the sexes in occurrence and/or severity of knee OA? 3) Does reproductive status affect prevalence and severity of knee OA in females? 4) How do occurrence and severity of knee OA in baboons compare to prevalence of this disease in humans?
Methods
SNPRC/TBRI baboon colony
The sample came from a larger colony of Papio hamadryas ssp. that resides in San Antonio, TX at SNPRC/TBRI. The baboons are fed primarily commercially available Purina monkey diet with a high carbohydrate (67%), low fat (14%), and 19% protein content supplemented with fresh fruits and vegetables. Animals are housed outdoors in social groups in enclosures with concrete-bottoms and chain link walls. Baboons habitually walk quadrupedally, and occasionally walk or stand bipedally (both in captivity and in the wild), particularly when hand-carrying objects or when surveying their surroundings29. Various substrates are provided to allow for a range of normal locomotor behavior, climbing, and environmental enrichment. Housing based on species-appropriate social structure allows for behavior and interactions that are typical for wild baboons, and has facilitated efficient collection of biomedical data in an environment that is healthy for the animals30. Many behavioral and clinical data are routinely collected by animal care and veterinary staff in accordance with accepted protocols by the Institutional Animal Care and Use Committee and were available for this study (i.e., female reproductive data, birth and death records, weights).
Baboon sample
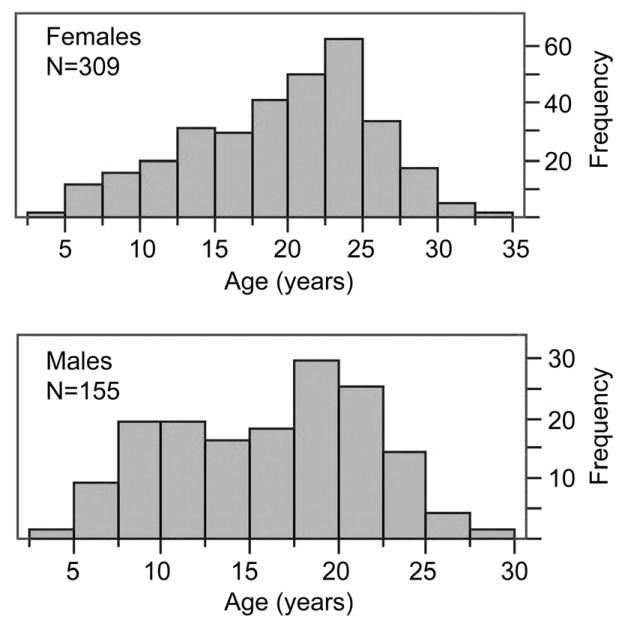
We visually examined right distal femora of 464 baboons (309 females, 155 males) that were opportunistically collected at routine necropsy of animals that died in the period from 2004 to 2011. Exact ages of the baboons are known from colony records. Our sample age range was 4.5 to 33 years of age (Fig. 1, Table 2), roughly developmentally equivalent to 14 to 100 years in humans based on average lifespan comparisons between baboons and humans31. Animal weight (kg) was recorded at time of death.
Fig. 1.
Histograms showing age distributions for female and male baboons used in this study.
Table 2.
Descriptive statistics for age, weight, and osteophyte occurrence by sex and OA severity.
| age (yrs.) | weight (kg) | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| OA severity | N (% of sex) (CI lower, upper) |
% grade with osteophytes (CI lower, upper) |
mean | sd | min-max | mean | sd | min-max |
| ♀ Grade 1 | 59 (19%) (9%, 29%) |
24% (13%, 35%) |
17.28 | 6.68 | 4.9—32.45 | 16.32 | 3.35 | 10.04—26.4 |
| ♀ Grade 2 | 76 (25%) (15%, 34%) |
12% (5%, 19%) |
17.13 | 5.76 | 6.0—33.27 | 17.16 | 3.38 | 10.14—29.6 |
| ♀ Grade 3 | 97 (31%) (22%, 41%) |
55% (45%, 65%) |
20.29 | 5.44 | 6.13—30.46 | 18.20 | 4.76 | 10.40—32.05 |
| ♀ Grade 4 | 77 (25%) (15%, 35%) |
97% (90%, 99%) |
22.71 | 4.71 | 6.08—30.14 | 19.09 | 4.64 | 11.40—34.48 |
|
| ||||||||
| ♂ Grade 1 | 17 (11%) (3%, 36%) |
24% (3%, 44%) |
15.77 | 6.07 | 4.47—25.52 | 28.19 | 7.37 | 14.58—45.21 |
| ♂ Grade 2 | 65 (42%) (30%, 54%) |
26% (15%, 37%) |
13.32 | 5.04 | 5.7—23.81 | 27.31 | 4.95 | 17.12—38.90 |
| ♂ Grade 3 | 53 (34%) (21%, 47%) |
49% (36%, 62%) |
18.41 | 5.09 | 7.62—28.14 | 27.36 | 4.91 | 19.41—43.60 |
| ♂ Grade 4 | 20 (13%) (4%, 35%) |
95% (72%, 99%) |
18.55 | 4.30 | 9.74—24.23 | 28.82 | 3.60 | 24.54—36.76 |
One author (TEM) scored each femur using the grading system described below. Specimens were visually examined under a Fisher Hamilton SafeAire Biosafety Cabinet with a recessed double-lamp T8 fluorescent light fixture with a GE cool white 34W bulb without magnification. The scorer was blinded to animal age, sex, and reproductive status. Specimens were read randomly with respect to all variables. Five percent of specimens were scored in duplicate for assessment of intraobserver error. Overall agreement between the first and second observations was 87% (kappa coefficient32 of 0.82 indicating excellent agreement).
Macroscopic grading system
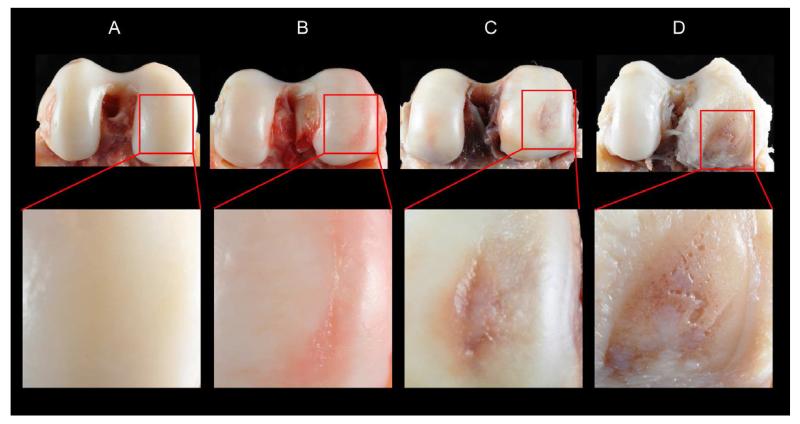
Each femur was assigned an OA severity score of increasing severity from 1 to 4 based on macroscopic inspection of the cartilage of the distal articular surface (Table 3, Fig. 2). Our system compares to a modified Outerbridge33 classification (Table 3) and to the gross pathological OA system for assessing knee OA in rhesus macaques21.
Table 3.
Macroscopic OA severity grading system used in this paper for articular cartilage of baboon knees. The corresponding stages in the modified Outerbridge system33 used for humans are also indicated.
| Grade | Severity | Modified Outerbridge system33 |
Gross observations |
|---|---|---|---|
| 1 | Unaffected | 0 | No signs of any cartilage degradation |
| 2 | Mild OA | 1, 2 | Presence of cartilage fibrillation (i.e., obvious macroscopic alterations of at least the superficial layer of the articular cartilage that manifest as surface roughening or striations), but no cartilage lesions |
| 3 | Moderate OA |
3 | Presence of cartilage degradation to the point of lesion development (i.e., clear indication of erosion of articular cartilage, regardless of lesion size) but no eburnation |
| 4 | Advanced OA |
4 | Presence of any amount of eburnation (i.e., devoid of cartilage, polished subchondral bone surface – indicative of bone-on-bone contact) |
Fig. 2.
Posterior views of distal femora illustrating the four OA severity grades: A, Grade 1 = unaffected; B, Grade 2 = mild OA (cartilage fibrillation); C, Grade 3 = moderate OA (cartilage lesion); D, Grade 4 = advanced OA (eburnation). Higher magnification view of affected area is provided below each specimen. Images differ slightly in scale and orientation to provide the best view of the area of interest.
We also recorded presence/absence of osteophytes because of their importance in radiographic diagnosis of OA in humans34. We documented all osteophytes that occurred anywhere on the distal femora. Typical locations included the patellar groove and margins of the articular surface. We did not include osteophytes in our macroscopic grading system because of their incomplete association with OA in humans35 (and in baboons, see below). We analyzed the correlation between osteophyte occurrence and cartilage degradation to inform the translation of knee OA in this animal model to disease in humans.
Age-matched subset
Because OA is a progressive disease with a strong age dependency, and because the mean age for female baboons in our sample is significantly older than that for males, we confirmed tests for sex effects in a sample subset (N = 306) in which we age-matched females to males (153 of each sex).
Reproductive status in females
It is generally understood that human females are at higher risk of OA during and after peri-menopause (irregular menstrual cycles) and post-menopause (cessation of menstrual cycles)36,37. For this reason we examined reproductive status in our female baboon sample with respect to knee OA occurrence and severity. Reproductive status was based on a review of behavioral data during the last year of the animals’ lives. These data included records of turgescence (the swelling of perineal [sex] skin in response to estrogen), vaginal bleeding, lactation, and pregnancies. Turgescence data were collected regularly throughout life and resulted from visual observations of the perineal skin comprising an index from 0 (minimum turgescence [menstruation]) to 4 (maximum turgescence [ovulation])6. These data were collected by trained animal behavior staff three of every seven days for most animals. Reliable data were available for 287 of 309 females in our sample.
Following Martin et al.38 a female was considered post-menopausal at the time of death if she exhibited no vaginal bleeding and a turgescence index (TI) = 0 for at least one year prior to death. Peri-menopausal females did not reach maximum turgescence (TI = 4) during the 6-month period prior to death, but did have some TI values greater than 0 during this period. A female was also classified as peri-menopausal if she experienced >2 cycles that were abnormal in length relative to her average cycle length for the prior 12 months. Cycles were omitted if affected by pregnancy or lactation. To address the interaction of age and reproductive status we examined a subset of our adult female sample (aged 20 to 25) for which we had representatives across all three reproductive groups (N = 20, 103, 2 for pre-, peri-, and post-menopausal baboons, respectively). This allowed for examination of the relationship between OA severity and reproductive status in a subset of females of comparable age.
Statistical analyses
Statistical analyses were performed using JMP (version 9.0; SAS Institute Inc., 2010). Chi-square analyses were used to test for independence between categorical data (e.g., sex vs. OA severity). Student’s T-tests and ANOVA were used for comparisons of means and ANCOVA were used for comparisons of continuous variables (e.g., age or weight) with categorical data (e.g., stage of OA, sex, reproductive status). A p-value of ≤ 0.05 was required for significance.
Results
Occurrence of OA severity grades by sex, as well as descriptive statistics for age and body weight, are presented in Table 2. Information on occurrence of osteophytes is also presented.
Age effects
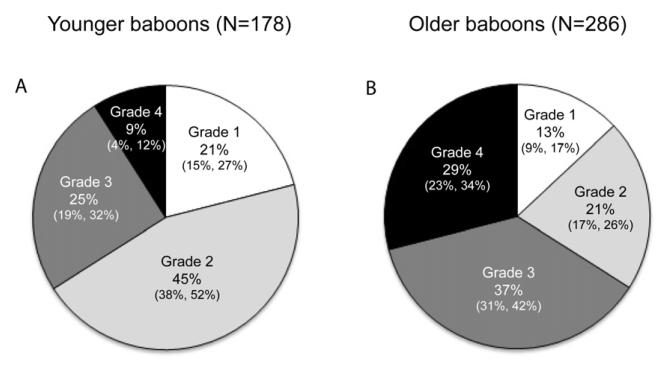
As expected knee OA is more frequent and severe in older animals (those ≥ 17 years – roughly equivalent to 50+ years of age in humans; p < 0.0001). Older baboons show higher prevalence of Grades 3 and 4 OA and lower frequencies of Grades 1 and 2 than do younger baboons (Fig. 3). For both sexes, age is correlated with OA stage (ANCOVAs, p < 0.0001). In addition osteophytes occur in 57% (CI 51%, 63%) of the older animals vs. 30% (CI 24%, 37%) of younger ones (p < 0.0001).
Fig. 3.
Pie charts illustrating differences in relative proportions of OA severity grades in younger vs. older baboons for the entire sample (N = 464). Younger baboons are those < 17 years of age. Older baboons are those ≥ 17 years (~50 years and older in humans). 95% confidence intervals are presented below frequencies using the following format: (lower, upper).
There is considerable age variation within each OA severity grade. Females as young as 6.0 years of age (roughly equivalent to an 18-year-old human) are affected with OA that has progressed to the point of eburnation. On the other hand, females as old as 32.45 years (~97 years in humans) show no evidence of knee OA. Likewise, mild knee OA (Grade 2) is evident in males as young as 5.7 years (~17-year-old human) and eburnation is evident as early as 9.74 years (~29 years in humans). Unaffected males (Grade 1), however, include animals as old as 25.52 years (~77 years in humans).
Body weight and OA severity
Mean weight is significantly higher in males than females (27.62 vs. 17.81 kg, respectively; p < 0.0001), as expected in this sexually dimorphic species. However, there is a sex difference in the relationship between weight and OA severity grade. Mean weight differs significantly among OA severity grades in females (p = 0.0007), showing a positive trend with increasing severity grade (Table 2). There is no such relationship in males (p = 0.6399).
Sex differences in OA progression
OA incidence shows statistically significant sex effects in baboons that may be attributable to quicker progression to advanced stages of the disease in females. An initial comparison between males and females shows that the proportions of animals in each severity grade differs significantly by sex (p < 0.0001) with the most obvious differences being the larger percentage (42%) of males with “mild” (Grade 2) OA vs. females (25%), and the larger percentage of females (25%) with “advanced” (Grade 4) OA vs. males (13%) (Table 2). It is important to note, however, that our sample includes many more old females than old males, as reflected in a significantly (p < 0.0001) older mean age for females (19.5 yrs. vs. 16.0 yrs. in males). As explained above this led us to reexamine observed sex differences in a subset of our sample for which we were able to age-match females to males. In this sample subset, the sex effect remained statistically significant (p = 0.05).
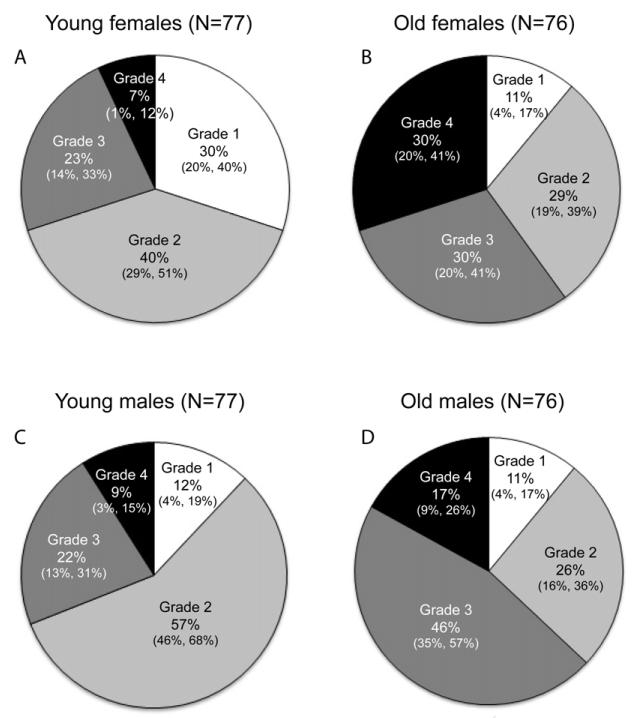
Sex effects by age group
Tests for sex effects within the younger and older age groups of the age matched sample, suggest that males develop knee OA earlier in life, but that females may progress more quickly to advanced stages of the disease (Fig. 4). OA occurrence is higher in young males than in young females (Chi-square: p = 0.0054). The most striking difference in the younger animals is the higher percentage of males (57%) relative to females (40%) that have Grade 2 OA. Older male and female baboons show no difference in knee OA occurrence, but show very different patterns of severity. Affected older females are nearly equally distributed among the three stages of severity, but males show a higher proportion of “moderate” disease (46% vs. 30%) than do females and a lower relative proportion of “advanced” disease (17% vs. 30%).
Fig. 4.
Pie charts illustrating differences in relative proportions of OA severity grades in younger vs. older baboons by sex for the age-matched sample (N = 306). Younger baboons are < 17 years of age. Older baboons are ≥ 17 years (~50 years and older in humans). 95% confidence intervals are presented below frequencies using the following format: (lower, upper).
Reproductive status and OA occurrence
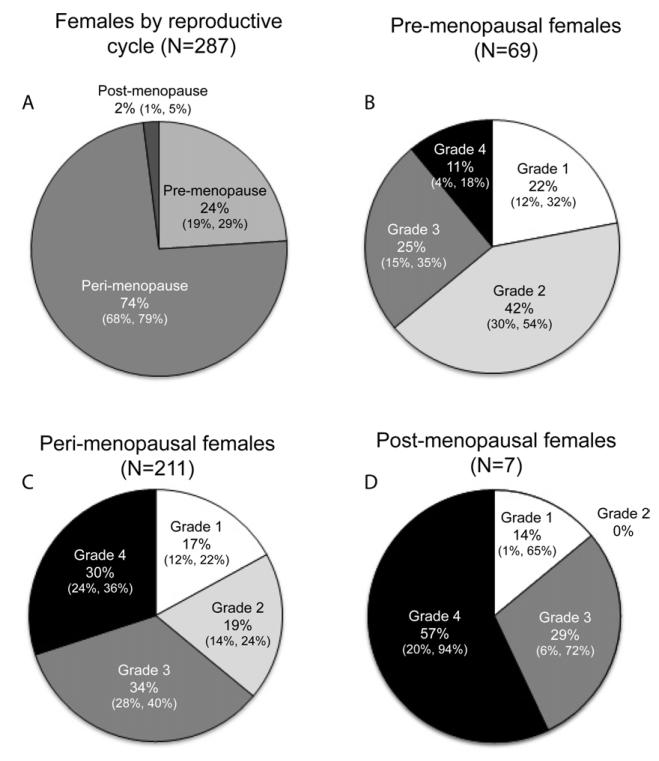
There is a strong relationship between reproductive status and OA severity grade in females. Figure 5 shows the mean ages and proportion of females in our sample in each reproductive status category and, for each category, the proportion of females with each OA severity grade. Disease severity differs significantly by reproductive status (Chi-square: p = 0.0005). The smaller percentage of females in the Grade 2 OA category across the pre-, peri-, to post-menopausal, complemented by the opposite trend in the Grade 4 category illustrates the relationship between reproductive status and OA grade in these baboon females.
Fig. 5.
Pie charts illustrating the relative proportions of A, pre-, peri-, and post-menopausal females; B, pre-menopausal females in each of the OA severity grades (age range: 6.0—26.12 yrs., x̄ = 16.28 yrs.); C, Peri-menopausal females in each of the OA severity grades (age range: 8.53—33.27 yrs., x̄ = 21.26 yrs.); D, Post-menopausal females in each of the OA severity grades (age range: 22.6—29.47 yrs., x̄ = 26.5 yrs. 95% confidence intervals are presented below frequencies using the following format: (lower, upper).
Examination of a subset of females aged 20-25 to address the interaction of age and reproductive status revealed no significant difference in age among the reproductive groups (p = 0.16). In this subset there remained a significant effect of reproductive status on OA severity score among the three groups (p = 0.011), and between the pre- and peri-menopausal groups (p = 0.016) when the menopausal group is omitted from the analysis. The persistence of a significant effect of reproductive status on OA severity grade in this subset suggests that this effect is independent of age.
Osteophytes and knee OA
Osteophytes were present in 47% (CI 42%, 51%) of all animals, and were not reliably associated with OA, particularly in milder disease. Details on osteophyte occurrence by sex and OA severity grade are provided in Tables 2 and 3. Only18% (CI 12%, 25%) of animals with Grade 2 OA had osteophytes, but this proportion is much higher at 53% (CI 45%, 61%) for those with Grade 3 OA. In those with Grade 4 OA, 97% (CI 91%, 99%) had osteophytes. Osteophytes were also evident in 24% (CI 14%, 33%) of individuals that showed no macroscopic evidence of cartilage degradation (Grade 1). A test for correlation between age and the presence of osteophytes in the unaffected animals revealed no relationship (ANOVA: p = 0.5061). Osteophytes were present in 43% (CI 35%, 50%) of males and 49% (CI 43%, 54%) of females, a non-significant difference (Chi-square: p = 0.2005). The strong effect of reproductive status on osteophyte occurrence was also evident (Chi-square: p = 0.0003), with the proportion of those with osteophytes at 33% (CI 22%, 44%) for pre-menopausal females, 55% (CI 48%, 61%) for peri-menopausal females, and 100% for post-menopausal females.
Discussion
Our results show that naturally-occurring knee OA is common in adult baboons, and that rates of occurrence and severity are influenced not only by age, as expected, but also by reproductive status and body weight in females, and by sex with regard to patterns of disease progression. Additionally, our results provide insight into mild OA suggesting that this baboon model could be of particular value in the study of early, non-radiographically detectible knee OA.
Body weight and OA severity
Higher mean weight is evident for increasing grade of OA severity in the baboon females, but this relationship is not evident in males. However, although weight (i.e. obesity) is a known risk factor for OA in humans, nonhuman primates do not show the same range of extreme variation in body weight39 that is apparent in some modern human populations, so may not be expected to exhibit the same relationships between body weight and OA severity.
Reproductive status and knee OA in females
In humans it is widely understood that women are at higher risk of knee OA than men, and that this risk is highest in post-menopausal women36,37. A variety of potentially sex-specific hormonal and environmental factors may explain women’s apparent higher susceptibility to OA. Our results show that female baboons with irregular menstrual cycles (peri-menopausal) and those with no menstrual cycles (post-menopausal) have a higher prevalence of moderate and advanced OA than do the regularly cycling, pre-menopausal baboons. This may point to the importance of reproductive status in OA pathogenesis in primates, including humans. Recent reviews40,41 note that attempts to clarify the roles of estrogen and other hormones in sex-differences in human OA have provided frustratingly inconsistent results, likely because of heterogeneity in study design and amongst study subjects in confounding factors such as activity levels and dietary habits, comorbidities, body composition, and access to healthcare. This large baboon colony provides an opportunity, especially in light of our initial findings, to more thoroughly examine the relationship between variability in hormonal status and OA susceptibility in a primate population in which the environmental heterogeneity inherent in human populations is vastly reduced.
Prevalence of knee OA and sex differences in knee OA progression
As expected, moderate and advanced OA (Grades 3 & 4) were common in older baboons (65%; CI 59%, 71%). These grades presumably represent stages of baboon OA that correspond to radiographically detectable OA in humans – a presumption supported by preliminary comparison between radiographs and macroscopic grades of baboon knees. In humans 59% of Americans that are 65 years of age or older are reported to be affected by radiographically detectable OA36. This proportion is within our 95% CI, and is likely lower than the actual rate of OA among humans because one can expect that not all cases in humans are diagnosed and recorded. Osteoarthritis clearly progresses with age in baboons as in humans but many older baboons remain unaffected or only affected by mild disease showing that OA is not an inevitable consequence of aging in primates. Similarly, more than 30% of humans aged 70-90 years who were tissue donors showed no cartilage degradation or other morphological signs of OA in their knee joints, and only 54% of centenarians showed evidence of hip, knee, shoulder, or spine OA42.
Sex differences in OA prevalence suggest that male baboons begin to show articular cartilage degeneration (Grade 2 OA) earlier in life, but that females progress more rapidly to advanced stages of disease. In our age-matched sample subset, the older age group shows no sex difference in the proportion of unaffected individuals, and little difference in the proportion with mild Grade 2 OA (Fig. 4). Significant differences are evident, however, with regard to moderate and advanced OA. This is in spite of the fact that in the young group, females show a much larger proportion of unaffected animals and a smaller proportion of animals with mild OA than do adult males (Fig. 4). Nonetheless, given that mild OA is more prevalent in the younger adult males than younger females (Fig. 4), one might expect that the more severe stages of the disease would occur in higher proportions in males among the older adults. The opposite, though, is the case, with a higher percentage of females in the most advanced disease stage (Fig. 4), perhaps because of the strong effect of reproductive status discussed above.
Regarding the incidence of mild OA in young males, it is important to note that only 18% of all baboons with this OA Grade 2 have osteophytes and the degeneration at this stage involves slight roughening or fibrillation limited to the most superficial aspect of cartilage. It is reasonable to expect that these changes represent OA that would be “radiographically undetectable” and are very early in the disease progression compared to what is diagnosed in humans in most clinical situations. This could explain why higher prevalence of radiographic knee OA is not known for human males.
Osteophytes and knee OA
Our grading system, like others focused on the macroscopic examination of the articular surface21,33, is based on cartilaginous indications of OA. We do, however, take into consideration bony manifestations of the disease in the form of osteophytes, the role of which in OA pathogenesis is an active area of research1,35.
Approximately 70% of those baboons with cartilage lesions or eburnation on their distal femora also have osteophytes, a frequency that corroborates well with a study on human knees comparing osteophyte frequency based on radiography data and presence of cartilaginous lesions diagnosed from magnetic resonance imaging43. Furthermore, osteophytes are not as frequent in younger baboons as older ones (30% vs. 57%, respectively), suggesting that age is correlated with osteophyte frequency in this species as in humans44 (Table 1).
Radiographic, histological, and painful OA in SNPRC/TBRI baboons
This first systematic characterization and test for sex effects on normal population-level variation on knee OA in a large captive colony of baboons conducted through direct visual examination of articular cartilage is part of a broader research program devoted to the study of pathogenesis of common, complex age-related skeletal disorders. In this initial study we examined the knee because of its clinical relevance to humans. Radiographs and veterinary confirmations of OA in vivo are only opportunistically available for the animals on which this retrospective study was conducted and, unfortunately, we do not have the complete skeleton to examine OA in all joints, but a thorough search of examination records revealed that OA, not surprisingly, does occur in other joints (e.g., hip, shoulder, vertebral column). A number of arthritic conditions of the spine have been noted in these baboons and may be the focus of future investigations13. At present it is unclear if baboons suffer from generalized OA (i.e., disease in three or more joints in the same animal), but this is another area for future research. In addition to macroscopic evidence of knee OA, we have obtained histological sections for a small number of animals revealing that subchondral bone remodeling occurs with advanced macroscopic grades of OA.
Assessment of the degree of pain in OA is challenging in any animal model. Some baboons’ clinical records contain observations of behaviors (e.g., favoring a limb) or rapid weight loss that can indicate pain. Notes made by veterinarians during clinical examinations often indicate symptomatic OA in the form of limited range of motion and crepitation in the baboon’s knees. Palpitation of knee joints was also commonly used to initially evaluate presence of osteophytes during clinical examinations. In presumed severe cases of OA, radiographs were taken during the veterinary examinations, and many of these baboons showed radiographic evidence of knee OA (e.g., presence of osteophytes, subchondral bone erosion). Subsequent necropsies confirmed that these baboons had macroscopic evidence of cartilage lesions or eburnation (indicating Grade 3 & 4 OA) and osteophytes in their right knees.
The baboon and other animal models in OA research
Our results support the potential of baboons in general and the SNPRC/TBRI colony in particular to address an ongoing critical need for appropriate animal models for human OA. Because this colony was established to support the study of common complex disease genetics, its size, genetic make-up, age distribution, and thorough genetic characterization enable studies of contributors to population-level normal variation in OA susceptibility at a scale that is not yet possible in other non-human primate models.
Our efforts have produced a unique skeletal resource consisting of an age-continuous series of knees, thereby allowing for examination of all disease stages including unaffected and very early stage knees that are both critical to advancing our understanding of idiopathic knee OA etiology, and logistically and ethically impossible to collect routinely and reliably in humans. The standardized documentation of a range of OA conditions in an animal model that closely resembles the human disease without a chemical or surgical perturbation has tremendous potential to significantly advance research aimed at determining causes of idiopathic OA, and identifying mechanisms on which to base prevention and therapeutic strategies.
No one animal model is adequate for modeling all aspects (e.g., structural changes, pain, symptomatic conditions) and sub-types of human OA, so examination of the disease in multiple species is most appropriate45. Many other animal models (e.g., dogs, rats, sheep, rabbits), are widely available and valuable for generating and testing many OA-related hypotheses, but do not allow for investigations of population-level variability in idiopathic OA susceptibility and progression such as that presented here. Indeed our ongoing studies indicate that in spite of obvious differences in habitual locomotion between baboons and humans, cartilaginous manifestations of knee OA follow a similar topographic pattern of occurrence and progression between the two species (unpublished data). Many other models rely on disease development via surgical or chemical induction2,46. Although others (e.g., guinea pigs, some strains of mice, Syrian hamsters) do spontaneously develop OA46, those are distantly-related species that do not replicate the disease process in humans as faithfully as do these closely related primates.
Acknowledgments
Many thanks go to Jennifer Harris, Courtney Roush, and Elizabeth Dick (all from Texas Biomedical Research Institute [TBRI]) for help with specimen acquisition and processing. We thank Dr. Mark Sharp and Linda Freeman-Shade (both from TBRI) for assistance in extracting and organizing the turgescence and clinical data from the CAMP (Computerized Animal Management Program) database at TBRI. Dr. Cassie Bauer (TBRI) was very helpful with information about clinical and radiographic diagnosis of OA in baboons. Kristyn Mathewson and Noel Shaheen (both from St. Mary’s University) aided in data collection. The authors also thank Rob Healey (University of California at San Diego) for his technical assistance in characterizing OA in the baboon knee.
Role of the funding source
An NIH MARC U*STAR grant (5T34GM008073-27) to St. Mary’s University funded the participation of Daniel J. Araujo and Tanya Lerma in this research. Cristine D. Saks was funded by a Biaggini Research Fellowship (St. Mary’s University, Department of Biological Sciences) and a Summer Undergraduate Research Fellowship (Undergraduate Research Office, St. Mary’s University). Heather B. Coan, Lorena M. Havill, and Shayna M. Levine were supported by a grant from the Society for Women’s Health Research Isis Fund Network on Sex Differences in Musculoskeletal Health. Coan and Havill were also supported by a grant from the Max and Minnie Tomerlin Voelcker Fund. This investigation used resources which were supported by the SNPRC grant P51 RR013986 from the NCRR of the NIH and which are currently supported by the Office of Research Infrastructure Programs through P51 OD013986. This investigation was conducted in facilities constructed with support from Research Facilities Improvement Program Grant Numbers 1 C06 RR014578, 1 C06 RR013556, 1 C06 RR015456, and 1 C06 RR017515 from the National Center for Research Resources, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
Lorena M. Havill (LMH), Richard D. Coutts (RDC), Daniel P. Nicolella (DPN), Todd L. Bredbenner (TLB), Thomas E. Macrini (TEM), and Cristine D. Saks (CDS) acquired funding for this research. LMH, Heather B. Coan (HBC), TEM, Daniel J. Araujo (DJA), Shayna M. Levine (SML), RDC, and DPN contributed to the conception and design of this study. DJA, Tanya Lerma (TL), CDS, SML and TEM acquired data. All authors contributed to the analysis and interpretation of these data. TEM, HBC, and LMH drafted the manuscript. All authors reviewed, revised, and provided final approval of this manuscript prior to submission.
Conflict of interest
Richard D. Coutts has consulting arrangements with Exactech, Carticept, and Synthasome regarding cartilage repair. He is also on the scientific advisory boards for Tigenix, Exactech, and Carticept regarding cartilage repair. The other authors have no conflicts of interest to disclose in relation to this manuscript.
References
- 1.Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis and Rheumatism. 2012;64:1697–707. doi: 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldring MB, Goldring SR. Osteoarthritis. Journal of Cellular Physiology. 2007;213:626–34. doi: 10.1002/jcp.21258. [DOI] [PubMed] [Google Scholar]
- 3.Hunter DJ. Risk stratification for knee osteoarthritis progression: a narrative review. Osteoarthritis and Cartilage. 2009;17:1402–7. doi: 10.1016/j.joca.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 4.Blagojevic M, Jinks C, Jeffery A, Jordan KP. Risk factors for onset of osteoarthritis of the knee in older adults: a systematic review and meta-analysis. Osteoarthritis and Cartilage. 2010;18:24–33. doi: 10.1016/j.joca.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Meulenbelt I. Osteoarthritis year 2011 in review: genetics. Osteoarthritis and Cartilage. 2012;20:218–22. doi: 10.1016/j.joca.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Hochberg MC, Yerges-Armstrong L, Mitchell BD. Osteoarthritis susceptibility genes continue trickling in. Lancet. 2012 doi: 10.1016/S0140-6736(12)60818-6. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conroy MB, Kwoh CK, Krishnan E, Nevitt MC, Boudreau R, Carbone LD, et al. Muscle strength, mass, and quality in older men and women with knee osteoarthritis. Arthritis Care and Research. 2012;64:15–21. doi: 10.1002/acr.20588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shively CA, Clarkson TB. The unique value of primate models in translational research. American Journal of Primatology. 2009;71:715–21. doi: 10.1002/ajp.20720. [DOI] [PubMed] [Google Scholar]
- 9.DeRousseau CJ, Rawlins RG, Denlinger JL. Aging in the musculoskeletal system of rhesus monkeys: I. Passive joint excursion. American Journal of Physical Anthropology. 1983;61:483–94. doi: 10.1002/ajpa.1330610411. [DOI] [PubMed] [Google Scholar]
- 10.DeRousseau CJ. Aging in the musculoskeletal system of rhesus monkeys: II. Degenerative joint disease. American Journal of Physical Anthropology. 1985;67:177–84. doi: 10.1002/ajpa.1330670303. [DOI] [PubMed] [Google Scholar]
- 11.Carlson CS, Loeser RF, Purser CB, Gardin JF, Jerome CP. Osteoarthritis in cynomolgus macaques III: effects of age, gender, and subchondral bone thickness on the severity of disease. Journal of Bone and Mineral Research. 1996;11:1209–17. doi: 10.1002/jbmr.5650110904. [DOI] [PubMed] [Google Scholar]
- 12.Balena R, Toolan BC, Shea M, Markatos A, Myers ER, Lee SC, et al. The effects of 2-year treatment with the aminobisphosphonate alendronate on bone metabolism, bone histomorphometry, and bone strength in ovariectomized nonhuman primates. Journal of Clinical Investigation. 1993;92:2577–86. doi: 10.1172/JCI116872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Havill LM, Mahaney MC, Czerwinski SA, Carey KD, Rice K, Rogers J. Bone mineral density reference standards in adult baboons (Papio hamadryas) by sex and age. Bone. 2003;33:877–88. doi: 10.1016/s8756-3282(03)00231-x. [DOI] [PubMed] [Google Scholar]
- 14.Havill LM, Levine SM, Newman DE, Mahaney MC. Osteopenia and osteoporosis in adult baboons (Papio hamadryas) Journal of Medical Primatology. 2008;37:146–53. doi: 10.1111/j.1600-0684.2007.00270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kessler MJ, Turnquist JE, Pritzker KPH, London WT. Reduction of passive extension and radiographic evidence of degenerative knee joint diseases in cage-raised and free-ranging aged rhesus monkeys (Macaca mulatta) Journal of Medical Primatology. 1986;15:1–9. [PubMed] [Google Scholar]
- 16.Châteauvert JMD, Pritzker KPH, Kessler MJ, Grynpas MD. Spontaneous osteoarthritis in rhesus macaques. I. Chemical and biochemical studies. Journal of Rheumatology. 1989;16:1098–104. [PubMed] [Google Scholar]
- 17.Châteauvert JMD, Grynpas MD, Kessler MJ, Pritzker KPH. Spontaneous osteoarthritis in rhesus macaques. II. Characterization of disease and morphometric studies. Journal of Rheumatology. 1990;17:73–83. [PubMed] [Google Scholar]
- 18.Pritzker KPH, Châteauvert J, Grynpas MD, Renlund RC, Turnquist J, Kessler MJ. Rhesus macaques as an experimental model for degenerative arthritis. Puerto Rico Health Sciences Journal. 1989;8:99–102. [PubMed] [Google Scholar]
- 19.Carlson CS, Loeser RF, Jayo MJ, Weaver DS, Adams MR, Jerome CP. Osteoarthritis in cynomolgus macaques: a primate model of naturally occurring disease. Journal of Orthopaedic Research. 1994;12:331–9. doi: 10.1002/jor.1100120305. [DOI] [PubMed] [Google Scholar]
- 20.Carlson CS, Loeser RF, Johnstone B, Tulli HM, Dobson DB, Caterson B. Osteoarthritis in cynomolgus macaques II. Detection of modulated proteoglycan epitopes in cartilage and synovial fluid. Journal of Orthopaedic Research. 1995;13:399–409. doi: 10.1002/jor.1100130314. [DOI] [PubMed] [Google Scholar]
- 21.Gahunia HK, Babyn P, Lemaire C, Kessler MJ, Pritzker KPH. Osteoarthritis staging: comparison between magnetic resonance imaging, gross pathology and histopathology in the rhesus macaque. Osteoarthritis and Cartilage. 1995;3:169–80. doi: 10.1016/s1063-4584(05)80051-2. [DOI] [PubMed] [Google Scholar]
- 22.Lim KKT, Kessler MJ, Pritzker KPH, Turnquist JE, Dieppe PA. Osteoarthritis of the hand in nonhuman primates: a clinical, radiographic, and skeletal survey of Cayo Santiago rhesus macaques. Journal of Medical Primatology. 1996;25:301–8. doi: 10.1111/j.1600-0684.1996.tb00214.x. [DOI] [PubMed] [Google Scholar]
- 23.Miller LM, Novatt JT, Hamerman D, Carlson CS. Alterations in mineral composition observed in osteoarthritic joints of cynomolgus monkeys. Bone. 2004;35:498–506. doi: 10.1016/j.bone.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 24.Duncan AE, Colman RJ, Kramer PA. Longitudinal study of radiographic spinal osteoarthritis in a macaque model. Journal of Orthopaedic Research. 2011;29:1152–1160. doi: 10.1002/jor.21390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hunneyball IM, Harrison GBL, Stanworth DR. The production of experimental arthritis in baboons. IRCS Journal of Medical Science. 1979;7:517. [Google Scholar]
- 26.Gardner DL, Skelton-Stroud PN, Fitzmaurice RJ. Akute Muramyl-Dipeptid-induzierte Arthritis beim Pavian Papio cynocephalus. Zeitschrift fur Rheumatologie. 1991;50:86–92. [PubMed] [Google Scholar]
- 27.Athanasiou KA, Agarwal A, Muffoletto A, Dzida FJ, Constantinides G, Clem M. Biomechanical properties of hip cartilage in experimental animal models. Clinical Orthopaedics and Related Research. 1995;316:254–66. [PubMed] [Google Scholar]
- 28.Malinin T, Ouellette EA. Articular cartilage nutrition is mediated by subchondral bone: a long-term autograph study in baboons. Osteoarthritis and Cartilage. 2000;8:483–91. doi: 10.1053/joca.1999.0324. [DOI] [PubMed] [Google Scholar]
- 29.Rose MD. Postural behaviour of olive baboons (Papio anubis) and its relationship to maintenance and social activities. Primates. 1977;18:59–116. [Google Scholar]
- 30.Brent L. The study of captive baboon behavior. In: VandeBerg JL, Williams-Blangero S, Tardif SD, editors. The Baboon in Biomedical Research. Springer; New York: 2009. pp. 21–34. [Google Scholar]
- 31.VandeBerg JL. Introduction. In: VandeBerg JL, Williams-Blangero S, Tardif SD, editors. The Baboon in Biomedical Research. Springer; New York: 2009. pp. xvii–xxiii. [Google Scholar]
- 32.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74. [PubMed] [Google Scholar]
- 33.Roach HI, Tilley S. The pathogenesis of osteoarthritis. In: Bronner F, Farach-Carson MC, editors. Bone and Osteoarthritis. Springer; London: 2007. pp. 1–18. [Google Scholar]
- 34.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Annals of the Rheumatic Diseases. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van der Kraan PM, van den Berg WB. Osteophytes: relevance and biology. Osteoarthritis and Cartilage. 2007;15:237–44. doi: 10.1016/j.joca.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 36.O’Connor MI. Osteoarthritis of the hip and knee: sex and gender differences. Orthopedic Clinics of North America. 2006;37:559–68. doi: 10.1016/j.ocl.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 37.Srikanth VK, Fryer JL, Zhai G, Winzenberg TM, Hosmer D, Jones G. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthritis and Cartilage. 2005;13:769–81. doi: 10.1016/j.joca.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 38.Martin LJ, Carey KD, Comuzzie AG. Variation in menstrual cycle length and cessation of menstruation in captive baboons. Mechanisms of Ageing and Development. 2003;124:865–71. doi: 10.1016/s0047-6374(03)00134-9. [DOI] [PubMed] [Google Scholar]
- 39.Leigh SR. Growth and development of baboons. In: VandeBerg JL, Williams-Blangero S, Tardif SD, editors. The Baboon in Biomedical Research. Springer; New York: 2009. pp. 57–88. [Google Scholar]
- 40.Maleki-Fischbach M, Jordan JM. Sex differences in magnetic resonance imaging-based biomarkers and in those of joint metabolism. Arthritis Research & Therapy. 2010;12:212–9. doi: 10.1186/ar3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stevens-Lapsley JE, Kohrt WM. Osteoarthritis in women: effects of estrogen, obesity and physical activity. Women’s Health. 2010;6:601–15. doi: 10.2217/whe.10.38. [DOI] [PubMed] [Google Scholar]
- 42.Loeser RF, Shakoor N. Aging or osteoarthritis: which is the problem? Rheumatic Diseases Clinics of North America. 2003;29:653–73. doi: 10.1016/s0889-857x(03)00062-0. [DOI] [PubMed] [Google Scholar]
- 43.Boegård T, Rudling O, Petersson IF, Jonsson K. Correlation between radiographically diagnosed osteophytes and magnetic resonance detected cartilage defects in the tibiofemoral joint. Annals of the Rheumatic Diseases. 1998;57:401–7. doi: 10.1136/ard.57.7.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hernborg J, Nilsson BE. The relationship between osteophytes in the knee joint, osteoarthritis and aging. Acta Orthopaedica Scandinavica. 1973;44:69–74. doi: 10.3109/17453677308988675. [DOI] [PubMed] [Google Scholar]
- 45.Little CB, Zaki S. What constitutes an “animal model of osteoarthritis” - the need for consensus? Osteoarthritis and Cartilage. 2012;20:261–7. doi: 10.1016/j.joca.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 46.Bendele AM. Animal models of osteoarthritis. Journal of Musculoskeletal and Neuronal Interactions. 2001;1:363–76. [PubMed] [Google Scholar]
- 47.Pritzker KPH, Gay S, Jimenez SA, Ostergaard K, Pelletier JP, Revell PA, et al. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis and Cartilage. 2006;14:13–29. doi: 10.1016/j.joca.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 48.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis and Rheumatism. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Colman RJ, Kemnitz JW, Lane MA, Abbott DH, Binkley N. Skeletal effects of aging and menopausal status in female rhesus macaques. Journal of Clinical Endocrinology and Metabolism. 1999;84:4144–8. doi: 10.1210/jcem.84.11.6151. [DOI] [PubMed] [Google Scholar]
- 50.Ham KD, Loeser RF, Lindgren BR, Carlson CS. Effects of long-term estrogen replacement therapy on osteoarthritis severity in cynomolgus monkeys. Arthritis and Rheumatism. 2002;46:1956–64. doi: 10.1002/art.10406. [DOI] [PubMed] [Google Scholar]