Abstract
Introduction
Gene delivery from hydrogel biomaterials provides a fundamental tool for a variety of clinical applications including regenerative medicine, gene therapy for inherited disorders and drug delivery. The high water content and mild gelation conditions of hydrogels support their use for gene delivery by preserving activity of lentiviral vectors and acting to shield vectors from any host immune response.
Areas Covered
Strategies to control lentiviral entrapment within and retention/release from hydrogels are reviewed. We discuss the ability of hydrogel design parameters to control the transgene expression profile and the capacity of hydrogels to protect vectors from (and even modulate) the host immune response.
Expert Opinion
Delivery of genetic vectors from scaffolds provides a unique opportunity to capitalize on the potential synergy between the biomaterial design for cell processes and gene delivery. Hydrogel properties can be tuned to directly control the events that determine the tissue response to controlled gene delivery, which include the extent of cell infiltration, preservation of vector activity and vector retention. While some design parameters have been identified, numerous opportunities for investigation are available in order to develop a complete model relating the biomaterial properties and host response to gene delivery.
Keywords: gene therapy, hydrogels, lentivirus, tissue engineering
1. Introduction
Delivery of genetic vectors represents a promising approach to treat a wide range of diseases and disorders. For example, gene therapies for Parkinson’s disease, hemophilia B, muscular dystrophy, and 1-antitrypsin deficiency are in phase I and phase II clinical trials [1–4]. In the preclinical settings, gene delivery has shown promise for regenerative medicine applications where induced expression of a transgene has been employed to promote the formation of tissues, such as bone [5,6], spinal cord [7–12] or the eye [13]. Gene delivery can provide either short-term or long-term expression of the transgene at specific sites, which can have either local or systemic effects. Notably, gene therapy allows for changes to the gene sequence or delivery of multiple genes without redesigning the delivery system. From a research perspective, this flexibility makes biomaterial-mediated gene delivery a versatile tool with which to identify the appropriate factor or combination of factors that yield the most therapeutic benefit. Efficient delivery systems has proved challenging for many applications, with virus activity and dose and the host immune response contributing to limited gene expression and threatening patient safety. The majority of strategies to improve gene delivery have focused on vector optimization [14]. Alternatively, biomaterial platforms provide an opportunity to improve gene transfer by enhancing vector stability and shielding the immune system, while promoting and/or controlling cell-vector interactions in order to modulate the location and duration of transgene expression.
Viral vectors have demonstrated better clinical potential because of their ability to more efficiently transfer therapeutic genes and achieve long-term transgene expression in vivo compared to their non-vial counterparts [15–19]. Non-viral approaches include direct delivery of naked plasmids or oligonucleotides, and the complexation of these nucleic acids with cationic polymers or lipids. While complexation can enhance delivery efficiency, maintenance of vector stability of the complexes and establishment of long-term transgene expression represent significant challenges [17–19]. Viral vectors are derived from viral pathogens, in which the harmful sequences have been removed and therapeutic sequences have been inserted [14,19,20]. Several viral vectors are currently being tested in clinical trials for various therapies, including retroviruses, lentiviruses, adenoviruses, and adeno-associated viruses [1–4,14,21–26]. Improved safety and efficacy of lentiviral vectors in recent years has greatly enhanced their feasibility for clinical use over other viral vectors [14, 19]. Lentiviral vectors infect both dividing and non-dividing cell populations, integrate the delivered gene into host chromosomes to enable long-term expression, and are relatively easy to produce [14,16,18,19]. For an extensive review of recent advances and translational potential of lentiviral vectors, please refer to Sakuma, et al. 2012 [14]. Their efficiency and considerable clinical potential has motivated their delivery from biomaterials; however, many reports are descriptive with regards to biomaterial design. We have supplemented our discussion of biomaterial design with reports that employed alternative vectors, with the objective of highlighting design parameters that should be considered for lentiviral vectors.
Compared to other biomaterial delivery systems (e.g., microporous scaffolds based on polylactide-co-glycolide (PLG) or ceramic materials), hydrogels provide a hydrated, tissue-like environment and typically mild, aqueous fabrication conditions that enable encapsulation of active vectors. While this review focuses on hydrogel-based delivery systems, other categories of materials have been reviewed for their potential to deliver viral [19] and non-viral [15,16] vectors. Hydrogels are formed by the crosslinking or self-assembly of hydrophilic polymers, which can be formed from naturally occurring (e.g., fibrin, chitosan and hyaluronan) or synthetic (e.g., polyethylene glycol (PEG) and polyvinyl alcohol) materials. Furthermore, hydrogels can be customized for many applications. For example, they can be designed to be injectable or environmentally responsive, to encourage infiltration of specific cell types and to acquire various geometries. Control over delivery of genetic vectors can be achieved by altering physical properties of the hydrogel carrier, such as pore size and degradation kinetics. Importantly, transgene expression can be designed to enhance or synergize with the intrinsic bioactivity of the scaffold and thereby create an environment that promotes tissue formation for regenerative medicine (Figure 1). For instance, the biomaterial provides a support for cell adhesion and an architecture that can serve to organize cells while transgene expression can target cellular processes (e.g., proliferation, differentiation) that complement these structural functions. Interactions between gene therapy vectors and biomaterial scaffolds can be tuned to modulate the release rate of vector, target specific internalization pathways, and potentially enhance intracellular trafficking [16,19,27]. As biomaterial delivery of lentiviral vectors is an emerging technology, most publications have been descriptive (i.e., demonstrating feasibility) or have investigated design of lentiviral vectors to modulate the host response. The following sections describe the established design parameters for gene delivery from hydrogels, and will focus on the emerging literature describing delivery of lentiviral vectors (Table 1).
Figure 1.
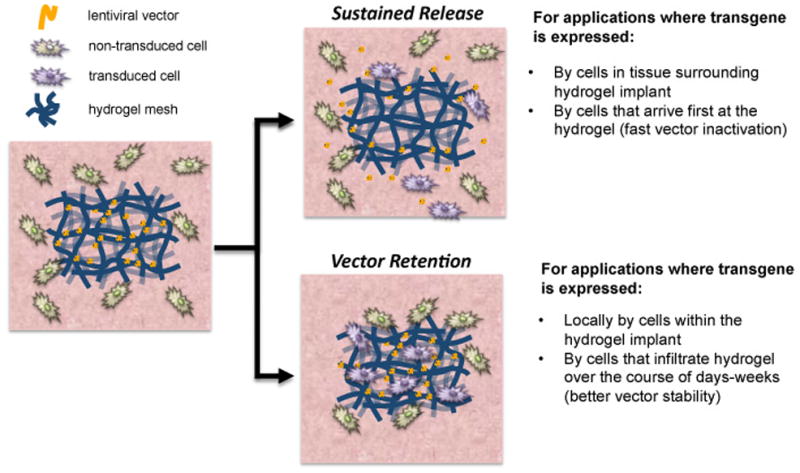
Gene delivery strategies using hydrogels. The hydrogel design parameters for delivery of gene therapy vectors can be modulated to achieve different transgene expression profiles. Hydrogels can be designed to enable sustained release of vectors to target the cells surrounding the hydrogel. Alternatively, hydrogels can be designed to retain vectors within the scaffold to target infiltrating cells and better preserve vector activity.
Table 1.
Hydrogels for Lentiviral Delivery
| Hydrogel Material | Strategy for Enhanced Transduction | Application | Reference |
|---|---|---|---|
| Collagen | Hydroxyapatite nanoparticles (non-specific vector retention) | Subcutaneous implant | [37] |
| Collagen | Encapsulation only | In vitro cell culture | [48] |
| Fibrin | Encapsulation only | In vitro cell microarrays | [35,70] |
| Fibrin | Hydroxyapatite nanoparticles (non-specific vector retention) | Subcutaneous implant | [39] |
| Fibrin | Fusion peptide/VSV-G envelope (conjugation to vector) | In vitro cell microarrays | [45] |
| Chitosan/β-Glycerol | Positive charge on chitosan (non-specific vector retention) | In vitro cell culture | [46] |
| Phosphate blend | |||
| PEG | Encapsulation only | 3D stem cell culture | [44] |
| PEG/Gelatin | Macropores (increased cell infiltration) | Subcutaneous gene transfer | [40] |
2. Lentiviral Vectors for Gene Therapy
Viral vectors available for gene therapy can be categorized as integrating or non-integrating. Integrating vectors such as adeno-associated virus (AAV) and retrovirus (including lentivirus) have the ability to insert their viral genome into the chromosomal DNA of host cells. Nonintegrating vectors such as adenovirus and herpes simplex virus type 1 (HSV-1) deliver their genomes into the nucleus of the host cell, but remain episomal. Because integrating vectors get inserted into the host genome, their expression can be long-term and potentially lifelong, whereas non-integrating vectors tend to have short-term expression [19, 28], though there are examples of expression that persists for years (e.g., AAV) [29]. The duration of expression is critical to the choice of vector, as applications such as wound healing may require transient expression, whereas hemophilia, sickle cell anemia, and other genetic disorders require life-long genetic modification.
Lentiviruses are a subset of retrovirus that have gained popularity due to their ability to infect both nondividing and dividing cells, broad tropism, integration into the host genome which enables long-term availability of the encoded therapeutic protein and relative ease of production and the availability of large libraries of constructs [14,19]. Retroviruses are enveloped viruses containing single-stranded RNA that replicate by reverse transcription of the viral RNA into linear double-stranded DNA and subsequent integration into the genome of the host cell [30]. However, for use in gene therapy applications, retroviruses must be engineered to be replication defective otherwise they will produce and release virions leading to pathogenic effects. For example, lentiviral vectors were developed based on the human immunodeficiency virus type 1 (HIV-1), but have been engineered to enhance patient safety [14,20]. Specifically, the genes required for virus replication, packaging, and export from an infected cell have been removed from the viral genome. Thus transient transfection systems employing packaging cell lines in vitro are employed to produce replication defective viruses [20,31].
In brief, the packaging cells are transfected with a vector expression plasmid that encodes for the transgene along with packaging constructs, which are plasmids that supply the packaging cells with the genes needed to produce and export the viral particles (e.g., reverse transcriptase, envelope proteins). To further increase patient safety, packaging constructs have been optimized to decrease risk of homologous recombination, which could lead to a replication competent virus. An attractive feature of this production scheme is the envelope proteins can be changed by switching out the plasmid that encodes for these proteins without altering the other packaging constructs, a process called pseudotyping. Since the envelope proteins dictate cell association and internalization that influences the target cell types, changing the envelope plasmid may allow for the targeting of specific cell types (tropism) [32]. While investigation into new viral envelopes is ongoing, most lentiviral vectors are pseudotyped with the vesicular stomatitis virus glycoprotein (VSV-G) due to its broad tropism [20,33].
3. Encapsulation of Lentiviral Particles within Hydrogels
The retention of vectors within hydrogels can facilitate gene transfer to cells infiltrating the scaffold, as opposed to released vector that may target cells adjacent to the scaffold (Figure 1). The method of scaffold fabrication and the material properties influence the extent to which vectors are retained. Vector release from hydrogels typically occurs by diffusion, with the rate of release influenced by scaffold design parameters such as porosity, tortuosity, hydrophobicity or hydrophilicity, rate and mechanism of degradation and interactions between the vector and the material. In addition to the degree of virus retention, the ability of cells to infiltrate hydrogels is a key determinant of transgene expression. Natural materials can be advantageous because of biocompatibility and their inherent biological interactions with cells that effect cell attachment, migration and differentiation. Alternatively, synthetic polymers offer the potential to create hydrogels with more precise control over the physical properties (e.g., mechanics, degradation) that directly affect both tissue formation and gene delivery. Hydrogel platforms for gene therapy have the potential for much improvement over other strategies and, relative to bolus injection, can enhance the extent and duration of transgene expression at an implantation site [34]. The overall efficiency of delivery is the sum of effects of the hydrogel on host response (e.g., cell infiltration) and the vector activity/accessibility (e.g., release rate), which combine to determine the overall efficiency of gene delivery. The entrapment of vectors within hydrogels is typically employed to provide a sustained release that will maintain elevated concentrations of the vectors locally to increase the opportunity for cellular internalization.
Both natural and synthetic hydrogels have been used to entrap gene therapy vectors as these hydrogels form under mild conditions that do not significantly impact vector activity (Table 1) [34–40]. The initial factor impacting retention versus release from the hydrogels is the mesh size. Hydrogels with a mesh size larger than the vector diameter (50–100 nm) typically exhibit a rapid release governed by diffusion, while those with a mesh size less than the vector diameter exhibit release rates that are highly dependent on the degradation rate of the hydrogel. The mesh size of both natural a synthetic hydrogels can be varied over a wide range (<50 nm to over 1500 nm) [41–43] and macropores (>10 μm) can be added to further increase mass transfer and cell infiltration [40]. Notably, imperfections in the hydrogel networks may enable diffusion of lentiviral vectors even in hydrogels with a theoretical mesh size smaller than the vector radius [44,45]. Environmentally responsive hydrogels (e.g., temperature or pH sensitive hydrogels), in which pore size changes in response to stimuli, have been used to achieve even greater dynamic control over vector release [46].
A second factor influencing release versus retention are interactions between vectors and hydrogels, which can prevent vector diffusion away from the hydrogel thereby increasing the local vector concentration that facilitates more effective gene transfer [38,39,45,47]. Additionally, the association of a vector with a biomaterial substrate that supports cell adhesion can co-localize virus and cells to enhance delivery. Either vectors or the hydrogel backbone can be modified prior to encapsulation to mediate non-specific or specific interactions. Hydrogels made from cationized gelatin, PEG modified with cationic peptides and positively charged chitosan have all been used to increase the non-specific retention of gene therapy vectors [10,38,46,48,49]. Alternatively, the specific interactions between avidin and biotin [50] or antibodies and vectors [51] have been exploited to provide vector-material interactions to promote vector retention. More recently, the lentiviral capsid proteins have been engineered to covalently bind fibrin during polymerization, which can release the vector as the hydrogel degrades [45]. Various strategies found in the literature to enhance the retention of lentiviral vectors encapsulated in hydrogels are summarized in Table 1. Importantly, the strength of interactions between scaffolds and vectors must be optimized so that vectors are adequately retained, but not so strong as to prevent vector dissociation, which is required for the vector to interact with the nearby cells and be internalized [38]. Similarly, the diffusion rate must be balanced to maintain spatial localization of gene transfer (Figure 1). If diffusion is too rapid, lentivirus may travel to a different area of tissue in which transgene expression is not desired or where target cells are inaccessible [35,45]. The addition of interconnected macropores into the hydrogels can further increase the probability that infiltrating cells will internalize vectors and thereby improve transgene expression (Figure 2) [40]. In addition, cells encapsulated within 3D hydrogels in the presence of adenoviral vectors expressed significantly higher levels of delivered transgene than those transduced in traditional cultures on polystyrene [44,47]. These results, which would likely translate to lentiviral transduction, suggest that the 3D geometry directly enhances transduction by ensuring a higher degree of co-localization entrapped cells.
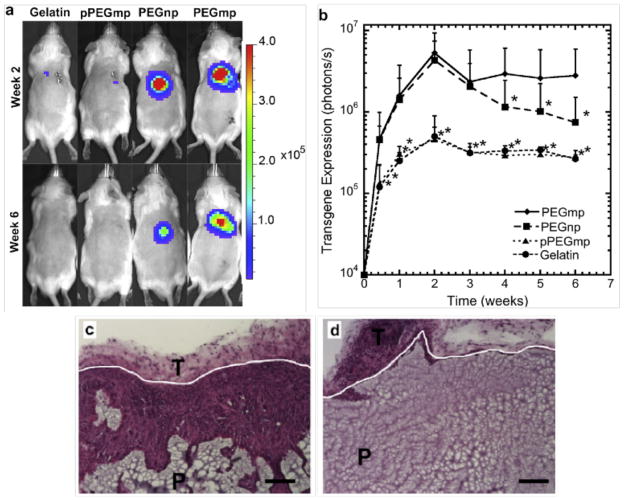
Figure 2.
In vivo transgene expression from lentivirus loaded hydrogels with and without macroporous structure. (a) Representative bioluminescence images following subcutaneous implantation of hydrogels. Three virus loading configurations into the PEG hydrogels are shown: (i) non-macroporous PEG (no gelatin) (PEGnp) hydrated with virus solution following gelation, (ii) PEG encapsulating gelatin microspheres (PEGmp) hydrated with virus solution following gelation, and (iii) PEG encapsulating gelatin microspheres that have been loaded with virus prior to gelation (pPEGmp) by swelling gelatin microspheres with virus solution. A gelatin only control is also shown. (b) Transgene expression was measured as integrated photon flux (photons/s) using an IVIS bioluminescence imaging system (b). Asterisks represent statistical difference (p < 0.05) relative to the PEGmp condition at each time point based on a KruskaleWallis test. (c), (d) Hematoxylin and eosin staining showing cell infiltration into PEGmp (c) and PEGnp (d) hydrogels 6 wks after subcutaneous implantation. The labels P and T denote PEG and tissue surrounding the implant, respectively. White lines denote hydrogel-tissue interface. Scale bars = 100 μm. Reprinted from Biomaterials, 33, Shepard JA, Virani FR, Goodman AG, Gossett TD, Shin S, Shea LD, Hydrogel macroporosity and the prolongation of transgene expression and the enhancement of angiogenesis, 7412–7421, 2012, with permission from Elsevier.
3.1 Natural Hydrogels
Hydrogels based on natural polymers readily support cell adhesion and migration and can be used to entrap lentiviral vectors. In fibrin hydrogels, low to medium fibrin concentrations, which partially determines mesh size, have been reported to allow for maximal levels of infection by encapsulated lentivirus in vivo and in vitro [34,35,39,52]. At higher fibrin concentrations, both vector diffusion and cell infiltration were relatively inefficient. Compared to collagen and alginate hydrogels, fibrin supports the highest levels of transgene expression for at least one month after intraperitoneal implantation (Figure 3) [34]. Fibrin contains multiple sites for cell adhesion and rapid cell infiltration was observed in vivo. Although this study reported that collagen supported similar levels of cell infiltration as fibrin in vivo, transgene expression was significantly reduced and instead resembled that of non-cell adhesive alginate [34]. This result is likely due to a faster degradation of fibrin, compared to collagen, in this implantation site. Taken together, these studies demonstrate that vector-material interactions may contribute to gene transfer beyond those that determine cell infiltration. These interactions may increase lentiviral stability or directly facilitate lentiviral infection [10,34,37,39].
Figure 3.
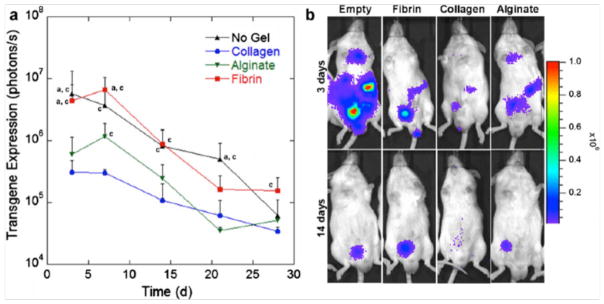
In vivo transgene expression from hydrogel-filled polylactide-glycolide scaffolds as a function of time. (a) Hydrogel (collagen, fibrin, or alginate) precursors were mixed with a lentivirus encoding for the reporter gene luciferase, loaded into the pores of a PLG scaffold, and implanted in the fat pad of mice. Transgene expression was then measured using an in vivo bioluminesence imaging. Letters indicate statistically significant differences (p < 0.05) compared to (a) alginate or (c) collagen analyzed by a Kruskal–Wallis test (n= 3). (b) Representative bioluminescence images from scaffolds at 3 and 14 days post-implantation. Reprinted with permission from Springer Science+Business Media: Drug Del Transl Res, Hydrogels to modulate lentivirus delivery in vivo from microporous tissue engineering scaffolds, 1, 2011, 91–101, Aviles MO, Shea LD, Figure 4.
3.2 Synthetic Hydrogels
Although these studies demonstrate the potential utility of natural hydrogels for localized gene delivery, the mode and dynamics of cell infiltration and hydrogel degradation can be more tightly controlled in hydrogels based on synthetic materials, such as PEG. For example, PEG hydrogels can be crosslinked via protease-sensitive peptides [43]. As cells migrate through the hydrogels, these crosslinks are locally degraded to allow for simultaneous cell infiltration and vector release. The use of synthetic hydrogels also allows for the addition of specific and protease-susceptible peptides that can be chosen to preferentially encourage the infection of specific cell types [36]. To enable robust cell infiltration, interconnected macropores can be incorporated into synthetic hydrogels using various methods [40,53–57]. For example, macroporous PEG hydrogels have been fabricated by embedding a secondary material (e.g., gelatin microspheres) that serves as a sacrificial template after hydrogel formation (Figure 2) [40]. Modulating the macropore size and density has the potenital to control cell infiltration (macroporous) independently of hydrogel degradation and vector release. Hydrogels can be designed to further encourage infiltration and transduction of specific cell types by incorporating short, bioactive peptides derived from the extracellular matrix (e.g., RGD) into scaffolds. In synthetic hydrogels, precise control over the density of adhesive sites can be used to modulate the strength of cell-scaffold interactions and the type of extracellular receptors that mediate these interactions. Along with the density of adhesion sites, the degradation rate of the matrix modulates cell migration through the material, which influences integration with host tissue. Integration of biomaterials tailored to present specific cues (e.g., chemical, mechanical, spatial) and gene therapy vectors represent a promising strategy to promote tissue formation and regeneration. In summary, the hydrogel provides a physical space containing a provisional matrix that promotes cell infiltration, transduction, and trophic factor expression that lead to tissue induction.
4. Hydrogels to Increase Lentivirus Stability
Many hydrogels can be formed under conditions that do not compromise activity; however, the stability of viral vectors remains a critical design parameter as hydrogels loaded with viral vectors may not used in applications that result in immediate cellular internalization of vectors. For example, hydrogels used for regenerative medicine may require days to weeks for cells to fully infiltrate the scaffold and thereby encounter the encapsulated vector. Hydrogel systems have been developed that are capable of providing sustained release of small molecules and proteins over the time scale of days to weeks. Sustained release of vectors from materials is capable of producing increasing levels of gene transfer with time [19,58]. In contrast, sustained release may have no impact at some implantation sites. For delivery from scaffolds to the mouse peritoneal fat pad [34], expression was determined by the amount of vector release shortly after implantation, with no observed effect of release rate. Lentiviral vectors have a reported half-life of approximately 24 hours, and strategies may need to be developed that maintain the virus activity for several days [17,34,37].
Vector entrapment within hydrogels has the potential to increase lentivirus stability through protection from extracellular degradative enzymes and camouflage from the immune system [39,59–61]. Gene transfer with lentiviral vectors entrapped within collagen or fibrin hydrogels has been increased by the addition of hydroxyapatite (HA) nanoparticles [37,39]. For collagen hydrogels, the hydroxyapatite association of the vector increased the half-life to more than 30 hours [37]. More recently, the incorporation of HA nanoparticles within fibrin hydrogels significantly enhanced the duration of transgene expression in vivo (at least two additional weeks), though the mechanism appeared to be related to cell infiltration and hydrogel stability rather than vector stability (Figure 4) [39]. Taken together, hydrogels can be combined with strategies to preserve lentiviral activity in order to enhance gene transfer.
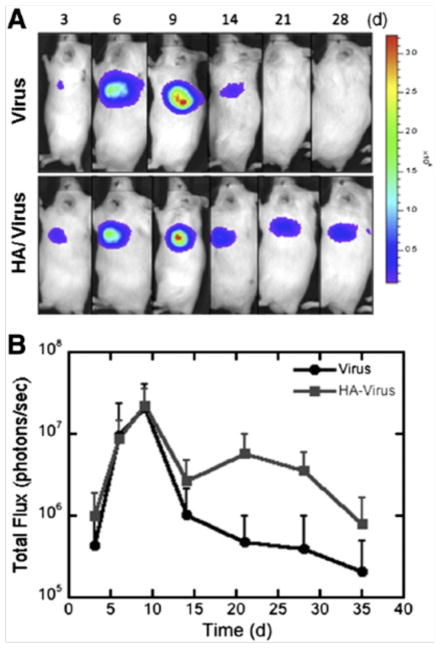
Figure 4.
In vivo transgene expression within fibrin hydrogels. Fibrin hydrogels containing either virus alone or HA/virus complexes were transplanted subcutaneously and expression profile was monitored for 28 days. (a) Represenative images of in vivo luciferase expression using bioluminescence imaging. (b) Quantification of luminescence as a function of time (n=6). Values at day 21 are statsitically different. Reprinted from J Control Rel, 157, Kidd ME, Shin S, Shea LD, Fibrin hydrogels for lentiviral gene delivery in vitro and in vivo, 80–85, 2012, with permission from Elsevier.
5. Immune Response and Lentiviral Vectors
The host response to the lentiviral vectors can significantly influence gene transfer and many researchers have focused on both understanding and attenuating the immune response to lentiviral vectors. Within hours of systemic injection, lentiviral vectors can trigger an inflammatory response characterized by a transient cytokine surges (e.g., interleukin-6 or tumor necrosis factor-α) and a type I interferon response that limits transgene expression [62,63]. This effect has been observed for multiple virus pseudotypes, and in vitro challenge of antigen-presenting cells suggested that plasmacytoid dendritic cells initiated the response. The mechanism of this response is hypothesized to involve activation of toll-like receptor 7 (TLR7) and/or TLR9, which recognize single-stranded RNA and unmethylated CpG, respectively. In addition to toll-like receptors, the complement system, a family of proteins that bind pathogens and aide in their clearance from the body, is well documented to target VSV-G envelope proteins, which leads to vector inactivation [64]. These studies indicate that stable transgene expression depends on the ability of the delivery system to protect the vector from the innate immune system and provides an opportunity to design and employ instructive hydrogels capable of blocking inflammation while delivering their viral vector payload to target cells.
The innate immune system may also facilitate an adaptive immune response to the vector, the transgene product or transduced cells. Some patients may have pre-existing antibodies to the envelope proteins used to pseudotype lentiviral vectors interfere with viral transduction and threaten patient safety, requiring patient screening prior to administration [14,28]. However, exposure to the lentiviral vector will cause the immune system to produce vector-specific antibodies that can neutralize the vector following re-administration, which may limit the therapeutic benefit. In addition, antibodies and antigen-specific T cells may develop recognition of the transgene product or viral vector components, leading to destruction of transduced cells that produce the therapeutic protein.
5.1. Hydrogels that Block Immune Recognition
Hydrogels can be utilized to protect vectors from neutralizing immune complexes and mitigate immune responses due to inflammatory cytokine release by immune cells. Vector encapsulation within hydrogels and attachment of hydrogels onto the vector surface are two potential strategies to address these goals. Hydrogels can be designed with a pore size to limit antibody and complement protein diffusion. Thus viral vectors are protected from the immune system simply by being encapsulated. Alternatively, individual viral particles can be masked from the immune system by modifying the envelope proteins that are recognized by host pattern recognition receptors (e.g., TLR7). For example, PEG can be covalently attached to a VSV-G psuedotyped lentiviral vector, a method known as PEGylation. PEGylation has been shown to extend the circulation half-life of the active vector in mice by a factor of 5 and reduced the rate of vector inactivation in the serum by a factor of 1000 without affecting the number of virus particles present in the circulation [65]. Furthermore, increased vector circulation time of the vector led to significantly enhanced transduction efficiency in the bone marrow and spleen. In sum, PEGylation protects lentivirial vectors from inactivation by serum compliment proteins and, as a result, improves the transduction efficiency of VSV-G pseudotyped lentiviral vectors in vivo [65].
5.2. Hydrogels for Local Immunosuppression
Hydrogels can be engineered to locally suppress inflammation and immunity within the matrix. Peptide sequences that bind and inactivate inflammatory cytokines or their receptors can be integrated into the hydrogel matrix in the same manner as adhesive peptides and degradable linkages (see Synthetic Hydrogels section) [66,67]. These strategies can result in decreased immune cell migration and activation within the hydrogel. Alternatively, ligands or antibodies that induce T cell apoptosis through Fas signaling can be incorporated into the hydrogel providing local immunosuppression [68].
Engineering hydrogels to locally suppress inflammation and immunity may be a promising strategy to promote long-term gene expression. This hypothesis is supported by long-term transgene expression in the liver following systemic vector delivery to mice deficient in the receptor for IFNα and IFNβ, key anti-viral cytokines [63]. Thus, immunomodulatory hydrogels could facilitate stable gene transfer to both immune and non-immune cells by suppressing activation of the immune system to the lentivirus or transduced cells. Alternatively, hydrogels may eventually be engineered to induce tolerance to both the vector and the transgene, thereby allowing for multiple administrations of the vector and long-term expression.
6. Conclusions
Hydrogels offer the opportunity to improve current gene delivery technologies for a range of applications in gene therapy and regenerative medicine. In contrast to bolus injection of naked lentiviral vectors, encapsulation of vectors within hydrogels or direct chemical modification of vectors with hydrogel materials can increase residence times in vivo and prevent vector inactivation by the immune system. Furthermore, these systems can maintain localized, high vector concentrations that overcome mass transport limitations to gene transfer. In addition, hydrogels can be engineered to present peptide motifs that support cell adhesion and vector retention. This strategy results in increased co-localization of vectors and target cells, thereby promoting gene transfer.
Hydrogel biomaterials have enormous potential for a range of applications in gene therapy and regenerative medicine. Hydrogels enhance the potential of lentiviral therapies by retaining vectors at local tissue sites, shielding vectors from the innate immune response and increasing transduction efficiency. For regenerative medicine and tissue engineering applications, hydrogels can be tuned to present cell adhesive, mechanical and biodegradation properties that mimic the tissue of interest in vivo. For detailed reviews of gene delivery from biomaterial scaffolds for tissue engineering applications, see De Laporte and Shea 2007 [69] and Gower and Shea 2012 [27]. Incorporation of lentiviral vectors further enhances the regenerative capacity of hydrogels by adding a mode by which to locally deliver transgenes that regulate direct expression of choice soluble factors and perturb intracellular signaling pathways that determine cell phenotype.
7. Expert Opinion
Hydrogel biomaterials offer the opportunity to control the level, duration, and cellular targets of transgene expression, which are major barriers for gene therapy. Encapsulation of vectors within hydrogels or modification of vectors with hydrogels can shield the vector from clearance or inactivation by the immune system. Furthermore, hydrogels can be modified with biomimetic features to encourage adhesion and migration of specific cell types, thereby increasing transduction of this cell population. The design of hydrogel-based gene delivery systems for regenerative medicine should balance the need to minimize the immune response and limit vector clearance while promoting cell infiltration. Hydrogels with encapsulated vectors that are designed to limit immune cell activation may have limited gene transfer, which could result from the low cell recruitment due to minimal cell-matrix interactions typical of hydrogels that limit immune cell activation.
For regenerative medicine, the material can be designed to promote cellular processes associated with tissue formation, and vector delivery can further increase its bioactivity. Efficient gene transfer reflects a balance between vector retention versus release within the hydrogel, the rate of cell infiltration, and the stability of the vector within the system. Numerous opportunities remain for further refining these design parameters, developing technologies to maximize transduction, and providing spatial and temporal control over gene expression that can mimic the complex patterns of gene expression present during tissue development. Hydrogel biomaterials provide a unique set of tools that can be designed to synergize the requirements for gene transfer with those for promoting tissue formation.
Highlights.
Lentiviral vectors are attractive for their relative ease of production, availability of extensive transgene libraries, and ability to infect both dividing and non-dividing cells.
For tissue engineering applications, the hydrogel provides a physical space that presents specific cues to promote cell infiltration, and access to entrapped lentiviral vectors encoding for inductive factors that influence tissue formation.
Delivery of lentiviral vectors from hydrogels can enable sustained, localized expression within the implantation site.
Hydrogels engineered to mediate non-specific and specific chemical interactions with lentiviral vectors can be designed to either retain vectors within the hydrogel, or release vectors from the hydrogel, which influences the distribution of transduced cells.
In combination with vector retention, the extent and location of transgene expression is also influenced by the rate of cell infiltration into the hydrogel.
Gene delivery is also influenced by the ability of the hydrogel to stabilize vector activity and shield the vector from the host immune response.
List of Abbreviations
- 3D
three-dimensional
- AAV
adeno-associated virus
- HA
hydroxyapatite
- HSV-1
herpes simplex virus-type 1
- IFNαβ
interferon-αβ
- PEG
poly(ethylene glycol)
- RGD
Arginine-glycine-aspartic acid peptide
- RNAi
interference RNA
- TLR
toll-like receptor
- VSV-G
vesicular stomatitis virus glycoproteins
Footnotes
Declaration of Interests
The authors thank the NIH (R01 EB005678, R01 EB003806, R21 EB006520 and PL1 EB008542, a P30 Biomaterials Core within the Oncofertility Consortium Roadmap Grant) for support.
References
- 1.Flotte TR, Trapnell BC, Humphries M, et al. Phase 2 clinical trial of a recombinant adeno-associated viral vector expressing alpha1-antitrypsin: Interim results. Human Gene Ther. 2011;22:1239–47. doi: 10.1089/hum.2011.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.LeWitt PA, Rezai AR, Leehey MA, et al. AAV2-GAD gene therapy for advanced Parkinson’s disease: A double-blind, sham-surgery controlled, randomised trial. Lancet Neurol. 2011;10:309–19. doi: 10.1016/S1474-4422(11)70039-4. [DOI] [PubMed] [Google Scholar]
- 3.Nathwani AC, Tuddenham EG, Rangarajan S, et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. New Engl J Med. 2011;365:2357–65. doi: 10.1056/NEJMoa1108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowles DE, McPhee SW, Li C, et al. Phase 1 gene therapy for Duchenne muscular dystrophy using a translational optimized AAV vector. Mol Ther. 2012;20:443–55. doi: 10.1038/mt.2011.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Partridge KA, Oreffo ROC. Gene delivery in bone tissue engineering: Progress and prospects using viral and nonviral strategies. Tissue Eng. 2004;10:295–307. doi: 10.1089/107632704322791934. [DOI] [PubMed] [Google Scholar]
- 6.Kofron MD, Laurencin CT. Bone tissue engineering by gene delivery. Adv Drug Delivery Rev. 2006;58:555–76. doi: 10.1016/j.addr.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 7.DeLaporte L, Yang Y, Zelivyanskaya M, et al. Plasmid releasing multiple channel bridges for transgene expression after spinal cord injury. Mol Ther. 2009;80:432–9. doi: 10.1038/mt.2008.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeLaporte L, Yan AL, Shea LD. Local gene delivery from ECM-coated poly(lactide-co-glycolide) multiple channel bridges after spinal cord injury. Biomaterials. 2009;30:2361–8. doi: 10.1016/j.biomaterials.2008.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeLaporte L, Huang A, Ducommon MM, et al. Patterned transgene expression in multiple channel bridges after spinal cord injury. Acta Biomat. 2010;6:2889–97. doi: 10.1016/j.actbio.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shin S, Tuinstra HM, Salvay DM, Shea LD. Phosphatidylserine immobilization of lentivirus for localized gene transfer. Biomaterials. 2010;31:4353–4359. doi: 10.1016/j.biomaterials.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeLaporte L, des Rieux LA, Zelivyanskaya ML, et al. VEGF and FGF-2 delivery from spinal cord bridges to enhance angiogenesis following injury. J Biomed Mater Res A. 2011;98:372–82. doi: 10.1002/jbm.a.33112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12•.Tuinstra H, Aviles MO, Shin S, et al. Bridges delivering neurotrophin encoding lentivirus enhance regeneration following spinal cord injury. Biomaterials. 2012;33:1618–26. doi: 10.1016/j.biomaterials.2011.11.002. One of the first examples demonstrating enhanced tissue regeneration in vivo using biomaterial-mediated delivery of lentiviral vectors encoding therapeutic proteins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lipinski DM, Thake M, MacIaren RE. Clinical applications of retinal gene therapy. Prog Retin Eye Res. 2012;32:22–47. doi: 10.1016/j.preteyeres.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 14•.Sakuma T, Barry MA, Ikeda Y. Lentiviral vectors: Basic to translational. Biochem J. 2012;443:603–18. doi: 10.1042/BJ20120146. Detailed review of recent advances in lentiviral vector design. [DOI] [PubMed] [Google Scholar]
- 15.Loser P, Huser A, Hillgenberg M, et al. Advances in the development of non-human viral DNA-vectors for gene delivery. Curr Gene Ther. 2002;2:161–71. doi: 10.2174/1566523024605555. [DOI] [PubMed] [Google Scholar]
- 16.Jang JH, Houchin TL, Shea LD. Gene delivery from polymer scaffolds for tissue engineering. Expert Rev Med Dev. 2004;1:127–38. doi: 10.1586/17434440.1.1.127. [DOI] [PubMed] [Google Scholar]
- 17.Pannier AK, Shea LD. Controlled release systems for DNA delivery. Mol Ther. 2004;10:19–26. doi: 10.1016/j.ymthe.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 18.McMahon J, Conroy S, Lyons M, et al. Gene transfer into rat mesenchymal stem cells: a comparative study of viral and nonviral vectors. Stem Cells Develop. 2006;15:87–96. doi: 10.1089/scd.2006.15.87. [DOI] [PubMed] [Google Scholar]
- 19.Jang JH, Schaffer DV, Shea LD. Engineering biomaterial systems to enhance viral vector gene delivery. Mol Ther. 2011;19:1407–15. doi: 10.1038/mt.2011.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20••.Dull T, Zufferey R, Kelly M, et al. A third-generation lentivirus vector with a conditional packaging system. J Virol. 1998;72:8463–71. doi: 10.1128/jvi.72.11.8463-8471.1998. Landmark publication that describes the modifications to third-generation lentiviral vectors that dramatically increased their safety and efficacy for future clinical use. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaplitt MG, Feigin A, Tang C, et al. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson’s disease: an open label, phase I trial. Lancet. 2007;369:2097–105. doi: 10.1016/S0140-6736(07)60982-9. [DOI] [PubMed] [Google Scholar]
- 22.Kay MA, Manno CS, Ragni MV, et al. Evidence for gene transfer and expression of factor IX in haemophila B patients treated with an AAV vector. Nature Genetics. 2000;24:257–61. doi: 10.1038/73464. [DOI] [PubMed] [Google Scholar]
- 23.Mah C, Fraites TJ, Zolotukhin I, et al. Improved method of recombinant AAV2 delivery for systemic targeted gene therapy. Mol Ther. 2002;6:106–12. doi: 10.1006/mthe.2001.0636. [DOI] [PubMed] [Google Scholar]
- 24••.Cartier N, Hacein-Bey-Abina S, Bartholomae CC, et al. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science. 2009;326:818–23. doi: 10.1126/science.1171242. Clinical study demonstrating the feasibility of lentiviral gene therapies in patients. [DOI] [PubMed] [Google Scholar]
- 25.Maguire AM, High KA, Auricchio A, et al. Age-dependent effects of RPE65 gene therapy for Leber’s congenital amaurosis: a phase 1 dose-escalation trial. Lancet. 2009;374:1597–605. doi: 10.1016/S0140-6736(09)61836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maguire AM, Simonelli F, Pierce EA, et al. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. New Engl J Med. 2008;358:2240–8. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27•.Gower RM, Shea LD. Biomaterial scaffolds for controlled, localized gene delivery of regenerative factors. Adv Wound Care. 2012 doi: 10.1089/wound2011.0325. published online January 2012. Review of recent advances in biomaterial scaffolds for sustained gene delivery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yi Y, Noh MJ, Lee KH. Current advances in retroviral gene therapy. Curr Gene Ther. 2011;11:218–28. doi: 10.2174/156652311795684740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cabrera-Salazar MA, Roskelley EM, Bu J, et al. Timing of therapeutic intervention determines functional and survival outcomes in a mouse model of late infantile batten disease. Mol Ther. 2007;15:1782–8. doi: 10.1038/sj.mt.6300249. [DOI] [PubMed] [Google Scholar]
- 30.Coffin J, Hughes S, Varmus H. Retroviruses. Cold Spring Harbor Laboratory Press; 1997. [PubMed] [Google Scholar]
- 31.Zhang X, Godbey W. Viral vectors for gene delivery in tissue engineering. Adv Drug Delivery Rev. 2006;58:515–34. doi: 10.1016/j.addr.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 32.Humbert JM, Frecha C, Amirache Bouafia F, et al. Measles virus glycoprotein-pseudotyped lentiviral vectors are highly superior to vesicular stomatitis virus G pseudotypes for genetic modification of monocyte-derived dendritic cells. J Virol. 2012;86:5192–203. doi: 10.1128/JVI.06283-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kootstra NA, Verma IM. Gene therapy with viral vectors. Ann Rev Pharm Toxicol. 2003;43:413–39. doi: 10.1146/annurev.pharmtox.43.100901.140257. [DOI] [PubMed] [Google Scholar]
- 34••.Aviles MO, Shea LD. Hydrogels to modulate lentivirus delivery in vivo from microporous tissue engineering scaffolds. Drug Del Transl Res. 2011;1:91–101. doi: 10.1007/s13346-010-0011-1. Important study demonstrating that when lentiviral vectors were delivered from hydrogels in vivo, transgene expression was determined by the amount of vector release shortly after implantation, rather than the release rate. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35••.Raut SD, Lei P, Padmashali RM, Andreadis ST. Fibrin-mediated lentivirus gene transfer: Implications for lentivirus microarrays. J Control Rel. 2010;144:213–20. doi: 10.1016/j.jconrel.2010.02.009. Important study demonstrating that cellular transduction by lentiviral vectors delivered from fibrin hydrogels depended the extent of cell-material interactions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shepard JA, Huang A, Shikanov A, Shea LD. Balancing cell migration with matrix degradation enhances gene delivery to cells cultured three-dimensionally within hydrogels. J Control Rel. 2010;146:128–35. doi: 10.1016/j.jconrel.2010.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shin S, Shea LD. Lentivirus immobilization to nanoparticles for enhanced and localized delivery from hydrogels. Mol Ther. 2010;18:700–6. doi: 10.1038/mt.2009.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shepard JA, Wesson PJ, Wang CE, et al. Gene therapy vectors with enhanced transfection based on hydrogels modified with affinity peptides. Biomaterials. 2011;32:5092–9. doi: 10.1016/j.biomaterials.2011.03.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39••.Kidd ME, Shin S, Shea LD. Fibrin hydrogels for lentiviral gene delivery in vitro and in vivo. J Control Rel. 2012;157:80–5. doi: 10.1016/j.jconrel.2011.08.036. Study demonstrating that hydrogel platforms capable stabilizing lentiviral vectors significantly enhanced transgene expression in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40••.Shepard JA, Virani FR, Goodman AG, et al. Hydrogel macroporosity and the prolongation of transgene expression and the enhancement of angiogenesis. Biomaterials. 2012;33:7412–21. doi: 10.1016/j.biomaterials.2012.06.081. Important study demonstrating that cell access to vectors embedded within hydrogels was the main determinate of cell transduction and transgene expression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baier Leach J, Bivens KA, Patrick CW, Jr, Schmidt CE. Photocrosslinked hyaluronic acid hydrogels: natural, biodegradable tissue engineering scaffolds. Biotech Bioeng. 2003;82:578–89. doi: 10.1002/bit.10605. [DOI] [PubMed] [Google Scholar]
- 42.Storm C, Pastore JJ, MacKintosh FC, et al. Nonlinear elasticity in biological gels. Nature. 2005;435:191–4. doi: 10.1038/nature03521. [DOI] [PubMed] [Google Scholar]
- 43.Raeber G, Lutolf M, Hubbell J. Molecularly engineered PEG hydrogels: A novel model system for proteolytically mediated cell migration. Biophys J. 2005;89:1374–88. doi: 10.1529/biophysj.104.050682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44•.Cho NJ, Elazar M, Xiong A, et al. Viral infection of human progenitor and liver-derived cells encapsulated in three-dimensional PEG-based hydrogel. Biomed Mater. 2008;4:011001–8. doi: 10.1088/1748-6041/25/1/011001. Study that explored the relationship between hydrogel architecture and transduction by lentiviral vectors. [DOI] [PubMed] [Google Scholar]
- 45••.Padmashali RM, Andreadis ST. Engineering fibrinogen-binding VSV-G envelope for spatially-and cell-controlled lentivirus delivery through fibrin hydrogels. Biomaterials. 2011;32:3330–9. doi: 10.1016/j.biomaterials.2011.01.035. Important study that used direct modification of the lentiviral envelope to increase vector retention and stability and transgene expression that was spatially localized to hydrogels. [DOI] [PubMed] [Google Scholar]
- 46•.McMahon SS, Nikolskaya N, Choileáin SN, et al. Thermosensitive hydrogel for prolonged delivery of lentiviral vector expressing neurotrophin-3 in vitro. J Gene Med. 2011;13:591–601. doi: 10.1002/jgm.1613. First report of a hydrogel platform capable of thermosensitive lentiviral delivery. [DOI] [PubMed] [Google Scholar]
- 47.Neumann AJ, Schroeder J, Alini M, et al. Enhanced adenovirus transduction of hMSCs using 3D hydrogel cell carriers. Mol Biotech. 2012 doi: 10.1007/s12033-012-9522-y. published online March 2012. [DOI] [PubMed] [Google Scholar]
- 48.Young S, Wong M, Tabata Y, Mikos AG. Gelatin as a delivery vehicle for the controlled release of bioactive molecules. J Control Rel. 2005;109:256–74. doi: 10.1016/j.jconrel.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 49.Kasper FK, Kushibiki T, Kimura Y, et al. In vivo release of plasmid DNA from composites of oligo (poly (ethylene glycol) fumarate) and cationized gelatin microspheres. J Control Rel. 2005;107:547–61. doi: 10.1016/j.jconrel.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 50.Segura T, Anderson BC, Chung PH, et al. Crosslinked hyaluronic acid hydrogels: a strategy to functionalize and pattern. Biomaterials. 2005;26:359–71. doi: 10.1016/j.biomaterials.2004.02.067. [DOI] [PubMed] [Google Scholar]
- 51.Zhang LH, Luo T, Zhang C, et al. Anti DNA antibody modified coronary stent for plasmid gene delivery: results obtained from a porcine coronary stent model. J Gene Med. 2011;13:37–45. doi: 10.1002/jgm.1529. [DOI] [PubMed] [Google Scholar]
- 52.Lee HH, Haleem AM, Yao V, et al. Release of bioactive adeno-associated virus from fibrin scaffolds: effects of fibrin glue concentrations. Tissue Eng Part A. 2011;17:1969–78. doi: 10.1089/ten.tea.2010.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Behravesh E, Jo S, Zygourakis K, Mikos AG. Synthesis of in situ cross-linkable macroporous biodegradable poly(propylene fumarate-co-ethylene glycol) hydrogels. Biomacromol. 2002;3:374–81. doi: 10.1021/bm010158r. [DOI] [PubMed] [Google Scholar]
- 54.Ford MC, Bertram JP, Hynes SR, et al. A macroporous hydrogel for the coculture of neural progenitor and endothelial cells to form functional vascular networks in vivo. Proc Natl Acad Sci USA. 2006;103:2512–7. doi: 10.1073/pnas.0506020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Keskar V, Marion NW, Mao JJ, Gemeinhart RA. In vitro evaluation of macroporous hydrogels to facilitate stem cell infiltration, growth, and mineralization. Tissue Eng Part A. 2010;15:1695–1707. doi: 10.1089/ten.tea.2008.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hwang Y, Sangaj N, Varghese S. Interconnected macroporous poly(ethylene glycol) cyrogels as a cell scaffold for cartilage tissue engineering. Tissue Eng Part A. 2010;16:3033–41. doi: 10.1089/ten.TEA.2010.0045. [DOI] [PubMed] [Google Scholar]
- 57.Scott EA, Nichols MD, Kuntz-Willits R, Elbert DL. Modular scaffolds assembled around living cells using poly(ethylene glycol) microspheres with macroporation via a non-cytotoxic porogen. Acta Biomater. 2010;6:29–38. doi: 10.1016/j.actbio.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jang JH, Shea LD. Controllable delivery of non-viral DNA from porous scaffolds. J Control Rel. 2003;86:157–68. doi: 10.1016/s0168-3659(02)00369-3. [DOI] [PubMed] [Google Scholar]
- 59•.Sailaja G, HogenEsch H, North A, et al. Encapsulation of recombinant adenovirus into alginate microspheres circumvents vector-specific immune response. Gene Ther. 2002;9:1722–9. doi: 10.1038/sj.gt.3301858. One of the first studies demonstrating that encapsulation of viral vectors within hydrogels can act to shield vectors from the host immune response. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mok H, Park JW, Park TG. Microencapsulation of PEGylated adenovirus within PLGA microspheres for enhanced stability and gene transfection efficiency. Pharm Res. 2007;24:2263–9. doi: 10.1007/s11095-007-9441-y. [DOI] [PubMed] [Google Scholar]
- 61.Gary DJ, Lee H, Sharma R, et al. Influence of nano-carrier architecture on in vitro siRNA delivery performance and in vivo biodistribution: Polyplexes vs micelleplexes. ACS Nano. 2011;5:3493–505. doi: 10.1021/nn102540y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62•.Vandendriessche T, Thorrez L, Acosta-Sanchez A, et al. Efficacy and safety of adeno-associated viral vectors based on serotype 8 and 9 vs. lentiviral vectors for hemophilia B gene therapy. J Thromb Haemost. 2007;5:16–24. doi: 10.1111/j.1538-7836.2006.02220.x. Clinical study that compared the safety and efficacy of lentiviral and adeno-associated vectors for gene therapy. [DOI] [PubMed] [Google Scholar]
- 63•.Brown BD, Sitia G, Annoni A, et al. In vivo administration of lentiviral vectors triggers a type I interferon response that restricts hepatocyte gene transfer and promotes vector clearance. Blood. 2007;109:2797–805. doi: 10.1182/blood-2006-10-049312. Results from this study demonstrate disabling a single component of the innate/immune network (interferon αβ receptor) concurrently with lentiviral administration was sufficient to establish persistent xenoantigen expression in the liver. [DOI] [PubMed] [Google Scholar]
- 64.DePolo NJ, Reed JD, Sheridan PL, et al. VSV-G pseudotyped lentiviral vector particles produced in human cells are inactivated by human serum. Mol Ther. 2000;2:218–22. doi: 10.1006/mthe.2000.0116. [DOI] [PubMed] [Google Scholar]
- 65.Croyle MA, Callahan SM, Auricchio A, et al. PEGylation of a vesicular stomatitis virus G pseudotyped lentivirus vector prevents inactivation in serum. J Virol. 2004;78:912–21. doi: 10.1128/JVI.78.2.912-921.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lin CC, Boyer PD, Aimetti AA, Anseth KS. Regulating MCP-1 diffusion in affinity hydrogels for enhancing immuno-isolation. J Control Rel. 2010;142:384–91. doi: 10.1016/j.jconrel.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Su J, Hu B-H, Lowe WL, Jr, et al. Anti-inflammatory peptide-functionalized hydrogels for insulin-secreting cell encapsulation. Biomaterials. 2010;31:308–14. doi: 10.1016/j.biomaterials.2009.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cheung CY, Anseth KS. Synthesis of immunoisolation barriers that provide localized immunosuppression for encapsulated pancreatic islets. Bioconj Chem. 2006;17:1036–42. doi: 10.1021/bc060023o. [DOI] [PubMed] [Google Scholar]
- 69•.De Laporte L, Shea LD. Matrices and scaffolds for DNA delivery in tissue engineering. Adv Drug Deliv Rev. 2007;59:292–307. doi: 10.1016/j.addr.2007.03.017. Detailed review of biomaterial scaffolds for gene delivery in tissue engineering. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lei P, Padmashali RM, Andreadis ST. Cell-controlled and spatially arrayed gene delivery from fibrin hydrogels. Biomaterials. 2009;30:3790–9. doi: 10.1016/j.biomaterials.2009.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]