Abstract
Focused library synthesis and structure-activity relationship development of 5,6,7-substituted pyrazolopyrimidines led to the discovery of 2-(5,7-diethyl-2-(4-(2-fluoroethoxy)phenyl)pyrazolo[1,5-a]pyrimidin-3-yl)-N,N-diethylacetamide (6b), a novel translocator protein (TSPO) ligand exhibiting a 36-fold enhancement in affinity compared to another pyrazolopyrimidine-based TSPO ligand, 6a (DPA-714). Radiolabeling with fluorine-18 (18F) facilitated production of 2-(5,7-diethyl-2-(4-(2-[18F]fluoroethoxy)phenyl)pyrazolo[1,5-a]pyrimidin-3-yl)-N,N-diethylacetamide (18F-6b) in high radiochemical yield and specific activity. In vivo studies of 18F-6b were performed which illuminated this agent as an improved probe for molecular imaging of TSPO-expressing cancers.
Introduction
There remains a critical need to develop and rigorously validate molecular imaging biomarkers that aid tumor diagnosis, predict clinical outcome, and quantify response to therapeutic interventions. Imaging techniques routinely used in clinical oncology include magnetic resonance imaging (MRI), X-ray computed tomography (CT), ultrasound imaging (US), and positron emission tomography (PET). Of these, the sensitivity and quantitative nature of PET, coupled with the ability to readily produce biologically active compounds bearing positron-emitting isotopes (e.g., 11C, 18F), renders PET imaging as one the most attractive techniques for detecting tumors and profiling their molecular features. By far, the most widely used PET tracer in clinical oncology is 2-deoxy-2-[18F]fluoro-D-glucose (18F-FDG), a probe that accumulates in tissue as a function of glucose utilization. PET using 18F-FDG is a powerful approach for tumor detection in many organ sites. However, not all tumors exhibit elevated glucose avidity, and 18F-FDG uptake can be affected by a plethora of normal metabolic processes. Furthermore, tumor imaging can be confounded by 18F-FDG uptake in normal tissues such as healthy brain. These issues highlight an unmet need to explore and validate additional molecular targets for cancer imaging.
Our laboratory has explored translocator protein (TSPO) expression as a target for molecular imaging of cancer.1–5 Formerly referred to as peripheral benzodiazepine receptor (PBR), TSPO is an 18-kDa protein typically localized to the outer mitochondria membrane. TSPO participates in the regulation of numerous cellular processes, including steroid biosynthesis, cholesterol metabolism, apoptosis, and cellular proliferation.6 In normal tissues, TSPO tends to be expressed in those that produce steroids and those that are mitochondrial-enriched such as myocardium, skeletal muscle, and renal tissue. Tissues such as liver and brain exhibit comparatively modest expression.6 While classically exploited as a target in neuroscience, elevated TSPO expression is also observed in many cancers.13 In oncology, TSPO expression is typically linked with disease progression and diminished survival and is a hallmark of aggressive and potentially metastatic tumors.13 For this reason, our laboratory has explored the use of TSPO imaging ligands within the context of colon cancer,1 breast cancer, 2 and glioma,4, 5 as these agents could potentially serve as useful cancer imaging biomarkers. We recently reported the first utilizations of the PET agents N-[18F]fluoroacetyl-N-(2,5-dimethoxybenzyl)-2-phenoxyaniline (18F-PBR06)5 and N,N-diethyl-2-(2-(4-(2-[18F]fluoroethoxy)phenyl)-5,7-dimethylpyrazolo[1,5-a]pyrimidin-3-yl)acetamide (18F-DPA-714)4 for quantitative assessment of TSPO expression in preclinical glioma. In these proof-of-principle PET imaging studies, tumors were detectable among surrounding normal brain and, importantly, TSPO levels could be quantitatively assayed in tumors using compartmental analysis of the PET data.4, 5 However, drawbacks were observed with both agents in this context, including tracer accumulation in normal brain that reached levels potentially sufficient to prevent detection of gliomas with modest TSPO expression. Both tracers also exhibited significant metabolism in vivo, which required correction of plasma input functions for quantitative analysis. While illustrating the potential of TSPO PET to detect tumors in brain, these studies prompted our desire to develop novel TSPO PET ligands with improved properties for cancer imaging.
The goal of this study was to determine whether optimization of the pyrazolopyrimidine scaffold, specifically at the 5-, 6-, and 7-positions, would yield TSPO ligands with greater affinity and potentially serve as more robust PET imaging ligands in vivo. These experiments led to the discovery of 2-(5,7-diethyl-2-(4-(2-fluoroethoxy)phenyl)pyrazolo[1,5-a]pyrimidin-3-yl)-N,N-diethylacetamide (6b), a novel TSPO-selective ligand that exhibits a surprising 36-fold enhancement in affinity compared to another pyrazolopyrimidine, 6a (DPA-714). An appropriate analog of 6b, compound 7, could be radiolabeled with fluorine-18 (18F) to yield the novel TSPO PET ligand 2-(5,7-diethyl-2-(4-(2-[18F]fluoroethoxy)phenyl)pyrazolo[1,5-a]pyrimidin-3-yl)-N,N-diethylacetamide (18F-6b), which was subsequently evaluated in vivo in healthy rats and a preclinical model of glioma. 18F-6b exhibited negligible accumulation in normal brain, yet robust accumulation in tumor tissue, which facilitated excellent imaging contrast. Overall, these studies illuminate 18F-6b as a promising, novel PET ligand for evaluating TSPO expression in tumors and potentially other diseases.
Results and Discussion
CHEMISTRY
MAOS of 5,6,7-substituted Pyrazolopyrimidine Library
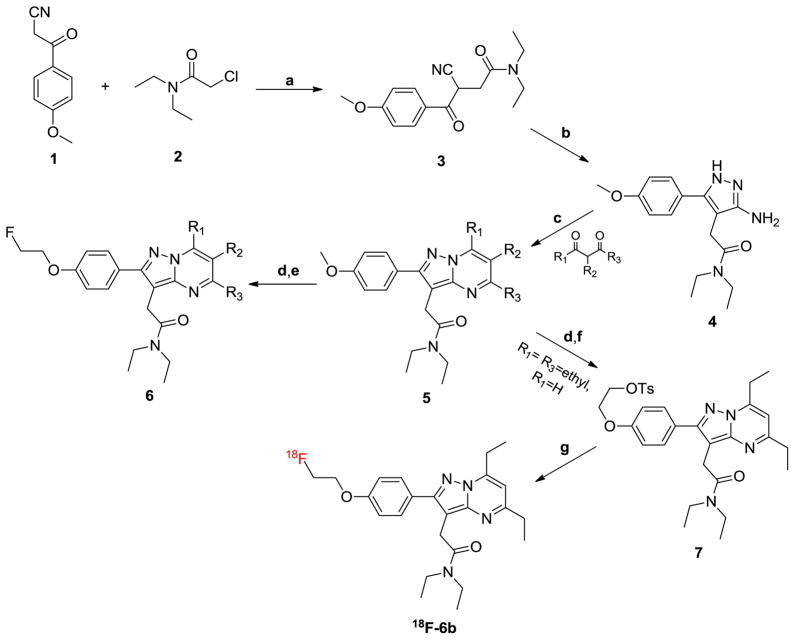
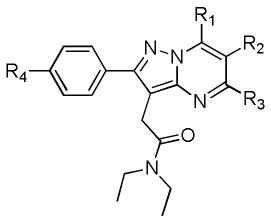
A library of structurally diverse pyrazolopyrimidines (Table 1) was assembled using a microwave-assisted organic synthesis (MAOS) method that prioritized points of divergence at the 5-R3, 6-R2, and 7-R1 positions on the core pyrazolopyrimidine scaffold. Members of this library were formed through condensation of a pyrazole core (4) with various substituted diones.11 Entries in Table 1 fall into two distinct series (5 and 6), with seven compounds (a–g) per series. Each compound within the series results from one of seven diones utilized, while each series is differentiated by the installation of a unique surrogate imaging handle at the 4-postion (R4) of the 2-phenyl group pendant to the core scaffold. Series 5 entries (5a–5g) feature a methoxy group at R4 and include a previously reported pyrazolopyrimidine, 5a (DPA-713),7–10 while series 6 entries (6a–6g) feature a 2-fluoroethoxy group at R4 and include 6a.7–9, 12 Given our ultimate goal of developing novel PET imaging ligands, coupled with an interest in expanding structure-activity relationships (SAR) around the 5,6,7-substituted pyrazolopyrimidine core, we rationalized that maintaining the surrogate imaging handles of future representative imaging probes would accelerate the development of novel agents.
Table 1.
Affinity, liphopilicity, and molecular weight of pyraxolopyrimidines
| |||||||
|---|---|---|---|---|---|---|---|
| Compd | R1 | R2 | R3 | R4 | MW | LogP7.5 | Ki (nM)δ |
| 5aα | -Me | -H | -Me | -OMe | 366.21 | 2.40 | 12.23 |
| 5b | -Et | -H | -Et | -OMe | 394.51 | 2.84 | 0.18 |
| 5cβ | -iPr | -H | -iPr | -OMe | 422.56 | 2.98 | >500 |
| 5d | -Me | -Me | -Me | -OMe | 380.48 | 2.45 | 93.75 |
| 5e | -Me | -Et | -Me | -OMe | 394.51 | 2.78 | 157.14 |
| 5f | -Me | -CH2C(O)CH3 | -Me | -OMe | 408.49 | 2.57 | 200.89 |
| 5g | -Me | -Cl | -Me | -OMe | 400.9 | 2.99 | 55.36 |
| 6aγ | -Me | -H | -Me | -OCH2CH2F | 398.47 | 2.12 | 9.73 |
| 6b | -Et | -H | -Et | -OCH2CH2F | 426.53 | 2.50 | 0.27ε |
| 6c | -iPr | -H | -iPr | -OCH2CH2F | 454.58 | 2.73 | >500 |
| 6d | -Me | -Me | -Me | -OCH2CH2F | 412.5 | 2.47 | 83.04 |
| 6e | -Me | -Et | -Me | -OCH2CH2F | 426.53 | 2.53 | 276.79 |
| 6f | -Me | -CH2C(O)CH3 | -Me | -OCH2CH2F | 440.51 | 2.07 | 251.79 |
| 6g | -Me | -Cl | -Me | -OCH2CH2F | 432.92 | 2.55 | 67.86 |
The overall synthetic methodology is presented in Scheme 1.11 Here, compound 4, bearing a 3-amino-1H-pyrazole core, was accessible in two steps using MAOS from commercially available phenylpropanenitrile 1 and chloroacetamide 2. From 4, the synthesis diverges at the condensation step with a series of unique diones to yield the “five” series of pyrazolopyrimidines (5a–5g) that mimic analogous 11C-based PET probes with an R4-OMe. Subsequently, compounds 6a–6g were achieved by cleavage of methoxy group followed by microwave–assisted ether synthesis with 2-fluoroethyl-4-methylbenzenesulfonate which yielded an R4-2-fluoroethoxy surrogate imaging handle to mimic 18F-labeled PET probes (Table 1). The library developed primarily explored the effects of R1, R2, and R3 substituents that varied in steric bulk. Generally, substituents at R1 and R3 mirrored one another and consisted of linear and branched alkyl substituents such as methyl, ethyl, and isopropyl moieties. These substituents were cross-matched with similar groups at the R2 position, which included hydrogen, methyl, ethyl, chloro, and 2-propanone.
Scheme 1. Synthetic methodology utilized to generate a focused library of 5,6,7-substituted pyrazolopyrimidines and 18F-6bα.
αReagents and conditions: (a) NaOH, NaI, microwave, 80 °C, 40 min, 80% EtOH in H2O, 70%; (b) hydrazine, AcOH, microwave, 90 °C, 40 min, EtOH, 42%; (c) microwave, 140–190 °C, 45min, EtOH, 10–90%; (d) HTPB, microwave, 110 °C, 40 min, aqueous HBr; (e) NaH, 2-fluoroethyl-4-methylbenzenesulfonate, microwave, 120 °C, 30 min, dry THF. (f) ethylene di(p-toluenesulfonate), NaH, microwave, 120 °C, 30 min, dry THF, 45%. (g) Fluoride-18 ion, K+-K2.2.2/K2CO3, 99 °C, 20 min, dry DMSO.
Lipophilicity
Lipophilicity of series 5 and 6 were evaluated at pH = 7.5 (log P7.5) using reversed phase HPLC14 and is tabulated in Table 1. The values for library entries varied from 2.0 to 3.0 for both series and correlated predictably with dione structure, where polarity and hydrocarbon content of the R1–R3 substituents appeared to be key determinants. In both series, analogs a, d, and f, with R1–R3 combinations of hydrogen, methyl, and 2-propanone, proved the least lipophilic. Introduction of ethyl groups at R1, R2, or R3 increased log P7.5 values accordingly, as with analogs b and e, which ranged from 2.50–2.84. The most lipophilic analogs proved to be c and g (log P7.5 = 2.55–2.99), with isopropyl groups at the R1 and R3 positions or chlorine at R2. Interestingly, of the two series, series 5 (R4 = OMe), tended to be more lipophilic than series 6 (R4 = OCH2CH2F). Overall, the library entries exhibited lipophilicities (2.07–2.99) suitable for in vivo imaging,15 where potential agents must be sufficiently membrane penetrant to bind intracellular targets such as TSPO.
BIOLOGICAL TESTING
Binding Affinity in Cancer Cell Lysate and SAR Analysis
To evaluate binding of the library entries to TSPO, radioligand displacement was carried out in rat glioma cell lysate (C6) using N-(sec-butyl)-1-(2-chlorophenyl)-N-methyl-3H-isoquinoline-3-carboxamide (3H-PK 11195)4, 5. Affinities of library members are expressed as Ki (nM) values in Table 1. Encompassing modifications at R1–R4, both series exhibited a spectrum of Ki values that spanned micromolar to sub-nanomolar affinity. As expected, replacing the 4-methoxy with 4-(2-fluoroethoxy) at the R4 position had minimal impact upon TSPO affinity, as similar SAR trends emerged within series 5 and 6. In contrast, R1, R2, and R3 modification led to major impacts upon affinity. Though the R1 and R3 positions showed intolerance to steric bulk beyond ethyl, a surprising level of improvement in affinity could be realized by replacing methyl with ethyl at the R1 and R3 positions. For example, subnanomolar affinities (Ki) were observed for analogs 5b (0.18 nM) and 6b (0.27 nM), which represent major improvements over their respective leads, 5a (12.23 nM) and 6a (9.73 nM) (Table 1), as well as another high-affinity TSPO ligand, N-(2-methoxybenzyl)-N-(4-phenoxypyridin-3-yl)acetamide (PBR28) (4.0 nM)16. Increasing the R1 and R3 to a bulkier isopropyl group proved detrimental, with Ki values for both 5c and 6c greater than 500 nM. Overall, the R2 position was less tolerant to steric or electron density deviations from hydrogen in these studies. Substitution of the nascent R2 hydrogen (5a, 6a) with methyl (5d, 93.75 nM; 6d, 83.04 nM) decreased affinity approximately 10-fold with respect to their parent compounds, 5a and 6b, respectively; this trend was more dramatic with ethyl substitution (5e, 157.14 nM; 6e, 276.79 nM). Introduction of polarity at the R2 position with 2-propanone (5f, 200.89 nM; 6f, 251.79 nM) bore similar adverse effects upon affinity. Somewhat less dramatic decreases in affinity were observed upon halogen substitution at R2 with chlorine (5g, 55.36 nM; 6g, 67.86 nM). Given the size of chlorine atom, lying between methyl and ethyl, it is reasonable that the Ki of entries 5g and 6g are only slightly worse than those with alkyl substituents at the R2 position (Table 1). Overall, these experiments illuminated 5,7-diethyl, 6-hydrogen substitution as the optimal pattern in this series to impart improved affinity. Additionally, the studies also suggested that an analog could be produced for PET imaging using either fluorine-18 (6b) or carbon-11 (5a) substitution on the R4 position with minimal effect on TSPO affinity and only minor effect on lipophilicity. Since fluorine-18 has a significantly longer half-life than carbon-11 (109.8 min vs. 20.4 min), we elected to carry compound 6b forward for further characterization. The selectivity of compound 6b for TSPO over the central benzodiazepine receptor (CBR) was evaluated using radioligand displacement of the CBR ligand 3H-flunitrazepam in rat cerebral cortex membranes (Table 1). Unlike the displacement studies with 3H-PK 11195, compound 6b demonstrated poor affinity for CBR (Ki > 10,000 nM), indicating TSPO selectivity.
RADIOCHEMISTRY
Radioligand Precursor Preparation and Radiosynthesis
To produce 18F-6b, we synthesized precursor (7), a tosylated intermediate prepared from 5b in two steps with an overall yield of approximately 45% (Scheme 1). Nucleophilic fluorination of 7 with fluorine-18 was then performed (Scheme 1) in anhydrous dimethyl sulfoxide at 165 °C for 5 minutes. Purification of 18F-6b was carried out with preparative HPLC using 10 mM sodium phosphate buffer (pH 6.7) in ethanol (47.5/52.5, v/v). The retention time of 18F-6b was 12 minutes according to gamma detection and corresponded to the UV retention time of non-radioactive 6b. Radiochemical purity was consistently greater than 99%, with specific activity consistently greater than 4203 Ci/mmol (156 TBq/mmol) (n = 33).
IMAGING STUDIES
Uptake of 18F-6b in C6 Glioma
The in vivo performance of 18F-6b was evaluated in glioma-bearing Wistar rats using microPET imaging, with a typical study shown in Figure 1. MRI (T2-weighted) was used to localize tumors and for registration of anatomical features with PET4, 5 (Figure 1A). Dynamic PET imaging with 18F-6b illustrated that the majority of tracer uptake in the brain was localized to the tumor, with very modest accumulation in the adjacent, normal brain (Figure 1B and 1C). The tumor-selective uptake characteristics of 18F-6b afforded excellent imaging contrast between tumor and normal tissue. In addition to the tumor, accumulation of 18F-6b was observed outside of the brain in olfactory epithelium and the Harderian glands, as well as the tongue, where TSPO expression is elevated.13 Figure 1D illustrates time-activity curves (TACs) that were typical of eight representative studies for tumor, normal brain, and plasma over a 90-minute dynamic acquisition. We found that 18F-6b was rapidly delivered to tumor and normal brain, but cleared tumor tissues at a much slower rate than normal brain. After an initial spike in radioactivity consistent with tracer injection and rapid distribution, 18F-6b quickly cleared from the plasma. To validate the PET, imaging-matched brains were processed for post mortem staining and immunohistochemistry. We observed close agreement between tumor tissue (H&E, Figure 1E), elevated TSPO expression by immunohistochemistry (Figure 1F), and tumor accumulation of 18F-6b accumulation (Figure 1C). To more quantitatively compare the performance of 18F-6b to 18F-6a, we conducted dynamic PET using 18F-6b in a cohort of tumor-bearing rats (n = 5) and carried out pharmacokinetic modeling of the PET data, similar to our previous studies4, 5. We found that 18F-6b demonstrated a higher ratio of total distribution volume (VT) between tumor and normal brain (6.0, n = 5), when compared to 18F-6a (3.9, n =11),4 which resulted in greater signal-to-noise between tumor and surround normal brain.
Figure 1.
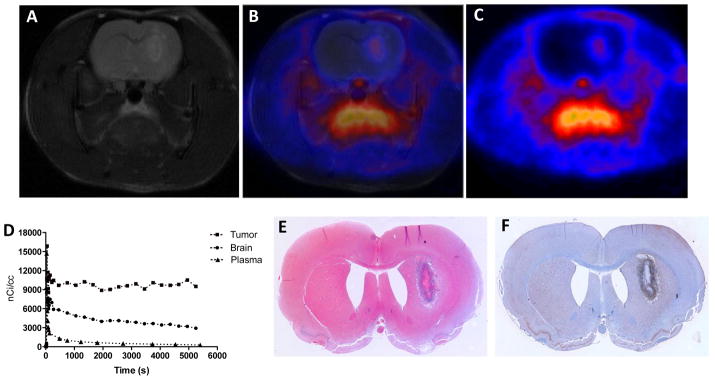
PET imaging of preclinical glioma using 18F-6b.
Characterization of 18F-6b Radiometabolites in Plasma
Analogous to our previous work,4 radio-HPLC was used to evaluate metabolism of 18F-6b in plasma at multiple time points during the PET scanning period (2, 12, 30, 60, 90 min) (Supplemental Figure 1). Parent compound (18F-6b) was the only radioactive species detected in plasma at all time points, suggesting a lack of circulating 18F-6b metabolites observed over a 90-minute period. This behavior contrasted the in vivo performance of 18F-6a, where we previously observed up to 30% metabolism within the first 12 minutes post-administration.4 Potentially, the apparent improved stability of 18F-6b over 18F-6a may stem from the rapid clearance of 18F-6b from plasma, rendering detection of low levels of metabolites challenging with HPLC.
Biodistribution and Specific Binding of 18F-6b in Rats
The biodistribution and TSPO-specificity of 18F-6b was evaluated in normal tissues. Tissue samples were harvested 60 minutes after infusion of the 18F-6b. The radioactivity of harvested tissue was measured using standard analytical methods and recorded as percentage of the injected dose per gram of tissue (%ID/g) (Supplemental Figure 2A). Consistent with known TSPO densities,13 we observed elevated accumulation of 18F-6b in spleen, heart, kidney, and lung.17 Moderate uptake was also observed in liver, colon, small bowel, and stomach. Very minor accumulation was observed in the brain, testes, skull, and skeletal muscle. At 60 minutes post-administration of tracer, very little radio-activity was observed in biological fluids, such as whole blood or urine.
To evaluate the specific binding of 18F-6b in normal tissue, we carried out a similar 60-minute biodistribution assay that included a bolus infusion of non-radioactive 6b (10 mg/kg) at 30 minutes (Supplemental Figure 2B). With displacement, we observed that activity in the organs exhibiting the greatest accumulation of 18F-6b, such as spleen, heart, kidney, and lung, was reduced to background levels, indicating near complete displacement and reversible binding in healthy tissue. Organs that exhibited low to moderate accumulation of 18F-6b without displacement, such as muscle or liver, were essentially unchanged with displacement, suggesting that the low to moderate tracer accumulation in these tissues may reflect non-specificity. Interestingly, activity found in the urine did not follow this trend and was elevated with displacement, suggesting that blocking TSPO with non-radioactive 6b altered the excretion profile of the agent. Overall, the results indicate a high degree of specific binding and reversibility of 18F-6b to TSPO in healthy tissues.
CRYSTALLOGRAPHY
6b was recrystallized from hot acetonitrile and ethanol (95%) by slow evaporation at room temperature and characterized by diffractometry. 6b demonstrated two ethyl branches at the 5- and 7-positions of the pyrazolopyrimidine ring, corresponding to the structure elucidated from NMR spectroscopy. The crystal structure also revealed three distinct planar entities, the pyrazolopyrimidine ring, the phenyl ring, and the amide group (Supplemental Figure 3).
Conclusion
In this study, we found that the 5,6,7-substitution pattern is a critical determinant of TSPO affinity among pyrazolopyrimidines. Experiments shown here led to the discovery of 6b, a TSPO ligand exhibiting significantly increased affinity compared to 6a and TSPO ligands derived from other chemical entities.18 Furthermore, in addition to improved TSPO affinity, compound 6b was TSPO-selective, exhibiting negligible binding affinity to CBR. Analog 7 of 6b could be radiolabeled with fluorine-18 to yield 18F-6b. Suggesting its potential as a probe for cancer imaging, preclinical imaging studies demonstrated robust accumulation of 18F-6b in tumor tissue and negligible accumulation in normal brain. Overall, these studies illuminate 18F-6b as a promising, novel PET ligand for evaluating TSPO expression in tumors and potentially other diseases.
Supplementary Material
Acknowledgments
Funding Sources
The authors acknowledge funding from the National Institutes of Health (K25 CA127349, P50 CA128323, S10 RR17858, U24 CA126588, 1R01 CA163806), The Kleberg Foundation, and The Lustgarten Foundation.
M. Noor Tantawy, George H. Wilson, Dan Colvin and Yiu-Yin Cheung are acknowledged.
Footnotes
Author Contributions
Dr. Manning directed and designed the study. Mr. Tang performed the synthetic chemistry and along with Mr. McKinley, Mr. Hight, and Dr. Buck, performed the imaging experiments. Dr. Nickels performed the radiochemistry, Dr. Uddin and Dr. Harp carried out the crystallography. All authors participated in writing or editing the manuscript.
Supporting Information. Synthetic and analytical results for all compounds; Methods; Supplemental Figures. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Deane NG, Manning HC, Foutch AC, Washington MK, Aronow BJ, Bornhop DJ, Coffey RJ. Targeted imaging of colonic tumors in smad3−/− mice discriminates cancer and inflammation. Mol Cancer Res. 2007;5:341–349. doi: 10.1158/1541-7786.MCR-06-0225. [DOI] [PubMed] [Google Scholar]
- 2.Wyatt SK, Manning HC, Bai M, Bailey SN, Gallant P, Ma G, McIntosh L, Bornhop DJ. Molecular imaging of the translocator protein (TSPO) in a pre-clinical model of breast cancer. Mol Imaging Biol. 2010;12:349–358. doi: 10.1007/s11307-009-0270-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manning HC, Goebel T, Thompson RC, Price RR, Lee H, Bornhop DJ. Targeted molecular imaging agents for cellular-scale bimodal imaging. Bioconjug Chem. 2004;15:1488–1495. doi: 10.1021/bc049904q. [DOI] [PubMed] [Google Scholar]
- 4.Tang D, Hight MR, McKinley ET, Fu A, Buck JR, Smith RA, Tantawy MN, Peterson TE, Colvin DC, Ansari MS, Nickels M, Manning HC. Quantitative preclinical imaging of TSPO expression in glioma using N,N-diethyl-2-(2-(4-(2-18F-fluoroethoxy) phenyl)-5,7-dimethylpyrazolo[1,5-a]pyrimi din-3-yl)acetamide. J Nucl Med. 2012;53:287–294. doi: 10.2967/jnumed.111.095653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buck JR, McKinley ET, Hight MR, Fu A, Tang D, Smith RA, Tantawy MN, Peterson TE, Colvin D, Ansari MS, Baldwin RM, Zhao P, Guleryuz S, Manning HC. Quantitative, preclinical PET of translocator protein expression in glioma using 18F-N-fluoroacetyl-N-(2,5-dimethoxybenzyl)-2-phenoxyaniline. J Nucl Med. 2011;52:107–114. doi: 10.2967/jnumed.110.081703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papadopoulos V, Baraldi M, Guilarte TR, Knudsen TB, Lacapere JJ, Lindemann P, Norenberg MD, Nutt D, Weizman A, Zhang MR, Gavish M. Translocator protein (18kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci. 2006;27:402–409. doi: 10.1016/j.tips.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Chauveau F, Van Camp N, Dolle F, Kuhnast B, Hinnen F, Damont A, Boutin H, James M, Kassiou M, Tavitian B. Comparative evaluation of the translocator protein radioligands 11C-DPA-713, 18F-DPA-714, and 11C-PK11195 in a rat model of acute neuroinflammation. J Nucl Med. 2009;50:468–476. doi: 10.2967/jnumed.108.058669. [DOI] [PubMed] [Google Scholar]
- 8.Doorduin J, Klein HC, Dierckx RA, James M, Kassiou M, de Vries EF. [11C]-DPA-713 [18F]-DPA-714 as new PET tracers for TSPO: a comparison with [11C]-(R)-PK11195 in a rat model of herpes encephalitis. Mol Imaging Biol. 2009;11:386–398. doi: 10.1007/s11307-009-0211-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.James ML, Fulton RR, Henderson DJ, Eberl S, Meikle SR, Thomson S, Allan RD, Dolle F, Fulham MJ, Kassiou M. Synthesis and in vivo evaluation of a novel peripheral benzodiazepine receptor PET radioligand. Bioorg Med Chem. 2005;13:6188–6194. doi: 10.1016/j.bmc.2005.06.030. [DOI] [PubMed] [Google Scholar]
- 10.Selleri S, Bruni F, Costagli C, Costanzo A, Guerrini G, Ciciani G, Costa B, Martini C. 2-Arylpyrazolo[1,5-a]pyrimidin-3-yl acetamides. New potent and selective peripheral benzodiazepine receptor ligands. Bioorg Med Chem. 2001;9:2661–2671. doi: 10.1016/s0968-0896(01)00192-4. [DOI] [PubMed] [Google Scholar]
- 11.Tang D, Buck JR, Hight MR, Manning HC. Microwave-assisted Organic Synthesis of a High-affinity Pyrazolo-pyrimidinyl TSPO Ligand. Tetrahedron Lett. 2010;51:4595–4598. doi: 10.1016/j.tetlet.2010.06.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin A, Boisgard R, Theze B, Van Camp N, Kuhnast B, Damont A, Kassiou M, Dolle F, Tavitian B. Evaluation of the PBR/TSPO radioligand [(18)F]DPA-714 in a rat model of focal cerebral ischemia. J Cereb Blood Flow Metab. 2010;30:230–241. doi: 10.1038/jcbfm.2009.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Batarseh A, Papadopoulos V. Regulation of translocator protein 18 kDa (TSPO) expression in health and disease states. Mol Cell Endocrinol. 2010;327:1–12. doi: 10.1016/j.mce.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waterhouse RN, Mardon K, Giles KM, Collier TL, O’Brien JC. Halogenated 4-(phenoxymethyl)piperidines as potential radiolabeled probes for sigma-1 receptors: in vivo evaluation of [123I]-1-(iodopropen-2-yl)-4-[(4-cyanophenoxy)methyl]pip eri dine. J Med Chem. 1997;40:1657–1667. doi: 10.1021/jm960720+. [DOI] [PubMed] [Google Scholar]
- 15.Pike VW. PET radiotracers: crossing the blood-brain barrier and surviving metabolism. Trends Pharmacol Sci. 2009;30:431–440. doi: 10.1016/j.tips.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Owen DR, Howell OW, Tang SP, Wells LA, Bennacef I, Bergstrom M, Gunn RN, Rabiner EA, Wilkins MR, Reynolds R, Matthews PM, Parker CA. Two binding sites for [3H]PBR28 in human brain: implications for TSPO PET imaging of neuroinflammation. J Cereb Blood Flow Metab. 2010;30:1608–1618. doi: 10.1038/jcbfm.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujimura Y, Kimura Y, Simeon FG, Dickstein LP, Pike VW, Innis RB, Fujita M. Biodistribution and radiation dosimetry in humans of a new PET ligand, (18)F-PBR06, to image translocator protein (18 kDa) J Nucl Med. 2010;51:145–149. doi: 10.2967/jnumed.109.068064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taliani S, Pugliesi I, Da Settimo F. Structural requirements to obtain highly potent and selective 18 kDa Translocator Protein (TSPO) Ligands. Curr Top Med Chem. 2011;11(7):860–886. doi: 10.2174/156802611795165142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.