Abstract
In utero stem cell transplantation, which promises treatment for a host of genetic disorders early in gestation before disease effect stems from Ray Owen’s seminal observation that self-tolerance, is acquired during gestation. To date, in utero transplantation (IUT) has proved useful in characterizing the hematopoietic stem cell. Recent observations support its use as an in vivo method to further understanding of self-tolerance. Preclinical development continues for its application as a treatment for childhood hematolymphoid diseases. In addition, IUT may offer therapeutic options in the treatment of diabetes among other diseases. Thus IUT serves as a technique or system important in both a basic and applied format. This review summarizes these findings.
Keywords: Self non-self discrimination, Immune ontogeny, Stem cell transplantation
INTRODUCTION
A variety of genetic disorders of the hematolymphoid system have been treated successfully by transplanting hematopoietic stem cell (HSC) into pediatric and adult patients. However, this procedure’s applicability is limited by compatible donor availability, the use of immunosuppression/cytoablation and the potential development of graft-vs-host disease (GVHD). Further, for some pediatric diseases (i.e., the glycogen storage diseases, thalassemia, etc.) significant disease progression begins during fetal development such that even at birth the patient is irreversibly compromised. In these conditions, postnatal bone marrow transplantation can ameliorate disease progression but is unable to correct the existing damage[1].
Successful allogeneic HSC transplantation is accomplished by donor reconstitution of hematopoiesis (usually after depletion of defective or undesirable host cells) following the infusion of donor HSC in anticipation of their homing to appropriate hematopoietic tissues. This requires overcoming the immune barrier of the host, limiting the ability of donor immune cells to mediate GVHD and facilitation of homing and engraftment of donor HSC where the inductive milieu will promote regulated reconstitution of hematopoiesis. In the clinical setting many of the complications of postnatal HSC transplantation are the result of procedures such as ionizing radiation and chemotherapy used to limit host immune rejection or deplete host marrow to allow adequate donor engraftment/expression.
In theory, intrauterine stem cell transplantation (IUSCT) presents significant advantages in comparison to postnatal stem cell (SC) transplantation, which if optimized, could present less toxic treatment protocols for many genetic/developmental diseases. Advances in molecular biological techniques and improvements in obstetric procedures such as chorionic villous sampling permit the collection of fetal material to diagnose these conditions early enough in gestation to allow the use of IUSCT for treatment prior to the onset of irreversible organ damage. This enthusiasm is founded on the concept that establishment of graft tolerance without cytoablation and without GVHD can be accomplished in a cost effective manner. Unfortunately, at present, the only clinical applications for which IUSCT has proven successful are in diseases associated with host immunodeficiency[2,3].
As we reported in our earlier review, intrauterine transplantation (IUT) as a method emanated from attempts in the first half of the 20th century to understand self-tolerance and accept the concept of autoimmunity[1,4]. While these observations ruled out genetic determinism and identified fetal developmental events as responsible, it became clear in the latter half of the last century lead by the experiments of Le Douarin in birds, that how cellular deletion and cellular suppression (the main mechanisms underlying self-tolerance formation and maintenance) were formed during development were and are poorly understood[5]. Indeed, only recently have we identified the importance of and probably of more interest, why gestational timing is crucial to utilizing IUT both clinically and experimentally[6].
Nonetheless, once the concept that introduction of foreign antigens (i.e., HSCs) could be introduced into the fetus and persist, clinicians were encouraged that this breakthrough might offer a path to cure. Supporting evidence that IUSCT could work comes from the observations on naturally occurring chimerism in dizygotic twins in both large animals and humans. Here, the mixing of HSC through the placental circulation throughout gestation may result in enhanced sibling cell expression (> 30%)[7]. Natural chimerism is common[8]. In some veterinary diseases amelioration of disease phenotype has been attributed to sibling cell expression[9-11]. Experimental work transplanting a variety of HSC into large and small animal fetuses was supportive[2].
Clinical experience has been disappointing. In part this may be due to inadequate understanding of immune ontogeny and the importance of time in rendering tolerance. As often, procedures were performed after our proposed gestational tolerance window (approximately weeks 12-16 gestation) closes. However there are well-documented examples of proper timing and administration of an adequate SC dosage to treat a disease that theoretically should benefit for which there is no engraftment or if engrafted no clinically meaningful expression is seen[12,13]. What we hope to show is given recent experimental observations; a successful protocol for treatment of even non-immunodeficiency diseases is within our grasp.
In this report, we would like to expand both experimentalists’ and clinicians’ appreciation of the power of this procedure (ramifications). Our observations on immune ontogeny reveal IUT’s utility in unlocking events fundamental to understanding self-tolerance. Also, we believe it possible that this fetal tolerance phase in a large animal may allow in vivo investigation of biologic systems that may permit the development of alternative solutions to a number of clinical problems. Herein, we review its experimental background, propose the antigen exposure model to explain developmental acquisition of self-tolerance, examine impediments and promising areas for optimizing the procedure for treatment of childhood diseases and finally speculate regarding this technique’s utility as in vivo platform for therapeutics.
BACKGROUND
The discovery of common placental circulation between dizygotic twins as the explanation for the freemartin coupled with the development of erythrocyte antigen profiling in cattle allowed Ray Owen to determine that dizygotic twins were chimeric with their sibling’s blood cells after birth. Thus, he concluded that self-tolerance is acquired during fetal development and not innate[1,14]. Subsequent experiments in mice, sheep and cattle confirm that imprinting (Rapid time-dependent irreversible behavioral learning that occurs during development originally described by Karl Lorenz in young geese.) during fetal development is responsible for immune tolerance in adult life[15-17]. In direct experiments, fetal transplantation with allogeneic HSC, xenogeneic HSC or RTV (Gene expression following retroviral vector transfer in utero follows similar kinetics to that observed after cellular transplantation. We believe this is due to the establishment of recipient transplantation tolerance to the gene product hence we use transplantation rather than transfer.) in sheep reveals a gestational window of receptivity to engraftment mirroring the acquisition of self-tolerance[6,18]. The SC xenografts are highly expandable and are associated with extensive differentiation. Indeed, besides normal hematopoietic lineages, differentiated cardiac, gastrointestinal, liver and pancreatic islet cell activity can be demonstrated years after transplantation[19,20]. This window occurs in mice later in gestation but successful long-term engraftment and expression of both allogeneic and xenogeneic HSC has been realized[1,21-23]. For example, in Figure 1 we note bi-lineage human chimerism in a mouse following IUT at the proper gestational age; significant expression required graft stimulation with human growth factors (see below). In summary, self non-self discrimination is relative and time dependent.
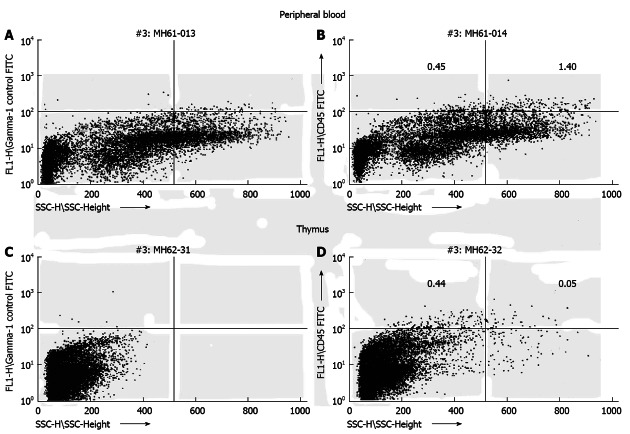
Figure 1.
Transplantation of human hematopoietic stem cell (CD34+) during the murine engraftment window results in bi-lineage expression and relevant cell migration of the differentiated human progeny 6 mo after transplantation. Human CD45 cells exhibit different side scatter characteristics (low side scatter: lymphocytes; high side scatter: granulocytes) dependent on organ tested (A, C: Control mouse; B, D: Experimental mouse)[23].
We have found performing allogeneic and xenogeneic IUT in sheep (a large animal) useful in the study of the HSC[24,25]. Formal study using timed gestational sheep identified the developmental event permissive for long-term engraftment receptivity as the period immediately following thymic demarcation (The timing of thymic demarcation into cortex and medulla varies with the size of the animal. In mice demarcation occurs at 66%, sheep 35% and humans 31% expiration of gestation. It is thought that the medulla is primarily responsible for deletional and cellular tolerance.). This phase is finite lasting no more than 30 d in sheep (term gestation 145 d) or 2 d in mice (term gestation 21 d). The ability to determine exactly when in gestation engraftment receptivity occurs permitted parallel experiments on lymphocyte ontogeny in sheep. These experiments identified the thymus as the site of immune activity (CD45 differentiation) during the transplant receptivity window. CD45 isoform differentiation occurring only in the thymus included all identifiable lineages: T cell, B cell and antigen presenting cell (APC). Unfortunately, ovine natural killer (NK) cell specific reagents were not available to track NK cell development. Evidence for thymic deletion of T and B cells is seen. It is important to note that these observations suggest B cell tolerogenesis does not occur in spleen, bone marrow or Peyer’s patches[6,26-30]. Studies using retroviral vector (RTV) transplantation in sheep demonstrate preferential thymic epithelial cell (TEC) expression of the gene product during this period with the development of specific lymphocyte unresponsiveness to the gene product and inability to generate viral specific antibody after birth[31,32]. Thus, B cell deletion (perhaps mediated by the developing “deletional TEC matrix”) is likely formed in the thymus during the window. Further, expression of the RTV gene product in the TECs in Hassall’s corpuscles (the proposed site Treg formation) and our phenotype experiments during this period (JSP, unpublished observations) suggest that, the onset of Treg formation occurs simultaneous with or near the conclusion of the tolerance window[31]. Reports by McCune and colleagues support this conclusion, as Tregs in humans are a predominant T cell phenotype after thymic demarcation. They further propose that fetal HSC may provide additional stimulus to this thymic differentiation cascade[33,34].
RAMIFICATIONS: DISSECTION OF SELF-TOLERANCE
These observations support a model for fetal tolerance induction where antigens (either autologous, allogeneic or xenogeneic) in the extracellular compartment of the developing thymus during this temporal phase are processed by the differentiating TEC progenitors and APC component of the thymic stroma and presented to developing immunocompetent cells (identifiable by changes in CD45 isoform expression) to form the tolerance repertoire. This two-stage antigen exposure model is presented in Figure 2. Presentation of surface antigens on cells intercalating the developing thymus during this finite period might also contribute. Endogenous gene derived presentation (to explain solid organ tolerance) is not consistent with our observations and unresponsiveness in the NK lineage must also be acquired during this period[35-42]. The extracellular antigen exposure model would explain the absence of rejection of both hematolymphoid and cellular components of solid organ xenografts and the marked expandability of grafts following transplantation[43,44]. It suggests that the NK cell compartment undergoes thymic imprinting during this critical phase to explain the absence of NK cell mediated rejection. Evidence supporting thymic processing and adaptability of NK cells is reported[42,45-47]. It seems unlikely that Treg(s) could account for the complete absence of NK cell mediated rejection (Figure 3)[48,49]. Thus these observations demonstrate the value of IUT as a method to explore mechanisms underlying induction of self non-self discrimination.
Figure 2.
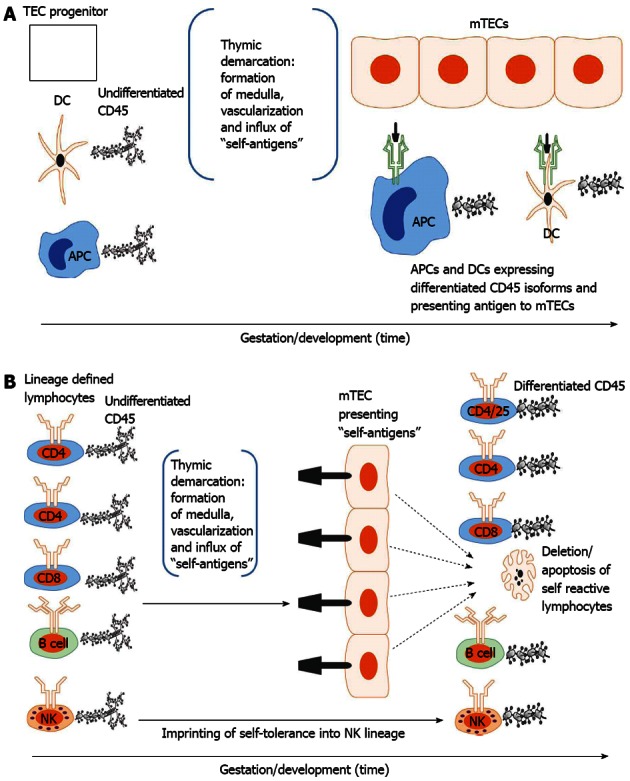
Developmental acquisition of self-tolerance: Antigen exposure model. A: Thymic epithelial (TEC) progenitors mature in intimate contact with dendritic cells (DCs) and likely other antigen presenting cells (APCs) thus forming the thymic medulla (i.e., thymic demarcation). With vascularization, self-antigens (i.e., antigens present during the finite tolerance window) from the periphery are processed and presented to medullary thymic epithelial cells (mTECs). The developing mTEC matrix is only receptive to antigen presentation during the tolerance window (time dependence) and subsequently exhibits on its surface the self-antigen repertoire (see 3. Ramifications: Dissection of immune tolerance); B: All lymphoid compartments [T, B and natural killer (NK) cell] enter the thymus expressing undifferentiated CD45 but are lineage defined. They then migrate through the thymus encountering mTECs expressing the self-antigen repertoire. CD4, CD8 and B cells reactive to self-antigens are deleted. CD4/CD25 cells reactive to self-antigens are rendered suppressive (Treg) in Hassal’s corpuscles. NK cells are imprinted in an undefined fashion (likely based on major histocompatibility complex exposure) to become tolerant to self. All differentiated lymphocytes (i.e., cells that have undergone CD45 isoform maturation and are not self-reactive or are tolerogenic (i.e.,Treg) then migrate to the spleen or other secondary lymphoid organs (see 3. Ramifications: Dissection of self-tolerance)[6,21,31-37,42].
Figure 3.
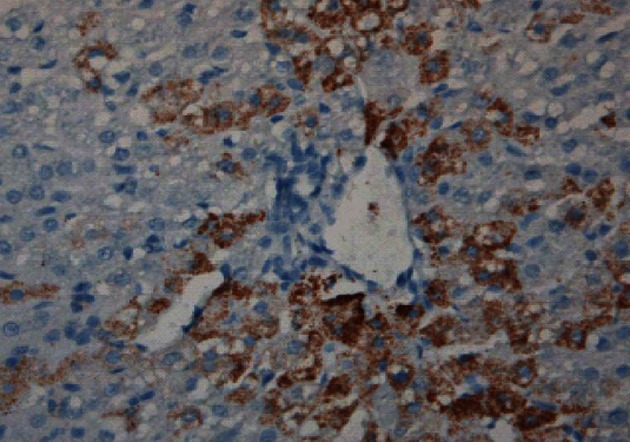
Induction of sheep tolerance to human hepatocytes by transplantation of human hematopoietic stem cells during the temporal engraftment window (note absence of sheep round cell infiltration). Sheep liver stained for human specific antibody 11 mo following transplantation[49].
RAMIFICATIONS: TREATMENT OF CHILDHOOD HEMATOLYMPHOID DISEASES
Xenotransplantation of human HSC following IUT in sheep and mice results in human cell engraftment and differentiation. In sheep this includes cardiac, liver, intestinal and pancreatic cells. Differentiated cellular function manifested by identification of circulating human proteins in transplanted sheep and mice is presented in Table 1[23,49-52]. The absence of rapid circulatory clearance speaks to the profound ability of IUT to alter the self non-self paradigm. IUSCT is thought to provide a number of therapeutic advantages over postnatal transplantation besides tolerance including expanding bone marrow space (or niche), sterile environment, proliferative environment and preempting clinical disease. Diseases thought amenable to IUT include hemoglobinopathies, immunodeficiency states and inborn errors of metabolism leading to storage diseases (mucopolysaccharidosis and mucolipidosis)[1].
Table 1.
Human proteins detected in circulation of animals transplanted in utero with human stem cells
| Animal transplanted | Human protein detected |
| Sheep | IgM1 |
| Sheep | Albumin[47,48] |
| Sheep | Factor VIII1 |
| Sheep | C-peptide[49,50] |
| Sheep | α-fetoprotein1 |
| Mouse | IgM[23] |
Unpublished observation by Esmail D Zanjani. IgM: Immunoglobulin M.
As Figures 4 and 5 reveal maximizing cell dosage and serial cell administrations can improve donor engraftment yet, there remain a set of problems in achieving adequate donor proliferation to ameliorate and/or cure these diseases[2]. The parameters proposed that may be limiting successful clinical application include: (1) failure to induce tolerance and/or an inadequate engraftment frequency (chimeric incidence); and (2) limited graft expression: gestational developmental impediments, maternal derived inhibition, lack of selective advantage (i.e., inadequate donor proliferation).
Figure 4.
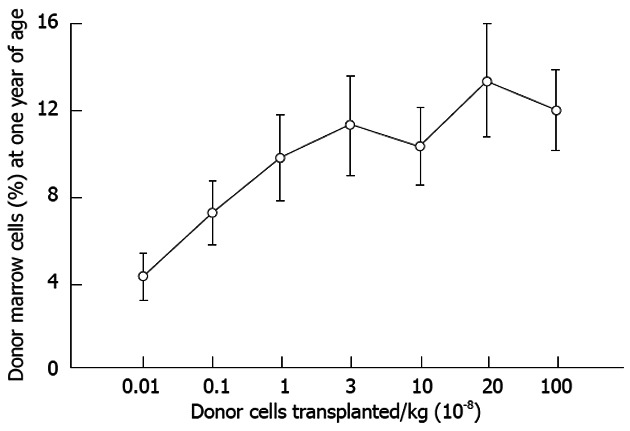
Levels of allogeneic engraftment plateau with transplantation of log-fold increases in donor cells in sheep. This is most consistent with a model of saturation kinetics with engraftment limited by available receptive sites. An identical curve is seen in the xenogeneic human-sheep model[2].
Figure 5.
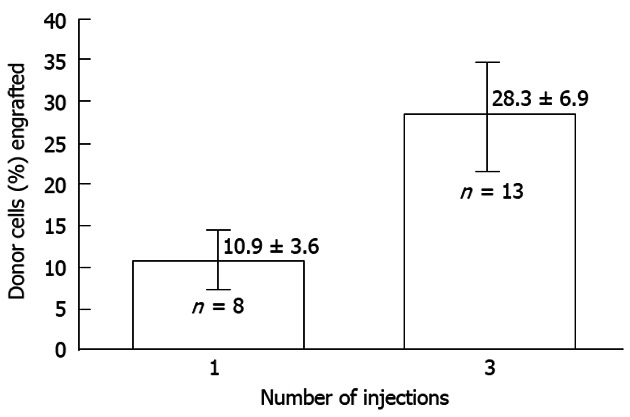
The effect of dividing cell dose on engraftment in sheep. Transplantation of the same total number of cells as a single injection vs a series of three injections given 1 wk apart is shown. The engraftment with divided doses is significantly higher supporting the concept that engraftment is limited at any particular time by the number of available receptive sites[2].
Tolerance/engraftment following IUT
Both natural and experimentally derived hematopoietic chimeras demonstrate immune tolerance using skin graft acceptance and mixed lymphocyte reactions. Deletional and cellular tolerance has been reported[16,43,53-55]. Marrow engraftment levels 60 d after HSC transplantation in sheep using xenogeneic or allogeneic HSC reveals a parallel kinetic profile with a peak level when transplantation is performed between days 65-70 gestation (Figure 6). This suggests accurate gestational timing may be critical to accessing the full panoply of immune tolerance mechanisms (i.e., deletion, regulatory cell formation and anergy among all immune competent lineages) forming during the window[56]. Transplantation before the onset of the window and maternal derived histoincompatibility may limit engraftment or expression but in general tolerance is achieved[6,54,55,57-60]. The short tolerance induction period in mice may contribute to occasional microchimerism and NK cell mediated rejection[21,57,58]. In sheep and mice, engraftment and/or expression can be augmented long after birth (i.e., further supporting the achievement of long-term immune tolerance and memory). In Table 2, groups of 6 sheep were assessed for tolerance to allogeneic HSC and then re-transplanted after birth with same donor HSC resulting in marked improvement in the level of engraftment. Flake has demonstrated similar findings in the allogeneic mouse model[43]. In a similar vein, mixed lymphocyte reaction unresponsiveness has been detected following xenogeneic in utero transplantation (unpublished observations).
Figure 6.
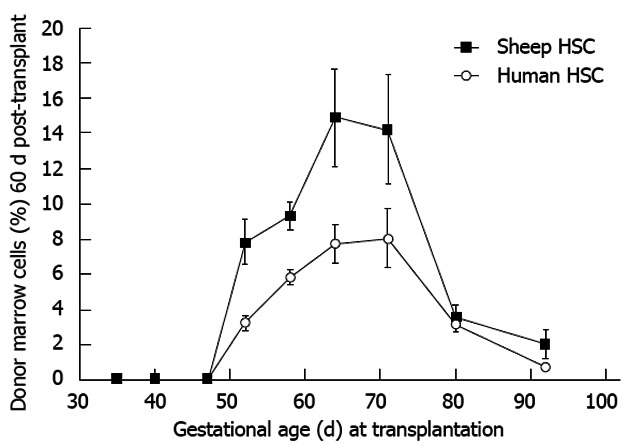
Allo- or xenogeneic stem cell bone marrow engraftment in sheep following transplantation during the temporal window when self non-self discrimination occurs (note parallel kinetics). Engraftment receptivity is gestational age-dependent[6].
Table 2.
Groups of 6 sheep were assessed for tolerance to allogeneic human stem cells and then re-transplanted after birth with same donor human stem cells
| Allogeneic sheep HSC render recipient sheep1 tolerant following in utero transplantation | ||
| Stimulator | Responder | Stimulation index2 |
| Donor | Donor | 0 |
| Recipient | Recipient | 0 |
| Donor | Recipient | 0-8 |
| Recipient | Donor | 58 ± 11 |
| Pooled | Donor | 69 ± 12 |
| Pooled | Recipient | 78 ± 12 |
| Postnatal infusion of allogeneic same donor HSC augments engraftment in tolerant sheep3 | ||
| % donor cells at birth | n | % increase4 |
| 6-10 | 4 | 86 ± 29 |
| 11-15 | 5 | 63 ± 22 |
| > 15 | 4 | 21 ± 11 |
As noted above, if performed during the proper phase in gestation, the SC engraftment incidence is high in both large and small animal models. Mouse models are hampered by the short induction period and multiple small fetuses to inject resulting in a diminished engraftment frequency. In large animals, the engraftment frequency is considerably higher. For example, less than exhaustive examination of the pancreas in sheep revealed a chimeric incidence of 79%[51].
Limited graft expression following IUT
Gestational developmental impediments: The course of hematopoietic ontogeny is associated with an orderly and predictable switch in primary sites of hematopoiesis. Mammalian hematopoietic sites are gestational age dependent starting in the yolk sac migrating to liver and spleen then finally lodging in the bone marrow. Yet, the marrow contributes little to circulating peripheral blood until just prior to birth. The mechanisms underlying this migration to marrow reflect bone and marrow ontogeny including formation of the osteoblastic niche. With regard to transplanted HSC, we reported a similar engraftment gradient dependent on bone and marrow maturity with little peripheral blood expression[61-63]. This osteoblastic niche (site of primitive HSC engraftment) matures by day 65 and may in part influence the peak in marrow engraftment seen following transplantation (Figures 6 and 7). But again, the liver/spleen remains the main site of hematopoiesis until just prior to birth. These represent serious homing and maturational impediments to achieving therapeutic levels of donor cell expression to treat diseases that are clinically evident during gestation.
Figure 7.
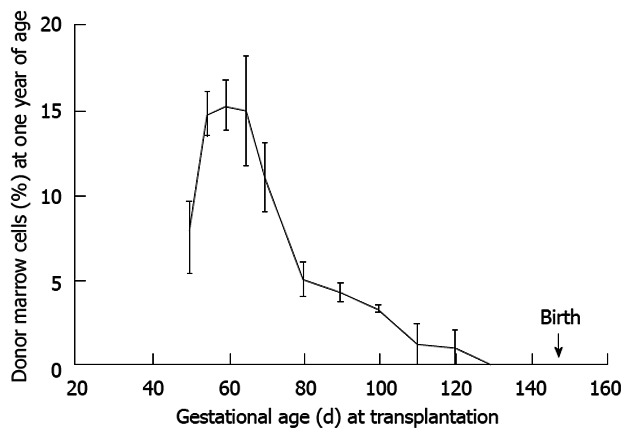
Maximal donor cell engraftment in sheep occurs during the mid-to-later stages of the tolerance induction period. This period follows thymic demarcation and formation of the osteoblastic niche. Further, engraftment is durable as the engraftment kinetics are similar to that noted at 60 d post transplant supporting our contention that the graft is viewed by the recipient as self (see Figure 6)[2].
Maternal-derived inhibition: Maternal derived inhibition either through histoincompatible maternal lymphocyte microchimerism or maternal alloantibodies may limit engraftment/expression. To address this issue, we observed that maternal histocompatible donor grafts from matched cord blood improved peripheral blood donor expression (2.75% vs 0.93%) in comparison to relatively mismatched grafts[62].
Inadequate competitive advantage to promote donor expression: Allogeneic SCs can repair genetic hematologic defects due allograft responsiveness to endogenous growth factors[22,64-66]. Donor-species-specific growth factors improve graft expression in xenografts confirming establishment of tolerance and importance of donor stimulation in either an allogeneic or xenogeneic environment following IUT[1,44]. A promising approach that needs further examination is the use of autologous stroma. In our hands, co-transplantation improved graft expression (donor hemoglobin) during gestation suggesting this may be a useful tack to treat diseases overtly manifest during gestation (Figure 8)[67].
Figure 8.
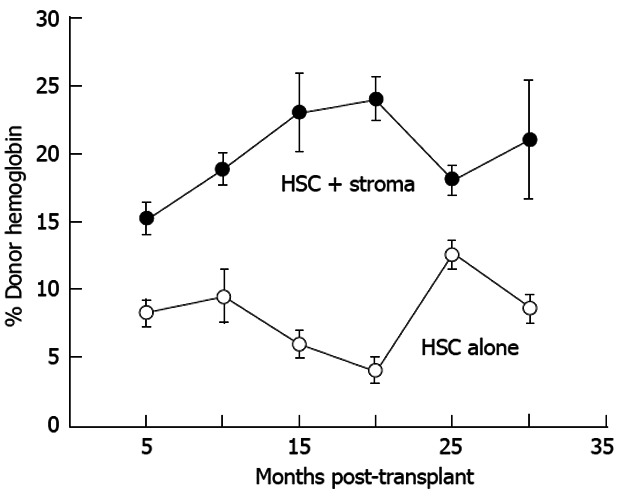
Co-transplantation with autologous adult sheep stromal cells improves allogeneic donor hematopoietic stem cell expression. Donor expression (circulating hemoglobin) persists more that 30 mo after transplantation. Donor hemoglobin levels in the peripheral blood of sheep co-transplanted with autologous adult bone marrow stroma (n = 4) are significantly higher (P < 0.01) than those transplanted with adult T-depleted bone marrow mononuclear cells alone (n = 3)[67]. HSC: Human stem cell.
The end of the tolerance induction phase in large animals is followed by exponential growth[6]. Thus, augmentation of donor engraftment/expression with transplantation of high dose donor SC and/or autologous stroma during this exponential growth phase to compete with endogenous/recipient SC differentiation and expansion should prove fruitful. Late retransplantation would mimic the mixing of hematopoietic cells in natural chimera that occurs through the placental circulation during and after closure of the gestational tolerance window and can result in the enhanced expression of sibling cells. The sheep is the maternal/fetal system most stable to test this hypothesis[68]. Studies in mice support this notion as endogenous marrow ablation after birth with retransplantation of donor HSC can essentially replace recipient cells[69].
Clinical experience and roadmap for implementation
Therapeutic success following IUT in fetuses diagnosed with a variety of genetic diseases has been disappointing. In repair of the severe combined immune deficiency (SCID) defect, the transplanted cells have a competitive advantage and a poorly populated functional organ in which differentiation may occur[3]. Yet, there are well-documented examples of proper timing and administration of an adequate SC dosage to treat a disease that theoretically should benefit for which limited graft expression is seen[12,13]. Moving forward will require a more coordinated approach, a clinical trial in an ideal candidate disease.
This coordinated approach should identify a candidate disease that will provide “proof principle” that our theories are correct in a clinical setting. While at first glance one might conjecture that transplantation in X-linked SCID was successful because the fetus is immunodeficient, an alternative and more likely explanation is that an under populated developing thymus provided important stimulus for expansion and expression of the donor graft (a proliferative stimulus similar to Mintz’s demonstration in anemic mice)[22]. For this reason thalasemia is a poor disease with which to test the feasibility of IUT. Here there is often marked over growth of endogenous marrow cells limiting donor cell engraftment during development[1]. In a similar fashion, failure to observe enhanced expression in mismatched allogeneic grafts in sheep suggest that either maternal derived inhibition limits donor expression or autologous stromal factors are required to augment graft expression. Likely mechanisms would be through either stromal major histocompatability complex matching to preferentially nurture the developing autologous SC or autocrine or paracrine factors which specifically stimulate autologous SC. Thus histocompatability is an important factor not due to potential recipient immune inhibition (i.e., failure of tolerance induction) but by providing a graft that is not prone to maternal immune inhibition or providing (as yet undefined) autologous factors supporting graft expression (evidenced by our report demonstrating improved peripheral blood expression during development in allogeneic grafts co-transplanted with autologous stroma, Figure 8)[67].
In summary, IUT recapitulates normal acquisition of self-tolerance. Experimentally, tolerance and engraftment are readily achieved. The problem remains identifying a disease that will promote graft expansion, limit maternal interference or selectively promote the graft through autologous stimulation. This likely requires retransplantation either late in gestation or shortly after birth (during exponential growth) to provide a dosing advantage to allow adequate numbers of donor HSC to compete with recipient HSC to ameliorate disease in this tolerant environment. Risks such as procedural, the possible need for multiple IUT procedures, infection and GVHD likely are not prohibitive[2]. Since the majority of the genetic disorders correctible with HSCT can be diagnosed early in gestation to allow for IUHSCT, it is important that attempts be made to correct these deficiencies before birth. Use of compatible donors, determining the optimal gestational age and co-transplantation of donor-derived stroma/mesenchymal SCs may help achieve this end.
Ramifications: In vivo platform for therapeutics
The formation of differentiated human cells/organs (without evidence for donor/recipient cell fusion) and functional human proteins following transplantation allows speculation as to the feasibility of IUT in applied therapeutics in an autologous or allogeneic setting. This would necessitate resolving problems such as cell/protein separation and isolation, as well as contamination with and transmission of infectious agents. Yet, development of effective methods to mitigate these impediments could unlock uncharted therapeutic possibilities. The finding of differentiated human functional proteins and evidence that engraftment/expression can be expanded before or after birth offers the possibility that IUT in a large animal could provide a source of autologous human proteins. In a similar fashion, effective cell isolation (hematopoietic cells seem the most feasible) might offer the possibility of autologous cell transfusion for clinical support.
While the above possible applications of IUT may be better served using ex vivo bioreactors or systems, in vivo use of sheep or an alternative animal of similar size to humans may be useful with regard to development of organs for transplantation. Our laboratory has been investigating this with regard to the liver and endocrine pancreas[49-52]. Our studies involving the endocrine pancreas have demonstrated long-term circulating human insulin following SC transplantation. The ease with which islets can be isolated for transplantation makes this an ideal organ to determine feasibility of IUT as a therapeutic modality for human organ transplantation. For both systems, the critical determinant will be the role of a single SC (or as Owen described them embryonal ancestral cells) in organ repair[1,70].
ACKNOWLEDGMENTS
The contents of this publication do not represent the views of the Department of Veterans Affairs or the United States Government. This report is the result of work partially supported by VA Sierra Nevada Health Care System (Reno, NV, United States) staff resources.
The representations of CD45 used in Figure 2 were obtained from Annual Review of Immunology 21 (2003) 107-37. CD45: a critical regulator of signaling thresholds in immune cells, Hermiston ML, Xu Z, Weiss A, authors; Sanford H Barsky for review of Figure 3; Richard Cacciato, Librarian, VA Sierra Nevada Health Care System.
Footnotes
Supported by Grant HL53955 from NHLBI, NIH, in part
P- Reviewer Guo ZK S- Editor Song XX L- Editor A E- Editor Li JY
References
- 1.Pixley JS, Mackintosh FR, Zanjani ED. Experimental and clinical basis of intrauterine stem cell transplantation. Rev Clin Exp Hematol. 1999;8:11–32. [Google Scholar]
- 2.Flake AW, Zanjani ED. In Utero Transplantation. In: Applebaum FR, Forman SJ, Negrin RS, Blume KG, editors. Thomas’ Hematopoietic Cell Transplantation. 4th ed. Oxford: Wiley-Blackwell Publishing; 2009. pp. 577–589. [Google Scholar]
- 3.Flake AW, Roncarolo MG, Puck JM, Almeida-Porada G, Evans MI, Johnson MP, Abella EM, Harrison DD, Zanjani ED. Treatment of X-linked severe combined immunodeficiency by in utero transplantation of paternal bone marrow. N Engl J Med. 1996;335:1806–1810. doi: 10.1056/NEJM199612123352404. [DOI] [PubMed] [Google Scholar]
- 4.Silverstein AM. Autoimmunity versus horror autotoxicus: the struggle for recognition. Nat Immunol. 2001;2:279–281. doi: 10.1038/86280. [DOI] [PubMed] [Google Scholar]
- 5.Coutinho A. The Le Douarin phenomenon: a shift in the paradigm of developmental self-tolerance. Int J Dev Biol. 2005;49:131–136. doi: 10.1387/ijdb.041965ac. [DOI] [PubMed] [Google Scholar]
- 6.Skopal-Chase JL, Pixley JS, Torabi A, Cenariu MC, Bhat A, Thain DS, Frederick NM, Groza DM, Zanjani ED. Immune ontogeny and engraftment receptivity in the sheep fetus. Fetal Diagn Ther. 2009;25:102–110. doi: 10.1159/000203399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hansen HE, Niebuhr E, Lomas C. Chimeric twins. T.S. and M.R. reexamined. Hum Hered. 1984;34:127–130. doi: 10.1159/000153448. [DOI] [PubMed] [Google Scholar]
- 8.van Dijk BA, Boomsma DI, de Man AJ. Blood group chimerism in human multiple births is not rare. Am J Med Genet. 1996;61:264–268. doi: 10.1002/(SICI)1096-8628(19960122)61:3<264::AID-AJMG11>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 9.Jolly RD, Thompson KG, Murphy CE, Manktelow BW, Bruere AN, Winchester BG. Enzyme replacement therapy--an experiment of nature in a chimeric mannosidosis calf. Pediatr Res. 1976;10:219–224. doi: 10.1203/00006450-197604000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Jones MZ, Cavanagh KT, Kranich R, Traviss C, Fujita Y, Ohta M, Matsuura F. Possible beta-mannosidosis chimera. Altered expression of metabolic perturbations. J Inherit Metab Dis. 1993;16:1012–1023. doi: 10.1007/BF00711519. [DOI] [PubMed] [Google Scholar]
- 11.Howell JM, Dorling PR, Shelton JN, Taylor EG, Palmer DG, Di Marco PN. Natural bone marrow transplantation in cattle with Pompe’s disease. Neuromuscul Disord. 1991;1:449–454. doi: 10.1016/0960-8966(91)90008-g. [DOI] [PubMed] [Google Scholar]
- 12.Shields LE, Lindton B, Andrews RG, Westgren M. Fetal hematopoietic stem cell transplantation: a challenge for the twenty-first century. J Hematother Stem Cell Res. 2002;11:617–631. doi: 10.1089/15258160260194767. [DOI] [PubMed] [Google Scholar]
- 13.Touraine JL, Raudrant D, Golfier F, Rebaud A, Sembeil R, Roncarolo MG, Bacchetta R, D’Oiron R, Lambert T, Gebuhrer L. Reappraisal of in utero stem cell transplantation based on long-term results. Fetal Diagn Ther. 2004;19:305–312. doi: 10.1159/000077957. [DOI] [PubMed] [Google Scholar]
- 14.Owen RD. Immunogenetic consequences of vascular anastomoses between bovine twins. Science. 1945;102:400–401. doi: 10.1126/science.102.2651.400. [DOI] [PubMed] [Google Scholar]
- 15.Billingham RE, Brent L, Medawar PB. Actively acquired tolerance of foreign cells. Nature. 1953;172:603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- 16.Anderson D, Billingham RE, Lampkin GH, Medawar PB. The use of skin grafting to distinguish between monozygotic and dizygotic twins in cattle. Heredity. 1951;5:379–397. [Google Scholar]
- 17.Silverstein AM, Prendergast RA, Kraner KL. Fetal response to antigenic stimulus. iv. rejection of skin homografts by the fetal lamb. J Exp Med. 1964;119:955–964. doi: 10.1084/jem.119.6.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Porada CD, Park PJ, Almeida-Porada G, Liu W, Ozturk F, Glimp HA, Zanjani ED. Gestational age of recipient determines pattern and level of transgene expression following in utero retroviral gene transfer. Mol Ther. 2005;11:284–293. doi: 10.1016/j.ymthe.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 19.Liechty KW, MacKenzie TC, Shaaban AF, Radu A, Moseley AM, Deans R, Marshak DR, Flake AW. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat Med. 2000;6:1282–1286. doi: 10.1038/81395. [DOI] [PubMed] [Google Scholar]
- 20.Colletti EJ, Airey JA, Liu W, Simmons PJ, Zanjani ED, Porada CD, Almeida-Porada G. Generation of tissue-specific cells from MSC does not require fusion or donor-to-host mitochondrial/membrane transfer. Stem Cell Res. 2009;2:125–138. doi: 10.1016/j.scr.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fairchild PJ, Waldmann H. Extrathymic signals regulate the onset of T cell repertoire selection. Eur J Immunol. 2000;30:1948–1956. doi: 10.1002/1521-4141(200007)30:7<1948::AID-IMMU1948>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 22.Fleischman RA, Mintz B. Prevention of genetic anemias in mice by microinjection of normal hematopoietic stem cells into the fetal placenta. Proc Natl Acad Sci USA. 1979;76:5736–5740. doi: 10.1073/pnas.76.11.5736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pixley JS, Zanjani ED, Shaft DM, Porada C, Mackintosh FR. Prolonged hematopoietic chimerism in normal mice transplanted in utero with human hematopoietic stem cells. Pathobiology. 1998;66:230–239. doi: 10.1159/000028028. [DOI] [PubMed] [Google Scholar]
- 24.Zanjani ED, Almeida-Porada G, Livingston AG, Zeng H, Ogawa M. Reversible expression of CD34 by adult human bone marrow long-term engrafting hematopoietic stem cells. Exp Hematol. 2003;31:406–412. doi: 10.1016/s0301-472x(03)00051-1. [DOI] [PubMed] [Google Scholar]
- 25.Zanjani ED. The human sheep xenograft model for the study of the in vivo potential of human HSC and in utero gene transfer. Stem Cells. 2000;18:151. doi: 10.1634/stemcells.18-2-151. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz RH, Mueller DL. Immunological tolerance. In: Paul WE, editor. Fundamental Immunology. 6th ed. New York: Lippincott-Raven Press; 2003. pp. 898–942. [Google Scholar]
- 27.Hardy RR. B lymphocyte development and biology. In: Paul WE, editor. Fundamental Immunology. 6th ed. New York: Lippincott-Raven Press; 2003. pp. 237–269. [Google Scholar]
- 28.Hardy RR, Hayakawa K. B cell development pathways. Annu Rev Immunol. 2001;19:595–621. doi: 10.1146/annurev.immunol.19.1.595. [DOI] [PubMed] [Google Scholar]
- 29.Reynolds JD. Evidence of extensive lymphocyte death in sheep Peyer’s patches. I. A comparison of lymphocyte production and export. J Immunol. 1986;136:2005–2010. [PubMed] [Google Scholar]
- 30.Griebel PJ, Hein WR. Expanding the role of Peyer’s patches in B-cell ontogeny. Immunol Today. 1996;17:30–39. doi: 10.1016/0167-5699(96)80566-4. [DOI] [PubMed] [Google Scholar]
- 31.Colletti E, Lindstedt S, Park PJ, Almeida-Porada G, Porada CD. Early fetal gene delivery utilizes both central and peripheral mechanisms of tolerance induction. Exp Hematol. 2008;36:816–822. doi: 10.1016/j.exphem.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tran ND, Porada CD, Almeida-Porada G, Glimp HA, Anderson WF, Zanjani ED. Induction of stable prenatal tolerance to beta-galactosidase by in utero gene transfer into preimmune sheep fetuses. Blood. 2001;97:3417–3423. doi: 10.1182/blood.v97.11.3417. [DOI] [PubMed] [Google Scholar]
- 33.Mold JE, Michaëlsson J, Burt TD, Muench MO, Beckerman KP, Busch MP, Lee TH, Nixon DF, McCune JM. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science. 2008;322:1562–1565. doi: 10.1126/science.1164511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mold JE, Venkatasubrahmanyam S, Burt TD, Michaëlsson J, Rivera JM, Galkina SA, Weinberg K, Stoddart CA, McCune JM. Fetal and adult hematopoietic stem cells give rise to distinct T cell lineages in humans. Science. 2010;330:1695–1699. doi: 10.1126/science.1196509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodewald HR, Paul S, Haller C, Bluethmann H, Blum C. Thymus medulla consisting of epithelial islets each derived from a single progenitor. Nature. 2001;414:763–768. doi: 10.1038/414763a. [DOI] [PubMed] [Google Scholar]
- 36.Nishikawa Y, Hirota F, Yano M, Kitajima H, Miyazaki J, Kawamoto H, Mouri Y, Matsumoto M. Biphasic Aire expression in early embryos and in medullary thymic epithelial cells before end-stage terminal differentiation. J Exp Med. 2010;207:963–971. doi: 10.1084/jem.20092144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsumoto M. Contrasting models for the roles of Aire in the differentiation program of epithelial cells in the thymus. Eur J Immunol. 2011;41:12–17. doi: 10.1002/eji.201041024. [DOI] [PubMed] [Google Scholar]
- 38.Tykocinski LO, Sinemus A, Kyewski B. The thymus medulla slowly yields its secrets. Ann N Y Acad Sci. 2008;1143:105–122. doi: 10.1196/annals.1443.018. [DOI] [PubMed] [Google Scholar]
- 39.Tykocinski LO, Sinemus A, Rezavandy E, Weiland Y, Baddeley D, Cremer C, Sonntag S, Willecke K, Derbinski J, Kyewski B. Epigenetic regulation of promiscuous gene expression in thymic medullary epithelial cells. Proc Natl Acad Sci USA. 2010;107:19426–19431. doi: 10.1073/pnas.1009265107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yokoyama WM. Natural Killer Cells. In: Paul WE, editor. Fundamental Immunology. 6th ed. New York: Lippincott-Raven Press; 2003. pp. 483–517. [Google Scholar]
- 41.Yokoyama WM, Altfeld M, Hsu KC. Natural killer cells: tolerance to self and innate immunity to viral infection and malignancy. Biol Blood Marrow Transplant. 2010;16:S97–S105. doi: 10.1016/j.bbmt.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun JC, Lanier LL. NK cell development, homeostasis and function: parallels with CD8+ T cells. Nat Rev Immunol. 2011;11:645–657. doi: 10.1038/nri3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peranteau WH, Hayashi S, Hsieh M, Shaaban AF, Flake AW. High-level allogeneic chimerism achieved by prenatal tolerance induction and postnatal nonmyeloablative bone marrow transplantation. Blood. 2002;100:2225–2234. doi: 10.1182/blood-2002-01-0166. [DOI] [PubMed] [Google Scholar]
- 44.Almeida-Porada G, Porada C, Gupta N, Torabi A, Thain D, Zanjani ED. The human-sheep chimeras as a model for human stem cell mobilization and evaluation of hematopoietic grafts’ potential. Exp Hematol. 2007;35:1594–1600. doi: 10.1016/j.exphem.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Leary JG, Goodarzi M, Drayton DL, von Andrian UH. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol. 2006;7:507–516. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- 46.Spits H, Blom B, Jaleco AC, Weijer K, Verschuren MC, van Dongen JJ, Heemskerk MH, Res PC. Early stages in the development of human T, natural killer and thymic dendritic cells. Immunol Rev. 1998;165:75–86. doi: 10.1111/j.1600-065x.1998.tb01231.x. [DOI] [PubMed] [Google Scholar]
- 47.Vaz F, Srour EF, Almeida-Porada G, Ascensao JL. Human thymic stroma supports human natural killer (NK) cell development from immature progenitors. Cell Immunol. 1998;186:133–139. doi: 10.1006/cimm.1998.1303. [DOI] [PubMed] [Google Scholar]
- 48.Ghiringhelli F, Ménard C, Terme M, Flament C, Taieb J, Chaput N, Puig PE, Novault S, Escudier B, Vivier E, et al. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. J Exp Med. 2005;202:1075–1085. doi: 10.1084/jem.20051511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Almeida-Porada G, Porada CD, Chamberlain J, Torabi A, Zanjani ED. Formation of human hepatocytes by human hematopoietic stem cells in sheep. Blood. 2004;104:2582–2590. doi: 10.1182/blood-2004-01-0259. [DOI] [PubMed] [Google Scholar]
- 50.Chamberlain J, Yamagami T, Colletti E, Theise ND, Desai J, Frias A, Pixley J, Zanjani ED, Porada CD, Almeida-Porada G. Efficient generation of human hepatocytes by the intrahepatic delivery of clonal human mesenchymal stem cells in fetal sheep. Hepatology. 2007;46:1935–1945. doi: 10.1002/hep.21899. [DOI] [PubMed] [Google Scholar]
- 51.Ersek A, Pixley JS, Goodrich AD, Porada CD, Almeida-Porada G, Thain DS, Zanjani ED. Persistent circulating human insulin in sheep transplanted in utero with human mesenchymal stem cells. Exp Hematol. 2010;38:311–320. doi: 10.1016/j.exphem.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goodrich AD, Ersek A, Varain NM, Groza D, Cenariu M, Thain DS, Almeida-Porada G, Porada CD, Zanjani ED. In vivo generation of beta-cell-like cells from CD34(+) cells differentiated from human embryonic stem cells. Exp Hematol. 2010;38:516–525.e4. doi: 10.1016/j.exphem.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Picus J, Aldrich WR, Letvin NL. A naturally occurring bone-marrow-chimeric primate. I. Integrity of its immune system. Transplantation. 1985;39:297–303. doi: 10.1097/00007890-198503000-00018. [DOI] [PubMed] [Google Scholar]
- 54.Carrier E, Lee TH, Busch MP, Cowan MJ. Induction of tolerance in nondefective mice after in utero transplantation of major histocompatibility complex-mismatched fetal hematopoietic stem cells. Blood. 1995;86:4681–4690. [PubMed] [Google Scholar]
- 55.Kim HB, Shaaban AF, Milner R, Fichter C, Flake AW. In utero bone marrow transplantation induces donor-specific tolerance by a combination of clonal deletion and clonal anergy. J Pediatr Surg. 1999;34:726–729; discussion 729-730. doi: 10.1016/s0022-3468(99)90364-0. [DOI] [PubMed] [Google Scholar]
- 56.Tai X, Van Laethem F, Pobezinsky L, Guinter T, Sharrow SO, Adams A, Granger L, Kruhlak M, Lindsten T, Thompson CB, et al. Basis of CTLA-4 function in regulatory and conventional CD4(+) T cells. Blood. 2012;119:5155–5163. doi: 10.1182/blood-2011-11-388918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sefrioui H, Donahue J, Srivastava AS, Gilpin E, Lee TH, Carrier E. Alloreactivity following in utero transplantation of cytokine-stimulated hematopoietic stem cells: the role of recipient CD4(-) cells. Exp Hematol. 2002;30:617–624. doi: 10.1016/s0301-472x(02)00803-2. [DOI] [PubMed] [Google Scholar]
- 58.Durkin ET, Jones KA, Rajesh D, Shaaban AF. Early chimerism threshold predicts sustained engraftment and NK-cell tolerance in prenatal allogeneic chimeras. Blood. 2008;112:5245–5253. doi: 10.1182/blood-2007-12-128116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Merianos DJ, Tiblad E, Santore MT, Todorow CA, Laje P, Endo M, Zoltick PW, Flake AW. Maternal alloantibodies induce a postnatal immune response that limits engraftment following in utero hematopoietic cell transplantation in mice. J Clin Invest. 2009;119:2590–2600. doi: 10.1172/JCI38979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nijagal A, Wegorzewska M, Jarvis E, Le T, Tang Q, MacKenzie TC. Maternal T cells limit engraftment after in utero hematopoietic cell transplantation in mice. J Clin Invest. 2011;121:582–592. doi: 10.1172/JCI44907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tavian M, Péault B. The changing cellular environments of hematopoiesis in human development in utero. Exp Hematol. 2005;33:1062–1069. doi: 10.1016/j.exphem.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 62.Jeanblanc C, Colletti EJ, Porada CD, Almeida-Porada G, Zanjani E. Successful in utero hematopoietic stem cell transplantation likely depends on niche maturity, recipient /donor MHC compatibility and donor cell origin. Blood. 2010;116:A574. [Google Scholar]
- 63.Zanjani ED, Ascensao JL, Tavassoli M. Liver-derived fetal hematopoietic stem cells selectively and preferentially home to the fetal bone marrow. Blood. 1993;81:399–404. [PubMed] [Google Scholar]
- 64.Blazar BR, Taylor PA, Vallera DA. Adult bone marrow-derived pluripotent hematopoietic stem cells are engraftable when transferred in utero into moderately anemic fetal recipients. Blood. 1995;85:833–841. [PubMed] [Google Scholar]
- 65.Blazar BR, Taylor PA, Vallera DA. In utero transfer of adult bone marrow cells into recipients with severe combined immunodeficiency disorder yields lymphoid progeny with T- and B-cell functional capabilities. Blood. 1995;86:4353–4366. [PubMed] [Google Scholar]
- 66.Casal ML, Wolfe JH. In utero transplantation of fetal liver cells in the mucopolysaccharidosis type VII mouse results in low-level chimerism, but overexpression of beta-glucuronidase can delay onset of clinical signs. Blood. 2001;97:1625–1634. doi: 10.1182/blood.v97.6.1625. [DOI] [PubMed] [Google Scholar]
- 67.Almeida-Porada G, Flake AW, Glimp HA, Zanjani ED. Cotransplantation of stroma results in enhancement of engraftment and early expression of donor hematopoietic stem cells in utero. Exp Hematol. 1999;27:1569–1575. doi: 10.1016/s0301-472x(99)00090-9. [DOI] [PubMed] [Google Scholar]
- 68.Delano ML, Mischler SA, Underwood WJ. Biology and diseases of ruminants: sheep, goats and cattle. In: Fox JG, Anderson LC, Loew FM, Quimby FW, editors. Laboratory Animal Medicine. 2nd ed. New York, NY: Academic Press; 2002. pp. 519–614. [Google Scholar]
- 69.Ashizuka S, Peranteau WH, Hayashi S, Flake AW. Busulfan-conditioned bone marrow transplantation results in high-level allogeneic chimerism in mice made tolerant by in utero hematopoietic cell transplantation. Exp Hematol. 2006;34:359–368. doi: 10.1016/j.exphem.2005.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]