Abstract
AIM: To compare the phenotypic and neural differentiation potential of human bone marrow derived multipotent adult progenitor cells (MAPC) and mesenchymal stem cells (MSC).
METHODS: Cultures of MAPC and MSC were established in parallel from same samples of human bone marrow (n = 5). Both stem cell types were evaluated for expression of pluripotency markers including Oct-4 and Nanog by immunocytochemistry and reverse-transcription polymerase chain reaction (RT-PCR) and expression of standard mesenchymal markers including CD14, CD34, CD44, CD45, CD73, CD90, CD105 and human leukocyte antigen (HLA)-ABC by flow cytometry. After treatment with neural induction medium both MAPC and MSC were evaluated for expression of neural proteins [neuronal filament-200 (NF-200) and glial fibrillar acidic protein (GFAP)] by immunocytochemistry and Western blotting and neural genes [NF-200, GFAP, Tau, microtubule-associated protein (MAP)-1B, MAP-2, neuron-specific enolase (NSE) and oligodendrocyte-1 (Olig-1)] by quantitative real-time-PCR.
RESULTS: MAPC had small trigonal shaped while MSC had elongated spindle-shaped morphology. The MAPC expressed Oct-4 and Nanog both at gene and protein levels, whereas MSC were negative for these pluripotent markers. MAPC were negative for HLA-ABC while MSC had high expression of HLA-ABC. In addition, MAPC as compared to MSC had significantly lower expression of CD44 (36.56% ± 1.92% vs 98.23% ± 0.51%), CD73 (15.11% ± 2.24% vs 98.53% ± 2.22%) and CD105 (13.81% ± 3.82% vs 95.12% ± 5.65%) (P < 0.001, for all) MAPC cultures compared to MSC cultures treated with neural induction medium had significantly higher fold change expression of NF-200 (0.64), GFAP (0.52), Tau (0.59), MAP-2 (0.72), Olig-1 (0.18) and NSE (0.29) proteins (P < 0.01 for Olig-1 and P < 0.001 for rest) as well as higher fold change expression of genes of NF-200 (1.34), GFAP (1.12), Tau (1.08), MAP-1B (0.92), MAP-2 (1.14) and NSE (0.4) (P < 0.001 for all).
CONCLUSION: MAPC can be differentially characterized from MSC as Oct-4 and Nanog positive stem cells with no expression of HLA-ABC and low expression of mesenchymal markers CD44, CD73 and CD105 and when compared to MSC they possess greater predilection for differentiation into neuro-ectodermal lineage.
Keywords: Bone marrow, Human multipotent adult progenitor cells, Human mesenchymal, Stem cells, Phenotypic markers, Neural differentiation
INTRODUCTION
Multipotent adult progenitor cells (MAPC) and mesenchymal stem cells (MSC) are two predominant non-hematopoietic stem cell types of the bone marrow stroma, that have enormous therapeutic properties including regenerative therapy for neurodegenerative disorders[1,2]. The MAPC are pluripotent stem cells with capacity to differentiate into cells of all the three germ layers[3-5]. They have been variously described in literature as multipotent adult stem cells (MASC)[6], mesodermal/multipotent progenitor cells (MPC)[7], marrow-isolated multi-lineage inducible cells[8], adult pluripotent stem cells[9] and embryonic like stem cells (ELSC)[10] by different groups. The expression of pluripotent and neural markers in MAPC and their increased mobilization in patients with neurodegenerative diseases like stroke show potential role of these stem cells in neurogenesis[11-13]. The MSC are mesodermal progenitors that are committed to differentiate into cells of mesodermal lineage[14]. However, some studies have shown that in addition to their mesodermal commitment they also differentiate into cells of neuro-ectodermal lineage, claiming their role in neurogenesis[15,16]. However, it is not known whether MAPC or MSC possess superior neurogenic potential and very less information is available on phenotypic differences between MAPC from MSC so it is also difficult to distinguish them from each other.
Therefore, the aim of the present study was to evaluate the expression of pluripotency and mesenchymal markers and to carry out a parallel comparison of neural differentiation potential of MAPC and MSC derived from the same samples of human bone marrow.
MATERIALS AND METHODS
Isolation, culture and characterization of MAPC and MSC
Subjects included in the study (n = 5) consisted of 2 healthy donors for bone marrow transplant patients and 3 patients with suspected iron deficiency anemia where bone marrow (BM) was done to look for iron stores, who otherwise had a normal BM. After informed consent, 5mL of BM aspirate was collected from each individual for this study, and it was divided into two equal parts for growing MAPC and MSC from the same sample in parallel.
The MAPC were cultured using Verfaillie’s protocol[3]. Briefly, bone marrow mononuclear cells (BMNC) of the marrow aspirates were depleted of CD45 and GlyA positive cells were cultured in growth medium consisting of 53.8% 1.5 mg/mL bovine serum albumin (BSA) mixed Dulbecco’s modified Eagle’s medium (DMEM)-low glucose medium (Gibco, www.invitrogen.com), 40% MCDB-201 (Sigma, www.sigmaaldrich.com), 2% fetal bovine serum (FBS) (Hyclone, www.thermoscientific.com), 1% ITS+1 Supplement (Sigma), 0.5 μmol/L dexamethasone (Sigma), 0.1 mmol/L L-ascorbic acid (Sigma), 1% LA-BSA (Sigma), 1% penicillin/streptomycin (Gibco), 10 ng/mL each of platelet-derived growth factor-BB (R and D, www.rndsystems.com) and epidermal growth factor (R and D) under hypoxic condition. The sub-confluent cultures were trypsinized and further expanded under same culture conditions to get optimal number of cells. The MAPC were characterized by expression of Pluripotency markers Oct-4 and Nanog and their differentiation into cells of three germ layers viz. neuronal (ectodermal cells), endothelial (mesodermal cells) and hepatocytes (endodermal cells).
The culture of MSC was carried out using Prockop’s protocol[17]. Briefly, BMNC were cultured in complete medium consisting 88% of α-MEM Medium, 10% of FBS, 2 mmol/L of L-Glutamine and 100 units/mL of pen-strep (all from Gibco) under normoxic condition. The MSC were characterized by expression of conventional mesenchymal markers and their differentiation into mesodermal cell including bone and fat cells.
Flow-cytometry
The phenotypes of MAPC and MSC were analyzed by two color flow cytometry at 3rd passage using human leukocyte antigen (HLA)-ABC [fluorescein isothiocyanate (FITC)]/CD44 [phycoerythrin (PE)], CD34 (FITC)/CD73 (PE), CD14 (FITC)/CD105 (PE) and CD45 (FITC)/CD90 (PE) (all from Serotec, www.abdserotec.com). The flow-cytometer used was FACS-calibur (Becton Dickinson) and data analysis was done using FACS express software.
Reverse-transcription polymerase chain reaction
Expression of Oct-4 and Nanog genes was done by reverse-transcription polymerase chain reaction (RT-PCR). Total RNA of the cells was extracted using RNeasy mini RNA isolation kit (Invitrogen). Two μg of total RNA was reverse transcribed into cDNA using random hexamers (Invitrogen). The cDNA was normalized by amplification of β-actin, The PCR conditions included denaturation at 94 °C for 4 min, amplification cycles 35 and elongation temperature 72 °C for 30 s with annealing. The amplicons were resolved on 2% agarose gel (Sigma-Aldrich) and pictures acquired using gel documentation system (Alpha Imager, www.proteinsimple.com).
Immunocytochemistry
The expression of pluripotency genes Oct-4 and Nanog on MAPC and MSC was analyzed by immunocytochemistry. The cells were fixed with 4% para-formaldehyde (Sigma Aldrich) in PBS for 1 h at room temperature. The fixed cells were incubated overnight at 4 °C with following primary antibodies: Nanog and OCT-4 (1:200 dilution) (Chemicon, www.millipore.com) After washing with PBS, cells were incubated with 1:500 diluted goat anti-mouse immunoglobulin G (IgG) (Fab)2 FITC (Abcam, www.abcam.com) as secondary antibody and stained with Hoechst dye. The pictures were acquired by fluorescent microscope (Nikon 80i, Japan).
Differentiation into neuro-ectodermal cells
We used following protocol for differentiation of MAPC and MSC into neuronal cells. The cells were plated into 12-well plates at a density of 2000-2500 cells/cm2 and incubated in neuro-ectodermal induction medium consisting of 98% basal medium (57% DMEM low glucose, 40% FBS, 1% Pen-strip, 1% ITS+1, 0.1 mmol/L L-ascorbic acid, 0.5 µmol/L dexamethasone), 100 ng/mL basic fibroblast growth factor (R and D systems), 100 ng/mL Noggin (R and D systems), 20 ng/mL NT-3 (R and D systems), 10 ng/mL brain-derived neurotrophic factor (R and D systems), 10 ng/mL glial cell line-derived neurotrophic factor (R and D systems), 20 μmol/L RA (Sigma), 1X B-27 supplement (Gibco), 1X 2-ME (Gibco). The differentiated cells were characterized as neuronal cells by immunocytochemical detection of neuronal filament-200 (NF-200), microtubule-associated protein 2 (MAP-2) and glial fibrillary acidic protein (GFAP) on the cells using MAP-2 (Abcam), NF-200 and GFAP primary antibodies (Biovision). Goat anti-mouse IgG (Fab) 2 FITC (Abcam) as secondary antibody and Hoechst dye staining as described above.
Western blotting
Western blotting was done using primary antibody NF-200 (200 kDa), GFAP (51-52 kDa) (Biovision), Tau (52 kDa), MAP-2 (280 kDa), Olig-1 (28 kDa) and neuron-specific enolase (NSE) (47 kDa) (Abcam) and β-actin (42 kDa) (Abcam) and horseradish peroxidase conjugated corresponding secondary antibodies. The signals were detected using an enhanced chemiluminescence detection system (Amersham Biosciences. www.gelifesciences.com).
Real-time PCR
The quantification of neuronal gene expression in MAPC and MSC was carried out by real time PCR. Total mRNA was isolated from the undifferentiated and neuro-ectodermal differentiated cells following single step mRNA isolation method using RNA isolation kit (Invitrogen). Total mRNA (2 μg) was reverse transcribed to cDNA using RT-PCR kit (Applied Biosystems) following manufacturer’s instructions. Real time analysis for NF-200, GFAP, MAP-2, MAP-1B, Tau, oligodendrocyte-1 (Olig-1), NSE and normalizing gene glyceraldehyde 3-phosphate dehydrogenase (Table 1) was performed using Sybr Green Master mix as per the manufacturer’s instruction (Applied Biosytems), analysis were done on Light-cycler 480 (Roche) and fold changes in gene expression was calculated using 2-ΔΔCT method.
Table 1.
Sequence of primers used in reverse-transcription polymerase chain reaction and real time-polymerase chain reaction
| No. | Primer | Sequence | Accession number |
| 1 | 4-Oct | f: 5’-CGTGAAGCTGGAGAAGGAGAAGCTG–3’ | NM_002701.4 |
| r: 5’-CAAGGGCCGCAGCTTACACATGTTC-3’ | |||
| 2 | Nanog | f: 5’-GCCGAAGAATAGCAATGGTGTG-3' | NM_024865.2 |
| r: 5'-CCAGGACTGGATGTTCTGGGTC-3' | |||
| 3 | NF-200 | f: 5’-CAGAGCTGGAGGCACTGAA-3’ | NM_021076.3 |
| r: 5’-CATCTCCCACTTGGTGTTCC-3’ | |||
| 4 | GFAP | f: 5’-GAGTACCAGGACCTGCTCAA-3’ | NM_002055.4 |
| r: 5’-TTCACCACGATGTTCCTCTT-3’ | |||
| 5 | MAP-1B | f: 5’-GCGGAGACAGTACCTTCGGAG-3’ | NM_005909.3 |
| r: 5’-CCGACGACCACCAGCAAGTAG-3’ | |||
| 6 | MAP-2 | f: 5’-TCAGAGCCAATTCGCAGAG-3’ | NM_002374.3 |
| r: 5’-TGTTGTC TGTTGATCCGATTTT-3’ | |||
| 7 | Tau | f: 5’-TCATTAGGCAACATCCATCATA-3’ | NM_001203252.1 |
| r: 5’-CACCTCGTCAGCTAGCGT-3’ | |||
| 8 | NSE | f: 5’-TCTGCAGTCCCGAGATCCCAGC-3’ | NM_001975.2 |
| r: 5’-CTGATGAGGGCTGGCGCGAT-3’ | |||
| 9 | Olig-1 | f: 5’-GCCCCACCAAGTACCTGTCTC-3’ | NM_138983.2 |
| r: 5’-GGGACCAGATGCGGGAAC-3’ | |||
| 10 | β-actin | f: 5'-GCTCGTCGTCGACAACGGCTC-3’ | NM_001101.3 |
| r: 5'-CAAACATGATCTGGGTCATCTTCTC-3' | |||
| 11 | GAPDH | f: 5'-GATTTGGTCGTATTGGG-3’ | NM_002046.3 |
| r: 5'-TCCACGACGTACTCAGC-3' |
NF-200: Neuronal filament-200; GFAP: Glial fibrillar acidic protein; Olig-1: Oligodendrocyte-1; NSE: Neuron-specific enolase; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; MAP-1B: Microtubule-associated protein-1B; MAP-2: Microtubule-associated protein-2.
Statistical analysis
The results were calculated as mean ± SE. The statistical significance between MAPC and MSC comparisons was determined by using one-way analysis of variance test. P values < 0.05 were considered to be statistically significant.
RESULTS
Morphology and phenotypes
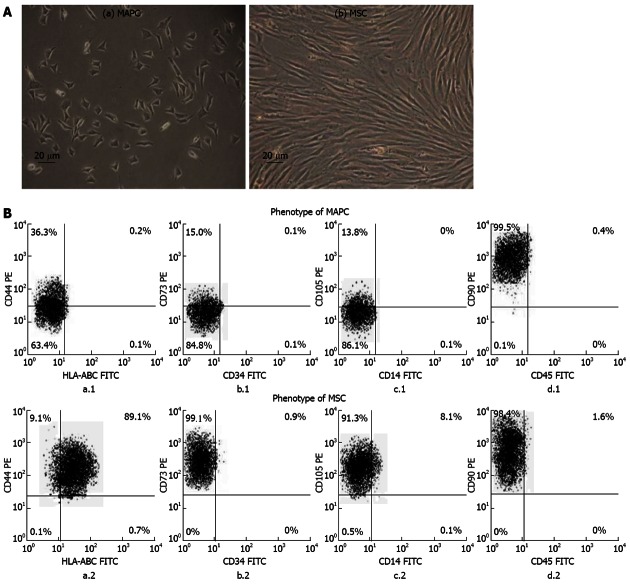
The MAPC and MSC both grew as adherent cells in culture but they were morphologically distinct from each other. The MAPC had small trigonal morphology while the MSC were large cells having elongated spindle shaped morphology (Figure 1A).
Figure 1.
Morphology and phenotypic markers of multipotent adult progenitor cells and mesenchymal stem cells. A: Representative photomicrographs (×10, 20 μm) of primary cultures showing (a) small trigonal morphology of multipotent adult progenitor cells (MAPC) and (b) large spindle shaped morphology of mesenchymal stem cells (MSC); B: Representative flow-cytometric dot-plots showing expression of (a.1 and a.2) HLA-ABC (FITC)/CD44 (PE), (b.1 and b.2) CD34 (FITC)/CD73 (PE), (c.1 and c.2) CD14 (FITC)/CD105 (PE), and (d.1 and d.2) CD45 (FITC)/CD90 (PE) on MAPC and MSC, respectively. FITC: Fluorescein isothiocyanate; PE: Phycoerythrin.
The MAPC had no expression of HLA-ABC (0%) while MSC had high expression of HLA-ABC (93.32% ± 2.58%). The MAPC compared to MSC had significantly lower expression of CD44 (36.56% ± 1.92 % vs 98.23% ± 0.51%), CD73 (15.11% ± 2.24% vs 98.53% ± 2.22%), CD105 (13.81% ± 3.82% vs 95.12% ± 5.65%) (P < 0.001 for all). Both MAPC and MSC had high expression of CD90 (99.80% ± 0.14% vs 99.47% ± 0.44%; P > 0.5) and no expression of CD14, CD34 and CD45 (Figure 1B).
Expression of pluripotency markers
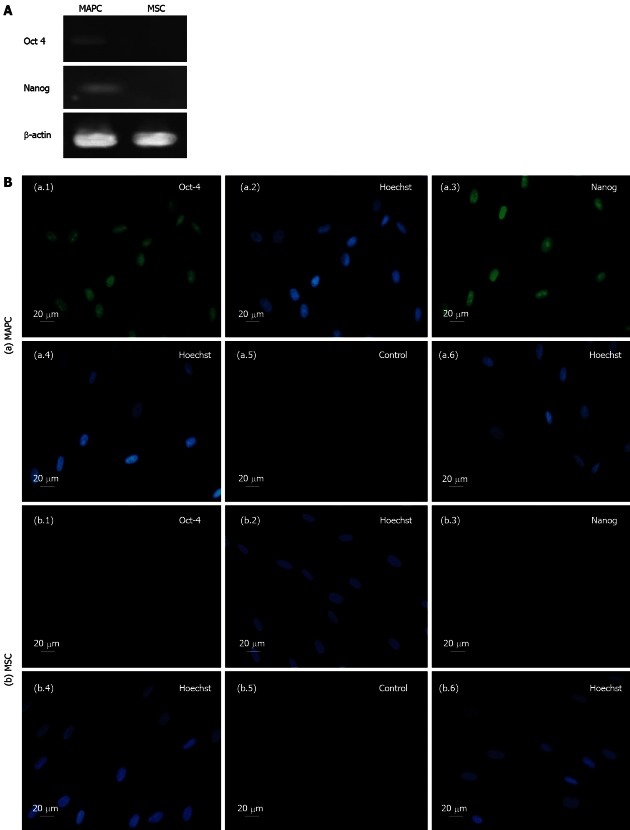
The MAPC expressed pluripotency markers Oct-4 and Nanog at gene and proteins levels while MSC expressed none of these markers either at gene or protein levels (Figure 2).
Figure 2.
Expression of embryonic markers by multipotent adult progenitor cells and by mesenchymal stem cells. A: Expression of Oct-4 and Nanog genes in multipotent adult progenitor cells (MAPC) and no expression of these genes in mesenchymal stem cells (MSC) as revealed by reverse-transcription polymerase chain reaction. β-actin represents the house keeping gene; B: Representative Immunocytochemistry photomicrographs (×40, 20 μm) of (a) MAPC showing fluorescein isothiocyanate (FITC) and Hoechst staining for Oct-4 (a.1 and a.2, respectively), Nanog (a.3 and a.4, respectively) and of controls, i.e., cells with no primary antibody (a.5 and a.6, respectively); and (b) MSC showing FITC and Hoechst staining for Oct-4 (b.1 and b.2, respectively) and Nanog (b.3 and b.4, respectively) and of controls, i.e., cells with no primary antibody (b.5 and b.6, respectively).
Neuro-ectodermal differentiation efficiency
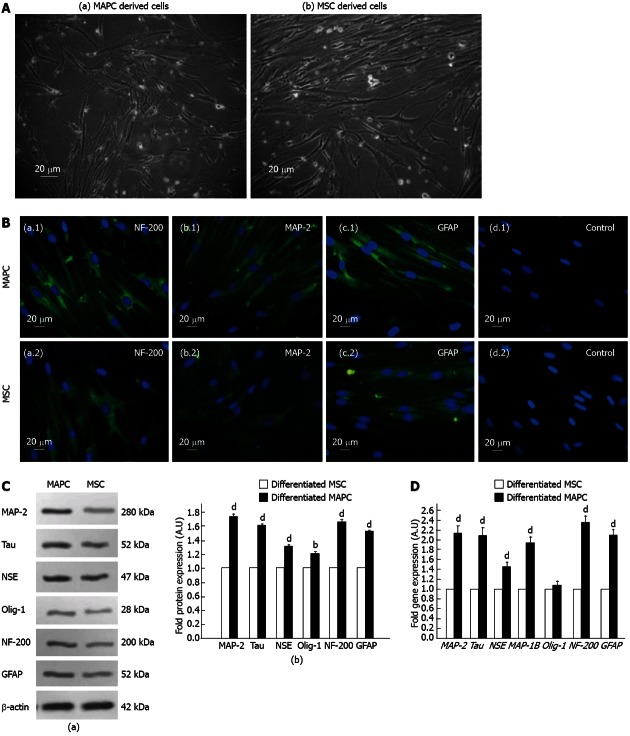
Following treatment with neurogenic induction medium, the cells which differentiated from both MAPC and MSC, had morphological characteristics of neuronal like cells as revealed by their bipolar elliptical shape and/or multiple branching points and neuritis (Figure 3A) and they expressed NF-200, MAP-2 and GFAP as revealed by immunocytochemistry (Figure 3B). The MAPC derived neuronal cells compared to those derived from MSC, showed a significantly higher fold change expression of NF-200 (0.64), GFAP (0.52), Tau (0.59), MAP-2 (0.72), Olig-1 (0.18) and NSE (0.29) (P < 0.01 for Olig-1 and P < 0.001 for rest) (Figure 3C). Similarly the fold change expression of NF-200 (1.34), GFAP (1.12), Tau (1.08), MAP-1B (0.92), MAP-2 (1.14) and NSE (0.4) genes were significantly higher in MAPC derived neuronal cells compared to those derived from MSC (P < 0.001 for all) but there was no difference in the fold change expression of Olig-1 gene (0.08) in neuronal cells derived from both stem cell types (P > 0.5) as revealed by real time quantitative PCR (Figure 3D).
Figure 3.
Neural differentiation potential of multipotent adult progenitor cells and of mesenchymal stem cell. A: Representative photomicrographs (×10, 20 μm) of neuronal cells differentiated from (a) multipotent adult progenitor cells (MAPC), and (b) mesenchymal stem cell (MSC); B: Representative immunocytochemical photomicrographs (×40, 20 μm) showing an overlay of fluorescein isothiocyanate (FITC) and Hoechst staining for (a.1 and a.2) neuronal filament-200 (NF-200), (b.1 and b.2) microtubule-associated protein 2 (MAP-2), and (c.1 and c.2) fibrillar acidic protein (GFAP) expression in neuronal cells differentiated from MAPC and MSC, respectively. Negative control represents cells with no treatment with neurogenic medium (d.1) MAPC and (d.2) MSC; C: Immunoblots analysis of neural proteins in MAPC and MSC derived neuronal cells. (a) Representative immunoblots of MAP-2, Tau, neuron-specific enolase (NSE), oligodendrocyte-1 (Olig-1), NF-200 and GFAP proteins in MAPC and MSC derived neuronal cells; (b) Error-bar diagrams showing fold change expression of proteins in MAPC (solid bars) and MSC (open bars) derived neuronal cells as revealed by densitometric quantification of immunoblots. bP < 0.01, dP < 0.001 vs MSC group; D: Error-bar diagrams showing expression of MAP-2, Tau, NSE, MAP-1B, Olig-1, NF-200 and GFAP genes in MAPC (solid bars) and MSC (open bars) derived neuronal cells as revealed by real-time quantitative polymerase chain reaction. The values of MSC and MAPC treated with neurogenic medium and their untreated counterparts were normalized to housekeeping glyceraldehyde-3-phosphate dehydrogenase and the fold increase values of MAPC compared to MSC have been expressed as mean ± SE, bP < 0.001 vs MSC group.
DISCUSSION
Our study shows that MAPC and MSC differ from each other in terms of morphology, phenotypic and pluripotency markers, and their neuro-ectodermal differentiation potential. Morphologically, MAPC are small trigonal cells while MSC are elongated spindle shaped cells. Phenotypically MAPC have no expression of HLA-ABC and low expression of CD44, CD73 and CD105 while MSC possess high expression of all these markers. In addition, MAPC express Oct-4 and Nanog both at gene and protein levels but MSC lack expression of these markers. The MAPC have higher neuro-ectodermal differentiation potential than MSC as revealed by their significantly higher expression of NF-200, GFAP, Tau, MAP-2, Olig-1 and NSE proteins and NF-200, GFAP, MAP-2, MAP-1B, Tau and NSE genes. To the best of our knowledge this is the first study showing a parallel comparison of phenotype, pluripotency markers and neuro-ectodermal differentiation potential of MAPC and MSC isolated from the same samples of human bone marrow.
The existence of MAPC in the stroma of adult bone marrow has been described previously[1,3-5] by different groups and most of these studies support our observation of small triangular morphology of MAPC. However despite this morphological difference, there are no well defined phenotypic markers distinguishing MAPC from MSC. We have observed that MAPC have low expression of CD44, CD73, and CD105 and no expression of HLA-ABC, while MSC have high expression of these markers.
Human embryonic stem cells also have no or negligibly low expressions of HLA-ABC highlighting that MAPC have properties similar to embryonic stem cells[18]. We found that MAPC express pluripotency markers Oct-4 and Nanog both at gene and protein levels but MSC entirely lacked expression of these pluripotency markers. The expression of Oct-4 and Nanog on MAPC corroborates with expression of these and other pluripotency markers in ELSC[10], MASC[6] and MPC[7]. A few studies have reported that Oct-4, Nanog and other pluripotency markers are also expressed in MSC derived from bone marrow and other adult tissues[19] and one study has shown that MSC express Nanog but not OCT-4[20]. In another study, it has been shown that culture conditions of low serum content, induce expression of Oct-4, Nanog and other pluripotency markers on MSC[21]. We have cultured MSC under standard serum conditions, and thus the difference in expression of pluripotency markers between our and these studies may be due to difference in culture conditions which either have induced expression of Oct-4 and Nanog on MSC or promoted the growth of a population of MAPC in the cultures. Similar to our observation in MAPC, the expression of Oct-4, Nanog and other pluripotency markers has been shown in fetal MSC, but not in adult MSC[22]. Thus lower expression of the conventional mesenchymal markers, no expression of HLA-ABC and expression of Oct-4 and Nanog, may be used as suitable markers to distinguish MAPC from MSC.
Bone marrow derived MSC have been reported to exhibit trans-differentiation into cells of neuronal lineage, thereby claiming for a role of these stem cells in therapy for neurological disorders[15,16]. More recently, MAPC have been shown to differentiate into neuronal cells and promote neuronal regeneration[12,13]. However, no data exists on comparative analysis of neural differentiation potential of MAPC and MSC. In the present study, we have carried out a parallel comparison of neuro-ectodermal potential of MAPC and MSC at protein and gene levels. We studied both stem cell types for gene and protein expression of markers of axons (NF-200 and Tau), astrocytes (GFAP), dendrocytes (MAP-1B, MAP-2 and Olig-1) and neurons (NSE) and observed that MAPC show significantly higher expression of NF-200, Tau, GFAP, MAP-1B, MAP-2 and NSE genes in comparison to MSC. Moreover, we compared the protein expression of NF-200, Tau, GFAP, Olig-1, MAP-2 and NSE, and similar to gene expression, we found significantly increased expression of these proteins in MAPC compared to MSC. Thus MAPC appear to have a greater predilection for neural differentiation, which needs to be therapeutically evaluated in vivo in pre-clinical animal models of neurological disorders.
In conclusion, our study showed that MAPC can be differentially characterized from MSC as Oct-4 and Nanog positive stem cells with no expression of HLA-ABC and low expression of mesenchymal markers CD44, CD73 and CD105 and they possess higher neuro-ectodermal differentiation potential than MSC indicating that MAPC may be more suitable cell type than MSC for cell based therapy for neurodegenerative disorders. Future studies directed towards the in vivo evaluation of the therapeutic potential of MAPC in pre-clinical models would lead to development MAPC based therapies for neurological diseases.
COMMENTS
Background
Multipotent adult progenitor cells (MAPC) and mesenchymal stem cells (MSC), the two predominant stem cell types of the bone marrow stroma, are currently being explored for cellular therapy of neurodegenerative disorders. However, there is no data on their phenotypic difference and it is also not yet known that which of these two possess a greater potential for neural differentiation and thus would be more suitable for therapeutic use.
Research frontiers
On the basis of a parallel comparison of the two stem cell types derived from the same sample of the bone marrow, this study reports that MAPC can be differentially characterized from MSC as Oct-4 and Nanog positive stem cells with no expression of human leukocyte antigen-ABC and low expression of other mesenchymal markers and with a greater predilection for differentiation into neuro-ectodermal lineage. Future studies comparing their in vivo therapeutic efficacy in pre-clinical animal models of neurodegenerative disorders would be important to confirm superiority of MAPC over MSC for therapeutic application in these diseases.
Innovations and breakthroughs
This is the first study reporting a parallel comparison of MAPC and MSC and demonstrating phenotypic differences between two stem cells types and a greater potential of MAPC than MSC towards neuro-ectodermal lineage.
Applications
This study offers a foundation for comparative studies on MAPC and MSC in experimental models of neurodegenerative disorders that in turn may lead to initiation of clinical studies on MAPC in these disease states.
Terminology
MAPC represent a primitive population of non-hematopoietic stem cells present in the bone marrow and other tissues. Their phenotype is not yet well defined but they express embryonal markers and give rise to cells of all the three germ layers.
Peer review
This is a great manuscript that compares MAPC with MSC. The presented data are significant to the field of research and adds to the knowledge about these stem cell types and their potential.
Footnotes
Supported by The Grant-in-Aid entitled “Stem cells for regenerative medicine: Isolation of Multipotent adult Progenitor Cells from Human Bone Marrow and their Clonal Expansion and Differentiation into Cardiomyocytes, Hepatocytes and Beta-islets” No. BT/PR6303/MED/14/776/2005, sanctioned by Department of Biotechnology, Government of India
P- Reviewers Bartold PM, Oudega M S- Editor Wen LL L- Editor A E- Editor Li JY
References
- 1.Kuçi S, Kuçi Z, Latifi-Pupovci H, Niethammer D, Handgretinger R, Schumm M, Bruchelt G, Bader P, Klingebiel T. Adult stem cells as an alternative source of multipotential (pluripotential) cells in regenerative medicine. Curr Stem Cell Res Ther. 2009;4:107–117. doi: 10.2174/157488809788167427. [DOI] [PubMed] [Google Scholar]
- 2.Chagastelles PC, Nardi NB, Camassola M. Biology and applications of mesenchymal stem cells. Sci Prog. 2010;93:113–127. doi: 10.3184/003685010X12708175591515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aranguren XL, Luttun A, Clavel C, Moreno C, Abizanda G, Barajas MA, Pelacho B, Uriz M, Araña M, Echavarri A, et al. In vitro and in vivo arterial differentiation of human multipotent adult progenitor cells. Blood. 2007;109:2634–2642. doi: 10.1182/blood-2006-06-030411. [DOI] [PubMed] [Google Scholar]
- 4.Kuroda Y, Kitada M, Wakao S, Nishikawa K, Tanimura Y, Makinoshima H, Goda M, Akashi H, Inutsuka A, Niwa A. Unique multipotent cells in adult human mesenchymal cell populations. Proceedings of the National Academy of Sciences. 2010;107:8639–8643. doi: 10.1073/pnas.0911647107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sohni A, Verfaillie CM. Multipotent adult progenitor cells. Best Pract Res Clin Haematol. 2011;24:3–11. doi: 10.1016/j.beha.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Beltrami AP, Cesselli D, Bergamin N, Marcon P, Rigo S, Puppato E, D’Aurizio F, Verardo R, Piazza S, Pignatelli A, et al. Multipotent cells can be generated in vitro from several adult human organs (heart, liver, and bone marrow) Blood. 2007;110:3438–3446. doi: 10.1182/blood-2006-11-055566. [DOI] [PubMed] [Google Scholar]
- 7.Pacini S, Carnicelli V, Trombi L, Montali M, Fazzi R, Lazzarini E, Giannotti S, Petrini M. Constitutive expression of pluripotency-associated genes in mesodermal progenitor cells (MPCs) PLoS One. 2010;5:e9861. doi: 10.1371/journal.pone.0009861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roger M, Clavreul A, Sindji L, Chassevent A, Schiller PC, Montero-Menei CN, Menei P. In vitro and in vivo interactions between glioma and marrow-isolated adult multilineage inducible (MIAMI) cells. Brain Res. 2012;1473:193–203. doi: 10.1016/j.brainres.2012.07.030. [DOI] [PubMed] [Google Scholar]
- 9.Mays RW, van’t Hof W, Ting AE, Perry R, Deans R. Development of adult pluripotent stem cell therapies for ischemic injury and disease. Expert Opin Biol Ther. 2007;7:173–184. doi: 10.1517/14712598.7.2.173. [DOI] [PubMed] [Google Scholar]
- 10.Feng G, Cui J, Zheng Y, Han Z, Xu Y, Li Z. Identification, characterization and biological significance of very small embryonic-like stem cells (VSELs) in regenerative medicine. Histol Histopathol. 2012;27:827–833. doi: 10.14670/HH-27.827. [DOI] [PubMed] [Google Scholar]
- 11.Paczkowska E, Kucia M, Koziarska D, Halasa M, Safranow K, Masiuk M, Karbicka A, Nowik M, Nowacki P, Ratajczak MZ, et al. Clinical evidence that very small embryonic-like stem cells are mobilized into peripheral blood in patients after stroke. Stroke. 2009;40:1237–1244. doi: 10.1161/STROKEAHA.108.535062. [DOI] [PubMed] [Google Scholar]
- 12.Busch SA, Hamilton JA, Horn KP, Cuascut FX, Cutrone R, Lehman N, Deans RJ, Ting AE, Mays RW, Silver J. Multipotent adult progenitor cells prevent macrophage-mediated axonal dieback and promote regrowth after spinal cord injury. J Neurosci. 2011;31:944–953. doi: 10.1523/JNEUROSCI.3566-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walker PA, Shah SK, Jimenez F, Gerber MH, Xue H, Cutrone R, Hamilton JA, Mays RW, Deans R, Pati S, et al. Intravenous multipotent adult progenitor cell therapy for traumatic brain injury: preserving the blood brain barrier via an interaction with splenocytes. Exp Neurol. 2010;225:341–352. doi: 10.1016/j.expneurol.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.García-Gómez I, Elvira G, Zapata AG, Lamana ML, Ramírez M, Castro JG, Arranz MG, Vicente A, Bueren J, García-Olmo D. Mesenchymal stem cells: biological properties and clinical applications. Expert Opin Biol Ther. 2010;10:1453–1468. doi: 10.1517/14712598.2010.519333. [DOI] [PubMed] [Google Scholar]
- 15.Bae KS, Park JB, Kim HS, Kim DS, Park DJ, Kang SJ. Neuron-like differentiation of bone marrow-derived mesenchymal stem cells. Yonsei Med J. 2011;52:401–412. doi: 10.3349/ymj.2011.52.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choong PF, Mok PL, Cheong SK, Leong CF, Then KY. Generating neuron-like cells from BM-derived mesenchymal stromal cells in vitro. Cytotherapy. 2007;9:170–183. doi: 10.1080/14653240701196829. [DOI] [PubMed] [Google Scholar]
- 17.Colter DC, Sekiya I, Prockop DJ. Identification of a subpopulation of rapidly self-renewing and multipotential adult stem cells in colonies of human marrow stromal cells. Proc Natl Acad Sci USA. 2001;98:7841–7845. doi: 10.1073/pnas.141221698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cabrera CM, Nieto A, Cortes JL, Montes RM, Catalina P, Cobo F, Barroso-Del-Jesus A, Concha A. The low rate of HLA class I molecules on the human embryonic stem cell line HS293 is associated with the APM components’ expression level. Cell Biol Int. 2007;31:1072–1078. doi: 10.1016/j.cellbi.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 19.Riekstina U, Cakstina I, Parfejevs V, Hoogduijn M, Jankovskis G, Muiznieks I, Muceniece R, Ancans J. Embryonic stem cell marker expression pattern in human mesenchymal stem cells derived from bone marrow, adipose tissue, heart and dermis. Stem Cell Rev. 2009;5:378–386. doi: 10.1007/s12015-009-9094-9. [DOI] [PubMed] [Google Scholar]
- 20.Pierantozzi E, Gava B, Manini I, Roviello F, Marotta G, Chiavarelli M, Sorrentino V. Pluripotency regulators in human mesenchymal stem cells: expression of NANOG but not of OCT-4 and SOX-2. Stem Cells Dev. 2011;20:915–923. doi: 10.1089/scd.2010.0353. [DOI] [PubMed] [Google Scholar]
- 21.Roche S, Richard MJ, Favrot MC. Oct-4, Rex-1, and Gata-4 expression in human MSC increase the differentiation efficiency but not hTERT expression. J Cell Biochem. 2007;101:271–280. doi: 10.1002/jcb.21185. [DOI] [PubMed] [Google Scholar]
- 22.Guillot PV, Gotherstrom C, Chan J, Kurata H, Fisk NM. Human first-trimester fetal MSC express pluripotency markers and grow faster and have longer telomeres than adult MSC. Stem Cells. 2007;25:646–654. doi: 10.1634/stemcells.2006-0208. [DOI] [PubMed] [Google Scholar]