Abstract
Patients with gallbladder carcinoma can present with a variety of paraneoplastic syndromes, including Cushing’s syndrome, hypercalcemia, acanthosis nigricans, bullous pemphigoid, dermatomyositis and the sign of Leser-Trélat. Surgical resection of the primary tumor results in resolution of these paraneoplastic syndromes. We present a 67-year old female with facial and cervical erythema who was initially diagnosed with dermatomyositis. However, an abdominal computed tomography (CT) and positron emission tomography-CT scan was suspicious for gallbladder carcinoma with lymph node metastasis. After surgical resection, her dermatomyositis was resolved. This case demonstrates that dermatomyositis may be a manifestation of preexisting gallbladder carcinoma.
Keywords: Dermatomyositis, Paraneoplastic syndromes, Gallbladder carcinoma
INTRODUCTION
Prior research has shown that paraneoplastic syndromes can be eliminated by surgical resection of the primary tumor. We present a case of a 67-year old female with dermatomyositis who underwent radical resection of a gallbladder carcinoma. After surgery, her dermatomyositis completely disappeared.
CASE REPORT
In February of 2012, a 67-year old woman, who presented with a two month history of facial and cervical erythema accompanied by pruritis and 20 d of arthralgia of the hands and morning stiffness, was admitted to our institution’s Department of Dermatology[1]. She had a past medical history of hypertension for about 5 years. She denied prolonged fever, malaise, anorexia, weight loss, recent vaccination, Raynaud’s phenomenon, cough, hemoptysis, hematemesis, melena, hematochezia, oral ulcer, alopecia, jaundice, drug intake (e.g., penicillamine, clofibrate, statins, corticosteroids, emetine, colchicine, chloroquine or zidovudine), breast lumps or abdominal pain. She was a nonsmoker, non-diabetic and without any history of exposure. There was no history of loss of consciousness, headache, vomiting, seizures, sensory dysfunction or involvement of distal limb muscles, bladder or bowels[2].
Physical examination revealed an elderly woman without pyrexia, jaundice, clubbing or lymphadenopathy[2]. Her pulse was 80/min, regular rate and rhythm, and her blood pressure was 130/86 mmHg, both supine and standing. Her temperature was 36.6 °C and respiratory rate 18/min. Her forehead, neck and upper eyelids were erythematous and moderately edematous. The skin lesions were distributed over the extensor side of the metacarpophalangeal joints of the hand. Erythema to the nail fold was seen (Figure 1). These lesions were of mixed character, like maculopapular eruptions. She did not have weakness of the forearms, arms, shoulder or pelvic girdle muscles.
Figure 1.
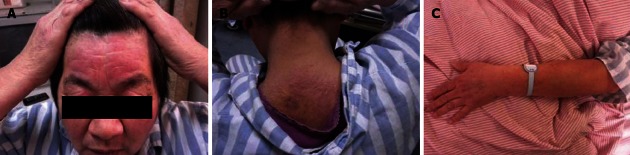
Physical examination. A: The skin lesions in the forehead and upper eyelids; B: The skin lesions in the neck; C: Erythema to the nail fold.
Laboratory examinations demonstrated a hemoglobin of 118 g/L, a total leucocyte count of 10200/mm3 (differential count N 88.6; L 6.4; M 4.9; E 0) and an erythrocyte sedimentation rate of 10 mm. Her serum bilirubin, urea and creatinine levels were 18.8 μmol/L, 6.31 mmol/L and 49.1 μmol/L, respectively. Her tests of synthetic liver function tests revealed an alkaline phosphatase of 51.1 U/L, aspartate aminotransferase of 58.0 U/L, alanine aminotransferase of 66.1 U/L, gamma-glutamyltransferase of 19.3 U/L, total protein of 58.8 g/dL, serum albumin of 35.1 g/dL and globulin of 23.7 g/dL. Chest X-ray, electrocardiogram and serum electrolytes (sodium, potassium and magnesium) were within normal limits. Her C-reactive protein was 14.1 mg/L, serum creatine kinase 61.7 U/L and lactate dehydrogenase 213 U/L. Her serum antinuclear antibody was positive at a titer of 1:320 with a speckled pattern. Serum tumor markers included cytokeratin-19 4.71 ng/mL and neuron-specific enolase 19.08 μg/L. Carcinoembryonic antigen, alpha-fetoprotein, carcinoembryonic antigen (CA)-125, CA-19-9 and CA-724 did not reveal any abnormalities.
Computed tomography of the abdomen and positron emission tomography-computed tomography scans were performed, which were suspicious for gallbladder calculi with associated soft tissue density and peripancreatic lymphadenopathy (Figure 2).
Figure 2.
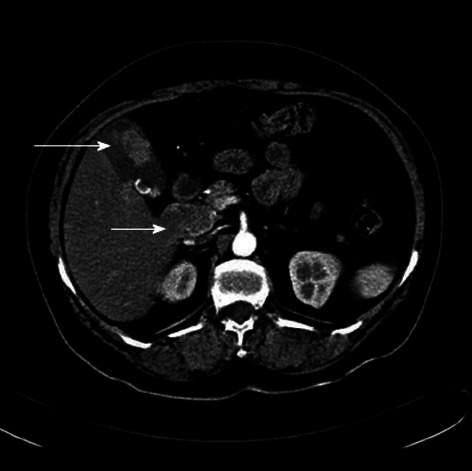
Abdominal computed tomography. Gallbladder carcinoma (long arrow) with lymph node metastasis (short arrow).
A biopsy of the skin was performed, which showed a mild degree of hyperkeratosis, hair follicle angle plug, epidermal atrophy, basal cell liquefaction and degeneration with more bite pigment cells, dermal papillary edema, agglomerate lymphocytic infiltration around the vessels and dermal adnexa, and a small amount of mucin between collagen fibers (Figure 3).
Figure 3.
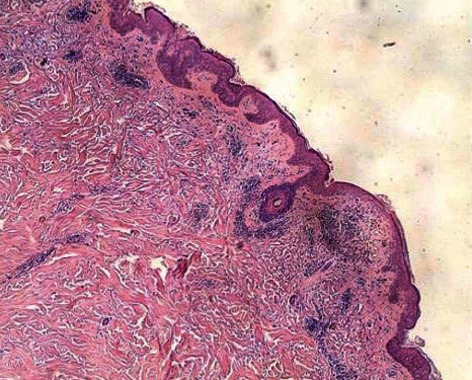
Biopsy of skin. Mild degree of hyperkeratosis, hair follicle angle plug, epidermal atrophy, basal cell liquefaction degeneration, dermal papilla edema, agglomerate lymphocytic infiltrate around vessels, and dermal adnexa.
She was treated with loratadine, cetirizine and hydroxychloroquine to reduce the sensitivity to ultraviolet rays and magnesium isoglycyrrhizinate as an anti-inflammatory. Vitamin C, calcium gluconate and coenzyme complex was used to relieve symptoms.
Radical cholecystectomy was performed on February 27, 2012. The gallbladder measured 8 cm × 6 cm × 4 cm and a hard, whitish mass on the fundus measured 4 cm, without clear borders. A hard, pale lump was seen on the liver surface, between the gallbladder and the inferior vena cava, with a clear boundary and a diameter of 2 cm. In addition, encapsulated and demarcated lumps were seen between the hepatoduodenal ligament and the inferior vena cava and duodenum, with a diameter of 6 cm; the tumor-node-metastasis classification for this patient is T4N2M0 (Figure 4). The gallbladder and tumor on the liver surface, with an additional 2 cm margin of normal liver parenchyma, were completely resected.
Figure 4.
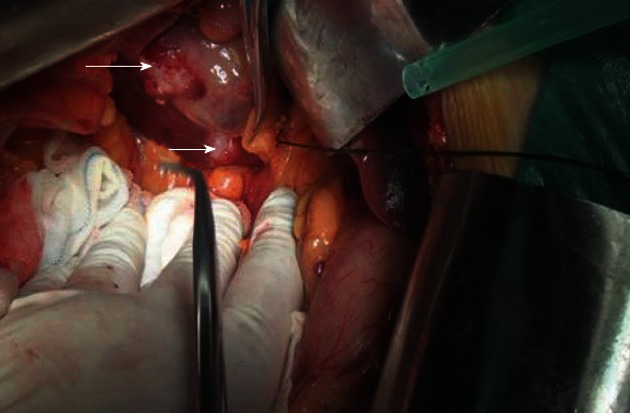
Radical resection of the gallbladder was performed. A hard, whitish mass located at the fundus of gallbladder (long arrow) and a hard, pale lump was seen in the liver surface (short arrow), between the gallbladder and the inferior vena cava.
On pathology, a well-differentiated gallbladder adenocarcinoma measuring 3 cm × 2 cm × 1.5 cm was found, involving all layers of the gallbladder wall, with perineural invasion. Mucosal glands showed moderate and high-grade dysplasia. The liver lesion was consistent with a 2 cm × 2 cm × 1 cm well-differentiated metastatic deposit. One lymph node was excised and was positive for adenocarcinoma (Figure 5). Immunohistochemical staining of the gallbladder tumor was negative for synaptophysin, chromogranin A and CD56, but immunoreactive for e-cadherin and vimentin. Some tumor cells were positive for cytokeratin 7. Liver tumor cells were negative for hepatocyte and cytokeratin 7.
Figure 5.

Histopathology of tumor. Low differentiated gallbladder adenocarcinoma, involving all layers of the gallbladder and infringing upon nerves.
The patient had an uncomplicated hospital course and was discharged on postoperative day 14. The skin lesions gradually abated. As of July 2012, there has been no evidence of cancer recurrence and the skin lesions have completely disappeared.
DISCUSSION
Dermatomyositis is an uncommon idiopathic inflammatory disorder of the skin and skeletal muscle that affects 10 adults per million and 3.2 children per million worldwide[3]. Cutaneous manifestations are classified as pathognomonic, characteristic or compatible[4]. Pathognomonic features, as in our patient, include small purple or red flat papules on the extensor surfaces, particularly the joints of the hand (Gottron papules). A skin or muscle biopsy is helpful in differentiating this condition from other papulosquamous diseases or contact or atopic dermatitis. Changes to the nail fold and a heliotropic rash are highly characteristic manifestations. For up to 40% of patients, skin findings may be the only manifestation[5].
Paraneoplastic syndromes are defined as a constellation of systemic symptoms in cancer patients remote from the tumor, either due to metastasis or caused by treatment[6]. Most of the symptoms are caused by a release of a constellation of tumor-related proteins, which can either be produced directly by the tumor itself or alternatively be secreted by the immune system[7].
A number of studies have suggested an association between inflammatory myopathy and malignancy, which appears stronger with dermatomyositis and weaker with polymyositis[8-11]. The cause of malignancy in patients with dermatomyositis is not clear, but compromised immunity or immunosuppressive or cytotoxic therapy may be involved. Secretion of hormones, such as adrenocorticotropic hormone, growth hormone and serotonin, has been previously described. With the exception of gastric and lung carcinoma, which seem to more commonly associated with dermatomyositis, the spectrum of cancers in patients with inflammatory myopathy is similar to that of the general population[10]. However, the association of dermatomyositis with gallbladder carcinoma is extremely rare. In this case of dermatomyositis associated with gallbladder carcinoma, her skin lesions completely disappeared after radical gallbladder cancer surgery. In conclusion, dermatomyositis can be considered as a paraneoplastic syndrome in gallbladder carcinoma, especially in adults over the age of 50 years. Abdominal ultrasound or computed tomography is necessary to make the diagnosis. Prognosis will be improved significantly through appropriate treatment of the primary tumor in the early stage.
Footnotes
P- Reviewer Shelat VG S- Editor Gou SX L- Editor Roemmele A E- Editor Li JY
References
- 1.Uribe-Uribe NO, Jimenez-Garduño AM, Henson DE, Albores-Saavedra J. Paraneoplastic sensory neuropathy associated with small cell carcinoma of the gallbladder. Ann Diagn Pathol. 2009;13:124–126. doi: 10.1016/j.anndiagpath.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Kundu AK, Karmakar PS, Bera AB, Pal SK. Carcinoma of the gall bladder presenting as dermatomyositis. J Assoc Physicians India. 2005;53:219–222. [PubMed] [Google Scholar]
- 3.Koler RA, Montemarano A. Dermatomyositis. Am Fam Physician. 2001;64:1565–1572. [PubMed] [Google Scholar]
- 4.Euwer RL, Sontheimer RD. Dermatologic aspects of myositis. Curr Opin Rheumatol. 1994;6:583–589. doi: 10.1097/00002281-199411000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Santmyire-Rosenberger B, Dugan EM. Skin involvement in dermatomyositis. Curr Opin Rheumatol. 2003;15:714–722. doi: 10.1097/00002281-200311000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Sacco E. Re: Paraneoplastic syndromes in patients with urological malignancies. Urol Int. 2010;85:483. doi: 10.1159/000321164. [DOI] [PubMed] [Google Scholar]
- 7.Kim HL, Belldegrun AS, Freitas DG, Bui MH, Han KR, Dorey FJ, Figlin RA. Paraneoplastic signs and symptoms of renal cell carcinoma: implications for prognosis. J Urol. 2003;170:1742–1746. doi: 10.1097/01.ju.0000092764.81308.6a. [DOI] [PubMed] [Google Scholar]
- 8.Barnes BE, Mawr B. Dermatomyositis and malignancy. A review of the literature. Ann Intern Med. 1976;84:68–76. doi: 10.7326/0003-4819-84-1-68. [DOI] [PubMed] [Google Scholar]
- 9.Manchul LA, Jin A, Pritchard KI, Tenenbaum J, Boyd NF, Lee P, Germanson T, Gordon DA. The frequency of malignant neoplasms in patients with polymyositis-dermatomyositis. A controlled study. Arch Intern Med. 1985;145:1835–1839. [PubMed] [Google Scholar]
- 10.Sigurgeirsson B, Lindelöf B, Edhag O, Allander E. Risk of cancer in patients with dermatomyositis or polymyositis. A population-based study. N Engl J Med. 1992;326:363–367. doi: 10.1056/NEJM199202063260602. [DOI] [PubMed] [Google Scholar]
- 11.Richardson JB, Callen JP. Dermatomyositis and malignancy. Med Clin North Am. 1989;73:1211–1220. doi: 10.1016/s0025-7125(16)30629-0. [DOI] [PubMed] [Google Scholar]


