Abstract
AIM: To investigate the effect of the establishment of in-house multidisciplinary team (MDT) availability (iMDTa) on survival in upper gastrointestinal cancer (UGI) patients.
METHODS: In 2001, a cancer centre with irradiation and chemotherapy facilities was established in the Norwegian county of West Agder with a change of iMDTa (WA/MDT-Change). “iMDTa”-status was defined according to the availability of the necessary specialists within one institution on one campus, serving the population of one county. We compared survival rates during 2000-2008 for UGI patients living in counties with (MDT-Yes), without (MDT-No), with a mix (MDT-Mix) and WA/MDT-Change. Survival was calculated with Kaplan-Meier method. Cox model was used to uncover differences between counties with different MDT status when adjusted for age, sex and stage.
RESULTS: We analyzed 395 patients from WA/MDT-Change and compared their survival to 12 135 UGI patients from four other Norwegian regions. Median overall survival for UGI patients in WA/MDT-Change increased from 129 to 300 d from 2000-2008, P = 0.001. The regions with the highest level of iMDTa achieved the largest decrease in risk of death for UGI cancers (compared to the county with MDT-Mix: MDT-Yes 11%, P < 0.05 and WA/MDT-Change 15%, P < 0.05). Analyzing the different tumour entities separately, patients living in the WA/MDT-Change county reached a statistically significant reduction in the risk of death [hazard ratios (HR)] compared to patients in the county with MDT-Mix for oesophageal and gastric, but not for pancreatic cancer. HR for the study period 2000-2004 are given first and then for the period 2005-2008: The HR for oesophageal cancers was reduced from [HR = 1.12; 95%CI: 0.75-1.68 to HR = 0.60, 95%CI: 0.38-0.95] and for gastric cancers from [HR = 0.87, 95%CI: 0.66-1.15 to HR = 0.63, 95%CI: 0.43-0.93], but not for pancreatic cancer [HR = 1.04-, 95%CI: 0.83-1.3 for 2000-2004 and HR = 1.01, 95%CI: 0.78-1.3 for 2005-2008]. UGI patients treated during the second study period in the county of WA/MDT-Change had a higher probability of receiving chemotherapy. In the first study period, only one out of 43 patients (2.4%, 95%CI: 0-6.9) received chemotherapy, compared to 18 of 42 patients diagnosed during 2005-2008 (42.9%, 95%CI: 28.0-57.8).
CONCLUSION: Introduction of iMDTa led to a two-fold increase of UGI patients, whereas no increase in survival was found in the MDT-No or MDT-Mix counties.
Keywords: Gastric cancer, Gastroesophageal cancer, Oesophageal cancer, Pancreatic cancer, Multidisciplinary treatment, Multidisciplinary team, Survival
INTRODUCTION
There is a lack of evidence that clinical decision making by a multidisciplinary team (MDT) leads to increased survival for oesophageal, gastric and pancreatic cancers[1-3].
The rationale for introducing MDTs is that modern cancer management has become increasingly complex, necessitating the involvement of various key professional groups in clinical decision making[4,5]. In addition, MDTs serve to monitor adherence to clinical guidelines and promote effective use of resources[1-3]. Evaluations of the effectiveness of a MDT on survival are warranted, but complicated to perform due to difficulties regarding: (1) its definition; (2) availability of valid measurement of its performance and, most importantly; and (3) the ethical and organizational hurdles of conducting prospective randomized studies of MDTs. Therefore, in this study we do not focus on the actual practice of MDTs but aim to analyse the effect of their in-house availability (iMDTa).
The most common upper-gastrointestinal (UGI) cancers in Norway are oesophageal, gastric and pancreatic[6]. Although these cancers have a dismal prognosis, the timely involvement of different medical specialists is advocated, based on a few studies on patients treated with curative intention[5,7-9]. However, these findings do not necessarily apply to palliative patients[10].
In 2001, a cancer centre with irradiation and chemotherapy facilities was established in the Norwegian county of West Agder with a change of iMDTa (WA/MDT-Change). Thus, iMDTa was established and the potential to work according to international MDT guidelines was created[4].
Our hypothesis was that the county of WA/MDT-Change, with its increasing iMDTa during the study period (from 2000-2008), would reach UGI cancer survival levels similar to those of a comparable Norwegian county with iMDTa. In addition, we hypothesized that the county of WA/MDT-Change would have favourable UGI cancer survival figures compared to a county without iMDTa during said period.
The primary objective was to evaluate the effect of the establishment of iMDTa on overall survival in a cohort of UGI cancer patients living in the Norwegian county of WA/MDT-Change.
MATERIALS AND METHODS
For detailed information about Norway’s health care system, the Cause of Death Registry, The Cancer Registry, the establishment of MDT-availability and the actual performance of MDT in the county of WA/MDT-Change, as well as a description of the other counties included for comparison, see electronic supplement. A short overview is given below.
Norway
Norway is a country with very little migration or socioeconomic disparity[11,12], amongst its 5 million inhabitants (Table 1). Health care coverage in Norway is provided through a single-payer universal government funded system. All persons residing in Norway are assigned a unique 11-digit identification number, making it possible to link information from various national registries.
Table 1.
Cox proportional hazards model adjusted for age and the different regions
| MDT-No | MDT-Change | MDT-Yes | Rest of Norway | ||
| All UGI cancers | 2000-2004 | HR 0.96, (0.83-1.10) | HR 0.96, (0.82-1.13) | HR 0.901, (0.80-1.0) | HR 0.96, (0.83-1.11) |
| 2005-2008 | HR 0.96, (0.82-1.13) | HR 0.811, (0.67-0.97) | HR 0.791, (0.70-0.89) | HR 0.851, (0.77-0.93) | |
| Oesophagus | 2000-2004 | HR 0.75, (0.49-1.15) | HR 1.12, (0.75-1.68) | HR 0.86, (0.63-1.17) | HR 0.89, (0.73-1.1) |
| 2005-2008 | HR 1.08, (0.72-1.61) | HR 0.601, (0.38-0.95) | HR 0.741, (0.53-1.02) | HR 0.84, (0.67-1.06) | |
| Gastric | 2000-2004 | HR 0.94, (0.70-1.26) | HR 0.87 (0.66-1.15) | HR 0.99, (0.84-1.12) | HR 0.94, (0.70-1.25) |
| 2005-2008 | HR 0.94, (0.70-1.23) | HR 0.631, (0.43-0.93) | HR 0.791, (0.65-0.97) | HR 0.821, (0.70-0.95) | |
| Pancreas | 2000-2004 | HR 0.97, (0.79-1.2) | HR 1.04, (0.83-1.3) | HR 0.90, (0.77-1.06) | HR 1.02, (1.02-1.03) |
| 2005-2008 | HR 0.92, (0.74-1.2) | HR 1.01, (0.78-1.3) | HR 0.841, (0.71-1.0) | HR 0.88, (0.78-1.0) |
UGI: Upper gastrointestinal cancer; MDT: Multidisciplinary team. Hazard ratios (HR) are given with 95% confidence interval, and the county of Oslo with MDT-Mixed serves as reference.
Statistically significant with P-value < 0.05.
Cause of Death Registry
Physicians are required by law to complete a death certificate for all deaths in Norway. The Cause of Death Registry[13] collects all death certificates for coding and registration of the cause of death.
The Cancer Registry
The Cancer Registry of Norway[6] (CRN), has collected data on all cancers that have occurred in Norway since 1953. Medical doctors are required by law to report these diagnoses, ensuring high levels of completeness[14]. Cancer type, date of diagnosis, extent or stage of the disease at diagnosis, and initial treatment in broad terms, are recorded.
Changes in WA/MDT-Change during 2000-2008
The Sørlandet Hospital Trust is the regional hospital in the county of WA/MDT-Change, which serves a stable population of approximately 170 000 inhabitants. In 2001, the Centre for Cancer Treatment was established at this hospital, thereby creating the potential for in-house MDTs. During the study period, the number of oncologists has increased from one consultant one day every fourth week to six full-time oncologists and two house officers. Prior to the establishment of the cancer centre, patients had to be referred for irradiation or complex chemotherapy to Oslo University Hospital, 300-500 km (a four to six hour drive) away.
In the ensuing years, increasing oncologic and palliative care expertise has developed and was practiced in conjunction with the already well-established pathological, radiological, gastrosurgical and gastroenterological specialties. Specifically, the following services were founded: A mobile palliative care team in 2002, an outpatient palliative care day centre in 2004 and an in-patient palliative care unit with ten beds in 2007. Prior to 2005, the management of cancer patients across specialties was discussed in informal and undocumented encounters between practitioners. From the summer of 2005 and onwards however, weekly MDT-meetings with a designated focus on gastrointestinal cancers have been held, with gastroenterologists, gastrointestinal surgeons, radiologists and oncologists present.
Other analyzed regions
Throughout the study period the inhabitants of the analysed regions selected for comparison had the same life expectancies and very similar socioeconomic conditions. Further details can be found in the electronic supplement (Table 1).
The choice of the Norwegian counties used for comparison to WA/MDT-Change in this study, is based on their stable status of iMDTa during the study period.
In this manuscript, we define “iMDTa” as a county’s theoretical possibility of MDT meetings within a single administrative institution with all departments on one campus (MDT-Yes). Thus, we measured the possibility of multidisciplinary in-house cooperation of necessary specialists, rather than the formal performance of MDTs. A county with stable iMDTa during the entire study period (MDT-Yes) was hypothesized to have the best survival figures for UGI patients. MDT-No describes a county with an absence of radiation units and medical oncologists within the hospital, where patients were referred to a tertiary university hospital for oncologic treatment during the entire study period. However, gastrointestinal surgeons, gastroenterologists, radiologists and, pathologists were available in such county. The population of the county of Oslo was treated partly in hospitals with all these services available and partly in hospitals without some of these services in the same institution. Therefore this region was defined as MDT-Mixed.
Patients
For the county of WA/MDT-Change, we used the hospital’s electronic database and confirmed and supplemented it with data from CRN. Further, we identified patients diagnosed with oesophageal, gastric or pancreatic cancers during the study period. Only patients with adenocarcinomas and squamous cell carcinomas were included. The clinical course of the disease of each patient was reviewed. Data regarding oncological, surgical and endoscopic interventions were collected. If surgery had been performed, it was characterized as curative or palliative. Survival figures between different regions were compared using data from CRN.
Ethics
The study was approved by the Regional Ethics Committee of Southern Norway. The anonymity of the patients included in the analysis was preserved according to the institutional guidelines of our hospital as well as those of the National Data Protection Commission of Norway.
Statistical analysis
Complete follow-up data were available on all patients. They were followed from the date of diagnosis to their death or the date of censoring (July 2011). Crude survival was calculated using the Kaplan-Meier method. Crude differences in survival were assessed with log-rank test. Further, to adjust for possible confounding multivariate Cox regression models were fitted. All models were adjusted for age, sex, stage and region and fitted separately for the two diagnostic periods. The results were presented as hazard ratios (HR) with 95% confidence intervals (CI). When assessing the regional differences, the county with MDT-Mix was used as a reference because its population was the largest (to ensure stability of the estimates). P-values of less than 0.05 were considered statistically significant. All analyses were performed with SPSS and Stata.
RESULTS
Patients
The annual incidences of oesophageal, gastric and pancreatic cancers in Norway from 2004 through 2008 were 4.1/100 000, 11.1/100 000, and 13.6/100 000, respectively. We analyzed 12 530 UGI patients living in five Norwegian regions, there of 395 patients in the county of WA/MDT-C. Median age at diagnosis was 74 years (17-98 years ) and median follow-up was 5 mo (0-138 mo).
The baseline characteristics of the patients are listed in Table 2.
Table 2.
Patient characteristics n(%)
|
Diagnosed Jan 2000-Dec 2004 |
Diagnosed Jan 2005-Dec 2008 |
|||||||||||||||
| MDT-Mix | MDT-No | MDT-Change | MDT-Yes | Other regions | MDT-Mix | MDT-No | MDT-Change | MDT-Yes | Other regions | |||||||
| Tumor type | ||||||||||||||||
| Oesophagus | 113 (15.4) | 31 (11.8) | 30 (14.6) | 68 (10.0) | 657 (12.9) | 91 (15.2) | 35 (16.2) | 30 (19.6) | 72 (12.8) | 571 (14.1) | ||||||
| Gastric | 258 (35.2) | 110 (41.8) | 77 (37.6) | 318 (47.0) | 2146 (42.2) | 214 (35.8) | 73 (33.8) | 45 (29.4) | 240 (42.6) | 1534 (38.0) | ||||||
| Pancreas | 362 (49.4) | 122 (46.4) | 98 (47.8) | 291 (43.0) | 2278 (44.8) | 293 (49.0) | 108 (50.0) | 78 (51.0) | 252 (44.7) | 1935 (47.9) | ||||||
| Total | 733 (100.0) | 263 (100.0) | 205 (100.0) | 677 (100.0) | 5081 (100.0) | 598 (100.0) | 216 (100.0) | 153 (100.0) | 564 (100.0) | 4040 (100.0) | ||||||
| Stage | ||||||||||||||||
| No metastasis | 109 (14.9) | 38 (14.4) | 28 (13.7) | 81 (12.0) | 694 (13.7) | 59 (9.9) | 30 (13.9) | 28 (18.3) | 71 (12.6) | 543 (13.4) | ||||||
| Lymph node metastasis | 155 (21.1) | 62 (23.6) | 49 (23.9) | 182 (26.9) | 1195 (23.5) | 125 (20.9) | 52 (24.1) | 44 (28.8) | 141 (25.0) | 926 (22.9) | ||||||
| Distant metastasis | 293 (40.0) | 104 (39.5) | 85 (41.5) | 303 (44.8) | 2083 (41.0) | 237 (39.6) | 97 (44.9) | 57 (37.3) | 237 (42.0) | 1642 (40.6) | ||||||
| Unknown | 176 (24.0) | 59 (22.4) | 43 (21.0) | 111 (16.4) | 1109 (21.8) | 177 (29.6) | 37 (17.1) | 24 (15.7) | 115 (20.4) | 929 (23.0) | ||||||
| Total | 733 (100.0) | 263 (100.0) | 205 (100.0) | 677 (100.0) | 5081 (100.0) | 598 (100.0) | 216 (100.0) | 153 (100.0) | 564 (100.0) | 4040 (100.0) | ||||||
MDT: Multidisciplinary team. No clinically relevant differences in stage distribution were revealed among the analyzed regions or between the two calendar periods. In addition, there were no clinically relevant differences in stage distribution among the studied counties.
No clinically relevant differences in stage distribution of UGI cancers were revealed among the analyzed regions or between the two calendar periods. Furthermore, the stage distribution remained stable during the whole study period. Roughly 40% of all UGI cancer patients had distant metastases at the time of their diagnosis.
The changes in survival over time are illustrated with Kaplan-Meier curves in Figure 1.
Figure 1.
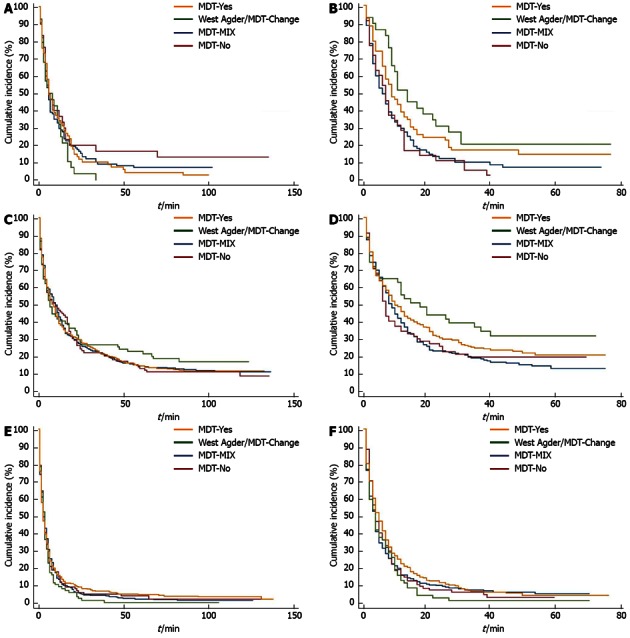
Increase in survival in counties with high in-house multidisciplinary team-availability during the second study period. A, B: Oesophagus; C, D: Stomach; E, F: Pancreas; A, C, E: 2000-2004; B, D, F: 2005-2008. MDT: Multidisciplinary team.
During the study period,the largest increases in survival were seen in the county of WA/MDT-Change, see green curve in Figure 1. Here, median survival for oesophageal cancer patients increased from 5 mo (3-12 mo) to 11 mo (9-23 mo) and from 7 mo (4-12 mo) to 15 mo (4-35 mo) for gastric cancer patients. However, these increases were not statistically significant. This numerical survival gain could not be observed in the MDT-No county (red curve) or in the MDT-Mix county (blue curve), whereas a survival gain could be observed in the MDT-Yes county (yellow curve).
After analyzing crude survival, survival was adjusted for age, region, sex and stage (even though no differences regarding sex and stage were found among the analysed regions). Comparing the two calendar periods of 2000-2004 and 2005-2008, the regions with the highest level of iMDTa achieved the largest decrease in risk of death for all UGI cancers (Table 1, compared to the county with MDT-Mix: MDT-Yes 11% and WA/MDT-Change 15%).
Analyzing the different tumour entities separately, the WA/MDT-Change county reached a statistically significant reduction in the risk of death (HR) compared to the county with MDT-Mix for oesophageal and gastric, but not for pancreatic cancer. HR for the study period 2000-2004 are given first and then for the period 2005-2008: The HR for oesophageal cancers was reduced from [HR = 1.12, 95%CI: 0.75-1.68 to HR = 0.60, 95%CI: 0.38-0.95] and for gastric cancers from [HR = 0.87, 95%CI: 0.66-1.15 to HR = 0.63, 95%CI: 0.43-0.93], but not for pancreatic cancer [HR = 1.04-, 95%CI: 0.83-1.3 for 2000-2004 and HR =1.01, 95%CI: 0.78-1.3 for 2005-2008].
Treatment and survival changes in the county of WA/MDT-Change
Hospital records for the region with changing status of iMDTa were analyzed to confirm the UGI cancer incidence numbers from the national registries, as well as the gain in survival. Further, we searched for changes in use of potentionall potentially life-prolonging oncologic interventions for the county WA/MDT-Change.
A total of 395 patients with UGI cancers were identified in the hospital records of the WA/MDT-Change county. These data are in full accordance with the incidence figures estimated by The National Cancer Registry[6].
The survival for all UGI cancer patients in the WA/MDT-Change county increased especially after 2004, when MDT meetings became more formalized. Median overall survival for all MDT-Change UGI cancer patients increased significantly from 129 d in the year 2000 to 300 d in 2008, P = 0.001. Also these data were in accordance with figures from CRN[6].
During the study period, several organizational changes were made at the Sørlandet Hospital Trust in the county of WA/MDT-Change. In line with the increased iMDTa, changes in the rates of curative surgery, oesophageal or bile duct stent placement, irradiation or chemotherapy were likely to have occurred and these were therefore analyzed.
UGI patients treated during the second study period had a higher probability of receiving chemotherapy. In the first study period, only one out of 43 patients in WA/MDT-Change (2.4%, 95%CI: 0-6.9) received chemotherapy, compared to 18 of 42 patients diagnosed during 2005-2008 (42.9%, 95%CI: 28.0-57.8).
The number of irradiation series did not increase for the diagnoses in question (data not shown).
During the study period, no major changes in surgical practice took place and there was no statistically significant increase in the number of curative UGI cancer surgeries (data not shown). No statistically significant increase in the use of gastro oesophageal or bile duct stents was observed during the two calendar periods of 2000-2004 and 2005-2008 (data not shown).
DISCUSSION
In this study, we found a more than two-fold increase in median survival for UGI cancer patients living in a Norwegian county during a time period in which in-house MDT has become available there. This increase in survival was not observed in counties without full iMDTa, but we saw a survival gain in both counties with iMDTa (MDT-Yes and MDT-Change).
The results of the described organizational changes are striking and clinically relevant, particularly in light of the limited advances in medical treatment of UGI cancer patients during the same time period[15].
This study is one of very few, that report a survival benefit of MDTs in cancer care. MDT meetings require a considerable amount of time from core specialists. Therefore, the need to confirm MDTs’ effectiveness on survival is of increasing importance, since there is an accelerating shortage of professional groups required for MDTs[16].
A major strength of the present study is its unique setting. Typically, before-after series[1-3] are confounded by concurrent changes in other factors, such as better treatments or different stage mixes, over the studied time period. In Norway, relatively few and stable socioeconomic differences are combined with an egalitarian public health service. In addition, high quality national cancer and death registries have been established decades ago. Furthermore, life expectancies were stable and similar in the analyzed regions throughout the study period. We were therefore able to analyze the un-confounded effect of changes in the organization of health care on survival of selected patient groups living in different regions.
Most importantly, survival outcomes can be attributed to patients’ residence, even if a few of them were operated or irradiated in other regions, thus indicating the quality of health care provided for the population living in a defined region. In addition, we have compared patient survival among regions between two time periods which were consequitive. Therefore, it is unlikely that significant changes in the possible confounding factors over a time period of 3-4 took place.
The precise role and composition of MDTs in cancer care vary throughout the world. Moreover, these variations exist even from hospital to hospital within the same region or country. Further hurdles in MDT research are the different interpretation of MDT-guidelines and the validity of documentation of the actual performance according to these guidelines[2]. Moreover, we are just starting to understand the individual factors of MDTs affecting the clinical outcome[17]. In the county of WA/MDT-Change, the MDTs were organized in line with international MDT guidelines[4], and aimed to perform accordingly.
While using registry data for patient identification prevents bias associated with clinician selection of patients, registry retrieved data has limitations with respect to the variables available for analysis. In addition to the CRN, we had complete hospital records for the region with changing status of iMDTa and could therefore analyze the changes in use of potentially life-prolonging oncologic interventions for the MDT-Change county. One measureable factor potentially contributing to the increase in survival in WA/MDT-Change may be the increased use of chemotherapy. This increase is higher than expected for this time period. A 50% increase in the use of chemotherapy for every year of the study period may be a result of more patients getting therapy. In that respect, MDT seems to result in increased referral of UGI patients to the medical oncologist. Travel distance to hospitals has been shown by others to be a barrier to treatment among patients with most types of cancer, including UGI cancers[18-20]. Furthermore, the EUROCARE working group found striking differences in gastric cancer survival and the quality of management logistics has been proposed as an important variable for patient survival[21]. In line with this argument, gastric cancer patients at district hospitals more often received adjuvant chemotherapy, than patients treated in university hospitals in Norway[22]. Unfortunately, we have not been able to analyze to what extent patients in other regions had received chemotherapy. However, in light of the striking survival gains, it appears that the increasing use of chemotherapy is unlikely to be the only reason for the survival gain seen in WA/MDT-Change.
The number of irradiation series or the use of gastro oesophageal or bile duct stents did not increase during the study period and we do not consider that the changes of the palliative services had a major impact on the life expectancy of the study patients, since the in-patient service was established at the very end of the study period.
The role of surgery for survival of the whole study cohort in this setting is more complex, since the group of UGI cancers as a whole has a low rate of curative surgery. Pancreatic and esophageal cancers are operable in less than one of five cases[15], and small changes in this ratio affect median overall survival to a limited extent. Concerning gastric cancer, 43% of cases are operated in Norway[22]. This proportion was allready higher before (data not shown), but stable throughout the study period, in our clinic. Thus, in light of a relatively high rate of surgery before iMDTa, the rate of surgery was not affected through the establishment of iMDTa in the WA/MDT-Change county. When looking at the results for gastric and esophageal cancers (Table 2), two findings are interesting: Both MDT-No and WA/MDT-Change centralized surgical treatment of esophageal, but not gastric cancer during the second interval of the study period to the MDT-Mix county of Oslo. In the WA/MDT-Change county, a survival benefit was seen for both entities, whereas the survival for these two diagnoses decreased in the MDT-No county (Table 2). These findings may support the theory that, in these particular geographic regions, the presence of oncologists in a hospital may have a greater impact than the place of curative surgery, at least on short term survival.
In this respect, the increased use of chemotherapy should be interpreted as an effect modifier for survival and the MDT members in WA/MDT-Change agree upon a during the study period gradually improved team spirit and more effective communication, although it seems easier to measure the results, rather than formally proof the process of such increased human interdependency.
A limitation of our study is the relatively low incidence of UGI cancers. We therefore analysed survival changes for all stages combined for each cancer type. As the main goal of our study was to assess changes in survival for the entire group of UGI cancer patients, we consider our results valid because the stage distribution for a given diagnosis in the different regions did not change during the course of the study period. In addition, the limited life expectancy for UGI cancer patients makes it possible to compare and assess results concerning improved patient outcome after treatment changes in a way that cannot be achieved in entities with long term survival. Seventy percent of UGI cancer patients live shorter than one year, making short term survival figures important both for the patients and health care administrators when considering organizational changes.
The explanation of the increased survival seen after the introduction of in-house MDT-availability is most likely multi-factorial. Future prospective studies should also analyze the communicative implications at play when a team is formed in-house over a period of several years and assessment tools for this purpose have been created[23]. Most importantly, it is not clear if the present findings for UGI cancer patients can be extrapolated to other cancer entities. This question should be addressed in future research.
In conclusion, we present one of the first studies showing a survival benefit for oesophageal and gastric cancer patients after the establishment of MDTs. We found a striking and more than two-fold increase in survival among patients with UGI cancers living in a Norwegian county with increasing iMDTa. During the analysed time period, no increase in survival was found in counties without consistent MDT availability. The survival gain might be partly explained by increased use of chemotherapy.
ACKNOWLEDGEMENTS
The authors express their gratitude to all members of the GI-cancer team at Sørlandet Hospital Trust for their continuous efforts for our patients. Especially, we would like to thank Geir Bøhler for his help with the UGI cancer database at the Southern Hospital trust and Marte Grønlie Cameron for her invaluable work in editing this manuscript. We thank the Norwegian Cancer Registry for providing us with the survival data.
COMMENTS
Background
There is a lack of evidence that clinical decision making by a multidisciplinary team (MDT) leads to increased survival for oesophageal, gastric and pancreatic cancers
Research frontiers
The rationale for introducing MDTs is that modern cancer management has become increasingly complex, necessitating the involvement of various key professional groups in clinical decision making. In addition, MDTs serve to monitor adherence to clinical guidelines and promote effective use of resources. Evaluations of the effectiveness of a MDT on survival are warranted, but complicated to perform due to difficulties regarding: (1) its definition; (2) availability of valid measurement of its performance and, most importantly; and (3) the ethical and organizational hurdles of conducting prospective randomized studies of MDTs.
Innovations and breakthroughs
In this study, authors did not focus on the actual practice of MDTs but aim to analyse the effect of their in-house availability. This is the first study to document a survival benefit of upper gastrointestinal (UGI) cancers after the implementation of MDT.
Applications
This study gives evidence to the wideheld belief of a survival benefit in UGI cancer patients, when treated in a setting of MDT.
Terminology
Here, the term “in-house MDT” was introduced and efined as a county’s theoretical possibility of MDT meetings within a single administrative institution with all departments on one campus (MDT-Yes).
Peer review
The authors examined the survival benefit of UGI cancer patients after the introduction of in-house MDT and compared the survival to the geographic regions with and without in-house MDT.
Footnotes
P- Reviewers Nelius T, Merrett N, Zullo A S- Editor Wen LL L- Editor A E- Editor Lu YJ
References
- 1.Fleissig A, Jenkins V, Catt S, Fallowfield L. Multidisciplinary teams in cancer care: are they effective in the UK? Lancet Oncol. 2006;7:935–943. doi: 10.1016/S1470-2045(06)70940-8. [DOI] [PubMed] [Google Scholar]
- 2.Hong NJ, Wright FC, Gagliardi AR, Paszat LF. Examining the potential relationship between multidisciplinary cancer care and patient survival: an international literature review. J Surg Oncol. 2010;102:125–134. doi: 10.1002/jso.21589. [DOI] [PubMed] [Google Scholar]
- 3.Taylor C, Munro AJ, Glynne-Jones R, Griffith C, Trevatt P, Richards M, Ramirez AJ. Multidisciplinary team working in cancer: what is the evidence? BMJ. 2010;340:c951. doi: 10.1136/bmj.c951. [DOI] [PubMed] [Google Scholar]
- 4.NHS, National Cancer Action Team. The Characteristics of an Effective Multidisciplinary Team (MDT) Available from: http: //wwwncinorguk/cancer_type_and_topic_specific_work/multidisciplinary_teams/mdt_developmentaspx 2010.
- 5.Ruhstaller T, Roe H, Thürlimann B, Nicoll JJ. The multidisciplinary meeting: An indispensable aid to communication between different specialities. Eur J Cancer. 2006;42:2459–2462. doi: 10.1016/j.ejca.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 6. Available from: http://www.kreftregisteret.no/en/
- 7.Katz MH, Wang H, Fleming JB, Sun CC, Hwang RF, Wolff RA, Varadhachary G, Abbruzzese JL, Crane CH, Krishnan S, et al. Long-term survival after multidisciplinary management of resected pancreatic adenocarcinoma. Ann Surg Oncol. 2009;16:836–847. doi: 10.1245/s10434-008-0295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pawlik TM, Laheru D, Hruban RH, Coleman J, Wolfgang CL, Campbell K, Ali S, Fishman EK, Schulick RD, Herman JM. Evaluating the impact of a single-day multidisciplinary clinic on the management of pancreatic cancer. Ann Surg Oncol. 2008;15:2081–2088. doi: 10.1245/s10434-008-9929-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stephens MR, Lewis WG, Brewster AE, Lord I, Blackshaw GR, Hodzovic I, Thomas GV, Roberts SA, Crosby TD, Gent C, et al. Multidisciplinary team management is associated with improved outcomes after surgery for esophageal cancer. Dis Esophagus. 2006;19:164–171. doi: 10.1111/j.1442-2050.2006.00559.x. [DOI] [PubMed] [Google Scholar]
- 10.Strong S, Blencowe NS, Fox T, Reid C, Crosby T, Ford HE, Blazeby JM. The role of multi-disciplinary teams in decision-making for patients with recurrent malignant disease. Palliat Med. 2012;26:954–958. doi: 10.1177/0269216312445296. [DOI] [PubMed] [Google Scholar]
- 11.Mackenbach JP, Stirbu I, Roskam AJ, Schaap MM, Menvielle G, Leinsalu M, Kunst AE. Socioeconomic inequalities in health in 22 European countries. N Engl J Med. 2008;358:2468–2481. doi: 10.1056/NEJMsa0707519. [DOI] [PubMed] [Google Scholar]
- 12.The Norwegian Healthe Directorate. Overarching Goals. Available from: http://www.helsedirektoratet.no/english/about-us/mission-and-role/Sider/default.aspx.
- 13. Available from: http://www.fhi.no/eway/default.aspx?pid=240&trg=Main_6664&Main_6664=6898:0:25,7838:1:0:0:::0:0.
- 14.Larsen IK, Småstuen M, Johannesen TB, Langmark F, Parkin DM, Bray F, Møller B. Data quality at the Cancer Registry of Norway: an overview of comparability, completeness, validity and timeliness. Eur J Cancer. 2009;45:1218–1231. doi: 10.1016/j.ejca.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 15.Jiang Y, Kimchi ET, Montero AJ, Staveley-O’Carroll KF, Ajani JA. Upper gastrointestinal tumors: current status and future perspectives. Expert Rev Anticancer Ther. 2008;8:975–991. doi: 10.1586/14737140.8.6.975. [DOI] [PubMed] [Google Scholar]
- 16.Bunnell CA, Shulman LN. Will we be able to care for cancer patients in the future? Oncology (Williston Park) 2010;24:1343–1348. [PubMed] [Google Scholar]
- 17.Lamb BW, Brown KF, Nagpal K, Vincent C, Green JS, Sevdalis N. Quality of care management decisions by multidisciplinary cancer teams: a systematic review. Ann Surg Oncol. 2011;18:2116–2125. doi: 10.1245/s10434-011-1675-6. [DOI] [PubMed] [Google Scholar]
- 18.Crawford SM, Sauerzapf V, Haynes R, Zhao H, Forman D, Jones AP. Social and geographical factors affecting access to treatment of lung cancer. Br J Cancer. 2009;101:897–901. doi: 10.1038/sj.bjc.6605257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones AP, Haynes R, Sauerzapf V, Crawford SM, Zhao H, Forman D. Travel time to hospital and treatment for breast, colon, rectum, lung, ovary and prostate cancer. Eur J Cancer. 2008;44:992–999. doi: 10.1016/j.ejca.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Stitzenberg KB, Sigurdson ER, Egleston BL, Starkey RB, Meropol NJ. Centralization of cancer surgery: implications for patient access to optimal care. J Clin Oncol. 2009;27:4671–4678. doi: 10.1200/JCO.2008.20.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lepage C, Sant M, Verdecchia A, Forman D, Esteve J, Faivre J. Operative mortality after gastric cancer resection and long-term survival differences across Europe. Br J Surg. 2010;97:235–239. doi: 10.1002/bjs.6865. [DOI] [PubMed] [Google Scholar]
- 22.Hølmebakk T, Frykholm G, Viste A. Introducing national guidelines on perioperative chemotherapy for gastric cancer in Norway: a retrospective audit. Eur J Surg Oncol. 2010;36:610–616. doi: 10.1016/j.ejso.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 23.Lamb BW, Sevdalis N, Mostafid H, Vincent C, Green JS. Quality improvement in multidisciplinary cancer teams: an investigation of teamwork and clinical decision-making and cross-validation of assessments. Ann Surg Oncol. 2011;18:3535–3543. doi: 10.1245/s10434-011-1773-5. [DOI] [PubMed] [Google Scholar]


