Abstract
AIM: To evaluate outcomes in resectable cholangiocarcinoma patients and to determine prognostic factors.
METHODS: A retrospective study was conducted among newly-diagnosed cholangiocarcinoma patients from January 2009 to December 2011 who underwent curative resection in Srinakarind Hospital (a 1000-bed university hospital). Two hundred and sixty-three cholangiocarcinoma patients with good performance were enrolled. These patients had pathological reports with clear margins or microscopic margins. Prognostic factors which included clinical factors, serum liver function test as well as serum tumor makers at presentation, tumor data, and receiving adjuvant chemotherapy were determined by uni- and multivariate analysis.
RESULTS: The median overall survival time was 17 mo (95%CI: 13.2-20.7); and 1-, 2-, and 3- year survival rates were 65.5%, 45.2% and 35.4%. Serum albumin levels, serum carcinoembryonic antigen (CEA) levels, staging classifications by American Joint Committee on cancer, pathological tumor staging, lymph node metastases, tumor grading, surgical margin status, and if adjuvant chemotherapy was administered, were shown to be significant prognostic factors of resectable cholangiocarcinoma by univariate analysis. Multivariate analysis, however, established that only abnormal serum CEA [hazard ratio (HR) 1.68; P = 0.027] and lymph node metastases (HR 2.27; P = 0.007) were significantly associated with a decrease in overall survival, while adjuvant chemotherapy (HR 0.71; P = 0.067) and surgical margin negative (HR 0.72; P = 0.094) tended to improve survival time.
CONCLUSION: Serum CEA and lymph node metastases which were associated with advanced stage tumors become strong negative prognostic factors in cholangiocarcinoma.
Keywords: Cholangiocarcinoma, Prognosis, Carcinoembryonic antigen, Lymph nodes, Neoplasm metastasis, Surgical margin status, Hepatectomy, Chemotherapy, Adjuvant, Survival rate
Core tip: Cholangiocarcinoma has a high prevalence in the Asian countries, particularly Thailand. Cholangiocarcinoma patients usually have a high mortality rate and poor treatment outcomes. Curative surgery is the only treatment for early stages of this cancer. Cholangiocarcinoma has a high rate of recurrence. This study aimed to evaluate outcomes in resectable cholangiocarcinoma patients and to determine prognostic factors. The results demonstrated serum carcinoembryonic antigen and lymph node metastases which were associated with advanced stage tumors become strong negative prognostic factors in cholangiocarcinoma, while additional treatment including adjuvant chemotherapy and adequate surgical resection may improve survival time.
INTRODUCTION
Cholangiocarcinoma is a malignant tumor of intrahepatic and extrahepatic bile duct epithelium[1]. It is a second most common malignancy of primary liver tumors worldwide[2]. The highest incidence is in the Northeast region of Thailand, while it is a rare tumor in Europe and America[3,4]. Opisthorchis viverrini infestation is a major risk factor in Thai patients, while primary sclerosing cholangitis, obesity, viral hepatitis B and viral hepatitis C infection are the risk factors in Western countries[5,6]. Cholangiocarcinoma is commonly classified into 3 groups based on the location of the tumor: intrahepatic, perihilar, or distal types[1].
Surgery with clear surgical margin is an important treatment for patients with local disease[7]. Standard surgery for cholangiocarcinoma depends on its location. Major hepatectomy is a surgical procedure for intrahepatic cholangiocarcinoma and perihilar cholangiocarcinoma, while pancreaticoduodenectomy is performed in distal cholangiocarcinoma[7,8].
Although most patients receive surgical treatment, the five-year survival rate is extremely low[9]. High locoregional recurrence and metastases are common causes of death in resectable patients[10]. Benefits of adjuvant therapy in achieving long-term survival in resectable cholangiocarcinoma patients are controversial[11]. Previous studies attempted to identify prognostic factors in this group[12-15]. Surgical margin status and lymph node involvement are important prognostic factors[9,11,16]. Other risk factors may be differentiation of tumor cells, preoperative tumor markers like carbohydrate antigen 19-9 (CA 19-9) and carcinoembryonic antigen (CEA), and site of tumor[13,17,18]. Data about prognosis in resectable cancer patients, however, are still limited. Moreover, only a few participants were enrolled in former reports. Therefore, this study aimed to determine prognostic factors in cholangiocarcinoma patients who underwent curative resection.
MATERIALS AND METHODS
Patients
A retrospective study was conducted among newly-diagnosed, cholangiocarcinoma patients from January 2009 to December 2011, who underwent curative surgery in Srinakarind Hospital, Khon Kaen University (a 1000-bed university hospital), Khon Kaen, Thailand. The study was reviewed and approved by the institutional review board (HE 551183). Curative resection was defined as a total excision of the entire tumor, including the primary tumor and the associated lymph node drainage fields. Two hundred and sixty-three cholangiocarcinoma patients with good performance status were enrolled. All patients with curative resection had pathological reports with a negative surgical margin or microscopic surgical margin. Demographic data including sex, age, underlying disease especially type 2 diabetes mellitus, body weight, height, and clinical manifestations were collected. Body mass index (BMI) was calculated from weight in kilograms divided by the square of the height in meters (kg/m2). BMI cutoffs were classified according to the World Health Organization criteria for Asian and Pacific populations (underweight, < 18.5 kg/m2; healthy, 18.5-22.9 kg/m2; at risk, 23-24.9 kg/m2; obese I, 25-29.9 kg/m2; and obese II, ≥ 30 kg/m2)[19]. Preoperative liver function status including total bilirubin, cholesterol, alanine transaminase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP), as well as serum tumor markers including CA 19-9 and CEA were evaluated.
Tumor data included tumor location, staging classification by the 7th edition of American Joint Committee on Cancer (AJCC), pathological tumor staging (pT), lymph node metastasis, tumor differentiation, and surgical margin status. All patients received the appropriate surgical procedure. Adjuvant chemotherapy was administered in patients who accepted the risk-benefit after a discussion with their physicians.
Statistical analysis
The survival time was defined as date of diagnosis to date of death from any cause. Patients’ characteristics and tumor data were summarized as mean and percentage. The cumulative survival rate is presented by the Kaplan-Meier curve. The following variable factors were analyzed: sex, age, diabetic status, hepatomegaly, BMI status, serum total bilirubin level, serum cholesterol level, serum albumin level, serum ALT level, serum AST level, serum ALP level, serum CEA level, serum CA 19-9 level, AJCC staging, tumor location, pT, lymph node status, tumor differentiation, surgical margin status and adjuvant chemotherapy. Differences in survival between subgroups were compared using the log-rank test. Univariate analysis was performed using the chi-squared testing. Multivariate analysis was performed with the Cox proportional hazard model. The statistical analyses were performed by using SPSS software version 20.0. A P-value of less than 0.05 was considered statistically significant. The database was closed for analysis in August 2012.
RESULTS
The patients’ characteristics and tumor data are presented in Tables 1 and 2. Abdominal pain was the most common clinical presentation. The majority of the patients had normal a BMI, level of serum total bilirubin below 10 mg/dL, level of serum albumin above 3 g/dL, elevation of serum liver enzymes as well as abnormal serum tumor markers, CA 19-9 and CEA. Intrahepatic cholangiocarcinoma was the most common site of tumor. Most patients were in an advanced stage, i.e., stage III or IV. One hundred and thirty-three patients received adjuvant chemotherapy of which the combination of fluorouracil and mitomycin C was the most administered regimen (60.9% of these patients). Other regimens included combination of gemcitabine and capecitabine, gemcitabine, fluorouracil, and capecitabine.
Table 1.
Baseline characteristics of 263 resectable cholangiocarcinoma patients n (%)
| Age, yr | |
| mean ± SD | 59.0 ± 8.9 |
| Range | 35-80 |
| Male | 181 (69.6) |
| DM | 19 (6.5) |
| BMI (mean ± SD), kg/m2 | |
| < 18.5 | 23 ± 8.7 |
| 18.5-22.9 | 127 ± 48.3 |
| 23-24.9 | 47 ± 17.9 |
| 25-29.9 | 48 ± 18.3 |
| ≥ 30 | 13 ± 4.9 |
| Not available | 5 ± 1.9 |
| Clinical manifestation | |
| Abdominal pain | 164 (62.4) |
| Jaundice | 54 (20.5) |
| Fever | 6 (2.3) |
| Cholangitis | 4 (1.5) |
| Weight loss | 1 (0.4) |
| Asymptomatic | 17 (6.5) |
| Hepatomegaly | 153 (58.2) |
| Total bilirubin (mg/dL) | |
| < 10 | 213 (81.0) |
| ≥ 10 | 50 (19.0) |
| Cholesterol (mg/dL) | |
| < 200 | 168 (63.9) |
| ≥ 200 | 95 (36.1) |
| Albumin (g/dL) | |
| < 3 | 42 (16.0) |
| ≥ 3 | 220 (83.7) |
| ALT (U/L) | |
| < 30 | 46 (17.5) |
| ≥ 30 | 151 (82.5) |
| AST (U/L) | |
| < 30 | 25 (9.5) |
| ≥ 30 | 238 (90.5) |
| ALP (U/L) | |
| < 100 | 82 (31.2) |
| ≥ 100 | 180 (68.5) |
| CA 19-9 (U/mL) | |
| < 35 | 108 (41.1) |
| ≥ 35 | 148 (56.3) |
| CEA (ng/mL) | |
| < 2.5 | 65 (24.7) |
| ≥ 2.5 | 183 (69.6) |
| Receiving adjuvant chemotherapy | |
| Yes | 138 (52.5) |
| No | 125 (47.5) |
CA 19-9: Carbohydrate antigen 19-9; CEA: Carcinoembryonic antigen; ALT: Alanine transaminase; AST: Aspartate aminotransferase; ALP: Alkaline phosphatase; BMI: Body mass index; DM: Diabetes mellitus.
Table 2.
Tumor data of 263 resectable cholangiocarcinoma n (%)
| Tumor location | |
| Intrahepatic | 166 (63.1) |
| Perihilar | 91 (34.6) |
| Distal | 6 (2.3) |
| AJCC staging | |
| 0 | 11 (4.2) |
| 1 | 37 (14.1) |
| 2 | 54 (20.5) |
| 3 | 89 (33.8) |
| 4 | 72 (27.4) |
| pT stage | |
| 0 | 10 (3.8) |
| 1 | 47 (17.9) |
| 2 | 85 (32.3) |
| 3 | 95 (36.1) |
| 4 | 25 (9.5) |
| pN stage | |
| 0 | 167 (63.5) |
| 1 | 96 (36.5) |
| Tumor grading | |
| Well diff | 198 (75.3) |
| Moderate diff | 14 (5.3) |
| Not available | 51 (19.4) |
| Margin surgical resection | |
| Free | 134 (51.0) |
| Not free | 129 (49.0) |
AJCC: American Joint Committee on Cancer; pN: Pathologic node; pT: Pathologic tumor.
Median overall survival of the entire cohort was 17 mo (95%CI: 13.2-20.7) as shown in Figure 1. One, two, and three-year survival rates were 65.5%, 45.2%, and 35.4%. Serum albumin, serum CEA, AJCC staging, pT staging, lymph node metastases and whether or nor having received adjuvant chemotherapy were significant prognostic factors in resectable cholangiocarcinoma by univariate analysis as shown in Table 3. Figure 2 revealed Kaplan-Meier survival curve regarding significant prognostic factors. Receiving adjuvant chemotherapy prolonged survival in resectable cholangiocarcinoma patients, however, the combination between fluorouracil and mitomycin C was not different other regimen to improve survival benefit [median survival time was 17.3 mo (95%CI: 12.8-21.7) vs 22.3 mo (95%CI: 20.3-24.3), respectively; P = 0.20]. Abnormal serum CEA and lymph node metastasis significantly impacted the overall survival in multivariate analysis (Table 4).
Figure 1.
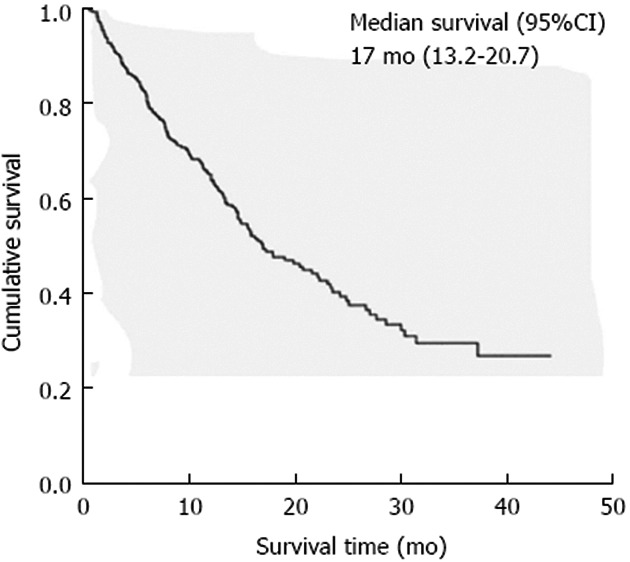
Kaplan-Meier survival curve used to analyze the overall survival time of 263 resectable cholangiocarcinoma.
Table 3.
Differences of survival time among significant variable factors when analyzed by univariate analysis
| Variable | Median survival (mo) | 95%CI | P value |
| Albumin (g/dL) | 0.04 | ||
| < 3 | 12.8 | 7.1-18.4 | |
| ≥ 3 | 19.1 | 14.6-23.5 | |
| CEA (ng/mL) | 0.02 | ||
| < 2.5 | 27.7 | 14.1-41.3 | |
| ≥ 2.5 | 16.5 | 13.0-20.0 | |
| AJCC staging | < 0.001 | ||
| 0 | Not reached | ||
| 1 | Not reached | ||
| 2 | 23.5 | 16.9-30.1 | |
| 3 | 12.8 | 10.6-15.1 | |
| 4 | 12.5 | 9.3-15.7 | |
| Tumor grading | 0.01 | ||
| Well differentiated | 17.9 | 12.6-23.2 | |
| Moderate differentiated | 7.7 | 0.0-21.7 | |
| Margin in resection group | 0.001 | ||
| Negative | 26.7 | 19.6-33.8 | |
| Positive | 14.1 | 11.9-16.4 | |
| pT stage | < 0.001 | ||
| 0 | Not reached | ||
| 1 | 28.6 | 23.1-34.1 | |
| 2 | 19.9 | 12.9-26.9 | |
| 3 | 12.8 | 9.4-16.3 | |
| 4 | 15.5 | 9.9-21.1 | |
| pN stage | < 0.001 | ||
| 0 | 25.1 | 20.0-30.1 | |
| 1 | 10.0 | 6.7-13.3 | |
| Receiving adjuvant chemotherapy | 0.01 | ||
| Yes | 21.6 | 16.9-26.4 | |
| No | 13.4 | 10.7-16.2 |
CEA: Carcinoembryonic antigen; AJCC: American Joint Committee on Cancer. pN: Pathologic node; pT: Pathologic tumor.
Figure 2.
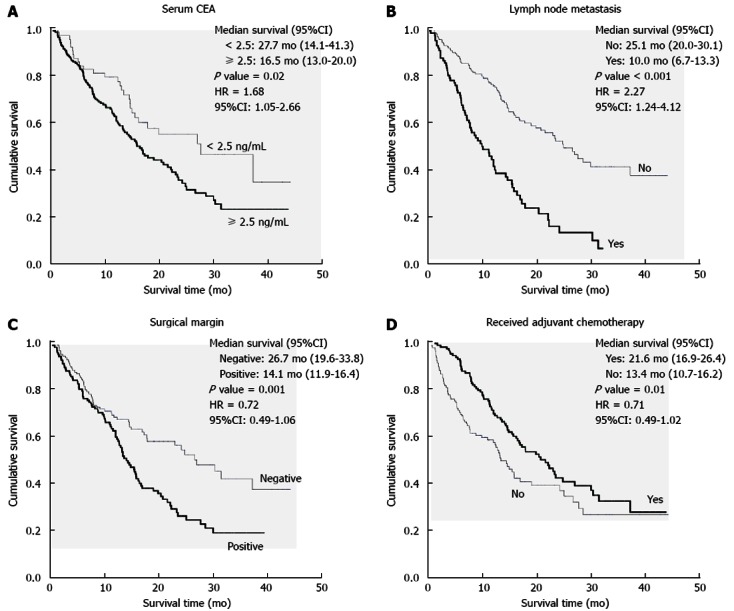
Kaplan-Meier survival curve showed significant difference in survival rate regarding prognostic factors. A: Serum carcinoembryonic antigen (CEA) level ≥ 2.5 ng/mL at presentation; B: Lymph node metastasis; C: Surgical margin; D: Receiving adjuvant chemotherapy. HR: Hazard ratio.
Table 4.
Significant prognostic factors by multivariate analysis
| Variable | HR | 95%CI | P value |
| Serum CEA (< 2.5 ng/mL ≥ 2.5 ng/mL) | 1.68 | 1.05-2.66 | 0.027 |
| Lymph node metastasis (yes vs no) | 2.27 | 1.24-4.12 | 0.007 |
| Receiving adjuvant chemotherapy (yes vs no) | 0.71 | 0.49-1.02 | 0.067 |
| Surgical margin (negative vs positive) | 0.72 | 0.49-1.06 | 0.094 |
CEA: Carcinoembryonic antigen; HR: Hazard ratio.
DISCUSSION
This cohort study had several similar and different characteristics from the previous reports[3,20,21]. Most patients in this study had a BMI below 23; whereas, the majority of patients in the cited previous report were overweight[20]. The tumor data showed that intrahepatic cholangiocarcinoma was the most common subtype, whereas, perihilar subtype was the most common location in other reports[3]. These findings were correlated with the first clinical presentation of abdominal pain and level of serum bilirubin below 10 mg/dL. Furthermore, this study found that asymptomatic presentation was more common in patients with intrahepatic cholangiocarcinoma than in other previous studies[21]. The authors’ results demonstrated serum albumin was a significant prognostic factor by univariate analysis. Serum albumin is marker of nutritional status in cancer patients[22]. A low level of serum albumin is usually found in malnourished patients, and associated with poor treatment outcomes such as postoperative infection and impaired wound healing[23,24]. Advanced stages of cancers, including cholangiocarcinoma, also lead to a decrease in serum albumin level[25]. Additionally, previous studies reported that low serum albumin was associated with an increased postoperative mortality in cholangiocarcinoma patients[26].
AJCC staging of cholangiocarcinoma, pT staging, and the differentiation of tumor cells were an associated prognostic factor, as well and were demonstrated in our results by univariate analysis. These results were similar with previous results[18,25]. A well-differentiated tumor histology was related to early staging and was a good prognostic factor from results of the previous studies[27,28].
The results showed that the level of serum CEA above 2.5 ng/mL and lymph node metastases were significant independent poor prognostic factors by univariate and multivariate analysis. CEA was demonstrated in fetal gut tissue and in tumors from the gastrointestinal tract[29]. Serum CEA in cancer patients was significantly higher than in healthy controls and may be a prognostic factor in several gastrointestinal cancers, including cholangiocarcinoma[30,31]. A previous study demonstrated that cancer patients with a high level of serum CEA was associated with an advanced stage of cancer and may signal poor prognosis[32,33]. This study demonstrated that cholangiocarcinoma patients with high level of serum CEA were associated high risk of death (HR 1.68, 95%CI: 1.05-2.66), which is similar to previous studies[25]. The preoperative serum CEA level in cholangiocarcinoma patients was correlated with the stage of cancer and could help determine their prognosis[32,34].
Lymphatic dissemination is a common metastatic pathway of cholangiocarcinoma. Previous studies demonstrated that up to 55% of cholangiocarcinoma patients who underwent operations had tumor cells in the regional lymph nodes[9]. Several studies showed that overall survival rate in cholangiocarcinoma patients with lymph node involvement was lower than other groups[35-37]. These findings were similar in both resectable and unresectable patients[25,26,38,39]. The findings of the present study also showed that lymph node metastases had an impact on survival.
Surgical margin status is a prognostic factor in several cancers, including cholangiocarcinoma. Previous studies showed overall survival rate in cholangiocarcinoma patients with positive surgical margin was lower than patients with negative surgical margin[15,28,40-42]. The present results demonstrated that a negative surgical margin was associated long-term survival time.
Adjuvant chemotherapy is a controversial issue in resectable cholangiocarcinoma. The present authors’ results showed that patients with adjuvant chemotherapy may have longer overall survival time than patients without adjuvant chemotherapy. Previous retrospective studies showed benefits of adjuvant chemotherapy[12,15,43]. Randomized studies, however, did not demonstrate a definite advantage in cholangiocarcinoma[44]. Recently, a meta-analysis showed that chemotherapy as a part of adjuvant therapy which included radiotherapy and concurrent chemoradiotherapy may be beneficial in resectable cholangiocarcinoma patients with high risk features, such as lymph node metastases and positive surgical margins[45]. In our institute, combination of 5-fluorouracil and mitomycin C was the most administered regimen. However, the survival of this combination was not significantly different from the other regimens.
In conclusion, serum CEA and lymph node metastasis which are associated with advanced tumor stages become strong negative prognostic factors in cholangiocarcinoma, while additional treatment including adjuvant chemotherapy and adequate surgical resection may improve survival time.
ACKNOWLEDGMENTS
This research involved with multidisciplinary team including surgeons, internists, medical oncologist, pathologists, diagnostic radiologists, and nurses. Technology Transfer Affairs, Khon Kaen University, for assistance with the English-language presentation of the manuscript.
COMMENTS
Background
Cholangiocarcinoma has a high prevalence in the Asian countries, particularly Thailand. Cholangiocarcinoma patients usually have a high mortality rate and poor treatment outcomes. Curative surgery is the only treatment for early stages of this cancer. Cholangiocarcinoma has a high rate of recurrence. This study aimed to evaluate outcomes in resectable cholangiocarcinoma patients and to determine prognostic factors.
Research frontiers
A retrospective study included newly-diagnosed 263 cholangiocarcinoma patients from January 2009 to December 2011 who underwent curative resection and had pathological reports with clear margins or microscopic margins in Srinakarind Hospital (a 1000-bed university hospital).
Innovations and breakthroughs
The results demonstrated serum carcinoembryonic antigen and lymph node metastases which were associated with advanced stage tumors become strong negative prognostic factors in cholangiocarcinoma, while additional treatment including adjuvant chemotherapy and adequate surgical resection may improve survival time.
Applications
Adjuvant chemotherapy and adequate surgical resection may improve survival time.
Terminology
Curative resection was defined as a total excision of the entire tumor, including the primary tumor and the associated lymph node drainage fields.
Peer review
This is an interesting study aimed to evaluate outcomes in resectable cholangiocarcinoma patients and to determine prognostic factors. The results are interesting and suggest that adjuvant chemotherapy which includes combination of fluorouracil and mitomycin C and other regimens may improve overall survival in resectable cholangiocarcinoma patients.
Footnotes
Supported by The Khon Kaen University Publication Clinic, Research
P- Reviewers Marin JJG, Kim JG S- Editor Gou SX L- Editor A E- Editor Xiong L
References
- 1.Nakeeb A, Pitt HA, Sohn TA, Coleman J, Abrams RA, Piantadosi S, Hruban RH, Lillemoe KD, Yeo CJ, Cameron JL. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg. 1996;224:463–473; discussion 473-475. doi: 10.1097/00000658-199610000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olnes MJ, Erlich R. A review and update on cholangiocarcinoma. Oncology. 2004;66:167–179. doi: 10.1159/000077991. [DOI] [PubMed] [Google Scholar]
- 3.Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004;24:115–125. doi: 10.1055/s-2004-828889. [DOI] [PubMed] [Google Scholar]
- 4.Green A, Uttaravichien T, Bhudhisawasdi V, Chartbanchachai W, Elkins DB, Marieng EO, Pairqjkul C, Dhiensiri T, Kanteekaew N, Haswell-Elkins MR. Cholangiocarcinoma in north east Thailand. A hospital-based study. Trop Geogr Med. 1991;43:193–198. [PubMed] [Google Scholar]
- 5.Watanapa P, Watanapa WB. Liver fluke-associated cholangiocarcinoma. Br J Surg. 2002;89:962–970. doi: 10.1046/j.1365-2168.2002.02143.x. [DOI] [PubMed] [Google Scholar]
- 6.Chapman RW. Risk factors for biliary tract carcinogenesis. Ann Oncol. 1999;10 Suppl 4:308–311. [PubMed] [Google Scholar]
- 7.Aljiffry M, Walsh MJ, Molinari M. Advances in diagnosis, treatment and palliation of cholangiocarcinoma: 1990-2009. World J Gastroenterol. 2009;15:4240–4262. doi: 10.3748/wjg.15.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kondo S, Takada T, Miyazaki M, Miyakawa S, Tsukada K, Nagino M, Furuse J, Saito H, Tsuyuguchi T, Yamamoto M, et al. Guidelines for the management of biliary tract and ampullary carcinomas: surgical treatment. J Hepatobiliary Pancreat Surg. 2008;15:41–54. doi: 10.1007/s00534-007-1279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zografos GN, Farfaras A, Zagouri F, Chrysikos D, Karaliotas K. Cholangiocarcinoma: principles and current trends. Hepatobiliary Pancreat Dis Int. 2011;10:10–20. doi: 10.1016/s1499-3872(11)60001-5. [DOI] [PubMed] [Google Scholar]
- 10.Weber SM, Jarnagin WR, Klimstra D, DeMatteo RP, Fong Y, Blumgart LH. Intrahepatic cholangiocarcinoma: resectability, recurrence pattern, and outcomes. J Am Coll Surg. 2001;193:384–391. doi: 10.1016/s1072-7515(01)01016-x. [DOI] [PubMed] [Google Scholar]
- 11.Anderson CD, Pinson CW, Berlin J, Chari RS. Diagnosis and treatment of cholangiocarcinoma. Oncologist. 2004;9:43–57. doi: 10.1634/theoncologist.9-1-43. [DOI] [PubMed] [Google Scholar]
- 12.Yubin L, Chihua F, Zhixiang J, Jinrui O, Zixian L, Jianghua Z, Ye L, Haosheng J, Chaomin L. Surgical management and prognostic factors of hilar cholangiocarcinoma: experience with 115 cases in China. Ann Surg Oncol. 2008;15:2113–2119. doi: 10.1245/s10434-008-9932-z. [DOI] [PubMed] [Google Scholar]
- 13.Cho SY, Park SJ, Kim SH, Han SS, Kim YK, Lee KW, Lee SA, Hong EK, Lee WJ, Woo SM. Survival analysis of intrahepatic cholangiocarcinoma after resection. Ann Surg Oncol. 2010;17:1823–1830. doi: 10.1245/s10434-010-0938-y. [DOI] [PubMed] [Google Scholar]
- 14.Saiura A, Yamamoto J, Kokudo N, Koga R, Seki M, Hiki N, Yamada K, Natori T, Yamaguchi T. Intrahepatic cholangiocarcinoma: analysis of 44 consecutive resected cases including 5 cases with repeat resections. Am J Surg. 2011;201:203–208. doi: 10.1016/j.amjsurg.2008.12.035. [DOI] [PubMed] [Google Scholar]
- 15.Murakami Y, Uemura K, Sudo T, Hashimoto Y, Nakashima A, Kondo N, Sakabe R, Ohge H, Sueda T. Prognostic factors after surgical resection for intrahepatic, hilar, and distal cholangiocarcinoma. Ann Surg Oncol. 2011;18:651–658. doi: 10.1245/s10434-010-1325-4. [DOI] [PubMed] [Google Scholar]
- 16.de Jong MC, Nathan H, Sotiropoulos GC, Paul A, Alexandrescu S, Marques H, Pulitano C, Barroso E, Clary BM, Aldrighetti L, et al. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol. 2011;29:3140–3145. doi: 10.1200/JCO.2011.35.6519. [DOI] [PubMed] [Google Scholar]
- 17.Jarnagin WR, Fong Y, DeMatteo RP, Gonen M, Burke EC, Bodniewicz BS J, Youssef BA M, Klimstra D, Blumgart LH. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234:507–517; discussion 517-519. doi: 10.1097/00000658-200110000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yusoff AR, Razak MM, Yoong BK, Vijeyasingam R, Siti ZM. Survival analysis of cholangiocarcinoma: a 10-year experience in Malaysia. World J Gastroenterol. 2012;18:458–465. doi: 10.3748/wjg.v18.i5.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The Asia-Pacific perspective: redefining obesity and its treatment. Sydney: Health Communications Australia Pty Ltd; 2000. [Google Scholar]
- 20.Farhat MH, Shamseddine AI, Tawil AN, Berjawi G, Sidani C, Shamseddeen W, Barada KA. Prognostic factors in patients with advanced cholangiocarcinoma: role of surgery, chemotherapy and body mass index. World J Gastroenterol. 2008;14:3224–3230. doi: 10.3748/wjg.14.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malhi H, Gores GJ. Cholangiocarcinoma: modern advances in understanding a deadly old disease. J Hepatol. 2006;45:856–867. doi: 10.1016/j.jhep.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valenzuela-Landaeta K, Rojas P, Basfi-fer K. [Nutritional assessment for cancer patient] Nutr Hosp. 2012;27:516–523. doi: 10.1590/S0212-16112012000200025. [DOI] [PubMed] [Google Scholar]
- 23.Pitt HA, Cameron JL, Postier RG, Gadacz TR. Factors affecting mortality in biliary tract surgery. Am J Surg. 1981;141:66–72. doi: 10.1016/0002-9610(81)90014-3. [DOI] [PubMed] [Google Scholar]
- 24.Blamey SL, Fearon KC, Gilmour WH, Osborne DH, Carter DC. Prediction of risk in biliary surgery. Br J Surg. 1983;70:535–538. doi: 10.1002/bjs.1800700910. [DOI] [PubMed] [Google Scholar]
- 25.Park J, Kim MH, Kim KP, Park do H, Moon SH, Song TJ, Eum J, Lee SS, Seo DW, Lee SK. Natural History and Prognostic Factors of Advanced Cholangiocarcinoma without Surgery, Chemotherapy, or Radiotherapy: A Large-Scale Observational Study. Gut Liver. 2009;3:298–305. doi: 10.5009/gnl.2009.3.4.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Su CH, Tsay SH, Wu CC, Shyr YM, King KL, Lee CH, Lui WY, Liu TJ, P’eng FK. Factors influencing postoperative morbidity, mortality, and survival after resection for hilar cholangiocarcinoma. Ann Surg. 1996;223:384–394. doi: 10.1097/00000658-199604000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jarnagin WR, Bowne W, Klimstra DS, Ben-Porat L, Roggin K, Cymes K, Fong Y, DeMatteo RP, D’Angelica M, Koea J, et al. Papillary phenotype confers improved survival after resection of hilar cholangiocarcinoma. Ann Surg. 2005;241:703–12; discussion 712-4. doi: 10.1097/01.sla.0000160817.94472.fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kloek JJ, Ten Kate FJ, Busch OR, Gouma DJ, van Gulik TM. Surgery for extrahepatic cholangiocarcinoma: predictors of survival. HPB (Oxford) 2008;10:190–195. doi: 10.1080/13651820801992575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gold P, Shuster J, Freedman SO. Carcinoembryonic antigen (CEA) in clinical medicine: historical perspectives, pitfalls and projections. Cancer. 1978;42:1399–1405. doi: 10.1002/1097-0142(197809)42:3+<1399::aid-cncr2820420803>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 30.Hammarström S. The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin Cancer Biol. 1999;9:67–81. doi: 10.1006/scbi.1998.0119. [DOI] [PubMed] [Google Scholar]
- 31.Gores GJ. Early detection and treatment of cholangiocarcinoma. Liver Transpl. 2000;6:S30–S34. doi: 10.1053/jlts.2000.18688. [DOI] [PubMed] [Google Scholar]
- 32.Juntermanns B, Radunz S, Heuer M, Hertel S, Reis H, Neuhaus JP, Vernadakis S, Trarbach T, Paul A, Kaiser GM. Tumor markers as a diagnostic key for hilar cholangiocarcinoma. Eur J Med Res. 2010;15:357–361. doi: 10.1186/2047-783X-15-8-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ni XG, Bai XF, Mao YL, Shao YF, Wu JX, Shan Y, Wang CF, Wang J, Tian YT, Liu Q, et al. The clinical value of serum CEA, CA19-9, and CA242 in the diagnosis and prognosis of pancreatic cancer. Eur J Surg Oncol. 2005;31:164–169. doi: 10.1016/j.ejso.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 34.Li FH, Chen XQ, Luo HY, Li YH, Wang F, Qiu MZ, Teng KY, Li ZH, Xu RH. [Prognosis of 84 intrahepatic cholangiocarcinoma patients] Ai Zheng. 2009;28:528–532. [PubMed] [Google Scholar]
- 35.Clark CJ, Wood-Wentz CM, Reid-Lombardo KM, Kendrick ML, Huebner M, Que FG. Lymphadenectomy in the staging and treatment of intrahepatic cholangiocarcinoma: a population-based study using the National Cancer Institute SEER database. HPB (Oxford) 2011;13:612–620. doi: 10.1111/j.1477-2574.2011.00340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagakawa T, Kayahara M, Ikeda S, Futakawa S, Kakita A, Kawarada H, Matsuno M, Takada T, Takasaki K, Tanimura H, et al. Biliary tract cancer treatment: results from the Biliary Tract Cancer Statistics Registry in Japan. J Hepatobiliary Pancreat Surg. 2002;9:569–575. doi: 10.1007/s005340200076. [DOI] [PubMed] [Google Scholar]
- 37.Nishio H, Nagino M, Nimura Y. Surgical management of hilar cholangiocarcinoma: the Nagoya experience. HPB (Oxford) 2005;7:259–262. doi: 10.1080/13651820500373010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guglielmi A, Ruzzenente A, Campagnaro T, Pachera S, Conci S, Valdegamberi A, Sandri M, Iacono C. Prognostic significance of lymph node ratio after resection of peri-hilar cholangiocarcinoma. HPB (Oxford) 2011;13:240–245. doi: 10.1111/j.1477-2574.2010.00277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rocha FG, Matsuo K, Blumgart LH, Jarnagin WR. Hilar cholangiocarcinoma: the Memorial Sloan-Kettering Cancer Center experience. J Hepatobiliary Pancreat Sci. 2010;17:490–496. doi: 10.1007/s00534-009-0205-4. [DOI] [PubMed] [Google Scholar]
- 40.Ellis MC, Cassera MA, Vetto JT, Orloff SL, Hansen PD, Billingsley KG. Surgical treatment of intrahepatic cholangiocarcinoma: outcomes and predictive factors. HPB (Oxford) 2011;13:59–63. doi: 10.1111/j.1477-2574.2010.00242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guglielmi A, Ruzzenente A, Campagnaro T, Pachera S, Valdegamberi A, Nicoli P, Cappellani A, Malfermoni G, Iacono C. Intrahepatic cholangiocarcinoma: prognostic factors after surgical resection. World J Surg. 2009;33:1247–1254. doi: 10.1007/s00268-009-9970-0. [DOI] [PubMed] [Google Scholar]
- 42.Silva MA, Tekin K, Aytekin F, Bramhall SR, Buckels JA, Mirza DF. Surgery for hilar cholangiocarcinoma; a 10 year experience of a tertiary referral centre in the UK. Eur J Surg Oncol. 2005;31:533–539. doi: 10.1016/j.ejso.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 43.Murakami Y, Uemura K, Sudo T, Hayashidani Y, Hashimoto Y, Nakamura H, Nakashima A, Sueda T. Adjuvant gemcitabine plus S-1 chemotherapy improves survival after aggressive surgical resection for advanced biliary carcinoma. Ann Surg. 2009;250:950–956. doi: 10.1097/sla.0b013e3181b0fc8b. [DOI] [PubMed] [Google Scholar]
- 44.Takada T, Amano H, Yasuda H, Nimura Y, Matsushiro T, Kato H, Nagakawa T, Nakayama T. Is postoperative adjuvant chemotherapy useful for gallbladder carcinoma? A phase III multicenter prospective randomized controlled trial in patients with resected pancreaticobiliary carcinoma. Cancer. 2002;95:1685–1695. doi: 10.1002/cncr.10831. [DOI] [PubMed] [Google Scholar]
- 45.Horgan AM, Amir E, Walter T, Knox JJ. Adjuvant therapy in the treatment of biliary tract cancer: a systematic review and meta-analysis. J Clin Oncol. 2012;30:1934–1940. doi: 10.1200/JCO.2011.40.5381. [DOI] [PubMed] [Google Scholar]


