Abstract
Study Objectives:
Changes in lymphocyte phenotype and functionality have been described in adult patients with obstructive sleep apnea (OSA). We hypothesized that OSA is associated with T lymphocyte alterations in children, particularly in T regulatory lymphocytes (T regs), and aimed to characterize circulating T lymphocyte subsets in children with OSA.
Design:
Cross-sectional.
Setting:
Kosair Children's Hospital (Louisville, KY, USA) and Comer Children's Hospital (Chicago, IL, USA).
Participants:
Consecutively recruited children being evaluated for habitual snoring.
Interventions:
N/A.
Measurements and Results:
Overnight polysomnography (PSG) was performed and a fasting blood sample was obtained from the patients. Flow cytometry was performed on peripheral blood mononuclear cells stained for CD3, CD4, CD8, CD25, FOXP3, interleukin-4 (IL-4), interferon-γ (IFN-γ), and IL-17. Patients were divided into three groups based on their PSG: controls (apnea-hypopnea indices [AHI] < 1/h total sleep time [TST]), mild OSA (1 ≤ AHI < 5/hTST), moderate-severe OSA (AHI ≥ 5/h TST). The percentage of CD4+ and T reg lymphocytes differed across groups. Children with moderate-severe OSA had significantly reduced T reg than control children (median [interquartile range] 4.8 [3.8-5.7% CD4+] versus 7.8 [7.0-9.2% CD4+]; P < 0.001). There were also significant differences in the percentage of T helper 1 (Th1) lymphocytes and in Th1:Th2 ratios between groups. Children with moderate-severe OSA had increased Th1 cells (P = 0.001) and Th1:Th2 ratios (P = 0.0026) compared with children with mild OSA and control children. Associations between AHI and T reg (P = 0.0003; r = -0.46), CD4+ lymphocytes (P = 0.0047; r = -0.37), and Th1:Th2 ratios (P = 0.0009; r = 0.43) emerged. In addition, the percentage of T reg was inversely correlated with Th1:Th2 ratios (P = 0.029; r = -0.29).
Conclusions:
Pediatric OSA is associated with reduced T reg population and altered Th1:Th2 balance toward Th1 predominance, suggesting a shift to a proinflammatory state. The changes in lymphocytic phenotypes associated with OSA may contribute to the variance in systemic inflammation and downstream morbidities associated with this condition.
Citation:
Tan HL; Gozal D; Wang Y; Bandla HPR; Bhattacharjee R; Kulkarni R; Kheirandish-Gozal L. Alterations in circulating t-cell lymphocyte populations in children with obstructive sleep apnea. SLEEP 2013;36(6):913-922.
Keywords: T cell lymphocytes, children, inflammation, sleep apnea
INTRODUCTION
It is now recognized that obstructive sleep apnea (OSA) in children is a common health problem with an estimated prevalence of up to 4%.1,2 Children with OSA experience repetitive episodes of increased upper airway resistance culminating in partial or complete obstruction of the upper airway during sleep, leading to major changes in intrathoracic pressures, recurrent arousals, and fragmented sleep, as well as episodic oxygen desaturations and hypercapnia.3,4
In addition to eliciting significant cardiovascular and metabolic morbidity,5–8 OSA imposes major detrimental effects on cognitive function9–14 that are likely mediated by increased oxidative stress and activation of inflammatory processes.15 Indeed, OSA can induce several inflammatory cascades, and elevated levels of proinflammatory cytokines such as interleukin-6 (IL-6), interferon-γ (IFN-γ), and tumor necrosis factor-α (TNF-α) have all been reported, albeit inconsistently, in children with OSA, whereas levels of the antiinflammatory cytokine IL-10 were reduced.16–19
Gozal20 proposed a working model, whereby the various physiologic alterations that characterize OSA elicit the activation of monocytes/macrophages, thereby inducing the secretion of cytokines and growth factors that promote vessel wall smooth muscle proliferation, endothelial dysfunction, and atherogenesis. However, the role of other inflammatory cells, such as lymphocytes, is less well explored in pediatric OSA, even though studies by Lavie and colleagues have clearly demonstrated altered T cell lymphocyte function in adult patients with OSA.21–24 Indeed, CD8+ lymphocytes exhibit increased expression of perforin, TNF-α, natural killer receptors CD56 and CD16, and their cytotoxicity against human umbilical vein endothelial cells and erythroleukemic cells in vitro is enhanced.22,23 Similarly, γδ T cells display increased expression of inhibitory natural killer B1 receptor, TNF-α, IL-8, and L-selectin, with decreased expression of IL-10 in adults with OSA.21 When tonsillar lymphoid tissues from children with OSA were compared with those obtained from children with recurrent tonsillitis, proliferative rates of CD3+, CD4+, and CD8+ T cell lymphocytes were higher in the OSA group, and expression of proinflammatory cytokines TNF-α, IL-6, and IL-1α was also increased.25,26 Furthermore, microarray analysis of the RNA derived from peripheral leukocytes in children with OSA showed recruitment of functionally relevant gene clusters, with prominent involvement of those underlying inflammatory pathways.27
The FOXP3 gene controls the transcriptional fate and differentiation of lymphocytes into T regulatory lymphocytes (T regs). The promoter region of the FOXP3 gene has been shown to be hypermethylated in pediatric OSA, and the degree of methylation correlates with OSA disease severity.28 T regs are a subset of T helper (Th) cells that are able to suppress the activation, proliferation, and effector functions of a wide range of immune cells.29 They are critical for the maintenance of self-tolerance and immune homeostasis, and their dysfunction can result in autoimmune disease, immunopathology, and allergy.30–34 Given their wide-ranging effects on the immune system, alterations in T reg differentiation may cause a secondary immunologic cascade resulting in aberrant inflammatory responses.
Based on aforementioned considerations, we hypothesized that OSA in children can elicit alterations in T cell lymphocytes, and that reductions in percentage of T reg along with imbalance of Th1:Th2 immunity would emerge.
MATERIALS AND METHODS
The research protocol was approved by the University of Louisville (protocol #474.99) and University of Chicago (protocol 09-115-B) human research ethics committees. Informed consent was obtained from the parents and age-appropriate assent from the children. Patients were recruited from the Sleep and Ear Nose and Throat clinics of Kosair Children's Hospital and Comer Children's Hospital, as well as by advertisement. Patients who had genetic or craniofacial syndromes and chronic diseases such as cardiac disease, diabetes, cerebral palsy, and chronic lung disease of prematurity were excluded.
There were 56 patients, age 5-12 y, who were recruited from the two sites, Louisville (n = 22) and Chicago (n = 34). They underwent standard overnight polysomnography (PSG) evaluation as previously described35 with assessment of eight standard electroencephalography channels, bilateral electrooculography, electromyography, two-lead electrocardiography, oronasal airflow measurement using a thermistor, nasal pressure transducer, and end-tidal carbon dioxide (ETCO2), chest and abdominal movement by respiratory inductance plethysmography, and pulse oximetry including pulse waveform. The PSG equipment used in Louisville was manufactured by Stellate (Montreal, Canada), and that used in Chicago by Polysmith (Nihon Kohden America Inc, Foothill Ranch, CA). The PSG studies were scored as per the 2007 American Association of Sleep Medicine guidelines for the scoring of sleep and associated events.36 The proportion of time spent in each stage of sleep was calculated as a percentage of total sleep time (TST). A respiratory event was scored as an obstructive apnea if it was associated with a > 90% decrease in signal amplitude for > 90% of the entire event compared with the baseline amplitude, the event lasted for at least two breaths, and there was continued or increased respiratory effort throughout the period of the event. A mixed apnea was scored if there was absent inspiratory effort during the initial part of the event, followed by resumption of inspiratory effort before the end of the event. A central apnea was scored if there was absent respiratory effort throughout the duration of the event, the event lasted for at least two missed breaths, and was associated with an arousal/ awakening or a ≥ 3% desaturation. A hypopnea was scored if the event was associated with a ≥ 50% decrease in amplitude of the nasal pressure transducer, lasted for at least two breaths, and was associated with an arousal/awakening or ≥ 3% desaturation. The apnea-hypopnea index (AHI) was calculated as the number of apneas and hypopneas per hour of TST.
A fasting blood sample was obtained the following morning.
Staining of Peripheral Blood Mononuclear Cells and Flow Cytometry
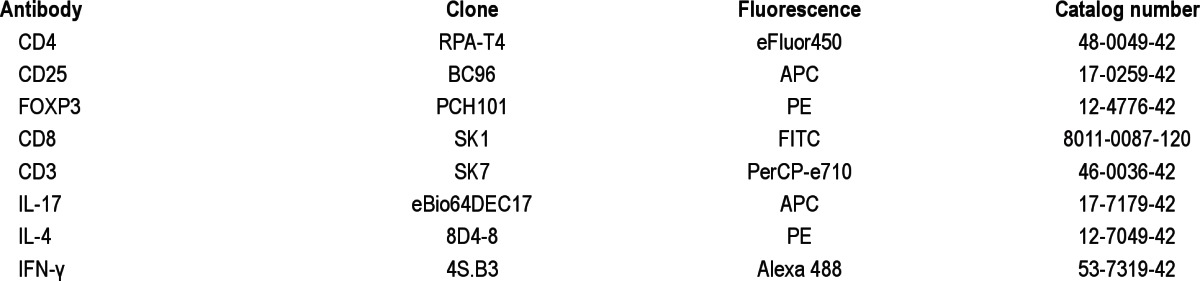
Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll gradient centrifugation (Ficoll-paque Premium, GE Healthcare, Barrington, IL, USA). The cells were washed three times in phosphate buffered saline and the resultant cell pellet was gently resuspended in freezing medium (100% fetal calf serum, 10% dimethyl sulfoxide). The cryovials were then placed in a Nalgene (Thermo Fisher Scientific, Waltham, MA) cell freezing container in the -80°C freezer, which ensured they were cooled gradually at a rate of 1°C/min to maximize successful cryopreservation of cells. When enough samples were collected to enable batch analysis, the cryovials were rapidly thawed in a 37°C water bath and RPMI medium with 5% fetal calf serum was added. The cells were washed. Viability was checked with Trypan blue and was consistently above 90%. The cells were stained for CD3, CD4, CD8, CD25, FOXP3, IL-4, IL-17, and IFN-γ. Table 1 shows the antibodies used (Ebio-science Inc, San Diego, CA).
Table 1.
Antibodies used for flow cytometry analysis of blood samples
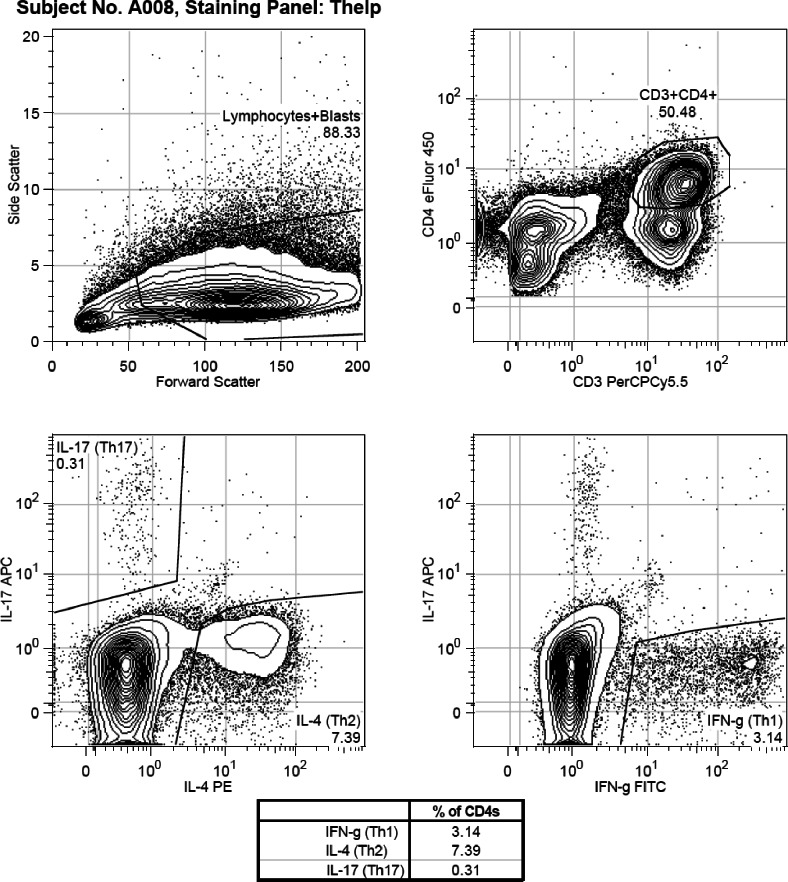
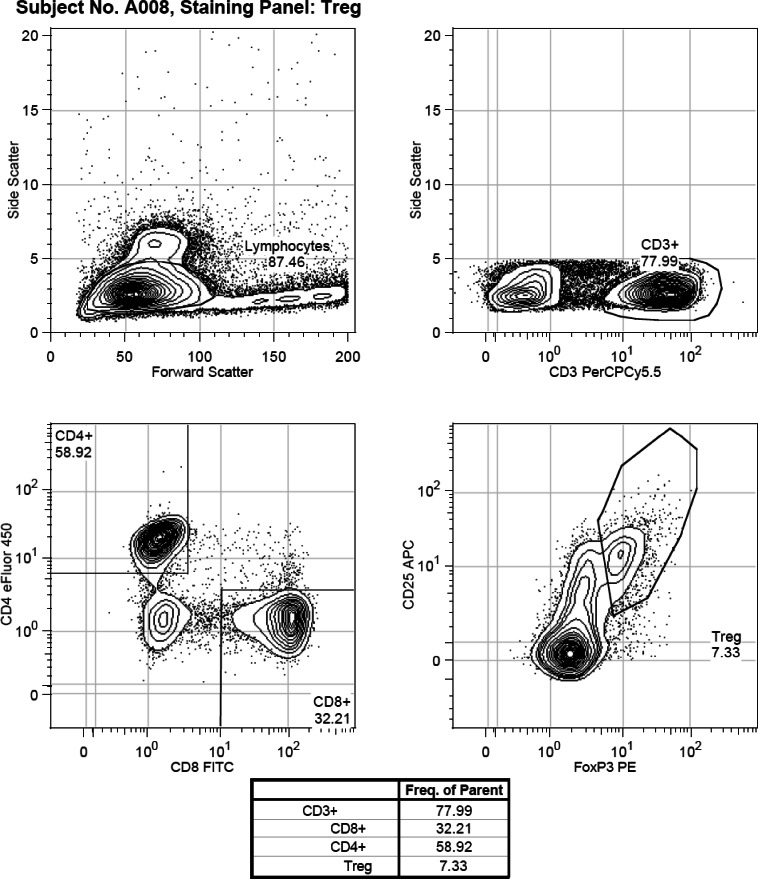
For extracellular staining of CD4, CD25, CD8, and CD3, antibodies were incubated for 30 min at 4°C. The FOXP3 staining buffer set (Ebioscience 00-5523-00) was used for FOXP3 staining per the manufacturer's instructions. For intracellular staining of IL-4, IL-17, and IFN-γ, the cells were “rested” for 18 h in the 37°C incubator after thawing, before they were stimulated with Cell Stimulation Cocktail (Ebioscience 00-4975-03), a cocktail of phorbol 12-myristate 13-acetate (PMA), ionomycin, brefeldin A, and monensin. PMA and ionomycin stimulate the cells to produce cytokines. Brefeldin A and monensin bring about the accumulation of secreted proteins in the endoplasmic reticulum and Golgi apparatus. The cells were then stained with the relevant intracellular antibody. Samples were analyzed in batches of 6-12. Flow cytometry analysis was performed using the Beckman-Coulter Gallios flow cytometer (Beckman Coulter Inc, Indianapolis, IN) and the results analyzed using Flowjo flow cytometry analysis software, version 7.6.5 (Treestar Inc, Ashland, OR). There were 100,000 events that were measured for each sample, and lymphocytes were gated based on their forward and side scatter. T reg lymphocytes were defined as cells that were CD4+CD25+ and FOXP3+. Th1 cells were defined as cells that were CD3+CD4+IFN-γ+, Th2 cells were defined as cells that were CD3+CD4+IL-4+, and Th17 cells were defined as cells that were CD3+CD4+IL-17+. Figures 1A and 1B show examples of the gating strategies used to differentiate the various cell subsets.
Figure 1A.
Flow cytometry assessments of T cell lymphocyte populations in peripheral blood of children. Example of gating strategy used for IL-4, IFN-γ and IL-17 positively labelled cells.
Figure 1B.
Flow cytometry assessments of T cell lymphocyte populations in peripheral blood of children. Example of gating strategy used for CD3, CD4, CD8, CD25 and FOXP3 positively labelled cells.
Immunophenotyping of PBMCs using flow cytometry has traditionally been performed on fresh samples. Over the past few years, the cryopreservation of PBMCs has proved to be an attractive alternative for the purpose of batching samples over time, particularly in human studies. This allows for future analysis, which has the added advantage of reducing interassay variation. Consortiums such as the Cryopreservation Working Group of the Paediatric AIDS Clinical Trials Group, and the Human Immunology Project have done much work to optimize and validate methods of PBMC cryopreservation for immuno-logic assays including flow cytometry where quality-control parameters have been established and techniques standardized.37,38 Our methodology was based on these recommendations. We also confirmed that there was no significant difference in the results between fresh and frozen samples in two initial trial experiments. In addition, at the start of the study, PBMCs from 200 mL of volunteer donor adult blood was processed and frozen. A cryovial from this control sample was analyzed in each batch and compared to the initial fresh sample to ensure interbatch consistency (please see Tables S1 and S2 for details).
Data Analysis
When data were normally distributed, they are expressed as mean ± standard deviation (SD), and the one-way or two-way analysis of variance (ANOVA) was used to compare differences between groups. To control for potential differences imposed by the presence of coexisting conditions such as asthma or allergies, separate analyses were conducted with comorbidities excluded, or by controlling for the presence of asthma and allergies as a covariate in the two-way ANOVA. When data were not normally distributed (e.g., T cell populations), they were expressed as median (interquartile range), and nonparametric statistical tests Kruskal-Wallis and Spearman correlation were used. Categorical data were analyzed using the chi-square test. Statistical analysis was performed using Graphpad Prism version 5.0 (Graphpad Software Inc, La Jolla, CA) and post hoc analyses for multiple comparisons were performed using the Dunn multiple comparison test.
RESULTS
Fifty-six patients were consecutively recruited and divided into three groups based on the AHI obtained during PSG. The control group consisted of patients with AHI < 1/h TST, children with 1 ≤ AHI < 5/h TST were classified as having mild OSA, whereas those with AHI ≥ 5/h TST were classified as having moderate-severe OSA. Their clinical characteristics are summarized in Table 2.
Table 2.
Clinical characteristics and PSG findings in 56 children
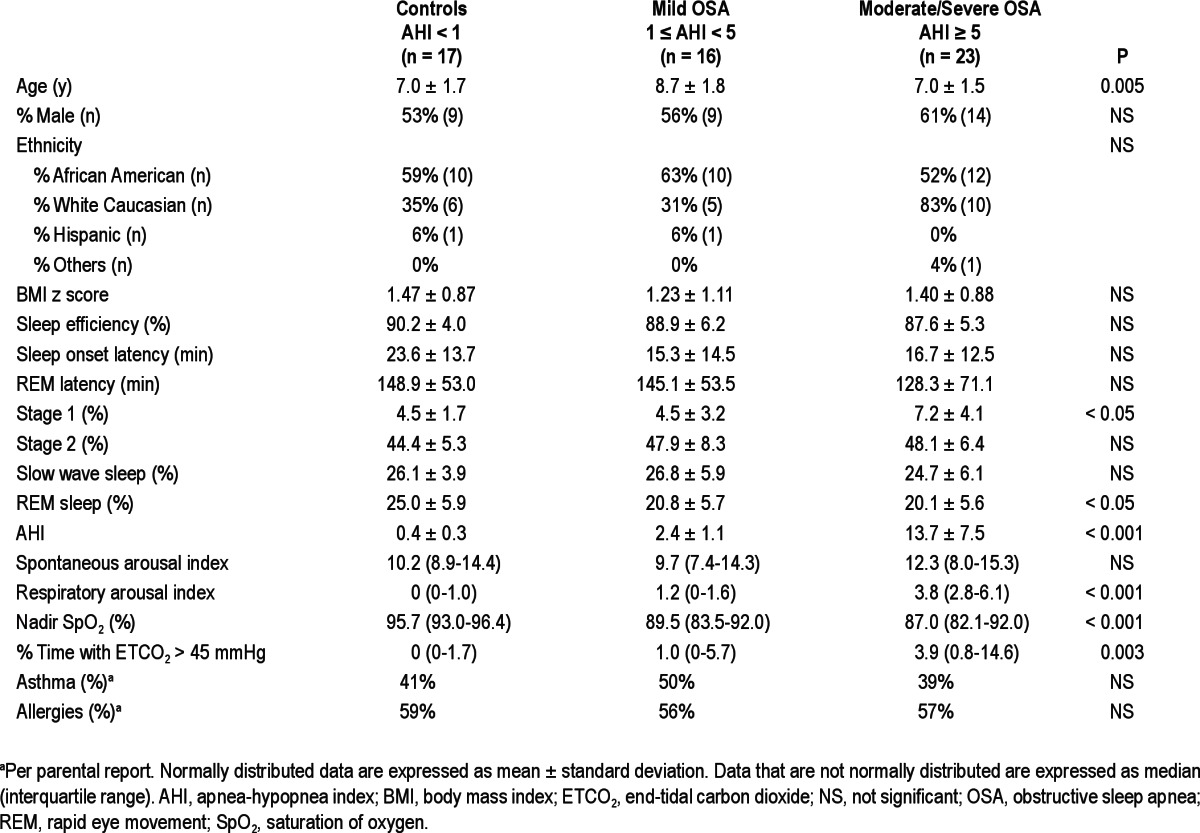
There were no significant differences in ethnicity, sex, or body mass index z- scores among the three groups. However, children with mild OSA (1 ≤ AHI < 5) were older than children in the other two groups (mean ± SD): 8.7 ± 1.8 versus 7.0 ± 1.7 and 7.0 ± 1.5 years; P = 0.005. There were no significant differences in sleep efficiency, sleep onset latency, or rapid eye movement sleep (REM) latency, percentage of time spent in Stage 2 sleep, and slow wave sleep between groups, although reduced percentage of time spent in REM sleep and increased percentage of time spent in Stage 1 sleep were noted in children with OSA (Table 2). Unsurprisingly, significant differences in AHI, oxygen saturation nadir, percent time with ETCO2 > 45 mmHg and respiratory arousal index emerged between groups (Table 2).
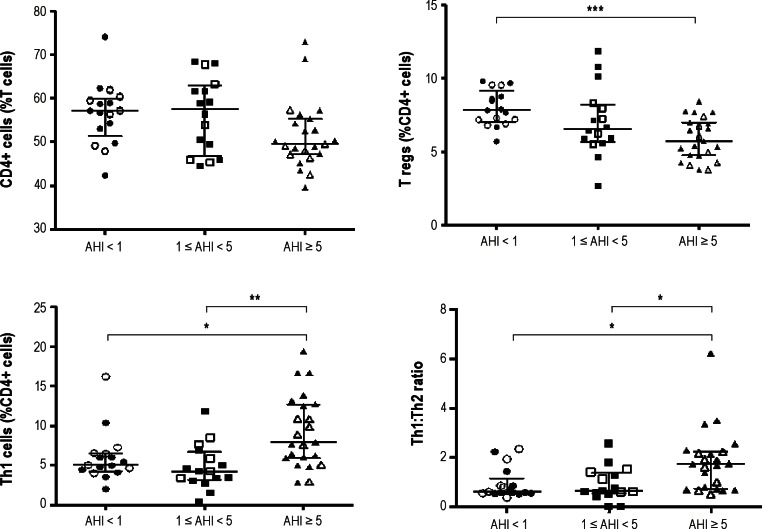
Our findings on T cell lymphocyte subsets are summarized in Table 3. There was a slight albeit significant difference in the percentage of CD4+ cells across groups (P = 0.039; Figure 2). The percentage of T reg lymphocytes markedly differed between groups (P = 0.0007), with children with moderate-severe OSA having a significantly lower percentage of T regs than control patients. In addition, there were significant differences in the percentage of Th1 lymphocytes and in Th1:Th2 ratios between groups (P = 0.001 and P < 0.003, respectively), with children with moderate-severe OSA having significantly higher percentage of Th1 cells and Th1:Th2 ratios compared with children with mild OSA and control patients (Figure 2).
Table 3.
T cell lymphocyte subsets in 56 children
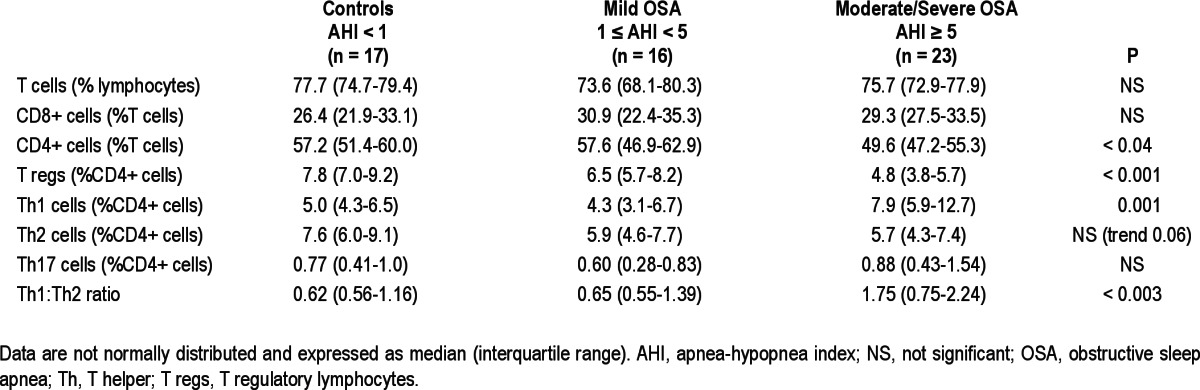
Figure 2.
Percentage of CD4+, Treg and Th1 cell lymphocytes, and Th1:Th2 ratios among children with and without OSA. The open labels indicate children without asthma or allergies whereas the closed labels are children with asthma and/or allergies. Circles refer to children with AHI<1/hrTST, squares to children with AHI between 1 and 5/hrTST, and triangles for children with AHI>5/hr/TST/. *P < 0.05, **P < 0.02, ***P < 0.01 ANOVA.
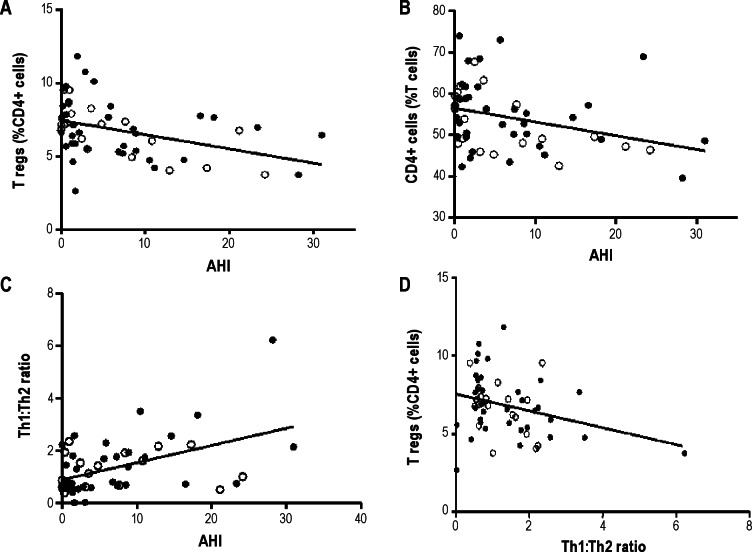
Inverse correlations between AHI and the percentage of T reg lymphocytes found in the peripheral blood, AHI and the percentage of CD4+ lymphocytes, and a positive correlation between the Th1:Th2 ratio and AHI were found (Figure 3). The percentage of T reg lymphocytes was negatively correlated with corresponding Th1:Th2 ratios (Figure 3). No significant associations were found between body mass index z scores and percentage of T reg cells, nor between age and percentage of T regs.
Figure 3.
Scatterplots and linear regression analysis between AHI and the percentage of T reg, CD4+ lymphocytes, Th1:Th2 ratios, and Th1:Th2 ratios plotted against T reg lymphocytes. The open circles are children without asthma or allergies and the closed circles refer to those children with asthma and/ or allergies. (A) Coefficient of correlation: r = -0.46, P = 0.0003. (B) Coefficient of correlation: r = -0.37, P = 0.0047. (C) Coefficient of correlation: r = 0.43, P = 0.0009. (D) Coefficient of correlation: r = -0.29, P = 0.029.
Subgroup analysis was performed on the children without asthma or allergies (n = 19). Despite the much smaller numbers, the percentage of T reg lymphocytes still differed between groups (P = 0.028), and similar to the pattern seen in the whole group, children with moderate-severe OSA had a significantly lower percentage of T regs than control children. The correlation between T regs and AHI remained statistically significant (P = 0.011, r = -0.57) as did the correlation between CD4+ cells and AHI (P = 0.021, r = -0.52).
DISCUSSION
In this study, we show that children with OSA are more likely to exhibit reductions in their circulating T reg lymphocytes along with a shift toward a Th1 proinflammatory phenotype, as suggested by increased Th1:Th2 ratios. To the best of our knowledge, this is the first report of such phenotypic changes in T lymphocytes in pediatric patients with OSA.
Before we address the potential implications of our findings, some limitations should be mentioned. First, our study was a cross-sectional study and observational in nature. Therefore, the putative associations identified in our cohort do not necessarily imply that causative mechanisms are operational. Alterations in T lymphocyte populations may have been affected not only by OSA, but also by other factors; for example, the patients in the three groups may have been exposed to different frequencies of external antigens that may modify the specific number of T regs and as well as Th1:Th2 balance. A large proportion of our patients had parent-reported asthma and allergies. However, even when subgroup analysis was performed on the relatively small number of children without these comorbidities, the significance of our findings remained unaltered. Further studies aiming to determine whether the changes observed in the T cell lymphocyte populations change after treatment of the OSA with adenotonsillectomy or noninvasive ventilation would be of considerable interest. Second, the potential mechanisms underlying the alterations in T reg and increases in Th1:Th2 ratios were not specifically explored. It is likely that epigenetic modifications of genes regulating T cell lymphocyte fate, such as FOXP3, may underlie the reductions in T reg populations.28 Indeed, hypermethylation of FOXP3 would be anticipated to diminish the expression of this critical transcription factor involved in T reg lymphocyte maturation and functionality, and therefore, both the number of T reg lymphocytes or their immune suppressive capacity might be altered by OSA.39,40 Alternatively, T reg lymphocytes could be more susceptible to the intermittent hypoxia and sleep fragmentation associated with OSA, and therefore undergo apoptotic changes. Thus, future studies should specifically address these issues. It should be mentioned that the functional study of T reg lymphocyte suppressive capacity is limited to in vitro coculture assays. The caveats to this approach include ethical considerations involving larger blood volume sampling in children, and the absence of conclusive evidence that in vitro assays reflect what happens in vivo.29
T reg lymphocytes can be natural T regs, the product of thymic development, or induced T regs, which are generated from conventional CD4+ cells in peripheral sites.41 The function of induced Treg cells seems to be distinct from that of natural T regs, with induced T reg cells being important in restraining allergic inflammation at mucosal surfaces, whereas natural T reg cells are thought to underlie the control of systemic and tissue specific autoimmunity. It has recently been shown that extrathymically generated T reg lymphocytes control Th2 inflammation at mucosal surfaces; specifically, mice deficient in induced T reg lymphocytes will spontaneously develop pronounced Th2-type pathologies in the gastrointestinal tract and lung, showing hallmarks of allergic inflammation and asthma.42 In humans, reduced numbers of T reg cells have been described in the bronchoalveolar lavage and blood of patients with asthma, as well as impaired chemotaxis of T reg cells to lung epithelial cells.43–45 We did not find significant differenc es in the parent-reported frequencies of asthma and allergies among the three severity groups in our cohort, thereby suggesting that the T reg lymphocyte alterations cannot be putatively ascribed to the presence or absence of these conditions, although further work with specific testing for allergies and pulmonary function testing is clearly necessary. However, we should also point out that an association between asthma and OSA has been reported.46–50 Indeed, the prevalence of OSA in children with poorly controlled asthma is higher than in the general pediatric population, and treatment of the OSA with adenotonsillectomy was associated with improvement in asthma symptoms.51 If OSA results in a decrease in the number of T reg cells, and T reg cells are needed for suppression of allergic inflammation, this could be a possible explanation for the link between OSA and asthma. It could also be argued that asthma is usually associated with a Th2 phenotype rather than a Th1 phenotype. In this study, we found evidence that the presence of OSA promoted a shift toward a Th1 phenotype. However, there is increasing evidence that asthma is a heterogeneous condition consisting of several different phenotypes,52 not all of which are associated with eosinophilia and a Th2 pheno-type. Furthermore, there could be compartmentalization of inflammation, and the inflammatory pattern seen locally within the airway mucosal surface may not reflect changes in peripheral blood. Our aim was to examine the relationship between OSA and T lymphocyte subsets, including T reg lymphocytes. Larger patient numbers with more definitive diagnostic tests such as lung function, skin prick tests, and immunoglobulin E levels would be needed to examine the potential relationships linking asthma/allergies, T reg cells, and OSA.
In recent years, it has become apparent that OSA can promote the activation and propagation of systemic inflammatory pathways leading to functional and structural disruption of the endothelium. In this context, the role of T reg lymphocytes in atherosclerosis has been the subject of intense investigation.53
Adoptive transfer of T reg cells has been shown to result in a reduction in atherosclerotic lesion development,54 whereas their depletion aggravated atherosclerotic lesion development.55
More recently, T reg lymphocytes were shown to suppress inflammatory responses and attenuate initial atherosclerosis development56 and their presence is critical to the prevention of endothelial activation and migration and adhesion of leukocytes.57 Of note, T reg cells also appear to mitigate the vascular consequences of angiotensin 2-mediated hypertension,58 and elevated blood pressures are frequently, albeit not universally, present in children with OSA.59 Based on aforementioned studies, we surmise that alterations in T reg lymphocytes in the context of pediatric OSA may underlie not only a shift toward systemic inflammation, but also promote vascular and potentially neurocognitive dysfunction.
To further complete our inventory of the findings, we should emphasize that other than T reg lymphocytes, other T lymphocytes have been shown to play a role in plaque development in atherosclerosis, not only by their direct effect on endothelial cells, but also via the cytokines they secrete.60,61 Th cells can be broadly divided into Th1 cells, which are involved in cell-mediated immunity, and Th2 cells, which drive antibody- mediated immunity.62 Although there have been recent modifications to the Th1:Th2 paradigm with the discovery of Th17 cells and the idea of Th cell plasticity, the concept that there can be reciprocal interactions between sets of T cell lymphocytes continues nonetheless to provide a framework for the understanding of immune regulation.63 Atherosclerotic lesions contain cytokines that promote a Th1 response.64,65 A suggested model is that this cytokine milieu causes activated T cells to differentiate into Th1 effector cells that produce IFN-γ, which acts synergistically with cytokines such as TNF-α and IL-1 from macrophages and vascular cells to promote atherosclerosis.66 The development of atherosclerosis is inhibited in ApoE-/- mice lacking the IFN-γ and IFN-γ receptor.67,68 When ApoE-/- mice were treated daily with pentoxifylline, an inhibitor of the Th1 differentiation pathway, the size of the atherosclerotic lesions they developed were reduced by 60% and histologically appeared as fatty streaks rather than the mature fibrofatty atherosclerotic lesions seen in mice not treated with pentoxifylline.69 Clinically, a Th1:Th2 imbalance with preference toward a Th1 response has been implicated in patients with coronary spastic angina.70
The shift toward a Th1 phenotype in children with OSA as described in the study could therefore provide additional insights into the link between OSA and the cardiovascular morbidities associated with this condition.
In summary, significant negative correlations between the severity of OSA and the percentage of T regulatory cells, along with a shift in the Th1:Th2 balance toward one of Th1 predominance, occur in the peripheral blood of children with OSA. These changes in lymphocytic phenotypes may contribute to the systemic inflammation seen in OSA, along with its attendant increased risk for multiple end-organ morbidity. The exact nature of the mechanisms underlying the immunologic pathways activated in OSA, and the determinants of the large variance in such processes remain to be identified. However, future exploration of these pathways may provide unique potential for therapeutic modifications.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Dr. Kheirandish-Gozal is supported by NIH grant K12 HL-090003; Dr. Gozal is supported by National Institutes of Health grants HL-065270 and HL-086662; Dr. Tan is supported by a European Respiratory Society Fellowship (STRTF fellowship no. 125-2011).
Role of each investigator: Dr. Tan performed subject recruitment, data analysis, and completed the first draft of the manuscript. Dr. Gozal and Dr. Wang assisted with project design, provided technical expertise, and critically reviewed the manuscript. Drs. Bandla and Bhattacharjee assisted with patient recruitment and with critical review of the manuscript. Dr. Kulkarni was responsible for coordination of ethics committee reports and patient recruitment. Dr. Kheirandish-Gozal provided the conceptual framework for the project, provided mentorship to Dr. Tan, was responsible for financial support and oversight of the project, and edited the manuscript for content.
SUPPLEMENTAL MATERIAL
Results of the control samplea from each batch
Comparison between fresh and cryopreserved sample
REFERENCES
- 1.Rosen CL, Larkin EK, Kirchner HL, et al. Prevalence and risk factors for sleep-disordered breathing in 8- to 11-year-old children: association with race and prematurity. J Pediatr. 2003;142:383–9. doi: 10.1067/mpd.2003.28. [DOI] [PubMed] [Google Scholar]
- 2.Lumeng JC, Chervin RD. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:242–52. doi: 10.1513/pats.200708-135MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gozal D. Obstructive sleep apnea in children. Minerva Pediatr. 2000;52:629–39. [PubMed] [Google Scholar]
- 4.Hakim F, Gozal D, Kheirandish-Gozal L. Sympathetic and catecholaminergic alterations in sleep apnea with particular emphasis on children. Front Neurol. 2012;3:7. doi: 10.3389/fneur.2012.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhattacharjee R, Kim J, Alotaibi WH, et al. Endothelial dysfunction in non-hypertensive children: potential contributions of obesity and obstructive sleep apnea. Chest. 2012;141:682–91. doi: 10.1378/chest.11-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kheirandish-Gozal L, Bhattacharjee R, Gozal D. Autonomic alterations and endothelial dysfunction in pediatric obstructive sleep apnea. Sleep Med. 2010;11:714–20. doi: 10.1016/j.sleep.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 7.Kheirandish-Gozal L, Bhattacharjee R, Kim J, et al. Endothelial progenitor cells and vascular dysfunction in children with obstructive sleep apnea. Am J Respir Crit Care Med. 2010;82:92–7. doi: 10.1164/rccm.200912-1845OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marcus CL, Greene MG, Carroll JL. Blood pressure in children with obstructive sleep apnea. Am J Respir Crit Care Med. 1998;157:1098–103. doi: 10.1164/ajrccm.157.4.9704080. [DOI] [PubMed] [Google Scholar]
- 9.Gozal D. Sleep-disordered breathing and school performance in children. Pediatrics. 1998;102:616–20. doi: 10.1542/peds.102.3.616. [DOI] [PubMed] [Google Scholar]
- 10.Gozal D, Pope DW., Jr Snoring during early childhood and academic performance at ages thirteen to fourteen years. Pediatrics. 2001;107:1394–99. doi: 10.1542/peds.107.6.1394. [DOI] [PubMed] [Google Scholar]
- 11.Gozal D, Kheirandish-Gozal L, Bhattacharjee R, et al. Neurocognitive and endothelial dysfunction in children with obstructive sleep apnea. Pediatrics. 2010;126:e1161–67. doi: 10.1542/peds.2010-0688. [DOI] [PubMed] [Google Scholar]
- 12.Gozal D, Kheirandish-Gozal L. Neurocognitive and behavioral morbidity in children with sleep disorders. Curr Opin Pulm Med. 2007;13:505–9. doi: 10.1097/MCP.0b013e3282ef6880. [DOI] [PubMed] [Google Scholar]
- 13.Gozal D. Obstructive sleep apnea in children: implications for the developing central nervous system. Semin Pediatr Neurol. 2008;15:100–6. doi: 10.1016/j.spen.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kheirandish L, Gozal D. Neurocognitive dysfunction in children with sleep disorders. Dev Sci. 2006;9:388–99. doi: 10.1111/j.1467-7687.2006.00504.x. [DOI] [PubMed] [Google Scholar]
- 15.Gozal D, Kheirandish-Gozal L. Cardiovascular morbidity in obstructive sleep apnea: oxidative stress, inflammation, and much more. Am J Respir Crit Care Med. 2008;177:369–75. doi: 10.1164/rccm.200608-1190PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gozal D, Serpero LD, Sans Capdevila O, et al. Systemic inflammation in non-obese children with obstructive sleep apnea. Sleep Med. 2008;9:254–9. doi: 10.1016/j.sleep.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gozal D, Serpero LD, Kheirandish-Gozal L, et al. Sleep measures and morning plasma TNF-alpha levels in children with sleep-disordered breathing. Sleep. 2010;33:319–25. doi: 10.1093/sleep/33.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tauman R, O'Brien LM, Gozal D. Hypoxemia and obesity modulate plasma C-reactive protein and interleukin-6 levels in sleep-disordered breathing. Sleep Breath. 2007;11:77–84. doi: 10.1007/s11325-006-0085-7. [DOI] [PubMed] [Google Scholar]
- 19.Tam CS, Wong M, McBain R, et al. Inflammatory measures in children with obstructive sleep apnoea. J Paediatr Child Health. 2006;42:277–82. doi: 10.1111/j.1440-1754.2006.00854.x. [DOI] [PubMed] [Google Scholar]
- 20.Gozal D. Sleep, sleep disorders and inflammation in children. Sleep Med. 2009;1:S12–6. doi: 10.1016/j.sleep.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Dyugovskaya L, Lavie P, Lavie L. Phenotypic and functional characterization of blood gammadelta T cells in sleep apnea. Am J Respir Crit Care Med. 2003;168:242–9. doi: 10.1164/rccm.200210-1226OC. [DOI] [PubMed] [Google Scholar]
- 22.Dyugovskaya L, Lavie P, Hirsh M, et al. Activated CD8+ T-lymphocytes in obstructive sleep apnoea. Eur Respir J. 2005;25:820–8. doi: 10.1183/09031936.05.00103204. [DOI] [PubMed] [Google Scholar]
- 23.Dyugovskaya L, Lavie P, Lavie L. Lymphocyte activation as a possible measure of atherosclerotic risk in patients with sleep apnea. Ann N Y Acad Sci. 2005;1051:340–50. doi: 10.1196/annals.1361.076. [DOI] [PubMed] [Google Scholar]
- 24.Lavie L, Dyugovskaya L, Polyakov A. Biology of peripheral blood cells in obstructive sleep apnea--the tip of the iceberg. Arch Physiol Biochem. 2008;114:244–54. doi: 10.1080/13813450802306701. [DOI] [PubMed] [Google Scholar]
- 25.Serpero LD, Kheirandish-Gozal L, Dayyat E, et al. A mixed cell culture model for assessment of proliferation in tonsillar tissues from children with obstructive sleep apnea or recurrent tonsillitis. Laryngoscope. 2009;119:1005–10. doi: 10.1002/lary.20147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim J, Bhattacharjee R, Dayyat E, et al. Increased cellular proliferation and inflammatory cytokines in tonsils derived from children with obstructive sleep apnea. Pediatr Res. 2009;66:423–8. doi: 10.1203/PDR.0b013e3181b453e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khalyfa A, Capdevila OS, Buazza MO, et al. Genome-wide gene expression profiling in children with non-obese obstructive sleep apnea. Sleep Med. 2009;10:75–86. doi: 10.1016/j.sleep.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 28.Kim J, Bhattacharjee R, Khalyfa A, et al. DNA methylation in inflamma-tory genes among children with obstructive sleep apnea. Am J Respir Crit Care Med. 2012;185:330–8. doi: 10.1164/rccm.201106-1026OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakaguchi S, Miyara M, Costantino CM, et al. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 30.Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101:455–8. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 31.Sakaguchi S, Yamaguchi T, Nomura T, et al. Regulatory T cells and immune tolerance. Cell. 2008;133:775–87. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 32.Sakaguchi S, Setoguchi R, Yagi H, et al. Naturally arising FOXP3-expressing CD25+CD4+ regulatory T cells in self-tolerance and autoimmune disease. Curr Top Microbiol Immunol. 2006;305:51–66. doi: 10.1007/3-540-29714-6_3. [DOI] [PubMed] [Google Scholar]
- 33.Sakaguchi S, Wing K, Miyara M. Regulatory T cells - a brief history and perspective. Eur J Immunol. 2007;37:S116–23. doi: 10.1002/eji.200737593. [DOI] [PubMed] [Google Scholar]
- 34.Sakaguchi S, Wing K, Onishi Y, et al. Regulatory T cells: how do they suppress immune responses? Int Immunol. 2009;21:1105–11. doi: 10.1093/intimm/dxp095. [DOI] [PubMed] [Google Scholar]
- 35.Montgomery-Downs HE, O'Brien LM, Gulliver TE, et al. Polysomno-graphic characteristics in normal preschool and early school-aged children. Pediatrics. 2006;117:741–53. doi: 10.1542/peds.2005-1067. [DOI] [PubMed] [Google Scholar]
- 36.Iber C A-IS, Chesson A, Quan S for the American Academy of Sleep Medicine. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. 2007 [Google Scholar]
- 37.Maecker HT, McCoy JP, Nussenblatt R. Standardizing immunophenotyping for the Human Immunology Project. Nat Rev Immunol. 2012;12:191–200. doi: 10.1038/nri3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weinberg A, Song LY, Wilkening C, et al. Optimization and limitations of use of cryopreserved peripheral blood mononuclear cells for functional and phenotypic T-cell characterization. Clin Vaccine Immunol. 2009;16:1176–86. doi: 10.1128/CVI.00342-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wieczorek G, Asemissen A, Model F, et al. Quantitative DNA methylation analysis of FOXP3 as a new method for counting regulatory T cells in peripheral blood and solid tissue. Cancer Res. 2009;69:599–608. doi: 10.1158/0008-5472.CAN-08-2361. [DOI] [PubMed] [Google Scholar]
- 40.Wilson CB, Rowell E, Sekimata M. Epigenetic control of T-helper-cell differentiation. Nat Rev Immunol. 2009;9:91–105. doi: 10.1038/nri2487. [DOI] [PubMed] [Google Scholar]
- 41.Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive FOXP3+ regulatory T cells: more of the same or a division of labor? Immunity. 2009;30:626–35. doi: 10.1016/j.immuni.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 42.Josefowicz SZ, Niec RE, Kim HY, et al. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature. 2012;482:395–9. doi: 10.1038/nature10772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nguyen KD, Vanichsarn C, Fohner A, et al. Selective deregulation in chemokine signaling pathways of CD4+CD25(hi)CD127(lo)/(-) regulatory T cells in human allergic asthma. J Allergy Clin Immunol. 2009;123:933–9. doi: 10.1016/j.jaci.2008.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hartl D, Koller B, Mehlhorn AT, et al. Quantitative and functional impairment of pulmonary CD4+CD25hi regulatory T cells in pediatric asthma. J Allergy Clin Immunol. 2007;119:1258–66. doi: 10.1016/j.jaci.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 45.Taylor A, Verhagen J, Akdis CA, et al. T regulatory cells and allergy. Microbes Infect. 2005;7:1049–55. doi: 10.1016/j.micinf.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 46.Ramagopal M, Mehta A, Roberts DW, et al. Asthma as a predictor of obstructive sleep apnea in urban African-American children. J Asthma. 2009;46:895–99. doi: 10.3109/02770900903229636. [DOI] [PubMed] [Google Scholar]
- 47.Ramagopal M, Scharf SM, Roberts DW, et al. Obstructive sleep apnea and history of asthma in snoring children. Sleep Breath. 2008;12:381–92. doi: 10.1007/s11325-008-0174-x. [DOI] [PubMed] [Google Scholar]
- 48.Desager KN, Nelen V, Weyler JJ, et al. Sleep disturbance and daytime symptoms in wheezing school-aged children. J Sleep Res. 2005;14:77–82. doi: 10.1111/j.1365-2869.2004.00432.x. [DOI] [PubMed] [Google Scholar]
- 49.Sulit LG, Storfer-Isser A, Rosen CL, et al. Associations of obesity, sleep-disordered breathing, and wheezing in children. Am J Respir Crit Care Med. 2005;171:659–64. doi: 10.1164/rccm.200403-398OC. [DOI] [PubMed] [Google Scholar]
- 50.Malakasioti G, Gourgoulianis K, Chrousos G, et al. Interactions of obstructive sleep-disordered breathing with recurrent wheezing or asthma and their effects on sleep quality. Pediatr Pulmonol. 2011;46:1047–54. doi: 10.1002/ppul.21497. [DOI] [PubMed] [Google Scholar]
- 51.Kheirandish-Gozal L, Dayyat EA, Eid NS, et al. Obstructive sleep apnea in poorly controlled asthmatic children: effect of adenotonsillectomy. Pediatr Pulmonol. 2011;46:913–8. doi: 10.1002/ppul.21451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fitzpatrick AM, Teague WG, Meyers DA, et al. Heterogeneity of severe asthma in childhood: confirmation by cluster analysis of children in the National Institutes of Health/National Heart, Lung, and Blood Institute Severe Asthma Research Program. J Allergy Clin Immunol. 2011;127:382–9. doi: 10.1016/j.jaci.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mor A, Planer D, Luboshits G, et al. Role of naturally occurring CD4+ CD25+ regulatory T cells in experimental atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:893–900. doi: 10.1161/01.ATV.0000259365.31469.89. [DOI] [PubMed] [Google Scholar]
- 54.Ait-Oufella H, Taleb S, Mallat Z, et al. Cytokine network and T cell immunity in atherosclerosis. Semin Immunopathol. 2009;31:23–33. doi: 10.1007/s00281-009-0143-x. [DOI] [PubMed] [Google Scholar]
- 55.van Es T, van Puijvelde GH, Foks AC, et al. Vaccination against FOXP3(+) regulatory T cells aggravates atherosclerosis. Atherosclerosis. 2010;209:74–80. doi: 10.1016/j.atherosclerosis.2009.08.041. [DOI] [PubMed] [Google Scholar]
- 56.Foks AC, Frodermann V, ter Borg M, et al. Differential effects of regulatory T cells on the initiation and regression of atherosclerosis. Atherosclerosis. 2011;218:53–60. doi: 10.1016/j.atherosclerosis.2011.04.029. [DOI] [PubMed] [Google Scholar]
- 57.Maganto-Garcia E, Bu DX, Tarrio ML, et al. FOXP3+-inducible regulatory T cells suppress endothelial activation and leukocyte recruitment. J Immunol. 2011;187:3521–9. doi: 10.4049/jimmunol.1003947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barhoumi T, Kasal DA, Li MW, et al. T regulatory lymphocytes prevent angiotensin II-induced hypertension and vascular injury. Hypertension. 2011;57:469–76. doi: 10.1161/HYPERTENSIONAHA.110.162941. [DOI] [PubMed] [Google Scholar]
- 59.Ng DK, Wong JC, Chan CH, et al. Ambulatory blood pressure before and after adenotonsillectomy in children with obstructive sleep apnea. Sleep Med. 2010;11:721–5. doi: 10.1016/j.sleep.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 60.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–95. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 61.Song L, Leung C, Schindler C. Lymphocytes are important in early atherosclerosis. J Clin Invest. 2001;108:251–9. doi: 10.1172/JCI11380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mosmann TR, Cherwinski H, Bond MW, et al. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–57. [PubMed] [Google Scholar]
- 63.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–93. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 64.Frostegard J, Ulfgren AK, Nyberg P, et al. Cytokine expression in advanced human atherosclerotic plaques: dominance of pro-inflamma-tory (Th1) and macrophage-stimulating cytokines. Atherosclerosis. 1999;145:33–43. doi: 10.1016/s0021-9150(99)00011-8. [DOI] [PubMed] [Google Scholar]
- 65.Uyemura K, Demer LL, Castle SC, et al. Cross-regulatory roles of interleukin (IL)-12 and IL-10 in atherosclerosis. J Clin Invest. 1996;97:2130–38. doi: 10.1172/JCI118650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Szabo SJ, Sullivan BM, Peng SL, et al. Molecular mechanisms regulating Th1 immune responses. Annu Rev Immunol. 2003;21:713–58. doi: 10.1146/annurev.immunol.21.120601.140942. [DOI] [PubMed] [Google Scholar]
- 67.Whitman SC, Ravisankar P, Daugherty A. IFN-gamma deficiency exerts gender-specific effects on atherogenesis in apolipoprotein E-/- mice. J Interferon Cytokine Res. 2002;22:661–70. doi: 10.1089/10799900260100141. [DOI] [PubMed] [Google Scholar]
- 68.Gupta S, Pablo AM, Jiang X, et al. IFN-gamma potentiates atherosclerosis in ApoE knock-out mice. J Clin Invest. 1997;99:2752–61. doi: 10.1172/JCI119465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Laurat E, Poirier B, Tupin E, et al. In vivo downregulation of T helper cell 1 immune responses reduces atherogenesis in apolipoprotein E-knockout mice. Circulation. 2001;104:197–202. doi: 10.1161/01.cir.104.2.197. [DOI] [PubMed] [Google Scholar]
- 70.Soejima H, Irie A, Miyamoto S, et al. Preference toward a T-helper type 1 response in patients with coronary spastic angina. Circulation. 2003;107:2196–2200. doi: 10.1161/01.CIR.0000066317.23972.CE. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Results of the control samplea from each batch
Comparison between fresh and cryopreserved sample